Abstract
A new lignan (1) and a new phenolic glycoside (2), together with eighteen known compounds (3–20), were isolated from Oxytropis ochrocephala. Their structures were unambiguously elucidated by spectroscopic techniques (UV, IR, 1D and 2D NMR), and HR-ESI-MS analysis, as well as by comparison with the literature. The insecticidal activity of these compounds was evaluated against Tetranychus urticae Koch, and the results showed that compounds 3, 9, 15, and 16 had a weak inhibitory effect at a concentration of 1 mg/mL after treatment for 24 h.
1. Introduction
Oxytropis ochrocephala Bunge is a perennial herb belonging to the genus Oxytropis and the Fabaceae family. This plant primarily grows in the pastoral regions of Qinghai, Xizang, Gansu, and Sichuan provinces in China [1]. O. ochrocephala is a common poisonous plant and can cause chronic accumulation poisoning after being consumed by livestock, and thus is commonly known as “locoweed”. Moreover, the degradation of other grassland plants caused by salinization and rodents has facilitated the proliferation of O. ochrocephala. As a result, this plant has emerged as a dominant species, significantly disrupting the ecological balance. Interestingly, O. ochrocephala is also commonly found in arid steppes and desert areas and simultaneously serves as a vital agent in sand stabilization and desertification control, embodying a dual-natured ecological role [2]. O. ochrocephala is commonly employed to reduce heat by promoting detumescence, bolstering physical strength, and improving immune function [3,4]. Over the past forty years, nearly 300 compounds have been identified in O. ochrocephala, including alkaloids, flavonoids, triterpenoids, and saponins. These compounds demonstrate significant biological activities such as anti-tumor, anti-hepatitis B virus, insecticidal, antibacterial, and anti-hypoxia effects [5]. In our ongoing research to discover bioactive compounds from O. ochrocephala, we isolated and identified two new compounds (1, 2) alongside eighteen known compounds (3–20) (Figure 1). The insecticidal properties of these compounds were evaluated.
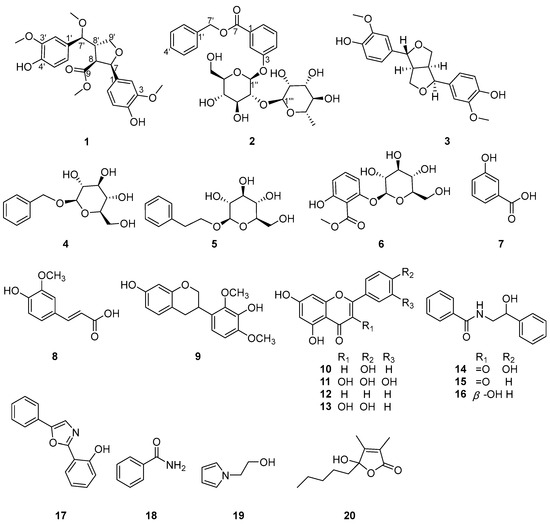
Figure 1.
Structures of compounds 1–20.
2. Results
2.1. Structural Elucidation
Compound 1 was acquired as a white solid powder. Its molecular formula was determined to be C22H26O8 via HR-ESI-MS (m/z 441.1522 [M + Na]⁺, calcd for C22H26O8Na, 441.1520) and 13C NMR (Table 1). The IR spectrum of 1 exhibited characteristic absorption bands for the aromatic ring (1517 cm−1), carbonyl (1728 cm−1), and hydroxy (3419 cm−1). The 1H NMR spectrum displayed signals for two methines [δH 3.12 (1H, dd, J = 9.5, 8.4 Hz, H-8) and δH 3.01 (1H, dddd, J = 10.5, 9.5, 7.7, 7.5 Hz, H-8′)], two oxymethine protons [δH 5.09 (1H, d, J = 8.4 Hz, H-7) and δH 4.19 (1H, d, J = 10.5 Hz, H-7′)], one oxymethylene group [δH 3.54 (1H, dd, J = 9.2, 7.7 Hz, H-9a) and δH 3.71 (1H, dd, J = 9.2, 7.5 Hz, H-9b)], four methoxyl groups [δH 3.88 (3-OCH3), 3.90 (3′-OCH3), 3.05 (7′-OCH3), 3.74 (9-OCH3)], and six aromatic protons [δH 6.85 (1H, d, J = 1.9 Hz, H-2), 6.89 (1H, d, J = 8.0 Hz, H-5), 6.82 (1H, dd, J = 8.0, 1.9 Hz, H-6); 6.84 (1H, d, J = 1.9 Hz, H-2′), 6.87 (1H, d, J = 8.0 Hz, H-5′); 6.81 (1H, dd, J = 8.0, 1.9 Hz, H-6′)] (Table 1). The 13C NMR spectrum combined with the HSQC spectrum of 1 exhibited 22 carbon resonances corresponding to two 1,3,4-trisubstituted benzene rings (δC 132.4, 108.4, 146.7, 145.9, 114.4, 119.1; 131.5, 109.1, 147.1, 145.5, 114.4, 121.1), four methoxy groups (δC 56.1 × 3. 51.9), an ester carbonyl carbon (δC 173.1), an oxymethylene carbon (δC 70.5), two oxymethine carbons (δC 82.2, 84.0), and two methine carbons (δC 50.3, 54.4). These NMR data suggested that compound 1 is typical of a tetrahydrofuranoid-type lignan [6].

