Furan Dissociation Induced by Collisions with H3+ and C+ Ions
Abstract
1. Introduction
2. Results
3. Discussion
- (i)
- Charge transfer (CT);
- (ii)
- Dissociative excitation;
- (iii)
- Dissociative ionization;
- (iv)
- Complexes formation.
4. Materials and Methods
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CIES | collision-induced emission spectroscopy |
| FWHM | full width at half maximum |
| CT | charge transfer |
| THF | tetrahydrofuran |
References
- González-Sánchez, L.; Sathyamurthy, N.; Gianturco, F.A. The role of small molecular cations in the chemical flow of the interstellar environments. Phys. Chem. Chem. Phys. 2023, 25, 23370–23383. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.I.; Hansen, N. An Aromatic Universe—A Physical Chemistry Perspective. J. Phys. Chem. A 2021, 125, 3826–3840. [Google Scholar] [CrossRef]
- Durante, M.; Cucinotta, F.A. Physical basis of radiation protection in space travel. Rev. Mod. Phys. 2011, 83, 1245–1281. [Google Scholar] [CrossRef]
- Cucinotta, F.; Durante, M. Cancer risk from exposure to galactic cosmic rays: Implications for space exploration by human beings. Lancet Oncol. 2006, 7, 431–435. [Google Scholar] [CrossRef]
- Durante, M.; Cucinotta, F.A. Heavy Ion Carcinogenesis and Human Space Exploration. Nat. Rev. Cancer 2008, 8, 465–472. [Google Scholar] [CrossRef]
- Schlathölter, T.; Poully, J.-C. Radiation-Induced Molecular Processes in DNA: A Perspective on Gas-Phase Interaction Studies. Chem. Eur. J. 2024, 30, e202400633. [Google Scholar] [CrossRef]
- Deng, Z.; Bald, I.; Illenberger, E.; Huels, M.A. Beyond the Bragg Peak: Hyperthermal Heavy Ion Damage to DNA Components. Phys. Rev. Lett. 2005, 95, 153201. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.; Hoekstra, R.; Morgenstern, R.; Schlathölter, T. Charge Driven Fragmentation of Nucleobases. Phys. Rev. Lett. 2003, 91, 053401. [Google Scholar] [CrossRef] [PubMed]
- Schlathölter, T.; Hoekstra, R.; Morgenstern, R. Charge Driven Fragmentation of Biologically Relevant Molecules. Int. J. Mass. Spectrom. 2004, 233, 173–179. [Google Scholar] [CrossRef]
- Wasowicz, T.J.; Pranszke, B. Interactions of protons with furan molecules studied by collision-induced emission spectroscopy at the incident energy range of 50–1000 eV. Eur. Phys. J. D 2016, 70, 175. [Google Scholar] [CrossRef][Green Version]
- Erdmann, E.; Aguirre, N.F.; Indrajith, S.; Chiarinelli, J.; Domaracka, A.; Rousseau, P.; Huber, B.A.; Bolognesi, P.; Richter, R.; Avaldi, L.; et al. A General Approach to Study Molecular Fragmentation and Energy Redistribution after an Ionizing Event. Phys. Chem. Chem. Phys. 2021, 23, 1859–1867. [Google Scholar] [CrossRef]
