Efficient Analytical Pretreatment of Cr(VI) in Ethylene Wastewater by Grafting g-C3N4 Material Based on Coupling Agent-Modified Basalt Matrix (Basalt–MTES/g-C3N4)
Abstract
1. Introduction
2. Results and Discussion
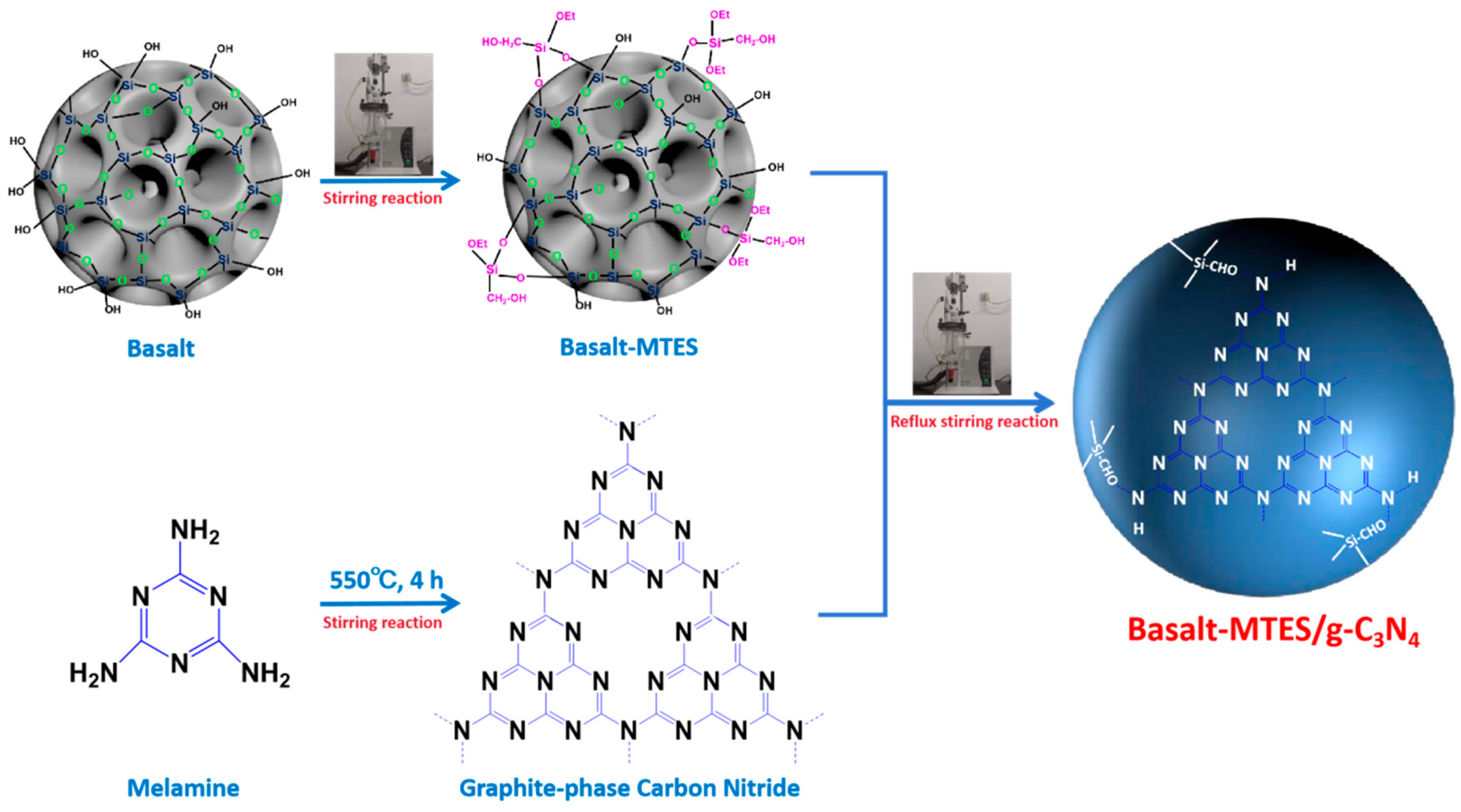
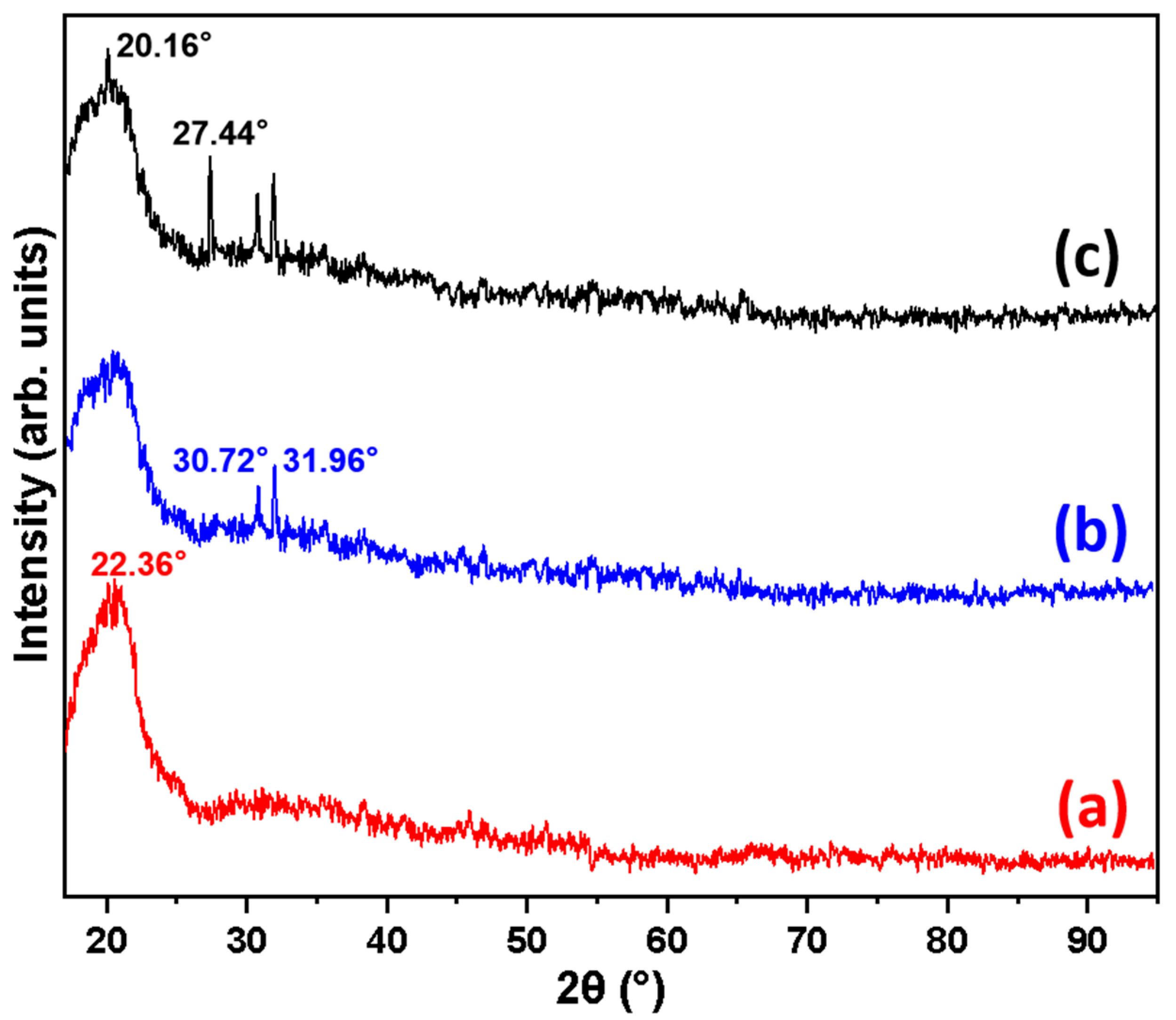
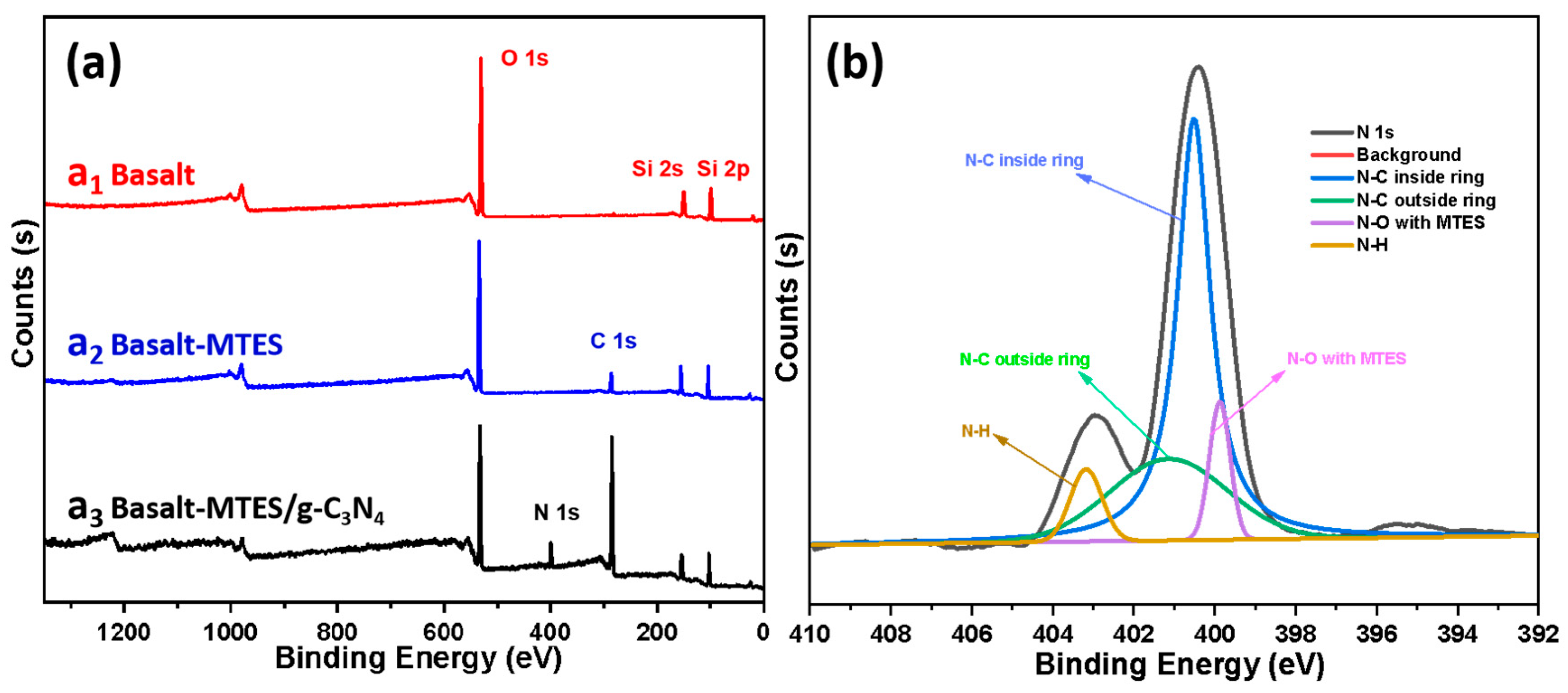
2.1. Characterization of Basalt, Basalt–MTES, and Basalt–MTES/g-C3N4
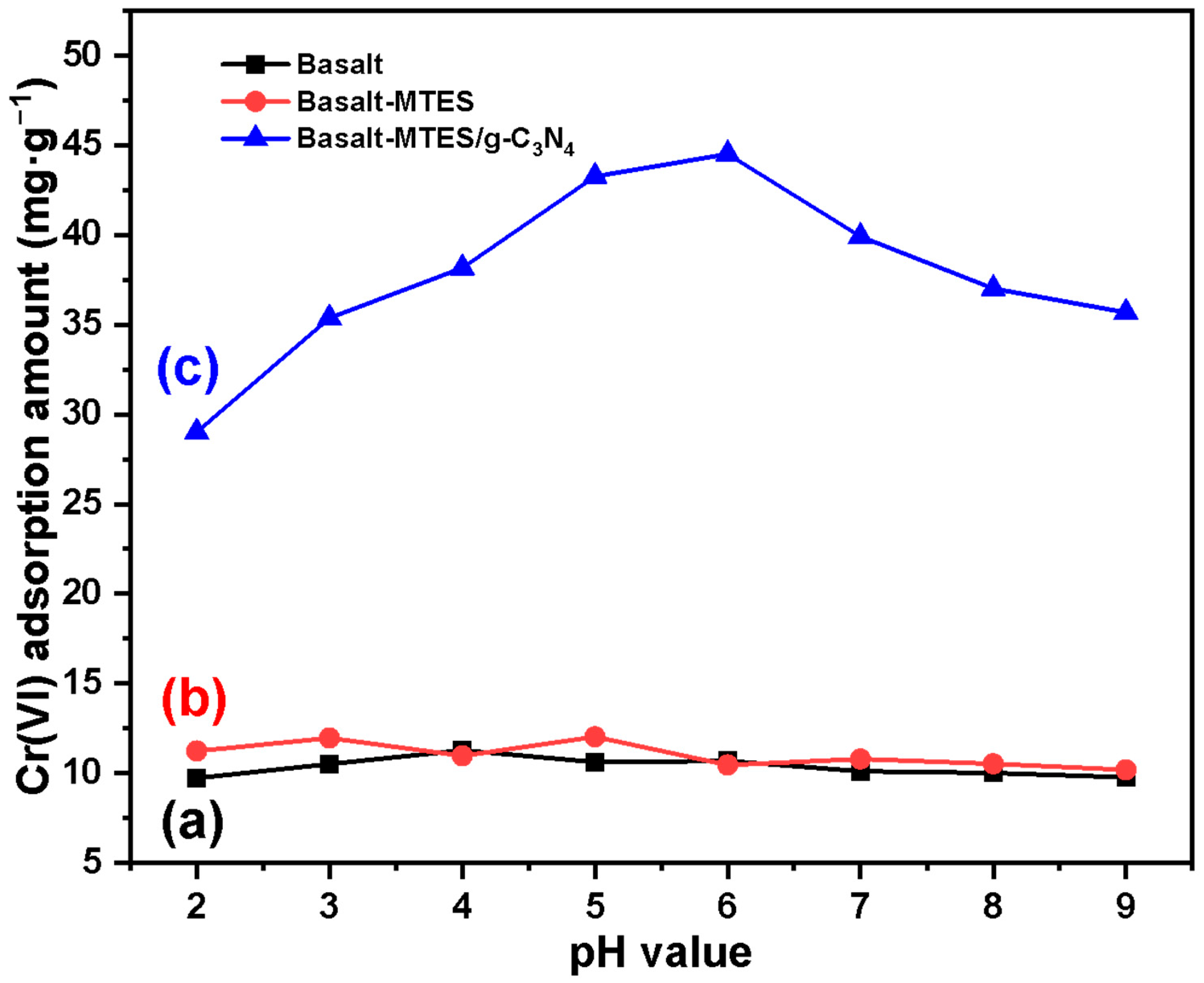
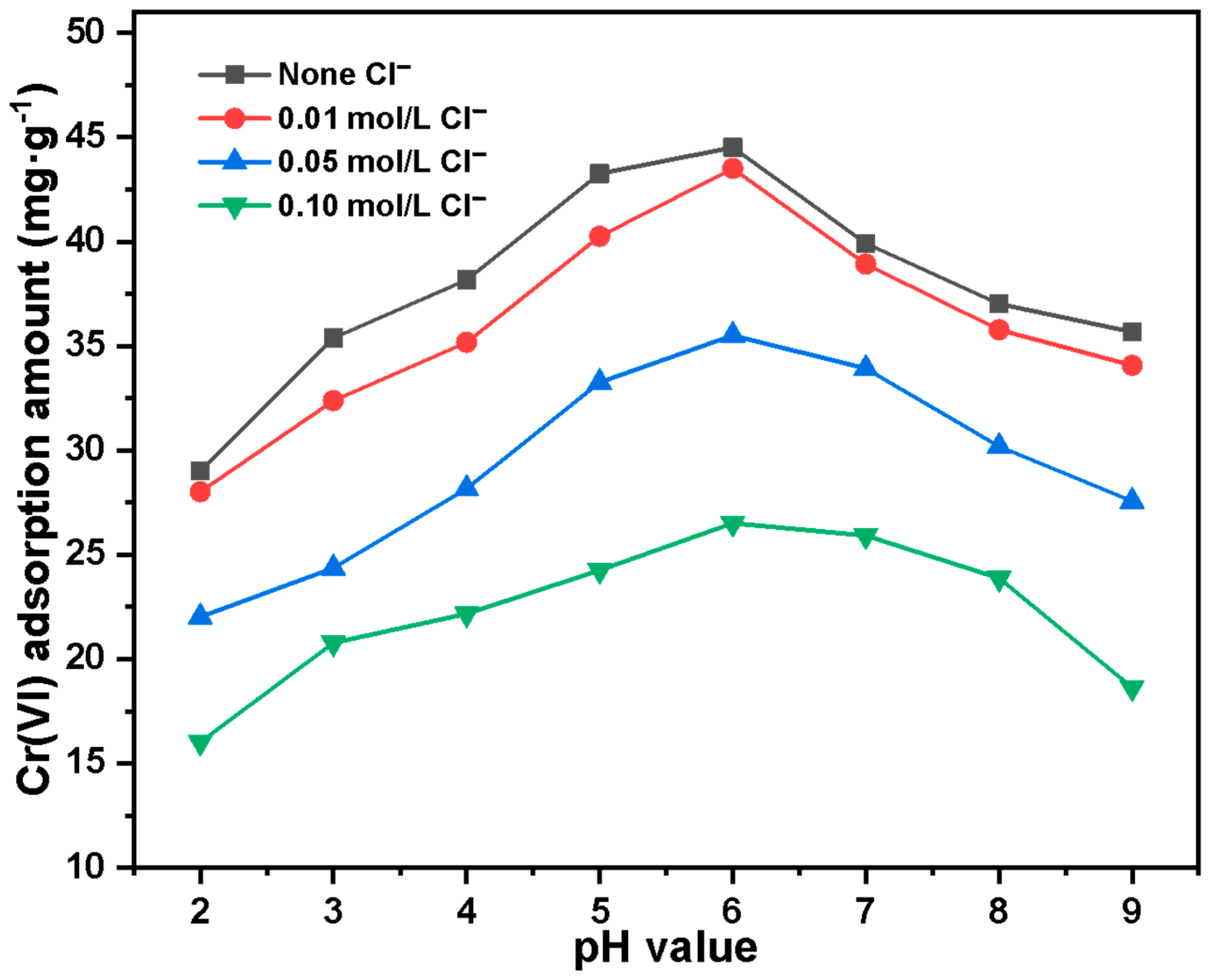
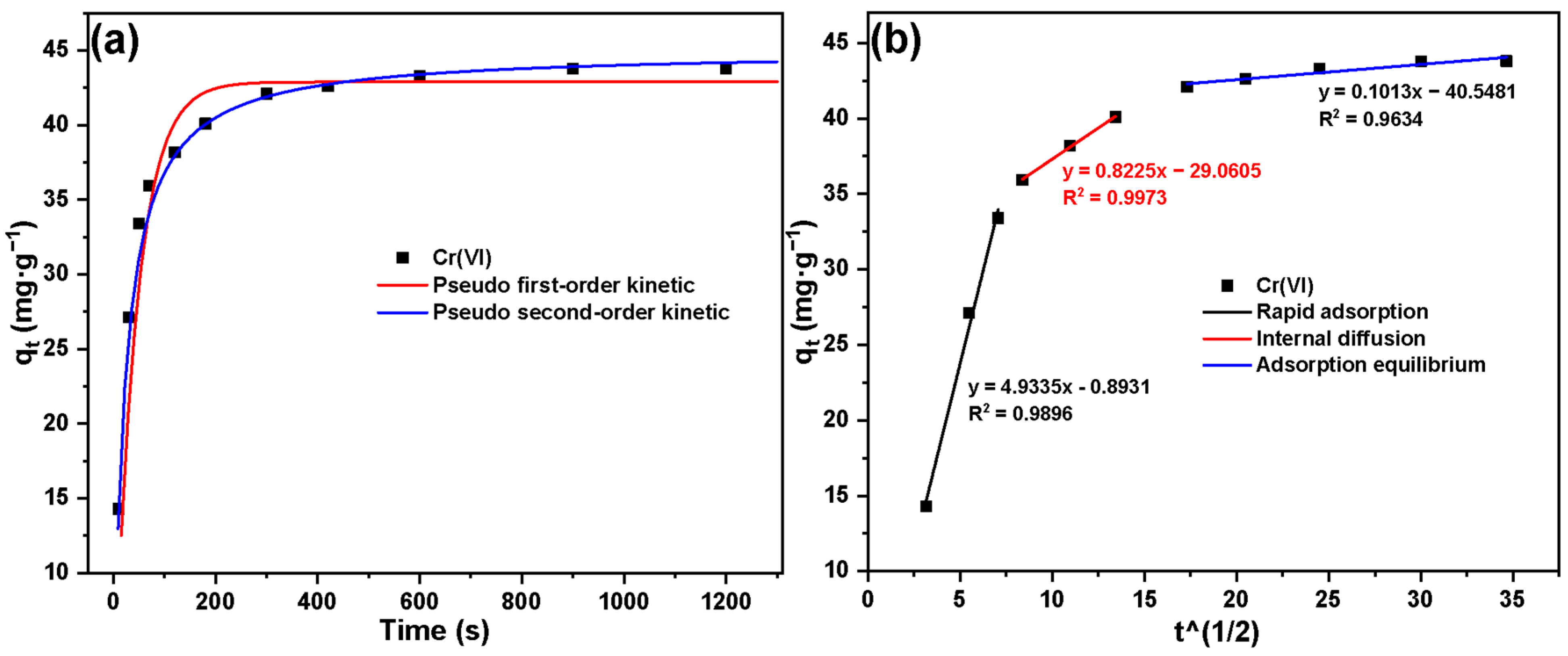
2.2. Adsorption Experiment Results of Cr(VI) by Basalt–MTES/g-C3N4
2.3. Analytical Pretreatment Experimental Result of Cr(VI) by Basalt–MTES/g-C3N4
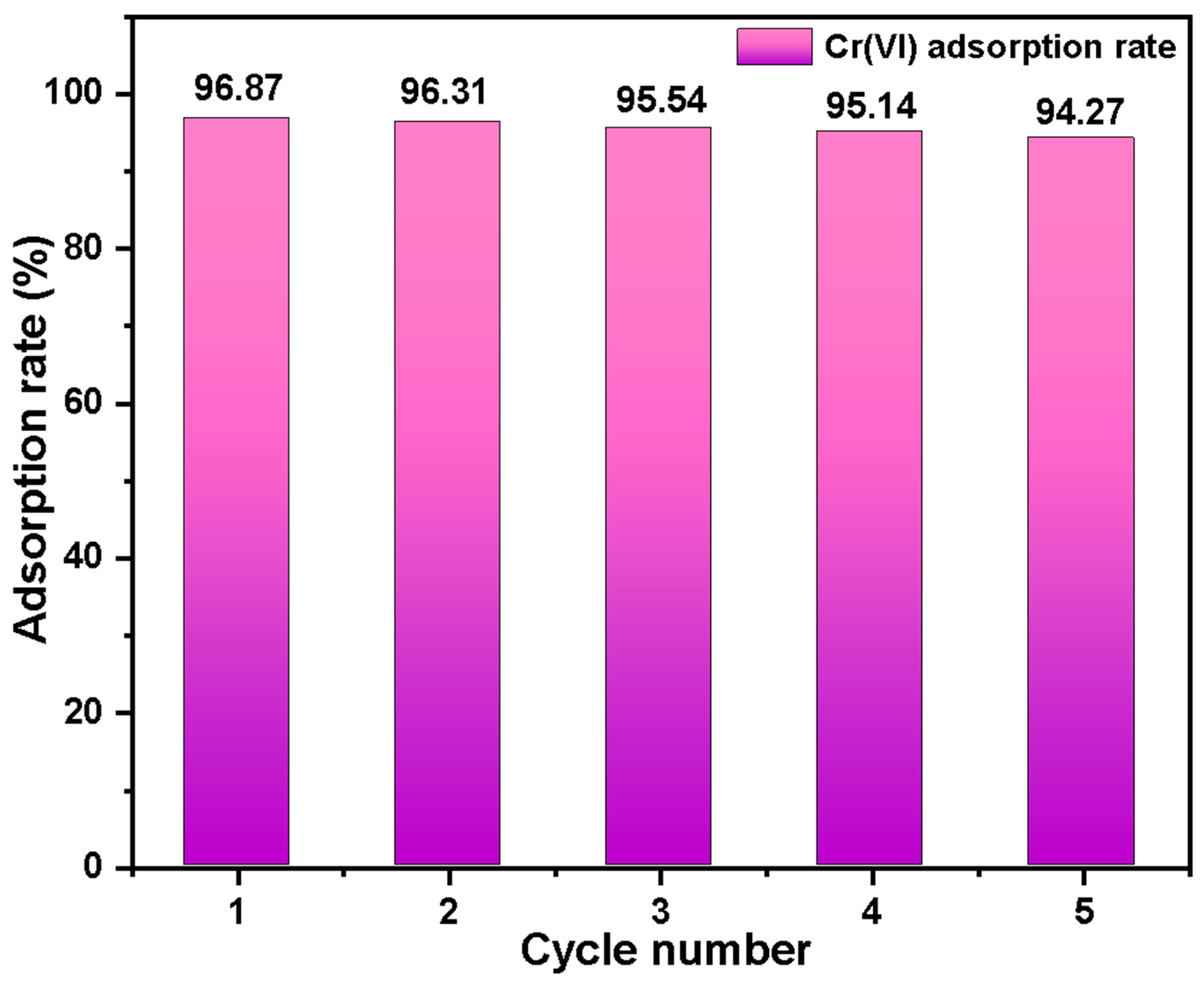
2.4. The Result of the Cyclic Regeneration Experiment
3. Materials and Methods
3.1. Materials
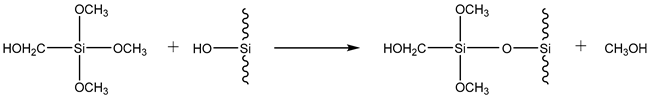
3.2. Preparation of Basalt Matrix-Grafted Graphitic Carbon Nitride Material (Basalt–MTES/g-C3N4)
3.2.1. Pretreatment of Basalt–MTES
3.2.2. Preparation of Basalt–MTES/g-C3N4
3.3. Characterization of Basalt–MTES/g-C3N4
3.4. Calculation Method of Adsorption Experiment
3.5. Adsorption Experiment of Cr(VI) on Basalt–MTES/g-C3N4
3.6. Analytical Pretreatment Experiment of Cr(VI)
3.7. Recycling and Regeneration Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Zhang, S.; Zhou, J.; Wang, Z. Toward carbon-neutral ethylene production: Assessment of the application potential of bio-ethylene production pathways in China. Biofuels Bioprod. Biorefining 2022, 16, 1568–1582. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, J.; Hu, X.; Zhu, F. Biomass production of carbohydrate-rich filamentous microalgae coupled with treatment and nutrients recovery from acrylonitrile butadiene styrene based wastewater: Synergistic enhancement with low carbon dioxide supply strategy. Bioresour. Technol. 2022, 349, 126829–126842. [Google Scholar] [CrossRef] [PubMed]
