Current Approaches to Microplastics Detection and Plastic Biodegradation
Abstract
1. Introduction
2. Analytical Techniques Used to Detect Biodegradation of Plastics
2.1. Microscopic Methods
2.1.1. Scanning Electron Microscopy
2.1.2. Transmission Electron Microscopy (TEM)
2.1.3. Atomic Force Microscopy
2.1.4. Fluorescence Microscopy/Nile Red (NR) Staining
2.2. Spectroscopic Methods
2.2.1. Raman Spectroscopy
2.2.2. X-Ray Photoelectron Spectroscopy (XPS)
2.2.3. X-Ray Diffraction (XRD)
2.2.4. Fourier Transform Infrared Spectroscopy
2.2.5. Nanoparticle Tracking Analysis (NTA)
2.2.6. Dynamic Light Scattering (DLS)
2.3. Chemical and Analytical Methods
2.3.1. Weight Loss Measurement
2.3.2. Clear Zone Formation
2.3.3. Contact Angle Measurement (CA)
2.3.4. Thermogravimetric Analysis (TGA)
2.3.5. Differential Scanning Calorimetry (DSC)
2.3.6. Pyrolysis-Gas Chromatography/Mass Spectrometry
2.3.7. High-Performance Liquid Chromatography (HPLC)/Ultra-Performance Liquid Chromatography (UPLC)
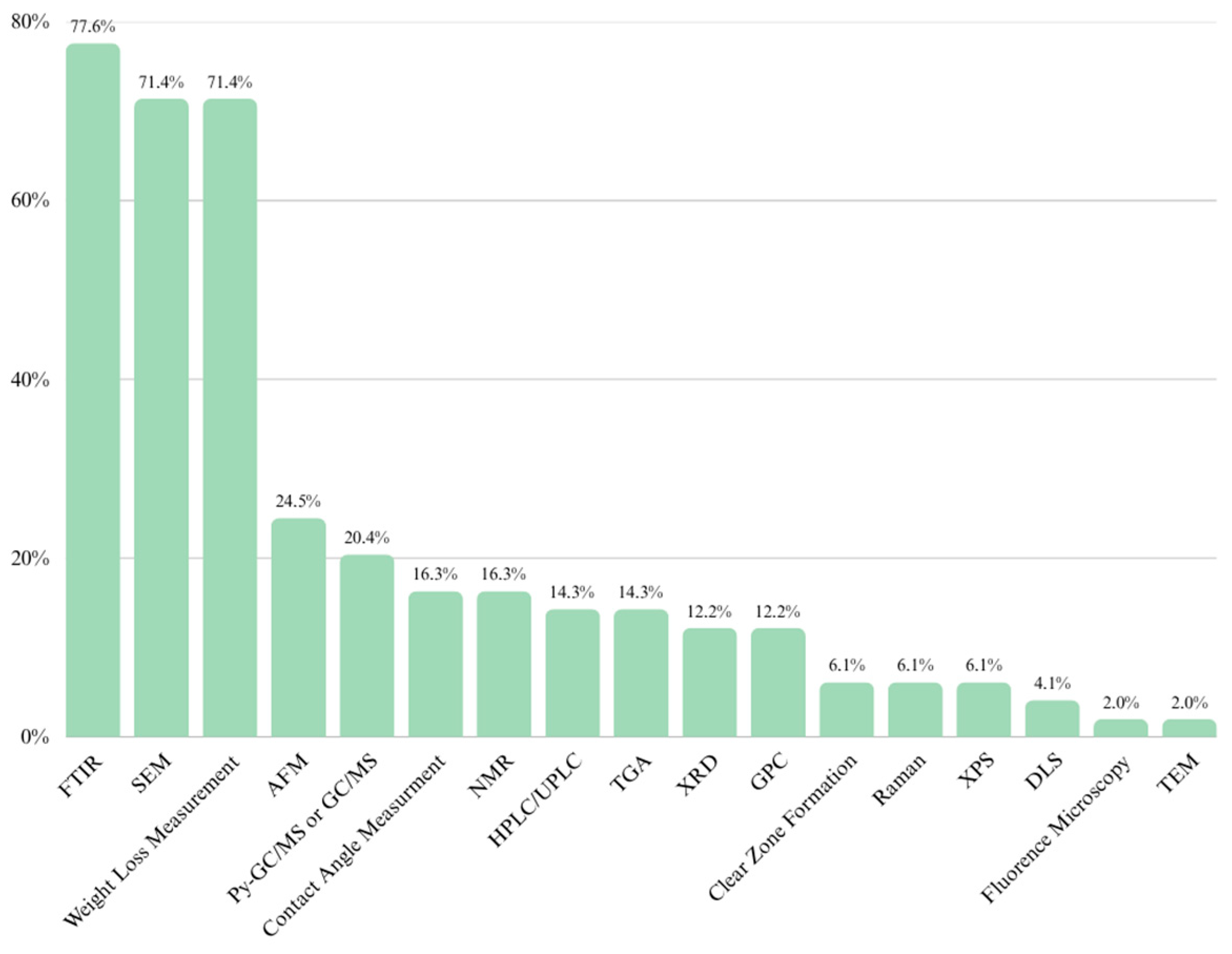
2.3.8. A Multi-Aspect Comparison of MPs Detection Methods
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MPs | microplastics |
| PET | polyethylene terephthalate |
| PE | polyethylene |
| LLDPE | linear low-density polyethylene |
| HDPE | high-density polyethylene |
