Exploring Salivary Thiocyanate as a Novel Biomarker of Physical Activity Response
Abstract
1. Introduction
2. Results and Discussion
2.1. Method Validation
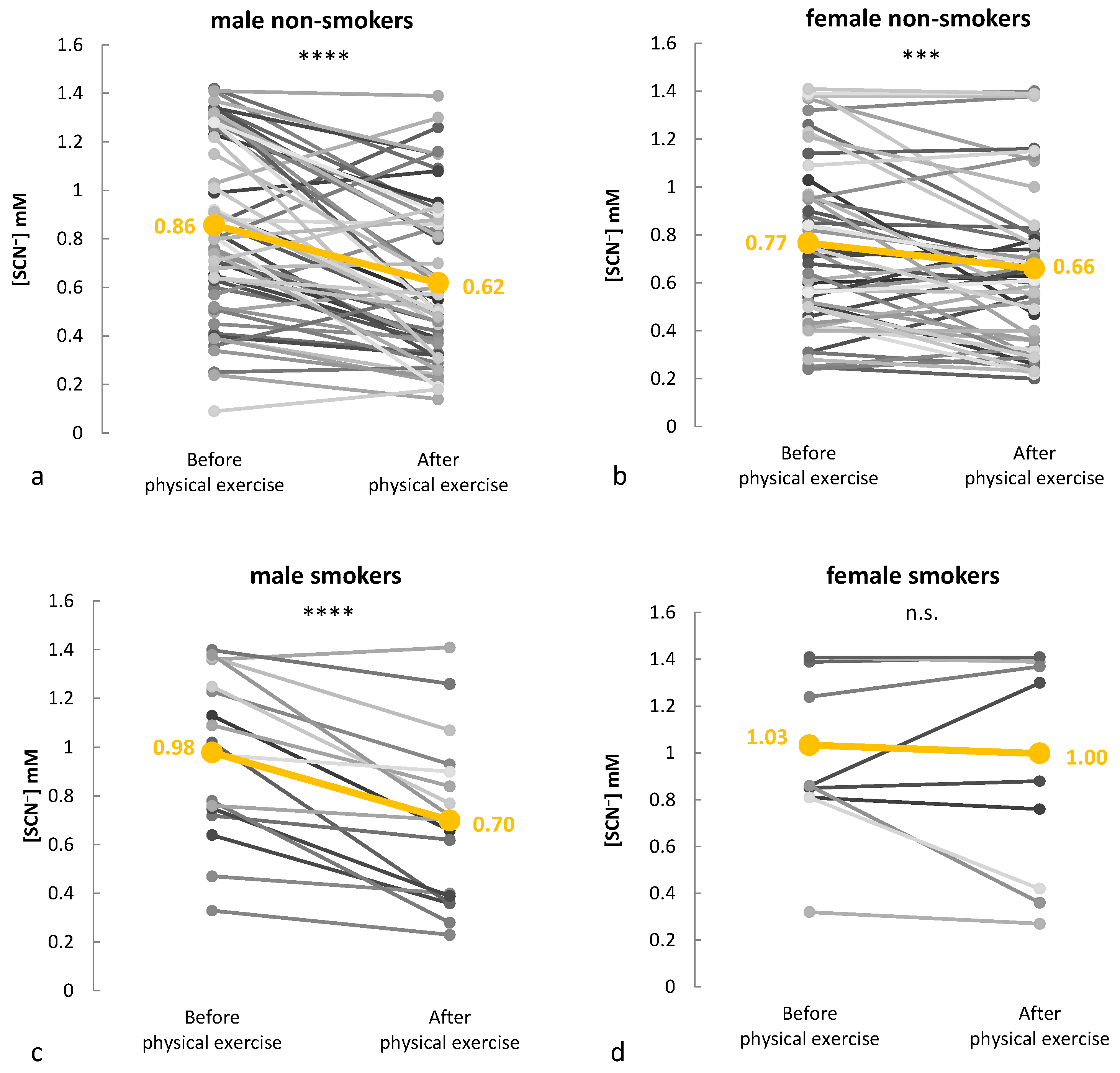
2.2. Thiocyanate Response to Graded Exercise
2.3. Monitoring Salivary Thiocyanate During Real-World Training Sessions
2.4. Biochemical Mechanisms of Exercise-Induced Thiocyanate Reduction
3. Materials and Methods
3.1. Method Development
3.2. Bioethical Approval
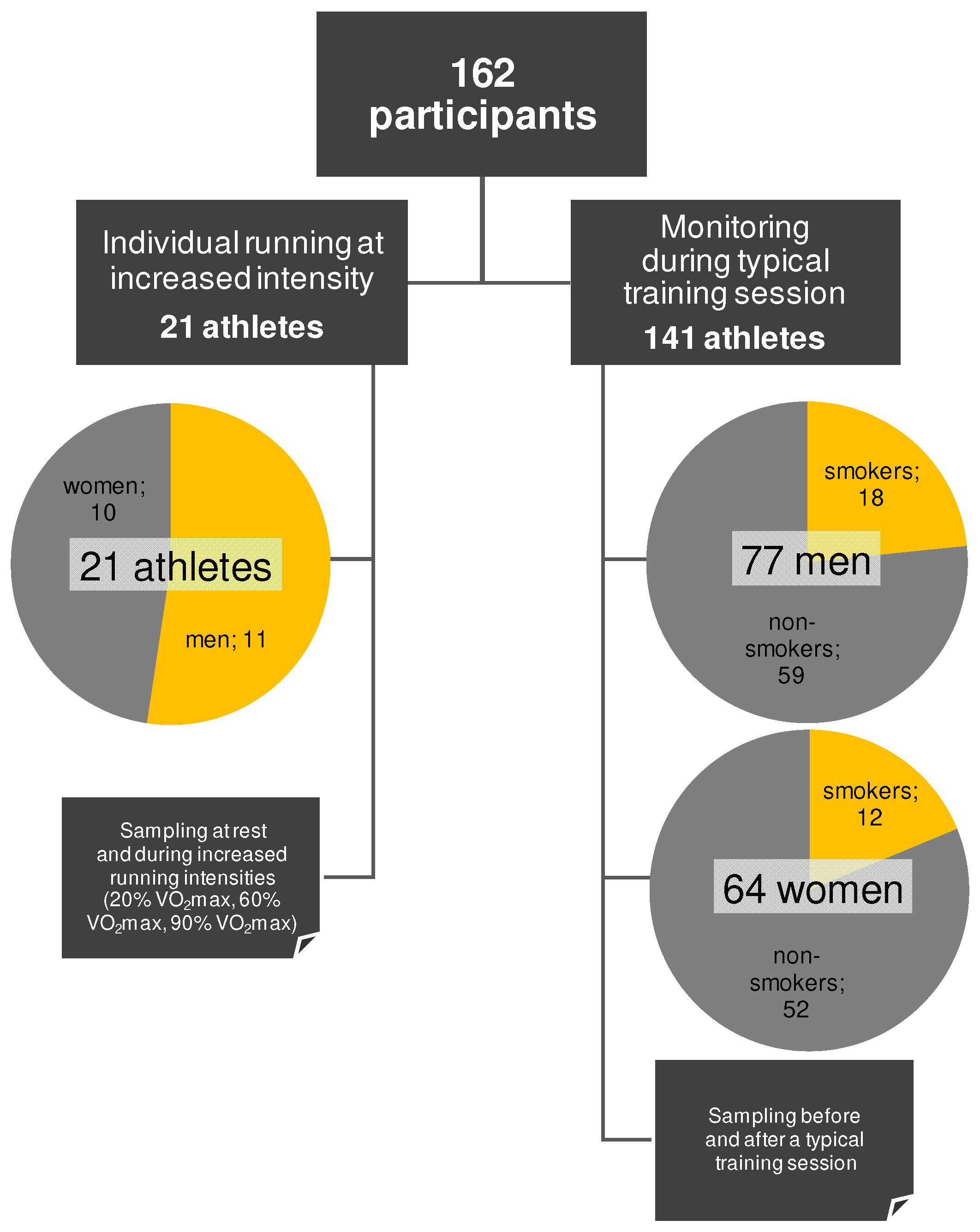
3.3. Participants
3.4. Cohort Management and Experimental Design
3.5. Saliva Collection, Preparation, and Analysis
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelly, R.S.; Kelly, M.P.; Kelly, P. Metabolomics, Physical Activity, Exercise and Health: A Review of the Current Evidence. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165936. [Google Scholar] [CrossRef] [PubMed]
- Eime, R.; Charity, M.; Harvey, J.; Westerbeek, H. Five-Year Changes in Community-Level Sport Participation, and the Role of Gender Strategies. Front. Sports Act. Living 2021, 3, 710666. [Google Scholar] [CrossRef] [PubMed]
- Menhas, R.; Dai, J.; Ashraf, M.A.; Noman, S.M.; Khurshid, S.; Mahmood, S.; Weng, Y.; Laar, R.A.; Sang, X.; Kamran, M.; et al. Physical Inactivity, Non-Communicable Diseases and National Fitness Plan of China for Physical Activity. Risk Manag. Healthc. Policy 2021, 14, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Guilhem, G.; Hanon, C.; Gendreau, N.; Bonneau, D.; Guével, A.; Chennaoui, M. Salivary Hormones Response to Preparation and Pre-Competitive Training of World-Class Level Athletes. Front. Physiol. 2015, 6, 333. [Google Scholar] [CrossRef]
- Furrer, R.; Hawley, J.A.; Handschin, C. The Molecular Athlete: Exercise Physiology from Mechanisms to Medals. Physiol. Rev. 2023, 103, 1693. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Bergeron, M.F.; Lee, E.C.; Mershon, J.E.; Armstrong, E.M. Overtraining Syndrome as a Complex Systems Phenomenon. Front. Netw. Physiol. 2021, 1, 794392. [Google Scholar] [CrossRef]
- Neves, R.S.; da Silva, M.A.R.; de Rezende, M.A.C.; Caldo-Silva, A.; Pinheiro, J.; Santos, A.M.C. Salivary Markers Responses in the Post-Exercise and Recovery Period: A Systematic Review. Sports 2023, 11, 137. [Google Scholar] [CrossRef]
- San-Millán, I. Blood Biomarkers in Sports Medicine and Performance and the Future of Metabolomics. Methods Mol. Biol. 2019, 1978, 431–446. [Google Scholar] [CrossRef]
- Bongiovanni, T.; Lacome, M.; Fanos, V.; Martera, G.; Cione, E.; Cannataro, R. Metabolomics in Team-Sport Athletes: Current Knowledge, Challenges, and Future Perspectives. Proteomes 2022, 10, 27. [Google Scholar] [CrossRef]
- Ferreira, J.; Jimenez, M.; Cerqueira, A.; da Silva, J.R.; Souza, B.; Berard, L.; Bachi, A.L.L.; Dame-Teixeira, N.; Coto, N.; Heller, D. Saliva as a Diagnostic Tool in Soccer: A Scoping Review. PeerJ 2024, 12, e18032. [Google Scholar] [CrossRef]
