Self-Standing Carbon Fiber Electrodes Doped with Pd Nanoparticles as Electrocatalysts in Zinc–Air Batteries
Abstract
1. Introduction
2. Experimental
2.1. Electrocatalyst Preparation
2.2. Physicochemical Characterization
2.3. Electrochemical Characterization
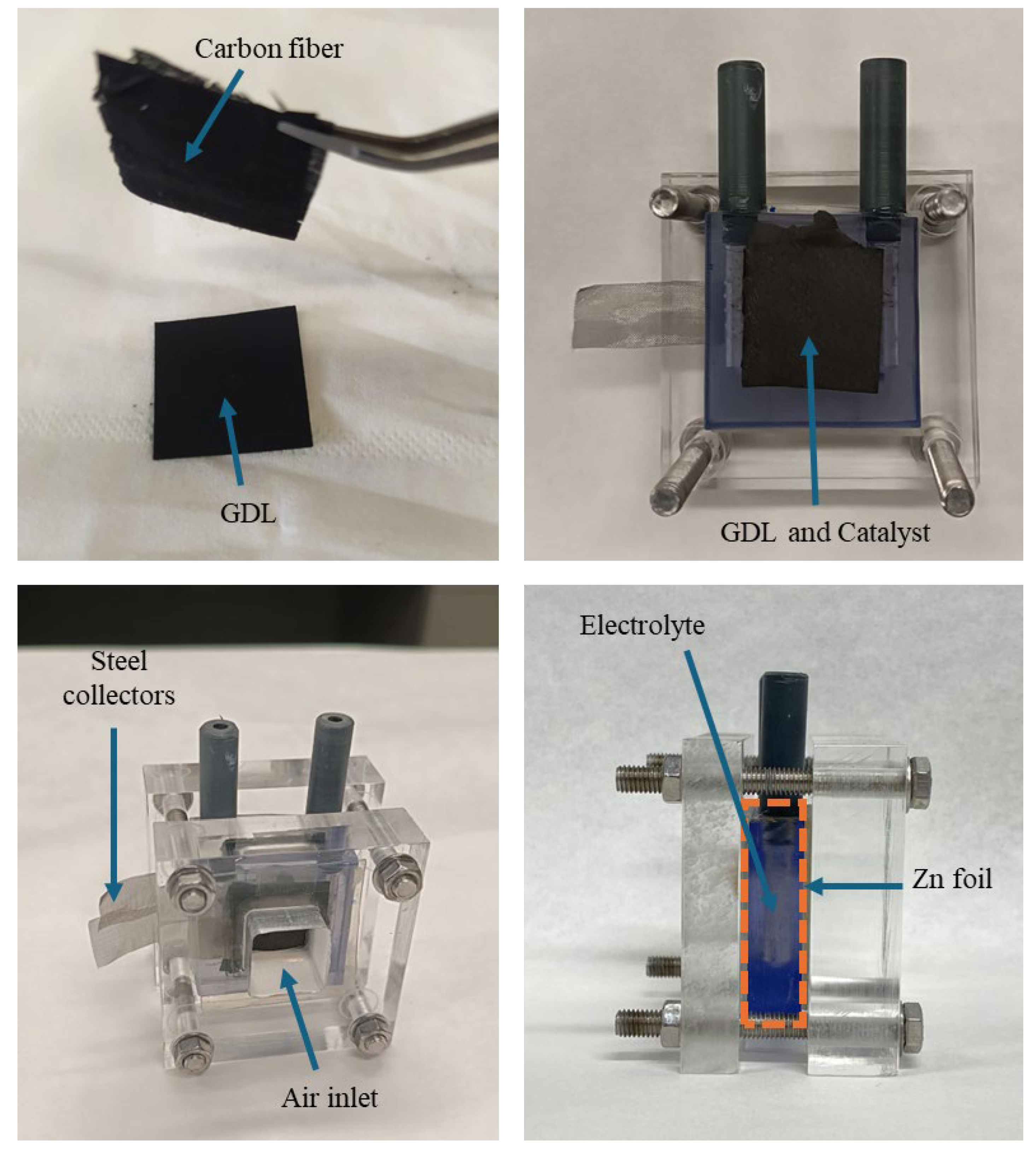
2.4. Zinc–Air Battery
3. Results and Discussion
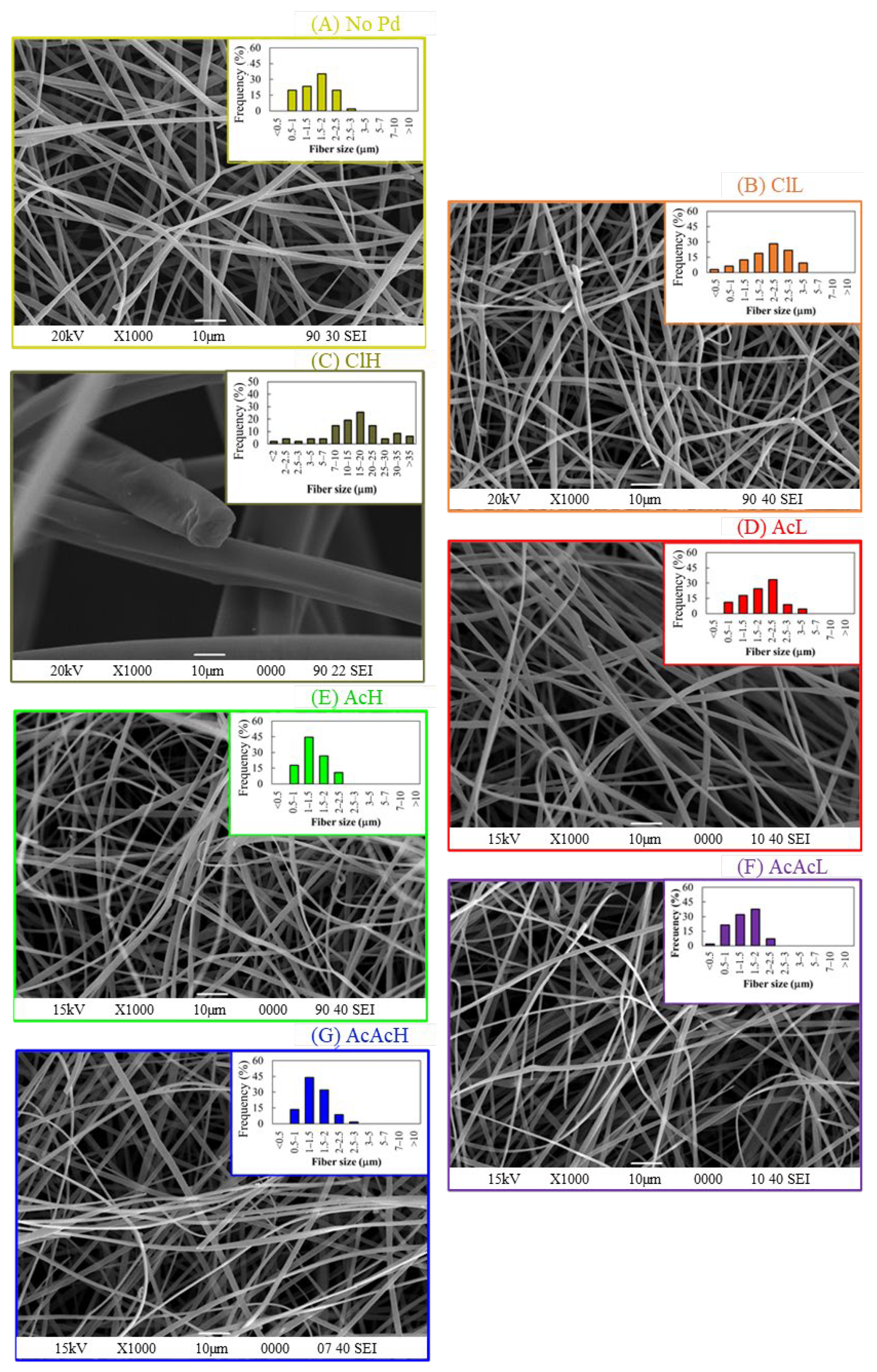
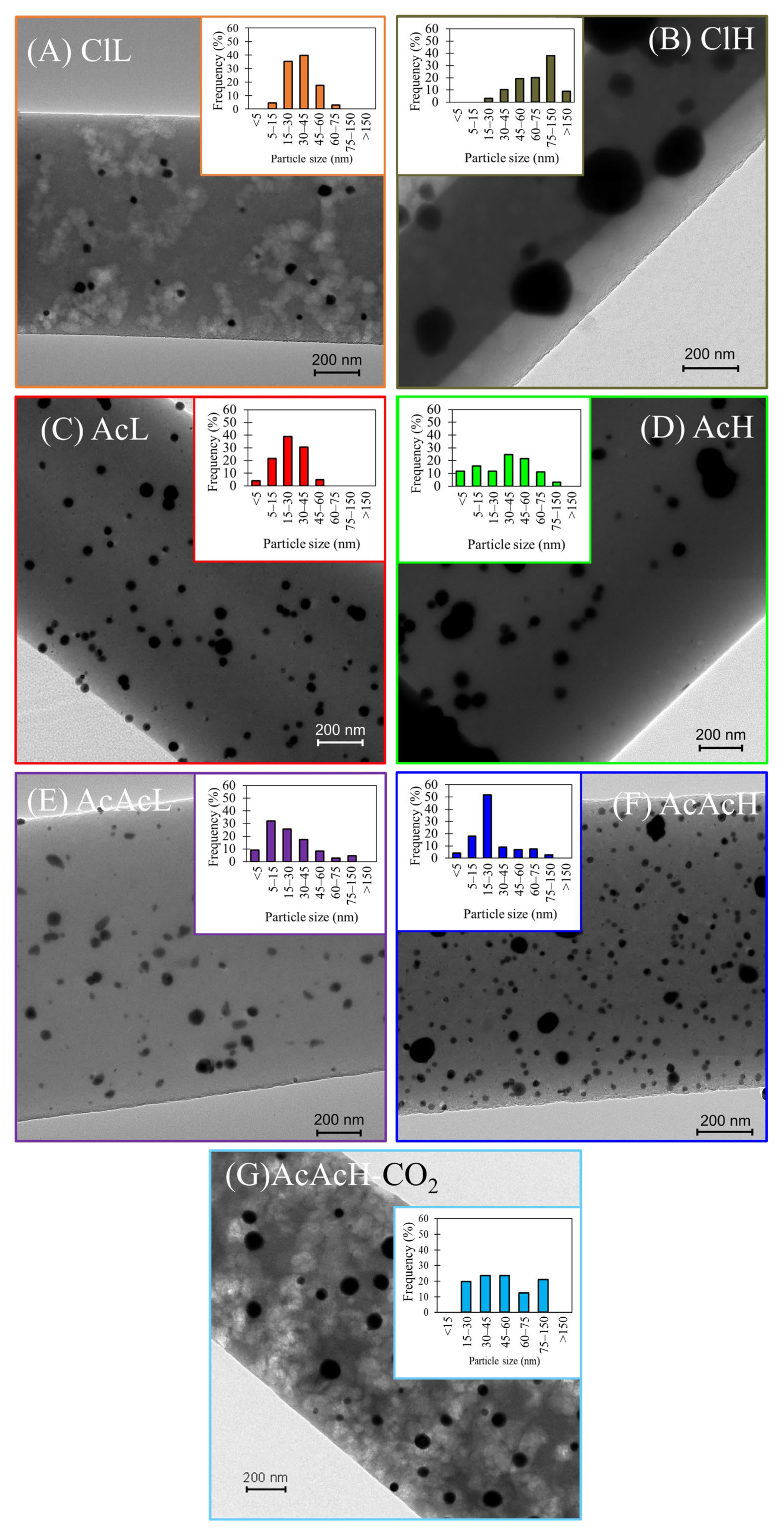
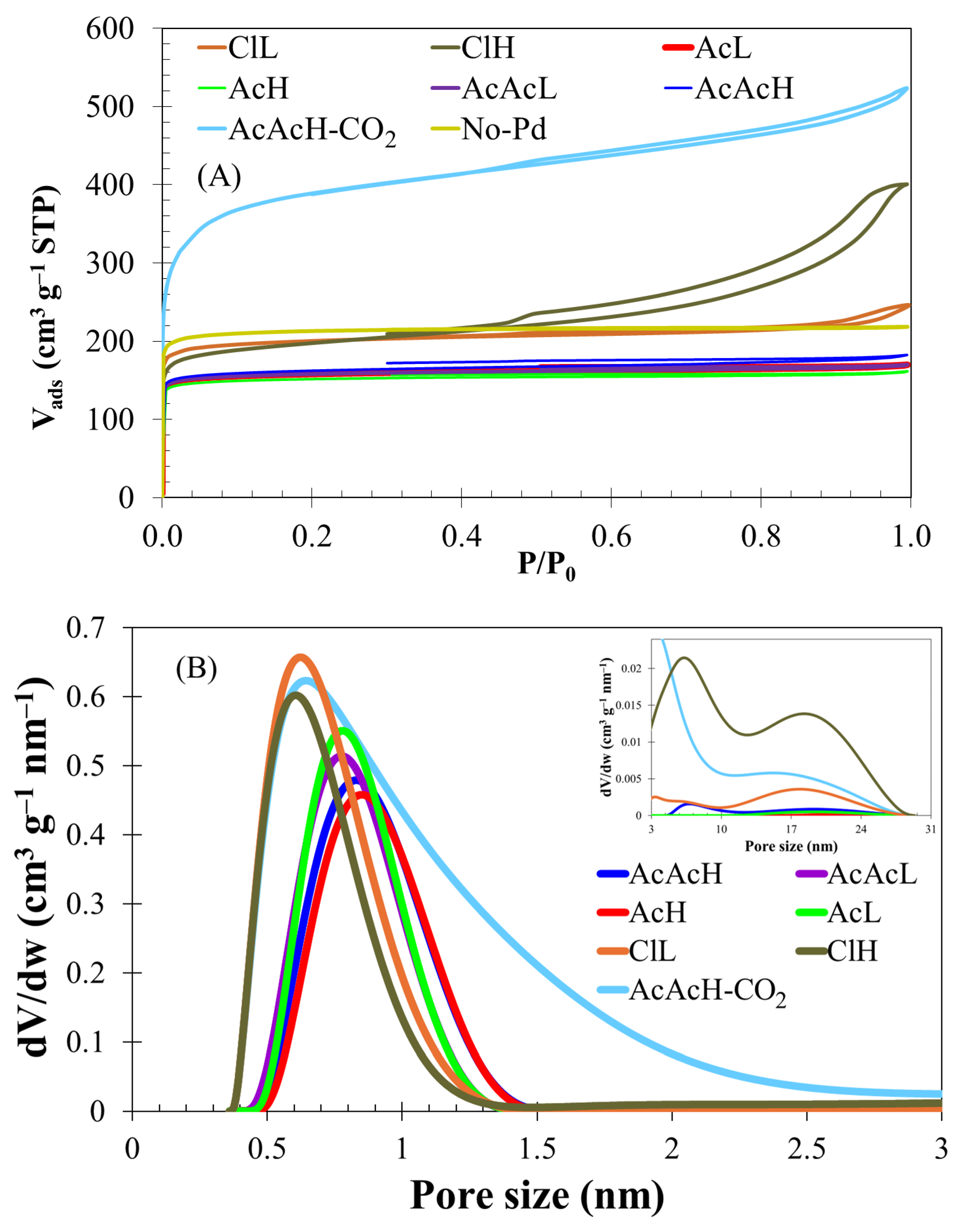
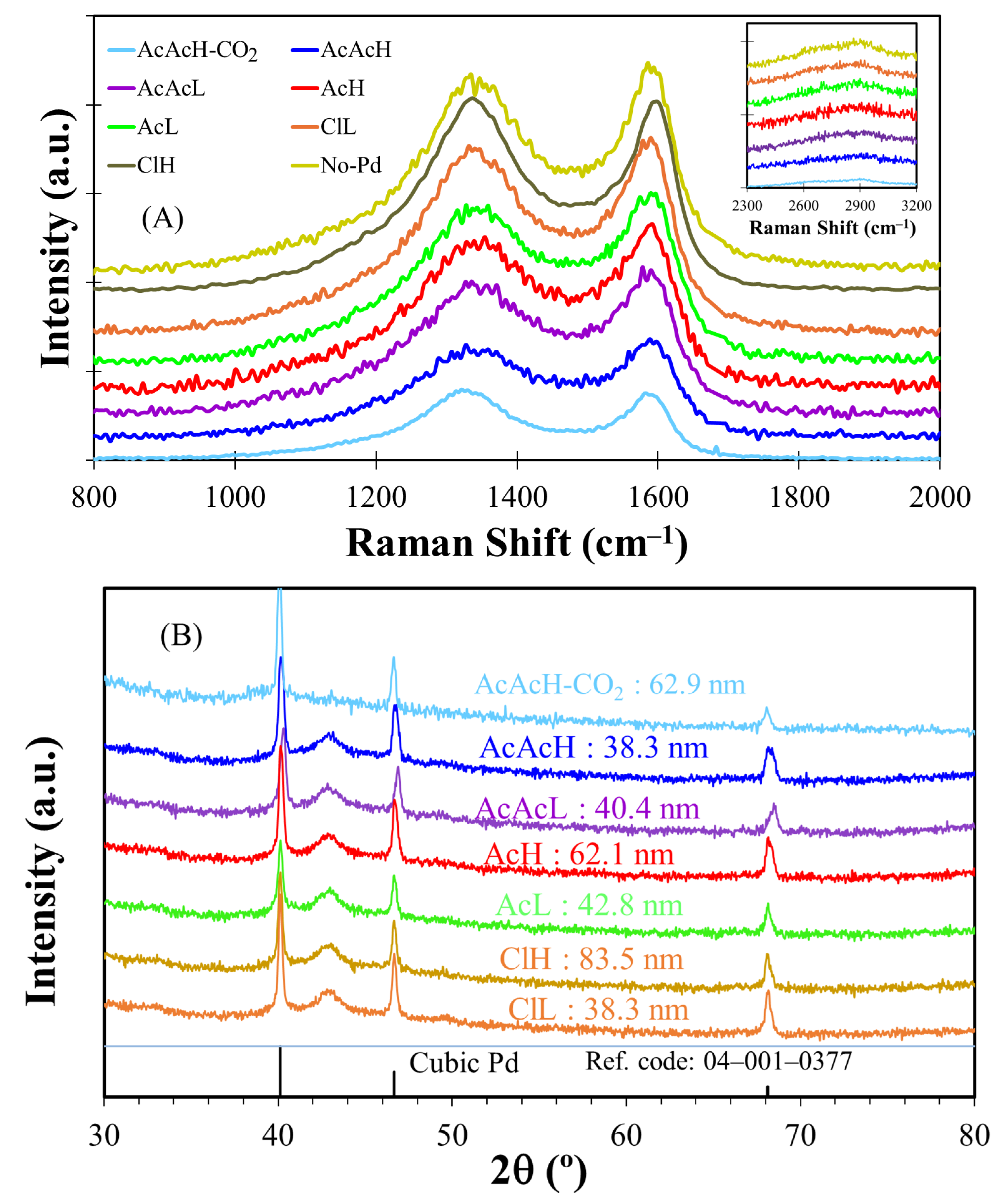
3.1. Carbon Fibers Preparation and Characterization
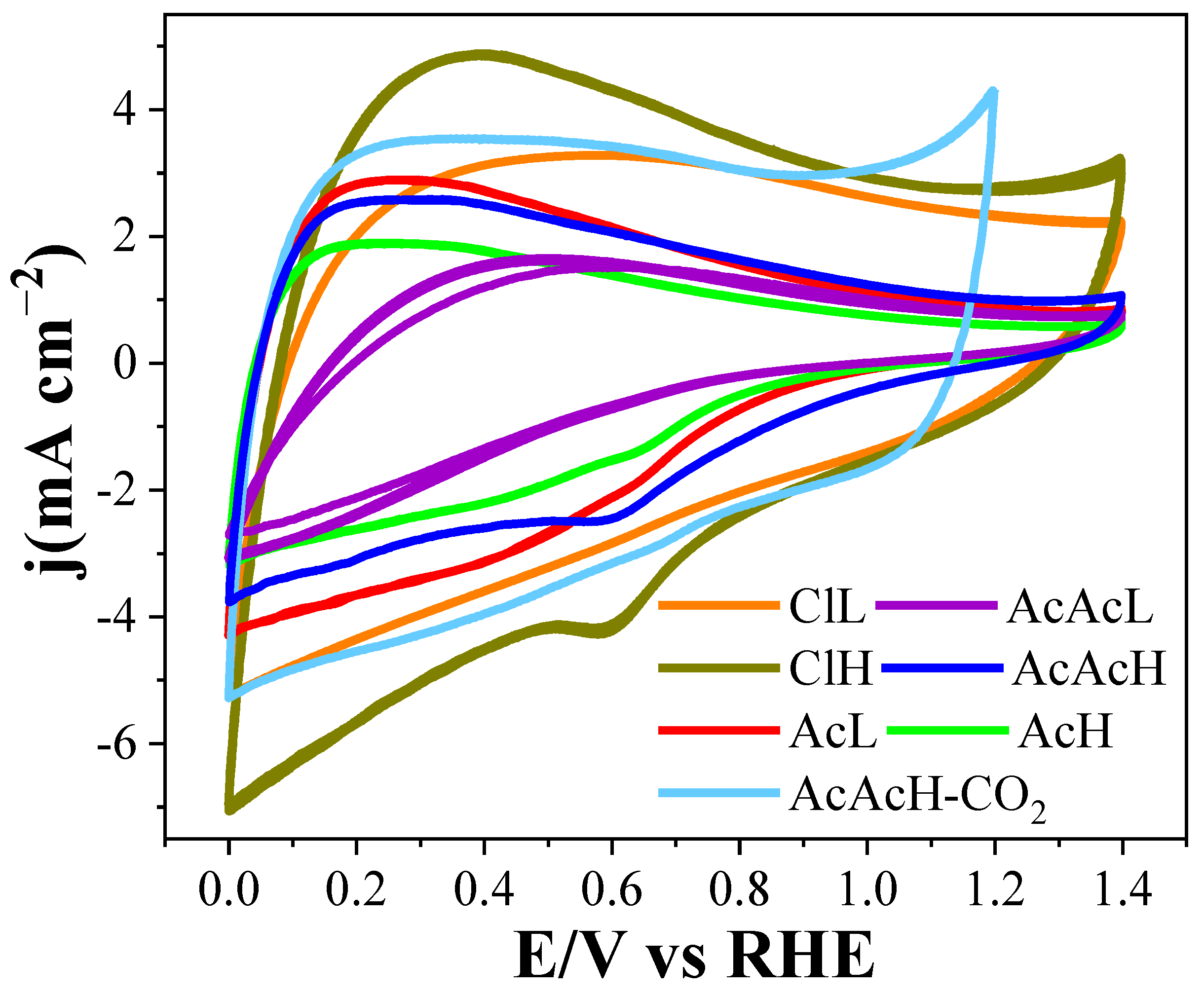
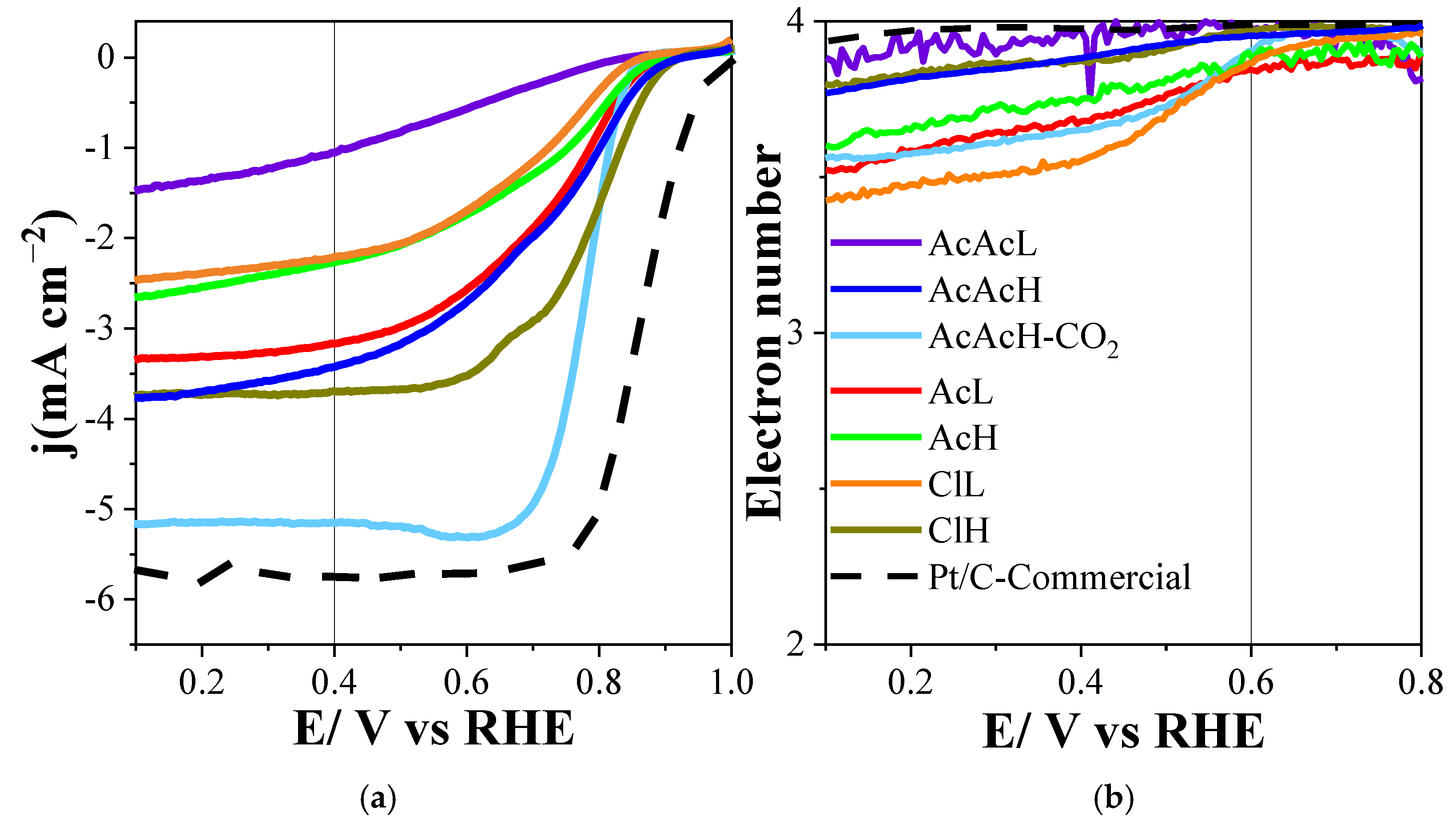
3.2. Electrochemical Characterization
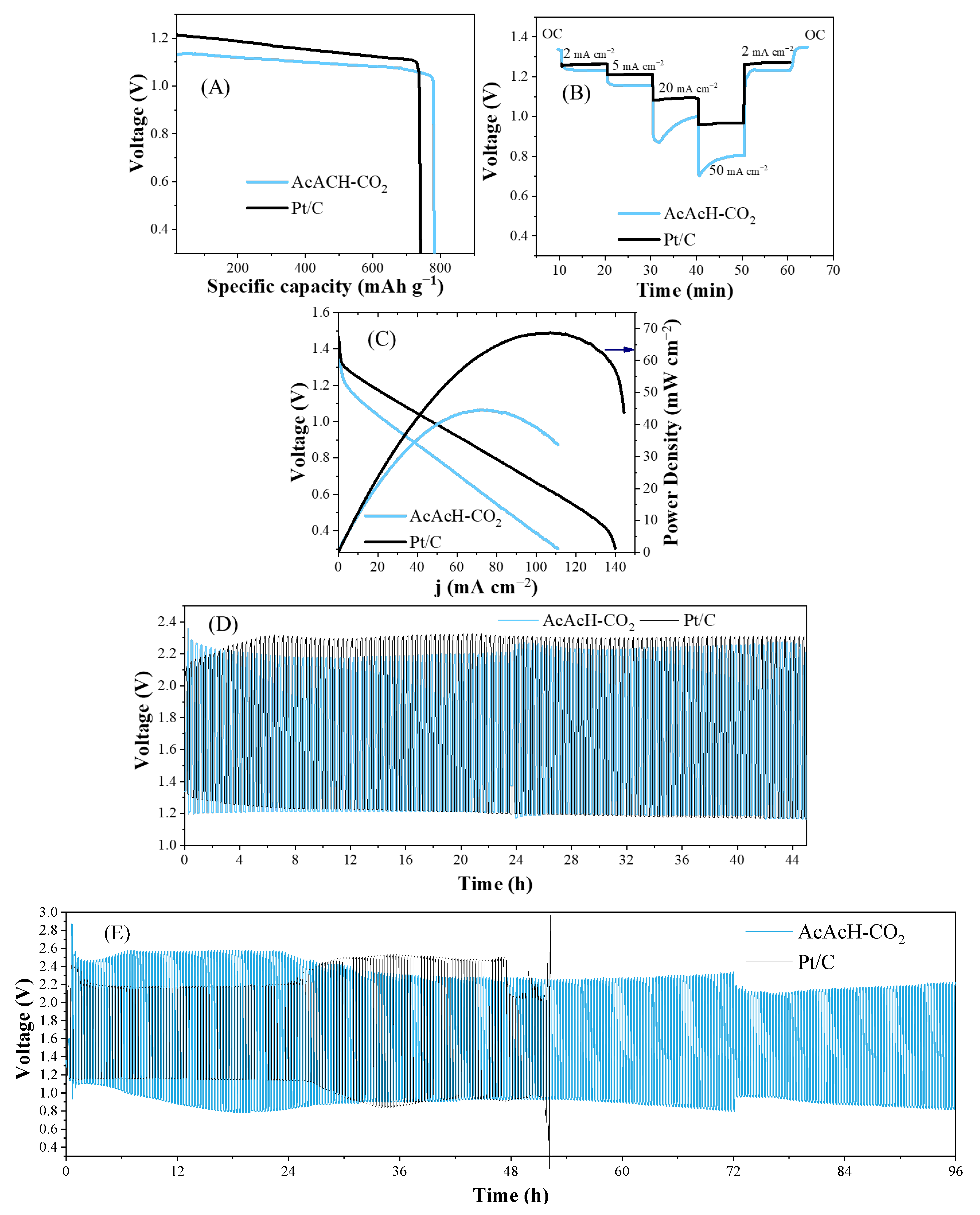
3.3. Zinc–Air Battery Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Saeed, G.; Zhu, L.; Hui, K.N.; Kim, N.H.; Lee, J.H. 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: A review. Chem. Eng. J. 2021, 403, 126352. [Google Scholar] [CrossRef]
- Frackowiak, E.; Béguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon N. Y. 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520. [Google Scholar] [CrossRef]
- Zhang, S.-J.; Yu, H.-Q.; Feng, H.-M. PVA-based activated carbon fibers with lotus root-like axially porous structure. Carbon N. Y. 2006, 44, 2059–2068. [Google Scholar] [CrossRef]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, Z.; Li, C.; Liu, M.; Jiang, L.; Zhou, Y.; Zhou, F.; Chen, S.; Jerrams, S.; Yu, J. A Highly Stretchable and Sensitive Strain Sensor Based on Dopamine Modified Electrospun SEBS Fibers and MWCNTs with Carboxylation. Adv. Electron. Mater. 2021, 7, 2100233. [Google Scholar] [CrossRef]
- Zhong, H.; Huang, J.; Wu, J.; Du, J. Electrospinning nanofibers to 1D, 2D, and 3D scaffolds and their biomedical applications. Nano Res. 2022, 15, 787–804. [Google Scholar] [CrossRef]
