Advances in the Exploration of Coordination Complexes of Vanadium in the Realm of Alzheimer’s Disease: A Mini Review
Abstract
1. Introduction
2. Coordination Chemistry and Biological Properties of Vanadium
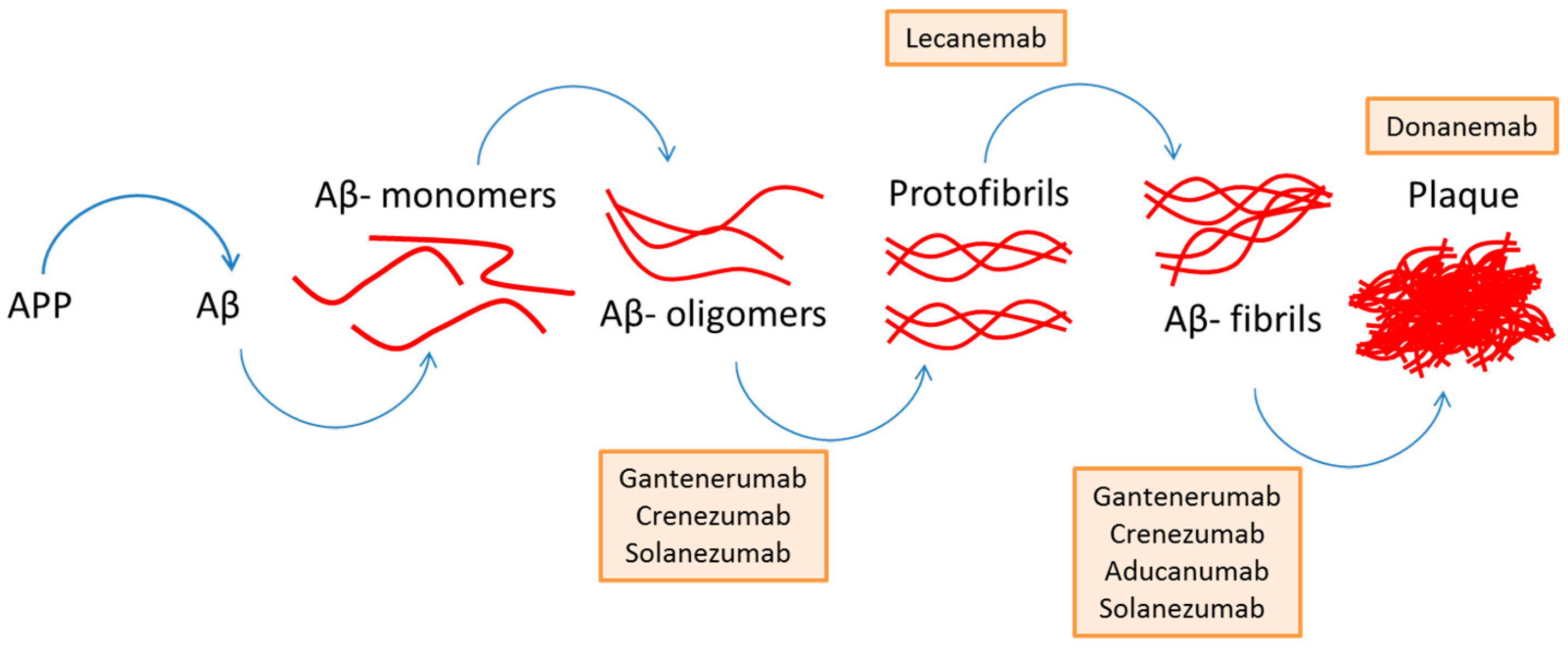
3. Amyloid-Beta Plaques in Alzheimer’s Disease
4. Recent Advances
In Vitro and In Vivo Evaluation of Vanadium Complexes
5. Outlook and Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-β |
| AChE | Acetylcholinesterase enzyme |
| AD | Alzheimer’s disease |
| Ala | Alanine |
| AMBER | Assisted Model Building with Energy Refinement |
| AMPKα | AMP-activated protein kinase α |
| Arg | Arginine |
| BACE1 | β-secretase |
| BEOV | bis(ethyl-maltolato)oxido-vanadium(IV) |
| Cys | Cysteine |
| ER | Endoplasmic reticulum |
| Gly | Glycine |
| Grp75 | Glucose-regulated protein 75 |
| Grp78 | Glucose regulated protein 78 |
| Hiapp | Human islet amyloid polypeptide |
| His | Histidine |
| InsR | Insulin receptor |
| INS-1 | Rat insulinoma |
| Ile | Isoleucine |
| JNK | c-Junk amino-terminal kinase |
| Met | Methionine |
| NF-κB | Nuclear factor-kappa B |
| PPARγ | Proliferator-activated receptor γ |
| PrP | Prion-protein |
| PTP1B | Protein-tyrosine phosphatase 1B |
| VAC | Vanadyl acetylacetonate |
References
- Saragea, P.D. Alzheimer’s Disease (AD): Environmental Modifiable Risk Factors. Int. J. Multidiscip. Res. 2024, 6, 1–12. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Porsteinsson, A.P.; Isaacson, R.S.; Knox, S.; Sabbagh, M.N.; Rubino, I. Diagnosis of Early Alzheimer’s Disease: Clinical Practice in 2021. J. Prev. Alzheimer’s Dis. 2021, 8, 371–386. [Google Scholar] [CrossRef]
- Saeed, A.; Lopez, O.; Cohen, A.; Reis, S.E. Cardiovascular Disease and Alzheimer’s Disease: The Heart–Brain Axis. J. Am. Heart Assoc. 2023, 12, e030780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Tian, Y.; Wang, Z.-T.; Ma, Y.-H.; Tan, L.; Yu, J.-T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimer’s Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimer’s Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef]
- Trejo-Lopez, J.A.; Yachnis, A.T.; Prokov, S. Neuropathology of Alzheimer’s Disease. Neurotherapeutics 2022, 19, 173–185. [Google Scholar] [CrossRef]
- Giri, M.; Kendre, P.N.; Bhalke, R.; Pande, V.; Autade, K.; Sumbe, R. Chapter 26—Combinatorial therapy in Alzheimer’s disease. In Alzheimer’s Disease and Advanced Drug Delivery Strategies; Prajapati, B.G., Chellappan, D.K., Kendre, P.N., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 439–461. [Google Scholar] [CrossRef]
