Unveiling the Biotechnological Potential of Cyanobacteria from the Portuguese LEGE-CC Collection Through Lipidomics and Antioxidant and Lipid-Lowering Properties
Abstract
1. Introduction
2. Results
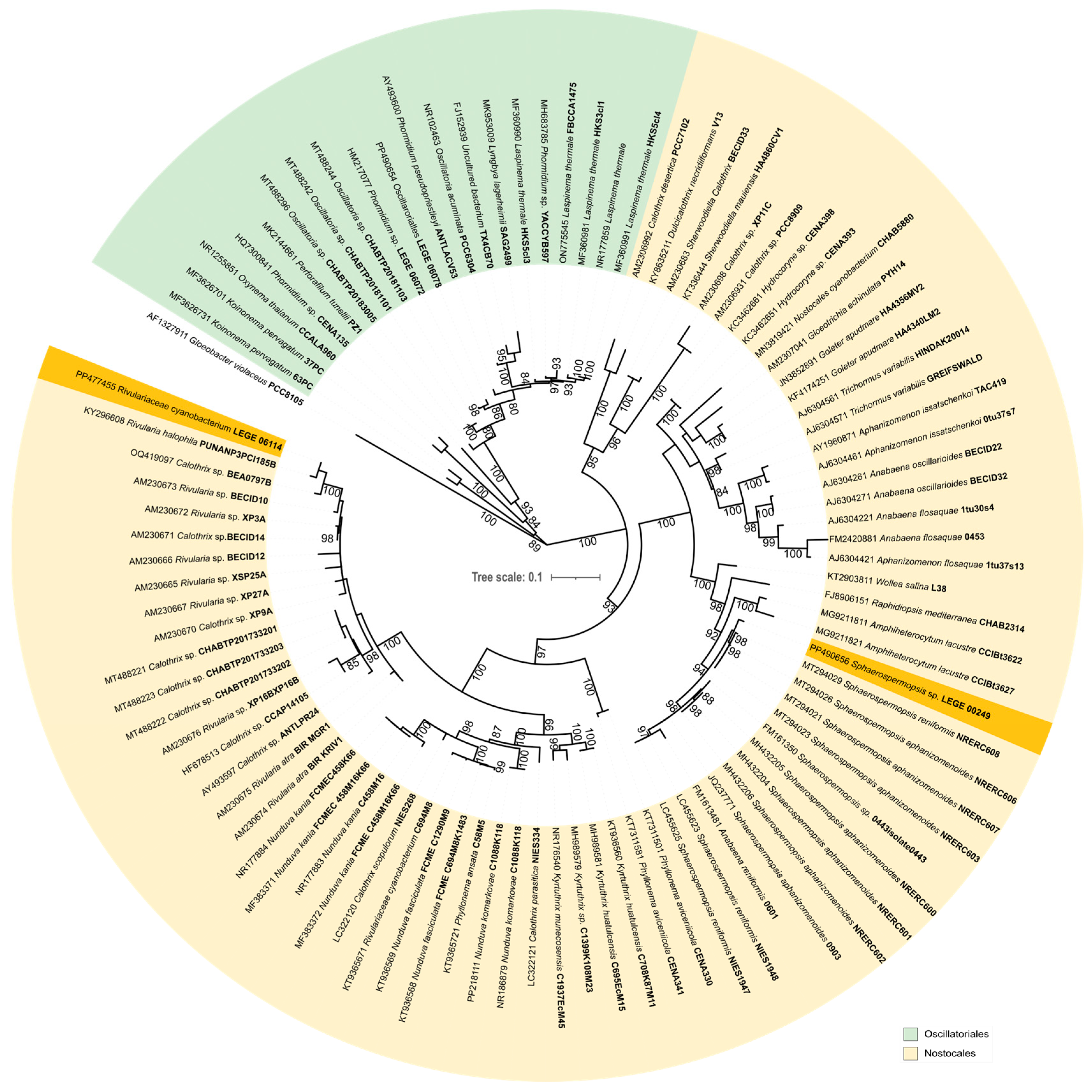
2.1. Phylogenetic Analysis and Potential for Cyanotoxin Production
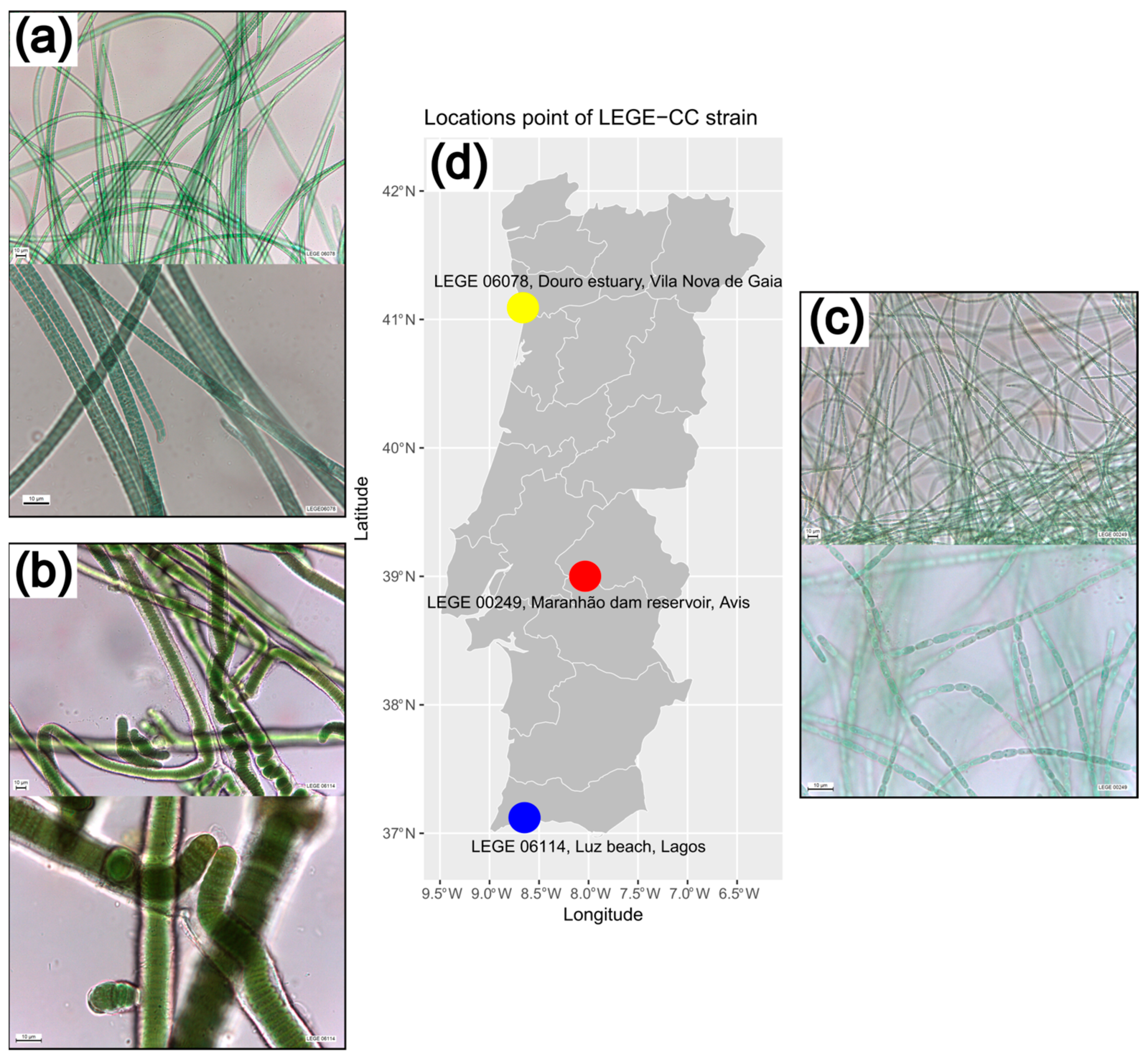
2.2. Morphological Characteristics of the Cyanobacteria Strains
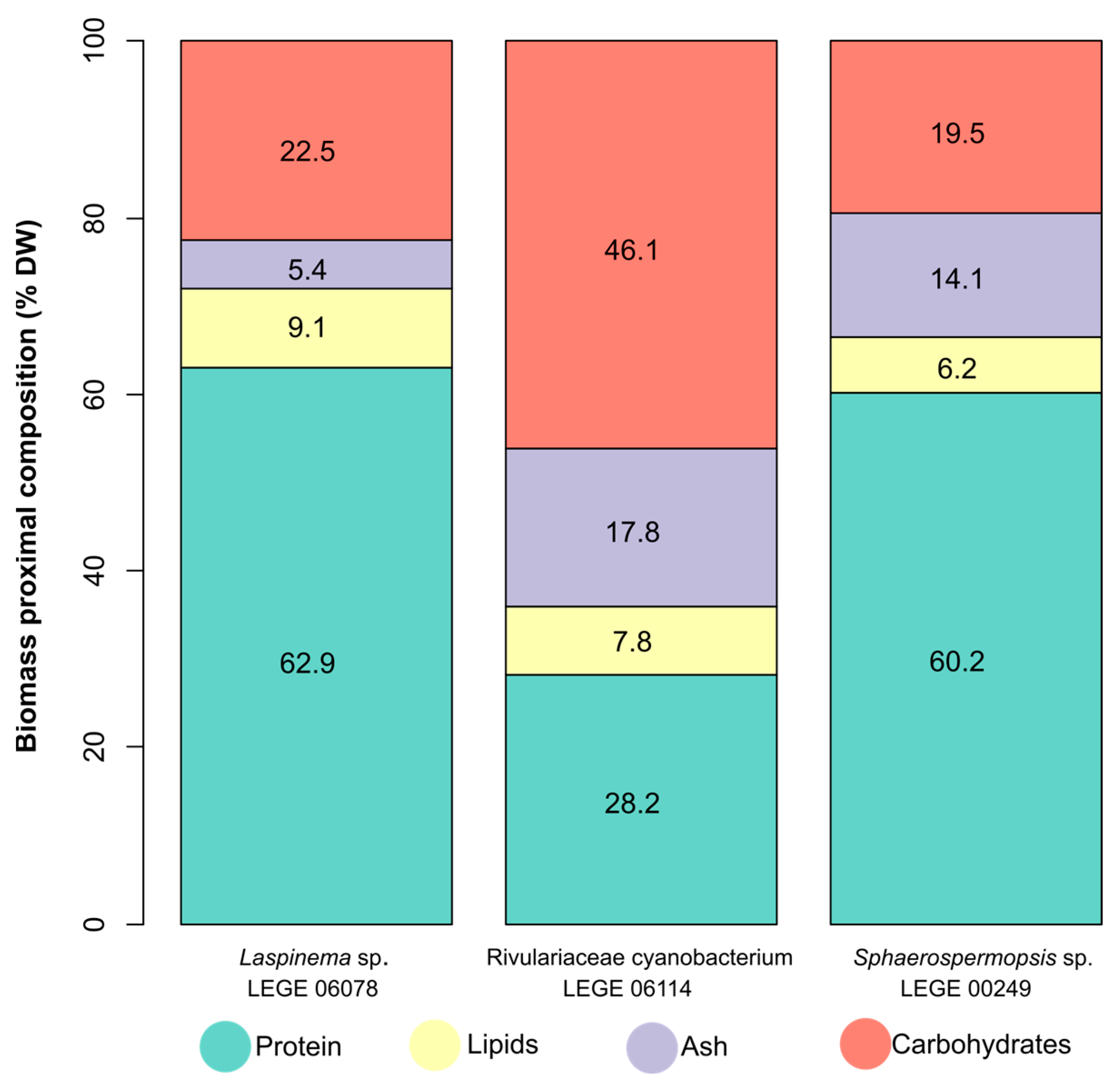
2.3. Biomass Proximate Composition Analysis
2.4. Fatty Acid Composition and Total Lipid Content of Cyanobacteria Strains
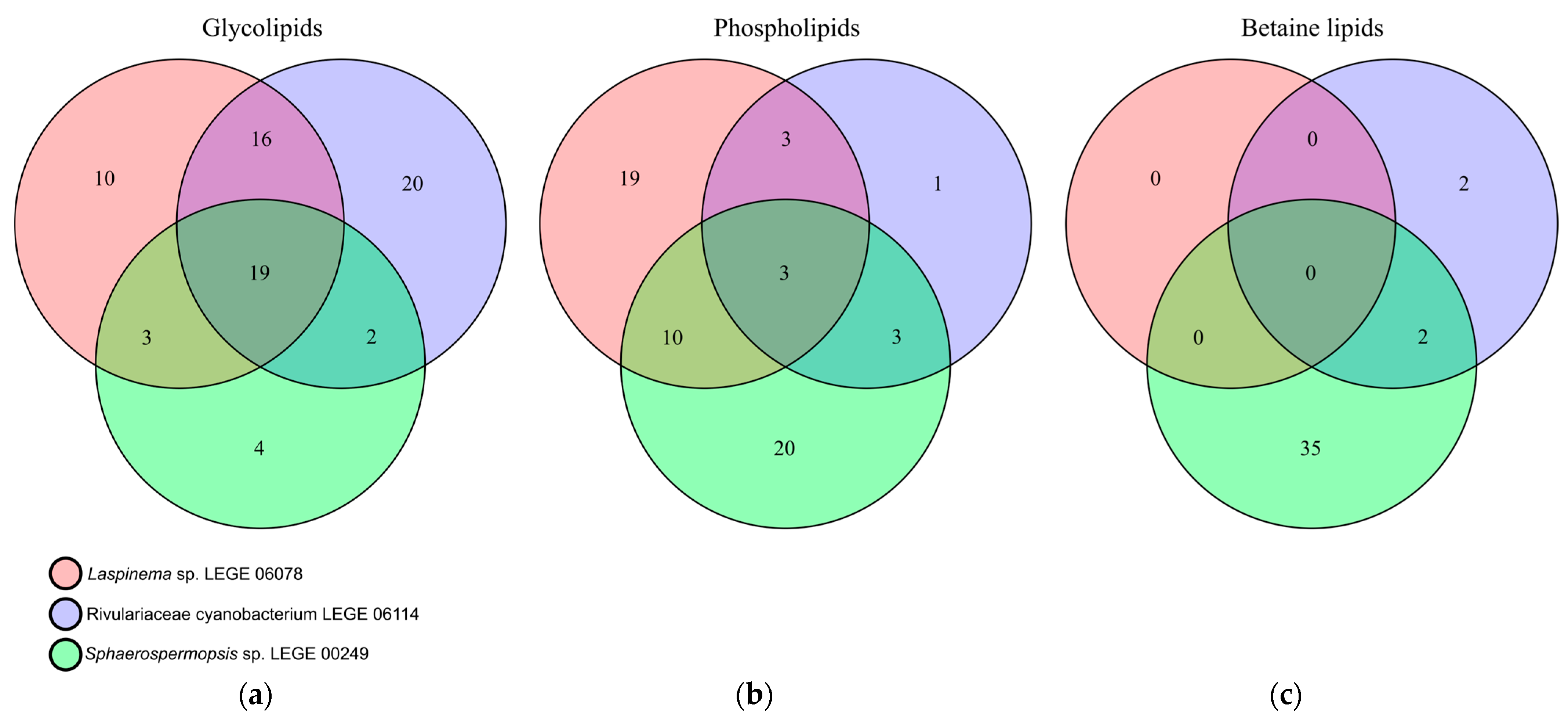
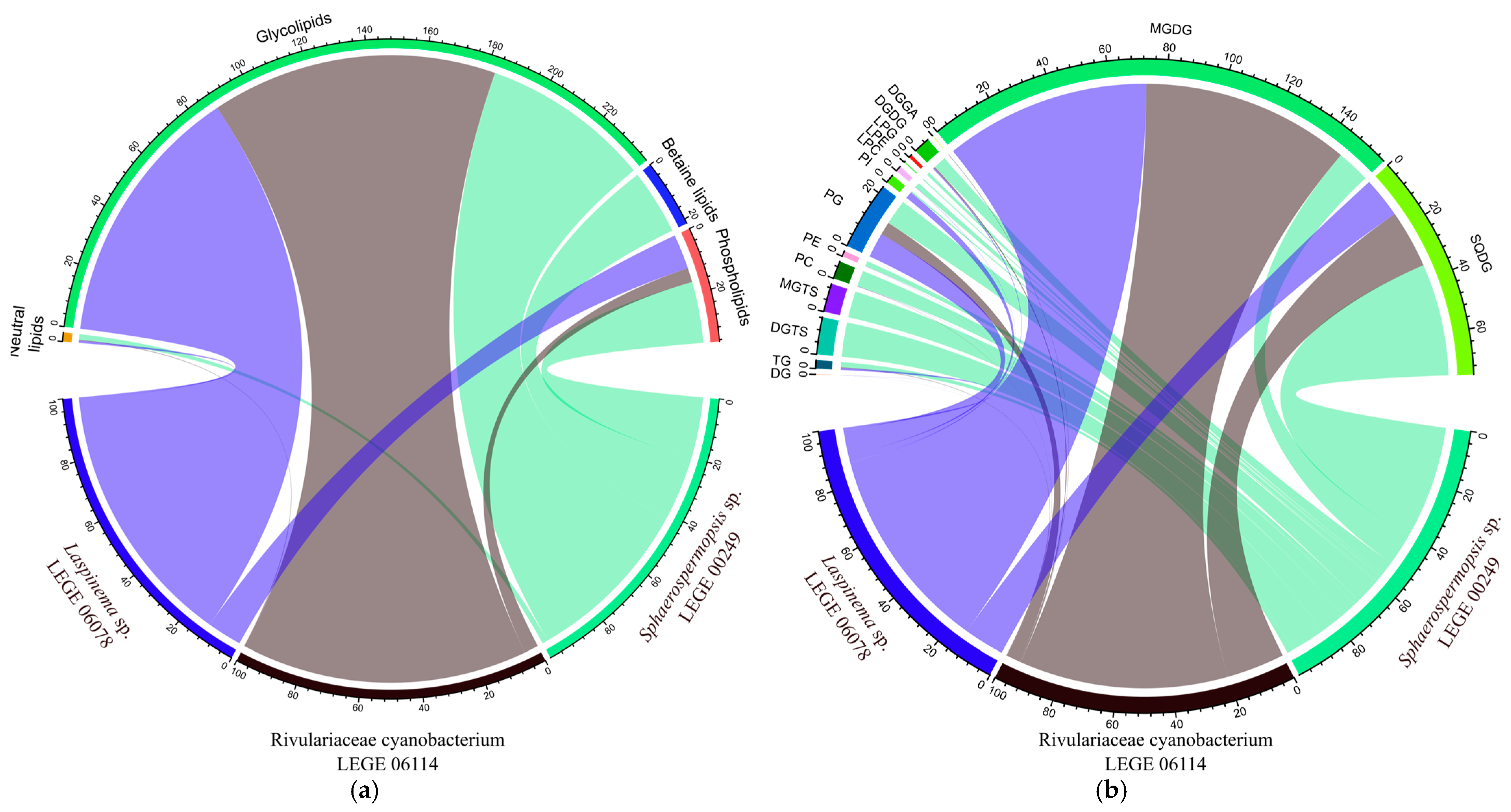
2.5. Identification of the Lipidome of the Cyanobacteria Strains
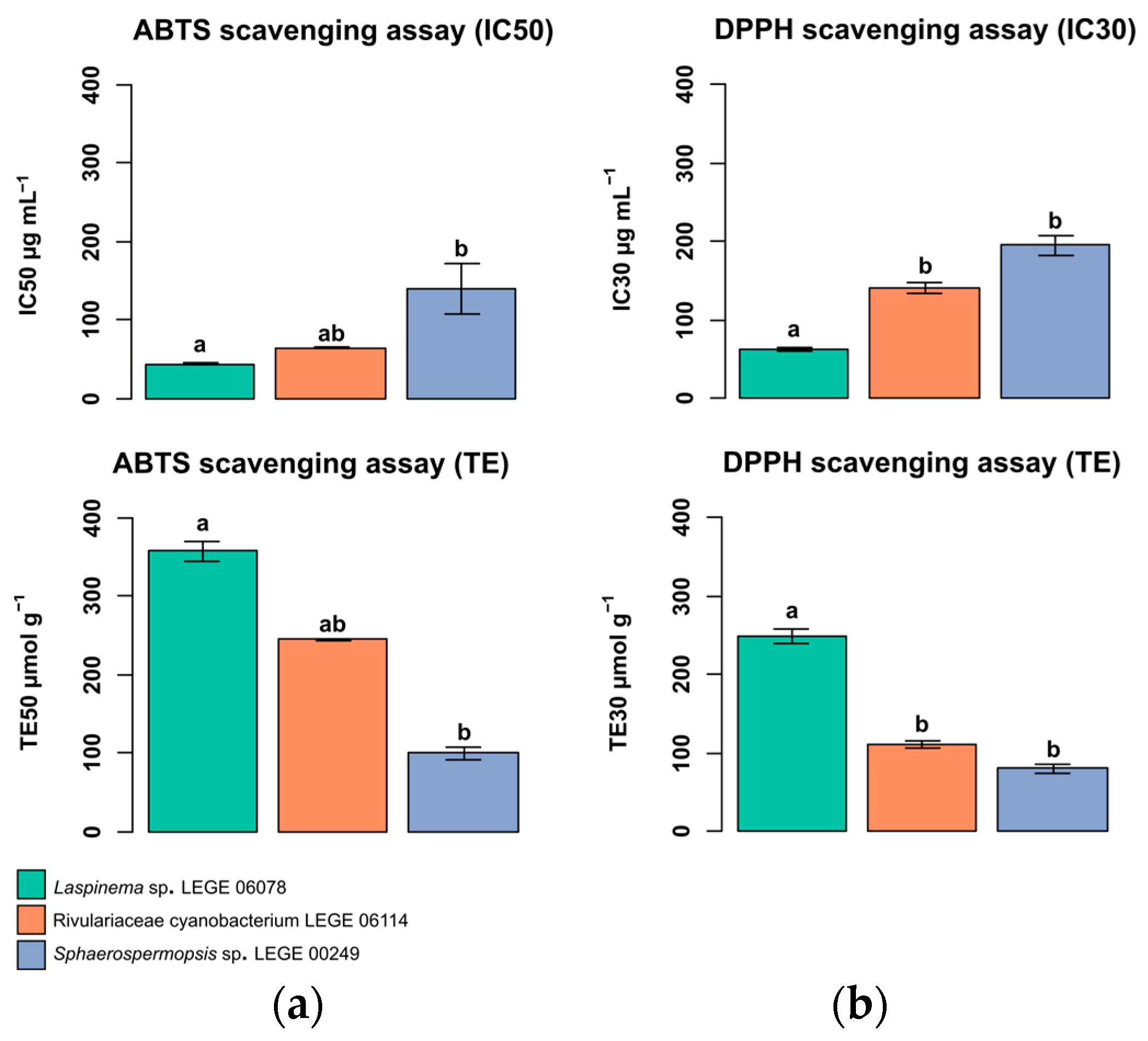
2.6. In Chemico Evaluation of Antioxidant Activity
2.7. Lipid-Lowering Activity
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cyanobacterial Material
4.2.1. Strains and Biomass Production
4.2.2. DNA Extraction, PCR Amplification, and Sequencing
4.2.3. Potential for Cyanotoxin Production
4.2.4. Phylogenetic Analysis
4.2.5. Morphological Analysis
4.3. Biomass Proximate Composition
4.4. Lipid Extraction Procedure
4.4.1. Fatty Acid Analysis by Gas Chromatography–Mass Spectrometry (GC–MS)
Data Acquisition
Data Analysis
4.5. Lipidomic Analysis
4.5.1. Data Acquisition
4.5.2. Data Analysis
4.6. Antioxidant Scavenging Activity
4.6.1. ABTS Cation Radical Scavenging Assay
4.6.2. DPPH Radical Scavenging Assay
4.7. Lipid-Lowering Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strunecký, O.; Ivanova, A.P.; Mareš, J. An Updated Classification of Cyanobacterial Orders and Families Based on Phylogenomic and Polyphasic Analysis. J. Phycol. 2023, 59, 12–51. [Google Scholar] [CrossRef] [PubMed]
- Kaštovský, J. Welcome to the Jungle!: An Overview of Modern Taxonomy of Cyanobacteria. Hydrobiologia 2024, 851, 1063–1077. [Google Scholar] [CrossRef]
- Moretto, J.A.; Berthold, D.E.; Lefler, F.W.; Huang, I.S.; Laughinghouse, H.D. Floridanema gen. nov. (Aerosakkonemataceae, Aerosakkonematales Ord. nov., Cyanobacteria) from Benthic Tropical and Subtropical Fresh Waters, with the Description of Four New Species. J. Phycol. 2025, 61, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Hentschke, G.S.; Ramos, V.; Pinheiro, Â.; Barreiro, A.; Costa, M.S.; Rego, A.; Brule, S.; Vasconcelos, V.M.; Leão, P.N. Zarconia navalis gen. nov., sp. nov., Romeriopsis navalis gen. nov., sp. nov. and Romeriopsis marina sp. nov., Isolated from Inter-and Subtidal Environments from Northern Portugal. Int. J. Syst. Evol. Microbiol. 2022, 72, 005552. [Google Scholar] [CrossRef]
- Luz, R.; Cordeiro, R.; Kaštovský, J.; Johansen, J.R.; Dias, E.; Fonseca, A.; Urbatzka, R.; Vasconcelos, V.; Gonçalves, V. New Terrestrial Cyanobacteria from the Azores Islands: Description of Venetifunis gen. nov. and New Species of Albertania, Kovacikia and Pegethrix. Phycologia 2023, 62, 483–498. [Google Scholar] [CrossRef]
- Luz, R.; Cordeiro, R.; Kaštovský, J.; Fonseca, A.; Urbatzka, R.; Vasconcelos, V.; Gonçalves, V. Description of Pseudocalidococcus azoricus gen. sp. nov. (Thermosynechococcaceae, Cyanobacteria), a Rare but Widely Distributed Coccoid Cyanobacteria. Diversity 2023, 15, 1157. [Google Scholar] [CrossRef]
- Hentschke, G.S.; Morais, J.; Oliveira, F.; Lopes, G.; Leão, P.N.; Barreiro, A.; Pinheiro, Â.; Brule, S.; Vasconcelos, V.M. Polyphasic Description of Ciimarium marinum gen. et sp. nov. (Prochlorococcaceae, Synechococcales): A New Picocyanobacterial Taxon from the Portuguese Coastal Ecosystem. Phytotaxa 2024, 637, 133–148. [Google Scholar] [CrossRef]
- Oliveira, F.L.; Hentschke, G.S.; Morais, J.; Silva, R.; Cruz, P.; Vasconcelos, V.M. Exploring the Cyanobacterial Diversity in Portugal: Description of Four New Genera from LEGE-CC Using the Polyphasic Approach. J. Phycol. 2024, 60, 1285–1304. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Cruz, J.D.; Vasconcelos, V. Legal Aspects of Microalgae in the European Food Sector. Foods 2024, 13, 124. [Google Scholar] [CrossRef]
- Galetović, A.; Azevedo, J.; Castelo-Branco, R.; Oliveira, F.; Gómez-Silva, B.; Vasconcelos, V. Absence of Cyanotoxins in Llayta, Edible Nostocaceae Colonies from the Andes Highlands. Toxins 2020, 12, 382. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Su, L.; Chen, S.; Zhao, L.; Wang, H.; Ding, F.; Chen, H.; Shi, R.; Wang, Y.; Huang, Z. Physicochemical Characterization and Functional Analysis of the Polysaccharide from the Edible Microalga Nostoc sphaeroides. Molecules 2018, 23, 508. [Google Scholar] [CrossRef] [PubMed]
- Lawande, S.; Bombe, P. The Spirulina Market Was Valued at $0.59 Billion in 2023. In This Market Is Expected to Reach $1.20 Billion by 2031 from an Estimated $0.64 Billion in 2024, at a CAGR of 9.4% During the Forecast Period of 2024 to 2031; Meticulous Research: Maharashtra, India, 2024. [Google Scholar]
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the Food and Functional Food Industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef]
