The Synthesis of B-Doped Porous Carbons via a Sodium Metaborate Tetrahydrate Activating Agent: A Novel Approach for CO2 Adsorption
Abstract
1. Introduction
2. Results and Discussion
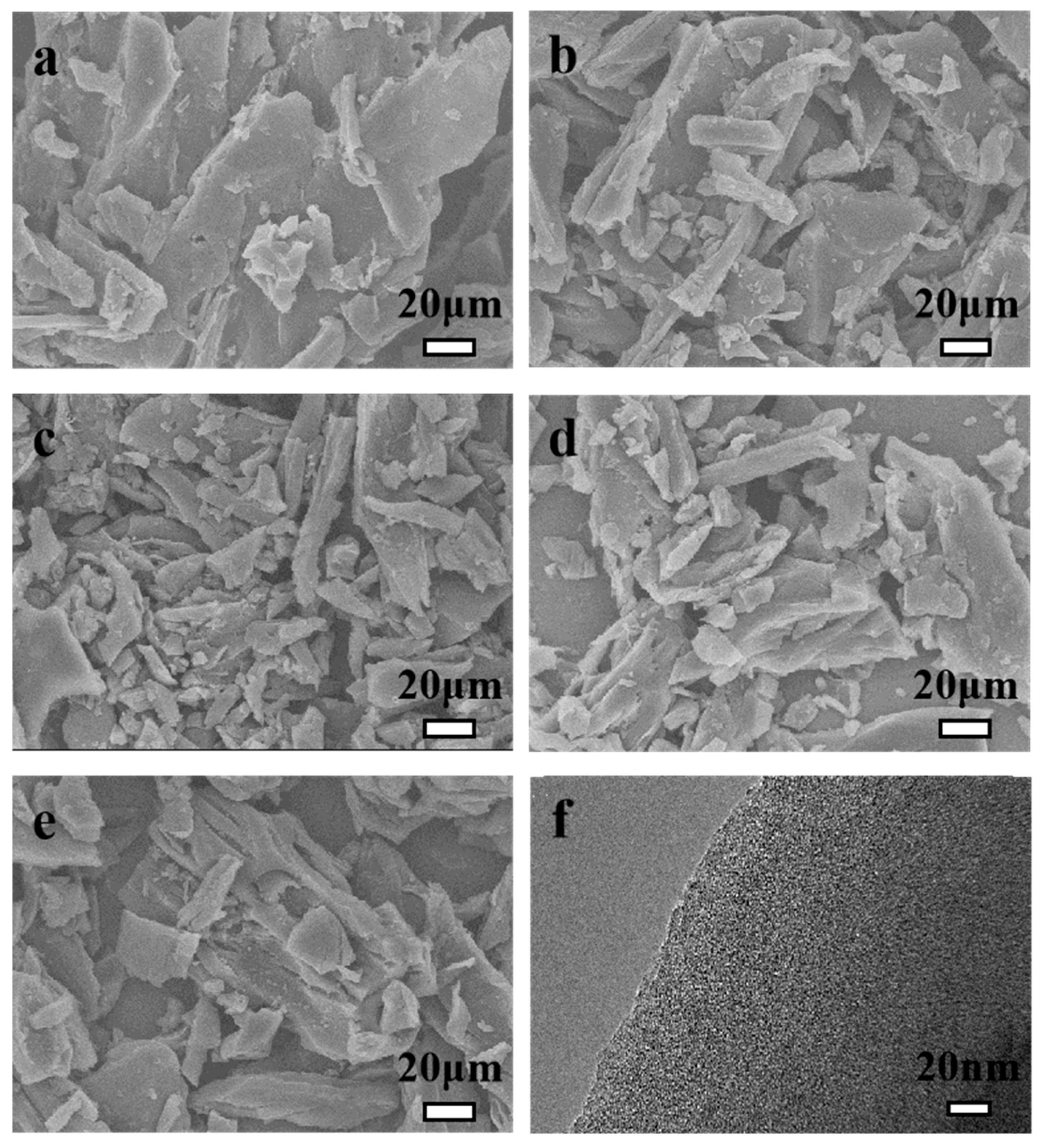
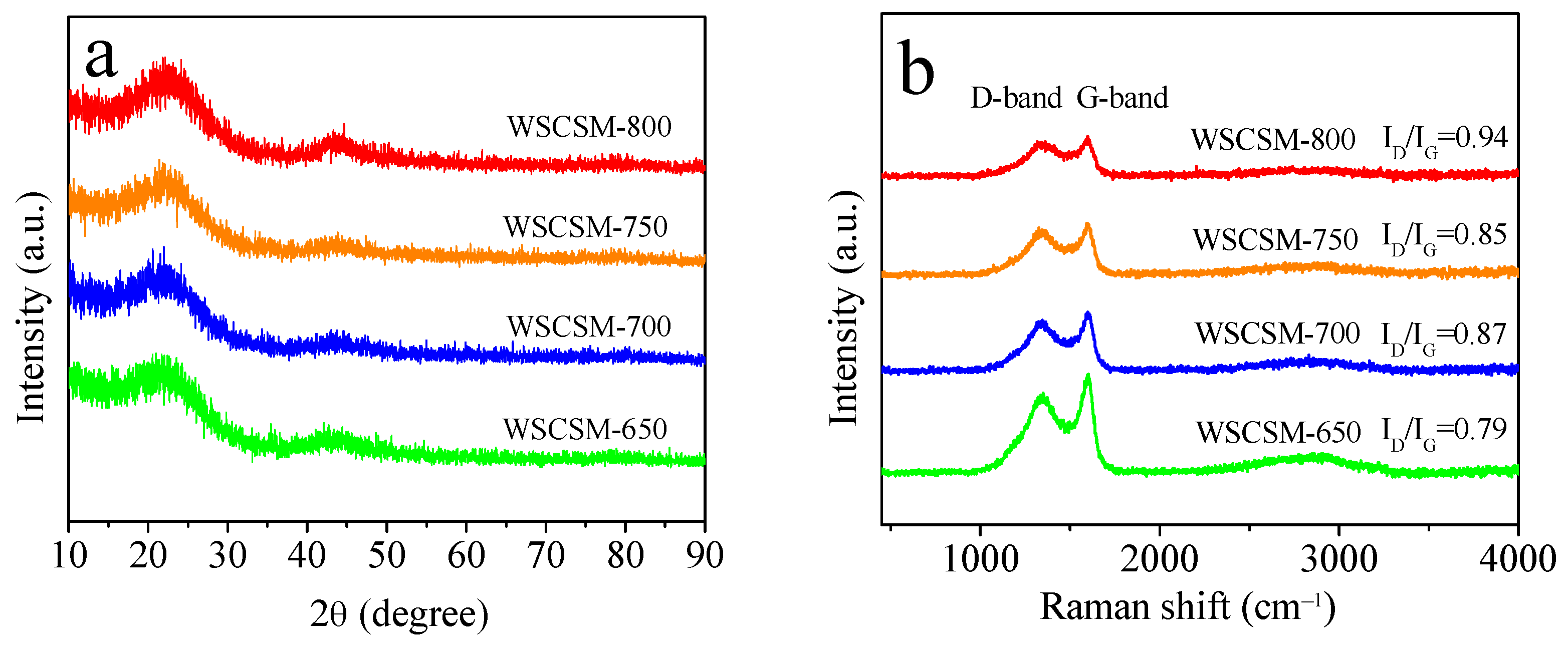
2.1. Morphology, Structural Phases, and Surface Chemistry Analysis
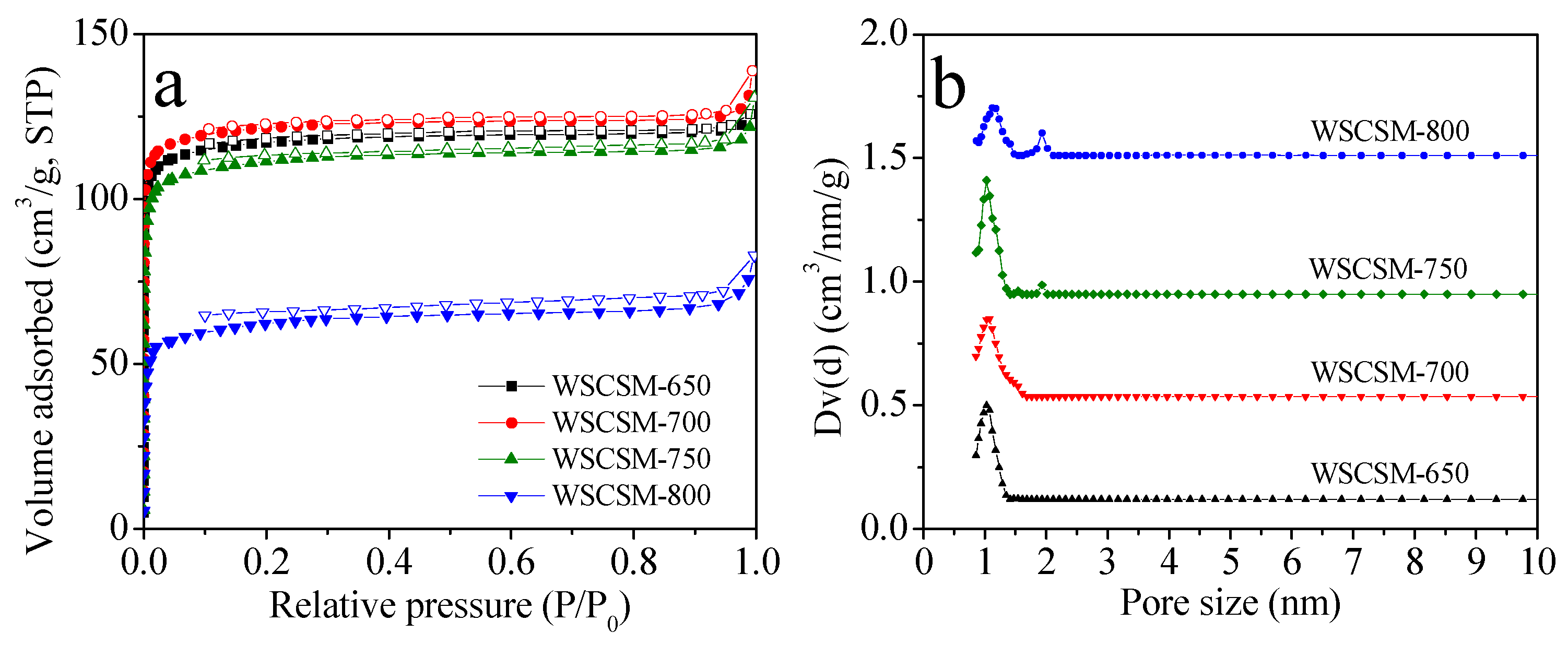
2.2. Textural Properties Analysis
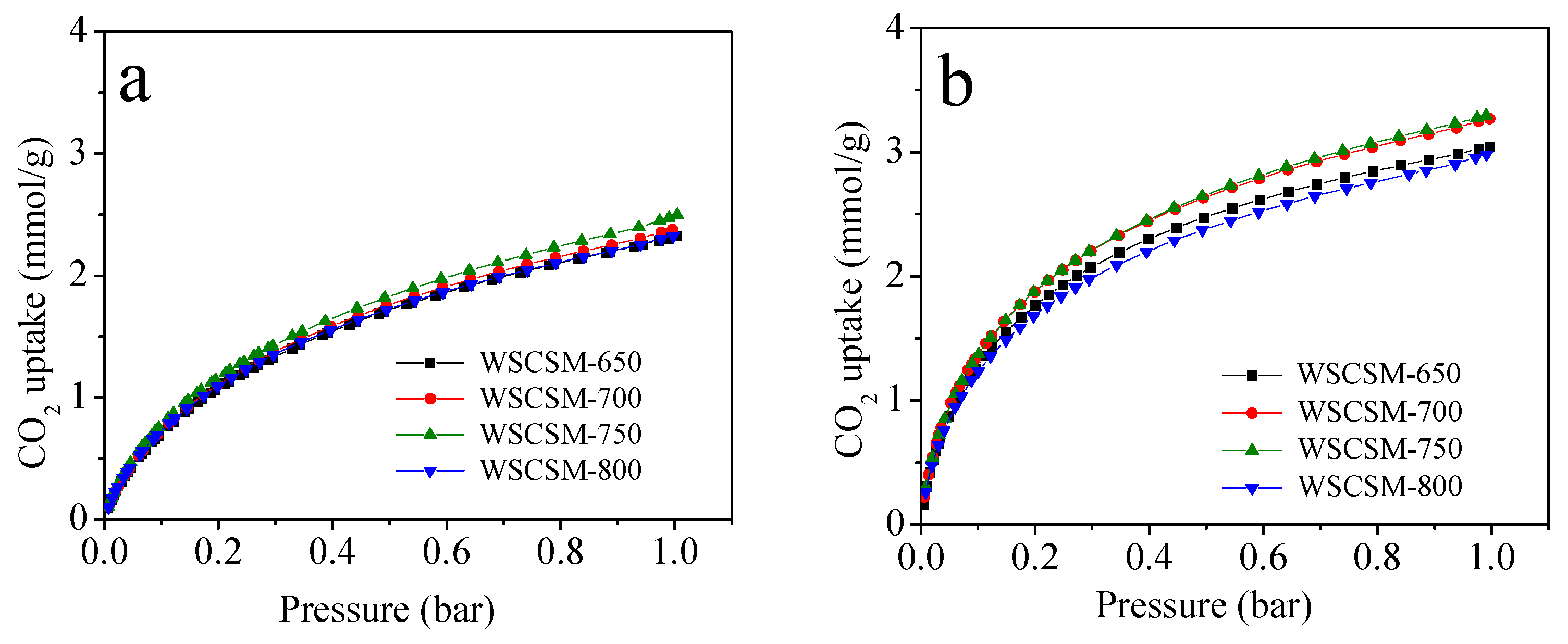
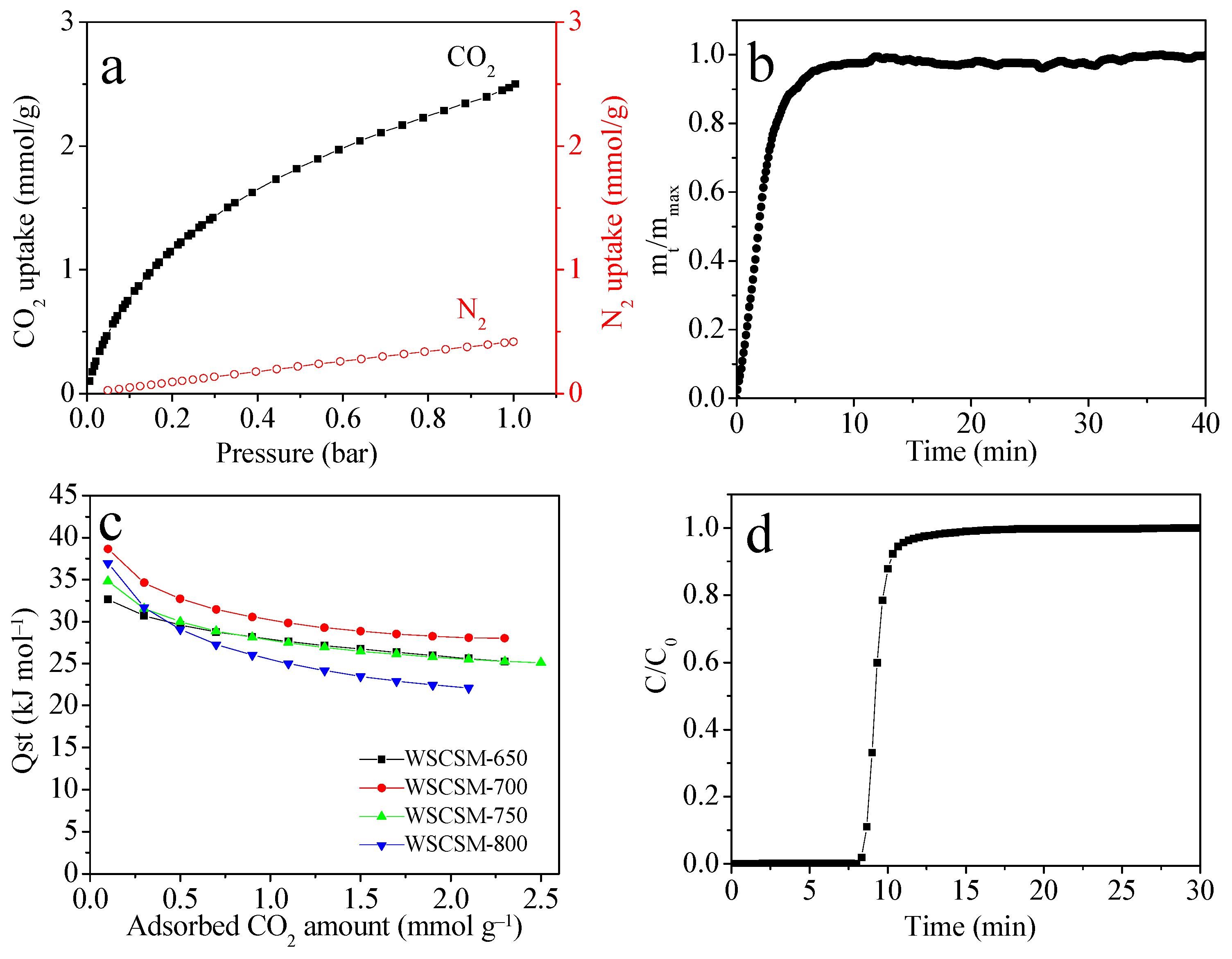
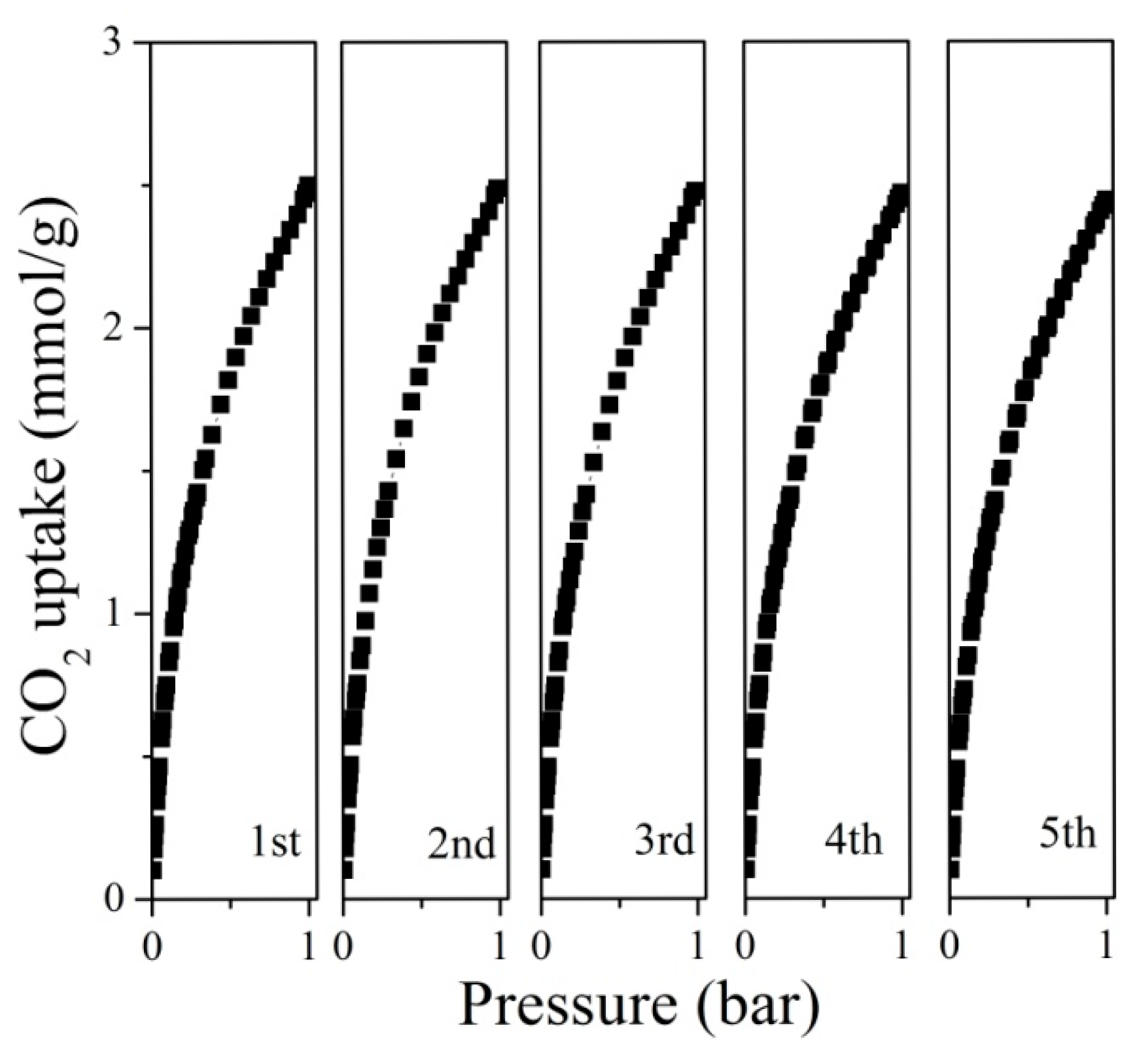
2.3. CO2 Adsorption Performance
3. Synthesis and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanifa, M.; Agarwal, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. A review on CO2 capture and sequestration in the construction industry: Emerging approaches and commercialised technologies. J. CO2 Util. 2023, 67, 102292. [Google Scholar] [CrossRef]
- Xu, W.-L.; Chen, H.-J.; Wang, Y.-C.; Liu, S.; Wan, X.-Y.; Peng, H.-L.; Huang, K. Chitin-derived fibrous carbon microspheres as support of polyamine for remarkable CO2 capture. Green Chem. Eng. 2022, 3, 267–279. [Google Scholar] [CrossRef]
- Sun, L.-B.; Kang, Y.-H.; Shi, Y.-Q.; Jiang, Y.; Liu, X.-Q. Highly Selective Capture of the Greenhouse Gas CO2 in Polymers. ACS Sustain. Chem. Eng. 2015, 3, 3077–3085. [Google Scholar] [CrossRef]
- Shao, L.; Li, Y.; Huang, J.; Liu, Y.-N. Synthesis of Triazine-Based Porous Organic Polymers Derived N-Enriched Porous Carbons for CO2 Capture. Ind. Eng. Chem. Res. 2018, 57, 2856–2865. [Google Scholar] [CrossRef]
- Sang, Y.F.; Cao, Y.W.; Wang, L.Z.; Yan, W.; Chen, T.W.; Huang, J.H.; Liu, Y.N. N-rich porous organic polymers based on Schiff base reaction for CO2 capture and mercury(II) adsorption. J. Colloid Interface Sci. 2021, 587, 121–130. [Google Scholar] [CrossRef]
- Guo, Y.F.; Tan, C.; Sun, J.; Li, W.L.; Zhang, J.B.; Zhao, C.W. Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption. Chem. Eng. J. 2020, 381, 122736. [Google Scholar] [CrossRef]
