A Metabolomics Exploration of Young Lotus Seeds Using Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging
Abstract
1. Introduction
2. Results
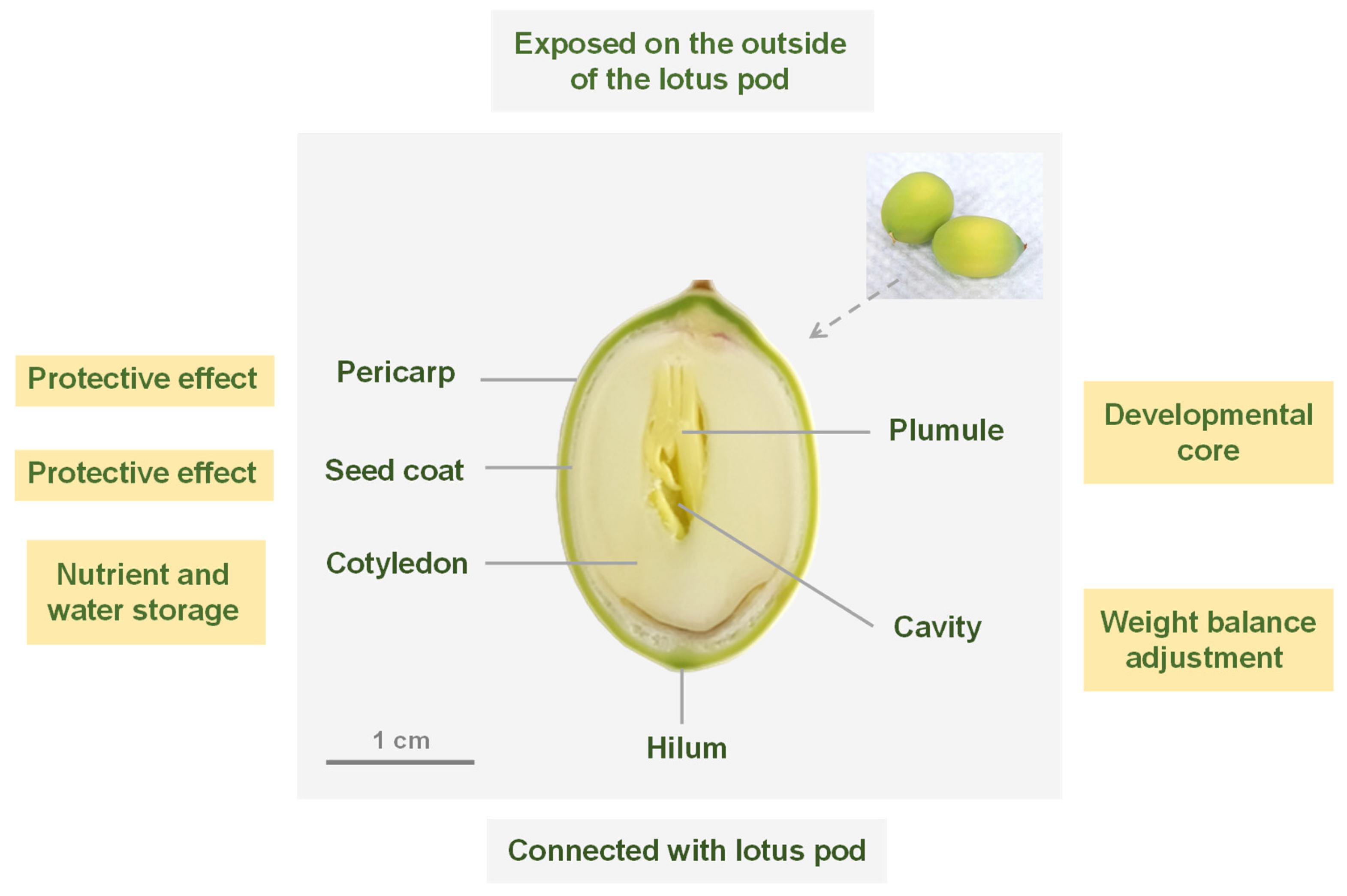
2.1. Structural Organization of Fresh Lotus Seed
2.2. Preparation of Frozen Section of Fresh Lotus Seed
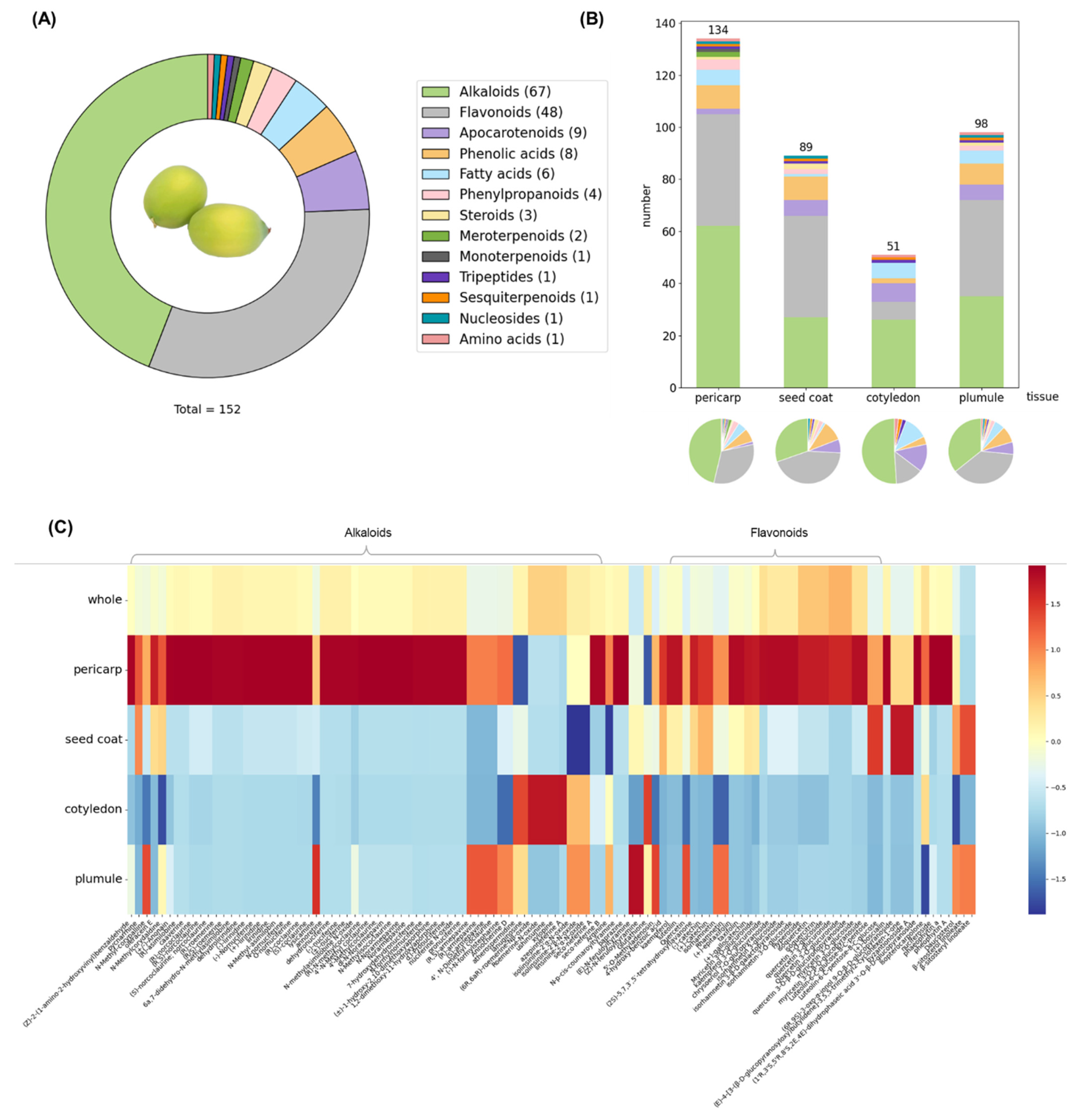
2.3. Metabolite Identification and Statistical Analysis of Metabolite Distribution
2.3.1. Establishment of In-House Compound Library for Lotus and Lotus Seed
2.3.2. Statistical Analysis of the Distribution of Secondary Metabolites in Lotus Seeds
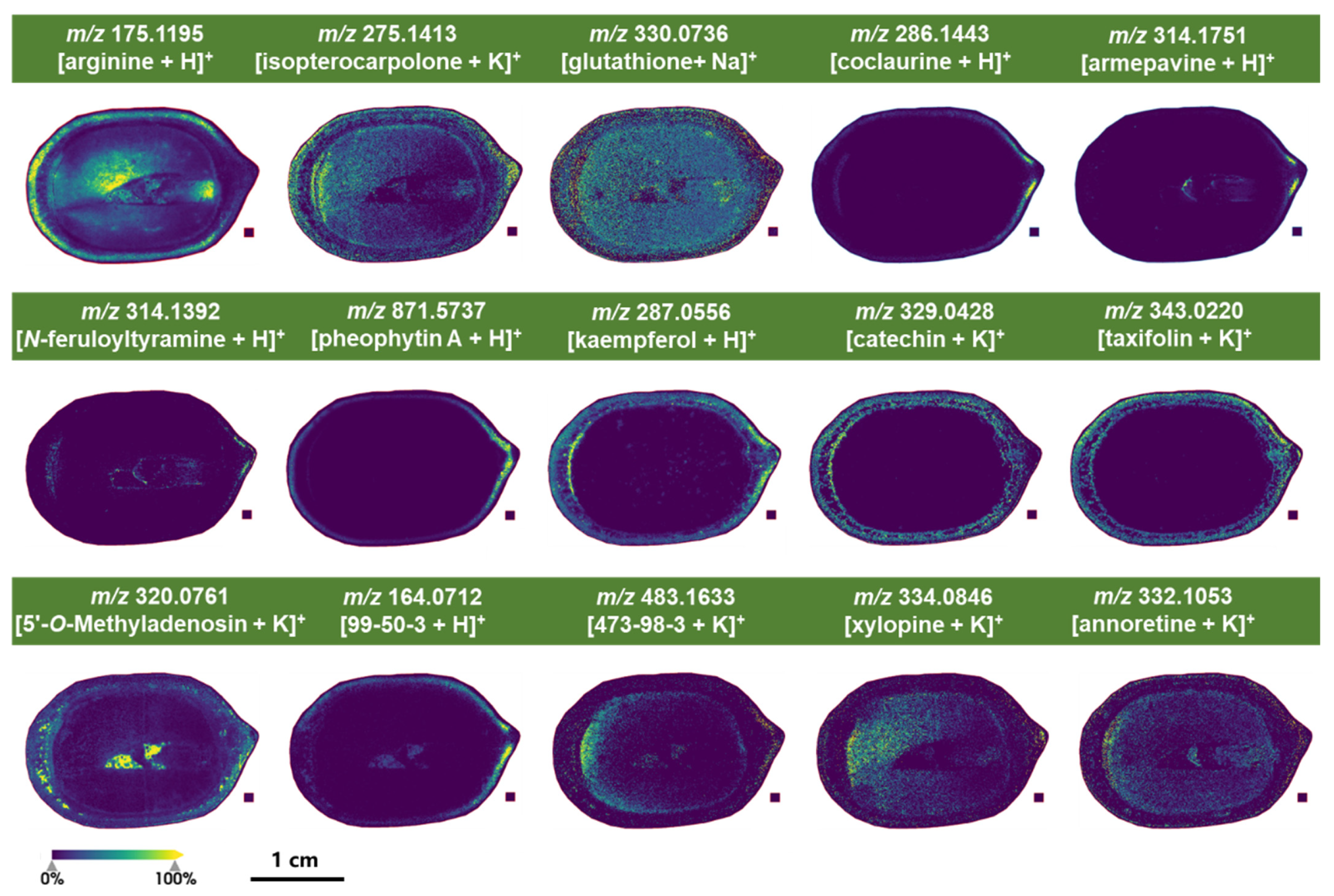
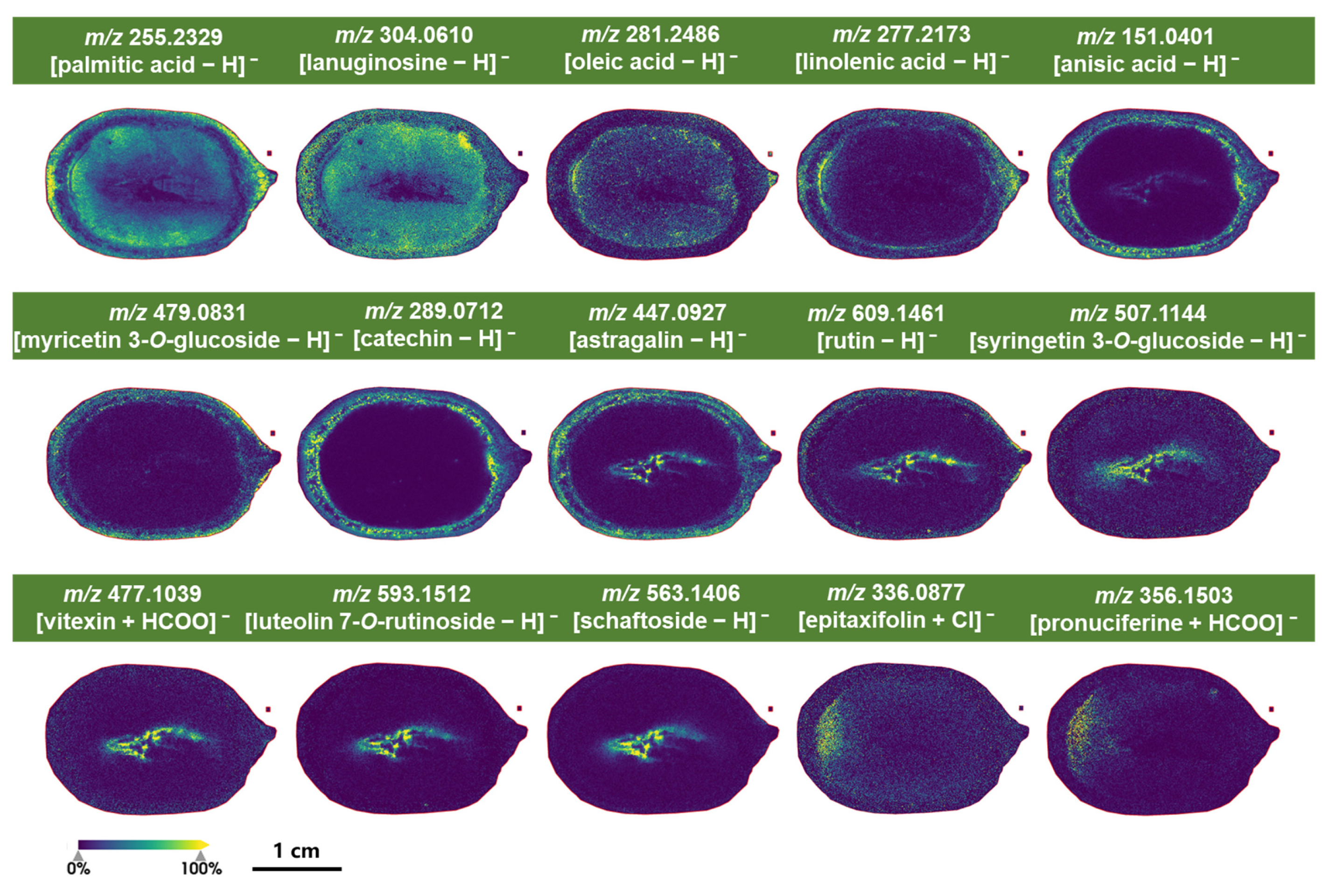
2.4. Identification and Localization of Characteristic Metabolites in Lotus Seeds
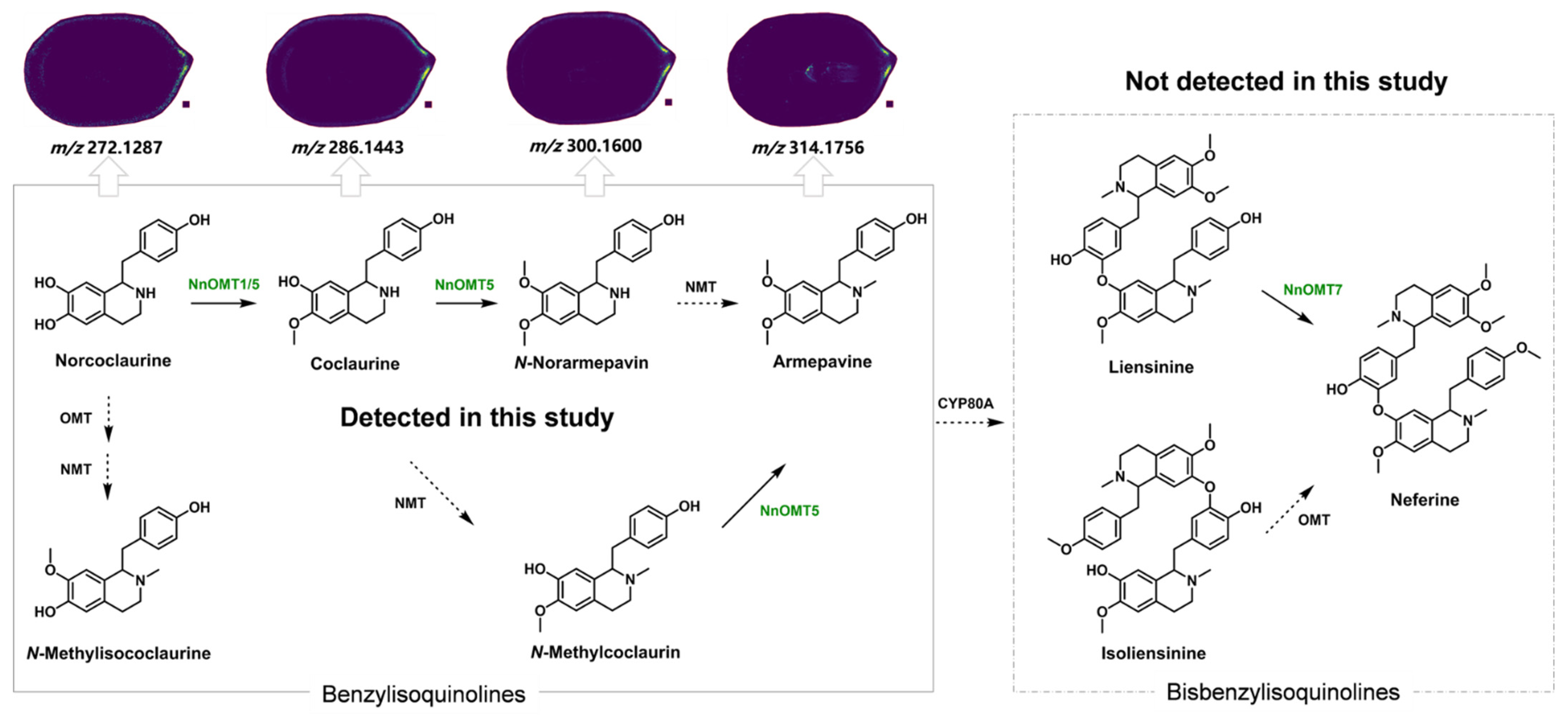
2.4.1. Characteristic Metabolites Identified Under Positive Ion Mode of MALDI-MSI Detection
2.4.2. Characteristic Metabolites Identified Under Negative Ion Mode of MALDI-MSI Detection
3. Discussion
4. Materials and Methods
4.1. Plant Material and Reagents
4.2. Sample Preparation for MALDI-MSI Analysis
4.3. Data Acquisition by MALDI-MSI and Data Analysis
4.4. Raw Data Processing of MSI
4.5. Semi-Quantification Approach
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MALDI-MSI | Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging |
| CMC-Na | Carboxymethylcellulose sodium |
| ITO | Indium–tin oxide |
| CHCA | α-Cyano-4-hydroxycinnamic acid |
| 9AA | 9-Aminoacridine |
References
- Teixidor-Toneu, I.; Jordan, F.M.; Hawkins, J.A. Comparative phylogenetic methods and the cultural evolution of medicinal plant use. Nat. Plants 2018, 4, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Jing, Q.; Wang, H.; Li, X.; Cui, D.; Zhao, Y.; Wang, W.; Hu, Y.; Jiang, M.; Gao, X.; Guo, D.; et al. State-of-the-art application of mass spectrometry imaging covering the medicinal and edible plants. TrAC-Trend. Anal. Chem. 2024, 179, 117878. [Google Scholar] [CrossRef]