Table 1.
1H NMR (500 MHz) and 13C NMR (126 MHz) data of compounds 1 and 2 (δ in ppm, J in Hz).
The planar structure of compound 1 was deduced from 1H–1H COSY cross-peaks and HMBC correlations. The cross-peaks of H-7/H-8/H-8′/H-7′(H-9′) in the 1H-1H COSY spectrum, combined with the HMBC correlations from H-9′ to C-7; H-7, H-8, H-8′ to C-9; H-7 to C-2/C-6; and H-7′ to C-2′/C-6′, confirmed a tetrahydrofuran ring and its substitutions as shown in Figure 2. The locations of the four methoxy groups at C-3′, C-3, C-7′, and C-9 were determined by the HMBC correlations from 3′-OCH3- to C-3′, 3-OCH3- to C-3, 7′-OCH3- to C-7′, H-7 to 7′-OCH3, and 9-OCH3- to C-9.
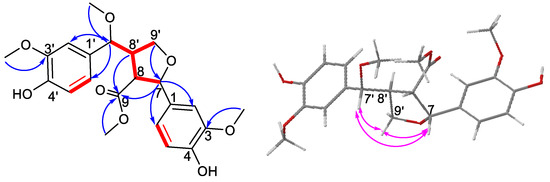
Figure 2.
1H–1H COSY (red bold), HMBC (blue arrows), and NOESY (pink double arrows) correlations of compound 1.
The relative configuration of compound 1 was determined by analysis of the coupling constants and NOESY spectrum (Figure 2). The NOESY correlations of H-7/H-9′α, H-7′/H-9′α suggested that these protons were co-facial and were randomly assigned as α-oriented. The large coupling constant of 3J7′,8′ = 10.5, 3J8,8′ = 9.4 Hz indicated a threo configuration. Consequently, H-8′ was β-oriented and H-8 was α-oriented. Thus, compound 1 was fully characterized as 7R*-methoxy-tanegool-9-methyl ester.
Compound 2 was obtained as a yellow oil. The HR-ESI-MS spectrum exhibited an ion peak at m/z 559.1785 [M + Na]+ (calcd for C26H32O12Na, 559.1786), from which the molecular formula of 2 was deduced to be C26H32O12, corresponding to 11 degrees of unsaturation. The IR spectrum indicated the presence of hydroxyl (3363 cm−1), ester carbonyl (1716 cm−1), aryl (1507 cm−1), and ether bond (1071 cm−1) groups. The 1H NMR (Table 1) showed signals for nine aromatic hydrogens (δH 7.74–7.30), two oxygenated methylenes (δH 5.36, 2H, d, J = 2.5 Hz, H-7′; 3.72, 3.86, m, H-6″), and two anomeric hydrogens (δH 5.10, 1H, d, J = 7.5 Hz, H-1″; δH 5.27, 1H, d, J = 1.5 Hz, H-1‴). The 13C NMR spectrum displayed twelve aromatic carbon signals (three quaternary and nine methine), one ester carbonyl carbon (δC 167.4, C-7), two oxygenated methylene carbons (δC 67.9, C-7′; 62.3, C-6″), and two anomeric carbons (δC 100.2, C-1″; 102.7, C-1‴).