- Ruiz-Rosas, R.; Bedia, J.; Lallave, M.; Loscertales, I.G.; Barrero, A.; Rodríguez-Mirasol, J.; Cordero, T. The production of submicron diameter carbon fibers by the electrospinning of lignin. Carbon N. Y. 2010, 48, 696–705. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Lallave, M.; Bedia, J.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T.; Otero, J.C.; Marquez, M.; Barrero, A.; Loscertales, I.G. Filled and Hollow Carbon Nanofibers by Coaxial Electrospinning of Alcell Lignin without Binder Polymers. Adv. Mater. 2007, 19, 4292–4296. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Ruiz-Rosas, R.; María Rosas, J.; Morallón, E.; Cazorla-Amorós, D.; Rodríguez-Mirasol, J.; Cordero, T. Activation of electrospun lignin-based carbon fibers and their performance as self-standing supercapacitor electrodes. Sep. Purif. Technol. 2020, 241, 116724. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Berenguer, R.; Valero-Romero, M.J.; Rodríguez-Mirasol, J.; Cordero, T. Phosphorus functionalization for the rapid preparation of highly nanoporous submicron-diameter carbon fibers by electrospinning of lignin solutions. J. Mater. Chem. A 2018, 6, 1219–1233. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Ruiz-Rosas, R.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Controlling the Composition, Morphology, Porosity, and Surface Chemistry of Lignin-Based Electrospun Carbon Materials. Front. Mater. 2019, 6, 114. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Ruiz-Rosas, R.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Phosphorus containing carbon (submicron)fibers as efficient acid catalysts. Catal. Today 2022, 383, 308–319. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Rosas, J.M.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. Highly porous and conductive functional carbon fibers from electrospun phosphorus-containing lignin fibers. Carbon N. Y. 2022, 200, 134–148. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, J.; Jiang, L.; Lubineau, G.; Payne, S.A.; Gutschmidt, D. Lignin-based carbon fibers: Carbon nanotube decoration and superior thermal stability. Carbon N. Y. 2014, 80, 91–102. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Cordero-Lanzac, T.; Berenguer, R.; Morallón, E.; Cazorla-Amorós, D.; Rodríguez-Mirasol, J.; Cordero, T. Lignin-derived Pt supported carbon (submicron)fiber electrocatalysts for alcohol electro-oxidation. Appl. Catal. B Environ. 2017, 211, 18–30. [Google Scholar] [CrossRef]
- Valero-Romero, M.J.; García-Mateos, F.J.; Kapteijn, F.; Rodríguez-Mirasol, J.; Cordero, T. Fischer-Tropsch synthesis over lignin-derived cobalt-containing porous carbon fiber catalysts. Appl. Catal. B Environ. 2023, 321, 122078. [Google Scholar] [CrossRef]
- Nie, G.; Zhao, X.; Luan, Y.; Jiang, J.; Kou, Z.; Wang, J. Key issues facing electrospun carbon nanofibers in energy applications: On-going approaches and challenges. Nanoscale 2020, 12, 13225–13248. [Google Scholar] [CrossRef]
- Hao, S.; Sheng, X.; Xie, F.; Sun, M.; Diao, F.; Wang, Y. Electrospun carbon nanofibers embedded with heterostructured NiFe2O4/Fe0.64Ni0.36 nanoparticles as an anode for high-performance lithium-ion battery. J. Energy Storage 2024, 80, 110412. [Google Scholar] [CrossRef]
- Jaimes-Paez, C.D.; García-Mateos, F.J.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T.; Morallón, E.; Cazorla-Amorós, D. Sustainable Synthesis of Metal-Doped Lignin-Derived Electrospun Carbon Fibers for the Development of ORR Electrocatalysts. Nanomaterials 2023, 13, 2921. [Google Scholar] [CrossRef]
- Gang, B.G.; Kwon, S. Design of an energy management technique for high endurance unmanned aerial vehicles powered by fuel and solar cell systems. Int. J. Hydrog. Energy 2018, 43, 9787–9796. [Google Scholar] [CrossRef]