- da Cruz, J.P.; dos Santos, F.N.; Rasteiro, F.M.; Marostegan, A.B.; Manchado-Gobatto, F.B.; Gobatto, C.A. A Metabolomic Approach and Traditional Physical Assessments to Compare U22 Soccer Players According to Their Competitive Level. Biology 2022, 11, 1103. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, T.; Dessì, A.; Noto, A.; Sardo, S.; Finco, G.; Corsello, G.; Fanos, V.; Pintus, R. Sportomics: Metabolomics Applied to Sports. The New Revolution? Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 11011–11019. [Google Scholar] [CrossRef] [PubMed]
- Prado, E.; Souza, G.H.M.F.; Pegurier, M.; Vieira, C.; Lima-Neto, A.B.M.; Assis, M.; Guedes, M.I.F.; Koblitz, M.G.B.; Ferreira, M.S.L.; Macedo, A.F.; et al. Non-Targeted Sportomics Analyses by Mass Spectrometry to Understand Exercise-Induced Metabolic Stress in Soccer Players. Int. J. Mass Spectrom. 2017, 418, 1–5. [Google Scholar] [CrossRef]
- Corrie, S.R.; Coffey, J.W.; Islam, J.; Markey, K.A.; Kendall, M.A.F. Blood, Sweat, and Tears: Developing Clinically Relevant Protein Biosensors for Integrated Body Fluid Analysis. Analyst 2015, 140, 4350–4364. [Google Scholar] [CrossRef]
- Malamud, D.; Rodriguez-Chavez, I.R. Saliva as a Diagnostic Fluid. Dent. Clin. N. Am. 2011, 55, 159. [Google Scholar] [CrossRef]
- Sohail, M.U.; Al-Mansoori, L.; Al-Jaber, H.; Georgakopoulos, C.; Donati, F.; Botrè, F.; Sellami, M.; Elrayess, M.A. Assessment of Serum Cytokines and Oxidative Stress Markers in Elite Athletes Reveals Unique Profiles Associated with Different Sport Disciplines. Front. Physiol. 2020, 11, 600888. [Google Scholar] [CrossRef]
- Pattinson, C.L.; Meier, T.B.; Guedes, V.A.; Lai, C.; Devoto, C.; Haight, T.; Broglio, S.P.; McAllister, T.; Giza, C.; Huber, D.; et al. Plasma Biomarker Concentrations Associated with Return to Sport Following Sport-Related Concussion in Collegiate Athletes—A Concussion Assessment, Research, and Education (CARE) Consortium Study. JAMA Netw. Open 2020, 3, e2013191. [Google Scholar] [CrossRef]
- Wanner, Z.R.; Southam, C.G.; Sanghavi, P.; Boora, N.S.; Paxman, E.J.; Dukelow, S.P.; Benson, B.W.; Montina, T.; Metz, G.A.S.; Debert, C.T. Alterations in Urine Metabolomics Following Sport-Related Concussion: A 1H NMR-Based Analysis. Front. Neurol. 2021, 12, 645829. [Google Scholar] [CrossRef]
- Pero, R.; Brancaccio, M.; Mennitti, C.; Gentile, L.; Arpino, S.; De Falco, R.; Leggiero, E.; Ranieri, A.; Pagliuca, C.; Colicchio, R.; et al. Urinary Biomarkers: Diagnostic Tools for Monitoring Athletes’ Health Status. Int. J. Environ. Res. Public Health 2020, 17, 6065. [Google Scholar] [CrossRef]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical Tattoo Biosensors for Real-Time Noninvasive Lactate Monitoring in Human Perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef]
- Chrimatopoulos, C.; Pavlou, E.; Kourkoumelis, N.; Sakkas, V. Discriminating the Salivary Profile of Athletes Using ATR-FTIR Spectroscopy and Chemometrics. Chemom. Intell. Lab. Syst. 2022, 230, 104660. [Google Scholar] [CrossRef]