The 1H-1H COSY correlations of H-4/H-5/H-6, combined with the HMBC correlations from H-2 (δH 7.73) to C-4 (δC 122.0), C-6 (δC 124.3), indicated that there was a 1,3-disubstituted benzene ring. Although the proton signals of the other benzene ring overlap, it can be inferred to be a monosubstituted benzene based on the remaining five aromatic protons. The HMBC correlations from H-2, H-6 to C-7 and H-7′ to C-3, C-6, C-7 confirmed a benzyl 3-hydroxy-benzoate structure as shown in Figure 3 [7]. Furthermore, the 1H-1H COSY cross-peaks of H-1″/H-2″/H-3″/H-4″/H-5″/H-6″ and H-1‴/H-2‴/H-3‴/H-4‴/H-5‴/H-6‴ and the HMBC correlations from H-4 to C-2, C-6; H-5 to C-1, C-3; H-6 to C-2, C-4, C-7; H-2′ to C-4′, C-7′; H-3′ to C-1′, C-4′; H-4′ to C-2′, C-7′; H-7′ to C-7, C-1′, C-4′; H-1″ to C-3, C-5″; H-2″ to C-1″, C-3″, C-1‴; H-4″ to C-5″; H-1‴ to C-2″, C-2‴, C-5‴; H-4‴ to C-3‴, C-5‴, C-6‴; and H-6‴ to C-4‴, C-5‴, as well as comparison of their spectroscopic data [Table S1] with those reported in the literature, suggested that compound 2 has the same sugar chain of α-l-rhamnopyranosyl-(1 → 2)-β-d-glucopyranosyl as japonicanoside A [8], which is connected to C-3 in compound 2. The relative configuration of the sugar chain was determined by the coupling constant (7.5 Hz) of H-1″/H-2″ and the characteristic signals of C-3‴ (δC 72.2), C-5‴ (δC 70.0), and C-6‴ (δC 18.2) [9], which was confirmed by the NOESY correlations of H-3‴/H-5‴, H-1″/H-3″, and H-1″/H-5″. To further verify the absolute configuration of sugar, acid hydrolysis and derivatization were performed according to the methods described in the literature [10]. The LC analysis results [Figure S53] showed that the retention times of the glycosyl derivatives of compound 2 were consistent with those of the reference substances of D-glucose and L-rhamnose, respectively. Therefore, they were identified as β-D-glucose and α-L-rhamnose. Thus, the structure of 2 was assigned as benzyl 3-O-α-l-rhamnopyranosyl-(1 → 2)-β-d-glucopyranosyloxybenzoate.
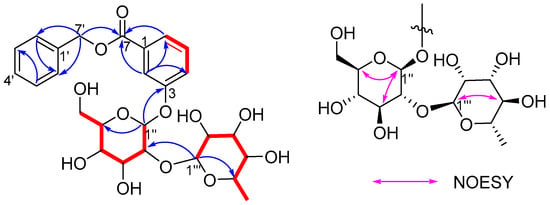
Figure 3.
1H–1H COSY (red bold), HMBC (blue arrows), and NOESY correlations of compound 2.
In addition, the other known compounds were identified as pinoresinol (3) [11], benzyl β-d-glucopyranoside (4) [12], phenylethyl β-d-glucopyranoside (5) [13], methyl 2-β-d-glucopyranosyloxy-6-hydroxybenzoate (6) [14], 3-hydroxybenzoic acid (7) [15], ferulic acid (8) [16], 3′,7-dihydroxy-2′,4′-dimethoxyisoflavan (9) [17], apigenin (10) [18], quercetin (11) [18], chrysin (12) [19], kaempferol (13) [18], N-benzoyl-β-hydroxyphenylethylamine (14) [20], (R)-2-hydroxy-N-phenethyl-2-phenylacetamide (15) [21], N-benzoyl-phenylethylamine (16) [20], 2-(2′-hydroxyphenyl)-5-phenyloxazole (17) [13], benzamide (18) [22], 1-(2-hydroxyethyl)pyrrole (19) [23], and hydroxydihydrobovolide (20) [24], respectively, by comparing their spectroscopic data with those reported in the literature.