- Qaretapeh, Z.; Zonoozi, H.; Azarmanesh, R. Biostimulation of styrene-containing wastewater in carrier-supported anaerobic bioreactor towards enhancing biogas production and styrene degradation. J. Water Process Eng. 2024, 64, 105708–105721. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Hu, M.; Li, P. Effect of hydraulic retention time (HRT) on the biodegradation of trichloroethylene wastewater and anaerobic bacterial community in the UASB reactor. Appl. Microbiol. Biotechnol. 2015, 99, 1977–1987. [Google Scholar] [CrossRef]
- Fasihi, M.; Rajabi, M.; Barfi, B. Efficacious and environmentally friendly deep eutectic solvent-based liquid-phase microextraction for speciation of Cr(III) and Cr(VI) ions in food and water samples. Int. J. Environ. Anal. Chem. 2020, 102, 1–13. [Google Scholar] [CrossRef]
- Vanda, D.; Atika, S.; Eka, Y. The effect of cetyltrimethylammonium bromide (CTAB) addition on green synthesis of porous N-doped TiO2 for photoreduction of heavy metal ion Cr(vi). RSC Adv. 2023, 13, 29645–29656. [Google Scholar]
- Stylianos, S.; Konstantinos, S.; Manassis, M. Reductive precipitation and removal of Cr(VI) from groundwaters by pipe flocculation-microfiltration. Environ. Sci. Pollut. Res. Int. 2018, 25, 12256–12262. [Google Scholar] [CrossRef]
- Hu, J.; Li, B.; Su, M. Highly sensitive and selective ratiometric fluorescence and colorimetric detection of Cr(VI) via in situ encapsulation of green/red carbon quantum dots in zeolite. Colloids Surf. A Physicochem. Eng. Asp. 2025, 705, 135565–135572. [Google Scholar] [CrossRef]
- Ali, K.; Setia, W.; Rinda, H. Quantification of the Trace Metal Element Cr in Stainless Steel Using Picosecond Laser-Induced Breakdown Spectroscopy at Atmospheric Pressure. Arab. J. Sci. Eng. 2023, 4, 8165–8172. [Google Scholar]
