Synthesis and Properties of Energetic MOFs Based on Bis(3-Nitro-1H-1,2,4-triazole-5-yl) Amine: Advancing High Thermal Stability and Low Sensitivity
Abstract
1. Introduction
2. Results and Discussion
2.1. Single-Crystal Structure
2.2. Thermal Behaviors
2.3. Energetic and Safety Characteristics
2.4. Hirshfeld Surface
3. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barton, L.M.; Edwards, J.T.; Johnson, E.C.; Bukowski, E.J.; Sausa, R.C.; Byrd, E.F.C.; Orlicki, J.A.; Sabatini, J.J.; Baran, P.S. Impact of Stereo- and Regiochemistry on Energetic Materials. J. Am. Chem. Soc. 2019, 141, 12531–12535. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.H.; Huang, B.B.; Li, H.Z.; Yan, Q.L. Nitramine-Based Energetic Cocrystals with Improved Stability and Controlled Reactivity. Cryst. Growth Des. 2020, 20, 8124–8147. [Google Scholar] [CrossRef]
- Tariq, Q.U.N.; Manzoor, S.; Ling, X.; Dong, W.S.; Lu, Z.J.; Wang, T.W.; Xu, M.Q.; Younis, M.A.; Yu, Q.Y.; Zhang, J.G. Fabrication, Characterization, and Performance Evaluation of Thermally Stable [5,6]-Fused Bicyclic Energetic Materials. ACS Appl. Mater. Interfaces 2024, 16, 52613–52623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Fei, T.; Meng, J.W.; Cai, J.X.; Zhang, L.; Pang, S.P.; He, C.L. Taming of Trinitromethyl-Oxadiazole to Access High Density and High Oxygen Balance via a Dual Modulation Strategy. Def. Technol. 2025, 43, 142–149. [Google Scholar]
- Li, Y.; Jiang, Z.Y.; Liu, Z.J.; Li, B.D. Constructing Single- or Dual-Layer Biomass Composite Energetic Material through Self-Assembly of Biomass Polyphenol Structural Materials. Langmuir 2024, 40, 12313–12321. [Google Scholar] [CrossRef]
- Imchen, S.; Amrit, P. Metal–Organic Framework (MOF)-based Single Atom Catalysts (SACs) for Photocatalytic Energy Conversion. ChemistrySelect 2024, 9, e202403516. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Lin, W.B. Metal–organic frameworks as a tunable platform for designing functional molecular materials. J. Am. Chem. Soc. 2013, 135, 13222–13234. [Google Scholar] [CrossRef]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, thermal and mechanical stabilities of metal–organic frameworks. Nat. Rev. Mater. 2016, 1, 15018. [Google Scholar] [CrossRef]