2.2. Biological Activity Analysis
Tetranychus urticae Koch belongs to the class Arachnida, family Tetranychidae, and genus Tetranychus [25]. This species is widely distributed globally and is characterized by its short generational cycle and high reproductive capacity. It inflicts damage on over 110 plant species across 32 families, making it one of the most detrimental mites in global agricultural and forestry production [26]. The prolonged use of chemical pesticides has led to increasingly prominent “3R” issues: residue, resistance, and resurgence. T. urticae has developed high resistance to more than 90 chemically active ingredients [27]. Therefore, the development of natural, highly effective, low-toxicity, and environmentally friendly bio-acaricides that are harmless to humans and livestock is of paramount importance. In light of this, we screened the contact toxicity activity of compounds 1–6 and 9–17 against T. urticae, with clofentezine used as a positive control. The results showed that compounds 3, 9, 15, and 19 only had a weak inhibitory effect at a concentration of 1 mg/mL after treatment for 24 h (Table 2), while the other compounds did not exhibit any activity.

Table 2.
The contact toxicity activity of the active compounds 3, 9, 15, and 19 against T. urticae.
3. Materials and Methods
3.1. General Experimental Procedures
A polarimeter (Rudolph Research Analytical, Hackettstown, NJ, USA) was used to obtain optical rotation. The infrared (IR) data were recorded on a Bruker Tensor 27 spectrometer (Rudolph Research Analytical, USA). High-resolution electrospray ionization mass spectrometry (HR-ESI-MS) data were obtained using a Thermo Scientific LTQ-Orbitrap Elite ETD mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Nuclear magnetic resonance (1H, 13C, and 2D NMR) data were collected on a Bruker Avance NEO 500 MHz spectrometer (Bruker, Karlsruhe, Germany). Preparative HPLC separations were performed on a semi-preparative high-performance liquid chromatography Shimadzu LC-2030 (Shimadzu Corporation, Kyoto, Japan) equipped with a YMC-pack C18 (ODS) column (10 × 250 mm, 10 μm, Mitsubishi Chemical Corp., Tokyo, Japan). Macroporous resin, MCI resin (75–150 μm, Mitsubishi Chemical Corp., Tokyo, Japan), Sephadex LH-20 dextran gel (GE Healthcare Bio-Sciences, Uppsala, Sweden), reverse-phase silica gel PR-C18, and normal-phase silica gel (Qingdao Marine Chemical Co., Ltd., Qingdao, China) were used for column chromatography (CC). GF254 fluorescent silica gel (Qingdao Marine Chemical Co., Ltd.) was used for thin-layer chromatography (TLC).
3.2. Plant Materials
The whole plant of Oxytropis ochrocephala was collected in August 2020 in Xining City, Qinghai Province, China. Professor Que Sheng, School of Nationalities and Normal Education, Qinghai Normal University, taxonomically identified the plant from a sample of a whole flowering plant. The plant specimen was deposited at the Natural Medicine Development Research Institute, Lanzhou Jiaotong University, with accession number HHJD-20200930.
3.3. Extraction and Isolation
The whole plant material of O. ochrocephala (15.8 kg) underwent triple percolation with 95% ethanol (EtOH, 3 × 50 L). The obtained crude extract (1.25 kg) was dissolved in warm water (1 L) at 50 °C. The solution was acidified to a pH range of 1–3 by the addition of a 2% hydrochloric acid (HCl) solution and was subsequently extracted three times with dichloromethane (CH2Cl2, 3 × 1 L), yielding an acidic extract weighing 330.12 g. This acidic extract was fractionated on a macroporous resin column and eluted with a water/methanol (H2O/MeOH) gradient (100:0 to 0:100, v/v), resulting in the formation of five fractions: F1 (0% MeOH, 5.65 g); F2 (30% MeOH, 9.81 g); F3 (50% MeOH, 16.02 g); F4 (80% MeOH, 87.53 g); and F5 (100% MeOH, 71.7 g). An additional fraction, F6 (124.72 g), was obtained through elution with acetone. Fractions F3 and F4 were chosen for further separation owing to their high compound diversity, as revealed by preliminary analysis. Fraction F3 (16.02 g) was chromatographed on a silica gel column and eluted with a CH2Cl2/MeOH gradient (100:0 to 0:100, v/v) to obtain sub-fractions