- Wasowicz, T.J.; Łabuda, M.; Pranszke, B. Charge Transfer, Complexes Formation and Furan Fragmentation Induced by Collisions with Low-Energy Helium Cations. Int. J. Mol. Sci. 2019, 20, 6022. [Google Scholar] [CrossRef]
- Wasowicz, T.J. Formation of OH radicals as evidence of intramolecular hydrogen migration in cation-induced dissociation of furan molecules. Rom. J. Phys. 2022, 67, 206. [Google Scholar]
- Ascenzi, D.; Erdmann, E.; Bolognesi, P.; Avaldi, L.; Castrovilli, M.C.; Thissen, R.; Romanzin, C.; Alcaraz, C.; Rabadán, I.; Méndez, L.; et al. H2O+ and OH+ reactivity versus furan: Experimental low energy absolute cross sections for modeling radiation damage. Phys. Chem. Chem. Phys. 2023, 25, 24643–24656. [Google Scholar] [CrossRef]
- Wasowicz, T.J. Probing luminescence in the collisions of furan molecules with dihydrogen cations using collision-induced emission spectroscopy. Rom. J. Phys. 2024, 69, 204. [Google Scholar] [CrossRef]
- Wasowicz, T.J.; Pranszke, B. Fragmentation of Tetrahydrofuran Molecules by H+, C+, and O+ Collisions at the Incident energy Range of 25−1000 eV. J. Phys. Chem. A 2015, 119, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Rudek, B.; Arndt, A.; Bennett, D.; Wang, M.; Rabus, H. Ion induced fragmentation cross-sections of DNA constituents. Eur. Phys. J. D 2015, 69, 237. [Google Scholar] [CrossRef]
- Wasowicz, T.J.; Pranszke, B. Optical spectroscopic studies of tetrahydrofuran fragmentation induced by collisions with dihydrogen cations. Acta Phys. Pol. A 2021, 140, 228. [Google Scholar] [CrossRef]
- Wasowicz, T.J. Photon luminescence studies of tetrahydrofuran following trihydrogen cations impact in the 20–1000 eV energy range. Rom. Rep. Phys. 2021, 73, 203. [Google Scholar]
- Fondren, L.D.; McLain, J.; Jackson, D.M.; Adams, N.G.; Babcock, L.M. Studies of Reactions of a Series of Ions With Nitrogen Containing Heterocyclic Molecules Using a Selected Ion Flow Tube. Int. J. Mass. Spectrom. 2007, 265, 60–67. [Google Scholar] [CrossRef]
- Wasowicz, T.J.; Pranszke, B. Observation of the hydrogen migration in the cation-induced fragmentation of the pyridine molecules. J. Phys. Chem. A 2016, 120, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Wasowicz, T.J. Neutral Dissociation of Pyridine Evoked by Irradiation of Ionized Atomic and Molecular Hydrogen Beams. Int. J. Mol. Sci. 2022, 23, 205. [Google Scholar] [CrossRef]
- Durante, M.; Debus, J.; Loeffler, J.S. Physics and biomedical challenges of cancer therapy with accelerated heavy ions. Nat. Rev. Phys. 2021, 3, 777–790. [Google Scholar] [CrossRef]
- Skuratov, V.A.; Gun, K.J.; Stano, J.; Zagorski, D.L. In situ luminescence as monitor of radiation damage under swift heavy ion irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B 2006, 245, 194–200. [Google Scholar] [CrossRef]
