Abstract
CO2-responsive polymers have emerged as a significant class of smart materials, distinguished by their ability to reversibly alter their properties upon exposure to CO2. Due to CO2’s abundant availability, low cost, non-toxicity, energy efficiency, and excellent biocompatibility, these polymers offer remarkable environmental and practical advantages. This review succinctly explores recent advancements in the synthesis, mechanisms, and applications of CO2-responsive polymers, emphasizing the pivotal roles of specific acidic and basic functional groups such as carboxylic acids, phenolic groups, amines, amidines, guanidines, and imidazoles. Advanced polymerization techniques including free radical polymerization (FRP), atom transfer radical polymerization (ATRP), reversible addition-fragmentation chain transfer (RAFT), and nitroxide-mediated polymerization (NMP) are critically evaluated for their precision and flexibility in polymer design. Significant applications in smart separation, carbon capture, drug delivery, desalination, emulsions, tissue engineering, and sensing technologies are discussed comprehensively. Although substantial progress has been made, ongoing challenges include enhancing response speed, durability, sustainability, and economic viability. Future research is recommended to focus on innovative polymer structures, computational modeling, hybrid materials, and greener synthesis methods. This review aims to inspire continued exploration and practical utilization of CO2-responsive polymers to address pressing environmental and technological needs.
1. Introduction
Stimuli-responsive materials demonstrate the capacity to react to external or internal stimuli through transitional behaviors, representing a significant area of interest within the field of smart systems [1,2,3,4]. Consequently, stimuli-responsive polymers have been receiving significant attention in recent years. Responsive polymers exhibit adaptive “smart” responses to subtle variations in environmental signals, including temperature [5,6], pH [7,8,9], light [10,11], ionic strength [12,13], enzymes [14,15,16], redox conditions [17,18], magnetic fields [19,20,21], and in mechanical forces [22,23]. The latest developments in the field of stimuli-responsive polymers offer potential applications in biomedical delivery [24], including drug and gene carriers and sensors, as well as in the development of “smart” surfaces, catalysts [25], and adhesives [26]. However, notable limitations are present in the application of these triggers, including economic and environmental costs, as well as product contamination, which render them less appealing for commercial use [27].
Notably, among these stimuli-sensitive polymeric systems, CO2 has garnered significant attention in recent years as a benign, inexpensive, abundant, and non-toxic trigger for stimuli-responsive materials. Because of the environmentally friendly attributes provided by the CO2 trigger, in comparison to the existing triggers, CO2 presents several distinct advantages [28,29,30,31,32,33,34].
Unlike temperature-responsive polymers that require heating or cooling to trigger changes, CO2-responsive polymers achieve transition behaviors simply through the addition or removal of CO2 gas, which alters the solution’s pH [28]. This approach is cost-effective due to CO2’s abundance and environmental friendliness, making it an increasingly attractive research topic [35,36]. Compared to pH-, ionic-, enzyme-, or redox-responsive polymers, which often require repeated chemical additions and produce by-products, CO2-responsive polymers enable reversible cycling by alternately purging CO2 and inert gases (argon or nitrogen) without contamination, maintaining high responsiveness over multiple cycles [37,38]. Moreover, because CO2 diffuses easily through water, it offers greater penetration depth than stimuli like light, magnetic fields, or mechanical forces, enabling deeper material responses. Finally, as a natural, biocompatible metabolite with excellent membrane permeability, CO2 enhances the biomedical potential of CO2-responsive polymers [35,39,40,41]. Therefore, CO2-responsive polymers have been extensively used in various applications, including oil–water separation systems [42,43], carbon capture and storage [44,45], drug delivery systems [45], forward osmosis desalination [46], tissue engineering [47], smart emulsions [48], CO2 sensing [49], and others [50].
CO2-responsive polymers can be synthesized by using different functional groups that enable reversible protonation, deprotonation, ionic interactions, or complexation in response to CO2 stimuli. These groups can be categorized into acidic moieties (such as carboxylic acids and phenols) and basic groups (including amines, amidines, guanidines, and imidazoles). They facilitate adjustable modifications in solubility, hydrophobicity, charge, and conformation, influenced by their type, distribution, and concentration [30]. Recent developments in synthetic methodologies, including free radical polymerization (FRP) [51], atom transfer radical polymerization (ATRP) [52], nitroxide-mediated polymerization (NMP) [53], and reversible addition-fragmentation chain transfer (RAFT) polymerization [54], have broadened the design possibilities for CO2-responsive polymers. Controlled and living radical polymerizations, such as ATRP, RAFT, and NMP, enable the precise customization of polymer architectures. This capability facilitates the synthesis of complex structures, including block copolymers and graft copolymers, which enhances their functional responsiveness and expands their range of applications.
Due to the numerous advantages of CO2 as a stimulus compared to other triggers, the number of publications on CO2-switchable (or CO2-responsive) materials has increased significantly in recent years. Although some review articles were published on this topic between 2015 and 2019, they are now considered outdated given the rapid advancements in this field [30,31,55]. Therefore, there remains a lack of a recent comprehensive review article specifically dedicated to CO2-responsive polymers. In particular, there is a need for a review that focuses on advanced synthetic methodologies and comparative analyses of different polymerization techniques, as well as a detailed exploration of their state-of-the-art applications across diverse technological domains. This review aims to fill that gap by providing a systematic and critical analysis of the latest developments, offering insights into current achievements, identifying prevailing challenges, and proposing future research directions. This work aspires to stimulate further innovation and guide ongoing efforts toward the full realization of the potential of CO2-responsive polymers in advanced applications.
2. CO2-Responsive Polymer Mechanisms
2.1. Acidic Functional Groups
This group was initially revealed by Moore and Lefevre, who showed that exposure to CO2 could trigger coagulation in latex resins stabilized by fatty acids [56]. Acidic functional groups endure modifications in their protonation state upon exposure to CO2, hence affecting the polymer’s charge and solubility characteristics [57]. The most acidic functional groups in CO2-responsive polymers are carboxylic acids and phenolic groups [58]. After contact with CO2, these groups become protonated, transitioning the polymer from a more hydrophobic to a hydrophilic state, as shown in Figure 1a [59].
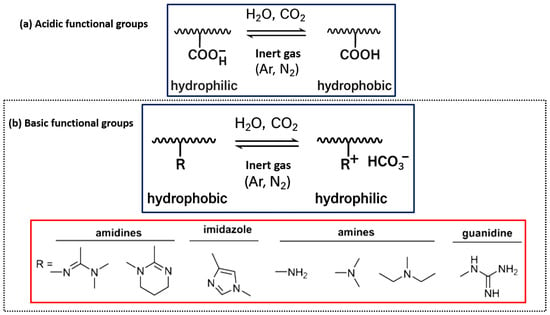
Figure 1.
Various types of CO2-responsive polymers. (a) Acidic groups (e.g., carboxylic acids, phenols) and (b) basic groups (e.g., amines, imidazoles, amidines, guanidines) that undergo reversible changes under CO2 exposure.
Carboxylic acids, represented by poly(acrylic acid) (PAA) and poly(methacrylic acid) (PMAA), are the most deeply investigated CO2-responsive acidic functional groups. Carboxylic acids, in their neutral state, are weak acids that can deprotonate at boosted pH levels to yield carboxylate anions (–COO−) [60]. Exposure to CO2 triggers a decline in the solution’s pH caused by the generation of carbonic acid, which protonates the carboxylate group, enhancing its hydrophilicity. The change in charge enhances the polymer’s water solubility, perhaps resulting in modifications to the polymer’s swelling behavior and self-assembly characteristics [58,61].
2.2. Basic Functional Groups
Basic functional groups can generate stable salts with CO2, leading to increased hydrophilicity of the polymer [62]. The primary functional groups that confer CO2-responsiveness to polymers include amines, imidazoles, amidines, and guanidines, as illustrated in Figure 1b. These groups can collaborate with CO2 to produce ammonium salts, carbamates, or bicarbonates, leading to substantial alterations in the polymer’s characteristics [55].
2.2.1. Amidines Groups
The CO2-responsive properties of amidine functional groups were first discovered by Jessop et al. (2006) [63], marking a significant advancement in the field of dynamic and switchable materials. Amidines are characterized by their relatively high basicity, which is stronger than that of amines but lower than guanidines, which enables efficient interaction with CO2 in aqueous environments [64]. In the absence of N–H bonds, amidines reversibly react with CO2 and water to form bicarbonate salts, which can subsequently regenerate the neutral amidine upon CO2 removal. However, when N–H bonds are present, the formation of carbamate salts may occur either alongside or instead of bicarbonates, altering the switching pathway [60].
One notable advantage of amidines is their faster conversion to bicarbonate salts compared to tertiary amines. However, the regeneration of the neutral form is a more thermodynamically demanding process owing to their higher basicity, making CO2 release slower and more energy-intensive [54]. The basicity and thus the CO2-responsiveness of amidines can be finely tuned through structural modifications, such as the incorporation of aromatic substituents on the nitrogen or central carbon atoms. These modifications lower the basicity, resulting in amidine systems with switching behavior more akin to tertiary amines [65]. Due to their rapid response to CO2 and structural tunability, amidines have emerged as promising candidates for incorporation into CO2-switchable (co)polymers, offering the potential for a wide range of applications in stimuli-responsive materials and environmentally adaptive systems [66].
2.2.2. Imidazole Groups
Imidazole is a heterocyclic compound containing nitrogen atoms that is capable of reversible protonation when exposed to CO2. When exposed to CO2 in aqueous media, imidazole confronts protonation, resulting in the formation of imidazolium bicarbonate, which enhances the hydrophilicity of the polymer. This characteristic renders imidazole a flexible functional group in CO2-responsive systems. Polymers with imidazole groups show variations in solubility and require volume phase transitions during CO2–N2 cycling [67]. Microgels synthesized with 1-vinyl imidazole (VIM) demonstrate reversible swelling in water when exposed to CO2, attributed to protonation, and they contract upon treatment with N2. This behavior enhances applications in responsive soft materials and catalysis. Furthermore, imidazole-functionalized microgels have served as catalysts for the cycloaddition of CO2 to epoxides. Their capacity to capture CO2 and adjust catalytic activity under mild conditions emphasizes their dual function as both a responsive and functional group [54,67].
2.2.3. Amino Groups
Amines are among the most prevalent basic functional groups for CO2-responsive polymers. Amines have lower basicity compared to alkylguanidines and alkylamidines. A prevalent criterion to determine basicity is the dissociation constant (pKaH) of the conjugate acid; a higher pKaH indicates a more potent base [68]. Molecules bearing amine functionalities, such as tertiary amines and sterically hindered primary and secondary amines, are capable of interacting with CO2 in aqueous media to form the corresponding bicarbonate salts. The relatively mild basicity of tertiary amines facilitates enhanced reversibility in CO2 capture–release cycles, allowing for CO2 desorption at ambient temperature in contrast to primary and secondary amines, which require thermal input for effective CO2 release [69]. Although tertiary amines exhibit lower basicity compared to stronger organic bases such as amidines and guanidines, their resulting quaternary ammonium bicarbonate salts demonstrate superior hydrolytic stability following CO2 purging [70].
Tertiary amine-containing molecules demonstrate excellent ionic conversion efficiency in aqueous environments, facilitating complete separation within water–tetrahydrofuran (THF) cosolvent systems. However, their application at elevated temperatures is limited due to a decrease in percent protonation, which necessitates increased CO2 pressure to maintain effective switching behavior at higher temperatures [27]. For instance, Darabi et al. (2014) [71] explored nitroxide-mediated polymerization (NMP) of 2-(diethylamino)ethyl methacrylate (DEAEMA) under a CO2 atmosphere in water. While DEAEMA was readily protonated at room temperature via CO2 bubbling, it underwent rapid deprotonation upon increasing the reaction temperature to 90 °C. This ease of deprotonation in bicarbonate salts derived from tertiary amines represents a significant advantage in applications requiring rapid reversion to the neutral amine form [27]. In addition to their favorable switching kinetics, tertiary amines benefit from commercial availability and relatively low cost. Bulky primary and secondary amines are also capable of CO2-switching behavior through bicarbonate salt formation, with steric hindrance playing a critical role in preventing carbamate formation. The presence of a single bulky alkyl substituent, such as isopropyl, on the nitrogen atom is typically sufficient to inhibit carbamate formation, allowing for rapid switching. Interestingly, these bulky secondary amines often exhibit faster switching dynamics than tertiary amines; however, the incorporation of two bulky groups, as in the case of diisopropylamine, diminishes this kinetic advantage. Moreover, secondary and primary amines offer an additional benefit of enhanced biodegradability compared to their tertiary counterparts [72].
2.2.4. Guanidines Groups
Guanidines represent the most basic class of CO2-switchable functional groups, and consequently, the regeneration of their neutral form from the corresponding bicarbonate salts necessitates greater energy input, typically involving higher temperatures and extended heating durations [68]. The incorporation of aromatic substituents can attenuate the inherent basicity of guanidines. Notably, guanidines possessing N–H bonds may form carbamates either exclusively or concurrently with bicarbonate salts upon exposure to CO2 [27]. Guanidine–alcohol mixtures have demonstrated CO2 sensitivity, functioning as switchable solvents; these mixtures transition into ionic liquids under a CO2 atmosphere and revert to their neutral state upon heating in a nitrogen environment [73]. This reversible behavior has inspired the design of guanidine-based CO2-responsive polymers. Although the higher temperatures required to revert guanidinium bicarbonate or carbamate salts to their neutral counterparts may pose limitations in certain systems, this thermal stability can be advantageous in applications necessitating elevated operational temperatures [32].
The choice of these functional groups amines, amidines, imidazoles, and guanidines derives from their robust and reversible interaction with CO2 under mild conditions. These groups facilitate dynamic alterations in the solubility, conformation, or assembly behavior of polymers upon exposure to CO2. Their efficacy, along with synthetic accessibility and compatibility with polymerization methods, positions them as optimal candidates for the development of responsive materials in diverse applications [30,31].
3. Synthesis Methods of CO2-Responsive Polymers
CO2-responsive polymers can be synthesized through a range of radical polymerization techniques, which are generally classified into two categories: conventional free radical polymerization (FRP) and controlled/living radical polymerization methods. While FRP is straightforward and widely used for large-scale production, it offers limited control over molecular weight and architecture. In contrast, controlled radical techniques such as atom transfer radical polymerization (ATRP), reversible addition-fragmentation chain transfer (RAFT), and nitroxide-mediated polymerization (NMP) enable precise control over polymer structure, block formation, and functional group placement. The following sections discuss these techniques with representative examples relevant to CO2-responsive applications [55,74].
