pH-Sensitive Multiliposomal Containers for Encapsulation and Rapid Release of Bioactive Substances
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characteristics of Cationic and Anionic Liposomes
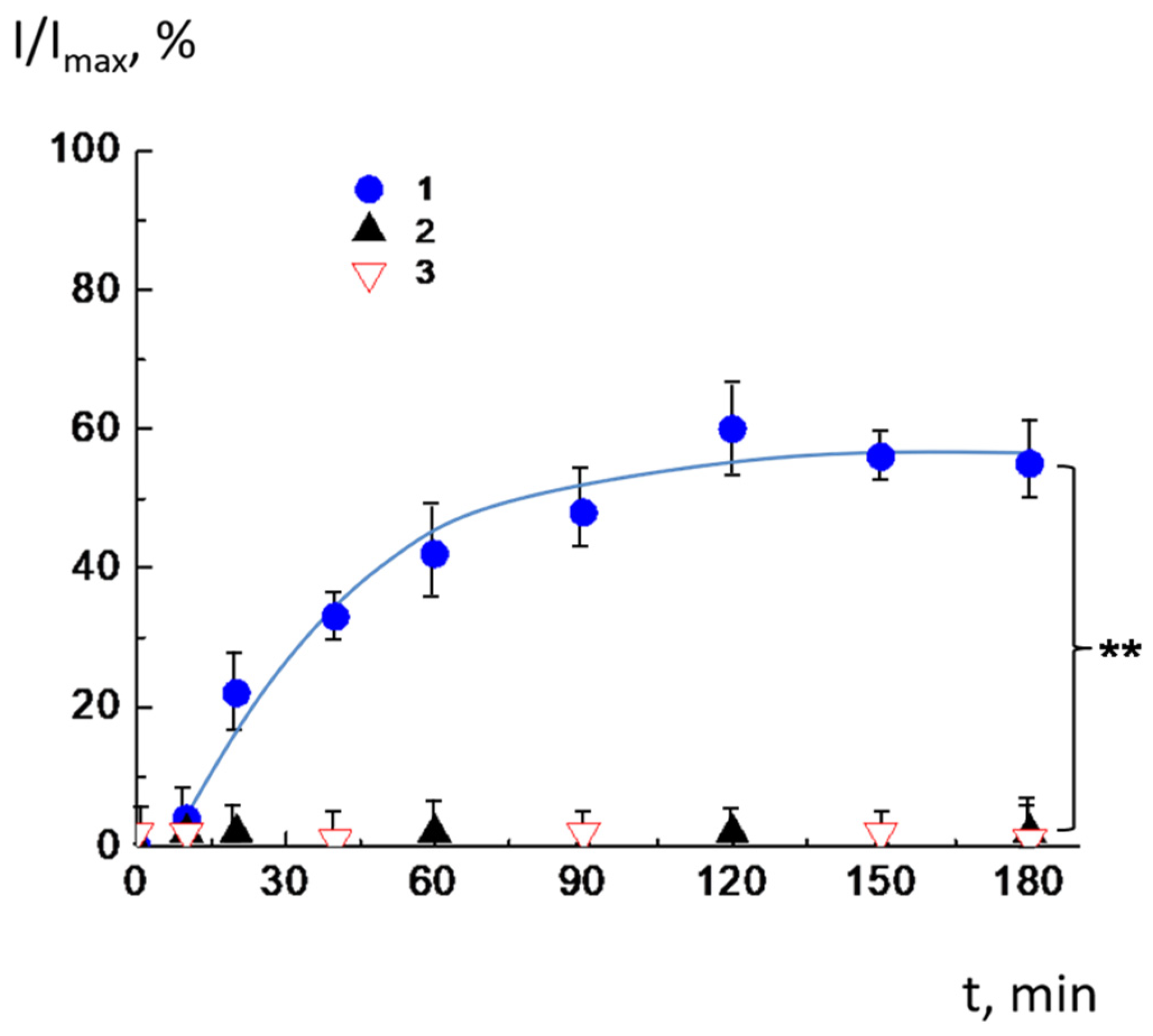
2.2. Release of the Cargo by CL2−/DOPC/AMS Liposomes Under Changes in pH
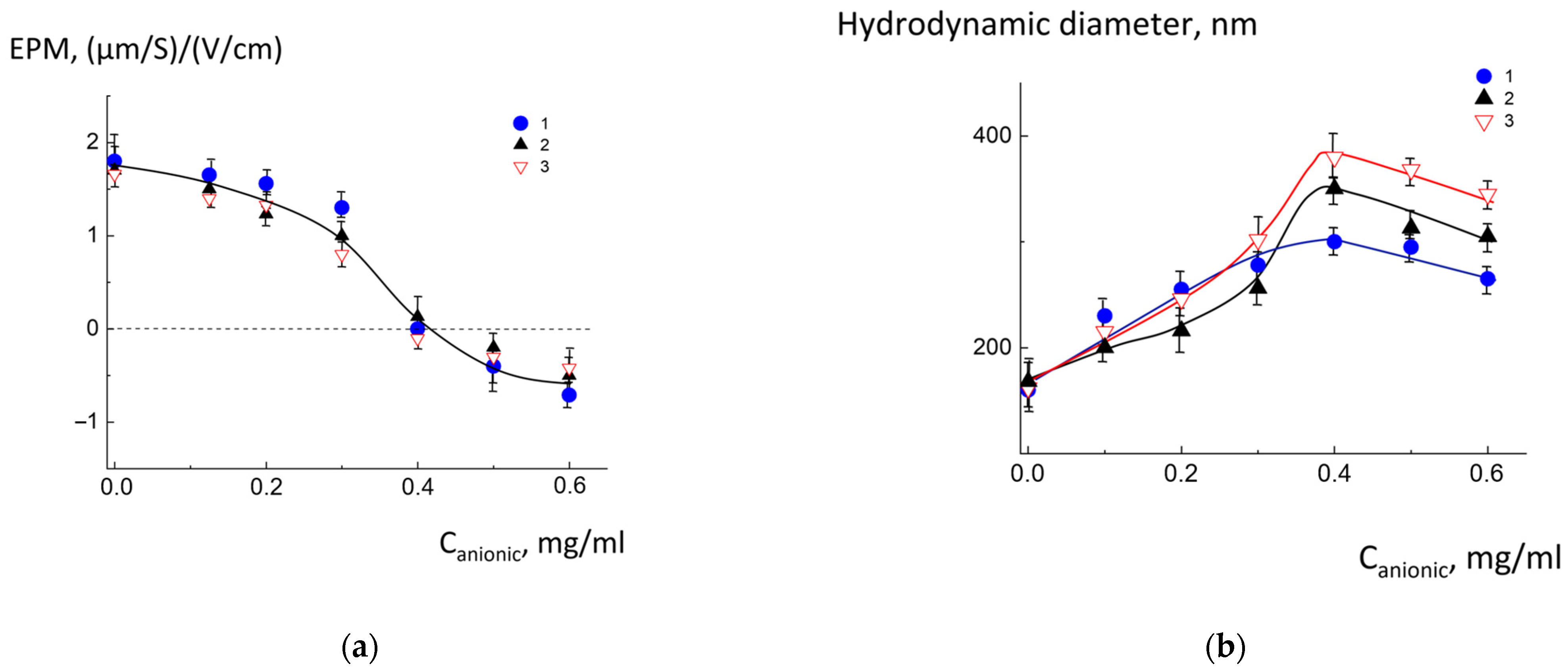
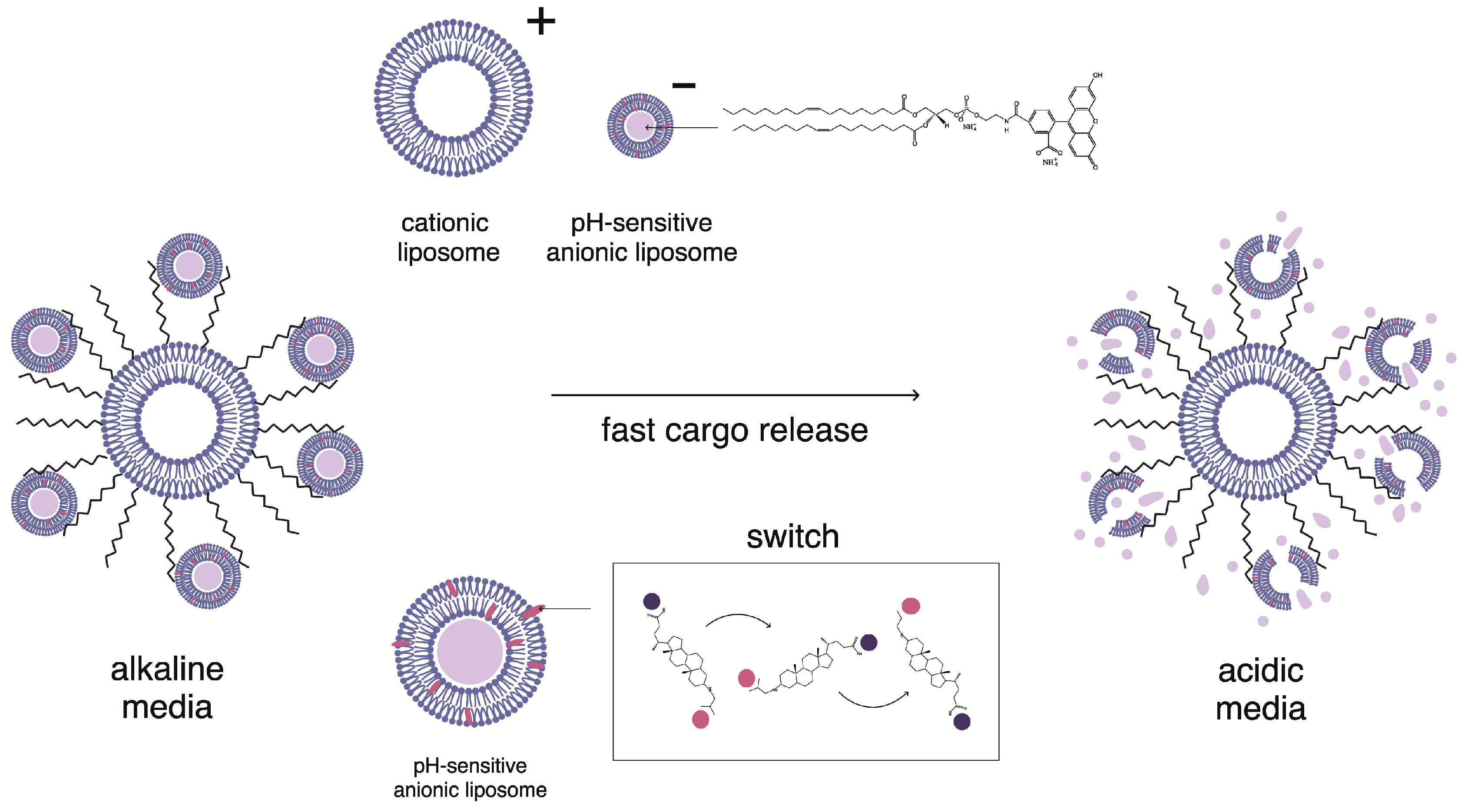
2.3. Complexation of pH-Sensitive Anionic Liposomes with Cationic Liposomes
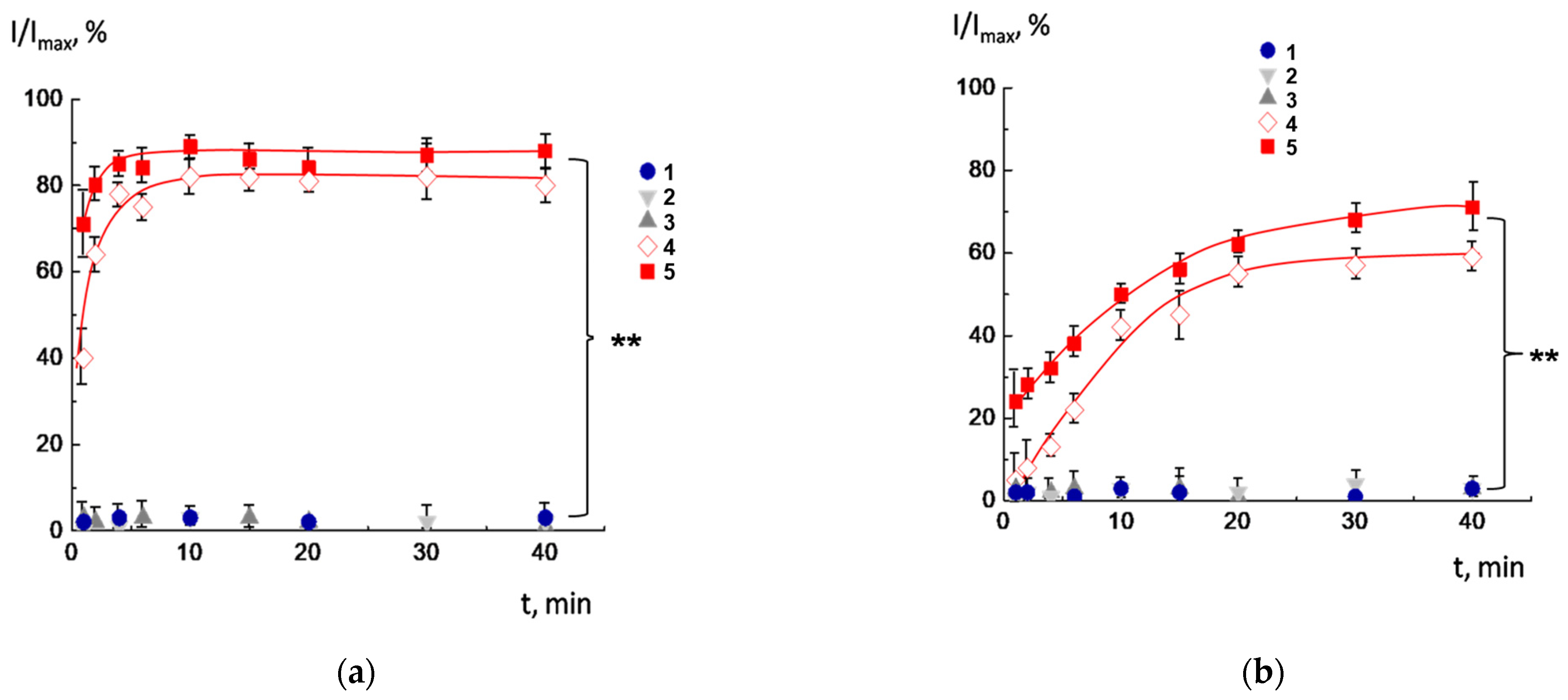
2.4. Integrity of CL2−/DOPC/AMS Lipid Membranes in Multiliposomal Complexes
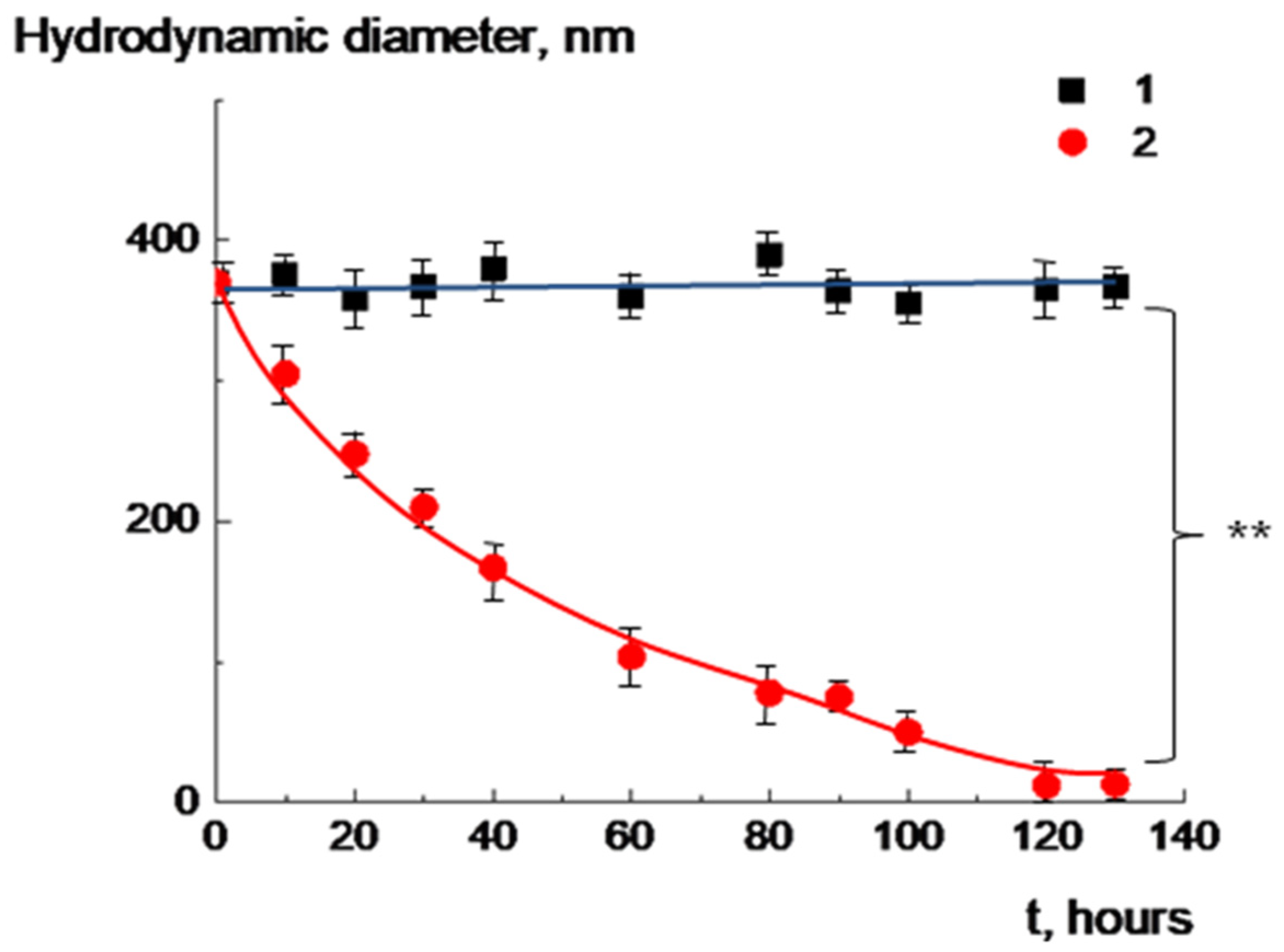
2.5. Biodegradation of Multiliposomal Complexes
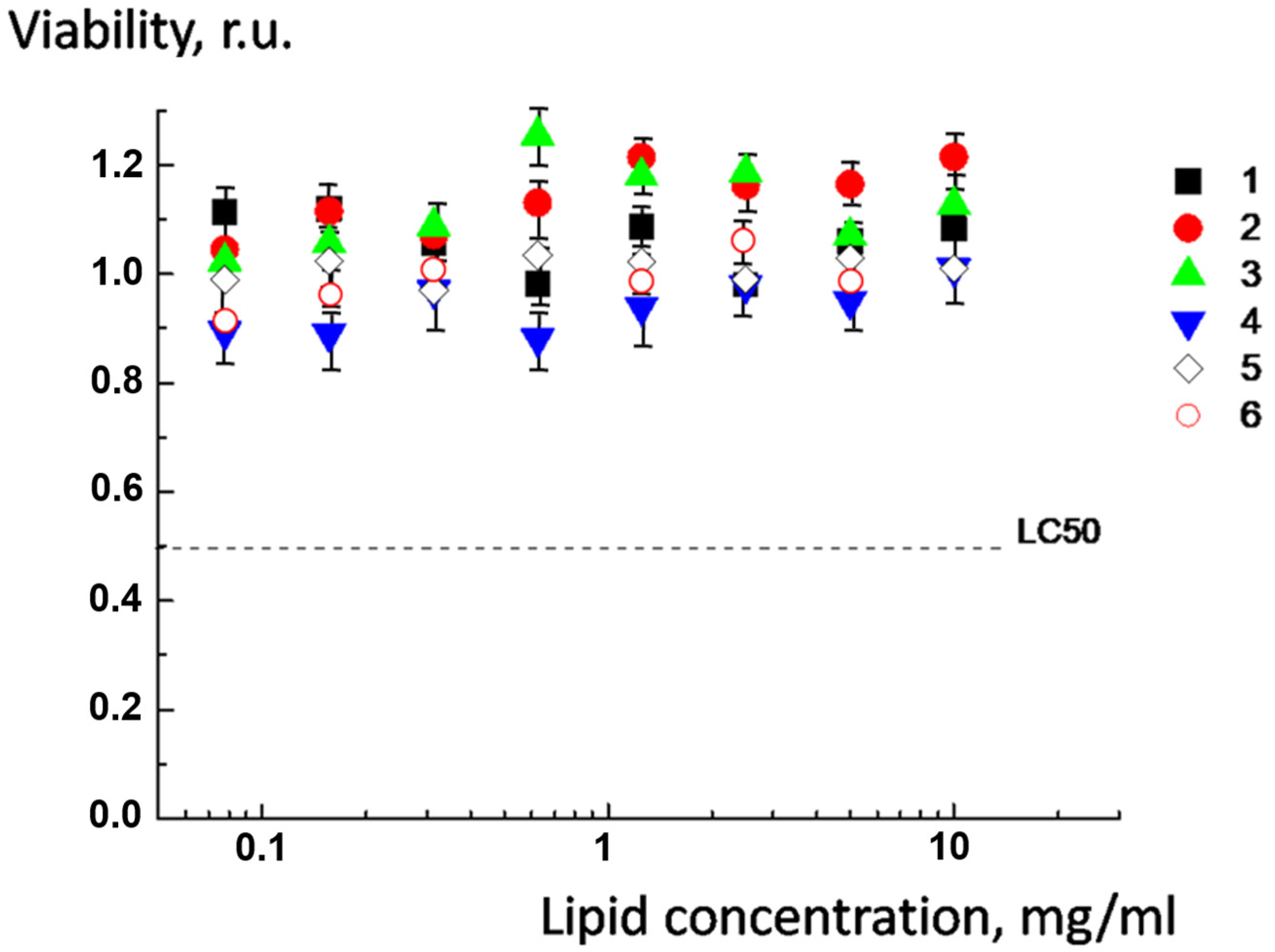
2.6. Cytotoxicity of pH-Sensitive Multiliposomal Complexes and the Products of Their Biodegradation
3. Materials and Methods
3.1. Chemicals
3.2. General Procedures
3.2.1. Synthesis of pH-Sensitive Anionic Liposomes
3.2.2. Synthesis of Cationic Liposomes
3.3. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, X.; Berger, M.F.; Solit, D.B. Precision oncology: Current and future platforms for treatment selection. Trends Cancer 2024, 10, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Bhardwaj, A.; Gupta, S. Cancer treatment therapies: Traditional to modern approaches to combat cancers. Mol. Biol. Rep. 2023, 50, 9663–9676. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Greeny, A.; Nandan, A.; Sah, R.K.; Jose, A.; Dyawanapelly, S.; Junnuthula, V.; KV, A.; Sadanandan, P. Advanced drug delivery and therapeutic strategies for tuberculosis treatment. J. Nanobiotechnol. 2023, 21, 414. [Google Scholar] [CrossRef]
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4. [Google Scholar] [CrossRef]
- Liu, W.; Ye, A.; Han, F.; Han, J. Advances and challenges in liposome digestion: Surface interaction, biological fate, and GIT modeling. Adv. Colloid Interface Sci. 2019, 263, 52. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640. [Google Scholar] [CrossRef]
- Basak, S.; Das, T.K. Zwitterionic, Stimuli-Responsive Liposomes for Curcumin Drug Delivery: Enhancing M2 Macrophage Polarization and Reducing Oxidative Stress through Enzyme-Specific and Hyperthermia-Triggered Release. ACS Appl. Bio Mater. 2025, 8, 726–740. [Google Scholar] [CrossRef]
- Zhao, Z.; Feng, Y.; Xiang, J.; Liu, J.; Piao, Y.; Shao, S.; Tang, J.; Zhou, Z.; Shen, Y. Screening of Zwitterionic Liposomes with Red Blood Cell-Hitchhiking and Tumor Cell-Active Transporting Capability for Efficient Tumor Entrance. Adv. Funct. Mater. 2023, 33, 2214369. [Google Scholar] [CrossRef]
- Karanth, H.; Murthy, R.S. pH-sensitive liposomes-principle and application in cancer therapy. J. Pharm. Pharmacol. 2007, 59, 469–483. [Google Scholar] [CrossRef]