| PP | polypropylene |
| PUR | polyurethane |
| PS | polystyrene |
| PVC | polyvinyl chloride |
| PLA | polylactic acid |
| PBS | polybutylene succinate |
| PHA | polyhydroxyalkanoates |
| PCL | polycaprolactone |
| SEM | scanning electron microscopy |
| AFM | atomic force microscopy |
| TEM | transmission electron microscopy |
| HPLC | high-performance liquid chromatography |
| UPLC | ultra-performance liquid chromatography |
| FTIR | Fourier transform infrared spectroscopy |
| Pyr-GC/MS | pyrolysis–gas chromatography–mass spectrometry |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| DSC | differential scanning calorimetry |
| CA | contact angle |
| TGA | thermogravimetric analysis |
| NTA | nanoparticle tracking analysis |
| DLS | dynamic light scattering |
| NR | Nile red |
References
- Kim, M.S.; Chang, H.; Zheng, L.; Yan, Q.; Pfleger, B.F.; Klier, J.; Nelson, K.; Majumder, E.L.; Huber, G.W. A Review of Biodegradable Plastics: Chemistry, Applications, Properties, and Future Research Needs. Chem. Rev. 2023, 123, 9915–9939. [Google Scholar] [CrossRef]
- PlasticEurope. Plastic-the Facts 2023. An Analysis of European Plastic Production, Demand and Waste Data. 2023. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/ (accessed on 6 March 2025).