- Szulinska, M.; Gibas-Dorna, M.; Miller-Kasprzak, E.; Suliburska, J.; Miczke, A.; Walczak-Gałezewska, M.; Stelmach-Mardas, M.; Walkowiak, J.; Bogdanski, P. Spirulina maxima Improves Insulin Sensitivity, Lipid Profile, and Total Antioxidant Status in Obese Patients with Well-Treated Hypertension: A Randomized Double-Blind Placebo-Controlled Study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2473–2481. [Google Scholar] [PubMed]
- Kallifidas, D.; Dhakal, D.; Chen, M.; Chen, Q.Y.; Kokkaliari, S.; Colon Rosa, N.A.; Ratnayake, R.; Bruner, S.D.; Paul, V.J.; Ding, Y.; et al. Biosynthesis of Dolastatin 10 in Marine Cyanobacteria, a Prototype for Multiple Approved Cancer Drugs. Org. Lett. 2024, 26, 1321–1325. [Google Scholar] [CrossRef]
- Fernández, F.G.A.; Reis, A.; Wijffels, R.H.; Barbosa, M.; Verdelho, V.; Llamas, B. The Role of Microalgae in the Bioeconomy. New Biotechnol. 2021, 61, 99–107. [Google Scholar] [CrossRef]
- Mondal, A.; Bose, S.; Banerjee, S.; Patra, J.K.; Malik, J.; Mandal, S.K.; Kilpatrick, K.L.; Das, G.; Kerry, R.G.; Fimognari, C.; et al. Marine Cyanobacteria and Microalgae Metabolites—A Rich Source of Potential Anticancer Drugs. Mar. Drugs 2020, 18, 476. [Google Scholar] [CrossRef]
- Tabarzad, M.; Atabaki, V.; Hosseinabadi, T. Anti-Inflammatory Activity of Bioactive Compounds from Microalgae and Cyanobacteria by Focusing on the Mechanisms of Action. Mol. Biol. Rep. 2020, 47, 6193–6205. [Google Scholar] [CrossRef]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavský, J.; Tsvetkova, I.V.; Ninova, M.S.; Kussovski, V.K. Antibacterial and Antifungal Activities of Selected Microalgae and Cyanobacteria. Int. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- Costa, B.; Mota, R.; Parreira, P.; Tamagnini, P.; Martins, M.C.L.; Costa, F. Broad-Spectrum Anti-Adhesive Coating Based on an Extracellular Polymer from a Marine Cyanobacterium. Mar. Drugs 2019, 17, 243. [Google Scholar] [CrossRef]
- Vega, J.; Bonomi-Barufi, J.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Cyanobacteria and Red Macroalgae as Potential Sources of Antioxidants and UV Radiation-Absorbing Compounds for Cosmeceutical Applications. Mar. Drugs 2020, 18, 659. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.C.; Huang, Y.W.; Wu, C.C.; Wang, J.J.; Chen, Y.T.; Singhania, R.R.; Chen, C.W.; Dong, C.D.; Hsieh, S.L. Anti-Obesity Effect of Nostoc commune Ethanol Extract In Vitro and In Vivo. Nutrients 2022, 14, 968. [Google Scholar] [CrossRef]
- UNICEF; WHO. The World Bank Levels and Trends in Child Malnutrition, Joint Child Malnutrition Estimates, Key Findings of the 2017 Edition; UNICEF: New York, NY, USA; WHO: Geneva, Switzerland; The World Bank: Washington, DC, USA, 2017; pp. 1–16. [Google Scholar]
- Palma dos Reis, C.R.; Serrano, F.; Fonseca, M.J.; Martins, A.T.; Campos, A.; McMahon, G.M.; Barros, H. The Fetal Origins of Disease: A Prospective Cohort Study on the Association of Preeclampsia and Childhood Obesity. J. Dev. Orig. Health Dis. 2022, 13, 68–74. [Google Scholar] [CrossRef]
- Conde, T.A.; Neves, B.F.; Couto, D.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, P.; Domingues, M.R. Microalgae as Sustainable Bio-Factories of Healthy Lipids: Evaluating Fatty Acid Content and Antioxidant Activity. Mar. Drugs 2021, 19, 357. [Google Scholar] [CrossRef]
- Khavari, F.; Saidijam, M.; Taheri, M.; Nouri, F. Microalgae: Therapeutic Potentials and Applications. Mol. Biol. Rep. 2021, 48, 4757–4765. [Google Scholar] [CrossRef]
- Tsvetanova, F.; Yankov, D. Bioactive Compounds from Red Microalgae with Therapeutic and Nutritional Value. Microorganisms 2022, 10, 2290. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Sangtani, R.; Parmar, H.S.; Bala, K. Algal Metabolites: Paving the Way towards New Generation Antidiabetic Therapeutics. Algal Res. 2023, 69, 102904. [Google Scholar] [CrossRef]
- Hentschke, G.S.; Hentschke, V.S.; Miguel, F.M. Cyanobacteria for Cardiomyocyte Protection against Miocardial Ischemia Injury: A Systematic Review of Animal and in Vitro Studies. Sci. Med. 2024, 34, e44970. [Google Scholar] [CrossRef]
- Amaro, H.M.; Catarina Guedes, A.; Preto, M.A.C.; Sousa-Pinto, I.; Xavier Malcata, F. Gloeothece sp. as a Nutraceutical Source-an Improved Method of Extraction of Carotenoids and Fatty Acids. Mar. Drugs 2018, 16, 327. [Google Scholar] [CrossRef]
- Amaro, H.M.; Barros, R.; Tavares, T.; Almeida, R.; Pinto, I.S.; Malcata, F.X.; Guedes, A.C. Gloeothece sp.—Exploiting a New Source of Antioxidant, Anti-Inflammatory, and Antitumor Agents. Mar. Drugs 2021, 19, 623. [Google Scholar] [CrossRef]
- Bruno, L.; Di Pippo, F.; Antonaroli, S.; Gismondi, A.; Valentini, C.; Albertano, P. Characterization of Biofilm-Forming Cyanobacteria for Biomass and Lipid Production. J. Appl. Microbiol. 2012, 113, 1052–1064. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.C.; Amaro, H.M.; Barbosa, C.R.; Pereira, R.D.; Malcata, F.X. Fatty Acid Composition of Several Wild Microalgae and Cyanobacteria, with a Focus on Eicosapentaenoic, Docosahexaenoic and α-Linolenic Acids for Eventual Dietary Uses. Food Res. Int. 2011, 44, 2721–2729. [Google Scholar] [CrossRef]
- Rey, F.; Melo, T.; Lopes, D.; Couto, D.; Marques, F.; Domingues, M.R. Applications of Lipidomics in Marine Organisms: Progress, Challenges and Future Perspectives. Mol. Omics 2022, 18, 357–386. [Google Scholar] [CrossRef]