- Antoniou, A.I.; Giofrè, S.; Seneci, P.; Passarella, D.; Pellegrino, S. Stimulus-responsive liposomes for biomedical applications. Drug Discov. Today 2021, 26, 1794–1824. [Google Scholar] [CrossRef]
- Helmlinger, G.; Sckell, A.; Dellian, M.; Forbes, N.S.; Jain, R.K. Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin. Cancer Res. 2002, 8, 1284–1291. [Google Scholar] [PubMed]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu. Rev. Biomed. Eng. 2006, 8, 343–375. [Google Scholar] [CrossRef] [PubMed]
- Yaroslavov, A.; Efimova, A.; Smirnova, N.; Erzunov, D.; Lukashev, N.; Grozdova, I.; Melik-Nubarov, N. A novel approach to a controlled opening of liposomes. Colloids Surf. B Biointerfaces 2020, 190, 110906. [Google Scholar] [CrossRef]
- Popov, A.S.; Efimova, A.A.; Kazantsev, A.V.; Erzunov, D.A.; Lukashev, N.V.; Grozdova, I.D.; Melik-Nubarov, N.S.; Yaroslavov, A.A. pH-Sensitive liposomes with embedded ampholytic derivatives of cholan-24-oic acid. Mendeleev Commun. 2021, 31, 827. [Google Scholar] [CrossRef]
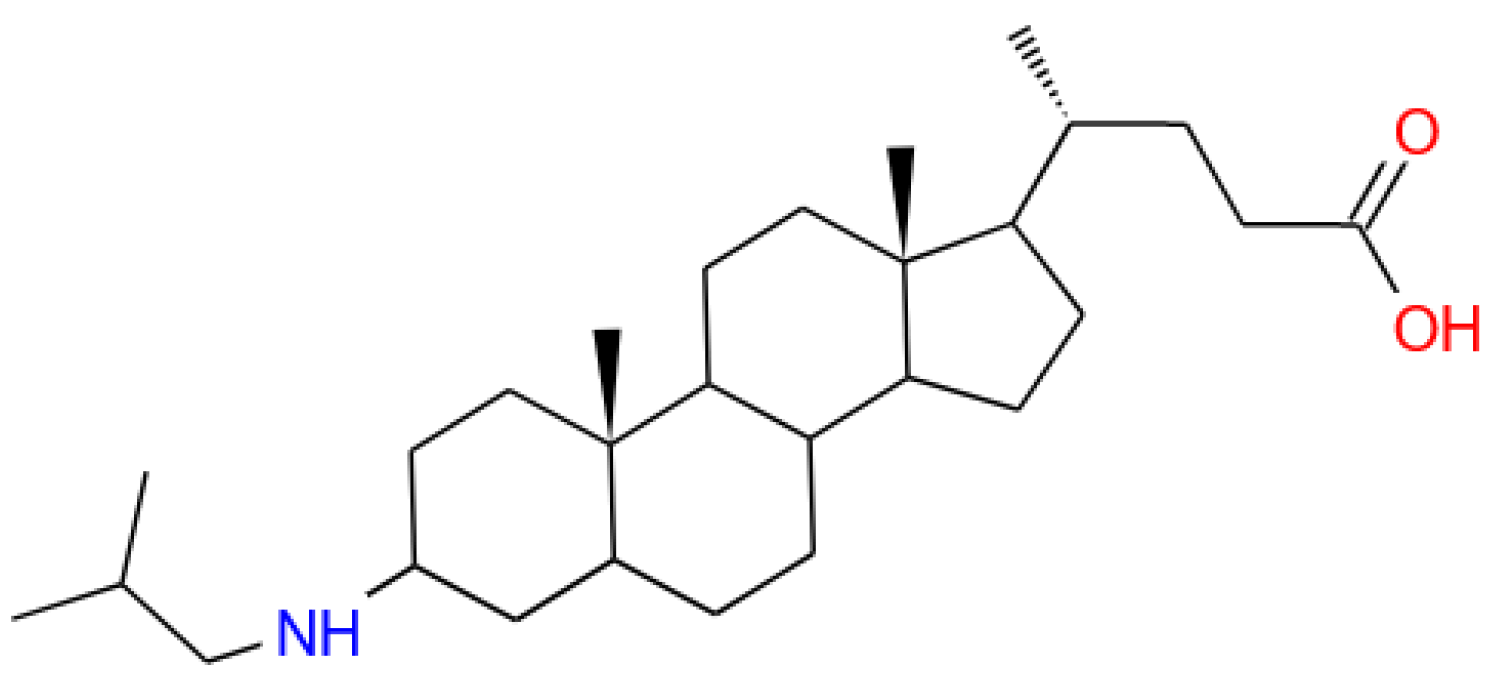
- Efimova, A.A.; Popov, A.S.; Kazantsev, A.V.; Semenyuk, P.I.; Le-Deygen, I.M.; Lukashev, N.V.; Yaroslavov, A.A. pH-Sensitive Liposomes with Embedded 3-(isobutylamino)cholan-24-oic Acid: What Is the Possible Mechanism of Fast Cargo Release? Membranes 2023, 13, 407. [Google Scholar] [CrossRef]