- Gebremariam, S.K.; Varghese, A.M.; Ehrling, S.; Wahedi, Y.A.; Hajaj, A.A.; Dumée, L.F.; Karanikolos, G.N. Hierarchically Porous Structured Adsorbents with Ultrahigh Metal–Organic Framework Loading for CO2 Capture. ACS Appl. Mater. Interfaces 2024, 16, 50758–50799. [Google Scholar] [CrossRef]
- Devendran, A.; Shahmirzaee, M.; Nagai, A. Recent Progress on Metal Organic Framework and Covalent Organic Framework Based Solid-State Electrolyte Membranes for Lithium Battery Applications. ChemElectroChem 2024, 11, e202300847. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.Y.; Feng, X.; Wang, B.; Yang, L. Explosives in the Cage: Metal-Organic Frameworks for High-Energy Materials Sensing and Desensitization. Adv. Mater. 2017, 29, 1701898. [Google Scholar] [CrossRef] [PubMed]
- Check, B.; Bairley, K.; Santarelli, J.; Pham, H.T.B.; Park, J. Applications of Electrically Conductive Metal–Organic Frameworks: From Design to Fabrication. ACS Mater. Lett. 2025, 7, 465–488. [Google Scholar] [CrossRef]
- Ye, B.C.; Li, W.H.; Zhang, X.; Chen, J.; Gao, Y.; Wang, D.S.; Pan, H.G. Advancing Heterogeneous Organic Synthesis with Coordination Chemistry-Empowered Single-Atom Catalysts. Adv. Mater. 2024, 36, 2402747. [Google Scholar] [CrossRef] [PubMed]
- Jerozal, R.T.; Pitt, T.A.; MacMillan, S.N.; Milner, P.J. High-concentration self-assembly of zirconium-and hafnium-based metal–organic materials. J. Am. Chem. Soc. 2023, 145, 13273–13283. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Q.; Liu, X.; Qu, X.; Wei, Q.; Chen, S.P.; Gao, S.L. High-energy metal–organic frameworks (HE-MOFs): Synthesis, structure and energetic performance. Coord. Chem. Rev. 2016, 307, 292–312. [Google Scholar] [CrossRef]
- Tan, B.J.; Dou, J.K.; Yang, X.; Li, W.J.; Zhang, J.; Zhang, P.F.; Mo, H.C.; Lu, X.M.; Wang, B.Z.; Liu, N. Application and prospects of EMOFs in the fields of explosives and propellants. Dalton Trans. 2024, 53, 13308–13319. [Google Scholar] [CrossRef]