- Hussain, M.S.; Mishra, A.K. Advancements in understanding and addressing Alzheimer’s disease: A comprehensive review. Int. J. Res. Med. Sci. 2024, 12, 1789–1795. [Google Scholar] [CrossRef]
- Zuliani, G.; Zuin, M.; Romagnoli, T.; Polastri, M.; Cervellati, C.; Brombo, G. Acetyl-cholinesterase-inhibitors reconsidered. A narrative review of post-marketing studies on Alzheimer’s disease. Aging Clin. Exp. Res. 2024, 36, 23. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Wang, Y.; Li, X.; Wang, S.; Wang, Z. Recent advance on carbamate-based cholinesterase inhibitors as potential multifunctional agents against Alzheimer’s disease. Eur. J. Med. Chem. 2022, 240, 114606. [Google Scholar] [CrossRef]
- Ansari, M.A.; Tripathi, T.; Venkidasamy, B.; Monziani, A.; Rajakumar, G.; Alomary, M.N.; Alyahya, S.A.; Onimus, O.; D’souza, N.; Barkat, M.A.; et al. Multifunctional Nanocarriers for Alzheimer’s Disease: Befriending the Barriers. Mol. Neurobiol. 2024, 61, 3042–3089. [Google Scholar] [CrossRef] [PubMed]
- Maurya, R.C.; Sutradhar, D.; Martin, M.H.; Roy, S.; Chourasia, J.; Sharma, A.K.; Vishwakarma, P. Oxovanadium (IV) complexes of medicinal relevance: Synthesis, characterization, and 3D-molecular modeling and analysis of some oxovanadium (IV) complexes in O,N-donor coordination matrix of sulfa drug Schiff bases derived from a 2-pyrazolin-5-one derivative. Arab. J. Chem. 2015, 8, 78–92. [Google Scholar] [CrossRef]
- Treviño, S.; Díaz, A.; Sánchez-Lara, E.; Sanchez-Gaytan, B.L.; Perez-Aguilar, J.M.; González-Vergara, E. Vanadium in Biological Action: Chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol. Trace Elem. Res. 2019, 188, 68–98. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Das, S.; Chatterjee, M.; Roy, K. Vanadium in Biological Systems. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 2293–2297. [Google Scholar] [CrossRef]
- Mukherjee, B.; Patra, B.; Mahapatra, S.; Banerjee, P.; Tiwari, A.; Chatterjee, M. Vanadium—An element of atypical biological significance. Toxicol. Lett. 2024, 150, 135–143. [Google Scholar] [CrossRef]
- Begum, A.; Vani, K.; Husain, A.; Chinnagalla, T.; Kumar, M.P.; Swapna, S.; Ayodhya, D.; Shaik, A. A comprehensive review of anti-diabetic activity of vanadium-based complexes via PTP-1B inhibition mechanism. Results Chem. 2023, 6, 101154. [Google Scholar] [CrossRef]
- Thompson, K.H.; Orvig, C. Coordination chemistry of vanadium in metallopharmaceutical candidate compounds. Coord. Chem. Rev. 2021, 219–221, 1033–1053. [Google Scholar] [CrossRef]