- Ahmadi, M.H.; Mohammadi, A.; Pourfayaz, F.; Mehrpooya, M.; Bidi, M.; Valero, A.; Uson, S. Thermodynamic analysis and optimization of a waste heat recovery system for proton exchange membrane fuel cell using transcritical carbon dioxide cycle and cold energy of liquefied natural gas. J. Nat. Gas Sci. Eng. 2016, 34, 428–438. [Google Scholar] [CrossRef]
- Tang, C.; Wang, B.; Wang, H.F.; Zhang, Q. Defect Engineering toward Atomic Co–Nx–C in Hierarchical Graphene for Rechargeable Flexible Solid Zn-Air Batteries. Adv. Mater. 2017, 29, 1703185. [Google Scholar] [CrossRef]
- Li, Y.; Gong, M.; Liang, Y.; Feng, J.; Kim, J.-E.; Wang, H.; Hong, G.; Zhang, B.; Dai, H. Advanced zinc-air batteries based on high-performance hybrid electrocatalysts. Nat. Commun. 2013, 4, 1805. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, Y.; Hou, H.; You, T. Simultaneous electrochemical determination of dopamine, uric acid and ascorbic acid using palladium nanoparticle-loaded carbon nanofibers modified electrode. Biosens. Bioelectron. 2008, 24, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, H. Recent advances in zinc–air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Han, X.; Li, L.; Chou, S.; Ji, D.; Huang, H.; Du, Y.; Liu, J.; Ramakrishna, S. Electronic and Defective Engineering of Electrospun CaMnO3 Nanotubes for Enhanced Oxygen Electrocatalysis in Rechargeable Zinc–Air Batteries. Adv. Energy Mater. 2018, 8, 1800612. [Google Scholar] [CrossRef]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically Rechargeable Zinc-Air Batteries: Progress, Challenges, and Perspectives. Adv. Mater. 2017, 29, 1604685. [Google Scholar] [CrossRef]
- Zheng, H.T.; Li, Y.; Chen, S.; Shen, P.K. Effect of support on the activity of Pd electrocatalyst for ethanol oxidation. J. Power Sources 2006, 163, 371–375. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, C.; Bai, S.; Jing, L.; Zhao, Z.; Liu, L. The performance of Pd-rGO electro-deposited PVDF/carbon fiber cloth composite membrane in MBR/MFC coupled system. Chem. Eng. J. 2019, 365, 317–324. [Google Scholar] [CrossRef]