- Kaur, P.; Singh, K.; Singh, B. Microplastics in soil: Impacts and microbial diversity and degradation. Pedosphere 2022, 32, 49–60. [Google Scholar] [CrossRef]
- Urbanek, A.K.; Rymowicz, W.; Mironczuk, A.M. Degradation of plastics and plastic-degrading bacteria in cold marine habitats. Appl. Microbiol. Biotechnol. 2018, 102, 7669–7678. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Gigault, J.; Halle, A.T.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Urbanek, A.K.; Kosiorowska, K.E.; Mironczuk, A.M. Current Knowledge on Polyethylene Terephthalate Degradation by Genetically Modified Microorganisms. Front. Bioeng. Biotechnol. 2021, 9, 771133. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly (ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- Wilkes, R.A.; Aristilde, L. Degradation and metabolism of synthetic plastics and associated products by Pseudomonas sp.: Capabilities and challenges. J. Appl. Microbiol. 2017, 123, 582–593. [Google Scholar] [CrossRef]
- Zhu, J.; Dong, G.; Feng, F.; Ye, J.; Liao, C.H.; Wu, C.H.; Chen, S.C. Microplastics in the soil environment: Focusing on the sources, its transformation and change in morphology. Sci. Total Environ. 2023, 896, 165291. [Google Scholar] [CrossRef]
- He, W.; Liu, S.; Zhang, W.; Yi, K.; Zhang, C.; Pang, H.; Huang, D.; Huang, J.; Li, X. Recent advances on microplastic aging: Identification, mechanism, influence factors, and additives release. Sci. Total Environ. 2023, 889, 164035. [Google Scholar] [CrossRef]
- Lei, K.; Qiao, F.; Liu, Q.; Wei, Z.; Qi, H.; Cui, S.; Yue, X.; Deng, Y.; An, L. Microplastics releasing from personal care and cosmetic products in China. Mar. Pollut. Bull. 2017, 123, 122–126. [Google Scholar] [CrossRef]
- Wagner, M.; Scherer, C.; Alvarez-Munoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef]
- Sekudewicz, I.; Dabrowska, A.M.; Syczewski, M.D. Microplastic pollution in surface water and sediments in the urban section of the Vistula River (Poland). Sci. Total Environ. 2021, 762, 143111. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Fu, Z.; Guan, D.; Zhang, D.; Zhang, H.; Zhang, Q.; Xie, J.; Sun, Y.; Wang, D. Innovative overview of the occurrence, aging characteristics, and ecological toxicity of microplastics in environmental media. Environ. Pollut. 2024, 346, 123623. [Google Scholar] [CrossRef]
- Schwabl, P.; Koppel, S.; Konigshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- He, D.; Zhang, Y.; Gao, W. Micro(nano)plastic contaminations from soils to plants: Human food risks. Curr. Opin. Food Sci. 2021, 41, 116–121. [Google Scholar] [CrossRef]
- Limonta, G.; Mancia, A.; Benkhalqui, A.; Bertolucci, C.; Abelli, L.; Fossi, M.C.; Panti, C. Microplastics induce transcriptional changes, immune response and behavioral alterations in adult zebrafish. Sci. Rep. 2019, 9, 15775. [Google Scholar] [CrossRef]