- Sumner, L.W.; Lei, Z.; Nikolau, B.J.; Saito, K. Modern plant metabolomics: Advanced natural product gene discoveries, improved technologies, and future prospects. Nat. Prod. Rep. 2015, 32, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Neumann, E.K.; Ge, J.; Gao, W.; Yang, H.; Li, P.; Sweedler, J.V. Interrogation of spatial metabolome of Ginkgo biloba with high-resolution matrix-assisted laser desorption/ionization and laser desorption/ionization mass spectrometry imaging. Plant Cell Environ. 2018, 41, 2693–2703. [Google Scholar] [CrossRef]
- Li, B.; Ge, J.; Liu, W.; Hu, D.; Li, P. Unveiling spatial metabolome of Paeonia suffruticosa and Paeonia lactiflora roots using MALDI MS imaging. New Phytol. 2021, 231, 892–902. [Google Scholar] [CrossRef]
- Sun, C.; Ma, S.; Li, L.; Wang, D.; Liu, W.; Liu, F.; Guo, L.; Wang, X. Visualizing the distributions and spatiotemporal changes of metabolites in Panax notoginseng by MALDI mass spectrometry imaging. J. Ginseng Res. 2021, 45, 726–733. [Google Scholar] [CrossRef]
- Wang, J.; Han, X.; Zheng, Y.; Zhao, Y.; Wang, W.; Ma, D.; Sun, H. Spatial Metabolomic Profiling of Pinelliae Rhizoma from Different Leaf Types Using Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging. Molecules 2024, 29, 4251. [Google Scholar] [CrossRef]
- Sarabia, L.D.; Boughton, B.A.; Rupasinghe, T.; van de Meene, A.M.L.; Callahan, D.L.; Hill, C.B.; Roessner, U. High-mass-resolution MALDI mass spectrometry imaging reveals detailed spatial distribution of metabolites and lipids in roots of barley seedlings in response to salinity stress. Metabolomics 2018, 14, 63. [Google Scholar] [CrossRef]
- Hu, H.; Qiu, K.; Hao, Q.; He, X.; Qin, L.; Chen, L.; Yang, C.; Dai, X.; Liu, H.; Xu, H.; et al. Electromagnetic Field-Assisted Frozen Tissue Planarization Enhances MALDI-MSI in Plant Spatial Omics. Anal. Chem. 2024, 96, 11809–11822. [Google Scholar] [CrossRef]