3.1. Free Radical Polymerization (FRP)
Free radical polymerization (FRP) is a significant commercial polymerization process including three primary stages: initiation, propagation, and termination. Free radical polymerization (FRP) is extensively used in diverse polymerization methods, such as bulk, solution, and emulsion polymerization. The flexibility, simplicity, and compatibility of FRP with a wide array of monomers make it a favored technique for manufacturing CO2-responsive polymers with customizable characteristics [74,75,76,77]. Early research on CO2-switchable polymer systems deploying FRP primarily concentrated on emulsion polymerization for the synthesis of latexes, incorporating CO2 responsiveness via surface-active moieties. Fowler et al. (2011) [78] developed CO2-switchable polystyrene (PS) and PMMA latexes stabilized by cationic surfactants like N′-hexadecyl-N,N-dimethylacetamidinium bicarbonate. The latexes demonstrated reversible aggregation and redispersion through the alternating bubbling of CO2 and N2, influenced by the protonation state of the switchable surfactant molecules. Similarly, Mihara and colleagues [79] developed redispersible latexes using imidazole-functionalized initiators capable of reversible protonation when exposed to CO2, resulting in electrostatic repulsion strong enough to stabilize latex particles without the need for additional surfactants. Nonetheless, even with their reversible behavior, these systems frequently faced limitations due to low solid content (<7 wt%), incomplete protonation at extreme temperatures, and the hydrolysis of functional groups.
A further advancement was achieved through the integration of tertiary amine-based comonomers, including 2-(diethylamino)ethyl methacrylate (DEAEMA) and N,N-dimethylaminoethyl methacrylate (DMAEMA), into emulsion formulations. The incorporation of these monomers led to notable enhancements in CO2 sensitivity and particle stability. For instance, DEAEMA was utilized in the fabrication of CO2-switchable Pickering emulsions and reactive latexes, resulting in materials that could be easily dispersed or aggregated by altering the gas environment. Researchers reported that latexes prepared with VA-044 as the initiator fully protonated under CO2 conditions achieved solid contents of over 20% without the need for any comonomer or surfactant, highlighting a significant advancement in the formulation of redispersible latexes [61,76,80,81]. While these foundational studies established FRP as a reliable tool for CO2-responsive latex fabrication, recent research has broadened its application scope far beyond emulsions. A prominent example is the work by Pashayev et al. (2024) [74], who synthesized the homopolymer poly(N-[3-(dimethylamino)propyl]acrylamide) (PDMAPAm) via conventional FRP. The monomer, DMAPAm, contains both secondary and tertiary amine functionalities, which undergo reversible protonation and deprotonation upon CO2–N2 exposure, enabling switchable hydrophilic–hydrophobic behavior. Despite a relatively high dispersity (Đ ≈ 3.8) compared to RAFT-derived analogs, the FRP-based polymer retained effective CO2-responsiveness. This highlights the strength of the functional monomer design in facilitating stimuli-responsive behavior, even when produced via less controlled radical methods. Figure 2 illustrates that the molecular weight distribution of the FRP-synthesized PDMAPAm is broader compared to the RAFT-synthesized versions. However, the functional performance regarding CO2-triggered switching remains intact, highlighting the potential of FRP for scalable and cost-effective production of CO2-responsive polymers.
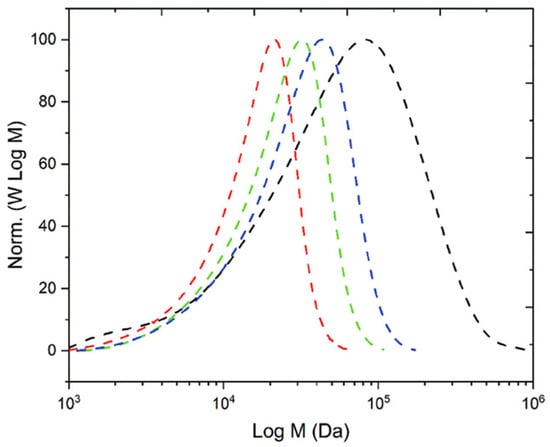
Figure 2.
Molecular weight distribution (MWD) profiles of PDMAPAm synthesized via RAFT (CDTPA:AIBN = 3:1 (---), 1.2:1 (---), 0.7:1 (---) and free radical polymerization (---) after 24 h [74].
In another significant study, Huang et al. (2024) [75] developed CO2-responsive polymers by applying aqueous free radical copolymerization of DMAEMA and polyether-modified methacrylates with acrylamide at 45 °C, as illustrated in Figure 3a. The resulting polymers featured tertiary amine groups and hydrophobic polyether side chains, which endowed them with gas-switchable solubility and dual responsiveness to both pH and CO2, making them attractive for smart hydrogel and coating applications. Likewise, Wu et al. (2023) [82] developed a CO2-responsive intelligent polymer sealant by using aqueous FRP of acrylamide and DMAPMA, which was cross-linked with polyethyleneimine (PEI) to improve its functionality (Figure 3b). The resulting polymer gel exhibited reversible swelling behavior and strong pH and CO2 responsiveness.
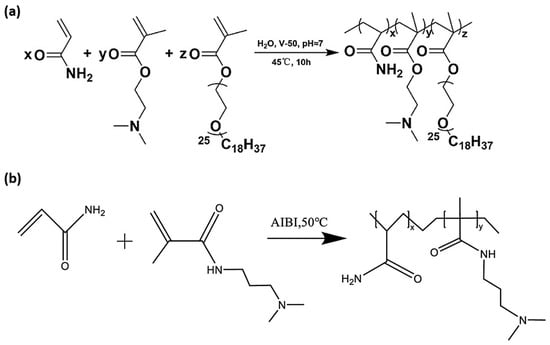
Figure 3.
(a) Synthesis of CO2-responsive polymer (a) via aqueous FRP of DMAEMA, AM, and polyether methacrylates [75] (b) by using gel using AM, DMAPMA, and PEI via aqueous FRP [82].
In the realm of nanostructured polymer systems, Yu et al. (2019) [83] revealed an innovative enzyme-assisted, photo-initiated FRP (photo-PISA) process carried out under open-air conditions. Cross-linked CO2-responsive polymer vesicles were synthesized through the copolymerization of HPMA and DMAEMA using a photoinitiator and an oxygen-scavenging enzyme system. The addition of the asymmetric cross-linker allyl methacrylate (AMA) provided mechanical stability, whereas DMAEMA units granted the vesicles CO2-switchable expansion behavior. Zhao et al. (2017) [48] investigated the new class of FRP-derived polymeric surfactants that include DEAEMA, demonstrating the ability to alter emulsification properties in oil–water systems based on the CO2–N2 gas environment. This study broadened the use of FRP-based CO2-responsive polymers in dynamic emulsion stabilization, an important field in materials chemistry. In another significant development, Ellis et al. (2019) [84] used FRP to synthesize nitrogen-rich polyamine draw solutes for forward osmosis, allowing for the modulation of osmotic pressure and CO2-responsiveness through the protonation of amine groups (Figure 4I). Furthermore, Gupta and Lee (2017) [85] developed a pyrene-functionalized polymeric probe through FRP for the fluorescence-based detection of nerve agent mimics (Figure 4II). The fluorescence of the material can be reversibly activated and deactivated through CO2–N2 purging, which is linked to the protonation state of amine groups affecting the photoinduced electron transfer (PET) pathway. Lastly, Fan et al. (2017) [86] developed single-chain CO2-responsive polymer nanoparticles (SCNPs) with free radical polymerization of copolymers, including DMAEMA (Figure 4III). Reversing swelling and shrinking characteristics of the nanoparticles allowed for gas-tunable control over their size and catalytic activity.
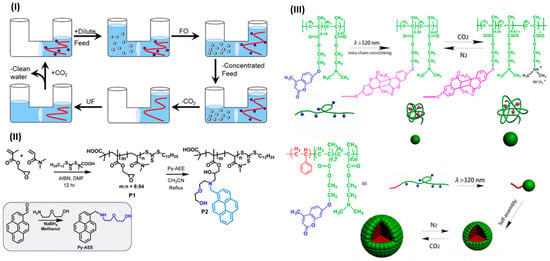
Figure 4.
(I–III) Representative examples of CO2-responsive polymer systems synthesized via free radical polymerization (FRP). (I) Forward osmosis–ultrafiltration (FO–UF) process using CO2-switchable nitrogen-rich polyamines as draw solutes [84]. (II) Synthesis of pyrene-functionalized polymeric probe P2 for CO2–pH-tunable fluorescence [85]. (III) Preparation and CO2–N2-responsive behavior of single-chain nanoparticles and micellar aggregates from DMAEMA-based copolymers [86].
Collectively, these contemporary examples illustrate that FRP is not merely a legacy method for latex production but a versatile and robust tool for constructing CO2-responsive polymers in various advanced formats. Despite its lack of molecular-level control compared to living radical polymerizations like RAFT or ATRP, FRP remains highly effective in producing functionally responsive materials, especially when precision in molecular weight is not a limiting factor for application.
3.2. Controlled Radical Polymerization Techniques
Reversible deactivation radical polymerization (RDRP), or controlled/living radical polymerization, has transformed polymer synthesis by permitting precise regulation of molecular weight, dispersity, and polymer architecture. In contrast to conventional living polymerizations, including anionic or cationic techniques, reversible-deactivation radical polymerization (RDRP) functions under more accessible and practical conditions [87]. In FRP, the lifespan of the developing chains is very rapid (under 1 s), resulting in all polymer chains becoming “dead” after the reaction. Consequently, the polymerization is non-living, and block copolymers cannot be produced by this process [88]. Anionic polymerization, as developed by Szwarc [89], reflects a true living system in which chain transfer and termination reactions are effectively eliminated. This can only be accomplished by removing all moisture and/or oxygen, requiring high vacuum conditions and very low temperatures (approximately −80 °C) [90]. By utilizing RDRP, or living radical polymerization, various monomers can be polymerized under milder conditions commonly used in FRP, while achieving a substantial level of control over the polymer structures. Additionally, RDRP exhibits lower sensitivity to water compared to anionic polymerization, which represents a significant advantage, as numerous industrial polymerizations occur in aqueous environments, such as emulsion polymerizations for latex production. In RDRP, the minimal occurrence of irreversible termination reactions allows for chains to remain active for extended durations, ideally throughout the entire polymerization process. This enables the incorporation of additional blocks, facilitating the preparation of block copolymers [89].
Subsequent sections will demonstrate that RDRP has emerged as the preferred polymerization technique for the synthesis of CO2-switchable polymers characterized by complex microstructures or mesostructures, such as di- and triblock copolymers and nanostructured particles. FRP is a more straightforward and cost-effective option compared to RDRP, particularly when precise control over molecular weight distribution and complex molecular architectures is not essential.
The three predominant types of reversible-deactivation radical polymerization (RDRP) are nitroxide-mediated polymerization (NMP), atom transfer radical polymerization (ATRP), and reversible addition-fragmentation transfer (RAFT) polymerization. Figure 5 provides a clear summary of these mechanisms, illustrating the activation–deactivation equilibria in NMP, ATRP, and RAFT polymerization pathways [87]. The primary distinction between RDRP and FRP lies in the significant reduction of termination reactions in RDRP, achieved through the incorporation of a control agent that facilitates the reversible deactivation of propagating chains. In NMP, nitroxide is introduced to the system to deactivate propagating radicals and reduce mutual termination. ATRP employs a transition metal catalyst, whereas RAFT utilizes a chain transfer agent to decrease the occurrence of termination reactions. NMP and ATRP utilize reversible termination, whereas RAFT employs reversible chain transfer [91]. Each of these controlling mechanisms can yield a polymer characterized by low dispersity (Đ < 1.5). Numerous reviews of RDRP across various polymerization systems, including dispersed systems, have been published [87,91,92,93]. The application of RDRP techniques in the production of switchable materials is emerging as a significant research focus. This paper highlights the synthesis of CO2-switchable polymers through reversible-deactivation radical polymerization (RDRP) in the subsequent sections.
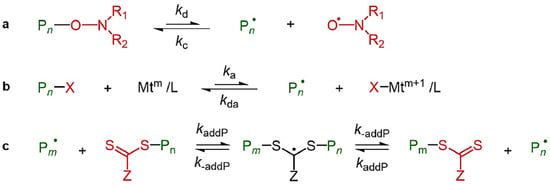
Figure 5.
Overview of simplified activation and deactivation mechanisms in reversible deactivation radical polymerization (RDRP). (a) Polymerization mediated by nitroxide radicals (NMP); (b) atom transfer radical polymerization (ATRP); (c) reversible addition-fragmentation chain transfer (RAFT) polymerization [87].
3.2.1. Atomic Transfer Radical Polymerization (ATRP)
Atom transfer radical polymerization (ATRP) is a prevalent method for the controlled radical polymerization of CO2-responsive polymers. In ATRP, there is an equilibrium between propagating radicals and dormant species [94]. The dormant species (Pn–X) are common and intermittently interact with transition metal complexes. Transition metal complexes function as activators in their lower oxidation state (Mtm/L), engaging with halide-terminated “dormant” chains to produce propagating radicals (Pn˙) and transition metal complexes in a higher oxidation state. In elevated oxidation states, transition metal complexes coordinate with halide ligands (X–Mtm+1/L) and function as deactivators. The deactivator interacts with propagating radicals to produce dormant species and the activator (Figure 6a). Consequently, the concentration of propagating radicals remains low in comparison to FRP, leading to a reduction in termination reactions. In certain applications, it may be necessary to remove the transition metal catalyst and ligand. This is clear for homogeneous systems; however, it can cause challenges in heterogeneous systems [95].