- Al-Khelaifi, F.; Diboun, I.; Donati, F.; Botrè, F.; Alsayrafi, M.; Georgakopoulos, C.; Suhre, K.; Yousri, N.A.; Elrayess, M.A. A Pilot Study Comparing the Metabolic Profiles of Elite-Level Athletes from Different Sporting Disciplines. Sports Med. Open 2018, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Alzharani, M.A.; Alshuwaier, G.O.; Aljaloud, K.S.; Al-Tannak, N.F.; Watson, D.G. Metabolomics Profiling of Plasma, Urine and Saliva after Short Term Training in Young Professional Football Players in Saudi Arabia. Sci. Rep. 2020, 10, 19759. [Google Scholar] [CrossRef] [PubMed]
- Luti, S.; Militello, R.; Pinto, G.; Illiano, A.; Amoresano, A.; Chiappetta, G.; Marzocchini, R.; Modesti, P.A.; Pratesi, S.; Pazzagli, L.; et al. Chronic Training Induces Metabolic and Proteomic Response in Male and Female Basketball Players: Salivary Modifications during In-Season Training Programs. Healthcare 2023, 11, 241. [Google Scholar] [CrossRef]
- Volodchenko, O.A.; Podrigalo, L.V.; Iermakov, S.S.; Zychowska, M.T.; Jagiełło, W. The Usefulness of Performing Biochemical Tests in the Saliva of Kickboxing Athletes in the Dynamic of Training. Biomed. Res. Int. 2019, 2019, 2014347. [Google Scholar] [CrossRef]
- Siekierzycka, A.; Radulska, A.; Woźniak, M.; Pelikant-Małecka, I.; Janaszak-Jasiecka, A.; Lewicka, E.; Kalinowski, L.; Olek, R.A. Plasma Cardiovascular Stress Biomarkers Response to Marathon Running. Sports Med. Health Sci. 2024. [Google Scholar] [CrossRef]
- Caetano Júnior, P.C.; Lemes, L.C.; Aguiar, J.C.; Strixino, J.F.; Raniero, L. Application of FT-IR Spectroscopy to Assess Physiological Stress in Rugby Players during Fatigue Test. Res. Biomed. Eng. 2016, 32, 123–128. [Google Scholar] [CrossRef]
- Bonne, N.J.; Wong, D.T.W. Salivary Biomarker Development Using Genomic, Proteomic and Metabolomic Approaches. Genome Med. 2012, 4, 82. [Google Scholar] [CrossRef]
- Hyvärinen, E.; Savolainen, M.; Mikkonen, J.J.W.; Kullaa, A.M. Salivary Metabolomics for Diagnosis and Monitoring Diseases: Challenges and Possibilities. Metabolites 2021, 11, 587. [Google Scholar] [CrossRef]
- Ferrari, E.; Gallo, M.; Spisni, A.; Antonelli, R.; Meleti, M.; Pertinhez, T.A. Human Serum and Salivary Metabolomes: Diversity and Closeness. Int. J. Mol. Sci. 2023, 24, 16603. [Google Scholar] [CrossRef]
- Gardner, A.; Carpenter, G.; So, P.W. Salivary Metabolomics: From Diagnostic Biomarker Discovery to Investigating Biological Function. Metabolites 2020, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Bellagambi, F.G.; Lomonaco, T.; Salvo, P.; Vivaldi, F.; Hangouët, M.; Ghimenti, S.; Biagini, D.; Di Francesco, F.; Fuoco, R.; Errachid, A. Saliva Sampling: Methods and Devices. An Overview. TrAC Trends Anal. Chem. 2020, 124, 115781. [Google Scholar] [CrossRef]
- Ashton, N.J.; Ide, M.; Zetterberg, H.; Blennow, K. Salivary Biomarkers for Alzheimer’s Disease and Related Disorders. Neurol. Ther. 2019, 8, 83–94. [Google Scholar] [CrossRef]
- Gleerup, H.S.; Hasselbalch, S.G.; Simonsen, A.H. Biomarkers for Alzheimer’s Disease in Saliva: A Systematic Review. Dis. Markers 2019, 2019, 4761054. [Google Scholar] [CrossRef]
- Wang, X.; Kaczor-Urbanowicz, K.E.; Wong, D.T.W. Salivary Biomarkers in Cancer Detection. Med. Oncol. 2017, 34, 7. [Google Scholar] [CrossRef]