- Rehder, D. The role of vanadium in biology. Metallomics 2015, 7, 730–742. [Google Scholar] [CrossRef]
- Butler, A.; Carrano, C.J. Coordination chemistry of vanadium in biological systems. Coord. Chem. Rev. 1991, 109, 61–105. [Google Scholar] [CrossRef]
- Sharfalddin, A.A.; Al-Younis, I.M.; Mohammed, H.A.; Dhahri, M.; Mouffouk, F.; Ali, H.A.; Anwar, M.J.; Qureshi, K.A.; Hussien, M.A.; Alghrably, M.; et al. Therapeutic Properties of Vanadium Complexes. Inorganics 2022, 10, 244. [Google Scholar] [CrossRef]
- Jakusch, T.; Kiss, T. In vitro study of the antidiabetic behavior of vanadium compounds. Coord. Chem. Rev. 2017, 351, 118–126. [Google Scholar] [CrossRef]
- Hiromura, M.; Adachi, Y.; Machida, M.; Hattori, M.; Sakurai, H. Glucose lowering activity by oral administration of bis(allixinato)oxidovanadium(iv) complex in streptozotocin-induced diabetic mice and gene expression profiling in their skeletal muscles. Metallomics 2009, 1, 92–100. [Google Scholar] [CrossRef]
- Amaral, L.M.P.F.; Moniz, T.; Silva, A.M.N.; Rangel, M. Vanadium Compounds with Antidiabetic Potential. Int. J. Mol. Sci. 2023, 24, 15675. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhou, R.; Zhou, Q.; Guo, X.; Yan, C.; Ke, M.; Lei, J.; Shi, Y. Structural basis of Notch recognition by human γ-secretase. Nature 2019, 565, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Holtzman, D.M. Three dimensions of the amyloid hypothesis: Time, space and ‘wingmen’. Nat. Neurosci. 2015, 18, 800–806. [Google Scholar] [CrossRef]
- Li, J.; Liao, W.; Huang, D.; Ou, M.; Chen, T.; Wang, X.; Zhao, R.; Zhang, L.; Mei, L.; Liu, J.; et al. Current strategies of detecting Aβ species and inhibiting Aβ aggregation: Status and prospects. Coord. Chem. Rev. 2023, 495, 215375. [Google Scholar] [CrossRef]
- Michaels, T.C.T.; Qian, D.; Šarić, A.; Vendruscolo, M.; Linse, S.; Knowles, T.P.J. Amyloid formation as a protein phase transition. Nat. Rev. Phys. 2023, 5, 379–397. [Google Scholar] [CrossRef]
- Tsoy, A.; Umbayev, B.; Kassenova, A.; Kaupbayeva, B.; Askarova, S. Pathology of Amyloid-β (Aβ) Peptide Peripheral Clearance in Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 10964. [Google Scholar] [CrossRef]
- Bezprozvanny, I. Alzheimer’s disease—Where do we go from here? Biochem. Biophys. Res. Commun. 2022, 633, 72–76. [Google Scholar] [CrossRef]
- Cai, W.; Wu, T.; Chen, N. The Amyloid-Beta Clearance: From Molecular Targets to Glial and Neural Cells. Biomolecules 2023, 13, 313. [Google Scholar] [CrossRef]