- Couto, D.; Conde, T.A.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, R.; Domingues, P. The Chemodiversity of Polar Lipidomes of Microalgae from Different Taxa. Algal Res. 2023, 70, 103006. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, J.; Hong, J.; Yoo, J.S.; Kim, M.S. Identification of Acylated Glycoglycerolipids from a Cyanobacterium. Lipids 1999, 34, 847–853. [Google Scholar] [CrossRef]
- Plohnke, N.; Seidel, T.; Kahmann, U.; Rögner, M.; Schneider, D.; Rexroth, S. The Proteome and Lipidome of Synechocystis sp. PCC 6803 Cells Grown under Light-Activated Heterotrophic Conditions. Mol. Cell. Proteom. 2015, 14, 572–584. [Google Scholar] [CrossRef]
- Couto, D.; Melo, T.; Conde, T.A.; Moreira, A.S.P.; Ferreira, P.; Costa, M.; Silva, J.; Domingues, R.; Domingues, P. Food Grade Extraction of Chlorella vulgaris Polar Lipids: A Comparative Lipidomic Study. Food Chem. 2022, 375, 131685. [Google Scholar] [CrossRef] [PubMed]
- Montero, O.; Porta, J.M.; Porta, J.; Martínez, G.; Lubián, L.M. Characterization of Two Synechococcus sp. PCC7002-Related Cyanobacterial Strains in Relation to 16S RDNA, CrtR Gene, Lipids and Pigments. Phycol. Res. 2011, 59, 147–155. [Google Scholar] [CrossRef]
- Colla, L.M.; Bertolin, T.E.; Costa, J.A.V. Fatty Acids Profile of Spirulina platensis Grown under Different Temperatures and Nitrogen Concentrations. Z. Für Nat. C 2004, 59, 55–59. [Google Scholar] [CrossRef]
- Couto, D.; Melo, T.; Conde, T.A.; Costa, M.; Silva, J.; Domingues, M.R.M.; Domingues, P. Chemoplasticity of the Polar Lipid Profile of the Microalgae Chlorella vulgaris Grown under Heterotrophic and Autotrophic Conditions. Algal Res. 2021, 53, 102128. [Google Scholar] [CrossRef]
- Sengupta, A.; Pakrasi, H.B.; Wangikar, P.P. Recent Advances in Synthetic Biology of Cyanobacteria. Appl. Microbiol. Biotechnol. 2018, 102, 5457–5471. [Google Scholar] [CrossRef] [PubMed]
- Freitas, S.; Silva, N.G.; Sousa, M.L.; Ribeiro, T.; Rosa, F.; Leão, P.N.; Vasconcelos, V.; Reis, M.A.; Urbatzka, R. Chlorophyll Derivatives from Marine Cyanobacteria with Lipid-Reducing Activities. Mar Drugs 2019, 17, 229. [Google Scholar] [CrossRef] [PubMed]
- Ramos, V.; Morais, J.; Castelo-branco, R.; Pinheiro, Â.; Martins, J.; Regueiras, A.; Pereira, A.L.; Lopes, V.R.; Frazão, B.; Gomes, D.; et al. Cyanobacterial Diversity Held in Microbial Biological Resource Centers as a Biotechnological Asset: The Case Study of the Newly Established LEGE Culture Collection. J. Appl. Phycol. 2018, 30, 1437–1451. [Google Scholar] [CrossRef]
- Mai, T.; Johansen, J.R.; Pietrasiak, N.; Bohunická, M.; Martin, M.P. Revision of the Synechococcales (Cyanobacteria) through Recognition of Four Families Including Oculatellaceae Fam. nov. and Trichocoleaceae Fam. nov. and Six New Genera Containing 14 Species. Phytotaxa 2018, 365, 1–59. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-Algae as a Source of Protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Pade, N.; Hagemann, M. Salt Acclimation of Cyanobacteria and Their Application in Biotechnology. Life 2015, 5, 25–49. [Google Scholar] [CrossRef]
- Yang, W.; Wang, F.; Liu, L.N.; Sui, N. Responses of Membranes and the Photosynthetic Apparatus to Salt Stress in Cyanobacteria. Front. Plant Sci. 2020, 11, 713. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, S.; Devendran, S.; Tsai, P.C.; Jayaraman, H.; Alagarsamy, V.; Pugazhendhi, A.; Ponnusamy, V.K. Metabolomics Integrated with Transcriptomics and Proteomics: Evaluation of Systems Reaction to Nitrogen Deficiency Stress in Microalgae. Process Biochem. 2020, 91, 1–14. [Google Scholar] [CrossRef]
- Carneiro, M.; Maia, I.B.; Cunha, P.; Guerra, I.; Magina, T.; Santos, T.; Schulze, P.S.C.; Pereira, H.; Malcata, F.X.; Navalho, J.; et al. Effects of LED Lighting on Nannochloropsis oceanica Grown in Outdoor Raceway Ponds. Algal Res. 2022, 64, 102685. [Google Scholar] [CrossRef]
- Cruz, J.D.; Delattre, C.; Felpeto, A.B.; Pereira, H.; Pierre, G.; Morais, J.; Petit, E.; Silva, J.; Azevedo, J.; Elboutachfaiti, R.; et al. Bioprospecting for Industrially Relevant Exopolysaccharide-Producing Cyanobacteria under Portuguese Simulated Climate. Sci. Rep. 2023, 13, 13561. [Google Scholar] [CrossRef]
- Aouir, A.; Amiali, M.; Bitam, A.; Benchabane, A.; Raghavan, V.G. Comparison of the Biochemical Composition of Different Arthrospira platensis Strains from Algeria, Chad and the USA. J. Food Meas. Charact. 2017, 11, 913–923. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.Y.; Raman, A.A.A.; Ibrahim, S. Microalgae Lipid and Biomass for Biofuel Production: A Comprehensive Review on Lipid Enhancement Strategies and Their Effects on Fatty Acid Composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232. [Google Scholar] [CrossRef]
- Banskota, A.H.; Sperker, S.; Stefanova, R.; McGinn, P.J.; O’Leary, S.J.B. Antioxidant Properties and Lipid Composition of Selected Microalgae. J. Appl. Phycol. 2019, 31, 309–318. [Google Scholar] [CrossRef]
- Zimba, P.V.; Shalygin, S.; Huang, I.S.; Momčilović, M.; Abdulla, H. A New Boring Toxin Producer—Perforafilum tunnelli gen. & ap. nov. (Oscillatoriales, Cyanobacteria) Isolated from Laguna Madre, Texas, USA. Phycologia 2021, 60, 10–24. [Google Scholar] [CrossRef]