- Zou, Y.; Tang, W.; Li, B. Exploring natural product biosynthesis in plants with mass spectrometry imaging. Trends Plant Sci. 2025, 30, 69–84. [Google Scholar] [CrossRef]
- Shan, F. Molecular Mechanism Study on Efficacy Difference of Lotus Leaf and Lotus Plumule. Ph.D. Dissertation, Chengdu University of Traditional Chinese Medicine, Chengdu, China, 2015. [Google Scholar]
- Mukherjee, P.K.; Mukherjee, D.; Maji, A.K.; Rai, S.; Heinrich, M. The sacred lotus (Nelumbo nucifera)–phytochemical and therapeutic profile. J. Pharm. Pharmacol. 2009, 61, 407–422. [Google Scholar] [PubMed]
- Punia Bangar, S.; Dunno, K.; Kumar, M.; Mostafa, H.; Maqsood, S. A comprehensive review on lotus seeds (Nelumbo nucifera Gaertn.): Nutritional composition, health-related bioactive properties, and industrial applications. J. Funct. Foods 2022, 89, 104937. [Google Scholar] [CrossRef]
- Chen, C.; Li, G.; Zhu, F. A novel starch from lotus (Nelumbo nucifera) seeds: Composition, structure, properties and modifications. Food Hydrocoll. 2021, 120, 106899. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, X.; Zeng, S.; Huang, X.; Guo, Z.; Zheng, Y.; Tian, Y.; Zheng, B. Nutritional composition, physiological functions and processing of lotus (Nelumbo nucifera Gaertn.) seeds: A review. Phytochem. Rev. 2015, 14, 321–334. [Google Scholar] [CrossRef]
- Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020; Volume 1, pp. 285–287.
- Wang, Z.; Li, Y.; Ma, D.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; Christian, M.; He, Z. Alkaloids from lotus (Nelumbo nucifera): Recent advances in biosynthesis, pharmacokinetics, bioactivity, safety, and industrial applications. Crit. Rev. Food Sci. 2023, 63, 4867–4900. [Google Scholar] [CrossRef]
- Kawamoto, T. Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch. Histol. Cytol. 2003, 66, 123–143. [Google Scholar] [CrossRef]