- Yang, F.; Cheng, K.; Wu, T.; Zhang, Y.; Yin, J.; Wang, G.; Cao, D. Au–Pd nanoparticles supported on carbon fiber cloth as the electrocatalyst for H2O2 electroreduction in acid medium. J. Power Sources 2013, 233, 252–258. [Google Scholar] [CrossRef]
- Tartakovsky, B.; Manuel, M.-F.; Neburchilov, V.; Wang, H.; Guiot, S.R. Biocatalyzed hydrogen production in a continuous flow microbial fuel cell with a gas phase cathode. J. Power Sources 2008, 182, 291–297. [Google Scholar] [CrossRef]
- Huang, J.; Wang, D.; Hou, H.; You, T. Electrospun Palladium Nanoparticle-Loaded Carbon Nanofibers and Their Electrocatalytic Activities towards Hydrogen Peroxide and NADH. Adv. Funct. Mater. 2008, 18, 441–448. [Google Scholar] [CrossRef]
- Ji, D.; Fan, L.; Li, L.; Peng, S.; Yu, D.; Song, J.; Ramakrishna, S.; Guo, S. Atomically Transition Metals on Self-Supported Porous Carbon Flake Arrays as Binder-Free Air Cathode for Wearable Zinc−Air Batteries. Adv. Mater. 2019, 31, 1808267. [Google Scholar] [CrossRef] [PubMed]
- Supchocksoonthorn, P.; Hrimchum, N.; Budsrirak, T.; Intaraprasit, S.; Thongsai, N.; Aussawasathien, D. Lignin based carbon fiber fabrics with hybrid doping approach as self-standing electrodes for supercapacitors. Electrochim. Acta 2023, 437, 141523. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. 2D-NLDFT adsorption models for carbon slit-shaped pores with surface energetical heterogeneity and geometrical corrugation. Carbon N. Y. 2013, 55, 70–80. [Google Scholar] [CrossRef]
- Calabuig-Mompó, S.; Cazorla-Amorós, D.; Morallón, E. Electrocatalysts based on graphene oxide and its buckypaper for enhanced Zn-air battery performance. J. Electroanal. Chem. 2024, 955, 118069. [Google Scholar] [CrossRef]
- Selishchev, D.S.; Kolobov, N.S.; Bukhtiyarov, A.V.; Gerasimov, E.Y.; Gubanov, A.I.; Kozlov, D.V. Deposition of Pd nanoparticles on TiO2 using a Pd(acac)2 precursor for photocatalytic oxidation of CO under UV-LED irradiation. Appl. Catal. B Environ. 2018, 235, 214–224. [Google Scholar] [CrossRef]