- Ratan, Y.; Rajput, A.; Maleysm, S.; Pareek, A.; Jain, V.; Pareek, A.; Kaur, R.; Singh, G. An Insight into Cellular and Molecular Mechanisms Underlying the Pathogenesis of Neurodegeneration in Alzheimer’s Disease. Biomedicines 2023, 11, 1398. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.C.; Kulig, W.; Poojari, C.; Rog, T.; Strodel, B. Physiologically-relevant levels of sphingomyelin, but not GM1, induces a β-sheet-rich structure in the amyloid-β(1-42) monomer. Biochim. Biophys. Acta-Biomembr. 2018, 1860, 1709–1720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef]
- Nagel-Steger, L.; Owen, M.C.; Strodel, B. An Account of Amyloid Oligomers: Facts and Figures Obtained from Experiments and Simulations. ChemBioChem 2016, 17, 657–676. [Google Scholar] [CrossRef]
- Iijima, K.; Liu, H.-P.; Chiang, A.-S.; Hearn, S.A.; Konsolaki, M.; Zhong, Y. Dissecting the pathological effects of human Aβ40 and Aβ42 in Drosophila: A potential model for Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 6623–6628. [Google Scholar] [CrossRef]
- Hampel, H.; Au, R.; Mattke, S.; van der Flier, W.M.; Aisen, P.; Apostolova, L.; Chen, C.; Cho, M.; De Santi, S.; Gao, P.; et al. Designing the next-generation clinical care pathway for Alzheimer’s disease. Nat. Aging 2022, 2, 692–703. [Google Scholar] [CrossRef]
- Lahiri, D.K.; Maloney, B. Beyond the signaling effect role of amyloid-β42 on the processing of APP, and its clinical implications. Exp. Neurol. 2021, 225, 51–54. [Google Scholar] [CrossRef][Green Version]
- Buccellato, F.R.; D’Anca, M.; Tartaglia, G.M.; Del Fabbro, M.; Scarpini, E.; Galimberti, D. Treatment of Alzheimer’s Disease: Beyond Symptomatic Therapies. Int. J. Mol. Sci. 2023, 24, 13900. [Google Scholar] [CrossRef]
- Kalantari, R.; Asadi, Z. DNA/BSA binding of a new oxovanadium (IV) complex of glycylglycine derivative Schiff base ligand. J. Mol. Struct. 2020, 1219, 128664. [Google Scholar] [CrossRef]
- Jeremic, D.; Jiménez-Díaz, L.; Navarro-López, J.D. Past, present and future of therapeutic strategies against amyloid-β peptides in Alzheimer’s disease: A systematic review. Ageing Res. Rev. 2021, 72, 101496. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Vogt, A.-C.S.; Jennings, G.T.; Mohsen, M.O.; Vogel, M.; Bachmann, M.F. Alzheimer’s Disease: A Brief History of Immunotherapies Targeting Amyloid β. Int. J. Mol. Sci. 2023, 24, 3895. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, X.; Zhu, D.; Zhao, C.; Du, W. Methionine oxidation of amyloid peptides by peroxovanadium complexes: Inhibition of fibril formation through a distinct mechanism. Metallomics 2015, 7, 1562–1572. [Google Scholar] [CrossRef]