The versatile nature of ATRP has allowed for the development of a wide spectrum of CO2-switchable polymer systems. Classical copper-mediated ATRP has been utilized to synthesize diverse diblock, triblock, star-like, and surface-grafted CO2-responsive polymers [96]. Poly(ethylene oxide)-block-poly((N-amidino)dodecyl acrylamide) (PEO-b-PAD) was synthesized using ATRP at room temperature using CuBr/PMDETA, providing a well-defined diblock structure containing CO2-switchable amidine moieties. Figure 6b illustrates the synthesis route for the preparation of PEO-b-PAD. The reaction was conducted in anhydrous methanol for 12 h, deploying pentamethyldiethylenetriamine (PMDETA) as the ligand and copper bromide (CuBr) as the catalyst [96]. Likewise, triblock copolymers like PEO-b-PS-b-PDEAEMA have been synthesized using sequential ATRP, using the tertiary amine functionality of DEAEMA for CO2-responsiveness. The PEO macroinitiator was prepared by conducting the ATRP of mono-methoxyl poly(ethylene oxide) in dichloromethane (CH2Cl2) with triethylamine (TEA) at ambient temperature for 24 h, using 2-bromo-isobutyryl bromide (BiBB) as the initiator. The macroinitiator (O–Br) was used in the ATRP of styrene in bulk using copper bromide (CuBr) as the catalyst and PMDETA as the ligand (Figure 6c). The reaction persisted for 16 h at 110 °C to yield the OS macroinitiator for the third stage. The last phase was the ATRP of DEAEMA conducted in toluene with an OS macroinitiator, a combination of copper chlorides (CuCl and CuCl2) as the catalyst, and the PMDETA ligand at 60 °C for 24 h. The PMDETA ligand was used in the production of a PDMAEMA-b-PEO-b-PDMAEMA triblock copolymer, functioning as a CO2-switchable nanocomposite via ATRP using a Br–PEO–Br initiator [97].
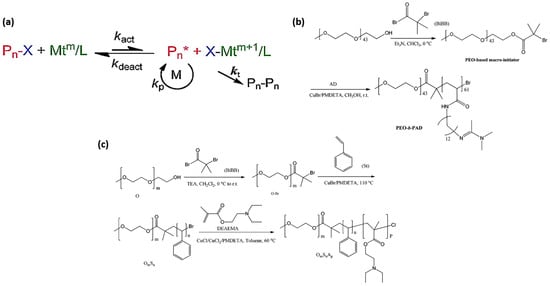
Figure 6.
(a–c) ATRP-based synthetic approaches for constructing CO2-responsive polymer architectures. (a) Illustration of the ATRP equilibrium mechanism [95]. (b) Schematic synthesis pathway of the diblock copolymer PEO-b-PAD [96]. (c) Stepwise ATRP strategy for preparing the triblock copolymer PEO-b-PS-b-PDEAEMA [98].
Likewise, Xu et al. (2013) [99] synthesized a fluorophore–polymer conjugate, PBI–PDMAEMA, which consists of two CO2-switchable PDMAEMA arms and a central perylene-3,4,9,10-tetracarboxylic acid bisimide (PBI) fluorophore. This was achieved through the ATRP of DMAEMA using N,N′-bis{2-[2-[(2-bromo-2-methylpropanoyl)oxy]ethoxy]ethyl}perylene-3,4,9,10-tetracarboxylic acid bisimide initiators. Following the synthesis of the PBI–Br macroinitiator (ATRP macroinitiator), it was applied in the ATRP of DMAEMA with CuBr as the catalyst, PMDETA as the ligand, and THF as the solvent.
In a recent study, Rezvani et al. (2019) [52] synthesized CO2-responsive polymers using the ATRP method. The process commenced with the extraction of cellulose nanocrystals (CNC) from microcrystalline cellulose (MCC) via acid hydrolysis. The CNCs were subsequently functionalized through the grafting of an ATRP initiator, α-Bromoisobutyryl bromide (BiBB), leading to the formation of CNC-Br. This step offered the essential spots for starting polymerization. The subsequent phase entailed the synthesis of copolymers through the grafting of poly(2-dimethylaminoethyl methacrylate) (PDMAEMA) and coumarin derivatives onto the CNCs. The reaction took place in tetrahydrofuran (THF), with DMAEMA and a coumarin derivative (CSA) added as monomers, while a catalyst system consisting of CuBr and PMDETA was used to initiate the polymerization (Figure 7). The polymerization took place at 70 °C for 24 h, leading to the production of both free copolymers and CNC-grafted copolymers.
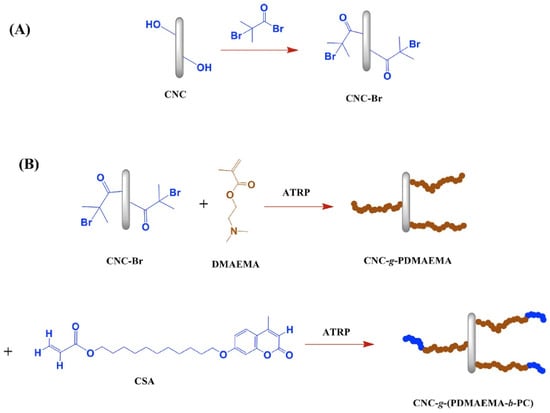
Figure 7.
Synthesis of (A) CNC-Br and (B) CNC-g-(PDMAEMA-b-PC) [52].
A recent innovation is the development of organocatalyzed ATRP (O-ATRP), which removes the necessity for metal catalysts. Su et al. (2019) [100] introduced an innovative method for synthesizing both hydrophobic and hydrophilic polymers through organocatalyzed atom transfer radical polymerization (O-ATRP). This approach uses a CO2-switchable photocatalyst capable of reversible transitions between hydrophobic and hydrophilic states, contingent on the presence or absence of CO2. This catalyst’s primary characteristic is its ability to be flipped by the addition or removal of CO2, allowing for reuse in future polymerization processes. The photocatalyst, functionalized with tertiary amine groups, exhibits hydrophobic properties in its neutral state (CO2-absent) and transitions to a hydrophilic state upon protonation in the presence of CO2. The polymerization can be conducted in either organic or aqueous media, depending on the state of the catalyst (Figure 8a). The development of the CO2-switchable photocatalyst (compound 1) included the functionalization of diphenyldihydrophenazine with tertiary amines to facilitate CO2 responsiveness. The catalyst demonstrates efficacy in hydrophobic polymerizations using solvents such as toluene, as well as in hydrophilic polymerizations conducted in carbonated water. The authors demonstrated that the catalyst could be effectively recycled and reused across multiple cycles, with minimal residual catalyst present in the final polymer (less than 15 ppb).
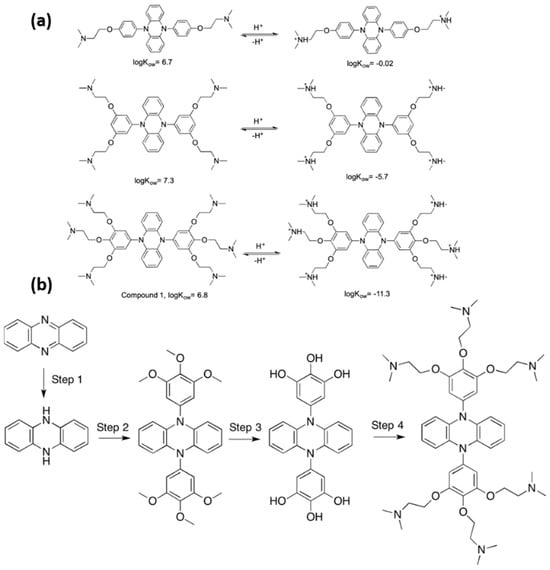
Figure 8.
(a) Molecular structures and predicted log Kow values for three potential CO2−switchable photocatalysts with varying numbers of tertiary amine groups in their neutral and protonated forms. (b) Synthesis of the CO2−switchable photoinitiated catalyst (compound 1): 2,2′,2″,2‴,2⁗,2′′′′′-[Phenazine-5,10−diyl bis [(benzene-5,1,2,3-tetrayl)tris(oxy)]] hexakis (N,N-dimethylethan−1-amine) [100].
The O-ATRP process was conducted using styrene (St) in toluene and hydroxyethyl methacrylate (HEMA) in carbonated water, demonstrating effective control over polymerization and resulting in narrow molecular weight distributions. The authors investigated the recycling process, in which the catalyst was extracted from the polymerization mixture relying on CO2-saturated water, and following the removal of CO2, it was employed again in later polymerizations. Figure 8b illustrates the synthesis of the CO2-switchable photocatalyst (compound 1), which highlights the recycling process of the catalyst following polymerization. This novel method offers a more sustainable approach to ATRP by enabling the reuse of the catalyst and reducing environmental impact, especially through the utilization of CO2 as a trigger for transitioning between hydrophilic and hydrophobic states [100].
3.2.2. Reversible Addition-Fragmentation Chain Transfer (RAFT) Polymerization
RAFT polymerization is a highly versatile and widely used technique in the field of reversible deactivation radical polymerization (RDRP) methods, particularly effective for the synthesis of CO2-responsive polymers with exact structural control. RAFT, established in the late 1990s, uses thiocarbonylthio-based chain transfer agents (CTAs) to promote a degenerative chain transfer process involving dormant and active chains. This promotes consistent chain growth, even at higher conversions, resulting in polymers characterized by narrow molecular weight distributions and regulated architectures [101]. Figure 9 illustrates a standard kinetic scheme for RAFT polymerization. Reaction I serves as the initiation step in RAFT, analogous to FRP. During the pre-equilibrium step (reaction II), the transfer of the RAFT agent to a propagating chain results in the formation of a macroRAFT agent. Reaction IV illustrates the principal RAFT equilibrium, in which the RAFT agent is exchanged among advancing polymer chains. Propagation (reaction III) and termination (reaction V) occur in a way comparable to the FRP mechanism. RAFT accommodates a broader range of monomers compared to NMP or ATRP, making it a commonly employed technique for synthesizing well-defined (co)polymers characterized by narrow molecular weight distributions and specific end functionalities.
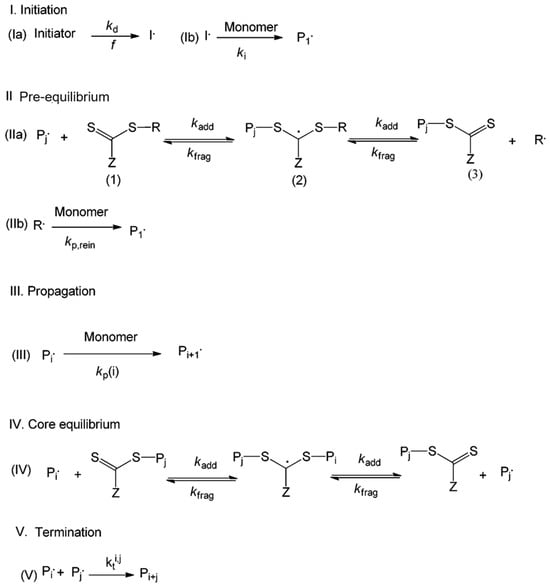
Figure 9.
Schematic depiction of the RAFT polymerization mechanism [101]. (Ia) Initiation; (Ib) formation of propagating radicals. (IIa,b) Pre-equilibrium step forming macro-RAFT agents. (III) Chain propagation. (IV) Main RAFT equilibrium involves chain transfer between active and dormant species. (V) Termination [101].
The RAFT agent can introduce color to the product, and residual odor can be a concern, which requires the post-reaction removal of the RAFT agent from the polymer chain ends [101].
Lin et al. (2016) [102] explained a straightforward grafting method in their paper that integrates RAFT polymerization with post-polymerization modification for the synthesis of CO2-responsive graft copolymers. A linear copolymer of poly(N,N-dimethylacrylamide-co-pentafluorophenyl acrylate), referred to as P(DMA-co-PFPA), was synthesized using RAFT polymerization. The backbone featured reactive pentafluorophenyl ester groups, providing the subsequent grafting of amine-terminated poly(2-(diethylamino)ethyl methacrylate) (PDEAEMA), a recognized CO2-responsive polymer, along with optional poly(methyl methacrylate) (PMMA) side chains. The homo-graft (PDMA-g-PDEAEMA, P4) and hetero-graft (PDMA-g-(PDEAEMA; PMMA), P5) copolymers demonstrated self-assembly into vesicular structures in aqueous media. When CO2 was bubbled through, the vesicles expanded as a result of the protonation of tertiary amine groups on the PDEAEMA side chains. P4 demonstrated reversible swelling and shrinking when subjected to alternating purging with CO2 and Ar, whereas P5 exhibited irreversible aggregation attributed to the presence of hydrophobic PMMA segments.
In another study by Guo et al. (2022) [103], a new approach to switchable RAFT polymerization was developed to fabricate CO2-responsive gradient copolymers, allowing for precise control over the sequence distribution of the monomers. This method involved a proton-switchable RAFT agent that could selectively polymerize more-activated monomers (MAMs) or less-activated monomers (LAMs) by switching between their active states through an acid–base treatment. Using this technique, the researchers synthesized a triblock copolymer, PBzMA-b-P(DEAEMA-grad-NVP)-b-PNVP, where the middle block showed a gradient from hydrophobic DEAEMA to hydrophilic NVP. This gradient structure led to the formation of compartmentalized micelles with CO2-responsive properties. These copolymers exhibited unique morphologies compared to typical block copolymers, offering the potential for tunable self-assembly and morphological changes when exposed to CO2.
In a similar vein, Chen et al. (2018) [104] presented a second-generation CO2-responsive system based on frustrated Lewis pair (FLP) chemistry, also synthesized via RAFT polymerization. In their system, two complementary block copolymers were created: one with bulky borane groups (Lewis acid) and the other with phosphine groups (Lewis base). When CO2 was bubbled through the mixture, these components formed cross-linked micelles via dative bonding, with CO2 acting as a bridge between the two blocks. These FLP-based micelles demonstrated an impressively fast CO2-responsiveness (less than 20 s), as well as thermal reversibility and recyclability. Moreover, the micelles were used as nanocatalysts in the conversion of C1 feedstocks, opening up new possibilities in sustainable chemistry. The process of self-assembly and catalysis with these FLP-based micelles is depicted in Figure 10.