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T.W. Variations of Oral Microbiota Are Associated with Pancreatic Diseases Including Pancreatic Cancer. Gut 2012, 61, 582–588. [Google Scholar] [CrossRef]
- Wong, D.T. Towards a Simple, Saliva-Based Test for the Detection of Oral Cancer. Expert Rev. Mol. Diagn. 2006, 6, 267–272. [Google Scholar] [CrossRef]
- Li, X.; Yang, T.; Lin, J. Spectral Analysis of Human Saliva for Detection of Lung Cancer Using Surface-Enhanced Raman Spectroscopy. J. Biomed. Opt. 2012, 17, 037003. [Google Scholar] [CrossRef]
- Miller, C.S.; King, C.P.; Langub, M.C.; Kryscio, R.J.; Thomas, M.V. Salivary Biomarkers of Existing Periodontal Disease: A Cross-Sectional Study. J. Am. Dent. Assoc. 2006, 137, 322–329. [Google Scholar] [CrossRef]
- Kaufman, E.; Lamster, I.B. Analysis of Saliva for Periodontal Diagnosis. J. Clin. Periodontol. 2000, 27, 453–465. [Google Scholar] [CrossRef]
- Ntovas, P.; Loumprinis, N.; Maniatakos, P.; Margaritidi, L.; Rahiotis, C. The Effects of Physical Exercise on Saliva Composition: A Comprehensive Review. Dent. J. 2022, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd-Markwick, K.; Starck, C.; Dulson, D.K.; Ali, A. Salivary Diagnostic Markers in Males and Females during Rest and Exercise. J. Int. Soc. Sports Nutr. 2017, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Pitti, E.; Petrella, G.; Di Marino, S.; Summa, V.; Perrone, M.; D’ottavio, S.; Bernardini, A.; Cicero, D.O. Salivary Metabolome and Soccer Match: Challenges for Understanding Exercise Induced Changes. Metabolites 2019, 9, 141. [Google Scholar] [CrossRef]
- Chrimatopoulos, C.; Chrimatopoulos, G.; Sakkas, V. Investigating Oral Biofluid Markers as a Tool for the Chemometric Discrimination of Different Physical Exercise Intensities Utilizing ATR-FTIR Spectroscopy. Chemom. Intell. Lab. Syst. 2023, 242, 104990. [Google Scholar] [CrossRef]
- Tékus, É.; Kaj, M.; Szabó, E.; Szénási, N.L.; Kerepesi, I.; Figler, M.; Gábriel, R.; Wilhelm, M. Comparison of Blood and Saliva Lactate Level after Maximum Intensity Exercise. Acta Biol. Hung. 2012, 63, 89–98. [Google Scholar] [CrossRef]
- Dame, Z.T.; Aziat, F.; Mandal, R.; Krishnamurthy, R.; Bouatra, S.; Borzouie, S.; Guo, A.C.; Sajed, T.; Deng, L.; Lin, H.; et al. The Human Saliva Metabolome. Metabolomics 2015, 11, 1864–1883. [Google Scholar] [CrossRef]
- Bosman, P.; Pichon, V.; Acevedo, A.C.; Chardin, H.; Combes, A. Development of Analytical Methods to Study the Salivary Metabolome: Impact of the Sampling. Anal. Bioanal. Chem. 2022, 414, 6899–6909. [Google Scholar] [CrossRef]
- Vitorino, R.; Lobo, M.J.C.; Ferrer-Correira, A.J.; Dubin, J.R.; Tomer, K.B.; Domingues, P.M.; Amado, F.M.L. Identification of Human Whole Saliva Protein Components Using Proteomics. Proteomics 2004, 4, 1109–1115. [Google Scholar] [CrossRef]
- Nakamura, M.; Slots, J. Salivary Enzymes. J. Periodontal Res. 1983, 18, 559–569. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Sarf, E.A.; Loginova, A.I. Diagnostic Value of Salivary Amino Acid Levels in Cancer. Metabolites 2023, 13, 950. [Google Scholar] [CrossRef]