- Yaroslavov, A.A.; Efimova, A.A.; Mulashkin, F.D.; Rudenskaya, G.N.; Krivtsov, G.G. Biodegradable liposome–chitosan complexes: Enzyme-mediated release of encapsulated substances. Mendeleev Commun. 2018, 28, 140. [Google Scholar] [CrossRef]
- Yaroslavov, A.A.; Efimova, A.A.; Krasnikov, E.A.; Trosheva, K.S.; Popov, A.S.; Melik-Nubarov, N.S.; Krivtsov, G.G. Chitosan-based multi-liposomal complexes: Synthesis, biodegradability and cytotoxicity. Int. J. Biol. Macromol. 2021, 177, 455. [Google Scholar] [CrossRef]
- Yaroslavov, A.A.; Efimova, A.A.; Abramova, T.A.; Pozdyshev, D.V.; Muronetz, V.I. Multi-compartment containers from a mixture of natural and synthetic lipids. Mendeleev Commun. 2023, 33, 221. [Google Scholar] [CrossRef]
- Knidri, H.E.; Dahmani, J.; Addaou, A.; Laajeb, A.; Lahsini, A. Rapid and efficient extraction of chitin and chitosan for scale-up production: Effect of process parameters on deacetylation degree and molecular weight. Int. J. Biol. Macromol. 2019, 139, 1092. [Google Scholar] [CrossRef]
- Ishihara, H. Current status and prospects of polyethyleneglycol-modified medicines. Biol. Pharm. Bull. 2013, 36, 883. [Google Scholar] [CrossRef]
- Zalba, S.; Ten Hagen, T.L.M.; Burgui, C.; Garrido, M.J. Stealth nanoparticles in oncology: Facing the PEG dilemma. J. Control. Release 2022, 351, 22. [Google Scholar] [CrossRef] [PubMed]