- Mak, C.W.; Ching-Fong Yeung, K.; Chan, K.M. Acute toxic effects of polyethylene microplastic on adult zebrafish. Ecotoxicol. Environ. Saf. 2019, 182, 109442. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.; Wu, W.M.; Zhao, J.; Jiang, L. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ. Sci. Technol. 2014, 48, 13776–13784. [Google Scholar] [CrossRef]
- Kosiorowska, K.E.; Biniarz, P.; Dobrowolski, A.; Leluk, K.; Mironczuk, A.M. Metabolic engineering of Yarrowia lipolytica for poly(ethylene terephthalate) degradation. Sci. Total Environ. 2022, 831, 154841. [Google Scholar] [CrossRef]
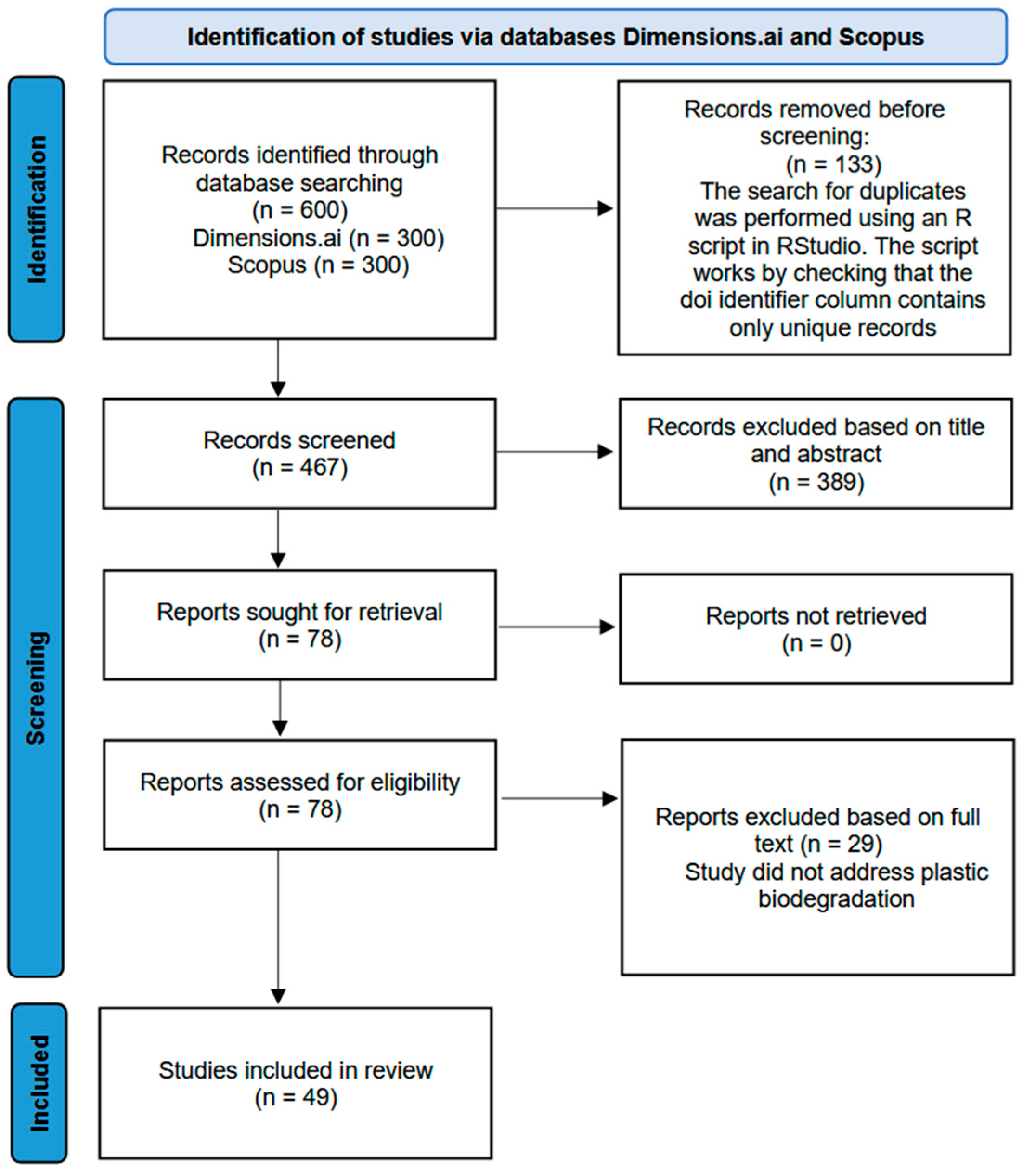
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Mariano, S.; Tacconi, S.; Fidaleo, M.; Rossi, M.; Dini, L. Micro and Nanoplastics Identification: Classic Methods and Innovative Detection Techniques. Front. Toxicol. 2021, 3, 636640. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Jayanthi, B.; Fauziah, S.H. Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull. 2018, 127, 15–21. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Y.; Zhang, H.; Wu, H.; Liu, Q.; Yang, F.; Hou, M.; Qi, Y.; Zhang, W. Exploitation of Enterobacter hormaechei for biodegradation of multiple plastics. Sci. Total Environ. 2024, 907, 167708. [Google Scholar] [CrossRef]
- Huber, M.J.; Ivleva, N.P.; Booth, A.M.; Beer, I.; Bianchi, I.; Drexel, R.; Geiss, O.; Mehn, D.; Meier, F.; Molska, A.; et al. Physicochemical characterization and quantification of nanoplastics: Applicability, limitations and complementarity of batch and fractionation methods. Anal. Bioanal. Chem. 2023, 415, 3007–3031. [Google Scholar] [CrossRef]
- Caputo, F.; Vogel, R.; Savage, J.; Vella, G.; Law, A.; Della Camera, G.; Hannon, G.; Peacock, B.; Mehn, D.; Ponti, J.; et al. Measuring particle size distribution and mass concentration of nanoplastics and microplastics: Addressing some analytical challenges in the sub-micron size range. J. Colloid Interface Sci. 2021, 588, 401–417. [Google Scholar] [CrossRef]
- Correia, M.; Loeschner, K. Detection of nanoplastics in food by asymmetric flow field-flow fractionation coupled to multi-angle light scattering: Possibilities, challenges and analytical limitations. Anal. Bioanal. Chem. 2018, 410, 5603–5615. [Google Scholar] [CrossRef]
- Song, C.; Liu, Z.; Wang, C.; Li, S.; Kitamura, Y. Different interaction performance between microplastics and microalgae: The bio-elimination potential of Chlorella sp. L38 and Phaeodactylum tricornutum MASCC-0025. Sci. Total Environ. 2020, 723, 138146. [Google Scholar] [CrossRef]