- Utke, I.; Hoffmann, P.; Melngailis, J. Gas-Assisted Focused Electron Beam and Ion Beam Processing and Fabrication. J. Vac. Sci. Technol. B 2008, 26, 1197–1276. [Google Scholar] [CrossRef]
- He, S.; Tian, R.; Wu, W.; Li, W.-D.; Wang, D. Helium-Ion-Beam Nanofabrication: Extreme Processes and Applications. Int. J. Extrem. Manuf. 2021, 3, 012001. [Google Scholar] [CrossRef]
- Hayles, M.F.; De Winter, D.A.M. An introduction to cryo-FIB-SEM cross-sectioning of frozen, hydrated Life Science samples. J. Microsc. 2021, 281, 138–156. [Google Scholar] [CrossRef]
- Thomas, R.D. When electrons meet molecular ions and what happens next: Dissociative recombination from interstellar molecular clouds to internal combustion engines. Mass. Spectrom. Rev. 2008, 27, 485–530. [Google Scholar] [CrossRef]
- Eraslan, A.N.; Brown, R.C. Chemiionization and ion-molecule reactions in fuel-rich acetylene flames. Combust. Flame 1988, 74, 19–37. [Google Scholar] [CrossRef]
- Gnanasekaran, L.; Priya, A.K.; Thanigaivel, S.; Tuan, K.A.H.; Moscoso, M.S. The conversion of biomass to fuels via cutting-edge technologies: Explorations from natural utilization systems. Fuel 2023, 33, 125668. [Google Scholar] [CrossRef]
- Sulzer, P.; Ptasinska, S.; Zappa, F.; Mielewska, B.; Milosavljevic, A.R.; Scheier, P.; Märk, T.D.; Bald, I.; Gohlke, S.; Huels, M.A.; et al. Dissociative Electron Attachment to Furan, Tetrahydrofuran, and Fructose. J. Chem. Phys. 2006, 125, 044304. [Google Scholar] [CrossRef]
- Guler, L.P.; Yu, Y.Q.; Kenttämaa, H.I. An Experimental and Computational Study of the Gas-Phase Structures of Five-Carbon Monosaccharides. J. Phys. Chem. A 2002, 106, 6754–6764. [Google Scholar] [CrossRef]
- Furukawa, Y.; Chikaraishi, Y.; Ohkouchi, N.; Ogawa, N.O.; Glavin, D.P.; Dworkin, J.P.; Abe, C.; Nakamura, T. Extraterrestrial ribose and other sugars in primitive meteorites. Proc. Natl. Acad. Sci. USA 2019, 116, 24440–24445. [Google Scholar] [CrossRef]
- Randaccio, L.; Geremia, S.; Demitri, N.; Wuerges, J. Vitamin B12: Unique Metalorganic Compounds and the Most Complex Vitamins. Molecules 2010, 15, 3228–3259. [Google Scholar] [CrossRef]
- Wang, L.J.; Yang, Y.; Zhang, C.Y. Phosphorylation-directed assembly of a single quantum dot based nanosensor for protein kinase assay. Anal. Chem. 2015, 87, 4696–4703. [Google Scholar] [CrossRef] [PubMed]
- Wijma, R.A.; Huttner, A.; Koch, B.C.P.; Mouton, J.W.; Muller, A.E. Review of the pharmacokinetic properties of nitrofurantoin and nitroxoline. J. Antimicrob. Chemother. 2018, 73, 2916–2926. [Google Scholar] [CrossRef]
- Chung, M.C.; Ferreira, E.I.; Santos, J.L.; Giarolla, J.; Rando, D.G.; Almeida, A.E.; Bosquesi, P.L.; Menegon, R.F.; Blau, L. Prodrugs for the Treatment of Neglected Diseases. Molecules 2008, 13, 616–677. [Google Scholar] [CrossRef] [PubMed]