- Zaima, N.; Goto-Inoue, N.; Hayasaka, T.; Setou, M. Application of imaging mass spectrometry for the analysis of Oryza sativa rice. Rapid Commun. Mass Spectrom. 2010, 24, 2723–2729. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.; Lee, Y.J.; Lee, T.G.; Yoon, S. Sample Preparation of Corn Seed Tissue to Prevent Analyte Relocations for Mass Spectrometry Imaging. J. Am. Soc. Mass Spectrom. 2017, 28, 1729–1732. [Google Scholar] [CrossRef]
- Pei, H. Chemical Constituents of Differents Parts from Nelumbo nucifera Gaertn. and Sedative Effect of Nelumbinis Semen. Master’s Dissertation, Jilin University, Changchun, China, 2021. [Google Scholar]
- Kim, H.W.; Wang, M.; Leber, C.A.; Nothias, L.-F.; Reher, R.; Kang, K.B.; van der Hooft, J.J.J.; Dorrestein, P.C.; Gerwick, W.H.; Cottrell, G.W. NPClassifier: A Deep Neural Network-Based Structural Classification Tool for Natural Products. J. Nat. Prod. 2021, 84, 2795–2807. [Google Scholar] [CrossRef]
- Horn, P.J.; Chapman, K.D. Imaging plant metabolism in situ. J. Exp. Bot. 2023, 75, 1654–1670. [Google Scholar] [CrossRef]
- Feng, C.-Y.; Li, S.-S.; Yin, D.-D.; Zhang, H.-J.; Tian, D.-K.; Wu, Q.; Wang, L.-J.; Su, S.; Wang, L.-S. Rapid determination of flavonoids in plumules of sacred lotus cultivars and assessment of their antioxidant activities. Ind. Crops Prod. 2016, 87, 96–104. [Google Scholar] [CrossRef]
- Chen, S.; Fang, L.; Xi, H.; Guan, L.; Fang, J.; Liu, Y.; Wu, B.; Li, S. Simultaneous qualitative assessment and quantitative analysis of flavonoids in various tissues of lotus (Nelumbo nucifera) using high performance liquid chromatography coupled with triple quad mass spectrometry. Anal. Chim. Acta 2012, 724, 127–135. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, T.; Guo, M. Current Advances in the Metabolomics Study on Lotus Seeds. Front. Plant Sci. 2016, 7, 891. [Google Scholar] [CrossRef] [PubMed]