- Samaridou, E.; Walgrave, H.; Salta, E.; Álvarez, D.M.; Castro-López, V.; Loza, M.; Alonso, M.J. Nose-to-brain delivery of enveloped RNA—Cell permeating peptide nanocomplexes for the treatment of neurodegenerative diseases. Biomaterials 2020, 230, 119657. [Google Scholar] [CrossRef]
- Mosiane, K.S.; Nweke, E.E.; Balogun, M.; Fru, P.N. Polyethyleneglycol-Betulinic Acid (PEG-BA) Polymer-Drug Conjugate Induces Apoptosis and Antioxidation in a Biological Model of Pancreatic Cancer. Polymer 2023, 15, 448. [Google Scholar] [CrossRef]
- Tenchov, R.; Sasso, J.M.; Zhou, Q.A. PEGylated Lipid Nanoparticle Formulations: Immunological Safety and Efficiency Perspective. Bioconjug. Chem. 2023, 34, 941. [Google Scholar] [CrossRef]
- Sadzuka, Y.; Kishi, K.; Hirota, S.; Sonobe, T. Effect of polyethyleneglycol (PEG) chain on cell uptake of PEG-modified liposomes. J. Liposome Res. 2003, 13, 157. [Google Scholar] [CrossRef]
- Zhang, J.X.; Zalipsk, Y.S.; Mullah, N.; Pecha, R.M.; Allen, T.M. Pharmaco attributes of dioleoylphosphatidylethanolamine/cholesterylhemisuccinate liposomes containing different types of cleavable lipopolymers. Pharmacol. Res. 2004, 49, 185. [Google Scholar] [CrossRef]
- Izumi, K.; Ji, J.; Koiwai, K.; Kawano, R. Long-Term Stable Liposome Modified by PEG-Lipid in Natural Seawater. ACS Omega 2024, 9, 10958. [Google Scholar] [CrossRef]