- Shi, J.; Cui, H.; Xu, J.; Yan, N.; Liu, Y. Design and fabrication of hierarchically porous carbon frameworks with Fe2O3 cubes as hard template for CO2 adsorption. Chem. Eng. J. 2020, 389, 124459. [Google Scholar] [CrossRef]
- Shi, S.; Liu, Y. Nitrogen-doped activated carbons derived from microalgae pyrolysis by-products by microwave/KOH activation for CO2 adsorption. Fuel 2021, 306, 121762. [Google Scholar] [CrossRef]
- Rehman, A.; Nazir, G.; Rhee, K.Y.; Park, S.-J. A rational design of cellulose-based heteroatom-doped porous carbons: Promising contenders for CO2 adsorption and separation. Chem. Eng. J. 2021, 420, 130421. [Google Scholar] [CrossRef]
- Zhi, Y.; Yin, Y.; Ciren, Q.; Xiao, Q.; Zhao, L.; Demir, M.; Simsek, U.B.; Hu, X.; Wang, L. One-step synthesis of K3PO4-activated phosphorus-enriched carbons for enhanced carbon capture. J. Environ. Chem. Eng. 2025, 13, 116694. [Google Scholar] [CrossRef]
- Chatterjee, S.; Jeevanandham, S.; Mukherjee, M.; Vo, D.-V.N.; Mishra, V. Significance of re-engineered zeolites in climate mitigation–A review for carbon capture and separation. J. Environ. Chem. Eng. 2021, 9, 105957. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal-Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xie, L.-H.; Li, J.-R.; Ma, Y.; Seminario, J.M.; Balbuena, P.B. CO2 Capture and Separations Using MOFs: Computational and Experimental Studies. Chem. Rev. 2017, 117, 9674–9754. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Yuan, X.; Zhang, T.C.; Ouyang, L.; Yuan, S. Nitrogen-doped porous carbon for excellent CO2 capture: A novel method for preparation and performance evaluation. Sep. Purif. Technol. 2022, 298, 121602. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Tang, M.; Liu, J.; Yuan, J.; Zhao, Y.; Zhang, G. Preparation of N, S co-doped carbon nanotubes composites by coal pyrolysis for the CO2 capture. J. Environ. Chem. Eng. 2024, 12, 114452. [Google Scholar] [CrossRef]
