1. Introduction
The physical properties of most polymeric materials are highly sensitive to environmental conditions, which poses significant challenges for their practical applications. In particular, the presence of moisture can induce plasticization and softening of polymeric materials owing to water absorption [
1], making this a major concern for commodity plastics, excluding polyolefins, which exhibit relatively low moisture affinity. Because it is practically impossible to achieve complete moisture removal from solid-state polymers, environmental deterioration owing to water absorption must be taken into account for practical applications. A pragmatic strategy for mitigating this issue is to regulate the absorbed moisture content in the polymer, thereby achieving the desired mechanical performance. To this end, systematic investigations into the effects of water absorption on polymer mechanics are essential. However, maintaining and controlling a fixed moisture content within water-absorbed polymer solids remain technically challenging. In previous studies [
2], we successfully controlled the water uptake of glassy poly(methyl methacrylate) (PMMA) by leveraging the strong water affinity of lithium through the addition of lithium salts to the polymer. In this study, we use this system to investigate the mechanism by which water absorption enhances the drawability of glassy polymers during the stretching process.
PMMA is a typical glassy polymer that is well known for its outstanding optical transparency among commodity plastics. It is widely used in applications such as optical lenses and aquariums, and it even shows potential for automotive glazing. However, the polar ester groups within PMMA readily form hydrogen bonds with water molecules [
3]. This makes PMMA susceptible to significant reductions in stiffness and glass transition temperature upon moisture uptake, resulting in pronounced plasticization and softening effects. Numerous studies have investigated the effect of environmental humidity on the mechanical properties of PMMA, including its tensile properties [
4,
5,
6,
7,
8], scratch resistance [
9], crack propagation behavior [
10,
11,
12], fracture characteristics [
13], and fatigue resistance [
14]. These studies collectively highlight the instability of the mechanical performance of PMMA under varying moisture conditions [
15]. Nevertheless, from an application-oriented perspective, the moisture sensitivity of PMMA could be leveraged to overcome its intrinsic brittleness, thereby broadening its functional versatility.
In general, the incorporation of metal salts in polymer matrices has been extensively reported as a strategy for inducing ionic cross-linking, which modulates the mechanical and viscoelastic behavior of ionomers. For example, Yoshikawa et al. [
16] demonstrated that the viscosity of ionized polystyrene/polyamide 6 blends is influenced by the type of ionic species. Takahashi et al. [
17] showed that the elongational viscosity in poly(ethylene-co-methacrylic acid) ionomers is enhanced in the presence of ionic interactions with added metal salts. Storey and co-workers [
18] reported that metal salts can act as ionic plasticizers in ionomer systems. Miyagawa et al. [
14] extended this approach to glassy polymers, showing that doping PMMA with LiCF
3SO
3 increases its glass transition temperature via ion–dipole interactions while preserving high transparency. In previous works, we systematically studied the effects of incorporating various lithium salts—including LiCF
3SO
3, LiC
4F
9SO
3, LiClO
4, LiN(CF
3SO
2)
2, LiBr, LiCl, and LiI—in PMMA. These salts were found to associatively interact with the polymer chains and to finely disperse within the PMMA matrix as “pinning sites”, leading to an increase in the glass transition temperature [
15,
19,
20,
21,
22,
23]. Furthermore, owing to their high hygroscopicity, these salts concentrate absorbed water in their vicinity, enabling control of the water content—and consequently the mechanical properties—of PMMA by adjusting the type and concentration of the salt. We have also reported that the water absorption resulting from the addition of salts leads to the plasticization of PMMA.
The aim of this study is to investigate the tensile properties of PMMA samples with different moisture content levels and to elucidate the mechanism underlying the changes in the stress–strain behavior induced by water absorption. For this purpose, we applied a nonequilibrium constitutive model in which the water domains surrounding salt-rich local domains diffuse into the dried PMMA matrix during stretching to PMMA samples doped with salts composed of different cationic and anionic species.
Understanding and controlling such moisture-responsive behavior not only provide insights into the fundamental mechanics of hydrated polymers but also open up avenues for practical applications. In particular, this behavior is promising for applications in wearable sensors [
24], where controlled ductility and environmental sensitivity are advantageous.
3. Theoretical Framework
Here, we introduce a nonequilibrium constitutive model to describe the effects of moisture on the stress–strain behavior of salt-doped PMMA. Under the initial conditions, water-rich regions (hydrated domains) are assumed to form around salt-rich regions. As tensile deformation progresses, these hygroscopic regions are expected to gradually spread throughout the specimen via strain-induced diffusion. Taking this mechanism into account, we constructed a constitutive equation in which the dried or initial PMMA matrix is modeled as an incompressible, highly rigid material that undergoes brittle fracture, whereas the fully moisturized matrix in equilibrium is assumed to behave as a plastically deformable fluid.