- Grozdova, I.; Melik-Nubarov, N.; Efimova, A.; Ezhov, A.; Krivtsov, G.; Litmanovich, E.; Yaroslavov, A. Intracellular delivery of drugs by chitosan-based multi-liposomal complexes. Colloids Surf. B 2020, 193, 111062. [Google Scholar] [CrossRef]
- Cocucci, E.; Kim, J.Y.; Bai, Y.; Pabla, N. Role of Passive Diffusion, Transporters, and Membrane Trafficking-Mediated Processes in Cellular Drug Transport. Clin. Pharmacol. Ther. 2017, 101, 121. [Google Scholar] [CrossRef] [PubMed]
- Mackarel, A.J.; Russell, K.J.; Brady, C.S.; FitzGerald, M.X.; O’Connor, C.M. Interleukin-8 and leukotriene-B(4), but not formylmethionyl leucylphenylalanine, stimulate CD18-independent migration of neutrophils across human pulmonary endothelial cells in vitro. Am. J. Respir. Cell Mol. Biol. 2000, 23, 154. [Google Scholar] [CrossRef] [PubMed]
- Borbás, E.; Sinkó, B.; Tsinman, O.; Tsinman, K.; Kiserdei, É.; Démuth, B.; Balogh, A.; Bodák, B.; Domokos, A.; Dargó, G.; et al. Investigation and Mathematical Description of the Real Driving Force of Passive Transport of Drug Molecules from Supersaturated Solutions. Mol. Pharm. 2016, 13, 3816. [Google Scholar] [CrossRef]
- Efimova, A.A.; Mulashkin, F.D.; Rudenskaya, G.N.; Evtushenko, E.G.; Orlov, V.N.; Melik-Nubarov, N.S.; Krivtsov, G.G.; Yaroslavov, A.A. Biodegradable Electrostatic Complexes of Chitosan Cationic Microparticles and Anionic Liposomes. Polym. Sci. Ser. B 2018, 60, 84. [Google Scholar] [CrossRef]