- Greco, N.J.; Tor, Y. Furan Decorated Nucleoside Analogues as Fluorescent Probes: Synthesis, Photophysical Evaluation, and Site-Specific Incorporation. Tetrahedron 2007, 63, 3515–3527. [Google Scholar] [CrossRef]
- Ponnappa, S.P.; Macleod, J.; Umer, M.; Soda, N.; Pannu, A.S.; Shiddiky, M.J.A.; Ayoko, G.A.; O’Mullane, A.P.; Sonar, P. Electropolymerized Porous Polymer Films on Flexible Indium Tin Oxide Using Trifunctional Furan Substituted Benzene Conjugated Monomer for Biosensing. ACS Appl. Polym. Mater. 2020, 2, 351–359. [Google Scholar] [CrossRef]
- Risangud, N.; Mama, J.; Sungkhaphan, P.; Pananusorn, P.; Termkunanon, O.; Arkana, M.S.; Sripraphot, S.; Lertwimol, T.; Thongkham, S. Synthesis and Characterization of Furan-Based Methacrylate Oligomers Containing the Imine Functional Group for Stereolithography. ACS Omega 2024, 9, 30771–30781. [Google Scholar] [CrossRef]
- Zhu, P.; Shi, M.; Shen, Z.; Liao, X.; Chen, Y. Electrocatalytic Conversion of Biomass-Derived Furan Compounds: Mechanisms, Catalysts and Perspectives. Chem. Sci. 2024, 15, 4723–4756. [Google Scholar] [CrossRef] [PubMed]
- Mascal, M.; Nikitin, E.B. Direct, High-Yield Conversion of Cellulose into Biofuel. Angew. Chem. 2008, 120, 8042–8044. [Google Scholar] [CrossRef]
- Oh, J.; Lee, Y.; Lee, K.G. Analytical methods, risk assessment, and mitigation strategies for furan in processed foods in various countries. Food Sci. Biotechnol. 2024, 33, 2427–2440. [Google Scholar] [CrossRef]
- Moro, S.; Chipman, J.K.; Wegener, J.W.; Hamberger, C.; Dekant, W.; Mally, A. Furan in heat-treated foods: Formation, exposure, toxicity, and aspects of risk assessment. Mol. Nutr. Food Res. 2012, 56, 1197–1211. [Google Scholar] [CrossRef]
- Luque, J.; Crosley, D.R. Lifbase: Database and Spectral Simulation (Version 1.5), SRI International Report MP 99-009. 1999. Available online: https://www.sri.com/case-studies/lifbase-spectroscopy-tool/ (accessed on 29 October 2021).
- Bass, A.M.; Broida, H.P. A Spectrophotometric Atlas of the Spectrum of CH from 3000 to 5000A; US Department of Commerce, National Bureau of Standards: Washington, DC, USA, 1961; Volume 24, pp. 1–20. [Google Scholar]
- Kramida, A.; Ralchenko, Y.; Reader, J.; NIST ASD Team. NIST Atomic Spectra Database, ver. 5.12; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2024. Available online: https://physics.nist.gov/asd (accessed on 26 March 2025).
- Bacchus-Montabonel, M.C. Charge Transfer in Molecular Systems. From Fusion Plasmas to Biological Systems. 2016. Available online: https://inis.iaea.org/records/rdf9d-2x132 (accessed on 26 March 2025).
- Bacchus-Montabonel, M.-C. Looking at Radiation Damage on Prebiotic Building Blocks. J. Phys. Chem. A 2013, 117, 14169–14175. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, M.; Sack, N.J.; Madey, T.E. Elastic and Inelastic Processes in the Interaction of l-10 eV Ions with Solids: Ion Transport through Surface Layers. Surf. Sci. Rep. 1997, 28, 177–245. [Google Scholar] [CrossRef]