- Yuan, X.; Kumar, N.M.; Brigljević, B.; Li, S.; Deng, S.; Byun, M.; Lee, B.; Lin, C.S.K.; Tsang, D.C.W.; Lee, K.B.; et al. Sustainability-inspired upcycling of waste polyethylene terephthalate plastic into porous carbon for CO2 capture. Green Chem. 2022, 24, 1494–1504. [Google Scholar] [CrossRef]
- Quesada-Plata, F.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Activated Carbons Prepared through H3PO4-Assisted Hydrothermal Carbonisation from Biomass Wastes: Porous Texture and Electrochemical Performance. ChemPlusChem 2016, 81, 1349–1359. [Google Scholar] [CrossRef]
- Pimentel, C.H.; Díaz-Fernández, L.; Gómez-Díaz, D.; Freire, M.S.; González-Álvarez, J. Separation of CO2 using biochar and KOH and ZnCl2 activated carbons derived from pine sawdust. J. Environ. Chem. Eng. 2023, 11, 111378. [Google Scholar] [CrossRef]
- Wang, L.; Sun, F.; Hao, F.; Qu, Z.; Gao, J.; Liu, M.; Wang, K.; Zhao, G.; Qin, Y. A green trace K2CO3 induced catalytic activation strategy for developing coal-converted activated carbon as advanced candidate for CO2 adsorption and supercapacitors. Chem. Eng. J. 2020, 383, 123205. [Google Scholar] [CrossRef]
- Singh, G.; Ismail, I.S.; Bilen, C.; Shanbhag, D.; Sathish, C.I.; Ramadass, K.; Vinu, A. A facile synthesis of activated porous carbon spheres from d-glucose using a non-corrosive activating agent for efficient carbon dioxide capture. Appl. Energy 2019, 255, 113831. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Park, S.-J. Valorization of shrimp shell biowaste for environmental remediation: Efficient contender for CO2 adsorption and separation. J. Environ. Manag. 2021, 299, 113661. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, J.; Feng, J.; Liu, C.; Xiao, Q.; Demir, M.; Simsek, U.B.; Kılıç, M.; Wang, L.; Hu, X. D-glucose-derived S-doped porous carbon: Sustainable and effective CO2 adsorption. Colloids Surf. A Phys. Eng. Asp. 2025, 709, 136054. [Google Scholar] [CrossRef]