F3.1–F3.5. Sub-fraction F3.3 (2.06 g) was chromatographed on a silica gel column, eluted with a gradient CH2Cl2/MeOH (100:0 to 0:100, v/v), and produced sub-fractions F3.3.1–F3.3.8, which were further purified by silica gel (petroleum ether/ethyl acetate, 2:1, v/v) and then underwent HPLC (MeOH/H2O, 60:40, v/v, flow rate: 2 mL/min), yielding compounds 7 (tR = 49.02 min, 5.2 mg), 15 (tR = 9.19 min, 5.8 mg), and 18 (tR = 16.28 min, 4.4 mg). F3.5 (4.36 g) was subjected to chromatographic separation on a reversed-phase C18 (RP-18) column and eluted with a gradient of H2O/MeOH ranging from 100:0 to 0:100 (v/v). This process led to the generation of sub-fractions F3.5.1–F3.5.11. These fractions were purified using Sephadex LH-20 (MeOH/CH2Cl2, 1:1, v/v), respectively, resulting in the isolation of compound 19 (2.6 mg), compound 8 (56.2 mg), and compound 5 (25.1 mg). Fraction F4 (87.53 g) was processed under the same chromatographic conditions as fraction F3, yielding sub-fractions F4.1–F4.10. F4.4 (6.79 g) was chromatographed on a silica gel column with a CH2Cl2/MeOH gradient from 100:0 to 0:100 (v/v), providing sub-fractions F4.4.1–F4.4.5. Sub-fraction F4.4.3 (1.43 g) was separated on an RP-18 column using a H2O/MeOH gradient of 100:0 to 0:100 (v/v), yielding sub-fractions F4.4.3.1–F4.4.3.8. Sub-fraction F4.4.3.2 (0.25 g) was purified on silica gel with a petroleum ether/ethyl acetate (30:1, v/v) eluent, yielding compounds 6 (3.5 mg), 11 (3.6 mg), 12 (2.3 mg), and 17 (12.3 mg). Sub-fraction F4.4.4 (1.76 g) was chromatographed on silica gel with a petroleum ether/ethyl acetate (10:1, v/v) eluent and further purified by semi-preparation HPLC with a mobile phase of MeOH/H2O (60:40, v/v, flow rate: 2 mL/min), leading to the isolation of compounds 2 (tR = 22.31 min, 20.2 mg), 4 (tR = 31.73 min, 4.2 mg), 14 (tR = 39.24 min, 2.6 mg), and 16 (tR = 24.62 min, 9.3 mg). Sub-fraction F4.5 (9.92 g) was separated on a silica gel column with a CH2Cl2/MeOH gradient (100:0 to 0:100, v/v), resulting in the generation of sub-fractions F4.5.1–F4.5.8. F4.5.3 (1.24 g) was chromatographed on a reversed-phase C18 (RP-18) column with a H2O/MeOH (70:30, v/v) and further purified on a Sephadex LH-20 column using a CH2Cl2/MeOH (1:1, v/v), leading to the isolation of compound 9 (3.3 mg) and compound 13 (5.0 mg). F4.5.4 (0.46 g) was purified on silica gel with a petroleum ether/ethyl acetate (10:1, v/v), yielding compound 10 (3.7 mg) and compound 20 (6.2 mg). F4.5.6 (0.37 g) was purified by silica gel chromatography using petroleum ether/ethyl acetate (5:1, v/v), followed by semi-preparation high-performance liquid chromatography (HPLC) with MeOH/H2O (70:30, v/v, flow rate: 2 mL/min), yielding compound 1 (tR = 26.39 min, 4.2 mg) and compound 3 (tR = 19.62 min, 3.6 mg).
3.4. Biology Assay
The insecticidal activity against the two-spotted spider mite, Tetranychus urticae Koch, was assessed using the capillary micro-droplet technique [27]. The compounds isolated from O. ochrocephala were dissolved in dimethyl sulfoxide (DMSO) (10 mg/mL) and prepared in three concentration levels: 10 mg/mL, 2 mg/mL, and 1 mg/mL. Healthy, wingless individuals of equal size were chosen for the tests. The test solutions were applied to the dorsal thorax of the mites using a capillary tube. Each concentration was evaluated in triplicate with 20 mites treated in each replicate. Concurrently, a control group was also set up. The treated mites were placed in 12 cm diameter Petri dishes lined with moist filter paper. These dishes were sealed with plastic wrap, punctured for air circulation, labeled, and kept at room temperature for incubation. The survival of the mites was checked every 24 h, and the number of dead mites was recorded. During this observation period, distilled water was added as necessary to keep the filter paper moist. Mortality was determined by a complete lack of movement when the legs and antennae were brushed. The mortality rate (%) was calculated as (number of dead mites/total number of mites) × 100, while the corrected mortality rate (%) was derived from [(control group survival rate − treatment group survival rate)/control group survival rate] × 100.