- Shariatgorji, M.; Nilsson, A.; Goodwin, R.J.A.; Källback, P.; Schintu, N.; Zhang, X.; Crossman, A.R.; Bezard, E.; Svenningsson, P.; Andren, P.E. Direct Targeted Quantitative Molecular Imaging of Neurotransmitters in Brain Tissue Sections. Neuron 2014, 84, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.J.S.; Dong, J.; Lian, C.; Qiao, F.; Zheng, J.; Ma, S. Visualization Analysis of Spatial Distribution of Alkaloids in Aconiti Radix Cocta During Processing Process by Matrix-Assisted Laser Desorption Ionization Mass Spectrometry Imaging. Chin. Pharm. J. 2022, 57, 834–839. [Google Scholar]
- Ma, X.; Fernández, F.M. Advances in mass spectrometry imaging for spatial cancer metabolomics. Mass Spectrom. Rev. 2024, 43, 235–268. [Google Scholar] [CrossRef]
- Taira, S.; Shimma, S.; Osaka, I.; Kaneko, D.; Ichiyanagi, Y.; Ikeda, R.; Konishi-Kawamura, Y.; Zhu, S.; Tsuneyama, K.; Komatsu, K. Mass Spectrometry Imaging of the Capsaicin Localization in the Capsicum Fruits. Int. J. Biotechnol. Wellness Ind. 2012, 1, 61–65. [Google Scholar] [CrossRef]
- Maia, M.; McCann, A.; Malherbe, C.; Far, J.; Cunha, J.; Eiras-Dias, J.; Cordeiro, C.; Eppe, G.; Quinton, L.; Figueiredo, A.; et al. Grapevine leaf MALDI-MS imaging reveals the localisation of a putatively identified sucrose metabolite associated to Plasmopara viticola development. Front. Plant Sci. 2022, 13, 1012636. [Google Scholar] [CrossRef]
- Gao, H. Arginine Metabolism and the Roles of NO in Root Development and Stress Response in Malus hupehensis (Pamp) Rehd. var Pinyiensis Jiang. Ph.D. Dissertation, Shandong Agricultural University, Tai’an, China, 2007. [Google Scholar]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef]
- Jiang, J.; Sun, C.; Wang, G.; Xu, Q.; Bian, Y.; Li, J.; Li, J.; Ding, R.; Lin, H.; Tian, W.; et al. C-13 Norisoprenoids and Eudesmanoids from Nelumbo nucifera Gaertn. Regulate the Lipid Metabolism via the AMPK/ACC/SREBP-1c Signaling Pathway. Chem. Biodivers. 2025, 22, e202401778. [Google Scholar] [CrossRef]