- Gugger, M.; Lyra, C.; Suominen, I.; Tsitko, I.; Humbert, J.F.; Salkinoja-Salonen, M.S.; Sivonen, K. Cellular Fatty Acids as Chemotaxonomic Markers of the Genera Anabaena, Aphanizomenon, Microcystis, Nostoc and Planktothrix (Cyanobacteria). Int. J. Syst. Evol. Microbiol. 2002, 52, 1007–1015. [Google Scholar] [CrossRef]
- Steinhoff, F.S.; Karlberg, M.; Graeve, M.; Wulff, A. Cyanobacteria in Scandinavian Coastal Waters—A Potential Source for Biofuels and Fatty Acids? Algal Res. 2014, 5, 42–51. [Google Scholar] [CrossRef]
- Jensen, N.; Jensen, H.E.; Aalbaek, B.; Blirup-Plum, S.A.; Soto, S.M.; Cepas, V.; López, Y.; Gabasa, Y.; Gutiérrez-del-Río, I.; Villar, C.J.; et al. Synthesis of the Cyanobacterial Halometabolite Chlorosphaerolactylate B and Demonstration of Its Antimicrobial Effect in Vitro and in Vivo. Front. Microbiol. 2022, 13, 950855. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.; Leikoski, N.; Wahlsten, M.; Azevedo, J.; Antunes, J.; Jokela, J.; Sivonen, K.; Vasconcelos, V.; Fewer, D.P.; Leão, P.N. Sphaerocyclamide, a Prenylated Cyanobactin from the Cyanobacterium Sphaerospermopsis sp. LEGE 00249. Sci. Rep. 2018, 8, 14537. [Google Scholar] [CrossRef]
- Abt, K.; Castelo-Branco, R.; Leão, P.N. Biosynthesis of Chlorinated Lactylates in Sphaerospermopsis sp. LEGE 00249. J. Nat. Prod. 2021, 84, 278–286. [Google Scholar] [CrossRef]
- Gutiérrez-Del-Río, I.; de Fraissinette, N.B.; Castelo-Branco, R.; Oliveira, F.; Morais, J.; Redondo-Blanco, S.; Villar, C.J.; JoséIglesias, M.; Soengas, R.; Cepas, V.; et al. Chlorosphaerolactylates A−D: Natural Lactylates of Chlorinated Fatty Acids Isolated from the Cyanobacterium Sphaerospermopsis Sp. LEGE 00249. J. Nat. Prod. 2020, 83, 1885–1890. [Google Scholar] [CrossRef]
- Moretto, J.A.; de Souza, A.O.; Berneira, L.M.; Brigagão, L.G.G.; de Pereira, C.M.P.; Converti, A.; Pinto, E. Microwave-Assisted Extraction of Fatty Acids from Cultured and Commercial Phytoplankton Species. Appl. Sci. 2022, 12, 2407. [Google Scholar] [CrossRef]
- Zapomělová, E.; Skácelová, O.; Pumann, P.; Kopp, R.; Janeček, E. Biogeographically Interesting Planktonic Nostocales (Cyanobacteria) in the Czech Republic and Their Polyphasic Evaluation Resulting in Taxonomic Revisions of Anabaena bergii Ostenfeld 1908 (Chrysosporum gen. nov.) and A. tenericaulis Nygaard 1949 (Dolichospermum tenericaule comb. nova). Hydrobiologia 2012, 698, 353–365. [Google Scholar] [CrossRef]
- Iliev, I.; Petkov, G.; Lukavsky, J.; Furnadzhieva, S.; Andreeva, R. Do cyanobacterial lipids contain fatty acids longer than 18 carbon atoms? Z. Fur Naturforschung Sect. C-A J. Biosci. 2014, 66, 267–276. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Shkrob, I.; Go, J.V. Dicarboxylic and Fatty Acid Compositions of Cyanobacteria of the Genus Aphanizomenon. Biochemistry 2001, 66, 92–97. [Google Scholar]
- Greque De Morais, E.; Nunes, I.L.; Druzian, J.I.; Greque De Morais, M.; Centeno Da Rosa, A.P.; Vieira Costa, J.A. Increase in Biomass Productivity and Protein Content of Spirulina sp. LEB 18 (Arthrospira) Cultivated with Crude Glycerol. Biomass Convers. Biorefinery 2020, 12, 597–605. [Google Scholar] [CrossRef]
- Tudor, C.; Gherasim, E.C.; Dulf, F.V.; Pintea, A. In Vitro Bioaccessibility of Macular Xanthophylls from Commercial Microalgal Powders of Arthrospira platensis and Chlorella pyrenoidosa. Food Sci. Nutr. 2021, 9, 1896–1906. [Google Scholar] [CrossRef]
- Boutarfa, S.; Senoussi, M.M.; González-Silvera, D.; López-Jiménez, J.Á.; Aboal, M. Fatty Acids Profile of Mastigocladus laminosus Cohn Ex Kichner Isolated from Algerian Hot Springs as a Biofuel Feedstock. Biocatal. Agric. Biotechnol. 2022, 42, 102373. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish Consumption, Fish Oil, Omega-3 Fatty Acids, and Cardiovascular Disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Weylandt, K.H.; Serini, S.; Chen, Y.Q.; Su, H.M.; Lim, K.; Cittadini, A.; Calviello, G. Omega-3 Polyunsaturated Fatty Acids: The Way Forward in Times of Mixed Evidence. Biomed Res. Int. 2015, 2015, 143109. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory from the American Heart Association. Circulation 2019, 140, E673–E691. [Google Scholar] [CrossRef]
- Wu, J.H.Y.; Marklund, M.; Imamura, F.; Tintle, N.; Ardisson Korat, A.V.; de Goede, J.; Zhou, X.; Yang, W.S.; de Oliveira Otto, M.C.; Kröger, J.; et al. Omega-6 Fatty Acid Biomarkers and Incident Type 2 Diabetes: Pooled Analysis of Individual-Level Data for 39 740 Adults from 20 Prospective Cohort Studies. Lancet Diabetes Endocrinol. 2017, 5, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Marklund, M.; Wu, J.H.Y.; Imamura, F.; Del Gobbo, L.C.; Fretts, A.; De Goede, J.; Shi, P.; Tintle, N.; Wennberg, M.; Aslibekyan, S.; et al. Biomarkers of Dietary Omega-6 Fatty Acids and Incident Cardiovascular Disease and Mortality: An Individual-Level Pooled Analysis of 30 Cohort Studies. Circulation 2019, 139, 2422–2436. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V.; Bogdanović, T.; Poljak, V.; Petričević, S. Changes in Fatty Acid Composition, Atherogenic and Thrombogenic Health Lipid Indices and Lipid Stability of Bogue (Boops boops Linnaeus, 1758) during Storage on Ice: Effect of Fish Farming Activities. J. Food Compos. Anal. 2015, 40, 120–125. [Google Scholar] [CrossRef]