- Sandoval, G.; Herrera-López, E.J. Lipase, Phospholipase, and Esterase Biosensors (Review). Methods Mol. Biol. 2018, 1835, 391. [Google Scholar]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell Proliferation and Cytotoxicity Assays. Cur. Pharm. Biotechnol. 2016, 17, 1213–1221. [Google Scholar] [CrossRef]
- van Tonder, A.; Joubert, A.M.; Cromarty, A.D. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res. Notes 2015, 8, 47. [Google Scholar] [CrossRef]
- Tolosa, L.; Donato, M.T.; Gómez-Lechón, M.J. General Cytotoxicity Assessment by Means of the MTT Assay. Methods Mol. Biol. 2015, 1250, 333–348. [Google Scholar] [CrossRef]
- Mendez, R. Sonication-Based Basic Protocol for Liposome Synthesis. Methods Mol. Biol. 2023, 2625, 365–370. [Google Scholar] [CrossRef]
- Dymek, M.; Sikora, E. Liposomes as biocompatible and smart delivery systems—The current state. Adv. Colloid Interface Sci. 2022, 309, 102757. [Google Scholar] [CrossRef]

| Composition of the Lipid Membrane | Hydrodynamic Diameter, nm | PDI | EPM, (μm/s)/(V/cm) |
|---|---|---|---|
| Anionic liposomes* | |||
| CL2−/DOPC/AMS (1/8/1) | 40 ± 10 | 0.17 ± 0.06 | −2.6 ± 0.2 |
| Cationic liposomes* | |||
| DOTAP1+/DPPC/DPPE-PEG (1/8.5/0.5) | 160 ± 17 | 0.11 ± 0.03 | +1.8 ± 0.2 |
| DOTAP1+/DPPC/DPPE-PEG (1/8/1) | 162 ± 19 | 0.10 ± 0.04 | +1.7 ± 0.2 |