- Xu, L.P.; Qiao, J.; Xu, S.Y.; Zhao, X.Y.; Gong, W.J.; Huang, T.Z. Constructing Strategies and Applications of Nitrogen-Rich Energetic Metal—Organic Framework Materials. Catalysts 2020, 10, 690. [Google Scholar] [CrossRef]
- Cao, Y.T.; Liu, Y.; Zhang, W.Q. Pentazolate Anion: A Rare and Preferred Five-Membered Ligand for Constructing Pentasil-Zeolite Topology Architectures. Angew. Chem. Int. Ed. 2024, 63, e202317355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Q.; Wang, K.C.; Li, J.C.; Lin, Z.E.; Song, S.W.; Huang, S.L.; Liu, Y.; Nie, F.D.; Zhang, Q.H. Stabilization of the Pentazolate Anion in a Zeolitic Architecture with Na20N60 and Na24N60 Nanocages. Angew. Chem. Int. Ed. 2018, 57, 2592–2595. [Google Scholar] [CrossRef]
- Gao, H.X.; Zhang, Q.H.; Shreeve, J.N.M. Fused Heterocycle-Based Energetic Materials (2012–2019). J. Mater. Chem. A 2020, 8, 4193–4216. [Google Scholar] [CrossRef]
- Sher, F.; Hayward, A.; Guerraf, E.A.; Ziani, I.; Hrnjić, H.; Boškailo, E.; Nemțanu, M.R. Advanced metal-organic frameworks for superior carbon capture, high-performance energy storage and environmental photocatalysis—A critical review. J. Mater. Chem. A 2024, 12, 27932–27973. [Google Scholar] [CrossRef]
- Lin, J.D.; Chen, F.; Xu, J.G.; Zheng, F.K.; Wen, N. Framework-Interpenetrated Nitrogen-Rich Zn (II) Metal-Organic Frameworks for Energetic Materials. ACS Appl. Nano. Mater. 2019, 2, 5116–5124. [Google Scholar] [CrossRef]
- Xu, R.; Yan, Z.Z.; Yang, L.; Wang, Q.Y.; Tong, W.C.; Song, N.M.; Han, J.M.; Zhao, Y. Nanoscale Homogeneous Energetic Copper Azides@Porous Carbon Hybrid with Reduced Sensitivity and High Ignition Ability. ACS Appl. Mater. Interfaces 2018, 10, 22545–22551. [Google Scholar] [CrossRef]
- Liu, G.R.; Bu, R.P.; Huang, X.; Zhong, K.; Jiao, F.B.; Wei, S.H.; Li, H.Z.; Zhang, C.Y. Energetic Cocrystallization as the Most Significant Crystal Engineering Way to Create New Energetic Materials. Cryst. Growth Des. 2022, 22, 954–970. [Google Scholar] [CrossRef]
- Cao, S.N.; Ma, X.F.; Ma, X.H.; Cen, P.P.; Wu, Y.W.; Yang, J.H.; Liu, X.Y.; Xie, G.; Chen, S.P. Modulating energetic performance through decorating the nitrogen-rich ligands in high-energy MOFs. Dalton Trans. 2020, 49, 2300–2307. [Google Scholar] [CrossRef]