- Liang, H.; Ding, W.; Zhang, H.; Peng, P. A novel lignin-based hierarchical porous carbon for efficient and selective removal of Cr(VI) from wastewater. Int. J. Biol. Macromol. 2022, 204, 310–320. [Google Scholar] [CrossRef]
- Tao, X.; Hu, X.; Wen, Z. Highly efficient Cr(VI) removal from industrial electroplating wastewater over Bi2S3 nanostructures prepared by dual sulfur-precursors: Insights on the promotion effect of sulfate ions. J. Hazard. Mater. 2022, 424, 127423–127436. [Google Scholar] [CrossRef] [PubMed]
- Ferhat, S.; Ounissa, S.; Stéphane, M. Polymer inclusion membranes based on CTA/PBAT blend containing Aliquat 336 as extractant for removal of Cr(VI): Efficiency, stability and selectivity. React. Funct. Polym. 2019, 139, 120–132. [Google Scholar]
- Zhang, Y.; Shi, D.; Xu, G. Efficient reduction of Cr(VI) over nitrogen defected 1D/2D CuO/g-C3N4 heterojunction photocatalysts. J. Porous Mater. 2024, 32, 651–664. [Google Scholar] [CrossRef]
- Pattnaik, P.; Mohanty, A.; Parida, K. A timely update on g-C3N4-based photocatalysts towards the remediation of Cr(VI) in aqueous streams. RSC Adv. 2024, 14, 36816–36834. [Google Scholar] [CrossRef]
- Özsoy, İ.; Fidan, S.; Bora, Ö.; Ürgün, S. Understanding the Damage Mechanisms of Basalt/Carbon Fiber Hybrid Composites Under Quasi-Static and Dynamic Loadings. Polymers 2025, 17, 866. [Google Scholar] [CrossRef]
- Liu, L.; Xiang, D.; Ma, J. Mechanical, electrical, and damage self-sensing properties of basalt fiber/carbon nanotube/poly (arylene ether nitrile) composites. J. Mater. Sci. Compos. 2025, 6, 2–9. [Google Scholar] [CrossRef]