According to Leonov [
26], the constitutive relation for such incompressible rigid materials can be derived from a strain energy density function
based on the second strain invariant:
where
is the extension ratio and
is the rigidity. Consequently, the constitutive relation for the initial salt-doped PMMA can be derived using
as follows:
Therefore, Young’s modulus of the initial PMMA/salt sample is given by 3
GD.
In contrast, the fully moisturized matrix (soft component) undergoes plastic deformation at a constant stress level
. Therefore, its stress–extension relationship can be reasonably approximated by a step function,
. Accordingly, the constitutive equation for water-saturated PMMA/salt samples can be expressed as
The value of
for each water-absorbed sample corresponds to the stress level or strength of the ductile region and decreases with increasing water content.
Here, we introduce the internal variable
to describe the nonequilibrium state in which the moisture surrounding the salt domains gradually diffuses into the PMMA matrix as the tensile deformation proceeds. The internal variable
represents the stress contribution from the dried matrix region; in other words,
corresponds to the contribution of the hygroscopic regions relative to the dried matrix. On the basis of the strategy in the Leonov model [
26], the simple constitutive relation for the moisturized sample can be expressed using parameter
α as follows
The spreading process of the hygroscopic regions around the salt over the entire sample specimen can be described using the strain dependence of the internal variable
. The
value represents the fraction of the dry component
and serves as a kinetic transfer parameter. As the hygroscopic regions grow during stretching, the fraction of the dry matrix accordingly decreases. Thus, the fraction
