Synthesis Amphiphilic One-Handed Helical Ladder Polymers with Circularly Polarized Luminescence
Abstract
1. Introduction
2. Results and Discussion
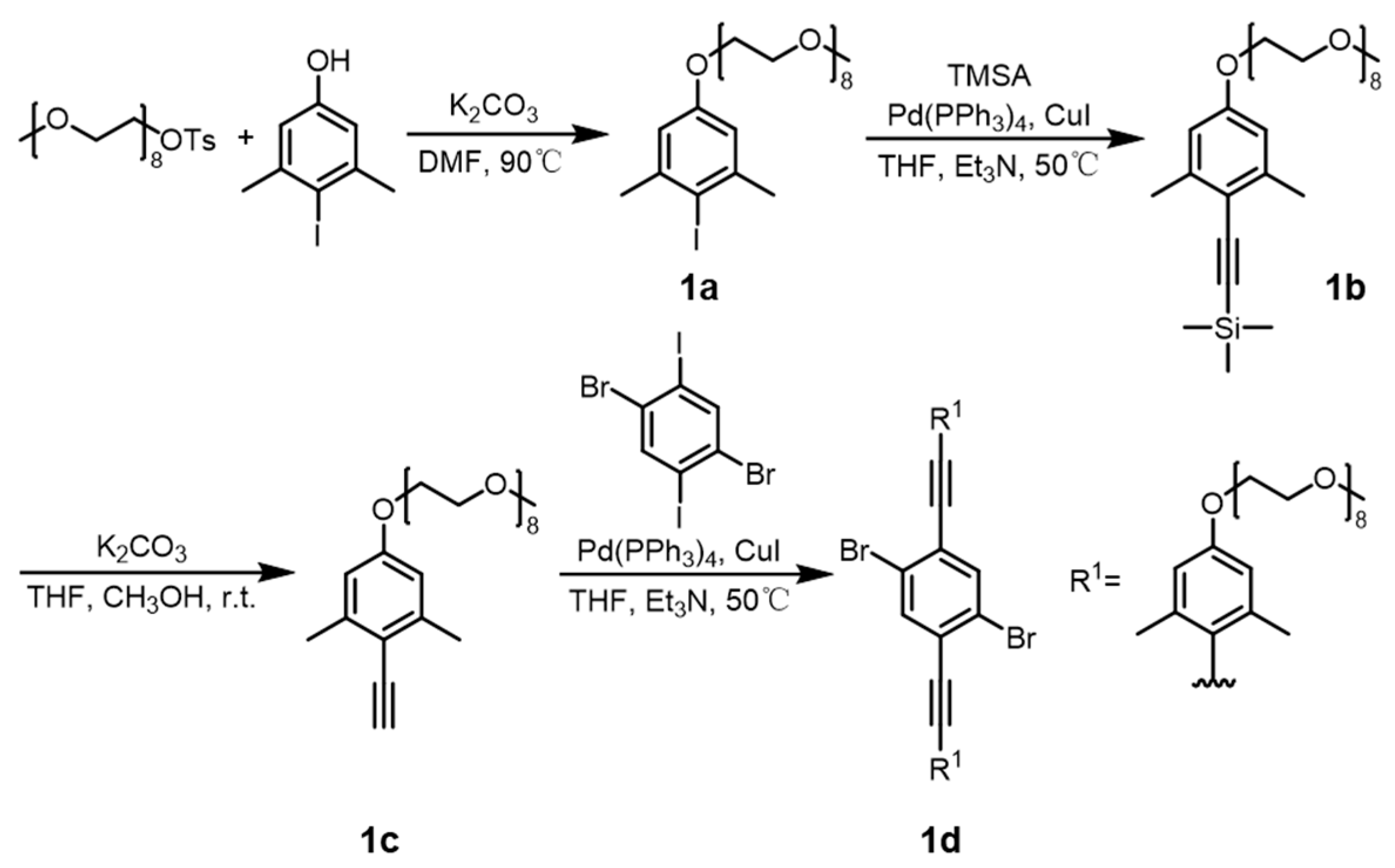
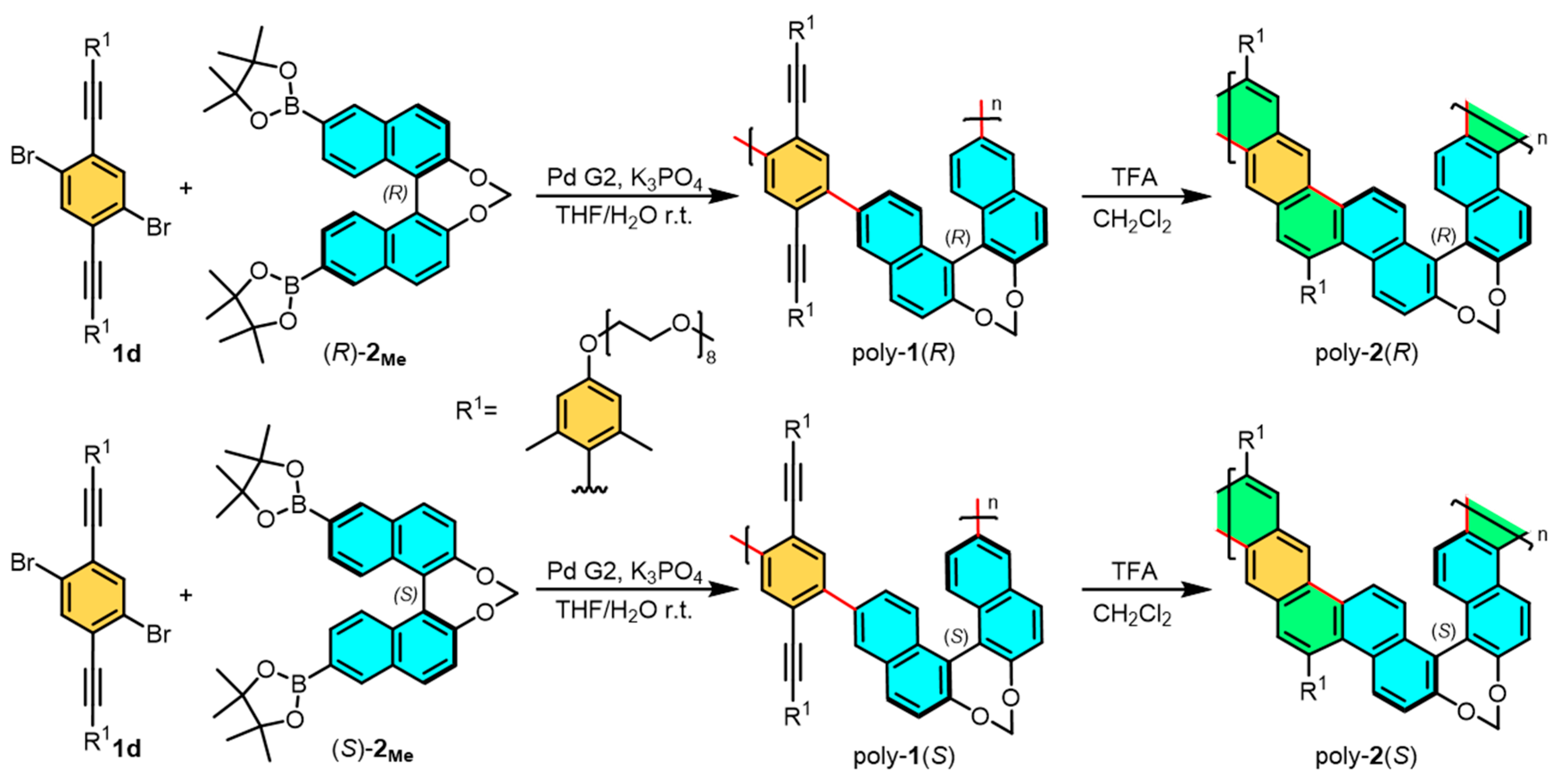
2.1. Synthesis of the Amphiphilic One-Handed Helical Ladder Polymers
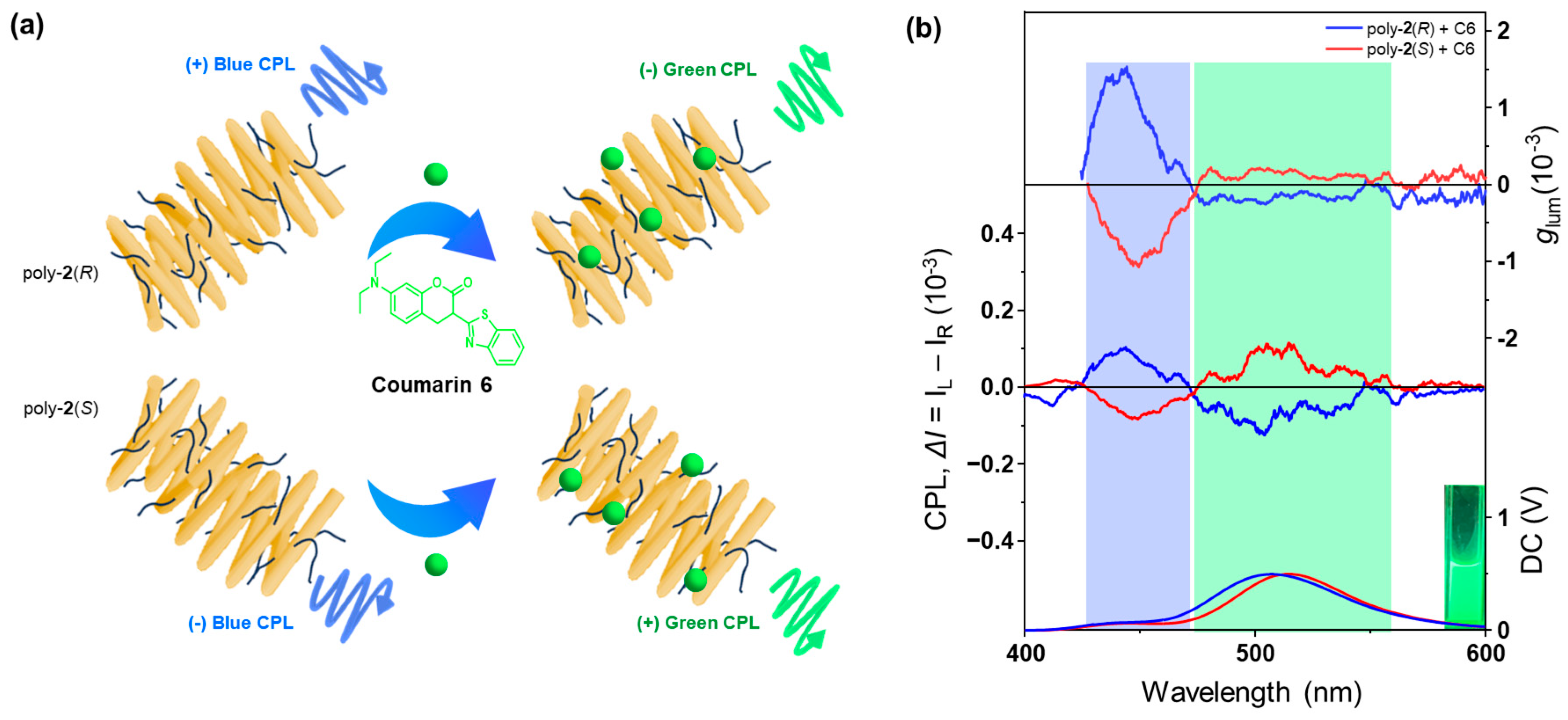
2.2. Chiroptical Properties of Amphiphilic One-Handed Helical Ladder Polymers
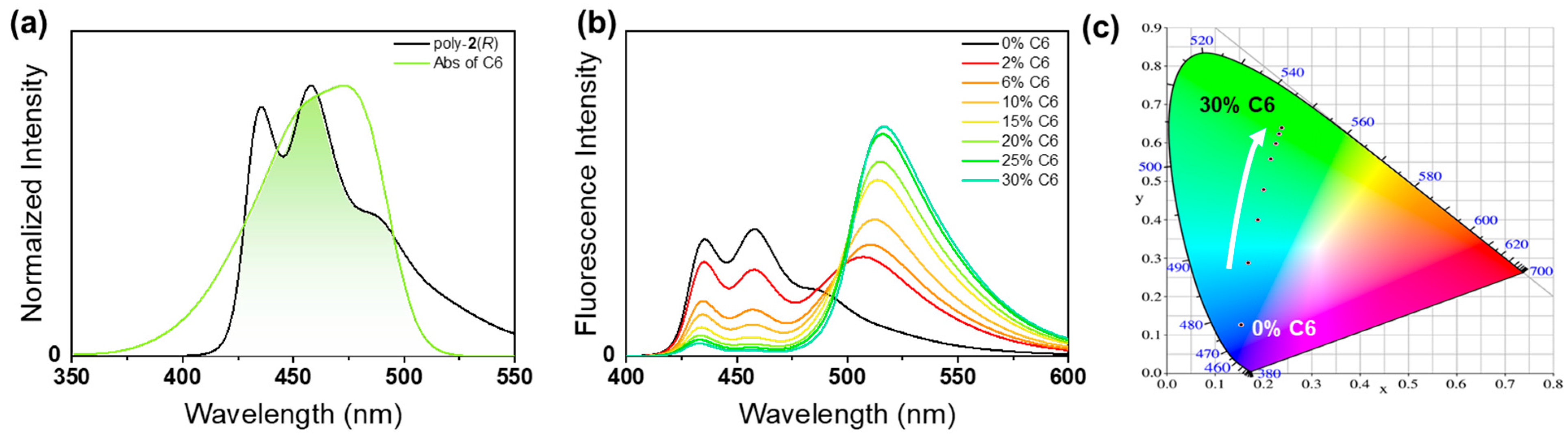
2.3. Construction and Optical Properties of Color-Tunable CPL Emission Systems Based on Amphiphilic One-Handed Helical Ladder Polymers
3. Instruments and Materials
3.1. Instruments
3.2. Materials
4. Synthetic Procedure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CD | Circular Dichroism |
| CPEL | Circularly Polarized Electroluminescence |
| CPL | Circularly Polarized Luminescence |
| DLS | Dynamic Light Scatter |
| SEM | Scanning Electron Microscope |
| TADF | Thermally Activated Delayed Fluorescence |
| TFA | Trifluoroacetic Acid |
| THF | Tetrahydrofuran |
| OEG | Oligo(Ethylene Glycol) |
| FRET | Förster Resonance Energy Transfer |
References
- Meng, Y.; Lyu, F.; Xu, X.; Zhang, L. Recent Advances in Chain Conformation and Bioactivities of Triple-Helix Polysaccharides. Biomacromol 2020, 21, 1653–1677. [Google Scholar] [CrossRef]
- Yashima, E.; Ousaka, N.; Taura, D.; Shimomura, K.; Ikai, T.; Maeda, K. Supramolecular Helical Systems: Helical Assemblies of Small Molecules, Foldamers, and Polymers with Chiral Amplification and Their Functions. Chem. Rev. 2016, 116, 13752–13990. [Google Scholar] [CrossRef]