- Massey, H. Atomic and Molecular Collisions; Taylor & Francis: London, UK, 1979. [Google Scholar]
- Tomaszewska, A.; Stepien, Z.M. H3+-synthesis on the surface of d-transition metals during the process of field desorption. Opt. Appl. 2002, 32, 283–289. [Google Scholar]
- Haris, K.; Kramida, A. Critically Evaluated Spectral Data for Neutral Carbon (C I). Astrophys. J., Suppl. Ser. 2017, 233, 16. [Google Scholar] [CrossRef]
- Lias, S.G. Ionization Energy Evaluation. In NIST Chemistry WebBook, NIST Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Schlathoölter, T.; Alvarado, F.; Bari, S.; Hoekstra, R. Ion-induced ionization and fragmentation of DNA building blocks. Phys. Scr. 2006, 73, C113. [Google Scholar] [CrossRef]
- Dampc, M.; Zubek, M. Dissociation and fragmentation of furan by electron impact. Int. J. Mass. Spectrom. 2008, 277, 52–56. [Google Scholar] [CrossRef]
- Linert, I.; Lachowicz, I.; Wasowicz, T.J.; Zubek, M. Fragmentation of Isoxazole Molecules by Electron Impact in the Energy Range 10-85 eV. Chem. Phys. Lett. 2010, 498, 27–31. [Google Scholar] [CrossRef]
- Wasowicz, T.J.; Linert, I.; Lachowicz, I.; Zubek, M. Electron impact fragmentation of pyrrole molecules studied by fluorescence emission spectroscopy. Photonics Lett. Pol. 2011, 3, 110–112. [Google Scholar]
- Wasowicz, T.J.; Kivimaki, A.; Dampc, M.; Coreno, M.; De Simone, M.; Zubek, M. Photofragmentation of Tetrahydrofuran Molecules in the Vacuum-Ultraviolet Region via Superexcited States Studied by Fluorescence Spectroscopy. Phys. Rev. A 2011, 83, 033411. [Google Scholar] [CrossRef]
- Wasowicz, T.J.; Kivimaki, A.; Coreno, M.; Zubek, M. Formation of CN(B2Σ+) Radicals in the Vacuum-Ultraviolet Photodissociation of Pyridine and Pyrimidine Molecules. J. Phys. B At. Mol. Opt. Phys. 2014, 47, 055103. [Google Scholar] [CrossRef]
- Kunert, T.; Schmidt, R. Excitation and Fragmentation Mechanisms in Ion-Fullerene Collisions. Phys. Rev. Lett. 2001, 86, 5258–5261. [Google Scholar] [CrossRef]
- Gatchell, M.; Zettergren, H. Knockout driven reactions in complex molecules and their clusters. J. Phys. B At. Mol. Opt. Phys. 2016, 49, 162001. [Google Scholar] [CrossRef]
- Chen, T.; Gatchell, M.; Stockett, M.H.; Alexander, J.D.; Zhang, Y.; Rousseau, P.; Domaracka, A.; Maclot, S.; Delaunay, R.; Adoui, L.; et al. Absolute fragmentation cross sections in atom-molecule collisions: Scaling laws for non-statistical fragmentation of polycyclic aromatic hydrocarbon molecules. J. Chem. Phys. 2014, 140, 224306. [Google Scholar] [CrossRef]