is reduced by the strain, reflecting the progressive release of water from the hygroscopic domains. The transfer process during extension is simply assumed to be the first-order approximate kinetics with respect to the current strain, where the driving force for the transfer process is assumed to be proportional to the internal variable:
Here, the rate constant
k corresponds to the diffusion rate of water within the hygroscopic regions around the salt in the dried PMMA matrix. The driving force for water diffusion may result from Poisson shrinkage in the direction perpendicular to the axis of elongation.
At the initial stage, the water pools are confined within the salt-rich domains, indicating that
α = 1 at
λ = 1. By integrating Equation (5) under the initial condition,
at
, we obtained the following equation:
indicating that the fraction
α exponentially decays with increasing extension. Consequently, by substituting Equations (2), (3), and (6) into Equation (4), we have the following nonequilibrium constitutive equation describing the spreading of the hygroscopic regions in the dried matrix:
This is a kinetically constitutive relation for describing the gradual change from brittle to ductile behavior during extension.
4. Results and Discussion
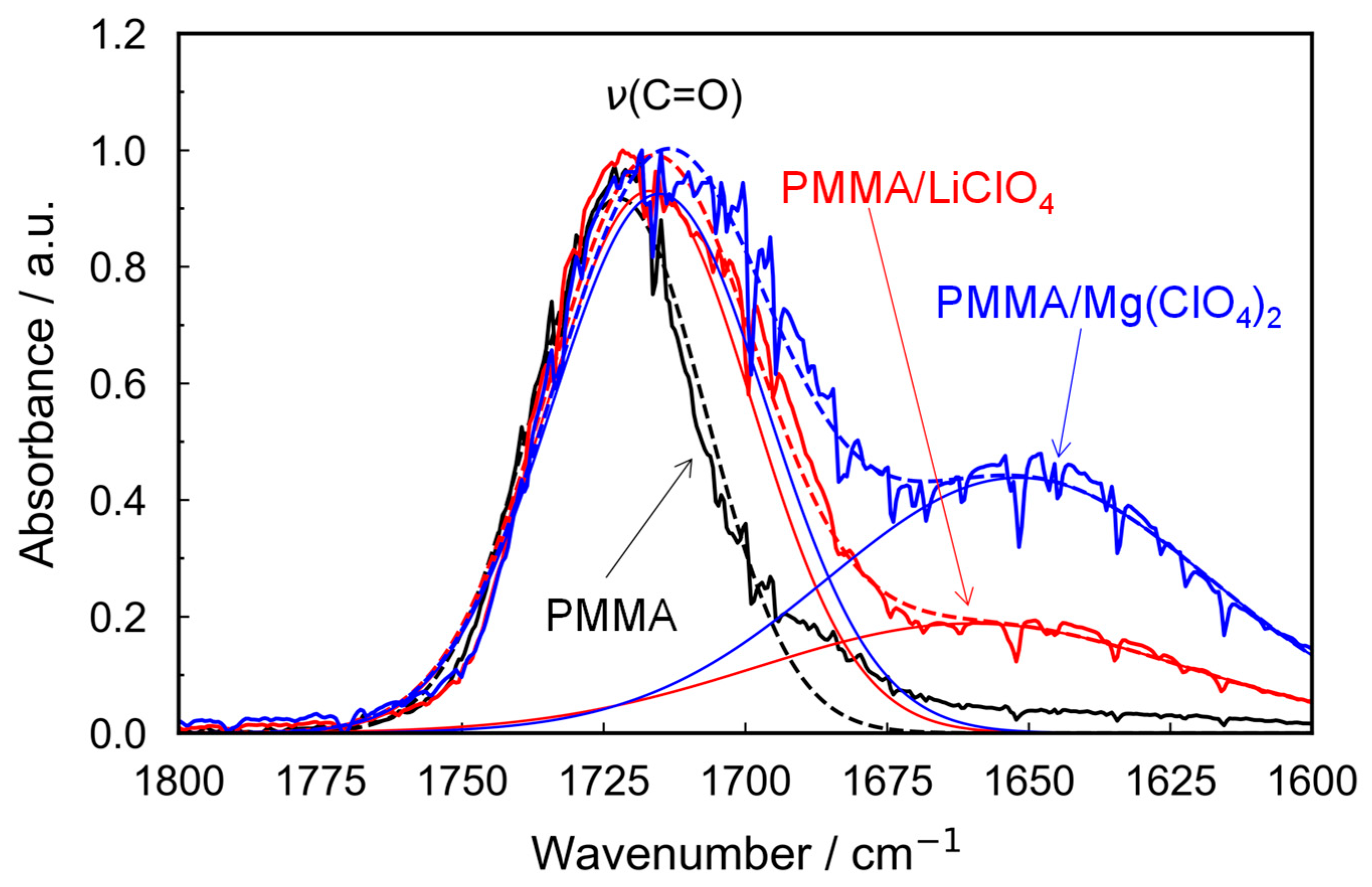
We compared the carbonyl absorption bands in the FT-IR spectra of the pristine PMMA and metal salt-doped PMMA samples (
Figure 1). The peak attributed to the carbonyl stretching vibration at 1720 cm
−1 extended toward lower wavenumbers. The red shift in the 1720 cm
−1 band indicates that the carbonyl groups experienced a stretching force owing to interactions with metal cations, suggesting stronger interactions in the case of Mg salt compared with those with monovalent Li salt. In addition, the salt-doped PMMA samples exhibited a novel broad peak at 1650 cm