- Matos, Â.P.; Feller, R.; Moecke, E.H.S.; de Oliveira, J.V.; Junior, A.F.; Derner, R.B.; Sant’Anna, E.S. Chemical Characterization of Six Microalgae with Potential Utility for Food Application. J. Am. Oil Chem. Soc. 2016, 93, 963–972. [Google Scholar] [CrossRef]
- Marques, B.; Lillebø, A.I.; Domingues, M.D.R.M.; Saraiva, J.A.; Calado, R. Effect of High-Pressure Processing (HPP) on the Fatty Acid Profile of Different Sized Ragworms (Hediste diversicolor) Cultured in an Integrated Multi-Trophic Aquaculture (IMTA) System. Molecules 2019, 24, 4503. [Google Scholar] [CrossRef]
- Lopes, D.; Melo, T.; Rey, F.; Meneses, J.; Monteiro, F.L.; Helguero, L.A.; Abreu, M.H.; Lillebø, A.I.; Calado, R.; Domingues, M.R. Valuing Bioactive Lipids from Green, Red and Brown Macroalgae from Aquaculture, to Foster Functionality and Biotechnological Applications. Molecules 2020, 25, 3883. [Google Scholar] [CrossRef]
- Rueda, F.M.; Hernández, M.D.; Egea, M.A.; Aguado, F.; García, B.; Martínez, F.J. Differences in Tissue Fatty Acid Composition between Reared and Wild Sharpsnout Sea Bream, Diplodus puntazzo (Cetti, 1777). Br. J. Nutr. 2001, 86, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Valfré, F.; Caprino, F.; Turchini, G.M. The health benefit of seafood. Vet. Res. Commun. 2003, 27 (Suppl. S1), 507–512. [Google Scholar] [CrossRef]
- Conde, T.A.; Couto, D.; Melo, T.; Costa, M.; Silva, J.; Domingues, M.R.; Domingues, P. Polar Lipidomic Profile Shows Chlorococcum amblystomatis as a Promising Source of Value-Added Lipids. Sci. Rep. 2021, 11, 4355. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Thelen, J.J.; Fedosejevs, E.; Harwood, J.L. The Lipid Biochemistry of Eukaryotic Algae. Prog. Lipid Res. 2019, 74, 31–68. [Google Scholar] [CrossRef]
- Okazaki, Y.; Otsuki, H.; Narisawa, T.; Kobayashi, M.; Sawai, S.; Kamide, Y.; Kusano, M.; Aoki, T.; Hirai, M.Y.; Saito, K. A New Class of Plant Lipid Is Essential for Protection against Phosphorus Depletion. Nat. Commun. 2013, 4, 1510. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Nishizawa, T.; Takano, K.; Ohnishi, M.; Mimura, T.; Saito, K. Induced Accumulation of Glucuronosyldiacylglycerol in Tomato and Soybean under Phosphorus Deprivation. Physiol. Plant. 2015, 155, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Reshef, V.; Mizrachi, E.; Maretzki, T.; Silberstein, C.; Loya, S.; Hizi, A.; Carmeli, S. New Acylated Sulfoglycolipids and Digalactolipids and Related Known Glycolipids from Cyanobacteria with a Potential to Inhibit the Reverse Transcriptase of HIV-1. J. Nat. Prod. 1997, 60, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Rossi, C.; Marcolongo, G.; Di Lena, A.; Venzo, A.; Berrie, C.P.; Corda, D. Selective in Vivo Anti-Inflammatory Action of the Galactolipid Monogalactosyldiacylglycerol. Eur. J. Pharmacol. 2005, 524, 159–168. [Google Scholar] [CrossRef]
- Ma, A.C.; Chen, Z.; Wang, T.; Song, N.; Yan, Q.; Fang, Y.C.; Guan, H.S.; Liu, H.B. Isolation of the Molecular Species of Monogalactosyldiacylglycerols from Brown Edible Seaweed Sargassum horneri and Their Inhibitory Effects on Triglyceride Accumulation in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2014, 62, 11157–11162. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.; Craigie, J.S.; Hafting, J.T. Lipids Isolated from the Cultivated Red Alga Chondrus crispus Inhibit Nitric Oxide Production. J. Appl. Phycol. 2014, 26, 1565–1571. [Google Scholar] [CrossRef]
- Falcón, L.I.; Magallón, S.; Castillo, A. Dating the Cyanobacterial Ancestor of the Chloroplast. ISME J. 2010, 4, 777–783. [Google Scholar] [CrossRef]
- Sato, N. Are Cyanobacteria an Ancestor of Chloroplasts or Just One of the Gene Donors for Plants and Algae? Genes 2021, 12, 823. [Google Scholar] [CrossRef]
- De Luca, M.; Pappalardo, I.; Limongi, A.R.; Viviano, E.; Radice, R.P.; Todisco, S.; Martelli, G.; Infantino, V.; Vassallo, A. Lipids from Microalgae for Cosmetic Applications. Cosmetics 2021, 8, 52. [Google Scholar] [CrossRef]
- Chai, C.; Park, J. Food Liposomes: Structures, Components, Preparations, and Applications. Food Chem. 2024, 432, 137228. [Google Scholar] [CrossRef]
- Lauritano, C.; Helland, K.; Riccio, G.; Andersen, J.H.; Ianora, A.; Hansen, E.H. Lysophosphatidylcholines and Chlorophyll-Derived Molecules from the Diatom Cylindrotheca closterium with Anti-Inflammatory Activity. Mar. Drugs 2020, 18, 166. [Google Scholar] [CrossRef] [PubMed]
- Breuer, G.; Lamers, P.P.; Martens, D.E.; Draaisma, R.B.; Wijffels, R.H. The Impact of Nitrogen Starvation on the Dynamics of Triacylglycerol Accumulation in Nine Microalgae Strains. Bioresour. Technol. 2012, 124, 217–226. [Google Scholar] [CrossRef]
- Klok, A.J.; Lamers, P.P.; Martens, D.E.; Draaisma, R.B.; Wijffels, R.H. Edible Oils from Microalgae: Insights in TAG Accumulation. Trends Biotechnol. 2014, 32, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Extons, J.H. Signaling through Phosphatidylcholine Breakdown. J. Biol. Chem. 1990, 265, 1–4. [Google Scholar] [CrossRef]
- Plouguerné, E.; da Gama, B.A.P.; Pereira, R.C.; Barreto-Bergter, E. Glycolipids from Seaweeds and Their Potential Biotechnological Applications. Front. Cell. Infect. Microbiol. 2014, 4, 174. [Google Scholar] [CrossRef] [PubMed]