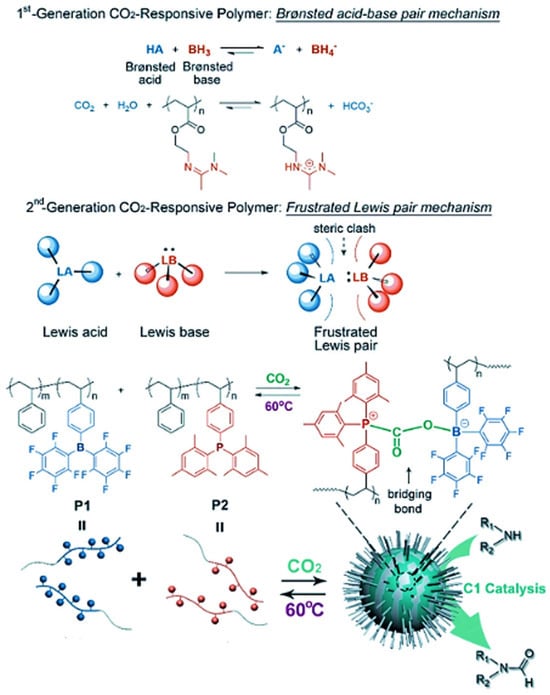
Figure 10.
(1st) Working principle of first-generation CO2-responsive polymers based on Brønsted acid–base pairs, with a typical example of amidine-containing polymers. (2nd) Frustrated Lewis pair (FLP) mechanism and the design of FLP-containing block copolymers (P1 and P2) as second-generation CO2-responsive systems for CO2-activated micellization and as recyclable nanocatalysts for CO2 catalytic conversion [104].
Amphiphilic block copolymers composed of poly(methacrylic acid) (PMAA) and poly(2-dimethylaminoethyl methacrylate) (PDMAEMA) serve as emulsifiers in the RAFT-mediated emulsion polymerization of PMMA latex particles. Through the modification of PDMAEMA block length and pH during polymerization, latex particles with surfaces enriched in either PDMAEMA or PMAA were synthesized in another study. The particles demonstrated bidirectional responsiveness to CO2; those with PDMAEMA surfaces precipitated at elevated pH levels and redispersed on CO2 exposure, but PMAA-surfaced particles exhibited the inverse behavior. This dual responsiveness helped reversible transitions between dispersion and precipitation, presenting a novel approach for intelligent colloidal systems [60].
Table 1 presents a summary of different CO2-responsive polymers synthesized through RAFT polymerization, a controlled and living radical polymerization method [105]. These polymers often include functional groups like tertiary amines, amidines, or guanidines that interact with CO2 via reversible chemical processes. RAFT allows for the accurate regulation of molecular weight, block composition, and end-group functionality, rendering it particularly effective for the synthesis of block copolymers, micelles, and nanostructures that experience CO2-induced phase transitions or self-assembly [103].

Table 1.
CO2-responsive polymers synthesized via RAFT polymerization.
3.2.3. Nitroxide-Mediated Polymerization (NMP)
Nitroxide-mediated polymerization (NMP) represents one of the earliest and strongest-established techniques among controlled radical polymerization (CRP) methods. NMP relies on a stable nitroxide radical, commonly TEMPO or analogous compounds, to reversibly deactivate growing polymer chains, which promotes polymerization with minimal termination. NMP offers a significant advantage over traditional free radical polymerization (FRP) by enabling precise control over molecular weight and producing narrow molecular weight distributions [115]. In the 1980s, Solomon et al. identified that radicals produced during free radical polymerization can be captured by nitroxides, which leads to the formation of controlled and living low molecular weight polymers [116]. In 1993, Georges and colleagues [117] applied 2,2,6,6-tetramethylpiperidinyl-N-oxyl (TEMPO) as a nitroxide in the living radical polymerization of styrene, leading to polystyrene with a narrow molecular weight distribution. This research established the basis for nitroxide-mediated polymerization. In NMP, an alkoxyamine encounters homolytic decomposition, resulting in the formation of nitroxide and initiator radicals [118].
Nitroxides are stable radicals that typically do not self-terminate, but they can react with growing radicals to deactivate them. The activation of a nitroxide-terminated polymer chain occurs roughly every 102 to 103 s, while deactivation happens quickly, around ∼10−3 s. During the brief period between activation and deactivation, a small number of monomers (usually 1 to 5) are added to the growing chains, resulting in polymers with a narrow molecular weight distribution (MWD) [90]. While nitroxides do not participate in irreversible termination reactions, propagating radicals may still terminate through mutual interactions. As conversion progresses, some irreversible termination occurs, increasing the ratio of nitroxide radicals to propagating radicals. Alkoxyamines typically require activation at elevated temperatures (>90 °C). Therefore, using nitroxide-mediated polymerization (NMP) to synthesize CO2-switchable polymers in a CO2 atmosphere is not practical, as CO2-switchable monomers would be protonated in that environment. High pressure would be needed to ensure sufficient CO2 dissolution. Alternatively, CO2-switchable groups can be protonated with strong acids, such as HCl, but this may cause the polymers to lose their CO2-switchable properties unless the acid is neutralized with a base and the residual salts are washed away.
Well-defined CO2-switchable polymers can be synthesized via NMP in a nitrogen atmosphere. For example, Zhou et al.(2009) [119] synthesized poly(p-chloromethylstyrene)-co-polystyrene (PCMS-co-PS) with low molar dispersity using NMP. They then modified this polymer to poly(p-azidomethylstyrene)-co-polystyrene (PAMS-co-PS) and introduced amidine groups through Staudinger ligation. This polymer demonstrated a neutral–charged–neutral transition in DMF with 0.5% H2O by alternately introducing CO2 and N2, which was confirmed by noticeable changes in the polymer’s conductivity (Figure 11).
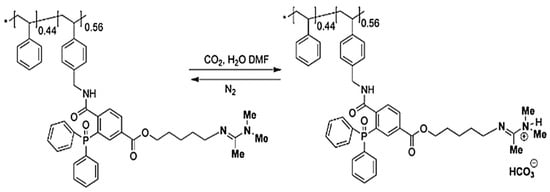
Figure 11.
Reversible protonation and deprotonation of p(“amidine”MS) −co−PS in DMF with 0.5% H2O following alternating CO2 and N2 purging [119].
Recently, Glasing et al. (2017) [120] used a grafting methodology to alter cellulose nanocrystals (CNC) with CO2-responsive polymers using nitroxide-mediated polymerization (NMP). This method involved the functionalization of the CNC surface with SG1-capped poly(2-dimethylaminoethyl methacrylate) (PDEAEMA) and poly(N-3-(dimethylamino)propyl methacrylamide) (PDMAPMAm) macroalkoxyamines. The synthesis of these CO2-responsive polymers was conducted in bulk via NMP, facilitating precise control over molecular weight, dispersity, and end-group fidelity. The grafting method enabled a comprehensive analysis of the preformed functional polymers before their attachment to the CNC surface, the initial step in the synthesis of PDEAEMA and PDMAPMAm, using NHS-BlocBuilder and 10 mol% styrene as a co-monomer in bulk at a temperature of 80 °C. This step indicates the polymerization of the monomers PDEAEMA and PDMAPMAm using the SG1-capped nitroxide as a radical initiator, facilitating well-controlled polymerization. Subsequently, CNC was modified with Glycidyl methacrylate (GMA), which is essential for introducing reactive sites on the CNC surface, thereby facilitating the subsequent grafting of CO2-responsive polymers. The modification of the CNC surface with GMA is essential for the subsequent Surface-Initiated NMP (SI-NMP) process, in which the functionalized CNC acts as a macro-initiator for the growth of CO2-responsive polymer chains. The CO2-responsive graft copolymers demonstrated reversible alterations in surface properties when exposed to CO2, shifting between hydrophobic and hydrophilic states. The ability to switch behavior presents opportunities for applications in nanocomposites, drug delivery systems, and wastewater treatment, where the modulation of surface properties is advantageous [120].
In a further investigation, surface-initiated nitroxide-mediated polymerization (SI-NMP) was chosen to graft CO2-responsive polymers onto cellulose nanocrystals (CNCs). CNCs were initially functionalized using an SG1-based alkoxyamine (CNC-BB), which acted as a macroinitiator. The functionalized CNCs underwent polymerization with 2-dimethylaminoethyl methacrylate (DMAEMA), diethylaminoethyl methacrylate (DEAEMA), and 3-(dimethylamino)propyl methacrylamide (PDMAPMAm) in a nitrogen atmosphere at 85 °C, utilizing styrene as a co-monomer to enhance polymerization control. The grafted CO2-responsive polymers demonstrated reversible transitions between hydrophilic and hydrophobic states when exposed to CO2. The full process starts with the synthesis of CNC–macroalkoxyamine (CNC–BB) and the subsequent grafting polymerization of DMAEMA, DEAEMA, and PDMAPMAm onto the CNC surface via SI-NMP. This method allows for the precise control of polymer grafting, enabling the creation of CO2-responsive CNC-based materials [53].
Table 2 provides a comparative analysis of the primary polymerization methods used for the synthesis of CO2-responsive polymers. Conventional free radical polymerization (FRP) continues to be popular because of its straightforward nature and affordability, particularly for the production of homopolymers or random copolymers. Nonetheless, it provides restricted control over molecular architecture. In contrast, controlled radical polymerization techniques like ATRP, RAFT, and NMP offer precise control over molecular weight, composition, and block architecture, which are essential for customizing CO2-responsiveness in self-assembled systems and stimuli-responsive applications. For example, ATRP facilitates the synthesis of precisely defined block copolymers with functional chain ends, whereas RAFT provides remarkable versatility for a wide array of monomers and reaction conditions. Consequently, these controlled methods are more appropriate when reproducibility, responsiveness, and functional complexity are essential in different applications. The comparative table effectively emphasizes their advantages and disadvantages [121,122].

Table 2.
Comparative analysis of polymerization methods for CO2-responsive polymers.
4. Applications of CO2-Responsive Polymers
The discovery of the potential for designing novel polymeric materials with CO2-responsiveness is going to contribute to the growth of innovative applications. This section investigates recent applications and identifies potential opportunities for the development of new materials. Few studies have been published that explore the potential applications of CO2-responsive polymers in different fields, which will be thoroughly discussed in this section.
4.1. Oil-Water Separation
Separation methods constitute a fundamental aspect of numerous chemical industries, typically executed through methods such as extraction, distillation, crystallization, membrane transport processes, and chromatography [123]. CO2-responsive polymers hold significant potential for application in advanced oil–water separation systems. Lei et al. (2017) [124] developed highly porous poly(styrene-co-N,N-(diethylamino)ethyl methacrylate) membranes characterized by “open-cell” structures and CO2-switchable wettability, applying water-in-oil high internal phase emulsion templates for their preparation (Figure 12I). These innovative membranes can alter their wettability from hydrophobic–superoleophilic to hydrophilic–superoleophobic via straightforward CO2 treatment in aqueous systems, providing gravity-driven and controllable oil–water separation. The practical implementation signifies a simple process in their original hydrophobic state, and these membranes permit selective oil penetration while obstructing water. Following CO2 treatment, the wettability transitions, allowing for water to permeate while obstructing oil (Figure 12II). This method offers a cost-effective method for the smart separation of large volumes.
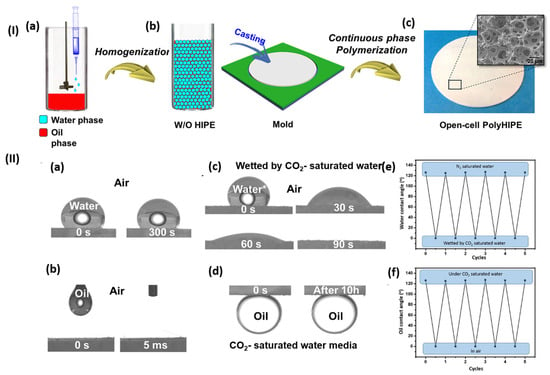
Figure 12.
Poly(St-co-DEA)-HIPE membrane: (I) synthesis and (II) wettability transitions. (I) Synthesis: (a) water phase added during oil phase homogenization for W/O HIPE preparation; (b) HIPF cast into a mold; (c) porous polyHIPE membrane formed by polymerizing the continuous phase. (II) Wettability measurements: (a) natural water contact angle at 0 and 300 s; (b) oil contact angle at 0 and 0.5 ms; (c) CO2-saturated water contact angle at 0, 30, 60, and 90 s; (d) oil contact angle under CO2-saturated water; (e) CO2-induced reversible water wettability; (f) CO2-induced reversible oil wettability [124].
Researchers at Queen’s University Canada have further advanced this technology by developing CO2-responsive polymer-grafted cotton using atom transfer radical polymerization (ATRP), demonstrating successful switching between hydrophobic and hydrophilic states when exposed to or removed from carbonated water [125]. Wang et al. (2023) [42] advanced the work of Lei et al. (2017) by developing scalable CO2-responsive membranes for the separation of oil and water. The membranes, composed of PMMA-co-PDEAEMA copolymers, demonstrate CO2-switchable wettability, shifting between hydrophobic–superoleophilic and superhydrophilic–underwater superoleophobic states in response to CO2–N2 stimulation. The membranes facilitate the gravity-driven separation of diverse oil–water systems, achieving separation efficiencies exceeding 99.9%. This innovation, observed through a capillary force-driven self-assembling method, provides a cost-effective and scalable solution for efficient and reversible oil–water separation. The membrane exhibits self-cleaning properties and high recyclability, rendering it appropriate for large-scale applications. Figure 13 demonstrates the CO2-induced wettability transition, which emphasizes the gas-switchable separation mechanism.
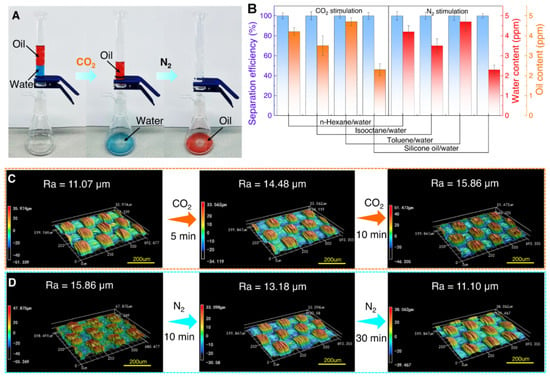
Figure 13.
Gas-switchable separation of immiscible oil–water mixtures. (A) Separation process un−er CO2–N2 stimulation at 25 °C. (B) Efficiency and oil–water content of PPFM−0.5 (150 μm gap) for four mixtures under CO2–N2. (C,D) Surface roughness changes of PPFM-0.5 under CO2–N2. Error bars show standard deviation from at least three samples [42].
Table 3 presents a summary of CO2-responsive polymers applied to oil–water separation applications, outlining the polymer materials, preparation methods, CO2-induced wettability alterations, oil–water separation efficacy, and significant findings.