| DOTAP1+/DPPC/DPPE-PEG (1/7/2) | 168 ± 15 | 0.12 ± 0.05 | +1.7 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efimova, A.A.; Abramova, T.A.; Yatsenko, I.V.; Kazantsev, A.V.; Pozdyshev, D.V.; Lukashev, N.V.; Muronets, V.I.; Yaroslavov, A.A. pH-Sensitive Multiliposomal Containers for Encapsulation and Rapid Release of Bioactive Substances. Molecules 2025, 30, 2608. https://doi.org/10.3390/molecules30122608
Efimova AA, Abramova TA, Yatsenko IV, Kazantsev AV, Pozdyshev DV, Lukashev NV, Muronets VI, Yaroslavov AA. pH-Sensitive Multiliposomal Containers for Encapsulation and Rapid Release of Bioactive Substances. Molecules. 2025; 30(12):2608. https://doi.org/10.3390/molecules30122608
Chicago/Turabian StyleEfimova, Anna A., Tatyana A. Abramova, Igor V. Yatsenko, Alexey V. Kazantsev, Denis V. Pozdyshev, Nikolay V. Lukashev, Vladimir I. Muronets, and Alexander A. Yaroslavov. 2025. "pH-Sensitive Multiliposomal Containers for Encapsulation and Rapid Release of Bioactive Substances" Molecules 30, no. 12: 2608. https://doi.org/10.3390/molecules30122608
APA StyleEfimova, A. A., Abramova, T. A., Yatsenko, I. V., Kazantsev, A. V., Pozdyshev, D. V., Lukashev, N. V., Muronets, V. I., & Yaroslavov, A. A. (2025). pH-Sensitive Multiliposomal Containers for Encapsulation and Rapid Release of Bioactive Substances. Molecules, 30(12), 2608. https://doi.org/10.3390/molecules30122608