- Morone, J.; Lopes, G.; Preto, M.; Vasconcelos, V.; Martins, R. Exploitation of Filamentous and Picoplanktonic Cyanobacteria for Cosmetic Applications: Potential to Improve Skin Structure and Preserve Dermal Matrix Components. Mar Drugs 2020, 18, 486. [Google Scholar] [CrossRef]
- Rodrigues, L.; Morone, J.; Hentschke, G.S.; Vasconcelos, V.; Lopes, G. Anti-Inflammatory Activity of Cyanobacteria Pigment Extracts: Physiological Free Radical Scavenging and Modulation of INOS and LOX Activity. Mar Drugs 2024, 22, 131. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Recent Developments in the Application of Seaweeds or Seaweed Extracts as a Means for Enhancing the Safety and Quality Attributes of Foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Silva, R.; Gonçalves, T.; Morone, J.; Moreira, G.A.; Morais, J.; Hentschke, G.S.; Álvarez-Gutiérrez, P.E.; Batista-García, R.A.; Vasconcelos, V.; Lopes, G. Pigments Profile and Antioxidant Potential of Extremophile Cyanobacteria Isolated from the Mexican Volcanic Lake Chichonal. Algal Res. 2024, 81, 103578. [Google Scholar] [CrossRef]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Zito, M.C.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef]
- Pan-utai, W.; Iamtham, S. Enhanced Microencapsulation of C-Phycocyanin from Arthrospira by Freeze-Drying with Different Wall Materials. Food Technol. Biotechnol. 2020, 58, 423–432. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, F.; De Troch, M.; Malanga, G.; Hernando, M. Differential Sensitivity of Fatty Acids and Lipid Damage in Microcystis aeruginosa (Cyanobacteria) Exposed to Increased Temperature. Comp. Biochem. Physiol. Part-C Toxicol. Pharmacol. 2020, 235, 108773. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Maddison, L.A.; Chen, W. Zebrafish as a Model for Obesity and Diabetes. Front. Cell Dev. Biol. 2018, 6, 379709. [Google Scholar] [CrossRef] [PubMed]
- Luz, R.; Gonçalves, V.; Vasconcelos, V.; Urbatzka, R. Metabolite Profiling and Bioactivity Assessment of Cyanobacteria from the Azores Reveals Unique Producers of Cytotoxic and Lipid Reducing Compounds. Algal Res. 2024, 83, 103703. [Google Scholar] [CrossRef]
- Costa, M.; Rosa, F.; Ribeiro, T.; Hernandez-Bautista, R.; Bonaldo, M.; Silva, N.G.; Eiríksson, F.; Thorsteinsdóttir, M.; Ussar, S.; Urbatzka, R. Identification of Cyanobacterial Strains with Potential for the Treatment of Obesity-Related Co-Morbidities by Bioactivity, Toxicity Evaluation and Metabolite Profiling. Mar Drugs 2019, 17, 280. [Google Scholar] [CrossRef]
- Yun, J.W. Possible Anti-Obesity Therapeutics from Nature—A Review. Phytochemistry 2010, 71, 1625–1641. [Google Scholar] [CrossRef]
- Castro, M.; Preto, M.; Vasconcelos, V.; Urbatzka, R. Obesity: The Metabolic Disease, Advances on Drug Discovery and Natural Product Research. Curr. Top. Med. Chem. 2016, 16, 2577–2604. [Google Scholar] [CrossRef]
- Yousefi, R.; Mottaghi, A.; Saidpour, A. Spirulina platensis Effectively Ameliorates Anthropometric Measurements and Obesity-Related Metabolic Disorders in Obese or Overweight Healthy Individuals: A Randomized Controlled Trial. Complement. Ther. Med. 2018, 40, 106–112. [Google Scholar] [CrossRef]
- Miczke, A.; Szulińska, M.; Hansdorfer-Korzon, R.; Kręgielska-Narożna, M.; Suliburska, J.; Walkowiak, J.; Bogdański, P.; Bogdanski, P. Effects of Spirulina consumption on body weight, blood pressure, and endothelial function in overweight hypertensive Caucasians: A double-blind, placebo-controlled, randomized trial. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 150–156. [Google Scholar]
- Dinicolantonio, J.J.; Bhat, A.G.; Okeefe, J. Effects of Spirulina on Weight Loss and Blood Lipids: A Review. Open Heart 2020, 7, e001003. [Google Scholar] [CrossRef]
- Moradi, S.; Ziaei, R.; Foshati, S.; Mohammadi, H.; Nachvak, S.M.; Rouhani, M.H. Effects of Spirulina Supplementation on Obesity: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Complement. Ther. Med. 2019, 47, 102211. [Google Scholar] [CrossRef]
- Jump, D.B. The Biochemistry of N-3 Polyunsaturated Fatty Acids. J. Biol. Chem. 2002, 277, 8755–8758. [Google Scholar] [CrossRef]
- Murakami, H.; Nobusawa, T.; Hori, K.; Shimojima, M.; Ohta, H. Betaine Lipid Is Crucial for Adapting to Low Temperature and Phosphate Deficiency in Nannochloropsis. Plant Physiol. 2018, 177, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gu, Y.; Pang, Q.; Yu, H.; Zhang, J. Dietary Betaine Regulates the Synthesis of Fatty Acids through MTOR Signaling in the Muscle of Zebrafish. J. Funct. Foods 2021, 85, 104610. [Google Scholar] [CrossRef]
- Silva, N.G.; Preto, M.; Vasconcelos, V.; Urbatzka, R. Reduction of Neutral Lipid Reservoirs, Bioconversion and Untargeted Metabolomics Reveal Distinct Roles for Vitamin K Isoforms on Lipid Metabolism. Food Funct. 2024, 15, 2170–2180. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Su, C.W.; Li, X.; Liu, Y.; Pan, Q.; Cao, T.; Kang, J.X. Lipid Extract From a Vegetable (Sonchus oleraceus) Attenuates Adipogenesis and High Fat Diet-Induced Obesity Associated With AMPK Activation. Front. Nutr. 2021, 8, 624283. [Google Scholar] [CrossRef]