Table 3.
Overview of CO2-responsive polymers in oil–water separation applications.
4.2. Carbon Capture and Storage
CO2-responsive polymers play a significant role in carbon capture and storage (CCS) technologies. Lu et al. [130] developed a more effective polymer, poly(N-heterocyclic carbenes-co-styrene), for CO2 capture. The findings indicate that this polymer exhibits enhanced CO2 capture rates at relatively low CO2 concentrations and demonstrates a more rapid CO2 release at elevated temperatures. Recently, Rieger’s group synthesized an acylated polyethyleneimine with amine groups, exhibiting thermal and CO2 responsive behaviors for CO2 capture and release. The results indicated that the thermal-responsive properties of the polymer facilitate CO2 release due to the formation of additional protons during the phase transition (LCST) on heating, which induced the decomposition of ammonium bicarbonate moieties, leading to the easier release of CO2 from the acylated polyethyleneimine solution [131].
A novel intelligent polymer sealant has been designed to reduce CO2 leakage in underground geological storage [82]. This sealant was generated through the cross-linking of CO2-responsive polymers, particularly acrylamide (AM) and N-[3-(dimethylamino) propyl] methacrylamide (DMAPMA), using polyethyleneimine (PEI) as the cross-linking agent. When exposed to CO2, the polymer system transitions from a sol to a gel state, resulting in a smooth surface and a uniformly porous three-dimensional network structure that effectively seals potential leakage pathways. The practical efficiency of these systems has been shown by core fluid displacement experiments, revealing a sealing efficiency of 73.6% for CO2 and a subsequent injection water sealing rate of 96.2%. The gelation mechanism signifies the binding of CO2 to water molecules, leading to the dissociation of H+ in aqueous solutions. This process generates an acidic environment that facilitates the attachment of tertiary amine groups to H+, thereby converting the gel from a nonionic to a cationic state (Figure 14). This transformation enhances hydrophilicity and increases electrostatic repulsion among polymer segments.
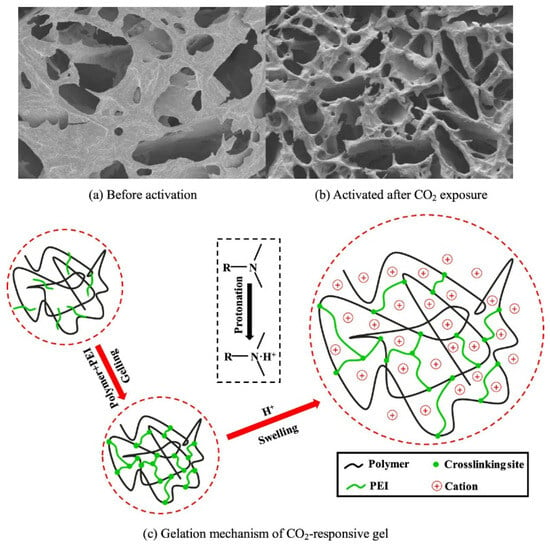
Figure 14.
Microscopic structure of the CO2 responsive polymer system for CO2 capture. (a) Before activation; (b) Activated after CO2 exposure; (c) Gelation mechanism of CO2-response gel [82].
4.3. Polymer-Assisted CO2 Reduction
Recent advancements indicate that the reduction of CO2 can be markedly improved by integrating molecular electrocatalysts with CO2-responsive polymer matrices [132,133,134]. A well-researched system includes the encapsulation of cobalt phthalocyanine (CoPc) within poly(4-vinylpyridine) (P4VP), demonstrating significant activity and selectivity for the electrochemical reduction of CO2 to carbon monoxide. This enhancement results from a synergistic interaction between the metal complex and the polymer microenvironment, influencing both the thermodynamics and kinetics of the catalytic cycle [135]. Figure 15 illustrates that the proposed mechanism initiates with the stepwise reduction of CoPc, succeeded by the protonation of the complex. In the presence of CO2, the reduced and protonated intermediate [CoPcH]− coordinates with CO2 to form a [CO2-CoPcH]− adduct. Following proton-coupled electron transfer, carbon monoxide is produced and the catalyst is regenerated. In the absence of CO2 coordination, an alternative pathway leads to H2 evolution via direct protonation. The P4VP polymer affects this mechanism through three primary effects: (1) axial coordination of pyridyl groups to the cobalt center increases CO2 binding affinity, (2) hydrogen bonding in the secondary coordination sphere stabilizes reactive intermediates, and (3) proton relays created by protonated pyridyl residues facilitate efficient proton delivery to the active site, which is essential for the rate-determining step. The interactions collectively change the rate-determining step from CO2 binding, as seen in bare CoPc systems, to the protonation of the coordinated intermediate in polymer-bound CoPc [136]. The observed shift has been validated through kinetic isotope effect (KIE) and proton inventory studies, indicating a significant reduction in KIE values when P4VP is present, aligning with polymer-mediated proton transfer mechanisms. The findings establish a mechanistic basis for the systematic design of multifunctional CO2-responsive polymers capable of both CO2 capture and its conversion under mild electrochemical conditions.
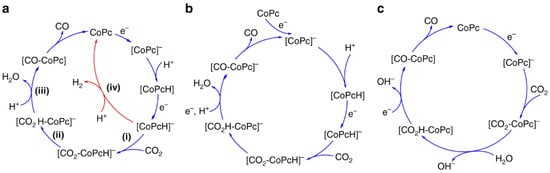
Figure 15.
Proposed CO2 reduction mechanisms of cobalt phthalocyanine (CoPc). (a) A mechanism showing sequential steps for competitive H2 generation: (i) first reduction of CoPc, (ii) protonation forming [CoPcH]−, (iii) CO2 binding to the reduced complex, and (iv) CO2 reduction to CO or competitive protonation leading to H2 generation. (b) Alternative mechanism proposed in organic solutions. (c) Mechanism proposed in low-concentration bicarbonate buffer under aqueous conditions [136].
4.4. Drug Delivery System
CO2-responsive polymers have transformed drug delivery systems by allowing for controlled release mechanisms that are triggered by stimuli. An exemplary case is the fabrication of CO2-responsive core–shell–corona structured magnetic Fe3O4@SiO2-poly(N,N-dimethylaminoethyl methacrylate) (PDMAEMA) nanocarriers. The hybrid magnetic nanoparticles exhibit a sandwich structure characterized by superparamagnetic properties and gas-responsive behavior, facilitating controlled drug release. Exposure to CO2 induces a change in the hydrodynamic radius of these nanoparticles via a switchable volume transition, leading to the release of encapsulated drugs such as doxorubicin [137]. CO2-responsive polymers, such as the amidine-containing block copolymer developed by Yuan et al. [96] can generate vesicles that exhibit a biomimetic “breathing” characteristic in response to CO2. This provides a modifiable membrane permeability in reaction to CO2 stimulation. Polymers such as poly((N,N-dimethylamino)ethyl methacrylate) and poly((N,N-diethylamino)ethyl methacrylate), which contain tertiary amine groups (Figure 16I), exhibit carbon dioxide responsiveness. This was demonstrated by Zhao et al. [98] through the design of triblock copolymers that can mimic gas-controllable organelle deformations (Figure 16II).
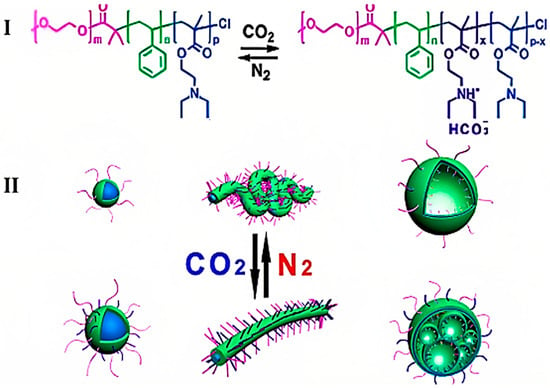
Figure 16.
CO2-switchable triblock copolymer (I) and CO2-driven controlled deformation of nanostructures (II) [98].
Functionalities that demonstrate responsiveness to CO2 include tertiary amines, amidines, and guanidines, which can undergo reversible protonation and deprotonation without generating side products. CO2-responsive polymers are garnering considerably more interest in the field of drug delivery compared to polymers responsive to other gas transmitters [31]. Increased CO2 levels in the body, referred to as hypercapnia, result in respiratory acidosis due to the excessive production of protons. The reduction in pH may provide advantages for patients with acute lung injury; however, the negative consequences significantly surpass the potential benefits [138]. Tian et al. recently investigated (2-diethylamino)ethyl methacrylate to develop a dual CO2- and photo-responsive system, implementing the reversible and dynamic characteristics of CO2-responsive systems. This system provided adaptability in volume and wall thickness for the delivery of curcumin. Significant changes in morphology were observed as the self-assemblies underwent different levels of protonation, resulting in a spectrum of morphologies including micelles, worm-like micelles, and small vesicles, corresponding to alterations in the hydrophilic fractions of the nanoaggregates. The protonation of tertiary amines by CO2, coupled with the dissociation of anthracene photodimers via UV light, supported the release of curcumin, which could be used for improved localization through external stimuli. This formulation demonstrates a responsive behavior to low pH, with accelerated drug release observed at pH 5.0 in comparison to pH 6.0 and pH 7.4 [139]. In conclusion, although the existing literature offers limited attention to this topic, it highlights considerable potential for diverse applications.
4.5. Other Applications
In addition to the previously discussed categories, a variety of innovative applications have recently emerged that harness CO2-responsive polymers, owing to the environmentally friendly nature of CO2 as a trigger. These applications span multiple fields, including forward osmosis desalination, tissue engineering, smart emulsions, extraction systems, and CO2 sensing technologies.
4.5.1. Forward Osmosis Desalination
CO2-responsive polymers have demonstrated significant potential in forward osmosis (FO) desalination [46]. Forward osmosis (FO) desalination involves the extraction of clean water from seawater or wastewater via a semipermeable membrane into a concentrated draw solution. An optimal draw solute should generate significant osmotic pressure while being easily removable, non-toxic, and stable [140,141]. A patent introduced CO2-switchable polymers as draw solutes, using the bicarbonate salt of a CO2-responsive polymer dissolved in carbonated water as the draw solution. Following water transport through osmosis, the elimination of CO2 through air or nitrogen bubbling results in polymer precipitation (if insoluble) or facilitates recovery through low-energy reverse osmosis (if soluble), producing fresh water [142]. Jessop and colleagues proposed the application of PDEAEMA, which, following neutralization, exhibits water-insolubility and is easily recoverable [143]. PDMAEMA is used as a draw solute, with significant water flux in forward osmosis. Following CO2 removal and moderate heating, PDMAEMA precipitates, providing straightforward separation while minimizing membrane degradation, back-diffusion, and toxicity [106].
Beyond water treatment applications, CO2-responsive polymers have been investigated for use in smart ion-channel devices. Jiang et al. [144] synthesized a CO2-responsive ion channel that is coated with PDEAEMA polymer brushes at both openings. These polymer films exhibit reversible transitions between hydrophobic and hydrophilic states in response to the addition or removal of CO2, providing voltage-independent regulation of their open and closed configurations. These systems exhibit potential applications in energy, filtration, and seawater desalination.
4.5.2. Tissue Engineering
In tissue engineering, CO2 serves as an important agent for polymer processing, particularly in the fabrication of scaffolds. Since the mid-1990s, CO2 has been used for the production of polymeric foams for applications in regenerative medicine [47,145]. Researchers have systematically optimized parameters to enhance pore interconnectivity and scaffold performance [145]. In addition to foaming, CO2 serves as a swelling agent for loading scaffolds with bioactive compounds and encapsulating plasmids for gene delivery. The application of low-pressure CO2 sintering for polymeric microspheres embedded with living cells represents a significant advancement, facilitating the one-step production of scaffolds devoid of organic solvents. This innovation enhances usability, reduces costs, and improves compatibility with polymers such as PLA, PGA, PEG, and PCL, thereby significantly broadening the scope of tissue engineering applications [146].
4.5.3. Smart Emulsions
Last but not least, CO2-responsive polymers have made significant advances in emulsion technologies. Chitosan-g-poly[(2-dimethylamino)ethyl methacrylate] has been developed as a CO2-switchable emulsifier, demonstrating reversible emulsification that is responsive to temperature and pH through the alternating bubbling of CO2 and N2. Stable emulsions of n-butanol and water can be generated through CO2 bubbling and disrupted by N2 bubbling. The development of two-way CO2-responsive polymer particles that use PMAA-b-PDMAEMA diblock copolymers represents an important milestone [147]. These particles promote reversible aggregation and dispersion across different pH levels, providing “green emulsion” technologies with programmable amphiphilic characteristics, which are beneficial for industries requiring precise emulsification and separation processes. CO2-responsive materials in analytical chemistry contributed to more environmentally friendly solid-phase extraction (SPE) techniques. Researchers have shown that CO2-responsive silica can effectively pre-concentrate analytes using solely water and carbonated water, thereby reducing the reliance on organic solvents and promoting sustainability, all while preserving high separation efficiency [60,148].
4.5.4. CO2 Sensing
Finally, CO2-responsive polymers are becoming more prevalent in CO2 sensing applications. Kang’s group designed a fluorophore-functionalized PDMAEMA polymer that exhibits reversible color changes resulting from the protonation or deprotonation of amine groups in response to the addition or removal of CO2 [99]. The solution transitions from dark red to orange, offering CO2 detection in aqueous solutions. Yung’s group developed an intuitive detection system by combining gold nanoparticles with a random copolymer (P(DMA-co-NAEAA)) that contains amidine groups. The dissolution of CO2 resulted in the protonation of amidine groups, leading to nanoparticle aggregation through electrostatic interactions, which manifested in plasmonic changes observable by the naked eye or through UV–Vis spectroscopy [149]. Additionally, Guo’s group developed CO2-responsive carbon nanotube (CNT) sensors that can detect dissolved CO2 in water, representing another significant application of CO2-responsive materials [150].