- Glosík, J.; Plašil, R.; Kotrík, T.; Dohnal, P.; Varju, J.; Hejduk, M.; Korolov, I.; Roučka, Š.; Kokoouline, V. Binary and ternary recombination of H3+ and D3+ ions with electrons in low temperature plasma. Mol. Phys. 2010, 108, 2253–2264. [Google Scholar] [CrossRef]
- Curtis, P.M.; Williams, B.W.; Porter, R.F. An experimental study of the energetics of fragmentation of H3. Chem. Phys. Lett. 1979, 65, 296–299. [Google Scholar] [CrossRef]
- Vogelhuber, K.M.; Wren, S.W.; Sheps, L.; Lineberger, W.C. The C–H Bond Dissociation Energy of Furan: Photoelectron Spectroscopy of the Furanide Anion. J. Chem. Phys. 2011, 134, 064302. [Google Scholar] [CrossRef]
- Wasowicz, T.J.; Dabkowska, I.; Kivimäki, A.; Coreno, M.; Zubek, M. Elimination and migration of hydrogen in the vacuum-ultraviolet photodissociation of pyridine molecules. J. Phys. B At. Mol. Opt. Phys. 2017, 50, 015101. [Google Scholar] [CrossRef]
- Wasowicz, T.; Pranszke, B. Unusual fragmentation pathways of tetrahydrofuran molecules induced by O+ impact. Sci. J. Gdyn. Marit. Univ. 2016, 96, 134–142. [Google Scholar]
- SenGupta, S.; Upadhyaya, H.P.; Kumar, A.; Naik, P.D.; Bajaj, P. Detection of OH radical in laser induced photo dissociation of tetrahydrofuran at 193 nm. J. Chem. Phys. 2005, 122, 124309. [Google Scholar] [CrossRef] [PubMed]
- Zubek, M.; Wasowicz, T.J.; Dabkowska, I.; Kivimaki, A.; Coreno, M. Hydrogen Migration in Formation of NH(A3Π) Radicals Via Superexcited States in Photodissociation of Isoxazole Molecules. J. Chem. Phys. 2014, 141, 064301. [Google Scholar] [CrossRef]
- Upadhyaya, H.P. Theoretical Study on the Dissociation Mechanism of Thiophene in the UV Photoabsorption, Ionization, and Electron Attachment Processes. Int. J. Quantum Chem. 2024, 124, e27503. [Google Scholar] [CrossRef]
- García-Moreno, I.; Moore, C.B. Spectroscopy of methylene: Einstein coefficients for CH₂(1B1–ã1A1) transitions. J. Chem. Phys. 1993, 99, 6429–6435. [Google Scholar] [CrossRef]
- Maclot, S.; Piekarski, D.G.; Domaracka, A.; Méry, A.; Vizcaino, V.; Adoui, L.; Martín, F.; Alcamí, M.; Huber, B.A.; Rousseau, P.; et al. Dynamics of Glycine Dications in the Gas Phase: Ultrafast Intramolecular Hydrogen Migration versus Coulomb Repulsion. J. Phys. Chem. Lett. 2013, 4, 3903–3909. [Google Scholar] [CrossRef]
- Bari, S.; Sobocinski, P.; Postma, J.; Alvarado, F.; Hoekstra, R.; Bernigaud, V.; Manil, B.; Rangama, J.; Huber, B.; Schlathölter, T. Fragmentation of α- and β-alanine molecules by ions at Bragg-peak energies. J. Chem. Phys. 2008, 128, 074306. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, L.; Yang, C.-L.; Yu, W.; Wang, B.; Yan, B.; Zou, Y.; Chen, L.; Wei, B. Formation of H₃⁺ from Hydrocarbon Dications Induced by Collisions with Charged Particles. Phys. Rev. A 2019, 100, 052706. [Google Scholar] [CrossRef]