3.5. Compound Characterization
Compound 1: white amorphous solid; −25.0 (c 0.01, MeOH); IR (KBr) νmax 3419, 2928, 1728, 1517, 1274 cm−1; 1H (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz), see Table 1; HR-ESI-MS m/z 441.1522 [M + Na]+ (calcd for C22H26O8Na, 441.1520).
Compound 2: yellow oil; +32.0 (c 0.01, MeOH); IR (KBr) νmax 3363, 1716, 1507, 1071 cm−1; 1H (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz), see Table 1; HR-ESI-MS m/z 559.1785 [M + Na]+ (calcd for C26H32O12Na, 559.1786).
4. Conclusions
In summary, our intensive phytochemical investigation of the plant O. ochrocephala identified twenty compounds, including a novel lignan (1), a phenolic glycoside (2), and eighteen known compounds: a lignan (3), five phenols (4–8), five flavonoids (9–13), six alkaloids (14–19), and another type compound (20). Their structures were unambiguously elucidated by spectroscopic techniques and HR-ESI-MS analysis, as well as by comparison with the literature. Insecticidal activity against Tetranychus urticae indicated that these compounds showed a weak inhibitory effect.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30122489/s1, The 1D and 2D NMR, IR spectra, and HR-ESI-MS data for 1 and 2, and the 1D NMR of 3–20 (PDF).
Author Contributions
Conceptualization, T.S. and Y.H.; writing—original draft preparation, G.L. and Z.L.; methodology, G.L.; investigation, G.L., J.X., Y.J. and L.W.; writing—review and editing T.S., Y.H. and H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 22467016), Gansu Provincial Agricultural Science and Technology Support Project (No. KJZC-2024-16), and the Research Foundation of the Education Bureau of Gansu Province (grant number 2022CYZC-35).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Academia Sinica. Flora of China; Science Press: Beijing, China, 1998; Volume 42, p. 21. [Google Scholar]
- Lu, H.; Wang, S.S.; Zhou, Q.W.; Zhao, Y.N.; Zhao, B.Y. Damage and control of major poisonous plants in the western grasslands of China-a review. Rangeland J. 2012, 34, 329–339. [Google Scholar] [CrossRef]
- Editorial Committee of Chinese Materia Medica. Chinese Materia Medica (The Volume of the Tibetan Medicines); Shanghai Science and Technology Press: Shanghai, China, 1999. [Google Scholar]
- Institute for Drug and Biological Products Control, the Ministry of Health. Medicinal Herbal in Chinese Nation; People’s Medical Publishing House: Beijing, China, 1984. [Google Scholar]
- Zhang, D.; Lei, J.; Hong, E.K.; Lu, D.; Yuan, W.; Yang, Z.; Ming, C. Anti-hypoxia effects of the ethanol extract of Oxytropis ochrocephala. Legume Res. 2016, 39, 914–920. [Google Scholar] [CrossRef]
- Zhou, K.S.; Tian, W.; Zhang, Z.; Tan, C.J. Research advances on chemical constituents of Oxytropis ochrocephala Bunge and its bioactivities. Nat. Prod. Res. Dev. 2020, 32, 161–171. [Google Scholar]
- Dang, P.H.; Nguyen, H.X.; Nguyen, H.H.T.; Vo, T.D.; Le, T.H.; Phan, T.H.N.; Nguyen, N.T. Lignans from the roots of Taxus wallichiana and their α-glucosidase inhibitory activities. J. Nat. Prod. 2017, 80, 1876–1882. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.Y.; Wu, X.D.; Jia, Y.N.; Fan, J.T.; Tan, N.H. A new phenolic glycoside from honey-fried Eriobotrya japonica. China J. Chin. Mater. Med. 2019, 44, 2806–2812. [Google Scholar]
- Kasai, R.; Okihara, M.; Asakawa, J.; Mizutani, K.; Tanaka, O. 13C NMR study of α-and β-anomeric pairs of d-mannopyranosides and l-rhamnopyranosides. Tetrahedron 1979, 35, 1427–1432. [Google Scholar] [CrossRef]