5. Challenges and Future Perspectives
CO2-responsive polymers represent a significant category of smart materials, characterized by their capacity to reversibly change physicochemical properties upon exposure to CO2. These polymers, which generally contain functional groups like amidines, amines, or carboxyls, present unique advantages compared to conventional stimuli-responsive materials, particularly through the use of CO2 as a safe, plentiful, cost-effective, and eco-friendly trigger [28,32,86]. This review has systematically investigated the fundamental mechanisms underlying the responsiveness of CO2-responsive polymers, with a particular emphasis on recent advancements in synthetic strategies and their expanding range of latest applications. These materials have been successfully applied across diverse fields, including oil–water separation, carbon capture and storage, drug delivery, tissue engineering, forward osmosis desalination, smart emulsions, extraction systems, and CO2 sensing. Their unique ability to reversibly switch between hydrophilic and hydrophobic states, combined with self-cleaning capabilities and operation under mild conditions, makes them highly promising candidates for the development of sustainable and energy-efficient technologies.
Despite these promising developments, several unresolved challenges must be addressed to advance the practical deployment of CO2-responsive polymers. First, precise control over switching kinetics and reversibility remains a major obstacle; rapid, low-energy responsiveness under fluctuating conditions is essential for real-time and long-term performance. Cyclic stability is another concern—many polymers experience mechanical degradation, fouling, or loss of activity after repeated switching cycles, particularly in membrane and separation technologies.
In biomedical applications such as drug delivery, achieving spatial and temporal control over CO2 levels in vivo is difficult and raises critical issues surrounding biocompatibility, degradation products, and off-target effects. Similarly, in forward osmosis and purification systems, challenges include the high viscosity of polymer solutions, membrane fouling, and limited recyclability. Environmental variables, such as temperature, humidity, pH, and coexisting species, can also interfere with polymer response, especially in real-world sensing or extraction contexts. Additionally, the tuning of thermal transitions (Tg, LCST) remains crucial to prevent unwanted aggregation or inconsistent behavior across applications.
Moving forward, a major research priority lies in developing scalable and environmentally sustainable synthesis strategies for CO2-responsive polymers. Future efforts should focus on refining controlled/living radical polymerization and adopting green chemistry approaches to enable low-cost, large-scale production. Designing polymers with enhanced architecture, such as block copolymers, grafts, or hyperbranched structures, can improve responsiveness, speed, and durability. Special emphasis should be placed on creating biodegradable or recyclable CO2-responsive systems, especially for biomedical and environmental applications.
The integration of computational modeling and machine learning can significantly accelerate the design of next-generation materials by predicting structure–function relationships and optimizing polymer formulations. Exploring hybrid materials, such as polymer–inorganic composites or multi-stimuli-responsive systems, may also yield synergistic properties suitable for complex operating environments. Furthermore, the industrial integration of CO2-responsive materials into continuous flow systems, programmable membranes, or CO2 capture platforms remains an open and important avenue for translation from laboratory innovation to large-scale impact. By addressing these open questions and interdisciplinary challenges, CO2-responsive polymers have the potential to drive sustainable innovations in energy, water, healthcare, and environmental remediation.
Author Contributions
M.S.: Writing—Original Draft, Conceptualization, Review and Editing. R.W.: Conceptualization, Writing—Review and Editing, Funding Acquisition, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Cooperative Research Exchange Project supported by the Ministry of Science and Technology of China (China-Bulgaria 2024, 18-14).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mrinalini, M.; Prasanthkumar, S. Recent Advances on Stimuli-Responsive Smart Materials and Their Applications. ChemPlusChem 2019, 84, 1103–1121. [Google Scholar] [CrossRef] [PubMed]
- Shafranek, R.T.; Millik, S.C.; Smith, P.T.; Lee, C.-U.; Boydston, A.J.; Nelson, A. Stimuli-Responsive Materials in Additive Manufacturing. Prog. Polym. Sci. 2019, 93, 36–67. [Google Scholar] [CrossRef]
- Theato, P.; Sumerlin, B.S.; O’Reilly, R.K.; Epps, T.H., III. Stimuli Responsive Materials. Chem. Soc. Rev. 2013, 42, 7055. [Google Scholar] [CrossRef] [PubMed]
- Moulin, E.; Faour, L.; Carmona-Vargas, C.C.; Giuseppone, N. From Molecular Machines to Stimuli-Responsive Materials. Adv. Mater. 2020, 32, 1906036. [Google Scholar] [CrossRef]
- Abdollahi, A.; Roghani-Mamaqani, H.; Razavi, B.; Salami-Kalajahi, M. The Light-Controlling of Temperature-Responsivity in Stimuli-Responsive Polymers. Polym. Chem. 2019, 10, 5686–5720. [Google Scholar] [CrossRef]
- Jochum, F.D.; Theato, P. Temperature- and Light-Responsive Smart Polymer Materials. Chem. Soc. Rev. 2013, 42, 7468–7483. [Google Scholar] [CrossRef]
- Ruan, C.; Zeng, K.; Grimes, C.A. A Mass-Sensitive PH Sensor Based on a Stimuli-Responsive Polymer. Anal. Chim. Acta 2003, 497, 123–131. [Google Scholar] [CrossRef]
- Guragain, S.; Bastakoti, B.P.; Malgras, V.; Nakashima, K.; Yamauchi, Y. Multi-Stimuli-Responsive Polymeric Materials. Chem.-A Eur. J. 2015, 21, 13164–13174. [Google Scholar] [CrossRef]
- Andrade, F.; Roca-Melendres, M.M.; Durán-Lara, E.F.; Rafael, D.; Schwartz, S. Stimuli-Responsive Hydrogels for Cancer Treatment: The Role of PH, Light, Ionic Strength and Magnetic Field. Cancers 2021, 13, 1164. [Google Scholar] [CrossRef]
- Zheng, R.; Wei, Y.; Zhang, Z.; Wang, Z.; Ma, L.; Wang, Y.; Huang, L.; Lu, Y. Stimuli-responsive Active Materials for Dynamic Control of Light Field. Responsive Mater. 2023, 1, 230017. [Google Scholar] [CrossRef]
- Roy, D.; Cambre, J.N.; Sumerlin, B.S. Future Perspectives and Recent Advances in Stimuli-Responsive Materials. Prog. Polym. Sci. 2010, 35, 278–301. [Google Scholar] [CrossRef]
- Hiratani, T.; Kose, O.; Hamad, W.Y.; MacLachlan, M.J. Stable and Sensitive Stimuli-Responsive Anisotropic Hydrogels for Sensing Ionic Strength and Pressure. Mater. Horiz. 2018, 5, 1076–1081. [Google Scholar] [CrossRef]
- Tan, B.H.; Tam, K.C.; Lam, Y.C.; Tan, C.B. Microstructure and Rheology of Stimuli-Responsive Nanocolloidal SystemsEffect of Ionic Strength. Langmuir 2004, 20, 11380–11386. [Google Scholar] [CrossRef]
- Qi, L.; Qiao, J. Design of Switchable Enzyme Carriers Based on Stimuli-Responsive Porous Polymer Membranes for Bioapplications. ACS Appl. Bio Mater. 2021, 4, 4706–4719. [Google Scholar] [CrossRef]
- Liu, Y.; Terrell, J.L.; Tsao, C.; Wu, H.; Javvaji, V.; Kim, E.; Cheng, Y.; Wang, Y.; Ulijn, R.V.; Raghavan, S.R.; et al. Biofabricating Multifunctional Soft Matter with Enzymes and Stimuli-Responsive Materials. Adv. Funct. Mater. 2012, 22, 3004–3012. [Google Scholar] [CrossRef]
- Wang, Y.; Shim, M.S.; Levinson, N.S.; Sung, H.; Xia, Y. Stimuli-Responsive Materials for Controlled Release of Theranostic Agents. Adv. Funct. Mater. 2014, 24, 4206–4220. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.-M.; Zhang, S.X.-A. Stimuli-Induced Reversible Proton Transfer for Stimuli-Responsive Materials and Devices. Acc. Chem. Res. 2021, 54, 2216–2226. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-Responsive Self-Healing Materials Formed from Host–Guest Polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef]
- Medeiros, S.F.; Santos, A.M.; Fessi, H.; Elaissari, A. Stimuli-Responsive Magnetic Particles for Biomedical Applications. Int. J. Pharm. 2011, 403, 139–161. [Google Scholar] [CrossRef]
- Zhang, W.; Choi, H. Stimuli-Responsive Polymers and Colloids under Electric and Magnetic Fields. Polymers 2014, 6, 2803–2818. [Google Scholar] [CrossRef]
- Manouras, T.; Vamvakaki, M. Field Responsive Materials: Photo-, Electro-, Magnetic- and Ultrasound-Sensitive Polymers. Polym. Chem. 2017, 8, 74–96. [Google Scholar] [CrossRef]
- Lu, S.; Sun, Y.-H.; Shi, R.; Clark, C.; Li, L.; Chiang, V.L. Novel and Mechanical Stress–Responsive MicroRNAs in Populus Trichocarpa That Are Absent from Arabidopsis. Plant Cell 2005, 17, 2186–2203. [Google Scholar] [CrossRef] [PubMed]
- Dolui, T.; Natarajan, T.S.; Aiswarya, S.; Chanda, J.; Ghosh, P.; Mukhopadhyay, R.; Wießner, S.; Heinrich, G.; Das, A.; Banerjee, S.S. Stimuli–Responsive Mechanoadaptive Elastomeric Composite Materials: Challenges, Opportunities, and New Approaches. Adv. Eng. Mater. 2023, 25, 2300584. [Google Scholar] [CrossRef]
- Mano, J.F. Stimuli-Responsive Polymeric Systems for Biomedical Applications. Adv. Eng. Mater. 2008, 10, 515–527. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Tang, K.; Verpoort, F.; Sun, T. Polymer-Based Stimuli-Responsive Recyclable Catalytic Systems for Organic Synthesis. Small 2014, 10, 32–46. [Google Scholar] [CrossRef]
- Blelloch, N.D.; Yarbrough, H.J.; Mirica, K.A. Stimuli-Responsive Temporary Adhesives: Enabling Debonding on Demand through Strategic Molecular Design. Chem. Sci. 2021, 12, 15183–15205. [Google Scholar] [CrossRef]
- Jessop, P.G.; Mercer, S.M.; Heldebrant, D.J. CO2-Triggered Switchable Solvents, Surfactants, and Other Materials. Energy Environ. Sci. 2012, 5, 7240. [Google Scholar] [CrossRef]
- Lin, S.; Theato, P. CO2-Responsive Polymers. Macromol. Rapid Commun. 2013, 34, 1118–1133. [Google Scholar] [CrossRef]
- Guo, Z.; Feng, Y.; He, S.; Qu, M.; Chen, H.; Liu, H.; Wu, Y.; Wang, Y. CO2-Responsive “Smart” Single-Walled Carbon Nanotubes. Adv. Mater. 2013, 25, 584–590. [Google Scholar] [CrossRef]
- Liu, H.; Lin, S.; Feng, Y.; Theato, P. CO2-Responsive Polymer Materials. Polym. Chem. 2017, 8, 12–23. [Google Scholar] [CrossRef]
- Darabi, A.; Jessop, P.G.; Cunningham, M.F. CO2-Responsive Polymeric Materials: Synthesis, Self-Assembly, and Functional Applications. Chem. Soc. Rev. 2016, 45, 4391–4436. [Google Scholar] [CrossRef] [PubMed]
- Schattling, P.; Pollmann, I.; Theato, P. Synthesis of CO2-Responsive Polymers by Post-Polymerization Modification. React. Funct. Polym. 2014, 75, 16–21. [Google Scholar] [CrossRef]
- Deng, J.-N.; Zhao, H.; Zheng, H.; Zhuang, Y.; Wei, K.; Yuan, H.; Deng, Z.; Gao, Y.; Zhou, X.; Yu, T.; et al. CO2-Responsive Preformed Particle Gels with High Strength for CO2 Conformance Control in Heterogeneous Reservoirs. Fuel 2025, 379, 133040. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, H.; Ji, S.; Lin, X.; Yang, Z.; Zhang, Z. CO2-Triggered Synergistic Self-Assembly of Cationic Polymer and Anionic Surfactant Systems for Enhanced Profile Control and Carbon Storage. Colloids Surf. A Physicochem. Eng. Asp. 2025, 713, 136538. [Google Scholar] [CrossRef]
- Hicks, J.C.; Drese, J.H.; Fauth, D.J.; Gray, M.L.; Qi, G.; Jones, C.W. Designing Adsorbents for CO2 Capture from Flue Gas-Hyperbranched Aminosilicas Capable of Capturing CO2 Reversibly. J. Am. Chem. Soc. 2008, 130, 2902–2903. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, T.; Wang, B.; Qiu, D.; Ma, N. Highly Sensitive CO2-Responsive Polymeric Microgels That Respond Within Seconds. Langmuir 2015, 31, 8138–8145. [Google Scholar] [CrossRef]