- Kalbasi, R.; Parishani, P.; Mazaheri, O. Encapsulation of Nickel Nanoparticles and Homopoly(Vinylsulfonic Acid) in Mesoporous Carbon CMK-3 as an Acid–Metal Bifunctional Catalyst for Tandem Reductive Amination. J. Clust. Sci. 2018, 29, 214–227. [Google Scholar]
- Çelikten, S.; Çavuşoğlu, B. Mechanical and microstructural properties of fiber-reinforced basalt rock cutting waste-based geopolymer composites exposed to high temperatures. Environ. Sci. Pollut. Res. Int. 2025, 32, 3629–3648. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, P.; Jalilian, M.; Dearn, D. Comparison of mechanical and physical behaviors of carbon/basalt/epoxy hybrid composite rod with carbon/glass/epoxy hybrid composite rod used in ACCC conductor core. Polym. Compos. 2024, 46, 5773–5787. [Google Scholar] [CrossRef]
- Taha, M.; Alenad, M.; Alhassan, S. Enhanced refractive index and nonlinear optical susceptibility in PVDF-ZrO2/g-C3N4 based nanocomposites for NLO applications. Opt. Quantum Electron. 2024, 56, 1429–1437. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.; Ding, L. Thermal and mechanical analysis of thermal break structure in balcony with basalt fiber reinforced polymer. J. Build. Eng. 2024, 98, 111298–111306. [Google Scholar] [CrossRef]
- Nováková, L.; Schnabl, P.; Büchner, J. The characterization of sunburn basalts and their magnetic and petrographic properties. J. Geosci. 2018, 63, 333–344. [Google Scholar] [CrossRef]
- Asfahani, J.; Ghani, A. Automated interpretation of nuclear and electrical well loggings for basalt characterization (case study from southern Syria). Appl. Radiat. Isot. 2012, 70, 2500–2506. [Google Scholar] [CrossRef] [PubMed]
- Purcar, V.; Rădiţoiu, V.; Dumitru, A. Antireflective coating based on TiO2 nanoparticles modified with coupling agents via acid-catalyzed sol-gel method. Appl. Surf. Sci. 2019, 4, 819–824. [Google Scholar] [CrossRef]