- Kanada, R.M.; Simionato, J.I.; Arruda, R.F.d.; Santin, S.M.d.O.; Souza, M.C.d.; Silva, C.C.d. N-trans-feruloyltyramine and flavonol glycosides from the leaves of Solanum sordidum. Rev. Bras. De Farmacogn. 2012, 22, 502–506. [Google Scholar] [CrossRef]
- Pearce, G.; Marchand, P.A.; Griswold, J.; Lewis, N.G.; Ryan, C.A. Accumulation of feruloyltyramine and p-coumaroyltyramine in tomato leaves in response to wounding. Phytochemistry 1998, 47, 659–664. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, T.; Zhang, C.; Guo, M. Flavonoids of Lotus (Nelumbo nucifera) Seed Embryos and Their Antioxidant Potential. J. Food Sci. 2017, 82, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Pheomphun, P.; Treesubsuntorn, C.; Thiravetyan, P. Effect of exogenous catechin on alleviating O3 stress: The role of catechin-quinone in lipid peroxidation, salicylic acid, chlorophyll content, and antioxidant enzymes of Zamioculcas zamiifolia. Ecotoxicol. Environ. Saf. 2019, 180, 374–383. [Google Scholar] [CrossRef]
- Jiang, C.; Ma, J.; Liu, Y.; Chen, J.; Ni, D.; Chen, L. Identification and distribution of a single nucleotide polymorphism responsible for the catechin content in tea plants. Hortic. Res. 2020, 7, 24. [Google Scholar] [CrossRef]
- Du, Y.; Peng, S.; Chen, H.; Li, J.; Huang, F.; Chen, W.; Wang, J.; Fang, X.; Liu, L.; Wei, L.; et al. Unveiling the Spatiotemporal Landscape of Ganoderma lingzhi: Insights into Ganoderic Acid Distribution and Biosynthesis. Engineering 2025, in press. [Google Scholar] [CrossRef]
- Shroff, R.; Schramm, K.; Jeschke, V.; Nemes, P.; Vertes, A.; Gershenzon, J.; Svatoš, A. Quantification of plant surface metabolites by matrix-assisted laser desorption–ionization mass spectrometry imaging: Glucosinolates on Arabidopsis thaliana leaves. Plant J. 2015, 81, 961–972. [Google Scholar] [CrossRef]