- Darmayanti, M.G.; Tuck, K.L.; Thang, S.H. Carbon Dioxide Capture by Emerging Innovative Polymers: Status and Perspectives. Adv. Mater. 2024, 36, 2403324. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-N.; Ilhami, F.B.; Cheng, C.-C. CO2-Responsive Water-Soluble Conjugated Polymers as a Multifunctional Fluorescent Probe for Bioimaging Applications. Biomacromolecules 2024, 25, 997–1008. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Lai, Y.-C.; Shieh, Y.-T.; Chang, Y.-H.; Lee, A.-W.; Chen, J.-K.; Lee, D.-J.; Lai, J.-Y. CO2-Responsive Water-Soluble Conjugated Polymers for In Vitro and In Vivo Biological Imaging. Biomacromolecules 2020, 21, 5282–5291. [Google Scholar] [CrossRef]
- Du, N.; Park, H.B.; Robertson, G.P.; Dal-Cin, M.M.; Visser, T.; Scoles, L.; Guiver, M.D. Polymer Nanosieve Membranes for CO2-Capture Applications. Nat. Mater. 2011, 10, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, S.; Zhang, J.; Chen, Z.; Zhu, B.; Li, J.; Liang, S.; Bai, Y.; Xu, J.; Rao, D.; et al. Scalable and Switchable CO2-Responsive Membranes with High Wettability for Separation of Various Oil/Water Systems. Nat. Commun. 2023, 14, 1108. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Liu, Y.; Gao, Q.; Tao, D.; Wang, Y.; Guo, J.; Yu, Y. CO2-Responsive Nanofibrous Membranes with Gas-Tunable Wettability for Switchable Oil/Water Separation. React. Funct. Polym. 2023, 182, 105481. [Google Scholar] [CrossRef]
- Lin, S.; Schattling, P.; Theato, P. Thermo- and CO2-Responsive Linear Polymers and Hydrogels as CO2 Capturing Materials. Sci. Adv. Mater. 2015, 7, 948–955. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, Y.; Wen, X.; Liu, Y.; Feng, M.; Rui, Z. Development and Applications of CO2-Responsive Gels in CO2 Flooding and Geological Storage. Gels 2023, 9, 936. [Google Scholar] [CrossRef]
- Frauholz, J.; Cunningham, M.F.; Jessop, P.G. Thermo- and CO2-Responsive Copolymers for Forward Osmosis Applications. Ind. Eng. Chem. Res. 2024, 63, 10713–10720. [Google Scholar] [CrossRef]
- Davies, O.R.; Lewis, A.L.; Whitaker, M.J.; Tai, H.; Shakesheff, K.M.; Howdle, S.M. Applications of Supercritical CO2 in the Fabrication of Polymer Systems for Drug Delivery and Tissue Engineering. Adv. Drug Deliv. Rev. 2008, 60, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ke, Y.; Hu, X.; Peng, F.; Yu, C.; Xing, L. Synthesis and Switchable Behavior of a CO2 Responsive Polymeric Surfactant Acting as Emulsifier. J. Dispers. Sci. Technol. 2021, 42, 407–415. [Google Scholar] [CrossRef]
- Ma, Y.; Promthaveepong, K.; Li, N. CO2-Responsive Polymer-Functionalized Au Nanoparticles for CO2 Sensor. Anal. Chem. 2016, 88, 8289–8293. [Google Scholar] [CrossRef]
- Zhang, Q.; Lei, L.; Zhu, S. Gas-Responsive Polymers. ACS Macro Lett. 2017, 6, 515–522. [Google Scholar] [CrossRef]
- Xie, T.; Zhang, H.; Bai, Y.; Mei, K.; Zheng, Y.; Zhang, C.; Cheng, X. Study on the Effect of CO2-Responsive Hydrogel Prepared by Free Radical Polymerization on the Self-Healing of Cement Paste Fractures: Application in CCUS Cementing. Constr. Build. Mater. 2024, 435, 136651. [Google Scholar] [CrossRef]
- Abousalman-Rezvani, Z.; Eskandari, P.; Roghani-Mamaqani, H.; Mardani, H.; Salami-Kalajahi, M. Grafting Light-, Temperature, and CO2-Responsive Copolymers from Cellulose Nanocrystals by Atom Transfer Radical Polymerization for Adsorption of Nitrate Ions. Polymer 2019, 182, 121830. [Google Scholar] [CrossRef]
- Garcia-Valdez, O.; Brescacin, T.; Arredondo, J.; Bouchard, J.; Jessop, P.G.; Champagne, P.; Cunningham, M.F. Grafting CO2-Responsive Polymers from Cellulose Nanocrystals via Nitroxide-Mediated Polymerisation. Polym. Chem. 2017, 8, 4124–4131. [Google Scholar] [CrossRef]
- Quek, J.Y.; Roth, P.J.; Evans, R.A.; Davis, T.P.; Lowe, A.B. Reversible Addition–Fragmentation Chain Transfer Synthesis of Amidine-based, CO2-responsive Homo and AB Diblock (Co)Polymers Comprised of Histamine and Their Gas-triggered Self-assembly in Water. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 394–404. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Y.; Huang, X.; Kang, T.; Severtson, S.J.; Wang, W.-J.; Liu, P. Tailoring CO2-Responsive Polymers and Nanohybrids for Green Chemistry and Processes. Ind. Eng. Chem. Res. 2019, 58, 15088–15108. [Google Scholar] [CrossRef]
- Lefevre, E.R.M.A. Method for Shear Coagulation of Latex Resins. U.S. Patent No. 4,623,678, 18 November 1986. [Google Scholar]
- Han, D.; Boissiere, O.; Kumar, S.; Tong, X.; Tremblay, L.; Zhao, Y. Two-Way CO2-Switchable Triblock Copolymer Hydrogels. Macromolecules 2012, 45, 7440–7445. [Google Scholar] [CrossRef]
- Fischer, V.; Landfester, K.; Muñoz-Espí, R. Molecularly Controlled Coagulation of Carboxyl-Functionalized Nanoparticles Prepared by Surfactant-Free Miniemulsion Polymerization. ACS Macro Lett. 2012, 1, 1371–1374. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, H.; Feng, Y. CO2-Responsive Anionic Wormlike Micelles Based on Natural Erucic Acid. Green Mater. 2014, 2, 95–103. [Google Scholar] [CrossRef]
- Shieh, Y.-T.; Yeh, Y.-C.; Cheng, C.-C. Two-Way CO2-Responsive Polymer Particles with Controllable Amphiphilic Properties. ACS Omega 2020, 5, 1862–1869. [Google Scholar] [CrossRef]
- Liu, P.; Lu, W.; Wang, W.-J.; Li, B.-G.; Zhu, S. Highly CO2 /N2-Switchable Zwitterionic Surfactant for Pickering Emulsions at Ambient Temperature. Langmuir 2014, 30, 10248–10255. [Google Scholar] [CrossRef]
- Cunningham, M.F.; Jessop, P.G. An Introduction to the Principles and Fundamentals of CO2-Switchable Polymers and Polymer Colloids. Eur. Polym. J. 2016, 76, 208–215. [Google Scholar] [CrossRef]
- Liu, Y.; Jessop, P.G.; Cunningham, M.; Eckert, C.A.; Liotta, C.L. Switchable Surfactants. Science 2006, 313, 958–960. [Google Scholar] [CrossRef]
- Scott, L.M.; Robert, T.; Harjani, J.R.; Jessop, P.G. Designing the Head Group of CO2-Triggered Switchable Surfactants. RSC Adv. 2012, 2, 4925. [Google Scholar] [CrossRef]
- Harjani, J.R.; Liang, C.; Jessop, P.G. A Synthesis of Acetamidines. J. Org. Chem. 2011, 76, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Quek, J.Y.; Davis, T.P.; Lowe, A.B. Amidine Functionality as a Stimulus-Responsive Building Block. Chem. Soc. Rev. 2013, 42, 7326. [Google Scholar] [CrossRef]
- Chen, S.; Lin, X.; Zhai, Z.; Lan, R.; Li, J.; Wang, Y.; Zhou, S.; Farooqi, Z.H.; Wu, W. Synthesis and Characterization of CO2-Sensitive Temperature-Responsive Catalytic Poly(Ionic Liquid) Microgels. Polym. Chem. 2018, 9, 2887–2896. [Google Scholar] [CrossRef]
- Fowler, C.I.; Jessop, P.G.; Cunningham, M.F. Aryl Amidine and Tertiary Amine Switchable Surfactants and Their Application in the Emulsion Polymerization of Methyl Methacrylate. Macromolecules 2012, 45, 2955–2962. [Google Scholar] [CrossRef]
- Barzagli, F.; Mani, F.; Peruzzini, M. A 13C NMR Study of the Carbon Dioxide Absorption and Desorption Equilibria by Aqueous 2-Aminoethanol and N-Methyl-Substituted 2-Aminoethanol. Energy Environ. Sci. 2009, 2, 322. [Google Scholar] [CrossRef]
- Mercer, S.M.; Jessop, P.G. “Switchable Water”: Aqueous Solutions of Switchable Ionic Strength. ChemSusChem 2010, 3, 467–470. [Google Scholar] [CrossRef]
- Darabi, A.; Shirin-Abadi, A.R.; Pinaud, J.; Jessop, P.G.; Cunningham, M.F. Nitroxide-Mediated Surfactant-Free Emulsion Copolymerization of Methyl Methacrylate and Styrene Using Poly(2-(Diethyl)Aminoethyl Methacrylate-Co-Styrene) as a Stimuli-Responsive Macroalkoxyamine. Polym. Chem. 2014, 5, 6163–6170. [Google Scholar] [CrossRef]
- Adekomi, A.A. Enhanced CO2 Capture and Desorption Using Surface-Active Tertiary Amine. Doctoral Dissertation, The University of Texas at Austin, Austin, TX, USA, 2024. [Google Scholar]
- Phan, L.; Chiu, D.; Heldebrant, D.J.; Huttenhower, H.; John, E.; Li, X.; Pollet, P.; Wang, R.; Eckert, C.A.; Liotta, C.L.; et al. Switchable Solvents Consisting of Amidine/Alcohol or Guanidine/Alcohol Mixtures. Ind. Eng. Chem. Res. 2008, 47, 539–545. [Google Scholar] [CrossRef]
- Pashayev, E.; Georgopanos, P. CO2-Responsive Copolymers for Membrane Applications, Synthesis, and Performance Evaluation. Macromol. Mater. Eng. 2025, 310, 2400290. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, M.; Su, X.; Feng, Y. CO2-Responsive Polymer Promoted by Polyether to Efficient Viscosity Increase for CO2 Plugging. Polymer 2024, 306, 127227. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.-J.; Lu, Y.; Li, B.-G.; Zhu, S. Reversibly Coagulatable and Redispersible Polystyrene Latex Prepared by Emulsion Polymerization of Styrene Containing Switchable Amidine. Macromolecules 2011, 44, 6539–6545. [Google Scholar] [CrossRef]
- Asua, J.M. (Ed.) Polymer Reaction Engineering; Wiley: Hoboken, NJ, USA, 2007; ISBN 9781405144421. [Google Scholar]
- Fowler, C.I.; Muchemu, C.M.; Miller, R.E.; Phan, L.; O’Neill, C.; Jessop, P.G.; Cunningham, M.F. Emulsion Polymerization of Styrene and Methyl Methacrylate Using Cationic Switchable Surfactants. Macromolecules 2011, 44, 2501–2509. [Google Scholar] [CrossRef]
- Mihara, M.; Jessop, P.; Cunningham, M. Redispersible Polymer Colloids Using Carbon Dioxide as an External Trigger. Macromolecules 2011, 44, 3688–3693. [Google Scholar] [CrossRef]
- Che, N.; Yang, S.; Kang, H.; Liu, R.; Li, Z.; Liu, Z.; Li, P.; Qu, X.; Huang, Y. Synthesis and Properties of CO2-Switchable Dex-g-PAHMA Copolymers. Polym. Chem. 2014, 5, 7109–7120. [Google Scholar] [CrossRef]
- Morse, A.J.; Armes, S.P.; Thompson, K.L.; Dupin, D.; Fielding, L.A.; Mills, P.; Swart, R. Novel Pickering Emulsifiers Based on PH-Responsive Poly(2-(Diethylamino)Ethyl Methacrylate) Latexes. Langmuir 2013, 29, 5466–5475. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lou, Y.; Zhai, X.; Li, Z.; Liu, B. Development and Characterization of CO2 –Responsive Intelligent Polymer Sealant. ACS Omega 2023, 8, 35066–35076. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Dai, X.; Xu, Q.; Zhang, L.; Tan, J. Open-Air Preparation of Cross-Linked CO2-Responsive Polymer Vesicles by Enzyme-Assisted Photoinitiated Polymerization-Induced Self-Assembly. Chem. Commun. 2019, 55, 11920–11923. [Google Scholar] [CrossRef]
- Ellis, S.N.; Riabtseva, A.; Dykeman, R.R.; Hargreaves, S.; Robert, T.; Champagne, P.; Cunningham, M.F.; Jessop, P.G. Nitrogen Rich CO2-Responsive Polymers as Forward Osmosis Draw Solutes. Ind. Eng. Chem. Res. 2019, 58, 22579–22586. [Google Scholar] [CrossRef]
- Gupta, M.; Lee, H. A Pyrene Derived CO2-Responsive Polymeric Probe for the Turn-On Fluorescent Detection of Nerve Agent Mimics with Tunable Sensitivity. Macromolecules 2017, 50, 6888–6895. [Google Scholar] [CrossRef]
- Fan, W.; Tong, X.; Farnia, F.; Yu, B.; Zhao, Y. CO2-Responsive Polymer Single-Chain Nanoparticles and Self-Assembly for Gas-Tunable Nanoreactors. Chem. Mater. 2017, 29, 5693–5701. [Google Scholar] [CrossRef]
- Corrigan, N.; Jung, K.; Moad, G.; Hawker, C.J.; Matyjaszewski, K.; Boyer, C. Reversible-Deactivation Radical Polymerization (Controlled/Living Radical Polymerization): From Discovery to Materials Design and Applications. Prog. Polym. Sci. 2020, 111, 101311. [Google Scholar] [CrossRef]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/Living Radical Polymerization: Features, Developments, and Perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- SZWARC, M. ‘Living’ Polymers. Nature 1956, 178, 1168–1169. [Google Scholar] [CrossRef]
- Cunningham, M.F. Controlled/Living Radical Polymerization in Aqueous Dispersed Systems. Prog. Polym. Sci. 2008, 33, 365–398. [Google Scholar] [CrossRef]
- Zetterlund, P.B.; Kagawa, Y.; Okubo, M. Controlled/Living Radical Polymerization in Dispersed Systems. Chem. Rev. 2008, 108, 3747–3794. [Google Scholar] [CrossRef] [PubMed]
- Asua, J.M. Miniemulsion Polymerization. Prog. Polym. Sci. 2002, 27, 1283–1346. [Google Scholar] [CrossRef]
- Qiu, J.; Charleux, B.; Matyjaszewski, K. Controlled/Living Radical Polymerization in Aqueous Media: Homogeneous and Heterogeneous Systems. Prog. Polym. Sci. 2001, 26, 2083–2134. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom Transfer Radical Polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Yan, Q.; Zhou, R.; Fu, C.; Zhang, H.; Yin, Y.; Yuan, J. CO2-Responsive Polymeric Vesicles That Breathe. Angew. Chem. Int. Ed. 2011, 50, 4923–4927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qian, J.; Fan, Y.; Feng, W.; Tao, Z.; Yang, H. A Facile CO2 Switchable Nanocomposite with Reversible Transition from Sol to Self-Healable Hydrogel. RSC Adv. 2015, 5, 62229–62234. [Google Scholar] [CrossRef]