- Sahebrao, S.; Subash, C. Application of airborne and ground geophysics to unravel the hydrogeological complexity of the Deccan basalts in central India. Hydrogeol. J. 2022, 30, 2097–2116. [Google Scholar]
- Shen, Y.; Liu, Z.; Bi, R.; Zhou, B.; Wang, Y.; Liu, J.; Wang, Z.; Han, B. Low-Crystallized Carbon as an Electron Mediator in g-C3N4/C/TiO2 for Enhancing Photocatalytic Degradation of Antibiotics. Nanomaterials 2025, 15, 365. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Hekmatara, H. MWCNT decorated Magnetic/Ceramic/Polymer (Fe2O3/Fe3O4/SiO2/PANI) nanocomposite with enhanced microwave absorption at an entire X and Ku frequency bands. Ceram. Int. 2024, 50, 11795–11803. [Google Scholar] [CrossRef]
- Wu, K.; Song, S.; Wu, H. Carbon quantum dots modified Bi2WO6 nanoflowers for enhancing photocatalytic activity: An experimental and DFT study. Micro Nano Lett. 2020, 15, 317–322. [Google Scholar] [CrossRef]
- Maybodi, S.; Hammood, O. Photodegradation of Polycyclic Aromatic Hydrocarbons Under Visible Light Using Modified g-C3N4 as Photocatalyst, Spectroscopic Studies. Polycycl. Aromat. Compd. 2025, 45, 192–204. [Google Scholar] [CrossRef]
- Li, H.; Pang, J.; Jaegers, R. Conversion of ethanol to 1,3–butadiene over Ag–ZrO2/SiO2 catalysts: The role of surface interfaces. J. Energy Chem. 2021, 54, 7–15. [Google Scholar] [CrossRef]
- Yuan, Y.; Yin, Y.; Yu, D. Effects of compound coupling agents on the properties of PTFE/SiO2 microwave composites. J. Mater. Sci. Mater. Electron. 2017, 28, 3356–3363. [Google Scholar] [CrossRef]