- Tan, C.; Dong, Y.; Wang, J.; Yang, X. Vanadyl acetylacetonate attenuates Aβ pathogenesis in APP/PS1 transgenic mice depending on the intervention stage. New J. Chem. 2019, 43, 17588–17594. [Google Scholar] [CrossRef]
- Xu, J.; Gong, G.; Huang, X.; Du, W. Schiff base oxovanadium complexes resist the assembly behavior of human islet amyloid polypeptide. J. Inorg. Biochem. 2018, 186, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, B.; Gong, G.; Huang, X.; Du, W. Inhibitory effects of oxidovanadium complexes on the aggregation of human islet amyloid polypeptide and its fragments. J. Inorg. Biochem. 2019, 197, 110721. [Google Scholar] [CrossRef]
- He, Z.; Wang, M.; Zhao, Q.; Li, X.; Liu, P.; Ren, B.; Wu, C.; Du, X.; Li, N.; Liu, Q. Bis(ethylmaltolato)oxidovanadium (IV) mitigates neuronal apoptosis resulted from amyloid-beta induced endoplasmic reticulum stress through activating peroxisome proliferator-activated receptor γ. J. Inorg. Biochem. 2020, 208, 111073. [Google Scholar] [CrossRef]
- He, Z.; Han, S.; Wu, C.; Liu, L.; Zhu, H.; Liu, A.; Lu, Q.; Huang, J.; Du, X.; Li, N.; et al. Correction: Bis(ethylmaltolato)oxidovanadium (IV) inhibited the pathogenesis of Alzheimer’s disease in triple transgenic model mice. Metallomics 2020, 12, 631. [Google Scholar] [CrossRef]
- He, Z.; Li, X.; Han, S.; Ren, B.; Hu, X.; Li, N.; Du, X.; Ni, J.; Yang, X.; Liu, Q. Bis(ethylmaltolato)oxidovanadium (IV) attenuates amyloid-beta-mediated neuroinflammation by inhibiting NF-κB signaling pathway via a PPARγ-dependent mechanism. Metallomics 2021, 13, mfab036. [Google Scholar] [CrossRef] [PubMed]
- Chaves, O.A.; de Oliveira, M.C.C.; de Salles, C.M.C.; Martins, F.M.; Iglesias, B.A.; Back, D.F. In vitro tyrosinase, acetylcholinesterase, and HSA evaluation of dioxidovanadium (V) complexes: An experimental and theoretical approach. J. Inorg. Biochem. 2019, 200, 110800. [Google Scholar] [CrossRef] [PubMed]
- Tavares, C.A.; Santos, T.M.R.; da Cunha, E.F.F.; Ramalho, T.C. Parameterization and validation of a new AMBER force field for an oxovanadium (IV) complex with therapeutic potential implications in Alzheimer’s disease. J. Mol. Graph. Model. 2023, 122, 108511. [Google Scholar] [CrossRef] [PubMed]

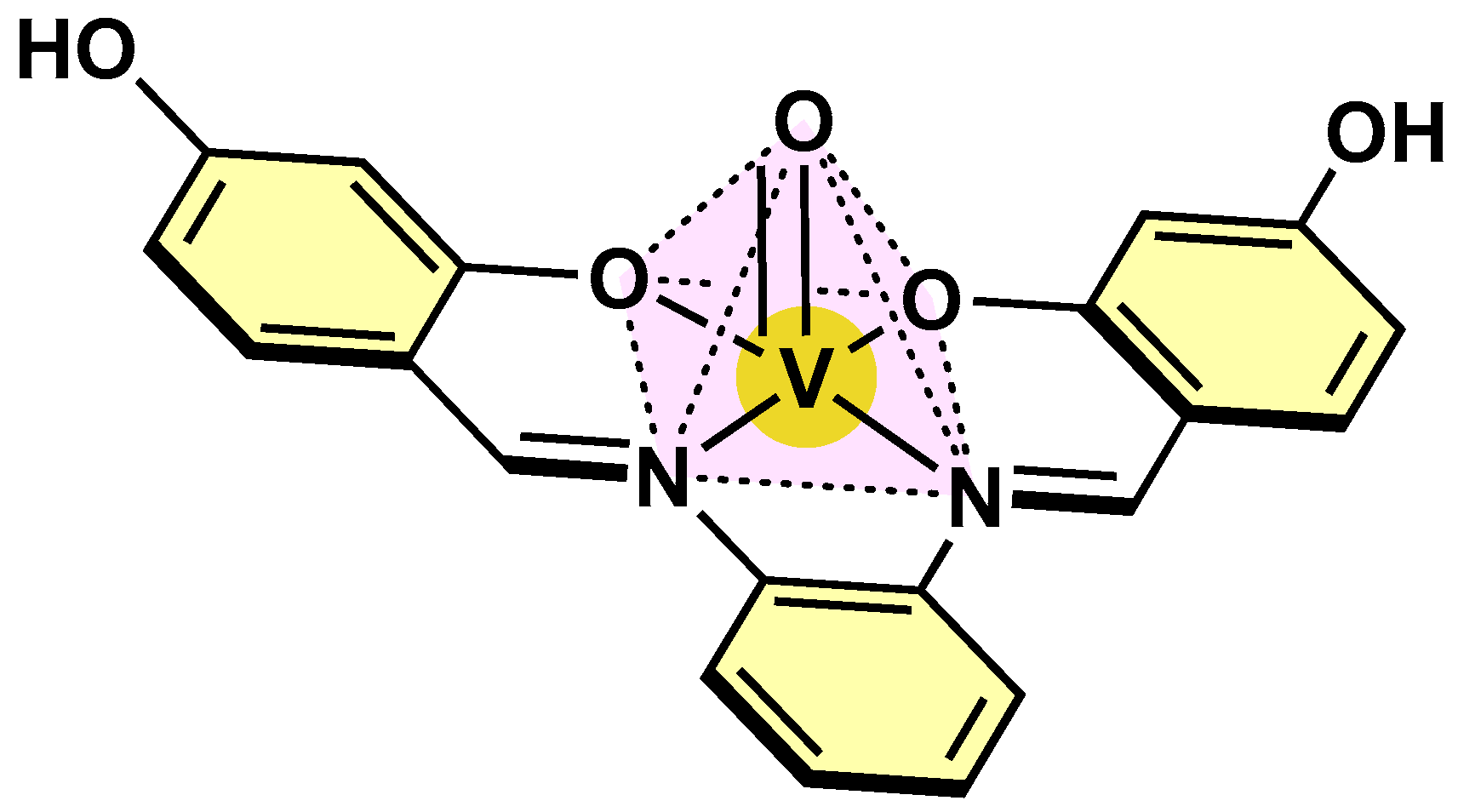



| Complex | Oxidation Statate | Activity Model | Biological Activity Summary | Reference |
|---|---|---|---|---|
| 1 | +5 | In vitro | Inhibits Aβ and PrP aggregation via oxidation of His111/Met residues; increases neuroblastoma cell viability | [47] |
| 2 | +5 | In vitro | Similar to 1; methionine oxidation delays aggregation | [47] |