- Xu, J.G.; Sun, C.; Zhang, M.J.; Liu, B.W.; Li, X.Z.; Liu, J.; Wang, S.H.; Zheng, F.K.; Guo, G.C. Coordination Polymerization of Metal Azides and Powerful Nitrogen-Rich Ligand toward Primary Explosives with Excellent Energetic Performances. Chem. Mater. 2017, 29, 9725–9733. [Google Scholar] [CrossRef]
- Xu, Z.; Hou, T.Y.; Zhang, X.P.; Yang, F.; Zhang, L.N.; Jiang, S.J.; Lu, M.; Xu, Y.G. Self-Assembly and Controllable Regulation of 1,5′-Bitetrazolate-2 N-oxide-Based EMOFs toward High Dimensionality. Cryst. Growth Des. 2024, 24, 461–470. [Google Scholar] [CrossRef]
- Ma, Q.; Huang, S.L.; Lu, H.C.; Nie, F.D.; Liao, L.Y.; Fan, G.J.; Huang, J.L. Energetic Cocrystal, Ionic Salt, and Coordination Polymer of a Perchlorate Free High Energy Density Oxidizer: Influence of pKa Modulation on Their Formation. Cryst. Growth Des. 2019, 19, 714–723. [Google Scholar] [CrossRef]
- Li, F.G.; Zhao, W.Y.; Chen, S.T.; Zhang, T.L.; Zhou, Z.N.; Yang, L. Nitrogen-Rich Alkali Metal Salts (Na and K) of [Bis (N, N-bis(1H-tetrazol-5-yl) amine)-zinc (II)] Anion: Syntheses, Crystal Structures, and Energetic Properties. Z. Anorg. Allg. Chem. 2015, 641, 911–916. [Google Scholar] [CrossRef]
- Banik, B.K.; Sahoo, B.M.; Kumar, B.V.V.R.; Panda, K.C.; Jena, J.; Mahapatra, M.K.; Borah, P. Green synthetic approach: An efficient eco-friendly tool for synthesis of biologically active oxadiazole derivatives. Molecules 2021, 26, 1163. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Jin, B.; Zhang, Q.C.; Shang, Y.; Guo, Z.C.; Tan, B.S.; Peng, R.F. Nitrogen-Rich Energetic Metal-Organic Framework: Synthesis, Structure, Properties, and Thermal Behaviors of Pb (II) Complex Based on N, N-Bis(1H-tetrazole-5-yl)-Amine. Materials 2016, 9, 681. [Google Scholar] [CrossRef]
- Zhang, G.F.; Hao, X.; Zou, Y.B.; Liu, S.C.; Wei, J.J.; Dong, Z.; Ye, Z.W. Towards Advanced N-rich Energetic Explosives: Based on Tetrazole and Triazole Groups with Large Conjugated Systems and Extensive Hydrogen Bonds. J. Mater. Chem. A 2024, 12, 33249–33256. [Google Scholar] [CrossRef]