−1 (the bending vibration mode of the hydroxy group), which is associated with water absorption. These results suggest that the divalent Mg
2+ ion interacts more strongly with water molecules than the monovalent Li
+ ion at the same molar ratio. The peak area around 1650 cm
−1 in the PMMA/Mg-salt system, as obtained from peak deconvolution analysis (see
Figure 1), was nearly twice that of the PMMA/Li-salt system. In the PMMA/Mg-salt system, the peak area observed at approximately 1650 cm
−1 using peak deconvolution (
Figure 1) was nearly twice that of the PMMA/Li-salt system. This result suggests that Mg
2+ ions promote greater water absorption than Li
+ ions, proportion to their cation valence at the same molar ratio, and further indicates that both salts are dispersed as dissociated ions within the PMMA matrix.
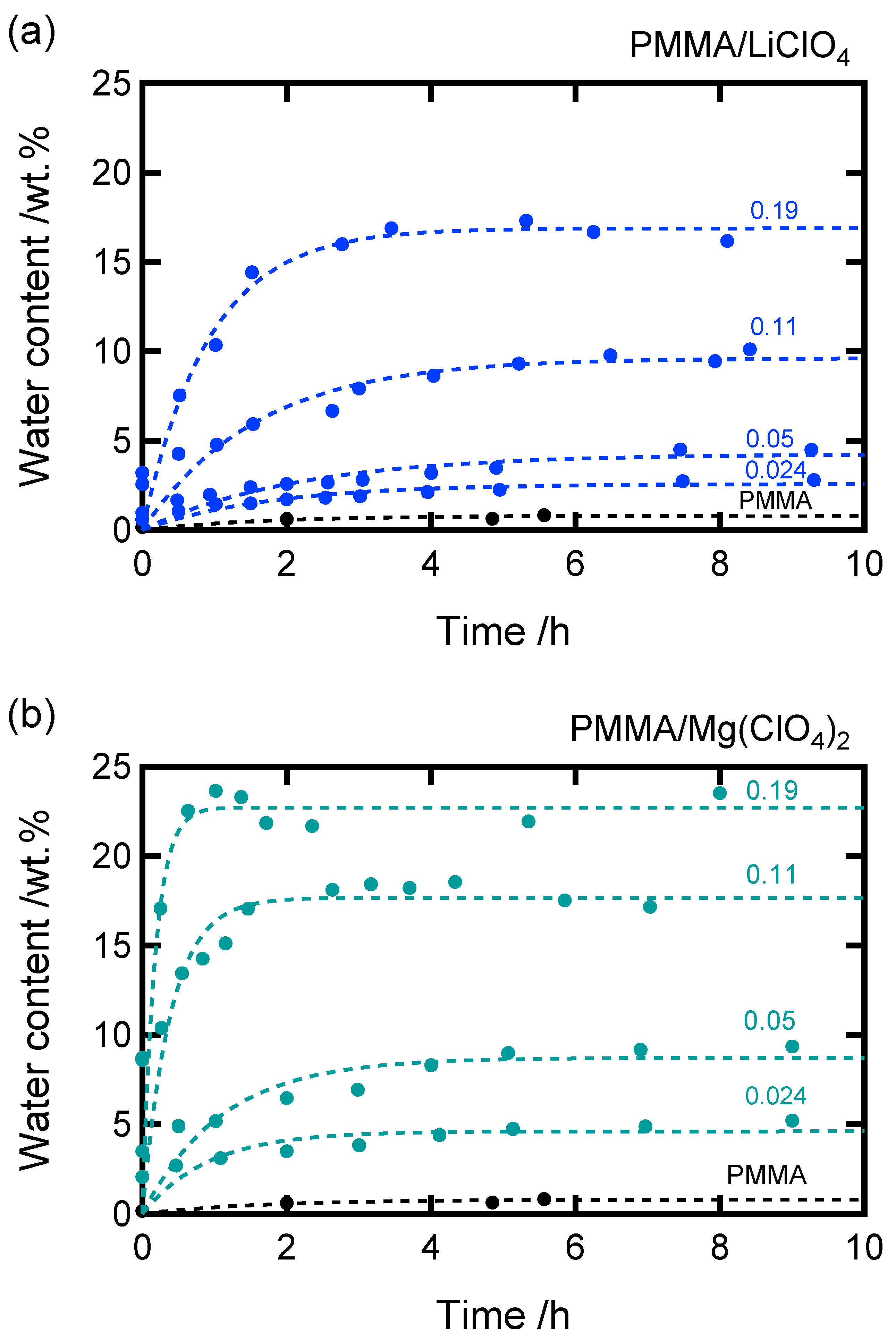
The time-dependent water absorption behavior of the samples is shown in
Figure 2. For both the Li- and Mg-doped systems, the water content increased with time and eventually reached a plateau, indicating water saturation. Assuming pseudo-first-order kinetics for water absorption, we can express the following equation representing the time dependence of the water concentration:
where
is the water concentration,
is the equilibrium concentration at
, and
τ is the inverse of the absorption rate. The theoretical curve derived from Equation (8) showed good agreement with the experimental data, as shown in
Figure 2. The curve fitting yielded the value of
.
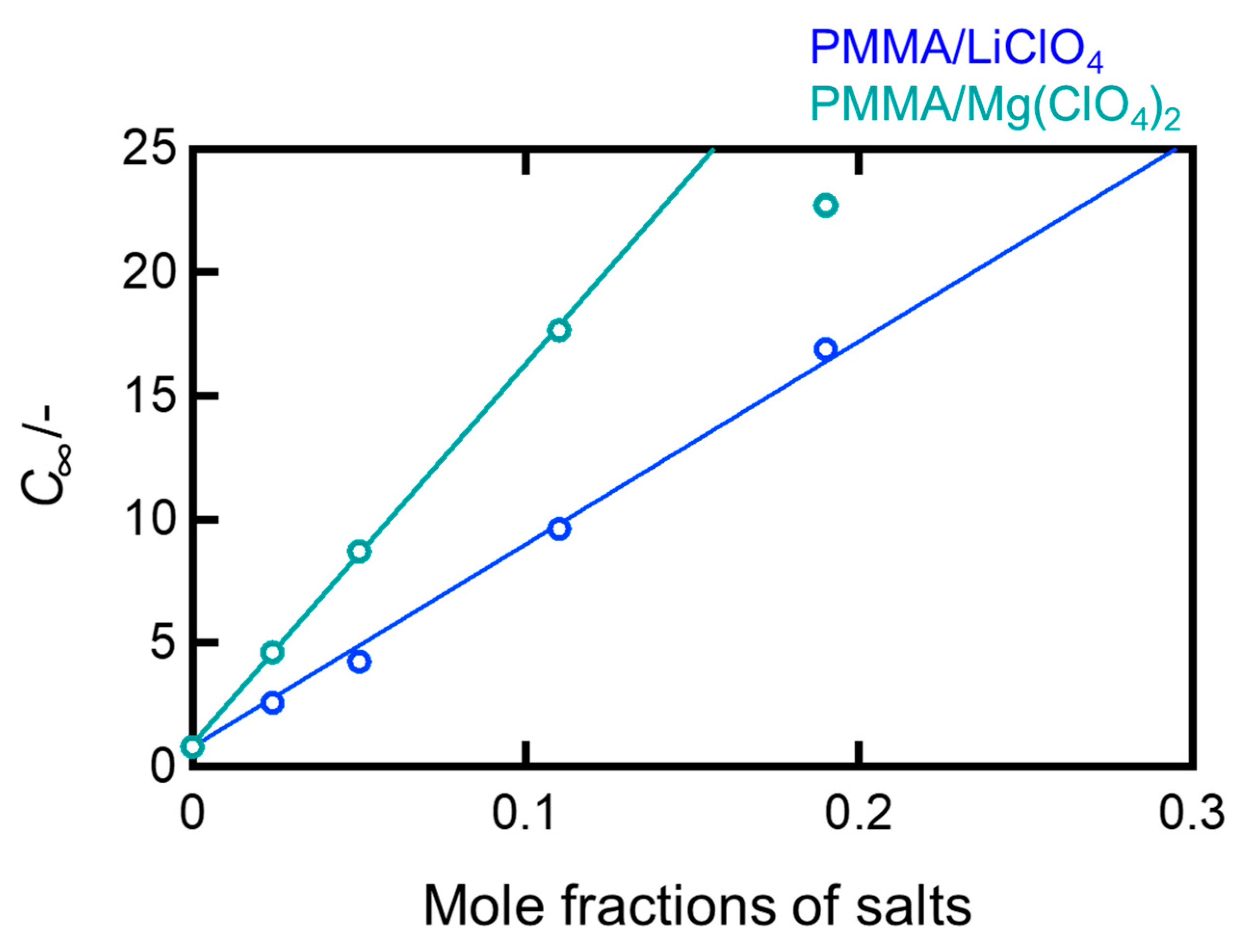
For the PMMA samples dopped with LiClO
4 and Mg(ClO
4)
2, the equilibrium concentration
is plotted against the molar fraction of the salt in
Figure 3. There was a linear relationship between
and the molar fraction of the salt. The regression lines were plotted excluding the PMMA/Mg(ClO
4)