- Caldwell, J.; Loussert-Fonta, C.; Toullec, G.; Heidelberg Lyndby, N.; Haenni, B.; Taladriz-Blanco, P.; Espina, B.; Rothen-Rutishauser, B.; Petri-Fink, A. Correlative Light, Electron Microscopy and Raman Spectroscopy Workflow To Detect and Observe Microplastic Interactions with Whole Jellyfish. Environ. Sci. Technol. 2023, 57, 6664–6672. [Google Scholar] [CrossRef]
- Kalaronis, D.; Ainali, N.M.; Evgenidou, E.; Kyzas, G.Z.; Yang, X.; Bikiaris, D.N.; Lambropoulou, D.A. Microscopic techniques as means for the determination of microplastics and nanoplastics in the aquatic environment: A concise review. Green Anal. Chem. 2022, 3, 100036. [Google Scholar] [CrossRef]
- Woo, S.; Song, I.; Cha Hyung, J. Fast and Facile Biodegradation of Polystyrene by the Gut Microbial Flora of Plesiophthalmus davidis Larvae. Appl. Environ. Microbiol. 2020, 86, e01361-20. [Google Scholar] [CrossRef]
- Roy, R.; Mukherjee, G.; Das Gupta, A.; Tribedi, P.; Sil, A.K. Isolation of a soil bacterium for remediation of polyurethane and low-density polyethylene: A promising tool towards sustainable cleanup of the environment. 3 Biotech 2021, 11, 29. [Google Scholar] [CrossRef]
- Fang, X.; Cai, Z.; Wang, X.; Liu, Z.; Lin, Y.; Li, M.; Gong, H.; Yan, M. Isolation and Identification of Four Strains of Bacteria with Potential to Biodegrade Polyethylene and Polypropylene from Mangrove. Microorganisms 2024, 12, 2005. [Google Scholar] [CrossRef]
- Li, W.; Luo, Y.; Pan, X. Identification and Characterization Methods for Microplastics Basing on Spatial Imaging in Micro-/Nanoscales. In Microplastics in Terrestrial Environments: Emerging Contaminants and Major Challenges; He, D., Luo, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 25–37. [Google Scholar]
- Obrador-Viel, T.; Zadjelovic, V.; Nogales, B.; Bosch, R.; Christie-Oleza, J.A. Assessing microbial plastic degradation requires robust methods. Microb. Biotechnol. 2024, 17, e14457. [Google Scholar] [CrossRef]
- Shruti, V.C.; Perez-Guevara, F.; Roy, P.D.; Kutralam-Muniasamy, G. Analyzing microplastics with Nile Red: Emerging trends, challenges, and prospects. J. Hazard. Mater. 2022, 423 Pt B, 127171. [Google Scholar] [CrossRef]
- Bianco, A.; Carena, L.; Peitsaro, N.; Sordello, F.; Vione, D.; Passananti, M. Rapid detection of nanoplastics and small microplastics by Nile-Red staining and flow cytometry. Environ. Chem. Lett. 2023, 21, 647–653. [Google Scholar] [CrossRef]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 mm to 20 μm) in Environmental Samples. Environ. Sci. Technol. 2017, 51, 13641–13648. [Google Scholar] [CrossRef]
- Shim, W.J.; Song, Y.K.; Hong, S.H.; Jang, M. Identification and quantification of microplastics using Nile Red staining. Mar. Pollut. Bull. 2016, 113, 469–476. [Google Scholar] [CrossRef]