- Aromi, G.; Barrios, L.A.; Roubeau, O.; Gamez, P. Triazoles and Tetrazoles: Prime Ligands to Generate Remarkable Coordination Materials. Coord. Chem. Rev. 2011, 255, 485–546. [Google Scholar] [CrossRef]
- Myers, T.W.; Brown, K.E.; Chavez, D.E.; Scharff, R.J.; Veauthuer, J.M. Laser Initiation of Fe (II) Complexes of 4-Nitro-pyrazolyl Substituted Tetrazine Ligands. Inorg. Chem. 2017, 56, 2297–2303. [Google Scholar] [CrossRef]
- Stetsiuk, O.; Alexandre, A.; Narcis, A. 1,2,4,5-Tetrazine based ligands and complexes. Dalton Trans. 2020, 49, 5759–5777. [Google Scholar] [CrossRef]
- Li, C.; Zhang, M.; Chen, Q.S.; Li, Y.Y.; Gao, H.Q.; Fu, W.; Zhou, Z.M. 1-(3,5-Dinitro-1H-pyrazol-4-yl)-3-nitro-1H-1,2,4-triazol-5-amine (HCPT) and its Energetic Salts: Highly Thermally Stable Energetic Materials with High-performance. Dalton Trans. 2016, 45, 17956–17965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.F.; Hao, X.; Zou, Y.B.; Liu, S.C.; Wei, J.J.; Dong, Z.; Ye, Z.W. Several energetic MOFs based on the N-rich energetic materials and alkali metals: Towards high detonation performances and good stabilities. CrystEngComm 2025, 27, 277–283. [Google Scholar] [CrossRef]
- Chen, D.; Jing, D.; Zhang, Q.; Xue, X.G.; Gou, S.H.; Li, H.Z.; Nie, F.D. Study of Six Green Insensitive High Energetic Coordination Polymers Based on Alkali/Alkali-Earth Metals and 4,5-Bis(tetrazol-5-yl)-2H-1,2,3-triazole. Chem. Asian J. 2017, 40, 3141–3149. [Google Scholar] [CrossRef]
- Finger, L.H.; Schröder, F.G.; Sundermeyer, J. Synthesis and Characterisation of 5,5′-Bistetrazolate Salts with Alkali Metal, Ammonium and Imidazolium Cations. Z. Anorg. Allg. Chem. 2013, 639, 1140–1152. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, Y.; Xiao, L.B.; Gao, H.X.; Zhao, F.Q.; Huang, J.; Lv, X.Q.; Ma, H.X. 4-(1H-Tetrazole-5-yl-Amino)-1,2,4,5-Tetrazin-1-one (TATzO) Metal Salts: Promising Pyrotechnic Agent. J. Therm. Anal. 2024, 149, 2697–2706. [Google Scholar] [CrossRef]
- Wang, T.W.; Zhou, J.Y.; Zhang, Q.; Zhang, L.; Zhu, S.G.; Li, Y. Novel 3D cesium(i)-based EMOFs of nitrogen-rich triazole derivatives as “green” orange-light pyrotechnics. New J. Chem. 2020, 44, 1278–1284. [Google Scholar] [CrossRef]