- Cao, J.; Xiao, G.; Xu, X.; Shen, D.; Jin, B. Study on carbonization of lignin by TG-FTIR and high-temperature carbonization reactor. Fuel Process. Technol. 2013, 106, 41–47. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Fleisch, T.H.; Zajac, G.W.; Schreiner, J.O.; Mains, G.J. An XPS study of the UV photoreduction of transition and noble metal oxides. Appl. Surf. Sci. 1986, 26, 488–497. [Google Scholar] [CrossRef]
- Schuepfer, D.B.; Badaczewski, F.; Guerra-Castro, J.M.; Hofmann, D.M.; Heiliger, C.; Smarsly, B.; Klar, P.J. Assessing the structural properties of graphitic and non-graphitic carbons by Raman spectroscopy. Carbon N. Y. 2020, 161, 359–372. [Google Scholar] [CrossRef]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martínez-Alonso, A.; Tascón, J.M.D. Raman microprobe studies on carbon materials. Carbon N. Y. 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Xu, L.; Wu, X.-C.; Zhu, J.-J. Green preparation and catalytic application of Pd nanoparticles. Nanotechnology 2008, 19, 305603. [Google Scholar] [CrossRef]
- Khezri, R.; Motlagh, S.R.; Etesami, M.; Pakawanit, P.; Olaru, S.; Somwangthanaroj, A.; Kheawhom, S. Balancing current density and electrolyte flow for improved zinc-air battery cyclability. Appl. Energy 2024, 376, 124239. [Google Scholar] [CrossRef]
- Lu, Q.; Zou, X.; Bu, Y. Introduction to Zinc–Air Batteries. In Zinc-Air Batteries; Wiley: Weinheim, Germany, 2022; pp. 1–34. ISBN 9783527837939. [Google Scholar]
- Chen, J.; Qiu, C.; Zhang, L.; Wang, B.; Zhao, P.; Zhao, Y.; Wang, H.; Yang, G.; Sun, A.; Fan, J.; et al. Wood-derived Fe cluster-reinforced asymmetric single-atom catalysts and weather-resistant organohydrogel for wide-temperature flexible Zn–air batteries. Energy Environ. Sci. 2024, 17, 4746–4757. [Google Scholar] [CrossRef]