- Seddigi, S.; Baig, U.; Khan, A. Visible Light Assisted CuO/g-C3N4 Nanocomposite Photocatalyst for Dye Degradation and In-Vitro Deactivation of Pseudomonas aeruginosa Bacteria. Arab. J. Sci. Eng. 2024, 50, 3925–3936. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, Y.; Wang, C. Studies on ZnO/g-C3N4 Heterojunction Photocatalyst for RhB Degradation Under Vis-Light in a MNB-Agitated Pilot Reactor. Arab. J. Sci. Eng. 2025, 50, 3751–3762. [Google Scholar] [CrossRef]
- Murugavel, K.; Vasanthakumar, V.; Pazhanivel, T. Exploring prompt photocatalytic degradation of MB dye using Cu 0.5 Co 0.5 WO4/g-C3N4 nanocomposite under visible light irradiation. Emergent Mater. 2024, 7, 987–998. [Google Scholar]
- Zhong, Q.; Lan, H.; Zhang, M. Preparation of heterostructure g-C3N4/ZnO nanorods for high photocatalytic activity on different pollutants (MB, RhB, Cr(VI) and eosin). Ceram. Int. 2020, 46, 12192–12199. [Google Scholar] [CrossRef]
- Yu, C. Removal of Cr(VI) from aqueous solutions by nZVI-loaded sludge-derived biochar: Performance and mechanism. Water Sci. Technol. 2022, 86, 2089–2105. [Google Scholar] [CrossRef] [PubMed]
- Al-Shannag, M.; Al-Qodah, Z.; Bani-Melhem, K. Heavy metal ions removal from metal plating wastewater using electrocoagulation: Kinetic study and process performance. Chem. Eng. J. 2015, 260, 749–756. [Google Scholar] [CrossRef]
- Örnek, A.; Özacar, M.; Şengil, A. Adsorption of lead onto formaldehyde or sulphuric acid treated acorn waste: Equilibrium and kinetic studies. Biochem. Eng. J. 2007, 37, 192–200. [Google Scholar] [CrossRef]
- Yang, W.; Cloete, S.; Morud, J. An Effective Reaction Rate Model for Gas-Solid Reactions with High Intra-Particle Diffusion Resistance. Int. J. Chem. React. Eng. 2016, 14, 331–342. [Google Scholar] [CrossRef]
- Lobo, C.; Castellari, J.; Lerner, C. Functional iron chitosan microspheres synthesized by ionotropic gelation for the removal of arsenic (V) from water. Int. J. Biol. Macromol. 2020, 164, 1575–1583. [Google Scholar] [CrossRef]