- Milard, M.; Laugerette, F.; Durand, A.; Buisson, C.; Meugnier, E.; Loizon, E.; Louche-Pelissier, C.; Sauvinet, V.; Garnier, L.; Viel, S.; et al. Milk Polar Lipids in a High-Fat Diet Can Prevent Body Weight Gain: Modulated Abundance of Gut Bacteria in Relation with Fecal Loss of Specific Fatty Acids. Mol. Nutr. Food Res. 2019, 63, e1801078. [Google Scholar] [CrossRef] [PubMed]
- Kotai, J. Instructions for Preparation of Modified Nutrient Solution Z8 for Algae. Nor. Inst. Water Res. Oslo 1972, 11, 5. [Google Scholar]
- Urbatzka, R.; Freitas, S.; Palmeira, A.; Almeida, T.; Moreira, J.; Azevedo, C.; Afonso, C.; Correia-da-Silva, M.; Sousa, E.; Pinto, M.; et al. Lipid Reducing Activity and Toxicity Profiles of a Library of Polyphenol Derivatives. Eur. J. Med. Chem. 2018, 151, 272–284. [Google Scholar] [CrossRef]
- Staub, V.R. Ernährungsphysiologisch-Autökologische Untersuchungen an Der Planktischen Blaualge Oscillatoria Rubescens DC. Schweiz. Z. Für Hydrologie 1961, 23, 82–198. [Google Scholar]
- Toerien, D.F.; Huang, C.H. Algal growth prediction using growth kinetic constants. Water Res. 1973, 7, 1673–1681. [Google Scholar] [CrossRef]
- Neilan, B.A.; Jacobs, D.; Del Dot, T.; Blackall, L.L.; Hawkins, P.R.; Cox, P.T.; Goodman, A.E. RRNA Sequences and Evolutionary Relationships among Toxic and Nontoxic Cyanobacteria of the Genus Microcystis. Int. J. Syst. Bacteriol. 1997, 47, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Taton, A.; Grubisic, S.; Brambilla, E.; De Wit, R.; Wilmotte, A. Cyanobacterial Diversity in Natural and Artificial Microbial Mats of Lake Fryxell (McMurdo Dry Valleys, Antarctica): A Morphological and Molecular Approach. Appl. Environ. Microbiol. 2003, 69, 5157–5169. [Google Scholar] [CrossRef]
- Firth, H.V.; Richards, S.M.; Bevan, A.P.; Clayton, S.; Corpas, M.; Rajan, D.; Van Vooren, S.; Moreau, Y.; Pettett, R.M.; Carter, N.P. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am. J. Hum. Genet. 2009, 84, 524–533. [Google Scholar] [CrossRef]
- Mihali, T.K.; Kellmann, R.; Muenchhoff, J.; Barrow, K.D.; Neilan, B.A. Characterization of the Gene Cluster Responsible for Cylindrospermopsin Biosynthesis. Appl. Environ. Microbiol. 2008, 74, 716–722. [Google Scholar] [CrossRef]
- Rantala-Ylinen, A.; Känä, S.; Wang, H.; Rouhiainen, L.; Wahlsten, M.; Rizzi, E.; Berg, K.; Gugger, M.; Sivonen, K. Anatoxin-a Synthetase Gene Cluster of the Cyanobacterium Anabaena sp. Strain 37 and Molecular Methods to Detect Potential Producers. Appl. Environ. Microbiol. 2011, 77, 7271–7278. [Google Scholar] [CrossRef]
- Lopes, V.R.; Ramos, V.; Martins, A.; Sousa, M.; Welker, M.; Antunes, A.; Vasconcelos, V.M. Phylogenetic, Chemical and Morphological Diversity of Cyanobacteria from Portuguese Temperate Estuaries. Mar. Environ. Res. 2012, 73, 7–16. [Google Scholar] [CrossRef]
- Jungblut, A.D.; Neilan, B.A. Molecular Identification and Evolution of the Cyclic Peptide Hepatotoxins, Microcystin and Nodularin, Synthetase Genes in Three Orders of Cyanobacteria. Arch. Microbiol. 2006, 185, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Benredjem, L.; Morais, J.; Hentschke, G.S.; Vasconcelos, V. First Polyphasic Study of Cheffia Reservoir (Algeria) Cyanobacteria Isolates Reveals Toxic Picocyanobacteria Genotype. Microorganisms 2023, 11, 2664. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the Sensitivity of Progressive Multiple Sequence Alignment through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Felsenstein, J. Evolutionary Trees from DNA Sequences: A Maximum Likelihood Approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Rodriguez, F.; Oliver, J.L.; Marin, A. The General Stochastic Model of Nucleotide Substitution The G4H Model. J. Theor. Biol. 1990, 142, 485–501. [Google Scholar] [CrossRef]
- Sugiura, N. Further Analysis of the Data by Anaike’ S Information Criterion and the Finite Corrections. Commun. Stat. Theory Methods 1978, 7, 13–26. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (ITOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Inkscape Project. Inkscape 2017 (Version 0.92). Available online: https://inkscape.org (accessed on 2 June 2025).
- Komárek, J.; Kaštovský, J.; Mareš, J.; Johansen, J.R. Taxonomic Classification of Cyanoprokaryotes (Cyanobacterial Genera) 2014, Using a Polyphasic Approach. Preslia 2014, 86, 295–335. [Google Scholar]
- Yarza, P.; Yilmaz, P.; Pruesse, E.; Glöckner, F.O.; Ludwig, W.; Schleifer, K.H.; Whitman, W.B.; Euzéby, J.; Amann, R.; Rosselló-Móra, R. Uniting the Classification of Cultured and Uncultured Bacteria and Archaea Using 16S RRNA Gene Sequences. Nat. Rev. Microbiol. 2014, 12, 635–645. [Google Scholar] [CrossRef]
- Hackmann, T.J. Setting New Boundaries of 16S RRNA Gene Identity for Prokaryotic Taxonomy. Int. J. Syst. Evol. Microbiol. 2025, 75, 006747. [Google Scholar] [CrossRef]
- The Joint FAO/WHO Expert Meetings on Nutrition (JEMNU): Nitrogen to Protein Conversion Factors for Soy-Based and Milk-Based Ingredients Used in Infant Formula and Follow-Up Formula. Report of the Meeting of the Expert Panel, Geneva, Switzerland, 16–17 July 2019. Geneva: World Health Organization and Food and Agriculture Organization of the United Nations; 2020. Licence: CC BY-NC-SA 3.0 IGO. Available online: https://www.who.int/publications/i/item/9789240000216 (accessed on 2 June 2025).