- De, S.; Rajput, J.; Roy, A.; Ghosh, P.N.; Safvan, C.P. Formation of H₃⁺ due to Intramolecular Bond Rearrangement in Doubly Charged Methanol. Phys. Rev. Lett. 2006, 97, 213201. [Google Scholar] [CrossRef]
- Hayakawa, K.; Matsumoto, J.; Shiromaru, H.; Achiba, Y. Dissociation of Methanol Dications Produced by Electron Capture with and without Transfer Ionization. J. Phys. B At. Mol. Opt. Phys. 2011, 44, 075207. [Google Scholar] [CrossRef]
- Glenewinkel-Meyer, T.; Muller, B.; Ottinger, C.; Tischer, H. Measurement of the HCl+(A2Σ+–X2Π) electronic transition moment using quasiresonant charge transfer at low energy. J. Chem. Phys. 1988, 88, 3475. [Google Scholar] [CrossRef]
- Furan. Available online: https://www.sigmaaldrich.com/PL/pl/product/aldrich/185922 (accessed on 12 February 2025).
- Brzozowski, J.; Bunker, P.; Elander, N.; Erman, P. Predissociation efects in the A, B, and C states of CH and the interstellar formation rate of CH via inverse predissociation. Astrophys. J. 1976, 207, 414–424. [Google Scholar] [CrossRef]
- Zachwieja, M. New Investigations of the A2Δ-X2Π Band System in the CH Radical and a New Reduction of the Vibration-Rotation Spectrum of CH from the ATMOS Spectra. J. Mol. Spectroc. 1995, 170, 285–309. [Google Scholar] [CrossRef]
- Luque, J.; Crosley, D.R. Electronic transition moment and rotational transition probabilities in CH. I. A2Δ-X2Π system. J. Chem. Phys. 1996, 104, 2146. [Google Scholar] [CrossRef]
- Ubachs, W.; Meyer, G.; Ter Meulen, J.; Dymanus, A. Hyperfine structure and lifetime of the C2Σ+, v=0 state of CH. J. Chem. Phys. 1986, 84, 3032–3041. [Google Scholar] [CrossRef]
- Jeffries, J.B.; Copeland, R.A.; Crosley, D.R. Transition probabilities in the C2Σ+- X2Π system of CH. J. Quant. Spectrosc. Radiat. Transf. 1987, 37, 419–423. [Google Scholar] [CrossRef]
- Luque, J.; Crosley, D.R. Transition probabilities in the A2Σ+ − X2Πi electronic system of OH. J. Chem. Phys. 1998, 109, 439–448. [Google Scholar] [CrossRef]
- Brzozowski, J.; Erman, P.; Lyyra, M. Precision Estimates of the Predissociation Rates of the OH A2Σ State (v ≤ 2). Phys. Scripta 1978, 17, 507. [Google Scholar]
- German, K.R. Radiative and predissociative lifetimes of the V′ = 0, 1, and 2 levels of the A 2Σ+ state of OH and OD. J. Chem. Phys. 1975, 63, 5252. [Google Scholar] [CrossRef]
- Steffens, K.L.; Luque, J.; Jeffries, J.B.; Crosley, D.R. Transition probabilities in OH A 2Σ+−X 2Πi: Bands with v′=2 and 3. J. Chem. Phys. 1997, 106, 6262. [Google Scholar] [CrossRef]
- Lamers, H.J.G.L.M.; Cassinelli, J.P. Introduction to Stellar Winds; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Cranmer, S.R. Coronal holes and the high-speed solar wind. Living Rev. Sol. Phys. 2009, 6, 3. [Google Scholar] [PubMed]