- Rajak, R.; Kumar, N.; Ghule, V.D.; Dharavath, S. Highly Dense N–N-Bridged Dinitramino Bistriazole-Based 3D Metal–Organic Frameworks with Balanced Outstanding Energetic Performance. ACS Appl. Mater. Interfaces 2024, 16, 20670–20680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Hou, T.Y.; Wu, Y.G.; Xu, Z.; Lin, Q.H.; Wang, P.C.; Xu, Y.G.; Lu, M. Crowding-out Strategy to Enhance Energetics of 3-nitro-1,2,4-triazol-5-one based E-MOFs at the Molecular Level through Adjusting pH. Chem. Eng. J. 2023, 466, 143091. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, W.S.; Lu, Z.J.; Wang, T.W.; Zhang, C.; Zhou, Z.N.; Zhang, J.G. Synthesis and Characterization of Thermally Stable Energetic Complexes with 3,5-diaminopyrazolone-4-oxime as a Nitrogen-rich Ligand. CrystEngComm 2022, 24, 5519–5526. [Google Scholar] [CrossRef]
- Cao, Y.T.; Wang, K.C.; Song, S.W.; Liu, Y.; Zhang, W.Q. Fabrication of Energetic Metal–Organic Frameworks: Potassium 5-carboxylato-3,4-dinitropyrazole and Potassium 5-(hydrazine carbonyl)-3,4-dinitropyrazole. Inorg. Chem. 2023, 62, 17199–17206. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, K.C.; Qi, X.J.; Jin, Y.H.; Zhang, Q.H. Construction of a Thermally Stable and Highly Energetic Metal—Organic Framework as Lead-free Primary Explosives. Cryst. Growth Des. 2018, 18, 1896–1902. [Google Scholar] [CrossRef]
- Rajak, R.; Kumar, P.; Dharavath, S. Mixed-Metallic Energetic Metal–Organic Framework: New Structure Motif for Potential Heat-Resistant Energetic Materials. Cryst. Growth Des. 2024, 24, 2142–2148. [Google Scholar] [CrossRef]
- Zhang, L.F.; Wang, Y.; Wang, Y.F.; Liu, S.; Zhang, N.; Yang, M.M.; Ma, H.X.; Guo, Z.Q. N-Methylene-C bridged Tetrazole and 1,2,4-triazole Energetic Salts as Promising Primary Explosives. CrystEngComm 2024, 26, 143–152. [Google Scholar] [CrossRef]
- Sućeska, M. EXPLO5 V6.05.02[CP]; Brodarski Institute: Zagreb, Croatia, 2019. [Google Scholar]
- Dacons, J.C.; Sitzmann, M.E. Synthesis of 2,4,6-trinitrophenyl Derivatives of Heterocyclic Compounds. J. Heterocycl. Chem. 1977, 14, 1151–1155. [Google Scholar] [CrossRef]