- Neyraud, E.; Tremblay-Franco, M.; Gregoire, S.; Berdeaux, O.; Canlet, C. Relationships between the Metabolome and the Fatty Acid Composition of Human Saliva; Effects of Stimulation. Metabolomics 2013, 9, 213–222. [Google Scholar] [CrossRef]
- Gatti, R.; De Palo, E.F. An Update: Salivary Hormones and Physical Exercise. Scand. J. Med. Sci. Sports 2011, 21, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Crewther, B.; Cronin, J.; Keogh, J.; Cook, C. The Salivary Testosterone and Cortisol Response to Three Loading Schemes. J. Strength Cond. Res. 2008, 22, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Park, N.J.; Li, Y.; Yu, T.; Brinkman, B.M.N.; Wong, D.T. Characterization of RNA in Saliva. Clin. Chem. 2006, 52, 988–994. [Google Scholar] [CrossRef]
- Zimmermann, B.G.; Noh, J.P.; Wong, D.T. Genomic Targets in Saliva. Ann. N. Y. Acad. Sci. 2007, 1098, 184–191. [Google Scholar] [CrossRef]
- Vissink, A.; Karst, F.; Spijkervet, L.; Van, A.; Amerongen, N. Aging and Saliva: A Review of the Literature. Spec. Care Dent. 1996, 16, 95–103. [Google Scholar] [CrossRef]
- Shern, R.J.; Fox, P.C.; Li, S.H. Influence of Age on the Secretory Rates of the Human Minor Salivary Glands and Whole Saliva. Arch. Oral. Biol. 1993, 38, 755–761. [Google Scholar] [CrossRef]
- Muro, C.K.; De Souza Fernandes, L.; Lednev, I.K. Sex Determination Based on Raman Spectroscopy of Saliva Traces for Forensic Purposes. Anal. Chem. 2016, 88, 12489–12493. [Google Scholar] [CrossRef]
- Singh, M.; Ingle, N.; Kaur, N.; Yadav, P.; Ingle, E. Effect of Long-Term Smoking on Salivary Flow Rate and Salivary pH. J. Indian Assoc. Public Health Dent. 2015, 13, 11. [Google Scholar] [CrossRef]
- Kondakova, I.; Lissi, E.A.; Pizarro, M. Total Reactive Antioxidant Potential in Human Saliva of Smokers and Non-Smokers. IUBMB Life 1999, 47, 911–920. [Google Scholar] [CrossRef]
- Wolff, A.; Joshi, R.K.; Ekström, J.; Aframian, D.; Pedersen, A.M.L.; Proctor, G.; Narayana, N.; Villa, A.; Sia, Y.W.; Aliko, A.; et al. A Guide to Medications Inducing Salivary Gland Dysfunction, Xerostomia, and Subjective Sialorrhea: A Systematic Review Sponsored by the World Workshop on Oral Medicine VI. Drugs RD 2016, 17, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Aps, J.K.M.; Martens, L.C. Review: The Physiology of Saliva and Transfer of Drugs into Saliva. Forensic Sci. Int. 2005, 150, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, K.M. The Salivary Peroxidase System: Thermodynamic, Kinetic and Antibacterial Properties. J. Oral. Pathol. Med. 1987, 16, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Dogon, I.L.; Amdur, B.H. Evidence for the Presence of Two Thiocyanate-Dependent Antibacterial Systems in Human Saliva. Arch. Oral. Biol. 1970, 15, 987-IN12. [Google Scholar] [CrossRef]
- Rodrigues, R.P.C.B.; Aguiar, E.M.G.; Cardoso-Sousa, L.; Caixeta, D.C.; Guedes, C.C.F.V.; Siqueira, W.L.; Maia, Y.C.P.; Cardoso, S.V.; Sabino-Silva, R. Differential Molecular Signature of Human Saliva Using ATR-FTIR Spectroscopy for Chronic Kidney Disease Diagnosis. Braz. Dent. J. 2019, 30, 437–445. [Google Scholar] [CrossRef]