- Vermillion-Salsbury, R.L.; Hercules, D.M. 9-Aminoacridine as a matrix for negative mode matrix-assisted laser desorption/ionization. Rapid Commun. Mass Spectrom. 2002, 16, 1575–1581. [Google Scholar] [CrossRef]
- Zhukov, A.V. Palmitic acid and its role in the structure and functions of plant cell membranes. Russ. J. Plant Physiol. 2015, 62, 706–713. [Google Scholar] [CrossRef]
- Pyne, M.E.; Gold, N.D.; Martin, V.J.J. Pathway elucidation and microbial synthesis of proaporphine and bis-benzylisoquinoline alkaloids from sacred lotus (Nelumbo nucifera). Metab. Eng. 2023, 77, 162–173. [Google Scholar] [CrossRef]
- Marthandam Asokan, S.; Mariappan, R.; Muthusamy, S.; Velmurugan, B.K. Pharmacological benefits of neferine—A comprehensive review. Life Sci. 2018, 199, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, X.; Guo, Y.; Liu, X.; Zhao, X.; Teka, T.; Lv, C.; Han, L.; Huang, Y.; Pan, G. Thirteen bisbenzylisoquinoline alkaloids in five Chinese medicinal plants: Botany, traditional uses, phytochemistry, pharmacokinetic and toxicity studies. J. Ethnopharmacol. 2021, 268, 113566. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.R.; Gautam, L.N.S.; Adhikari, D.; Karki, R. A Comprehensive Review on Chemical Profiling of Nelumbo Nucifera: Potential for Drug Development. Phytother. Res. 2017, 31, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Xu, L. Research and Application on Quality Characterisitics of Lotus seed in Different Maturity. Master’s Dissertation, Fujian Agriculure and Forestry Universiy, Fuzhou, China, 2015. [Google Scholar]
- Yu, Y. Identification and Functional Characterization of Methyltransferase Involved in Benzylisoquinoline Alkaloids Biosynthesis from Nelumbo nucifera. Ph.D. Disseration, China Academy of Chinese Medical Sciences, Beijing, China, 2023. [Google Scholar]