- Munawar, I.; Muhammad, S.; Zahid, A. Paracetamol and amoxicillin adsorptive removal from aqueous solution using phosphoric acid activated-carbon. Z. Phys. Chem. 2023, 237, 257–271. [Google Scholar]
- Mitra, A.; Mehran, H.; Arezoo, T. Chromium (VI) bioremoval from contaminated wastewater using Pseudomonas aeruginosa ATHA23 producing biofilm supported on clinoptilolite. Environ. Geochem. Health 2022, 45, 427–442. [Google Scholar]
- Khayyun, S.; Mseer, H. Comparison of the experimental results with the Langmuir and Freundlich models for copper removal on limestone adsorbent. Appl. Water Sci. 2019, 9, 170. [Google Scholar] [CrossRef]
- Spek, D. Selling a Theory: The Role of Molecular Models in J. H. van ‘t Hoff’s Stereochemistry Theory. Ann. Sci. 2006, 63, 157–177. [Google Scholar] [CrossRef]
- Zhong, L.; Ren, W.; Guo, W. A pilot scale test of ozonization treatment of ethene wastewater for reuse. Front. Chem. Eng. China 2008, 2, 191–195. [Google Scholar] [CrossRef]


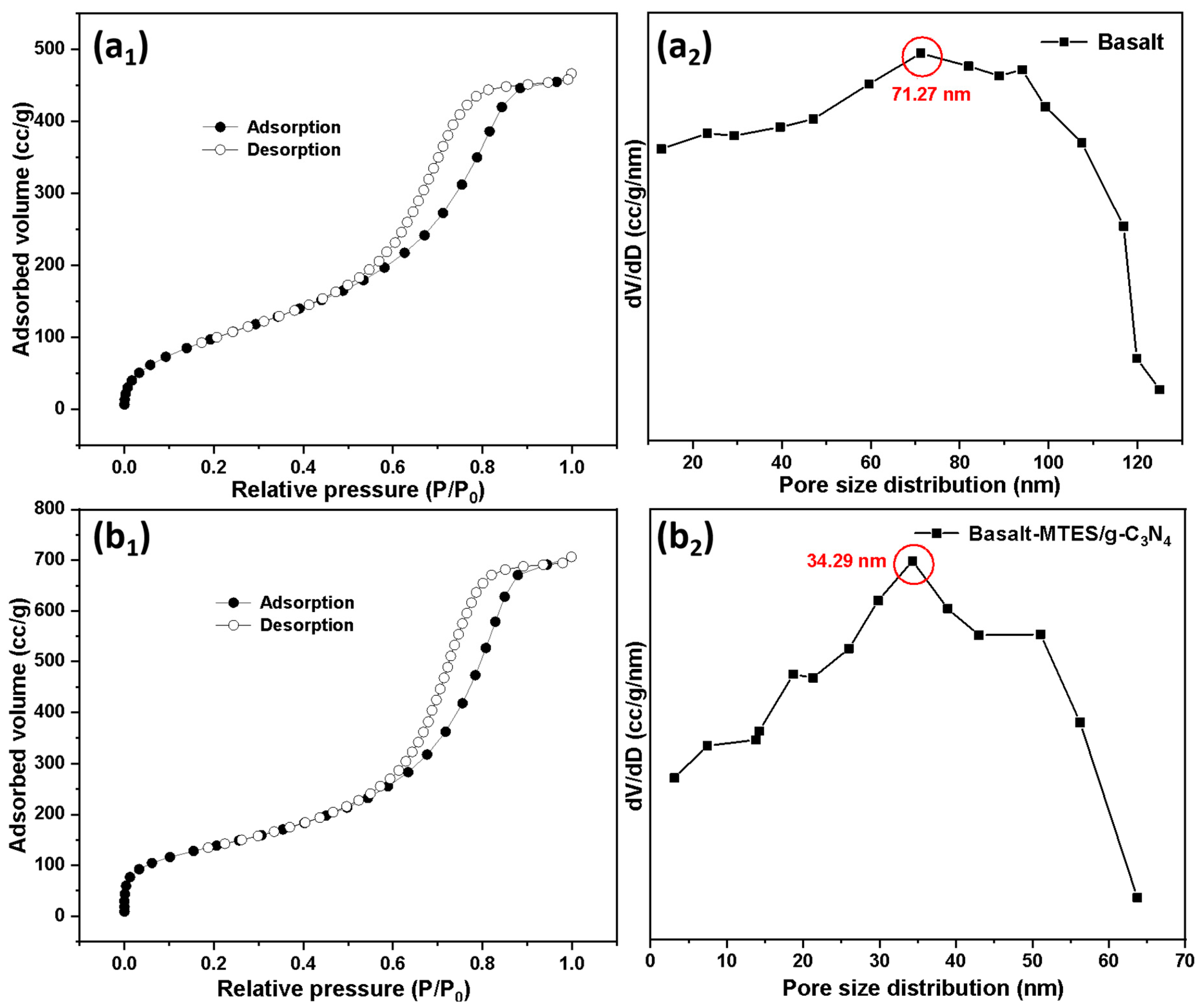

| Species | Pore Size (nm) | Specific Surface Area (m2·g−1) |
|---|---|---|
| Basalt | 71.27 | 563.25 |
| Basalt–MTES/g-C3N4 | 34.29 | 406.55 |
| Adsorption Kinetic Model | Parameters | Cr(VI) |
|---|---|---|
| Pseudo-first order | qe (mg·g−1) | 55.28 |
| K1 (s−1) | 0.014 | |
| R2 | 0.985 | |
| Pseudo-second order | qe (mg·g−1) | 42.62 |
| K2 (g·s·mg−1) | 0.086 | |
| R2 | 0.997 |
| Adsorption Isotherm Model | Parameters | Cr(VI) |
|---|---|---|
| qmax (mg·g−1) | 77.26 | |
| Langmuir | b (L·mg−1) | 0.093 |
| R2 | 0.992 | |
| Freundlich | Kf | 8.538 |
| nf | 0.354 | |
| R2 | 0.946 |
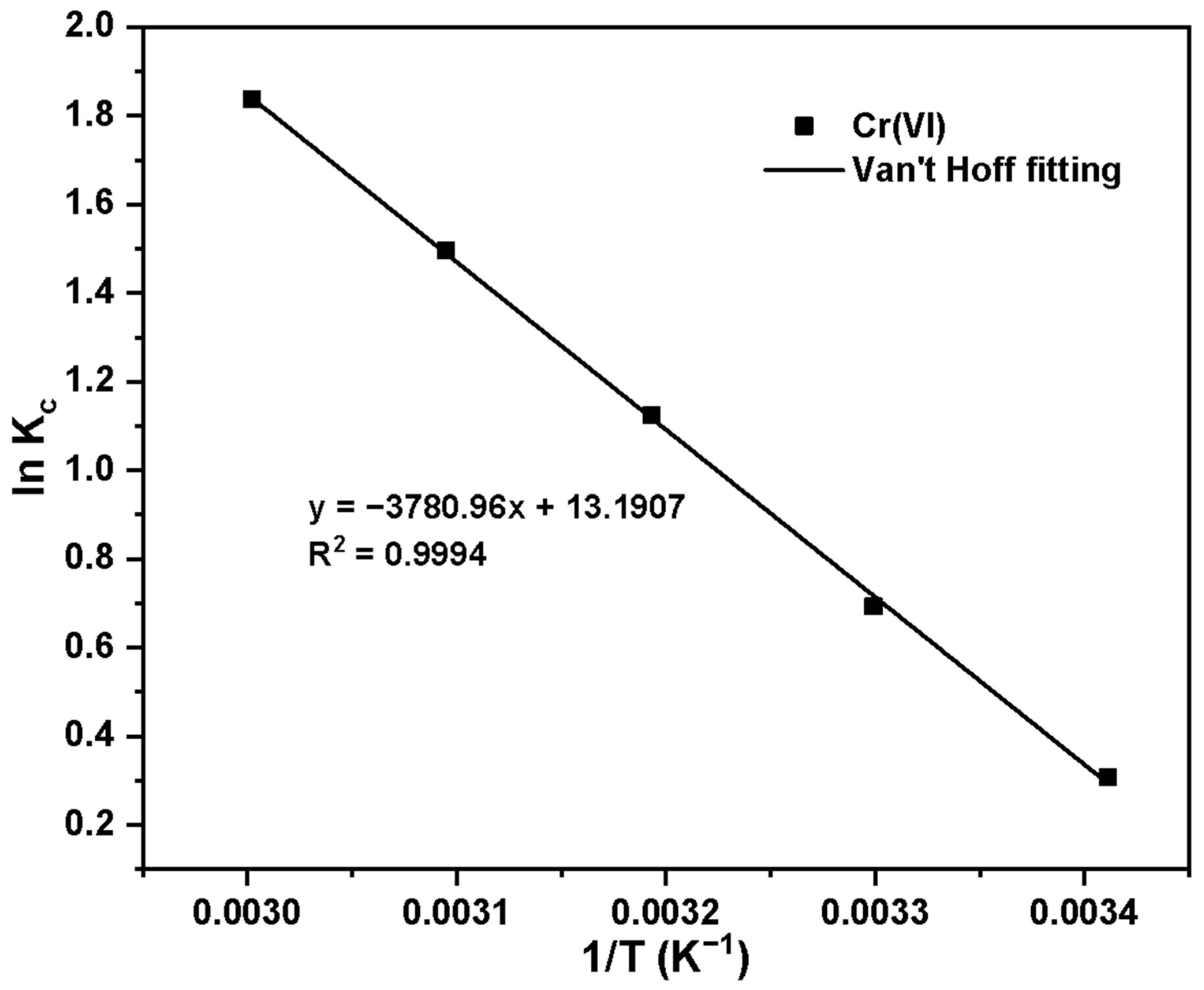
| Species | T (K) | ΔG (KJ·mol−1) | ΔH (KJ·mol−1) | ΔS (J·mol−1·K−1) | R2 |
|---|---|---|---|---|---|
| Cr(VI) | 293.15 | −0.71 | 31.43 | 109.67 | 0.9994 |