- Welk, A.; Rudolph, P.; Kreth, J.; Schwahn, C.; Kramer, A.; Below, H. Microbicidal Efficacy of Thiocyanate Hydrogen Peroxide after Adding Lactoperoxidase under Saliva Loading in the Quantitative Suspension Test. Arch. Oral. Biol. 2011, 56, 1576–1582. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Sarf, E.A. Biochemical Composition and Characteristics of Salivary FTIR Spectra: Correlation Analysis. J. Mol. Liq. 2021, 341, 117380. [Google Scholar] [CrossRef]
- Adolphe, Y.; Jacquot, M.; Linder, M.; Revol-Junelles, A.M.; Millière, J.B. Optimization of the Components Concentrations of the Lactoperoxidase System by RSM. J. Appl. Microbiol. 2006, 100, 1034–1042. [Google Scholar] [CrossRef]
- Madiyal, A.; Ajila, V.; Babu, S.G.; Hegde, S.; Kumari, S.; Madi, M.; Achalli, S.; Alva, P.; Ullal, H. Status of Thiocyanate Levels in the Serum and Saliva of Non-Smokers, Ex-Smokers and Smokers. Afr. Health Sci. 2018, 18, 727. [Google Scholar] [CrossRef]
- Luepker, R.V.; Pechacek, T.F.; Murray, D.M.; Johnson, C.A.; Hund, F.; Jacobs, D.R. Saliva Thiocyanate: A Chemical Indicator of Cigarette Smoking in Adolescents. Am. J. Public Health 1981, 71, 1320. [Google Scholar] [CrossRef]
- Pavelka, V.; Lazanas, A.C.; Sarigiannidou, M.; Hrbac, J.; Prodromidis, M.I. Nanostructured Cobalt(II) Phthalocyanine Modified Screen-Printed Electrodes for the Determination of Thiocyanate in Human Saliva. Electroanalysis 2024, 36, e202400011. [Google Scholar] [CrossRef]
- Mori, M.; Aoyagi, K.; Tomoda, T.; Ishikawara, F.; Sakamoto, S.; Myochin, H.; Kuga, M.; Kozaki, D.; Ohshima, N.; Izumi, T.; et al. Simultaneous Capillary Electrophoresis of Anions and Cations in a Single Injection Using an Anion Exchanger-Modified Capillary for Determination of Salivary Ions in Combination with Statistical Analyses. J. Chromatogr. A 2021, 1635, 461647. [Google Scholar] [CrossRef] [PubMed]
- Michigami, Y.; Fujii, K.; Ueda, K.; Yamamoto, Y. Determination of Thiocyanate in Human Saliva and Urine by Ion Chromatography. Analyst 1992, 117, 1855–1858. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.A.; Renaud, D.E.; Krishnasamy, S.; Meriç, P.; Buduneli, N.; Çetinkalp, Ş.; Liu, K.Z. Diabetes-Related Molecular Signatures in Infrared Spectra of Human Saliva. Diabetol. Metab. Syndr. 2010, 2, 48. [Google Scholar] [CrossRef]
- Schultz, C.P.; Ahmed, M.K.; Dawes, C.; Mantsch, H.H. Thiocyanate Levels in Human Saliva: Quantitation by Fourier Transform Infrared Spectroscopy. Anal. Biochem. 1996, 240, 7–12. [Google Scholar] [CrossRef]
- Cookeas, E.G.; Efstathiou, C.E. Flow Injection Amperometric Determination of Thiocyanate and Selenocyanate at a Cobalt Phthalocyanine Modified Carbon Paste Electrode. Analyst 1994, 119, 1607–1612. [Google Scholar] [CrossRef]
- Van Staden, J.F.; Botha, A. Spectrophotometric Determination of Thiocyanate by Sequential Injection Analysis. Anal. Chim. Acta 2000, 403, 279–286. [Google Scholar] [CrossRef]