- Bai, J.; Shao, J.; Yu, Q.; Demir, M.; Nazli Altay, B.; Muhammad Ali, T.; Jiang, Y.; Wang, L.; Hu, X. Sulfur-Doped porous carbon Adsorbent: A promising solution for effective and selective CO2 capture. Chem. Eng. J. 2024, 479, 147667. [Google Scholar] [CrossRef]
- Liu, C.; Zhi, Y.; Yu, Q.; Tian, L.; Demir, M.; Colak, S.G.; Farghaly, A.A.; Wang, L.; Hu, X. Sulfur-Enriched Nanoporous Carbon: A Novel Approach to CO2 Adsorption. ACS Appl. Nano Mater. 2024, 7, 5434–5441. [Google Scholar] [CrossRef]
- Quan, C.; Zhou, Y.; Wang, J.; Wu, C.; Gao, N. Biomass-based carbon materials for CO2 capture: A review. J. CO2 Util. 2023, 68, 102373. [Google Scholar] [CrossRef]
- Khan, M.H.; Akash, N.M.; Akter, S.; Rukh, M.; Nzediegwu, C.; Islam, M.S. A comprehensive review of coconut-based porous materials for wastewater treatment and CO2 capture. J. Environ. Manag. 2023, 338, 117825. [Google Scholar] [CrossRef]
- Karimi, M.; Shirzad, M.; Silva, J.A.C.; Rodrigues, A.E. Biomass/Biochar carbon materials for CO2 capture and sequestration by cyclic adsorption processes: A review and prospects for future directions. J. CO2 Util. 2022, 57, 101890. [Google Scholar] [CrossRef]
- Zhi, Y.; Shao, J.; Liu, C.; Xiao, Q.; Demir, M.; Al Mesfer, M.K.; Danish, M.; Wang, L.; Hu, X. High-performance CO2 adsorption with P-doped porous carbons from lotus petiole biomass. Sep. Purif. Technol. 2025, 361, 131253. [Google Scholar] [CrossRef]
- Cui, H.; Shi, J.; Xu, J.; Yan, N.; Liu, Y. Direct synthesis of N, S co-doped porous carbons using novel organic potassium salts as activators for efficient CO2 adsorption. Fuel 2023, 342, 127824. [Google Scholar] [CrossRef]