- Yan, Q.; Zhao, Y. CO2-Stimulated Diversiform Deformations of Polymer Assemblies. J. Am. Chem. Soc. 2013, 135, 16300–16303. [Google Scholar] [CrossRef]
- Xu, L.Q.; Zhang, B.; Sun, M.; Hong, L.; Neoh, K.-G.; Kang, E.-T.; Fu, G.D. CO2-Triggered Fluorescence “Turn-on” Response of Perylene Diimide-Containing Poly(N,N-Dimethylaminoethyl Methacrylate). J. Mater. Chem. A 2013, 1, 1207–1212. [Google Scholar] [CrossRef]
- Su, X.; Jessop, P.G.; Cunningham, M.F. Versatility of Organocatalyzed Atom Transfer Radical Polymerization and CO2-Switching for Preparing Both Hydrophobic and Hydrophilic Polymers with the Recycling of a Photocatalyst. Macromolecules 2019, 52, 6725–6733. [Google Scholar] [CrossRef]
- Barner-Kowollik, C. (Ed.) Handbook of RAFT Polymerization; Wiley: Hoboken, NJ, USA, 2008; ISBN 9783527319244. [Google Scholar]
- Lin, S.; Das, A.; Theato, P. CO2-Responsive Graft Copolymers: Synthesis and Characterization. Polym. Chem. 2017, 8, 1206–1216. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, T.; Wu, Y.; Shi, W.; Choi, B.; Feng, A.; Thang, S.H. Synthesis of CO2-Responsive Gradient Copolymers by Switchable RAFT Polymerization and Their Controlled Self-Assembly. Polym. Chem. 2020, 11, 6794–6802. [Google Scholar] [CrossRef]
- Chen, L.; Liu, R.; Yan, Q. Polymer Meets Frustrated Lewis Pair: Second-Generation CO2-Responsive Nanosystem for Sustainable CO2 Conversion. Angew. Chem. 2018, 130, 9480–9484. [Google Scholar] [CrossRef]
- Abel, B.A.; Sims, M.B.; McCormick, C.L. Tunable PH- and CO2-Responsive Sulfonamide-Containing Polymers by RAFT Polymerization. Macromolecules 2015, 48, 5487–5495. [Google Scholar] [CrossRef]
- Feng, A.; Zhan, C.; Yan, Q.; Liu, B.; Yuan, J. A CO2—And Temperature-Switchable “Schizophrenic” Block Copolymer: From Vesicles to Micelles. Chem. Commun. 2014, 50, 8958. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Feng, Y.; Wang, Y.; Wang, J.; Wu, Y.; Zhang, Y. A Novel Smart Polymer Responsive to CO2. Chem. Commun. 2011, 47, 9348. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, Z.; He, S.; Yin, H.; Fei, C.; Feng, Y. CO2-Driven Vesicle to Micelle Regulation of Amphiphilic Copolymer: Random versus Block Strategy. Polym. Chem. 2014, 5, 4756–4763. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.; Dreiss, C.A.; Feng, Y. CO2-Switchable Multi-Compartment Micelles with Segregated Corona. Soft Matter 2014, 10, 6387–6391. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, G.; Wang, W.-J.; Yuan, H.; Li, B.-G.; Zhu, S. Switchable Block Copolymer Surfactants for Preparation of Reversibly Coagulatable and Redispersible Poly(Methyl Methacrylate) Latexes. Macromolecules 2013, 46, 1261–1267. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Zhou, S.; Zhang, H.; Feng, A.-C.; Jian, C.; Hu, J.; Gao, W.; Yuan, J. Synthesis and Self-Assembly of CO2 –Temperature Dual Stimuli-Responsive Triblock Copolymers. Macromolecules 2014, 47, 2938–2946. [Google Scholar] [CrossRef]
- Hu, J.; Whittaker, M.R.; Li, Y.; Quinn, J.F.; Davis, T.P. The Use of Endogenous Gaseous Molecules (NO and CO2) to Regulate the Self-Assembly of a Dual-Responsive Triblock Copolymer. Polym. Chem. 2015, 6, 2407–2415. [Google Scholar] [CrossRef]
- Yuan, W.; Shen, J.; Zou, H. Amphiphilic Block Copolymer Terminated with Pyrene Group: From Switchable CO2-Temperature Dual Responses to Tunable Fluorescence. RSC Adv. 2015, 5, 13145–13152. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, G.; Wei, Z.; Li, X.; Tang, B. Preparation and Drug Release Property of CO2 Stimulus-Sensitive Poly(N, N-Dimethylaminoethyl Methacrylate)-b-Polystyrene Nanoparticles. Eur. Polym. J. 2013, 49, 3165–3170. [Google Scholar] [CrossRef]
- Wang, T.-T.; Wu, Y.-Y.; Luo, Z.-H.; Zhou, Y.-N. “Living” Polymer Dispersity Quantification for Nitroxide-Mediated Polymerization Systems by Mimicking a Monodispersed Polymer Blending Strategy. Macromolecules 2020, 53, 10813–10822. [Google Scholar] [CrossRef]
- Solomon, D.H. Genesis of the CSIRO Polymer Group and the Discovery and Significance of Nitroxide-mediated Living Radical Polymerization. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5748–5764. [Google Scholar] [CrossRef]
- Georges, M.K.; Veregin, R.P.N.; Kazmaier, P.M.; Hamer, G.K. Narrow Molecular Weight Resins by a Free-Radical Polymerization Process. Macromolecules 1993, 26, 2987–2988. [Google Scholar] [CrossRef]
- Bertin, D.; Gigmes, D.; Marque, S.R.A.; Tordo, P. Kinetic Subtleties of Nitroxide Mediated Polymerization. Chem. Soc. Rev. 2011, 40, 2189. [Google Scholar] [CrossRef]
- Zhou, K.; Li, J.; Lu, Y.; Zhang, G.; Xie, Z.; Wu, C. Re-Examination of Dynamics of Polyeletrolytes in Salt-Free Dilute Solutions by Designing and Using a Novel Neutral−Charged−Neutral Reversible Polymer. Macromolecules 2009, 42, 7146–7154. [Google Scholar] [CrossRef]
- Glasing, J.; Bouchard, J.; Jessop, P.G.; Champagne, P.; Cunningham, M.F. Grafting Well-Defined CO2-Responsive Polymers to Cellulose Nanocrystals via Nitroxide-Mediated Polymerisation: Effect of Graft Density and Molecular Weight on Dispersion Behaviour. Polym. Chem. 2017, 8, 6000–6012. [Google Scholar] [CrossRef]
- Zehm, D.; Laschewsky, A.; Liang, H.; Rabe, J.P. Straightforward Access to Amphiphilic Dual Bottle Brushes by Combining RAFT, ATRP, and NMP Polymerization in One Sequence. Macromolecules 2011, 44, 9635–9641. [Google Scholar] [CrossRef]
- Muzammal, S.; Ahmad, A.; Sheraz, M.; Kim, J.; Ali, S.; Hanif, M.B.; Hussain, I.; Pandiaraj, S.; Alodhayb, A.; Javed, M.S.; et al. Polymer-Supported Nanomaterials for Photodegradation: Unraveling the Methylene Blue Menace. Energy Convers. Manag. X 2024, 22, 100547. [Google Scholar] [CrossRef]
- Stastny, V.; Rudkevich, D.M. Separations Using Carbon Dioxide. J. Am. Chem. Soc. 2007, 129, 1018–1019. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, Q.; Shi, S.; Zhu, S. Highly Porous Poly(High Internal Phase Emulsion) Membranes with “Open-Cell” Structure and CO2-Switchable Wettability Used for Controlled Oil/Water Separation. Langmuir 2017, 33, 11936–11944. [Google Scholar] [CrossRef]
- Alex, J.P. Cormier CO2-Responsive Polymer Particles and Gels for Forward Osmosis; Queen’s University: Kingston, ON, Canada, 2023. [Google Scholar]
- Dou, B.; Lin, S.; Wang, Y.; Yang, L.; Yao, A.; Liao, H.; Tian, S.; Shang, J.; Lan, J. Versatile CO2-Responsive Sponges Decorated with ZIF-8 for Bidirectional Separation of Oil/Water and Controllable Removal of Dyes. ACS Appl. Mater. Interfaces 2023, 15, 37867–37883. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, L.; Grishkewich, N.; Tam, K.C.; Yuan, J.; Mao, Z.; Sui, X. CO2-Responsive Cellulose Nanofibers Aerogels for Switchable Oil–Water Separation. ACS Appl. Mater. Interfaces 2019, 11, 9367–9373. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Y.; Jiang, N.; Cheng, W.; Lu, D.; Zhang, T. Separate Reclamation of Oil and Surfactant from Oil-in-Water Emulsion with a CO2-Responsive Material. Environ. Sci. Technol. 2022, 56, 9651–9660. [Google Scholar] [CrossRef]
- Che, H.; Huo, M.; Peng, L.; Fang, T.; Liu, N.; Feng, L.; Wei, Y.; Yuan, J. CO2-Responsive Nanofibrous Membranes with Switchable Oil/Water Wettability. Angew. Chem. 2015, 127, 9062–9066. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, W.-Z.; Wang, Y.-M.; Qu, J.-P.; Lu, X.-B. N-Heterocyclic Carbene Functionalized Polymer for Reversible Fixation−Release of CO2. Macromolecules 2009, 42, 5419–5421. [Google Scholar] [CrossRef]
- Kainz, J.; Werz, P.D.L.; Troll, C.; Rieger, B. Temperature and CO2 Responsive Polyethylenimine for Highly Efficient Carbon Dioxide Release. RSC Adv. 2015, 5, 9556–9560. [Google Scholar] [CrossRef]
- Endo, T.; Nagai, D.; Monma, T.; Yamaguchi, H.; Ochiai, B. A Novel Construction of a Reversible Fixation−Release System of Carbon Dioxide by Amidines and Their Polymers. Macromolecules 2004, 37, 2007–2009. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, Z.; Thanneeru, S.; Meng, M.; Kruzyk, M.; Ung, G.; Liu, B.; He, J. A Polymer Solution To Prevent Nanoclustering and Improve the Selectivity of Metal Nanoparticles for Electrocatalytic CO2 Reduction. Angew. Chem. 2019, 131, 15981–15987. [Google Scholar] [CrossRef]
- Pham, T.H.M.; Zhang, J.; Li, M.; Shen, T.; Ko, Y.; Tileli, V.; Luo, W.; Züttel, A. Enhanced Electrocatalytic CO2 Reduction to C2+ Products by Adjusting the Local Reaction Environment with Polymer Binders. Adv. Energy Mater. 2022, 12, 2103663. [Google Scholar] [CrossRef]
- Soucy, T.L.; Dean, W.S.; Zhou, J.; Rivera Cruz, K.E.; McCrory, C.C.L. Considering the Influence of Polymer–Catalyst Interactions on the Chemical Microenvironment of Electrocatalysts for the CO2 Reduction Reaction. Acc. Chem. Res. 2022, 55, 252–261. [Google Scholar] [CrossRef]
- Liu, Y.; McCrory, C.C.L. Modulating the Mechanism of Electrocatalytic CO2 Reduction by Cobalt Phthalocyanine through Polymer Coordination and Encapsulation. Nat. Commun. 2019, 10, 1683. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Huo, M.; Peng, L.; Ye, Q.; Guo, J.; Wang, K.; Wei, Y.; Yuan, J. CO2-Switchable Drug Release from Magneto-Polymeric Nanohybrids. Polym. Chem. 2015, 6, 2319–2326. [Google Scholar] [CrossRef]
- Shigemura, M.; Lecuona, E.; Sznajder, J.I. Effects of Hypercapnia on the Lung. J. Physiol. 2017, 595, 2431–2437. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Huang, B.; Xiao, C.; Vana, P. Intelligent CO2—And Photo-Dual-Responsive Polymer Vesicles with Tunable Wall Thickness. Polym. Chem. 2019, 10, 1610–1618. [Google Scholar] [CrossRef]
- Rabiee, H.; Jin, B.; Yun, S.; Dai, S. Gas-Responsive Cationic Microgels for Forward Osmosis Desalination. Chem. Eng. J. 2018, 347, 424–431. [Google Scholar] [CrossRef]
- Riabtseva, A.; Ellis, S.N.; Champagne, P.; Jessop, P.G.; Cunningham, M.F. CO2-Responsive Branched Polymers for Forward Osmosis Applications: The Effect of Branching on Draw Solute Properties. Ind. Eng. Chem. Res. 2021, 60, 9807–9816. [Google Scholar] [CrossRef]
- Jessop, P.G.; Mercer, S.M.; Robert, T.; Brown, R.S.; Clark, T.J.; Mariampillai, B.E.; Resendes, R.; Wechsler, D. Systems and Methods for Use of Water with Switchable Ionic Strength. U.S. Patent No. 10,377,647, 13 August 2019. [Google Scholar]
- Cai, Y.; Shen, W.; Wang, R.; Krantz, W.B.; Fane, A.G.; Hu, X. CO2 Switchable Dual Responsive Polymers as Draw Solutes for Forward Osmosis Desalination. Chem. Commun. 2013, 49, 8377. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, E.; Wang, J. Gas-Breathing Polymer Film for Constructing Switchable Ionic Diodes. RSC Adv. 2015, 5, 35622–35630. [Google Scholar] [CrossRef]
- Bhamidipati, M.; Scurto, A.M.; Detamore, M.S. The Future of Carbon Dioxide for Polymer Processing in Tissue Engineering. Tissue Eng. Part B Rev. 2013, 19, 221–232. [Google Scholar] [CrossRef]
- Sheridan, M.; Shea, L.; Peters, M.; Mooney, D. Bioabsorbable Polymer Scaffolds for Tissue Engineering Capable of Sustained Growth Factor Delivery. J. Control. Release 2000, 64, 91–102. [Google Scholar] [CrossRef]
- Shieh, Y.-T.; Lin, Y.-T.; Cheng, C.-C. CO2-Switchable Behavior of Chitosan-g-Poly[(2-Dimethylamino)Ethyl Methacrylate] as an Emulsifier. Carbohydr. Polym. 2017, 170, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, S.; Mokhtari, R.; Hohl, L.; Miller, R.; Kraume, M. Advances in CO2-Switchable Surfactants towards the Fabrication and Application of Responsive Colloids. Adv. Colloid Interface Sci. 2023, 315, 102907. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yung, L.-Y.L. Detection of Dissolved CO2 Based on the Aggregation of Gold Nanoparticles. Anal. Chem. 2014, 86, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Wang, J.; Yin, Y.; Yuan, J. Breathing Polymersomes: CO2-Tuning Membrane Permeability for Size-Selective Release, Separation, and Reaction. Angew. Chem. Int. Ed. 2013, 52, 5070–5073. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).