- Wang, H.-F.; Tang, C.; Wang, B.; Li, B.-Q.; Cui, X.; Zhang, Q. Defect-rich carbon fiber electrocatalysts with porous graphene skin for flexible solid-state zinc–air batteries. Energy Storage Mater. 2018, 15, 124–130. [Google Scholar] [CrossRef]
- Li, Z.; Han, W.; Jia, P.; Li, X.; Jiang, Y.; Ding, Q. Co3O4 Nanoneedle Array Grown on Carbon Fiber Paper for Air Cathodes towards Flexible and Rechargeable Zn–Air Batteries. Nanomaterials 2021, 11, 3321. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Yan, F.; Zhu, C.; Geng, B.; Chen, Y.; Chou, S. Cobalt-Encapsulated Nitrogen-Doped Carbon Nanotube Arrays for Flexible Zinc–Air Batteries. Small Methods 2020, 4, 1900571. [Google Scholar] [CrossRef]
| Sample | Yield (wt%) | Diameter Carbon Fiber | Pd Particle Size | ||
|---|---|---|---|---|---|
| Stabilization | Carbonization | Total | (µm) | (nm) | |
| No Pd | 71 | 37 | 27 | 1.6 ± 0.2 | - |
| ClL | 65 | 36 | 23 | 1.4 ± 0.3 | 34.5 |
| ClH | 55 | 30 | 16 | 18.0 ± 0.8 | 85.4 |
| AcL | 50 | 48 | 24 | 1.6 ± 0.4 | 37.8 |
| AcH | 52 | 45 | 24 | 1.6 ± 0.2 | 59.7 |
| AcAcL | 43 | 42 | 18 | 1.4 ± 0.2 | 24.1 |
| AcAcH | 37 | 53 | 20 | 1.4 ± 0.1 | 27.2 |
| AcAcH-12 h | 61 | 48 | 29 | 2.1 ± 0.3 | 75.4 |
| AcAcH-CO2 | 37 | 36 | 13 | 1.3 ± 0.1 | 53.3 |
| Sample | Ads-Des N2 | XPS (wt%) | Pd Content | ||||
|---|---|---|---|---|---|---|---|
| ABET (m2/g) | Vmeso (cm3/g) | Vmicro (cm3/g) | C | O | Pd | (wt%) | |
| No Pd | 865 | <0.01 | 0.34 | 95.0 | 4.5 | - | - |
| ClL | 795 | 0.06 | 0.31 | 88.3 | 7.2 | 4.5 | 1.6 |
| ClH | 750 | 0.38 | 0.22 | 74.1 | 12.9 | 13.0 | 6.2 |
| AcL | 640 | 0.02 | 0.24 | 82.0 | 9.8 | 8.2 | 2.9 |
| AcH | 615 | 0.01 | 0.23 | 78.2 | 10.6 | 11.2 | 5.9 |
| AcAcL | 640 | 0.01 | 0.24 | 92.0 | 6.5 | 1.5 | 2.5 |
| AcAcH | 655 | 0.03 | 0.25 | 87.4 | 8.4 | 4.2 | 5.5 |
| AcAcH-12 h | 595 | 0.02 | 0.23 | 91.7 | 7.4 | 0.9 | 3.6 |
| AcAcH-CO2 | 1460 | 0.12 | 0.67 | 88.7 | 8.2 | 3.1 | 10.6 |