- Domínguez, A.; Siana, B.; Henry, A.L.; Scarlata, C.; Bedregal, A.G.; Malkan, M.; Atek, H.; Ross, N.R.; Colbert, J.W.; Teplitz, H.I. Dust Extinction from Balmer Decrements of Star-Forming Galaxies at 0.75 ≤ z ≤ 1.5 with Hubble Space Telescope/Wide-Field-Camera 3 Spectroscopy from the WFC3 Infrared Spectroscopic Parallel Survey. Astrophys. J. 2013, 763, 145. [Google Scholar] [CrossRef]
- Wang, S.; Lei, G.; Liu, D.; Yang, S. Balmer Hα, Hβ and Hγ Spectral Lines Intensities in High-Power RF Hydrogen Plasmas. Plasma Sci. Technol. 2014, 16, 219. [Google Scholar] [CrossRef]
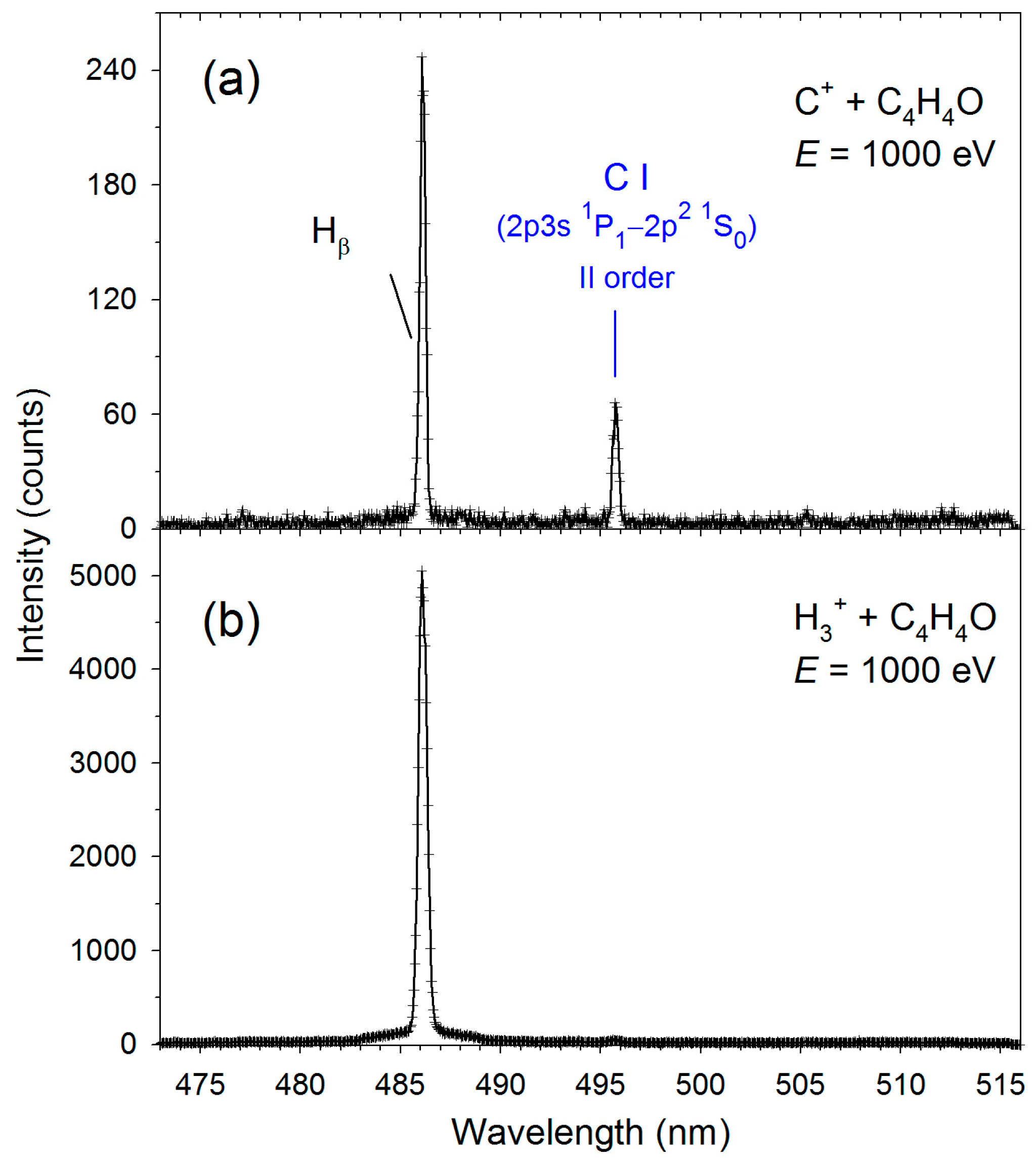
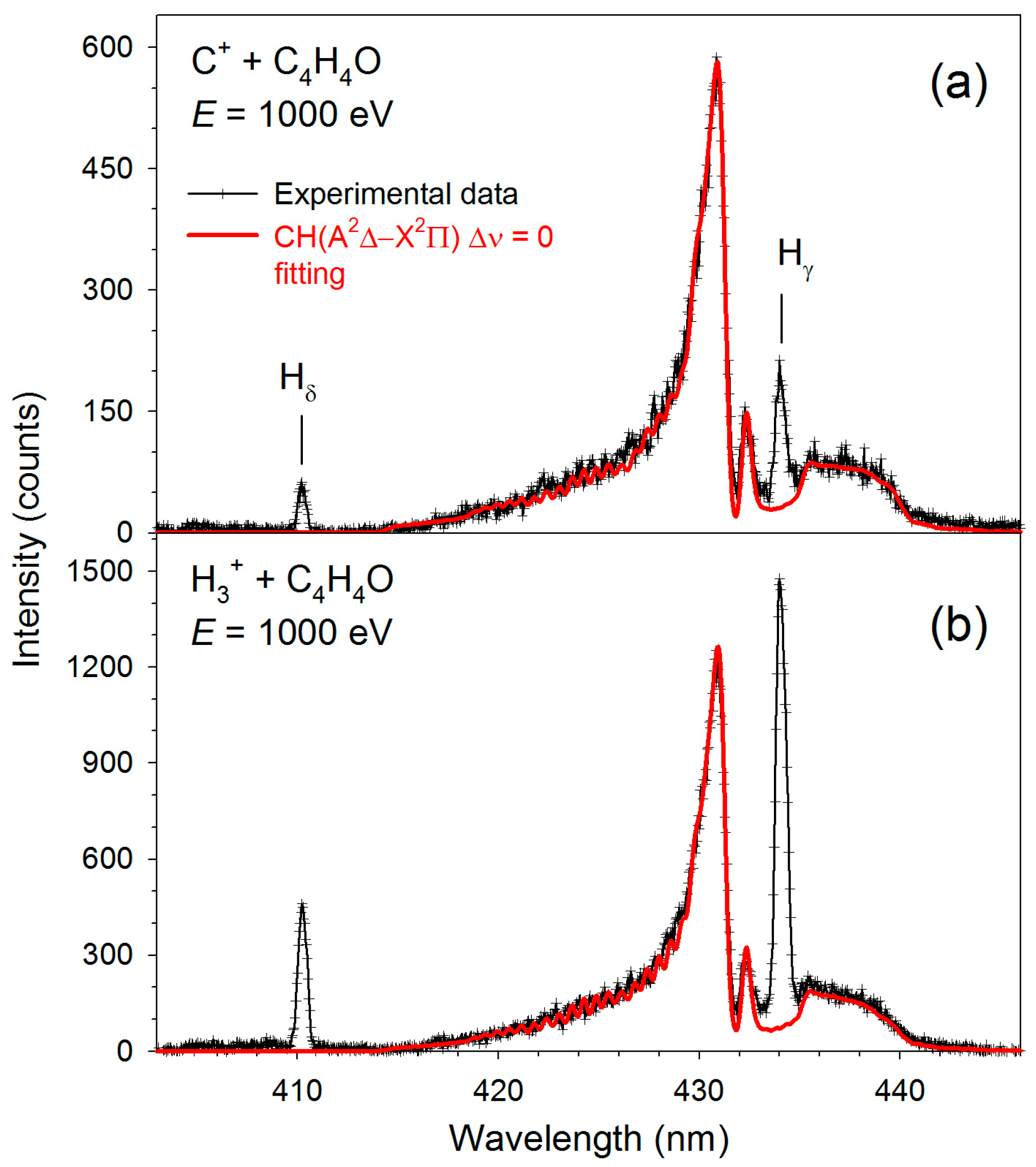

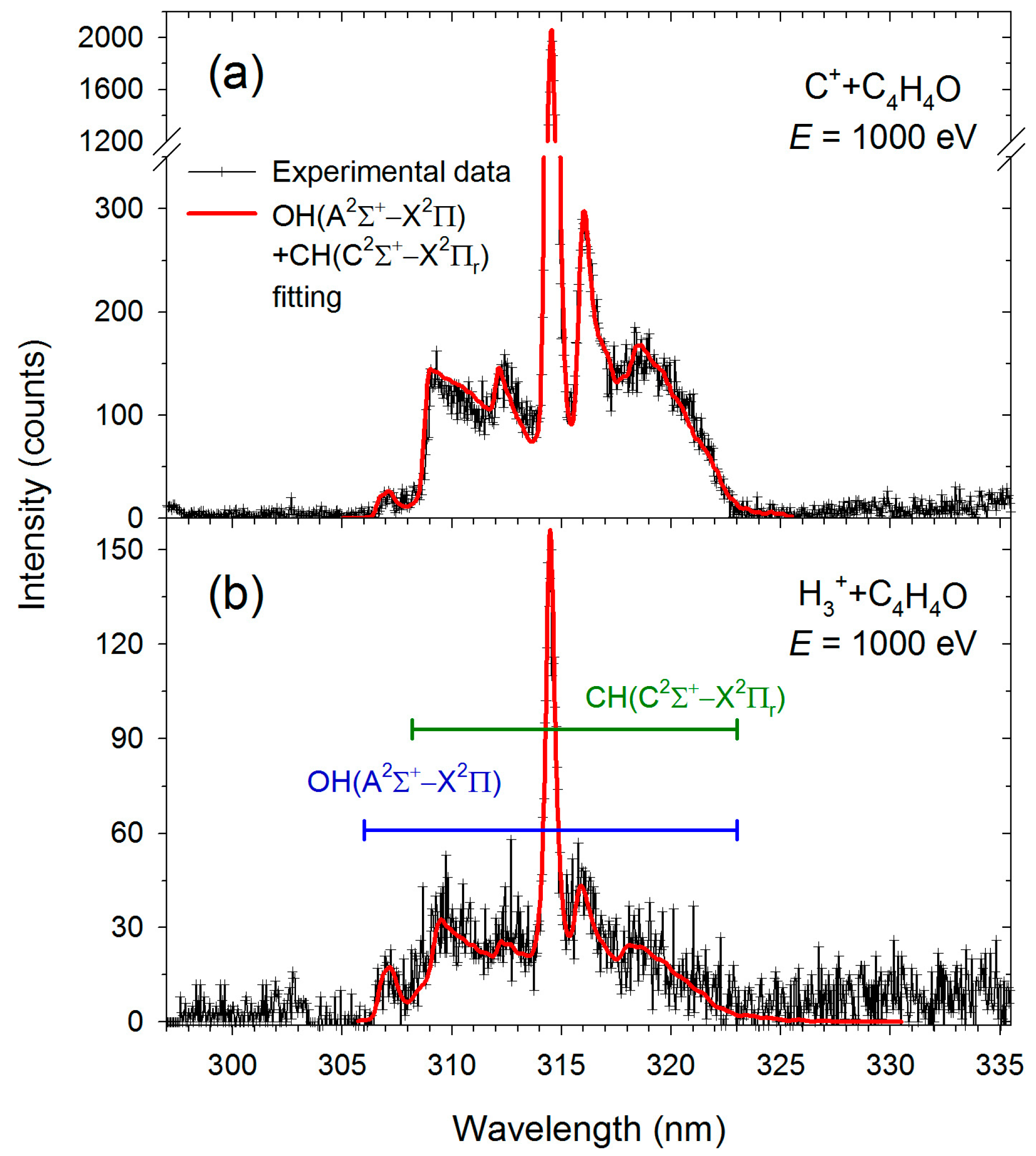

| Transition | H3+ + C4H4O | C+ + C4H4O | ||
|---|---|---|---|---|
| Tv [K] | TR [K] | Tv [K] | TR [K] | |
| CH(A2Δ→X2Πr) Δν = 0 | 7700 | 4150 | 8000 | 5000 |
| CH(B2Σ+→X2Πr) Δν = 0 | 3000 | 3500 | 3000 | 5000 |
| CH(C2Σ+→X2Πr) Δν = 0 | 4900 * | 5500 * | 5500 * | 6000 * |
| OH(A2Σ+→X2Π) Δν = 0 | 3900 * | 4800 * | 10,000 * | 12,000 * |
| Product | Electronic Transition | Vibrational Transition | λ (nm) | References |
|---|---|---|---|---|
| CH | A2Δ→X2Πr | (0;0) | 431.0 | [45,46] |
| (1;1) | 431.0 | |||
| (2;2) | 432.5 | |||
| (3;3) | 435.7 | |||
| B2Σ+→X2Πr | (0;0) | 386.5 | ||
| C2Σ+→X2Πr | (0;0) | 314.5 | ||
| (1;1) | 316.0 | |||
| OH | A2Σ+→X2Π | (0;0) | 306.5 | [45,46] |
| (1;1) | 312.2 | |||
| H | n = 4→2 | - | 486.1 | [47] |
| n = 5→2 | - | 434.0 | ||
| n = 6→2 | - | 410.2 | ||
| n = 7→2 | - | 397.0 | ||
| n = 8→2 | - | 386.0 | ||
| n = 9→2 | - | 379.0 | ||
| n = 10→2 | - | 373.0 | ||
| C | 2p3s 1P1→2p2 1D2 | - | 193.1 (386.2 IInd order) | [47] |
| 2p3s 1P1→2p2 1S0 | - | 247.9 (495.8 IInd order) |
| Furan (C4H4O) | Tetrahydrofuran (C4H8O) | Pyridine (C5H5N) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H+ | H2+ | H3+ | He++ | He+ | H+ | H2+ | H3+ | C+ | O+ | H+ | H2+ | He+ | |
| H(n = 4–7) | 58.3 | 46.3 | 37.6 | 47.9 | 8.4 | 88.8 | 76.2 | 67.3 | 4.8 | 17.6 | 61.4 | 45.9 | 10.8 |
| CH(A,B,C) | 39.9 | 51.0 | 59.4 | 52.1 | 82.1 | 11.2 | 23.8 | 32.7 | 36.2 | 69.6 | 26.9 | 40.4 | 42.2 |
| Other | 1.9 | 2.7 | 3.0 | - | 9.5 | - | - | - | 59.1 | 12.8 | 11.7 | 13.7 | 47.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasowicz, T.J. Furan Dissociation Induced by Collisions with H3+ and C+ Ions. Molecules 2025, 30, 2559. https://doi.org/10.3390/molecules30122559
Wasowicz TJ. Furan Dissociation Induced by Collisions with H3+ and C+ Ions. Molecules. 2025; 30(12):2559. https://doi.org/10.3390/molecules30122559
Chicago/Turabian StyleWasowicz, Tomasz J. 2025. "Furan Dissociation Induced by Collisions with H3+ and C+ Ions" Molecules 30, no. 12: 2559. https://doi.org/10.3390/molecules30122559
APA StyleWasowicz, T. J. (2025). Furan Dissociation Induced by Collisions with H3+ and C+ Ions. Molecules, 30(12), 2559. https://doi.org/10.3390/molecules30122559