- Bian, C.M.; Feng, W.J.; Lei, Q.Y.; Huang, H.F.; Li, X.; Wang, J.L.; Li, C.; Xiao, Z.L. A Facile Synthesis of Energetic Salts Based on Fully Nitroamino-Functionalized [1,2,4] triazolo [4,3-b] [1,2,4] triazole. Dalton Trans. 2020, 49, 368–374. [Google Scholar] [CrossRef]
- Brill, T.B.; Gongwer, P.E.; Williams, G.K. Thermal decomposition of energetic materials. 66. Kinetic compensation effects in HMX, RDX, and NTO. J. Phys. Chem. 1994, 98, 12242–12247. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Li, X.; Sun, Q.; Lin, Q.H.; Lu, M. [N-N=N-N]-Linked Fused Triazoles with π-π Stacking and Hydrogen Bonds: Towards Thermally Stable, Insensitive, and Highly Energetic Materials. Chem. Eng. J. 2021, 406, 126817. [Google Scholar] [CrossRef]
- Cao, Y.T.; Cai, Z.W.; Shi, J.H.; Zhang, Q.H.; Liu, Y.; Zhang, W.Q. A Heat-Resistant and Insensitive Energetic Material Based on the Pyrazolo-Triazine Framework. Energy Mater. Front. 2022, 3, 26–31. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Li, C.; Deng, C.L.; Wang, M. Structure Preparation Method and Performances of High-Energy Heat-Resistant Explosive CPTY. CN201711400638A, 7 September 2018. [Google Scholar]
- John, W.F.; Robert, D.C.; Richard, D.G. Facile Entry into the 3H,9H-Bis[1,2,4] Triazolo-[1,5-a:5′,1′-d] [1,3,5]Triazinium (5/6/5 Tricyclic NNN) System. Tetrahedron Lett. 2006, 47, 7707–7709. [Google Scholar]
- Li, Y.N.; Wang, B.; Chang, P.; Hu, J.J.; Chen, T.; Wang, Y.L.; Wang, B.Z. Novel Catenated N6 Energetic Compounds Based on Substituted 1,2,4-triazoles: Synthesis, Structures and Properties. RSC Adv. 2018, 8, 13755–13763. [Google Scholar] [CrossRef]
- Dippold, A.A.; Klapötke, T.M.; Winter, N. Insensitive Nitrogen-Rich Energetic Compounds Based on the 5,5′-Dinitro-3,3′-bi-1,2,4-triazol-2-ide Anion. Eur. J. Inorg. Chem. 2012, 2012, 3474–3484. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.; Schlegel, H.B.; Scuseria, G.E. Gaussian 09, Revision D. 01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Jenkins, H.D.B.; Tudela, D.; Glasser, L. Lattice Potential Energy Estimation for Complex Ionic Salts from Density Measurements. Inorg. Chem. 2002, 41, 2364–2367. [Google Scholar] [CrossRef]