- Luo, L.; Yang, C.; Liu, F.; Zhao, T. Heteroatom-N,S co-doped porous carbons derived from waste biomass as bifunctional materials for enhanced CO2 adsorption and conversion. Sep. Purif. Technol. 2023, 320, 124090. [Google Scholar] [CrossRef]
- Shao, J.; Wang, Y.; Che, M.; Liu, Y.; Jiang, Y.; Xiao, Q.; Demir, M.; Wang, L.; Hu, X. Sustainable CO2 Capture: N,S-Codoped Porous Carbons Derived from Petroleum Coke with High Selectivity and Stability. Molecules 2025, 30, 426. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, T.; Pan, H.; Liu, F.; Cao, J.; Lin, Q. Facile preparation of N-doped porous carbon from chitosan and NaNH2 for CO2 adsorption and conversion. Chem. Eng. J. 2022, 432, 134347. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.G.; Chen, B.Q.; Wang, L.Y.; Yang, J.; Wang, B.B. Nitrogen-doped hierarchically constructed interconnected porous carbon nanofibers derived from polyaniline (PANI) for highly selective CO2 capture and effective methanol adsorption. J. Environ. Chem. Eng. 2022, 10, 108847. [Google Scholar] [CrossRef]
- Shen, Z.; Song, Y.; Yin, C.; Luo, X.; Wang, Y.; Li, X. Construction of hierarchically porous 3D graphene-like carbon material by B, N co-doping for enhanced CO2 capture. Microporous Mesoporous Mater. 2021, 322, 111158. [Google Scholar] [CrossRef]
- Li, J.; Michalkiewicz, B.; Min, J.; Ma, C.; Chen, X.; Gong, J.; Mijowska, E.; Tang, T. Selective preparation of biomass-derived porous carbon with controllable pore sizes toward highly efficient CO2 capture. Chem. Eng. J. 2019, 360, 250–259. [Google Scholar] [CrossRef]
- Xue, R.; Xu, W.; Ma, X.; Guo, Y.; Zeng, Z.; Li, L.; Si, J. Targeted enhancement of ultra-micropore in highly oxygen-doped carbon derived from biomass for efficient CO2 capture: Insights from experimental and molecular simulation studies. Sep. Purif. Technol. 2025, 353, 128472. [Google Scholar] [CrossRef]