- Zhang, G.; Cheng, X.; Wang, Y.; Zhang, W. Supramolecular chiral polymeric aggregates: Construction and applications. Aggregate 2022, 4, e262. [Google Scholar] [CrossRef]
- Wu, G. Recent advances in helical polyacetylene derivatives used as coated chiral stationary phases for enantioseparation. Polym. Chem. 2022, 13, 3036–3047. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, B.; Guo, J.; Pan, K.; Deng, J. Stimuli-responsive circularly polarized luminescent films with tunable emission. J. Mater. Chem. C 2020, 8, 1459–1465. [Google Scholar] [CrossRef]
- Clauss, Z.S.; Wardzala, C.L.; Schlirf, A.E.; Wright, N.S.; Saini, S.S.; Onoa, B.; Bustamante, C.; Kramer, J.R. Tunable, biodegradable grafting-from glycopolypeptide bottlebrush polymers. Nat. Commun. 2021, 12, 6472. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. The innate and adaptive immune systems. Mol. Bio. Cell 2015, 1297–1342. [Google Scholar]
- Preda, G.; Pasini, D. One-Handed Covalent Helical Ladder Polymers: The Dawn of a Tailorable Class of Chiral Functional Materials. Angew. Chem. Int. Ed. 2024, 62, e202407495. [Google Scholar] [CrossRef]
- Nehls, B.S.; Galbrecht, F.; Brauer, D.J.; Lehmann, C.W.; Scherf, U.; Farrell, T. Synthesis and characterization of a helical step-ladder polyarylene. J. Polym. Sci. Polym. Chem. 2006, 44, 5533–5545. [Google Scholar] [CrossRef]
- Ikai, T.; Yoshida, T.; Shinohara, K.-I.; Taniguchi, T.; Wada, Y.; Swager, T.M. Triptycene-Based Ladder Polymers with One-Handed Helical Geometry. J. Am. Chem. Soc. 2019, 141, 4696–4703. [Google Scholar] [CrossRef]