| 3 | +4 | In vivo | Activates PPARγ and AMPKα; reduces Aβ deposition and tau phosphorylation; improves glucose metabolism | [48] |
| 4–7 | +4 | In vitro/in vivo | Inhibit hIAPP aggregation; 4 most effective; ligands less active alone | [49] |
| 8 | +4 | In vitro/in vivo | Interacts with PPARγ; reduces ER stress, Aβ and tau pathology, and neuroinflammation; improves cognition | [50,51,52,53] |
| 9 | +5 | In vitro | Effective inhibitor of hIAPP aggregation; strongest hydrophobic interactions among tested compounds | [50] |
| 10–12 | +5 | In sillico | Modulate AChE activity (activation by 10; inhibition by 11 and 12); interact via H-bonding and hydrophobic forces | [54] |
| 13 | +4 | In sillico | Interacts with PTP1B enzyme (via Gly183, Cys215, Arg221); strong binding energy (−104.8 kcal/mol) | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Navarro, J.A.; Delgado-Rangel, L.H.; Malpica-Calderón, R.; Sánchez-Mora, A.T.; Ponce-Bolaños, H.; González-Oñate, A.F.; Alí-Torres, J.; Colorado-Peralta, R.; Canseco-Gonzalez, D.; Reyes-Márquez, V.; et al. Advances in the Exploration of Coordination Complexes of Vanadium in the Realm of Alzheimer’s Disease: A Mini Review. Molecules 2025, 30, 2547. https://doi.org/10.3390/molecules30122547
Cruz-Navarro JA, Delgado-Rangel LH, Malpica-Calderón R, Sánchez-Mora AT, Ponce-Bolaños H, González-Oñate AF, Alí-Torres J, Colorado-Peralta R, Canseco-Gonzalez D, Reyes-Márquez V, et al. Advances in the Exploration of Coordination Complexes of Vanadium in the Realm of Alzheimer’s Disease: A Mini Review. Molecules. 2025; 30(12):2547. https://doi.org/10.3390/molecules30122547
Chicago/Turabian StyleCruz-Navarro, Jesús Antonio, Luis Humberto Delgado-Rangel, Ricardo Malpica-Calderón, Arturo T. Sánchez-Mora, Hugo Ponce-Bolaños, Andrés Felipe González-Oñate, Jorge Alí-Torres, Raúl Colorado-Peralta, Daniel Canseco-Gonzalez, Viviana Reyes-Márquez, and et al. 2025. "Advances in the Exploration of Coordination Complexes of Vanadium in the Realm of Alzheimer’s Disease: A Mini Review" Molecules 30, no. 12: 2547. https://doi.org/10.3390/molecules30122547
APA StyleCruz-Navarro, J. A., Delgado-Rangel, L. H., Malpica-Calderón, R., Sánchez-Mora, A. T., Ponce-Bolaños, H., González-Oñate, A. F., Alí-Torres, J., Colorado-Peralta, R., Canseco-Gonzalez, D., Reyes-Márquez, V., & Morales-Morales, D. (2025). Advances in the Exploration of Coordination Complexes of Vanadium in the Realm of Alzheimer’s Disease: A Mini Review. Molecules, 30(12), 2547. https://doi.org/10.3390/molecules30122547