- Umar, M.; Yusuf, B.O.; Aliyu, M.; Hussain, I.; Alhassan, A.M.; Awad, M.M.; Taialla, O.A.; Ali, B.; Alhooshani, K.R.; Ganiyu, S.A. Advancing frontiers in CO2 capture: The renaissance of biomass-derived carbon materials. Coord. Chem. Rev. 2025, 526, 216380. [Google Scholar] [CrossRef]
- Serafin, J.; Ouzzine, M.; Cruz, O.F.; Sreńscek-Nazzal, J.; Campello Gómez, I.; Azar, F.-Z.; Rey Mafull, C.A.; Hotza, D.; Rambo, C.R. Conversion of fruit waste-derived biomass to highly microporous activated carbon for enhanced CO2 capture. Waste Manag. 2021, 136, 273–282. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Lee, K.H.; Lee, S.K.; Chun, Y.; Kim, S.W.; Park, C.; Yoo, H.Y. Improvement of bioethanol production from waste chestnut shells via evaluation of mass balance-based pretreatment and glucose recovery process. Environ. Technol. Innov. 2022, 28, 102955. [Google Scholar] [CrossRef]
- Dziejarski, B.; Serafin, J.; Andersson, K.; Krzyżyńska, R. CO2 capture materials: A review of current trends and future challenges. Mater. Today Sustain. 2023, 24, 100483. [Google Scholar] [CrossRef]
- Sirinwaranon, P.; Sricharoenchaikul, V.; Vichaphund, S.; Soongprasit, K.; Rodchom, M.; Wimuktiwan, P.; Atong, D. Synthesis and characterization of the porous activated carbon from end-of-life tire pyrolysis for CO2 sequestration. J. Anal. Appl. Pyrolysis 2023, 174, 106139. [Google Scholar] [CrossRef]
- Jang, E.; Choi, S.W.; Lee, K.B. Effect of carbonization temperature on the physical properties and CO2 adsorption behavior of petroleum coke-derived porous carbon. Fuel 2019, 248, 85–92. [Google Scholar] [CrossRef]
- Goskula, S.; Siliveri, S.; Gujjula, S.R.; Adepu, A.K.; Chirra, S.; Narayanan, V. Development of activated sustainable porous carbon adsorbents from Karanja shell biomass and their CO2 adsorption. Biomass Convers. Biorefinery 2024, 14, 32413–32425. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Ikram, M.; Aslam, M.; Zhang, T.C.; Khalid, A.; Aftab, S.; Hussain, S.; Almukhlifi, H.A.; Abdel Hafez, A.A.; et al. Facile synthesis of inherently nitrogen-doped PAN-derived microporous carbons activated with NaNH2 for selective CO2 adsorption and separation. Chem. Eng. J. 2024, 497, 154704. [Google Scholar] [CrossRef]