- Schmid, C.; Dawid, C.; Peters, V.; Hofmann, T. Saponins from European licorice roots (Glycyrrhiza glabra). J. Nat. Prod. 2018, 81, 1734–1744. [Google Scholar] [CrossRef]
- Yi, B.; Hu, L.; Mei, W. Antioxidant phenolic compounds of cassava (Manihot esculenta) from Hainan. Molecules 2011, 16, 10157–10167. [Google Scholar] [CrossRef]
- Fujita, T.; Funayoshi, A.; Nakayama, M. A phenylpropanoid glucoside from Perilla frutescens. Phytochemistry 1994, 37, 543–546. [Google Scholar] [CrossRef]
- Banzragchgarav, O.; Murata, T.; Odontuya, G.; Buyankhishig, B.; Suganuma, K.; Davaapurev, B.O.; Inoue, N.; Batkhuu, J.; Sasaki, K. Trypanocidal activity of 2,5-diphenyloxazoles isolated from the roots of Oxytropis lanata. J. Nat. Prod. 2016, 79, 2933–2940. [Google Scholar] [CrossRef]
- Mimaki, Y.; Ishibashi, N.; Komatsu, M. Studies on the chemical constituents of Gloriosa rothschildiana and Colchicum autumnale. Jpn. J. Pharmacogn. 1991, 45, 255–260. [Google Scholar]
- Ran, Y.; Zhang, Y.; Wang, X.; Li, G. Nematicidal Metabolites from the Actinomycete Micromonospora sp. WH06. Microorganisms 2022, 10, 2274. [Google Scholar] [CrossRef] [PubMed]
- Čižmárik, J.; Matel, I. Examination of the chemical composition of propolis 2. Isolation and identification of 4-hydroxy-3-methoxycinnamic acid (ferulic acid) from propolis. J. Apic. Res. 1973, 12, 52–54. [Google Scholar] [CrossRef]
- Cui, B.L.; Nakamura, M.; Kinjo, J. Chemical constituents of Astragali semen. Chem. Pharm. Bull. 1993, 41, 178–182. [Google Scholar] [CrossRef]
- Hussein, I.A.; Srivedavyasasri, R.; Atef, A.; Mohammad, A.E.I.; Ross, S.A. Chemical constituents from Silene schimperiana Boiss. belonging to Caryophyllaceae and their chemotaxonomic significance. Biochem. Syst. Ecol. 2020, 92, 104113. [Google Scholar] [CrossRef]
- Rios, M.Y.; Estrada-Soto, S.; Flores-Morales, V.; Aguilar, M.I. Chemical constituents from Flourensia resinosa SF Blake (Asteraceae). Biochem. Syst. Ecol. 2013, 51, 240–242. [Google Scholar] [CrossRef]
- Kojima, K.S.; Purevsuren, S.; Narantuya, S. Alkaloids from oxytropis myriophylla (PALL) DC. Sci. Pharm. 2001, 69, 383–388. [Google Scholar] [CrossRef]
- Kelly, S.E.; LaCour, T.G. A one pot procedure for the synthesis of α-hydroxyamides from the corresponding α-hydroxyacids. Synth. Commun. 1992, 22, 859–869. [Google Scholar] [CrossRef]
- Cao, L.; Ding, J.; Gao, M.; Wang, Z.; Li, J.; Wu, A. Novel and direct transformation of methyl ketones or carbinols to primary amides by employing aqueous ammonia. Org. Lett. 2009, 11, 3810–3813. [Google Scholar] [CrossRef]
- Liu, Y.J.; Liu, Y.; Zhang, K.Q. Xanthothone, a new nematicidal N-compound from Coprinus xanthothrix. Chem. Nat. Compd. 2008, 44, 203–205. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhu, S.; Wu, G. Chemical constituents of Vernonia parishii. Chem. Nat. Compd. 2020, 56, 134–136. [Google Scholar] [CrossRef]
- Moraes, G.J.; Flechtmann, C.H.W. Manual de Acarologia Acarologia Básica e Ácaros de Plantas Cultivadas no Brasil; Holos: Ribeirão Preto, Brasil, 2008; p. 308. [Google Scholar]
- Jeppson, L.R.; Keifer, H.H.; Baker, E.W. Mites Injurious to Economic Plants; University of California Press: Berkeley, CA, USA, 1975. [Google Scholar]
- Grbić, M.; Van Leeuwen, T.; Clark, R.M.; Rombauts, S.; Rouzé, P.; Grbić, V.; Osborne, E.J.; Dermauw, W.; Thi Ngoc, P.C.; Ortego, F.; et al. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 2011, 479, 487–492. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).