2 (0.19) sample, which underwent deliquescence owing to an excessively high salt concentration. It is interesting to note that the slope of the curve for the Mg salt systems was approximately twice that for the Li salt systems, indicating that the divalent Mg
2+ ion induces roughly two times more water absorption than the monovalent Li
+ ion at the same molar ratio. As previously described, this also suggests that the salts likely dispersed as individual ions or small clusters in the PMMA matrix, rather than aggregating to form clusters.
We also investigated the tensile behavior of the salt-doped PMMA samples. The effects of moisture on the stress–strain curves for both systems are summarized in the
Supplementary Materials. Completely dry conditions were difficult to achieve, and even the dried samples retained a few percent moisture content. Both the PMMA/LiClO
4 (0.40) and PMMA/Mg(ClO
4)
2 (0.19) samples exhibited a brittle-to-ductile transition above 16% moisture content. The low moisture PMMA/salts used in this study exhibited brittle fracture behavior that was nearly independent of the salt content (see
Figure S1). The PMMA/LiClO
4 (0.11) system, containing only a small amount of salt, showed no ductilization regardless of the water content, confirming that the addition of salt led to embrittlement. Videos of the tensile deformation of dry PMMA, as well as both water-saturated PMMA/salts, are provided in the
Supporting Information (see
Videos S1–S3).
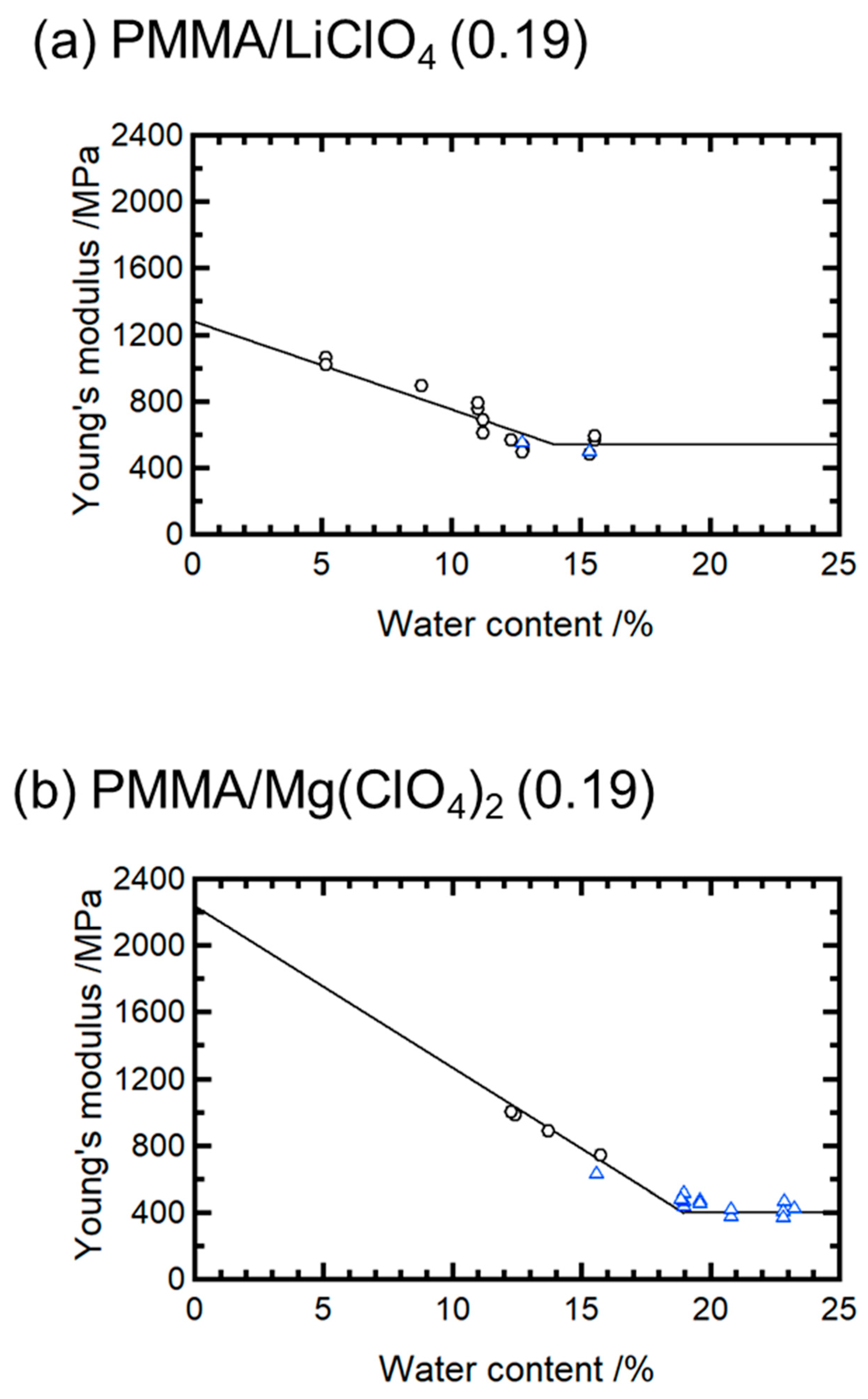
The dependence of Young’s modulus on the water content for PMMA/LiClO
4 (0.19) and PMMA/Mg(ClO
4)
2 (0.19) is shown in
Figure 4. Although small amounts of residual moisture remained after drying, their influence was consistent across samples and did not affect the relative trends observed in the ductility and modulus reduction. For both systems, the modulus decreased linearly with increasing the water content in the brittle state but plateaued after the transition to the ductile state. The slopes of the linear regions indicate that the modulus declines per 1% moisture increase, 53 MPa/% for Li