- Li, J.; Shi, C.; Bao, A.; Jia, J. Development of Boron-Doped Mesoporous Carbon Materials for Use in CO2 Capture and Electrochemical Generation of H2O2. ACS Omega 2021, 6, 8438–8446. [Google Scholar] [CrossRef]
- Dong, J.; Wang, R.; Wang, X.; Tan, S.; Zhao, Z.; Yin, Q.; Lu, X. Preparation of lignin-based porous carbon through thermochemical activation and nitrogen doping for CO2 selective adsorption. Carbon 2024, 229, 119530. [Google Scholar] [CrossRef]
- Shi, J.; Xu, J.; Cui, H.; Yan, N.; Yan, R.; Weng, Y. NaCl template synthesis of N-doped porous carbon from magnesium gluconate for efficient CO2 adsorption. Sep. Purif. Technol. 2025, 355, 129756. [Google Scholar] [CrossRef]
- Shao, J.; Wang, Y.; Liu, C.; Xiao, Q.; Demir, M.; Al Mesfer, M.K.; Danish, M.; Wang, L.; Hu, X. Advanced microporous carbon adsorbents for selective CO₂ capture: Insights into heteroatom doping and pore structure optimization. J. Anal. Appl. Pyrolysis 2025, 186, 106946. [Google Scholar] [CrossRef]
- Shao, J.; Wang, J.; Yu, Q.; Yang, F.; Demir, M.; Altinci, O.C.; Umay, A.; Wang, L.; Hu, X. Unlocking the potential of N-doped porous Carbon: Facile synthesis and superior CO2 adsorption performance. Sep. Purif. Technol. 2024, 333, 125891. [Google Scholar] [CrossRef]
- Wu, R.; Bao, A.L. Preparation of cellulose carbon material from cow dung and its CO2 adsorption performance. J. CO2 Util. 2023, 68, 102377. [Google Scholar] [CrossRef]
- Furukawa, H.; Yaghi, O.M. Storage of Hydrogen, Methane, and Carbon Dioxide in Highly Porous Covalent Organic Frameworks for Clean Energy Applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]