| Electrocatalyst | Eonset (V) (at j = −0.1 mA cm−2) | n (at 0.6 V) | j (mA cm−2) (at 0.4 V) | Half-Wave Potential (V) |
|---|---|---|---|---|
| ClL | 0.84 | 3.8 | −2.3 | 0.70 |
| ClH | 0.91 | 4 | −3.7 | 0.78 |
| AcL | 0.87 | 3.8 | −3.2 | 0.73 |
| AcH | 0.87 | 3.8 | −2.3 | 0.73 |
| AcAcL | 0.79 | 4 | −1 | 0.63 |
| AcAcH | 0.89 | 3.9 | −3.5 | 0.74 |
| AcAcH-CO2 | 0.86 | 3.9 | −5.2 | 0.78 |
| Pt/C | 0.98 | 3.9 | −5.7 | 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaimes-Paez, C.D.; García-Rollán, M.; García-Mateos, F.J.; Ruiz-Rosas, R.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T.; Morallón, E.; Cazorla-Amorós, D. Self-Standing Carbon Fiber Electrodes Doped with Pd Nanoparticles as Electrocatalysts in Zinc–Air Batteries. Molecules 2025, 30, 2487. https://doi.org/10.3390/molecules30122487
Jaimes-Paez CD, García-Rollán M, García-Mateos FJ, Ruiz-Rosas R, Rosas JM, Rodríguez-Mirasol J, Cordero T, Morallón E, Cazorla-Amorós D. Self-Standing Carbon Fiber Electrodes Doped with Pd Nanoparticles as Electrocatalysts in Zinc–Air Batteries. Molecules. 2025; 30(12):2487. https://doi.org/10.3390/molecules30122487
Chicago/Turabian StyleJaimes-Paez, Cristian Daniel, Miguel García-Rollán, Francisco José García-Mateos, Ramiro Ruiz-Rosas, Juana M. Rosas, José Rodríguez-Mirasol, Tomás Cordero, Emilia Morallón, and Diego Cazorla-Amorós. 2025. "Self-Standing Carbon Fiber Electrodes Doped with Pd Nanoparticles as Electrocatalysts in Zinc–Air Batteries" Molecules 30, no. 12: 2487. https://doi.org/10.3390/molecules30122487
APA StyleJaimes-Paez, C. D., García-Rollán, M., García-Mateos, F. J., Ruiz-Rosas, R., Rosas, J. M., Rodríguez-Mirasol, J., Cordero, T., Morallón, E., & Cazorla-Amorós, D. (2025). Self-Standing Carbon Fiber Electrodes Doped with Pd Nanoparticles as Electrocatalysts in Zinc–Air Batteries. Molecules, 30(12), 2487. https://doi.org/10.3390/molecules30122487