+ and 97 MPa/% for Mg
2+, suggesting a twofold greater reduction for Mg
2+ compared with that for Li
+.
In a previous study, we found that the embrittlement caused by the addition of salt is governed by the average relaxation time of the melt flow [
15]. Ion aggregation along the PMMA chains forms structural defects, degrading the toughness. The intercept of the extrapolated modulus in
Figure 4 represents the dry-state modulus. This value was approximately two times higher for the Mg systems than for the Li systems, indicating the stronger pinning effect of Mg
2+. Consistently, the stronger embrittlement observed in Mg systems is attributed to enhanced ionic pinning interactions along the PMMA backbone, which suppress molecular mobility and increase structural rigidity.
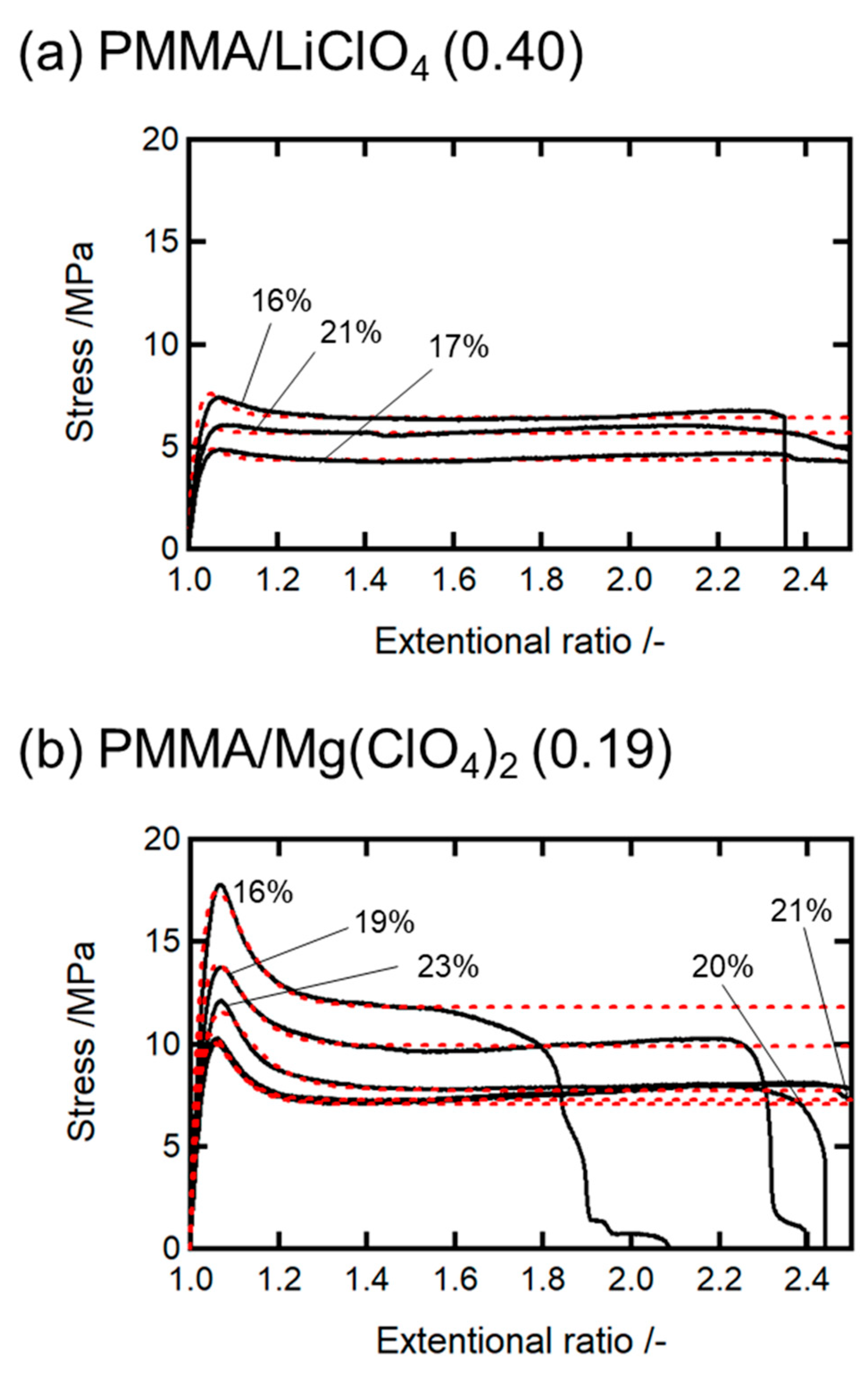
Here, we analyze the ductilization mechanism of the water-absorbed PMMA/salt systems based on the nonequilibrium constitutive formulation. The stress values in the ductile region relaxed at a constant level after exhibiting a maximum or yield peak, and the equilibrium level decreased with increasing water content (see
Figure 5). We consider that the water-rich domains, which were initially localized around the salt particles, gradually spread throughout the polymer matrix during tensile deformation. This redistribution process was modeled kinetically, because it led to a homogeneous water-absorbed state in the specimen, allowing for plastic deformation. We found that the constitutive equation (Equation (7)) reproduced the experimental stress–strain curves well for both the 20 and 30 wt.% salt-doped samples, as shown in
Figure 5a,b. The parameter
GD can be determined from the
EY values using
. The parameter