- Lahti, M.; Vilpo, J.; Hovinen, J. Spectrophotometric Determination of Thiocyanate in Human Saliva. J. Chem. Educ. 1999, 76, 1281–1282. [Google Scholar] [CrossRef]
- Stalikas, C.; Sakkas, V. From a Glimpse into the Key Aspects of Calibration and Correlation to Their Practical Considerations in Chemical Analysis. Microchim. Acta 2024, 191, 81. [Google Scholar] [CrossRef]
- Chrimatopoulos, C.; Nousis, L.; Diamanti, C.; Tsiostas, C.; Sakkas, V.; Tsilidis, K.K.; Ntzani, E. Development of Low-Cost Methodologies for the Determination of Total Phosphorus, Total Nitrogen and COD, as an Alternative to Commercially Available Kit Tests. Int. J. Environ. Anal. Chem. 2025, 1–21. [Google Scholar] [CrossRef]
- Molgat-Seon, Y.; Peters, C.M.; Sheel, A.W. Sex-Differences in the Human Respiratory System and Their Impact on Resting Pulmonary Function and the Integrative Response to Exercise. Curr. Opin. Physiol. 2018, 6, 21–27. [Google Scholar] [CrossRef]
- Inoue, H.; Ono, K.; Masuda, W.; Morimoto, Y.; Tanaka, T.; Yokota, M.; Inenaga, K. Gender Difference in Unstimulated Whole Saliva Flow Rate and Salivary Gland Sizes. Arch. Oral. Biol. 2006, 51, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Cendali, F.; D’Alessandro, A.; Nemkov, T. Dried Blood Spot Characterization of Sex-Based Metabolic Responses to Acute Running Exercise. Anal. Sci. Adv. 2023, 4, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Kawka, J.; Tatarczak-Michalewska, M. Levels of the Thiocyanate in the Saliva of Tobacco Smokers in Comparison to E-Cigarette Smokers and Nonsmokers Measured by HPLC on a Phosphatidylcholine Column. Molecules 2019, 24, 3790. [Google Scholar] [CrossRef]
- Magacz, M.; Kędziora, K.; Sapa, J.; Krzyściak, W. The Significance of Lactoperoxidase System in Oral Health: Application and Efficacy in Oral Hygiene Products. Int. J. Mol. Sci. 2019, 20, 1443. [Google Scholar] [CrossRef]
- Reiter, B. The Lactoperoxidase-Thiocyanate-Hydrogen Peroxide Antibacterium System. In Ciba Foundation Symposium 65-Oxygen Free Radicals and Tissue Damage; John Wiley & Sons: Chichester, UK, 1978; pp. 285–294. [Google Scholar] [CrossRef]
- Grzesiak-Gasek, I.; Kaczmarek, U. Influence of Swimming Training Session on Selected Saliva Components in Youth Swimmers. Front. Physiol. 2022, 13, 869903. [Google Scholar] [CrossRef]
- Damirchi, A.; Saati Zareei, A.; Sariri, R. Salivary Antioxidants of Male Athletes after Aerobic Exercise and Garlic Supplementation on: A Randomized, Double Blind, Placebo-Controlled Study. J. Oral Biol. Craniofacial Res. 2015, 5, 146–152. [Google Scholar] [CrossRef]
- Alves, R.C.C.; Ferreira, R.O.; Frazão, D.R.; de Souza Né, Y.G.; Mendes, P.F.S.; Marañón-Vásquez, G.; Royes, L.F.F.; Fagundes, N.C.F.; Maia, L.C.; Lima, R.R. The Relationship between Exercise and Salivary Oxidative Stress: A Systematic Review. Antioxidants 2022, 11, 1489. [Google Scholar] [CrossRef]
- de Bari, L.; Valenti, D.; Atlante, A.; Passarella, S. L-Lactate Generates Hydrogen Peroxide in Purified Rat Liver Mitochondria Due to the Putative l-Lactate Oxidase Localized in the Intermembrane Space. FEBS Lett. 2010, 584, 2285–2290. [Google Scholar] [CrossRef]