- Zheng, W.; Ikai, T.; Yashima, E. Synthesis of Single-Handed Helical Spiro-Conjugated Ladder Polymers through Quantitative and Chemoselective Cyclizations. Angew. Chem. Int. Ed. 2021, 60, 11294–11299. [Google Scholar] [CrossRef]
- Ammenhäuser, R.; Lupton, J.M.; Scherf, U. Chain-Length Dependence of the Optical Activity of Helical Triptycene-Based π-Conjugated Ladder Polymers. Adv. Optic. Mater. 2023, 12, 2301857. [Google Scholar] [CrossRef]
- Lee, J.; Kalin, A.J.; Yuan, T.; Al-Hashimi, M.; Fang, L. Fully conjugated ladder polymers. Chem. Sci. 2017, 8, 2503–2521. [Google Scholar] [CrossRef] [PubMed]
- Hirao, T.; Kishino, S.; Haino, T. Supramolecular chiral sensing by supramolecular helical polymers. Chem. Commun. 2023, 59, 2421–2424. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, W. Polymerization-induced Chiral Self-assembly for the In situ Construction, Modulation, Amplification and Applications of Asymmetric Suprastructures. Angew. Chem. Int. Ed. 2024, 63, 202414332. [Google Scholar] [CrossRef] [PubMed]
- Davydova, M.P.; Meng, L.; Rakhmanova, M.I.; Bagryanskaya, I.Y.; Sulyaeva, V.S.; Meng, H.; Artem’ev, A.V. Highly Emissive Chiral Mn(II) Bromide Hybrids for UV-Pumped Circularly Polarized LEDs and Scintillator Image Applications. Adv. Optic. Mater. 2023, 11, 2202811. [Google Scholar] [CrossRef]
- Xu, X.Q.; Li, W.J.; Zhang, D.Y.; Zhu, Y.; Xu, W.T.; Wang, Y.; Wang, X.Q.; Wang, W.; Yang, H.B. Chiral Rotaxane-Branched Dendrimers as Relays in Artificial Light-Harvesting Systems with Boosted Circularly Polarized Luminescence. Angew. Chem. Int. Ed. 2024, 64, e202419434. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Zhou, X.; Dai, H.; Wang, Y.; Xiao, Y. Thermoregulated CPL-Active Flexible Polymer/Perovskite Hybrid Materials with High Luminescence Dissymmetry Factor. Adv. Optic. Mater. 2024, 12, 2400066. [Google Scholar] [CrossRef]
- Ying, A.; Ai, Y.; Yang, C.; Gong, S. Aggregation-Dependent Circularly Polarized Luminescence and Thermally Activated Delayed Fluorescence from Chiral Carbene-CuI-Amide Enantiomers. Angew. Chem. Int. Ed. 2022, 61, e202210490. [Google Scholar] [CrossRef]
- Davydova, M.P.; Meng, L.; Rakhmanova, M.I.; Jia, Z.; Berezin, A.S.; Bagryanskaya, I.Y.; Lin, Q.; Meng, H.; Artem’ev, A.V. Strong Magnetically-Responsive Circularly Polarized Phosphorescence and X-Ray Scintillation in Ultrarobust Mn(II)–Organic Helical Chains. Adv. Mater. 2023, 35, e2303611. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, J.Y.; Xu, L.J.; Chen, Z.N. Chiral Copper(I) Iodide Cluster Hybrids Enabling Highly Efficient Circularly Polarized Electroluminescence. Adv. Funct. Mater. 2025, 2424704. [Google Scholar] [CrossRef]
- Davydova, M.P.; Xu, T.; Agafontsev, A.M.; Meng, L.; Wolff, M.; Petyuk, M.Y.; Bagryanskaya, I.Y.; Berezin, A.S.; Tkachev, A.V.; Meng, H.; et al. Toward Rhenium-Based Circularly Polarized OLEDs Using Tailored Chiral Re(CO)3 Emitters. Angew. Chem. Int. Ed. 2025, 64, e202419788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, G.; Li, D.; Jiang, Z.; Quan, Y.; Cheng, Y. Color-Tunable White Circularly Polarized Electroluminescence Triggered Using Chiral Co-Assembly-Sensitized Strategy. Laser Photonic Rev. 2024, 18, 2400223. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, Z.; Zhang, Y.; Xu, Z.; Li, H.; Cheng, Y.; Quan, Y. Deep Blue Circularly Polarized Luminescence Response Behavior of an Achiral Pyrene-Based Emitter Regulated by Chiral Co-assembly Helical Nanofibers. J. Phys. Chem. Lett. 2021, 12, 3767–3772. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, B.; Deng, J. Helical Polymer Working as a Chirality Amplifier to Generate and Modulate Multicolor Circularly Polarized Luminescence in Small Molecular Fluorophore/Polymer Composite Films. ACS Cent. Sci. 2023, 9, 1409–1418. [Google Scholar] [CrossRef]
- Ikai, T.; Mishima, N.; Matsumoto, T.; Miyoshi, S.; Oki, K.; Yashima, E. 2,2′-Tethered Binaphthyl-Embedded One-Handed Helical Ladder Polymers: Impact of the Tether Length on Helical Geometry and Chiroptical Property. Angew. Chem. Int. Ed. 2024, 63, 202318712. [Google Scholar] [CrossRef]
- Trilling, F.; Ausländer, M.-K.; Scherf, U. Ladder-Type Polymers and Ladder-Type Polyelectrolytes with On-Chain Dibenz[a,h]anthracene Chromophores. Macromol 2019, 52, 3115–3122. [Google Scholar] [CrossRef]
- Lee, J.; Li, H.; Kalin, A.J.; Yuan, T.; Wang, C.; Olson, T.; Li, H.; Fang, L. Extended Ladder-Type Benzo[k]tetraphene-Derived Oligomers. Angew. Chem. Int. Ed. 2017, 56, 13727–13731. [Google Scholar] [CrossRef]
- Sahoo, H. Förster resonance energy transfer—A spectroscopic nanoruler: Principle and applications. J. Photoch. Photobio. C Photochem. Rev. 2011, 12, 20–30. [Google Scholar] [CrossRef]