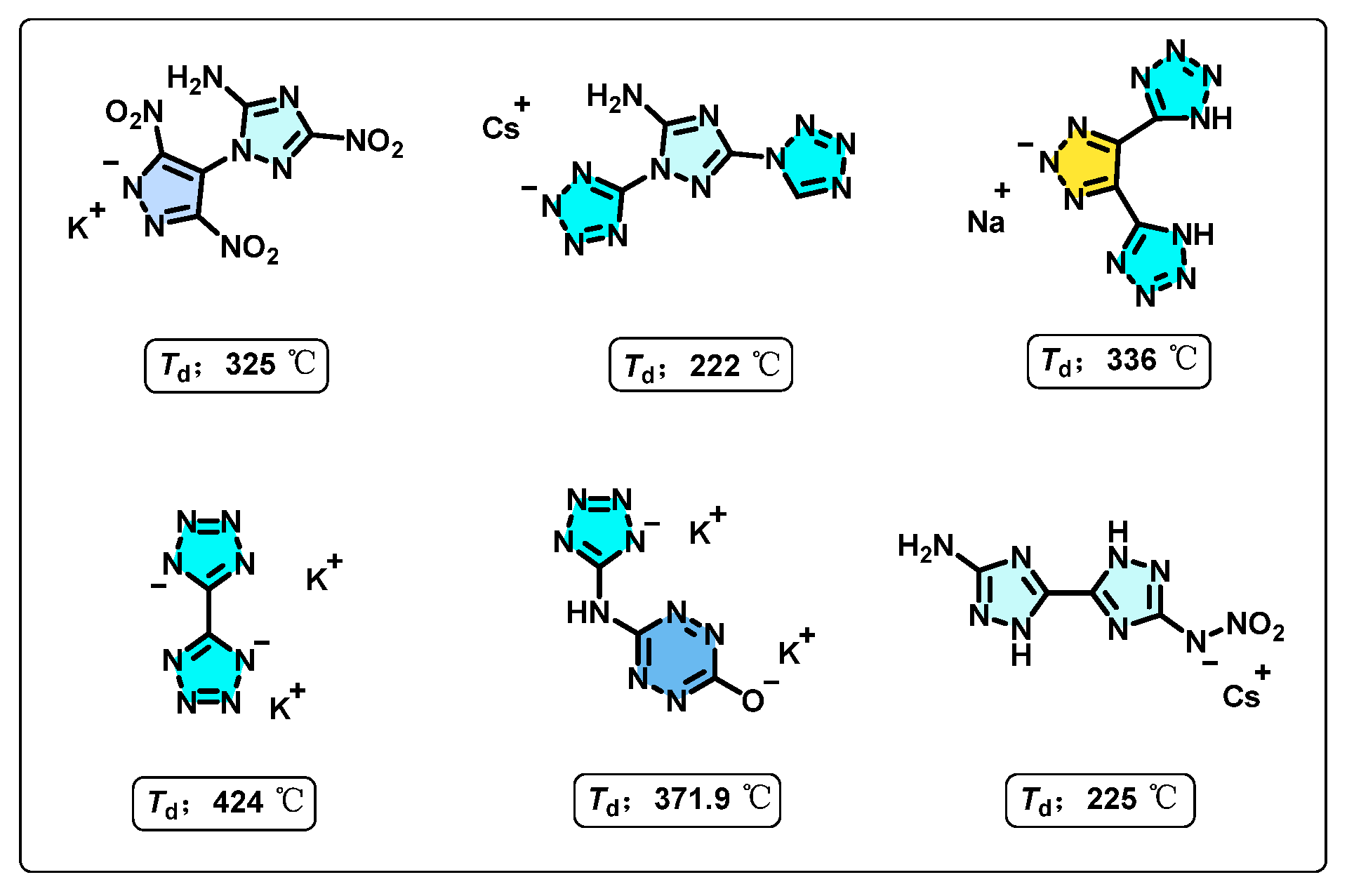
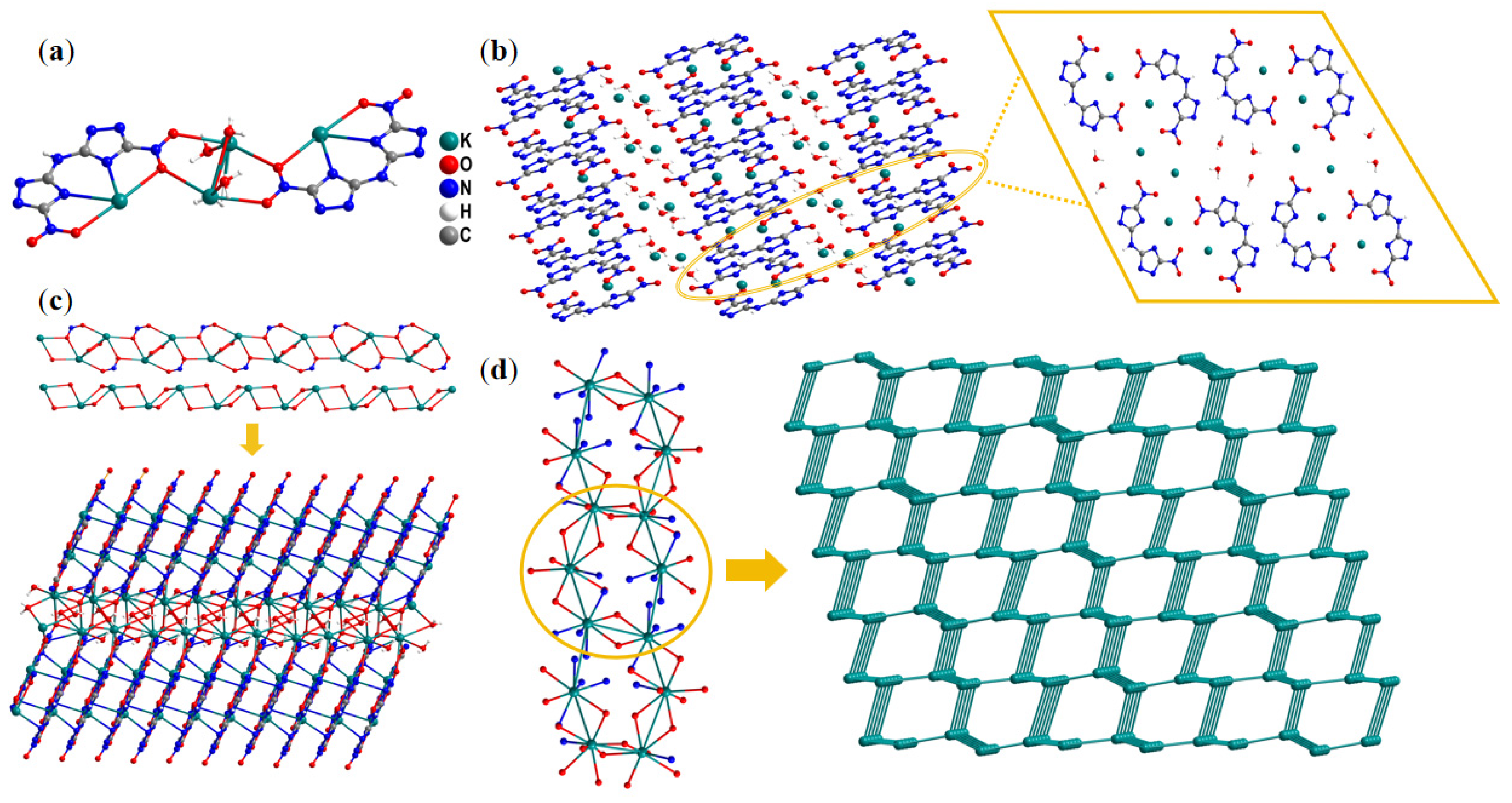
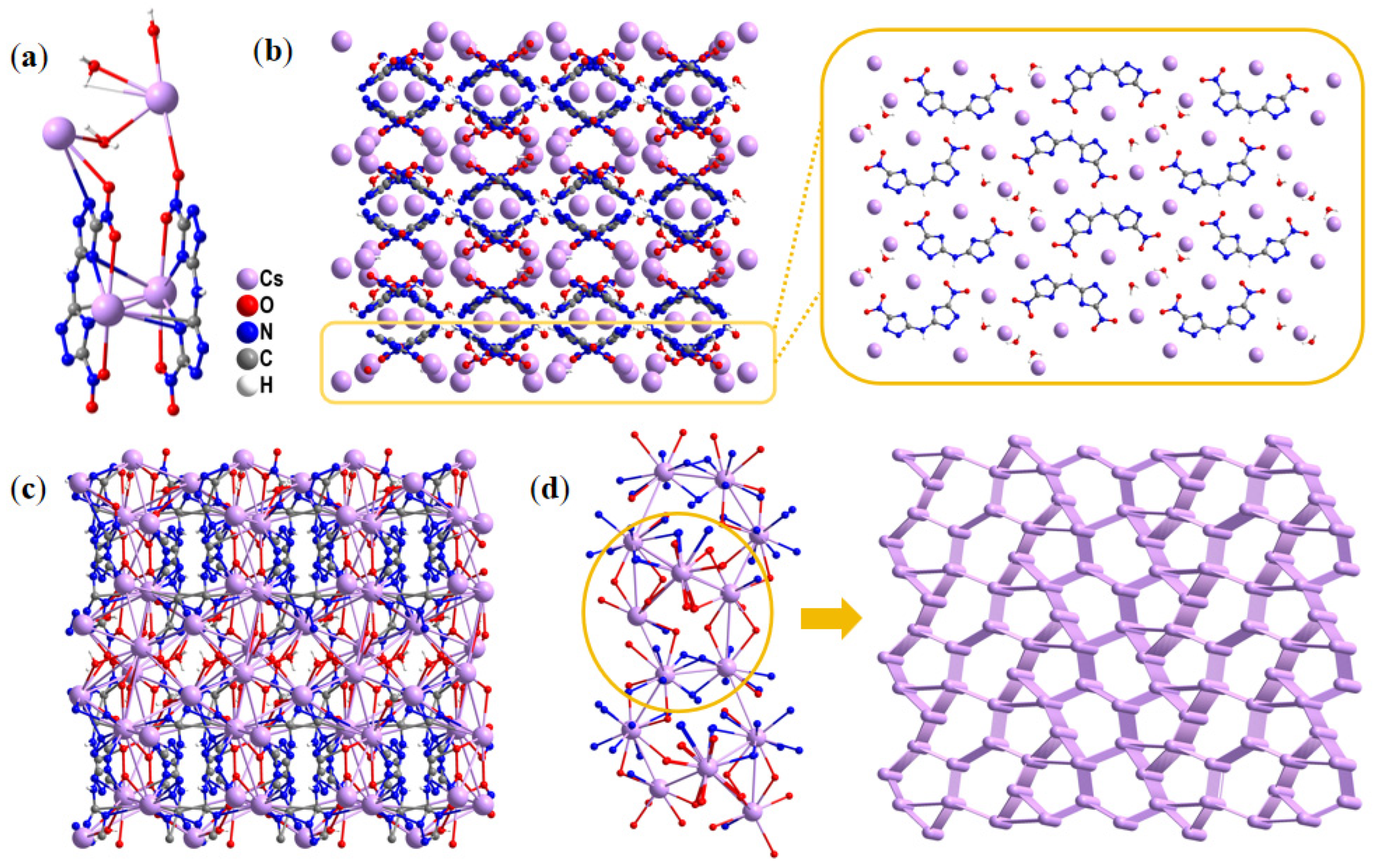
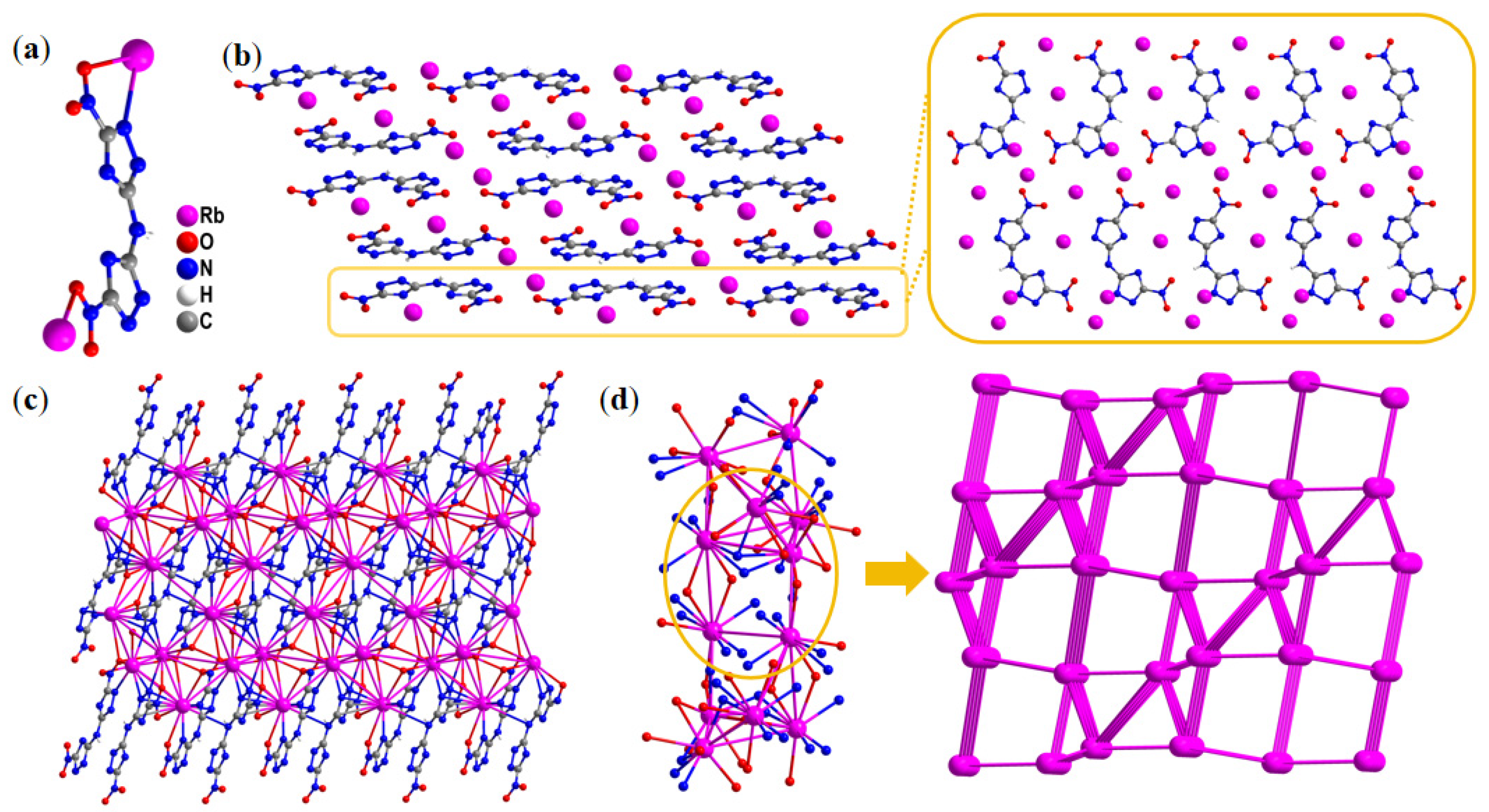
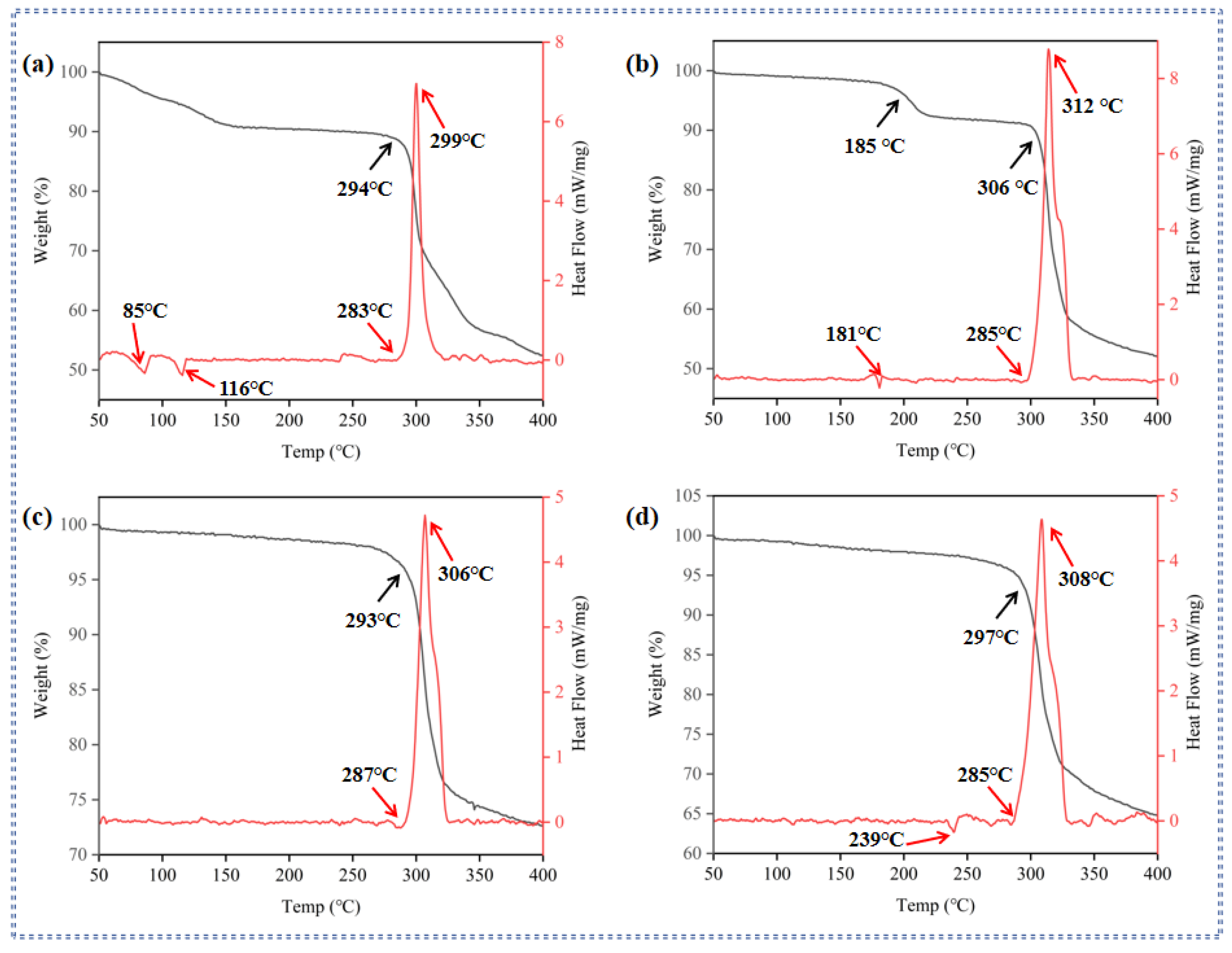
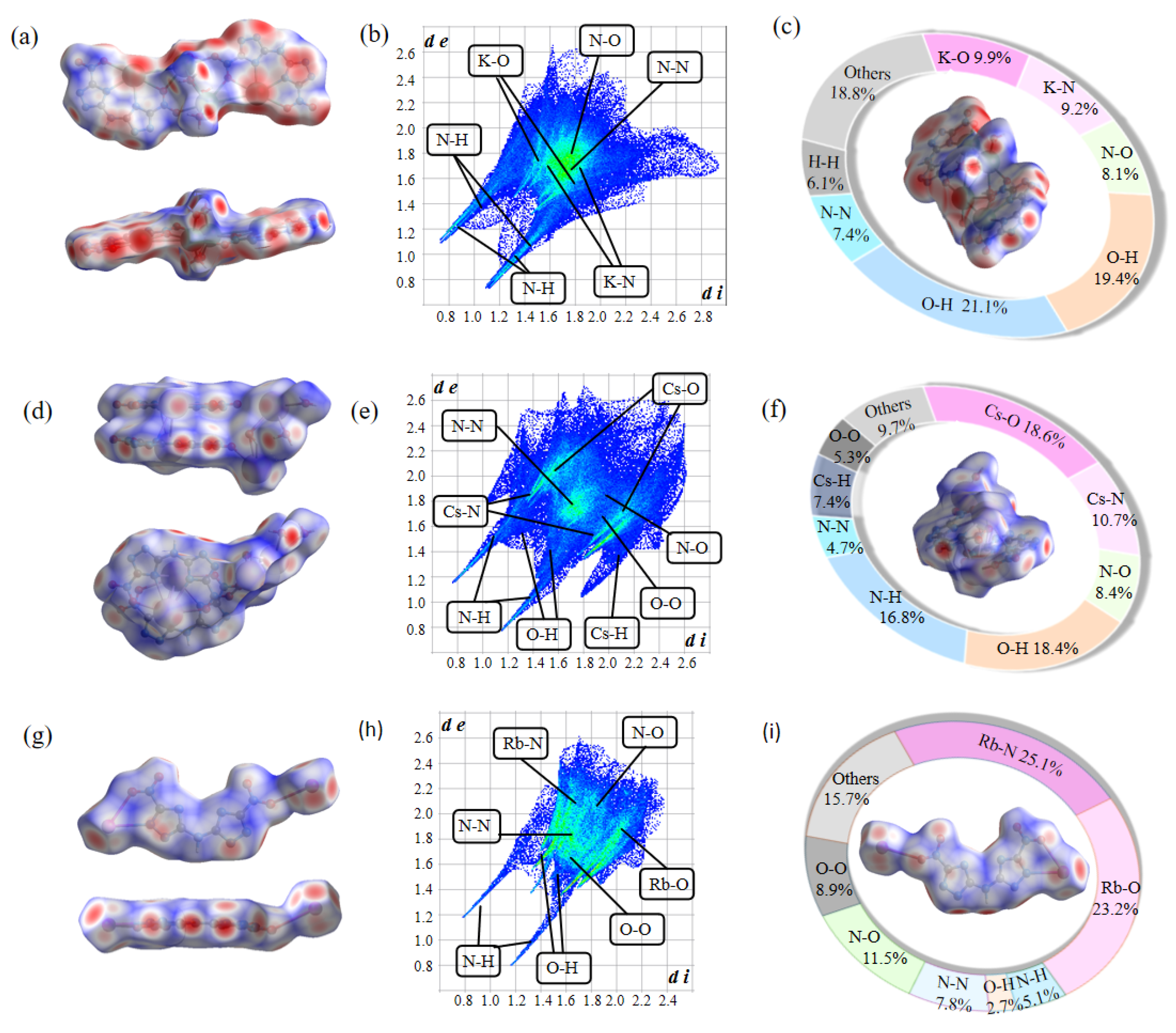
| Compd. | Td a [°C] | ρ b [g·cm−3] | ΔfH c [kJ·mol−1] | D d [m·s−1] | P e [GPa] | IS f [J] | FS g [N] |
|---|---|---|---|---|---|---|---|
| 2 | 283 | 1.860 | −273.4 | 7116 | 17.51 | >40 | >360 |
| 3 | 285 | 1.941 | −429.5 | 8844 | 26.88 | >40 | >360 |
| 4 | 287 | 2.756 | −485.0 | - | - | 15 | 240 |
| 5 | 285 | 2.582/2.556 h | −491.5 | - | - | 20 | 288 |
| [Na(H2BTT) (H2O)2] n [38] | 336 | 1.706 | - | 8120 | 22.83 | >40 | >360 |
| [Cs (ABTNA) H2O] n [41] | 225 | 2.413 | 974.05 | 6780 | 23.9 | 60 | 360 |
| TNT [50] | 295 | 1.65 | −67.0 | 6881 | 19.5 | 15 | 353 |
| RDX [51] | 205 | 1.81 | 86.3 | 8750 | 34.2 | 7.5 | 120 |
| HMX [52] | 279 | 1.90 | 116.1 | 9144 | 41.5 | 7 | 112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wang, J.; Cao, Y.; Wang, K.; Yang, H.; Liu, T. Synthesis and Properties of Energetic MOFs Based on Bis(3-Nitro-1H-1,2,4-triazole-5-yl) Amine: Advancing High Thermal Stability and Low Sensitivity. Molecules 2025, 30, 2478. https://doi.org/10.3390/molecules30122478
Chen S, Wang J, Cao Y, Wang K, Yang H, Liu T. Synthesis and Properties of Energetic MOFs Based on Bis(3-Nitro-1H-1,2,4-triazole-5-yl) Amine: Advancing High Thermal Stability and Low Sensitivity. Molecules. 2025; 30(12):2478. https://doi.org/10.3390/molecules30122478
Chicago/Turabian StyleChen, Shiluo, Jinxin Wang, Yuteng Cao, Kangcai Wang, Haijun Yang, and Tianlin Liu. 2025. "Synthesis and Properties of Energetic MOFs Based on Bis(3-Nitro-1H-1,2,4-triazole-5-yl) Amine: Advancing High Thermal Stability and Low Sensitivity" Molecules 30, no. 12: 2478. https://doi.org/10.3390/molecules30122478
APA StyleChen, S., Wang, J., Cao, Y., Wang, K., Yang, H., & Liu, T. (2025). Synthesis and Properties of Energetic MOFs Based on Bis(3-Nitro-1H-1,2,4-triazole-5-yl) Amine: Advancing High Thermal Stability and Low Sensitivity. Molecules, 30(12), 2478. https://doi.org/10.3390/molecules30122478