| Sample | SBET a (m2/g) | V0 b (cm3/g) | Vt c (cm3/g) | Vn d (cm3/g) | XPS (at. %) | CO2 Uptake (mmol/g) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | N | B | O | 25 °C | 0 °C | |||||
| WSC | 116 | 0.10 | 0.01 | 0.19 | 1.18 | 84.67 | 0.57 | 13.57 | 1.56 | 2.11 |
| WSCSM-650 | 464 | 0.19 | 0.18 | 0.24 | 2.30 | 87.62 | 0.29 | 9.78 | 2.32 | 3.05 |
| WSCSM-700 | 481 | 0.21 | 0.19 | 0.27 | 0.97 | 90.46 | 0.40 | 8.17 | 2.38 | 3.27 |
| WSCSM-750 | 437 | 0.19 | 0.15 | 0.27 | 0.95 | 88.43 | 0.79 | 9.83 | 2.51 | 3.30 |
| WSCSM-800 | 228 | 0.12 | 0.07 | 0.26 | 0.78 | 86.33 | 1.65 | 11.25 | 2.33 | 2.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, Y.; Liu, X.; Xiao, Q.; Demir, M.; Almesfer, M.K.; Colak, S.G.; Wang, L.; Hu, X.; Liu, Y. The Synthesis of B-Doped Porous Carbons via a Sodium Metaborate Tetrahydrate Activating Agent: A Novel Approach for CO2 Adsorption. Molecules 2025, 30, 2564. https://doi.org/10.3390/molecules30122564
Wang J, Wang Y, Liu X, Xiao Q, Demir M, Almesfer MK, Colak SG, Wang L, Hu X, Liu Y. The Synthesis of B-Doped Porous Carbons via a Sodium Metaborate Tetrahydrate Activating Agent: A Novel Approach for CO2 Adsorption. Molecules. 2025; 30(12):2564. https://doi.org/10.3390/molecules30122564
Chicago/Turabian StyleWang, Junting, Yingyi Wang, Xiaohan Liu, Qiang Xiao, Muslum Demir, Mohammed K. Almesfer, Suleyman Gokhan Colak, Linlin Wang, Xin Hu, and Ya Liu. 2025. "The Synthesis of B-Doped Porous Carbons via a Sodium Metaborate Tetrahydrate Activating Agent: A Novel Approach for CO2 Adsorption" Molecules 30, no. 12: 2564. https://doi.org/10.3390/molecules30122564
APA StyleWang, J., Wang, Y., Liu, X., Xiao, Q., Demir, M., Almesfer, M. K., Colak, S. G., Wang, L., Hu, X., & Liu, Y. (2025). The Synthesis of B-Doped Porous Carbons via a Sodium Metaborate Tetrahydrate Activating Agent: A Novel Approach for CO2 Adsorption. Molecules, 30(12), 2564. https://doi.org/10.3390/molecules30122564