| 303.15 | −1.81 | ||||
| 313.15 | −2.91 | ||||
| 323.15 | −4.02 | ||||
| 333.15 | −5.10 |
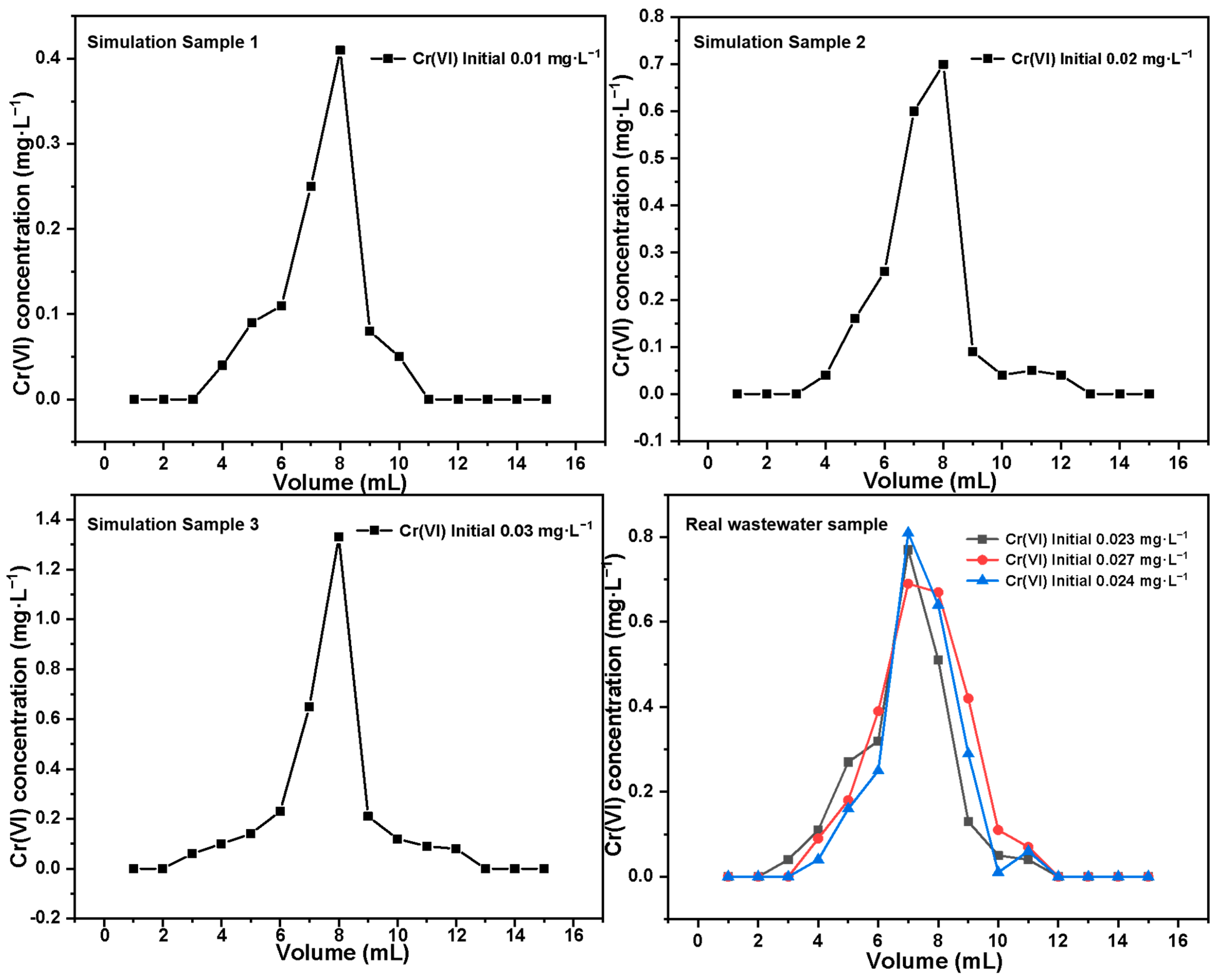
| Species | Initial Cr(VI) Concentration of the Sample | Enrichment Rate | |
|---|---|---|---|
| Cr(VI) | Simulated sample | 0.01 mg·L−1 | 99.21% |
| 0.02 mg·L−1 | 99.54% | ||
| 0.03 mg·L−1 | 99.33% | ||
| Real sample | 0.023 mg·L−1 | 97.31% | |
| 0.027 mg·L−1 | 98.02% | ||
| 0.024 mg·L−1 | 97.24% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Jia, M.; Ren, Y.; Ren, H.; Liang, S.; Sun, J.; Hao, S.; Li, J.; Li, H. Efficient Analytical Pretreatment of Cr(VI) in Ethylene Wastewater by Grafting g-C3N4 Material Based on Coupling Agent-Modified Basalt Matrix (Basalt–MTES/g-C3N4). Molecules 2025, 30, 2477. https://doi.org/10.3390/molecules30112477
Wang Z, Jia M, Ren Y, Ren H, Liang S, Sun J, Hao S, Li J, Li H. Efficient Analytical Pretreatment of Cr(VI) in Ethylene Wastewater by Grafting g-C3N4 Material Based on Coupling Agent-Modified Basalt Matrix (Basalt–MTES/g-C3N4). Molecules. 2025; 30(11):2477. https://doi.org/10.3390/molecules30112477
Chicago/Turabian StyleWang, Zheng, Mingchang Jia, Yi Ren, Hongmin Ren, Shuhao Liang, Jiaru Sun, Siqi Hao, Jinchuan Li, and He Li. 2025. "Efficient Analytical Pretreatment of Cr(VI) in Ethylene Wastewater by Grafting g-C3N4 Material Based on Coupling Agent-Modified Basalt Matrix (Basalt–MTES/g-C3N4)" Molecules 30, no. 11: 2477. https://doi.org/10.3390/molecules30112477
APA StyleWang, Z., Jia, M., Ren, Y., Ren, H., Liang, S., Sun, J., Hao, S., Li, J., & Li, H. (2025). Efficient Analytical Pretreatment of Cr(VI) in Ethylene Wastewater by Grafting g-C3N4 Material Based on Coupling Agent-Modified Basalt Matrix (Basalt–MTES/g-C3N4). Molecules, 30(11), 2477. https://doi.org/10.3390/molecules30112477