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet Food with Nutritional Health Benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Review Article—Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Artia, M. MS-DIAL: Data Independent MS/MS Deconvolution for Comprehensive. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular Framework for Processing, Visualizing, and Analyzing Mass Spectrometry-Based Molecular Profile Data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Automatic Method for Determination of Total Antioxidant Capacity Using 2,2-Diphenyl-1-Picrylhydrazyl Assay. Anal. Chim. Acta. 2006, 558, 310–318. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Barreiros, L.; Maia, M.A.; Reis, S.; Segundo, M.A. Rapid Assessment of Endpoint Antioxidant Capacity of Red Wines through Microchemical Methods Using a Kinetic Matching Approach. Talanta 2012, 97, 473–483. [Google Scholar] [CrossRef]
- Melo, T.; Marques, S.S.; Ferreira, I.; Cruz, M.T.; Domingues, P.; Segundo, M.A.; Domingues, M.R.M. New Insights into the Anti-Inflammatory and Antioxidant Properties of Nitrated Phospholipids. Lipids 2018, 53, 117–131. [Google Scholar] [CrossRef]
- Regueiras, A.; Huguet, Á.; Conde, T.; Couto, D.; Domingues, P.; Domingues, M.R.; Costa, A.M.; da Silva, J.L.; Vasconcelos, V.; Urbatzka, R. Potential Anti-Obesity, Anti-Steatosis, and Anti-Inflammatory Properties of Extracts from the Microalgae Chlorella vulgaris and Chlorococcum amblystomatis under Different Growth Conditions. Mar Drugs 2022, 20, 9. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]


| Relative Abundance (%) ± SD | |||
|---|---|---|---|
| Fatty Acids | Laspinema sp. LEGE 06078 | Rivulariaceae cyanobacterium LEGE 06114 | Sphaerospermopsis sp. LEGE 00249 |
| FA 14:0 | 1.9 ± 0.2 | 2.1 ± 0.4 | 4.2 ± 0.5 |
| FA 15:0 iso | 1.6 ± 0.2 | - | 3.6 ± 0.5 |
| FA 16:0 | 37.7 ± 1.8 | 29.0 ± 2.3 | 25.1 ± 3.0 |
| FA 16:1 n−9 | 2.5 ± 0.2 | 2.6 ± 0.4 | - |
| FA 16:1 n−7 | 5.9 ± 0.2 | 3.4 ± 0. | 10.2 ± 0.8 |
| FA 16:1 n−5 | - | 2.6 ± 0.5 | - |
| FA 16:2 n−4 | - | 5.0 ± 0.9 | - |
| FA 16:3 n−3 | - | 3.4 ± 0.6 | - |
| FA 17:0 | 2.6 ± 0.3 | - | - |
| FA 17:1 n−8 | 2.2 ± 0.2 | - | - |
| FA 18:0 | 5.9 ± 0.7 | 8.5 ± 0.9 | 15.1 ± 1.4 |
| FA 18:1 n−9 | 9.0 ± 0.6 | 10.3 ± 1.3 | 5.9 ± 0.4 |
| FA 18:1 n−7 | 2.6 ± 0.2 | - | - |
| FA 18:1 n−6 | - | 3.3 ± 0.3 | 6.5 ± 0. |
| FA 18:2 n−6 | 5.0 ± 0.4 | 9.5 ± 0.2 | 10.2 ± 1.1 |
| FA 18:3 n−6 | 3.7 ± 0.5 | 12.3 ± 1.2 | - |
| FA 18:3 n−3 | 8.9 ± 0.3 | 3.8 ± 0.5 | 11.0 ± 1.2 |
| FA 18:4 n−3 | 10.4 ± 0.3 | 4.2 ± 0.4 | - |
| FA 24:0 | - | - | 8.3 ± 1.0 |
| ∑ SFA | 49. ± 0.6 | 39.6 ± 1.7 | 56.3 ± 0.6 |
| ∑ MUFAs | 22.2 ± 0.2 | 22.2 ± 0.5 | 22.5 ± 0.4 |
| ∑ PUFAs | 28.1 ± 0.7 | 38.2 ± 1.4 | 21.2 ± 0.3 |
| ∑ n−3 | 19.3 ± 0.6 | 11.4 ± 1.6 | 11.0 ± 1.2 |
| ∑ n−6 | 8.8 ± 0.9 | 26.8 ± 0.6 | 10.2 ± 10.1 |
| n−6/n−3 ratio | 0.4 | 2.4 | 0.9 |
| AI | 0.9 ± 0.03 | 0.6 ± 0.03 | 1.0 ± 0.02 |
| TI | 0.6 ± 0.02 | 0.7 ± 0.09 | 0.9 ± 0.05 |
| (h/H) | 0.6 ± 0.01 | 0.8 ± 0.02 | 0.9 ± 0.01 |
| Number of Lipid Species | Major Species | |||||
|---|---|---|---|---|---|---|
| Lipid Classes | LEGE 06078 | LEGE 06114 | LEGE 00249 | LEGE 06078 | LEGE 06114 | LEGE 00249 |
| Glycolipids | 48 | 57 | 28 | |||
| MGDG | 25 | 25 | 9 | MGDG (34:4) | MGDG (34:3) | MGDG (34:4) |
| DGDG | 12 | 12 | 10 | DGDG (34:4) | DGDG (34:3) | DGDG (34:3) |
| DGGA | - | 9 | - | - | DGGA (35:1) | - |
| SQDG | 11 | 11 | 9 | SQDG (34:1) | SQDG (34:1) | SQDG (34:3) |
| Phospholipids | 35 | 10 | 36 | |||
| PC | 2 | 2 | 16 | PC (36:2) | PC (36:2) | PC (36:2) |
| LPC | 1 | - | 4 | LPC (18:1) | - | LPC (18:1) |
| PG | 18 | 6 | 8 | PG (34:1) | PG (34:1) | PG (34:1) |
| LPG | - | - | 1 | - | - | LPG (16:0) |
| PE | 5 | 2 | 5 | PE (36:2) | PE (30:0) | PE (32:1) |
| LPE | - | - | 1 | - | - | LPE (18:1) |
| PI | 9 | - | 1 | PI (34:1) | - | PI (34:1) |
| Betaine lipids | - | 4 | 37 | |||
| DGTS | - | 4 | 24 | - | DGTS (36:2) | DGTS (34:3) |
| MGTS | - | - | 13 | - | - | MGTS (16:0) |
| Neutral lipids | 42 | 35 | 12 | |||
| TG | 38 | 32 | 12 | TG (50:1) | TG (48:0) | TG (52:2) |
| DG | 4 | 3 | - | DG (34:4) | DG (34:3) | - |
| Total | 125 | 106 | 113 | |||
| Glycolipids | |||
| DGDG (32:0) | DGDG (34:4) | MGDG (34:1) | SQDG (30:0) |
| DGDG (32:1) | DGDG (34:5) | MGDG (34:2) | SQDG (34:1) |
| DGDG (34:1) | MGDG (32:0) | MGDG (34:3) | SQDG (34:3) |
| DGDG (34:2) | MGDG (32:1) | MGDG (34:4) | SQDG (36:0) |
| DGDG (34:3) | MGDG (32:2) | MGDG (36:2) | |
| Phospholipids | |||
| PC (36:2) | PG (34:1) | PG (34:2) | |
| Neutral lipids | |||
| TG (44:0) | TG (45:0) | TG (47:1) | TG (48:2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, F.; Conde, T.; Pinho, M.; Melo, T.; Hentschke, G.S.; Urbatzka, R.; Pereira, H.; Costa, M.; Vasconcelos, V.; Domingues, M.R. Unveiling the Biotechnological Potential of Cyanobacteria from the Portuguese LEGE-CC Collection Through Lipidomics and Antioxidant and Lipid-Lowering Properties. Molecules 2025, 30, 2504. https://doi.org/10.3390/molecules30122504
Oliveira F, Conde T, Pinho M, Melo T, Hentschke GS, Urbatzka R, Pereira H, Costa M, Vasconcelos V, Domingues MR. Unveiling the Biotechnological Potential of Cyanobacteria from the Portuguese LEGE-CC Collection Through Lipidomics and Antioxidant and Lipid-Lowering Properties. Molecules. 2025; 30(12):2504. https://doi.org/10.3390/molecules30122504
Chicago/Turabian StyleOliveira, Flavio, Tiago Conde, Marisa Pinho, Tânia Melo, Guilherme Scotta Hentschke, Ralph Urbatzka, Hugo Pereira, Monya Costa, Vitor Vasconcelos, and Maria Rosário Domingues. 2025. "Unveiling the Biotechnological Potential of Cyanobacteria from the Portuguese LEGE-CC Collection Through Lipidomics and Antioxidant and Lipid-Lowering Properties" Molecules 30, no. 12: 2504. https://doi.org/10.3390/molecules30122504
APA StyleOliveira, F., Conde, T., Pinho, M., Melo, T., Hentschke, G. S., Urbatzka, R., Pereira, H., Costa, M., Vasconcelos, V., & Domingues, M. R. (2025). Unveiling the Biotechnological Potential of Cyanobacteria from the Portuguese LEGE-CC Collection Through Lipidomics and Antioxidant and Lipid-Lowering Properties. Molecules, 30(12), 2504. https://doi.org/10.3390/molecules30122504