- Pan, M.; Zhang, G.; Ma, H.; Cheng, X.; Li, J.; Zhang, W. In situ thermoresponsive supramolecular assembly for switchable circularly polarized luminescence. Sci. Chi. Chem. 2024, 67, 2362–2372. [Google Scholar] [CrossRef]
- Yang, D.; Duan, P.; Zhang, L.; Liu, M. Chirality and energy transfer amplified circularly polarized luminescence in composite nanohelix. Nat. Commun. 2017, 8, 15727. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Liu, G. Full-Color Circularly Polarized Luminescence of Supramolecular Polymers with Handedness Inversion Regulated by Anion and Temperature. ACS Nano 2024, 18, 2279–2289. [Google Scholar] [CrossRef] [PubMed]
- Mitzel, F.; FitzGerald, S.; Beeby, A.; Faust, R. Acetylenic Quinoxalinoporphyrazines as Photosensitisers for Photodynamic Therapy. Chem. Eur. J. 2003, 9, 1233–1241. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Zheng, W. Synthesis Amphiphilic One-Handed Helical Ladder Polymers with Circularly Polarized Luminescence. Molecules 2025, 30, 2606. https://doi.org/10.3390/molecules30122606
Pan Z, Zheng W. Synthesis Amphiphilic One-Handed Helical Ladder Polymers with Circularly Polarized Luminescence. Molecules. 2025; 30(12):2606. https://doi.org/10.3390/molecules30122606
Chicago/Turabian StylePan, Ziheng, and Wei Zheng. 2025. "Synthesis Amphiphilic One-Handed Helical Ladder Polymers with Circularly Polarized Luminescence" Molecules 30, no. 12: 2606. https://doi.org/10.3390/molecules30122606
APA StylePan, Z., & Zheng, W. (2025). Synthesis Amphiphilic One-Handed Helical Ladder Polymers with Circularly Polarized Luminescence. Molecules, 30(12), 2606. https://doi.org/10.3390/molecules30122606