was also determined using the average values of the fracture stress.
The stress–strain curves for both systems were sufficiently well fitted by adjusting the parameter
k. The rate constant
k was obtained from the fitting curves shown in
Figure 5. The values of Young’s modulus, the plastic flow stress
, and the kinetic parameter
k values are listed in
Table 2. The
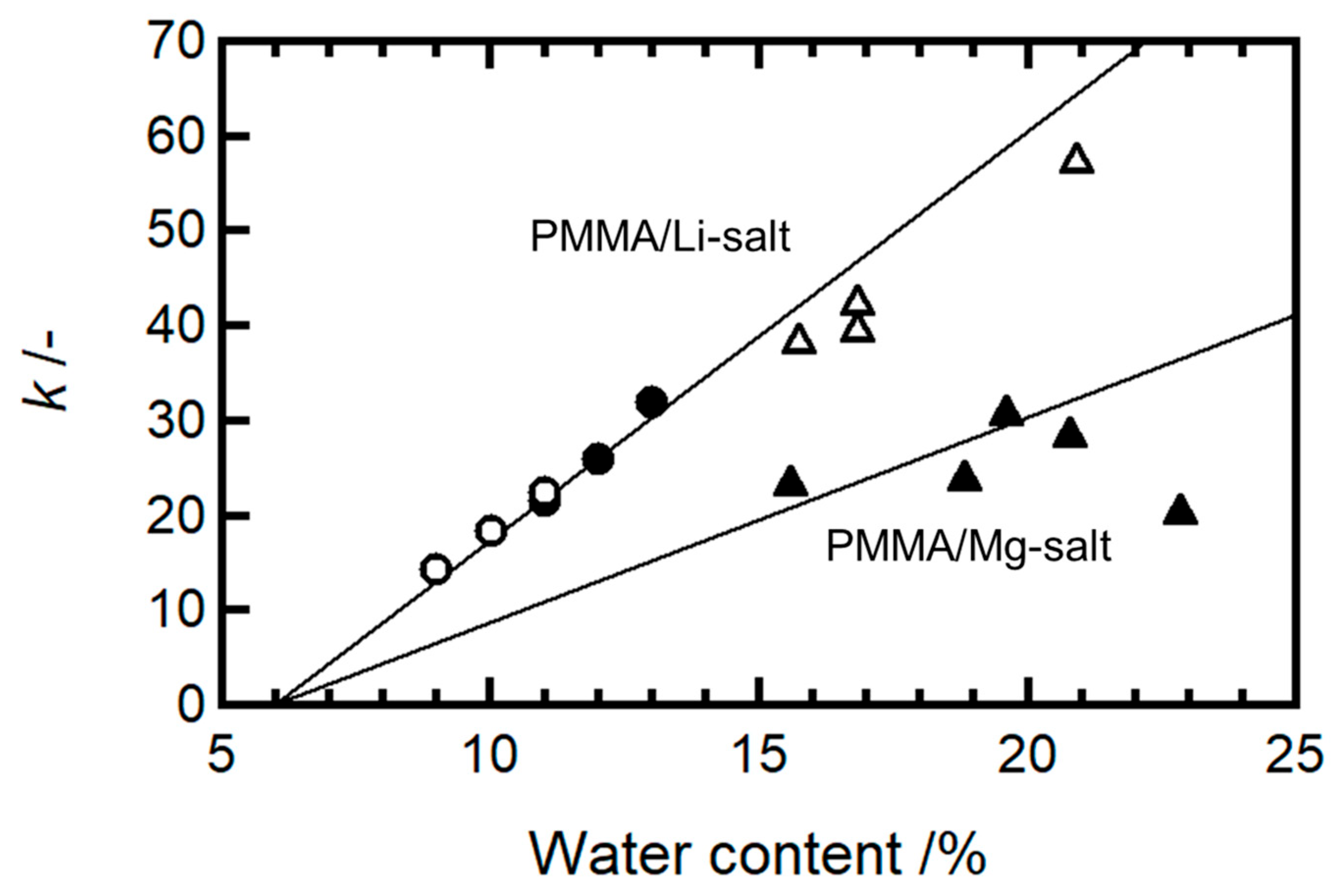
k values were then plotted as a function of the water content for both systems (
Figure 6). In the figure, the data of PMMA/LiCF3SO3 (0.16) and PMMA/LiCF3SO3 (0.28) previously published [
2] were included. The slope of
k for the Mg-salt systems was approximately half that of the Li salt systems, indicating that the interaction between Mg
2+ and water is twice as strong as that between Li
+ and water. Consequently, water diffusion is more suppressed in the Mg-salt-doped PMMA, indicating more limited redistribution of the moisture during deformation. This finding suggests that the kinetic parameter
k, which reflects water diffusion, is mainly governed by the cation species, and it is largely unaffected by the anion species.
5. Conclusions
In this study, we developed a nonequilibrium constitutive equation to describe the mechanical behavior of water-absorbed PMMA doped with various metal salts. By considering the strain-induced diffusion of water from the hygroscopic regions around the salt domains, the model successfully reproduced the experimentally observed transition from brittle to ductile behavior. The internal kinetic variable introduced in the model captures the progressive redistribution of moisture during deformation, allowing for a unified description of both the dry and hydrated states.