- Menéndez-Perdomo, I.M.; Facchini, P.J. Isolation and characterization of two O-methyltransferases involved in benzylisoquinoline alkaloid biosynthesis in sacred lotus (Nelumbo nucifera). J. Biol. Chem. 2020, 295, 1598–1612. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, L.; Fang, T.; Xiong, Y.; Ogutu, C.; Yang, D.; Vimolmangkang, S.; Liu, Y.; Han, Y. Investigation of benzylisoquinoline alkaloid biosynthetic pathway and its transcriptional regulation in lotus. Hortic. Res. 2018, 5, 29. [Google Scholar] [CrossRef]
- Qi, S.; Zhou, D. Lotus seed epicarp extract as potential antioxidant and anti-obesity additive in Chinese Cantonese Sausage. Meat Sci. 2013, 93, 257–262. [Google Scholar] [CrossRef]
- Yan, Z.; Luo, X.; Cong, J.; Zhang, H.; Ma, H.; Duan, Y. Subcritical water extraction, identification and antiproliferation ability on HepG2 of polyphenols from lotus seed epicarp. Ind. Crop. Prod. 2019, 129, 472–479. [Google Scholar] [CrossRef]
- Lu, C.-P.; Lin, K.-H.; Wu, C.-C.; Shih, M.-C.; Chang, W.-T.; Yu, Y.-P. Antioxidant and cytoprotective properties of seeds and seed by-products from lotus (Nelumbo nucifera). Not. Bot. Horti Agrobot. 2022, 50, 12711. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Xu, X.; Tang, C. A Metabolomics Exploration of Young Lotus Seeds Using Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging. Molecules 2025, 30, 3242. https://doi.org/10.3390/molecules30153242
Chen Y, Xu X, Tang C. A Metabolomics Exploration of Young Lotus Seeds Using Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging. Molecules. 2025; 30(15):3242. https://doi.org/10.3390/molecules30153242
Chicago/Turabian StyleChen, Ying, Xiaomeng Xu, and Chunping Tang. 2025. "A Metabolomics Exploration of Young Lotus Seeds Using Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging" Molecules 30, no. 15: 3242. https://doi.org/10.3390/molecules30153242
APA StyleChen, Y., Xu, X., & Tang, C. (2025). A Metabolomics Exploration of Young Lotus Seeds Using Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging. Molecules, 30(15), 3242. https://doi.org/10.3390/molecules30153242