- Passarella, S.; de Bari, L.; Valenti, D.; Pizzuto, R.; Paventi, G.; Atlante, A. Mitochondria and L-Lactate Metabolism. FEBS Lett. 2008, 582, 3569–3576. [Google Scholar] [CrossRef]
- Bartoloni, B.; Mannelli, M.; Gamberi, T.; Fiaschi, T. The Multiple Roles of Lactate in the Skeletal Muscle. Cells 2024, 13, 1177. [Google Scholar] [CrossRef]
- Barnard, J.P.; Stinson, M.W. Influence of Environmental Conditions on Hydrogen Peroxide Formation by Streptococcus gordonii. Infect. Immun. 1999, 67, 6558–6564. [Google Scholar] [CrossRef]
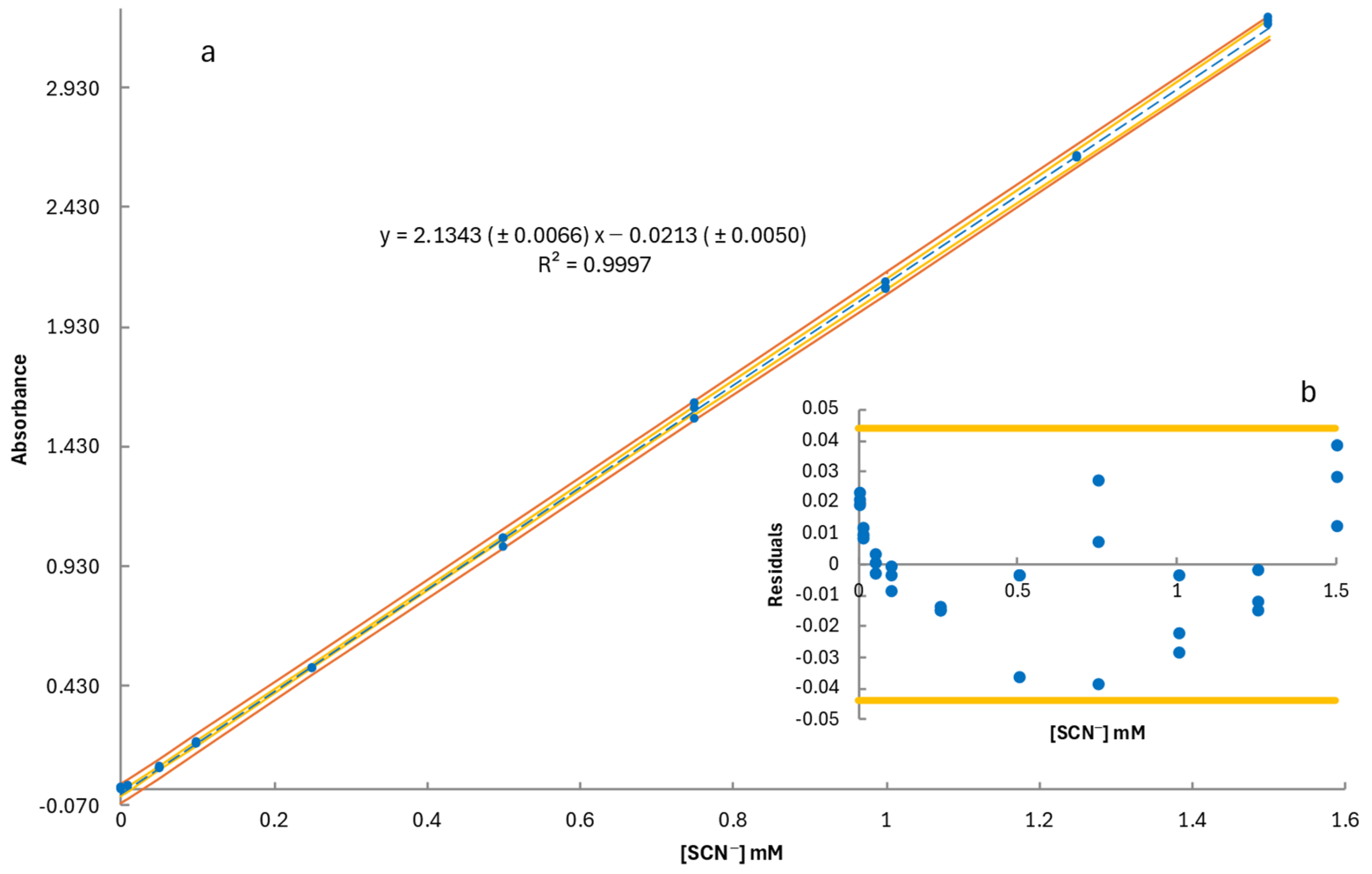

| Regression Statistics | |||||
|---|---|---|---|---|---|
| Multiple R | 0.999866794 | ||||
| R Square | 0.999733605 | ||||
| Adjusted R Square | 0.999724091 | ||||
| Standard Error | 0.018990959 a | ||||
| Observations | 30 | ||||
| ANOVA | |||||
| df | SS | MS | F | Significance F | |
| Regression | 1 | 37.89749192 | 37.89749192 | 105,079.1775 | 1.35487 × 10−51 |
| Residual | 28 | 0.010098383 | 0.000360657 | ||
| Total | 29 | 37.9075903 | |||
| Coefficients | Standard Error | t Stat | p-value | ||
| Intercept | −0.021333701 | 0.004970841 b | −4.291768989 | 0.000191323 | |
| X Variable 1 | 2.134258228 | 0.006583982 c | 324.1591855 | 1.35487 × 10−51 | |
| Lower 95% | Upper 95% | ||||
| Intercept | −0.031516007 | −0.011151395 | |||
| X Variable 1 | 2.120771552 | 2.147744903 | |||
| Analytical Parameters | ||
|---|---|---|
| LOD (mM) | 0.004 | |
| LOQ (mM) | 0.01 | |
| Working range (mM) | 0.01–1.5 | |
| Uncertainty (%) | 4.51 | |
| Accuracy (%) | 0.1 mM | 110.22 |
| 0.7 mM | 98.36 | |
| 1.25 mM | 97.57 | |
| Intra-day repeatability (% RSD) | 0.1 mM | 3.70 |
| 0.7 mM | 0.57 | |
| 1.25 mM | 0.54 | |
| Inter-day repeatability/reproducibility (% RSD) | 0.1 mM | 3.13 |
| 0.7 mM | 0.83 | |
| 1.25 mM | 0.56 | |
| ANOVA | Post-Hoc Test (Bonferroni) | |||
|---|---|---|---|---|
| Rest–20% VO2max (mL/kg/min) | 20–60% VO2max (mL/kg/min) | 60–90% VO2max (mL/kg/min) | ||
| Men | 0.013949 | 0.016197 | 0.001770 | 0.001243 |
| Women | 0.023837 | 0.009487 | 0.015651 | 0.001174 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrimatopoulos, C.; Papadopoulou, K.; Tsilouli, T.; Sakkas, V. Exploring Salivary Thiocyanate as a Novel Biomarker of Physical Activity Response. Molecules 2025, 30, 2476. https://doi.org/10.3390/molecules30112476
Chrimatopoulos C, Papadopoulou K, Tsilouli T, Sakkas V. Exploring Salivary Thiocyanate as a Novel Biomarker of Physical Activity Response. Molecules. 2025; 30(11):2476. https://doi.org/10.3390/molecules30112476
Chicago/Turabian StyleChrimatopoulos, Christoforos, Kalliopi Papadopoulou, Theodora Tsilouli, and Vasilios Sakkas. 2025. "Exploring Salivary Thiocyanate as a Novel Biomarker of Physical Activity Response" Molecules 30, no. 11: 2476. https://doi.org/10.3390/molecules30112476
APA StyleChrimatopoulos, C., Papadopoulou, K., Tsilouli, T., & Sakkas, V. (2025). Exploring Salivary Thiocyanate as a Novel Biomarker of Physical Activity Response. Molecules, 30(11), 2476. https://doi.org/10.3390/molecules30112476