The results revealed that the mechanical softening and ductilization of PMMA are strongly governed by the type and concentration of the metal salt. Divalent Mg2+ salts exhibited stronger interactions with water than monovalent Li+ salts, leading to higher water uptake but reduced mobility of absorbed water during deformation. This resulted in more pronounced embrittlement in the dry state and a slower transition to ductile behavior under moist conditions. The kinetic parameter k, representing moisture diffusion, was mainly influenced by the cation species, and it was less sensitive to the anion type. The proposed model is capable of extending to other hygroscopic polymer systems and serve as a predictive tool in the development of moisture-responsive materials.
These findings provide fundamental insights into the moisture-induced mechanical tuning of glassy polymers and offer a rational framework for designing polymer materials with tailored mechanical properties through ion–water–polymer interactions. We have previously pointed out that the molecular motion of hygroscopic domains induces molecular slippage and plastic flow of PMMA chains under tensile deformation in moisture-absorbed PMMA/salt systems [
2]. Such hydration around salt ions may also influence interfacial lubrication properties, particularly under sliding or contact conditions. Recent studies [
27] suggest that water enhances lubrication via hydration shells, which generate a hydration repulsive force capable of sustaining a large normal load and exhibiting a fluid response to shear between a hydrated silicon nitride ball and a sapphire substrate.
The results of this study indicate that the mechanical properties of polar glassy polymers, such as PMMA, can be effectively tailored by tuning the valence of the metal cation in the added salt. This framework provides a physical basis for interpreting the moisture-dependent mechanical behavior of PMMA-based systems, and it may be applicable to other polar glassy polymers subjected to humid environments.