- Meyers, N.; Catarino, A.I.; Declercq, A.M.; Brenan, A.; Devriese, L.; Vandegehuchte, M.; De Witte, B.; Janssen, C.; Everaert, G. Microplastic detection and identification by Nile red staining: Towards a semi-automated, cost- and time-effective technique. Sci. Total Environ. 2022, 823, 153441. [Google Scholar] [CrossRef] [PubMed]
- Elert, A.M.; Becker, R.; Duemichen, E.; Eisentraut, P.; Falkenhagen, J.; Sturm, H.; Braun, U. Comparison of different methods for MP detection: What can we learn from them, and why asking the right question before measurements matters? Environ. Pollut. 2017, 231 Pt 2, 1256–1264. [Google Scholar] [CrossRef]
- Ramanna, S.; Morozovskii, D.; Swanson, S.; Bruneau, J. Machine Learning of Polymer Types From the Spectral Signature of Raman Spectroscopy Microplastics Data. Adv. Artif. Intell. Mach. Learn. 2023, 3, 647–668. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Cabernard, L.; Roscher, L.; Lorenz, C.; Gerdts, G.; Primpke, S. Comparison of Raman and Fourier Transform Infrared Spectroscopy for the Quantification of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2018, 52, 13279–13288. [Google Scholar] [CrossRef] [PubMed]
- Anger, P.M.; von der Esch, E.; Baumann, T.; Elsner, M.; Niessner, R.; Ivleva, N.P. Raman microspectroscopy as a tool for microplastic particle analysis. TrAC Trends Anal. Chem. 2018, 109, 214–226. [Google Scholar] [CrossRef]
- Cai, L.; Wang, J.; Peng, J.; Wu, Z.; Tan, X. Observation of the degradation of three types of plastic pellets exposed to UV irradiation in three different environments. Sci. Total Environ. 2018, 628–629, 740–747. [Google Scholar] [CrossRef]
- Nava, V.; Frezzotti, M.L.; Leoni, B. Raman Spectroscopy for the Analysis of Microplastics in Aquatic Systems. Appl. Spectrosc. 2021, 75, 1341–1357. [Google Scholar] [CrossRef]
- Mather, R.R. 13—Surface modification of textiles by plasma treatments. In Surface Modification of Textiles; Wei, Q., Ed.; Woodhead Publishing: Cambridge, UK, 2009; pp. 296–317. [Google Scholar]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef]
- Melo-Agustin, P.; Kozak, E.R.; de Jesus Perea-Flores, M.; Mendoza-Perez, J.A. Identification of microplastics and associated contaminants using ultra high resolution microscopic and spectroscopic techniques. Sci. Total Environ. 2022, 828, 154434. [Google Scholar] [CrossRef]
- Bhagat, K.; Barrios, A.C.; Rajwade, K.; Kumar, A.; Oswald, J.; Apul, O.; Perreault, F. Aging of microplastics increases their adsorption affinity towards organic contaminants. Chemosphere 2022, 298, 134238. [Google Scholar] [CrossRef]
- Sobhani, Z.; Zhang, X.; Gibson, C.; Naidu, R.; Megharaj, M.; Fang, C. Identification and visualisation of microplastics/nanoplastics by Raman imaging (i): Down to 100 nm. Water Res. 2020, 174, 115658. [Google Scholar] [CrossRef]
- Moura, D.S.; Pestana, C.J.; Moffat, C.F.; Hui, J.; Irvine, J.T.S.; Lawton, L.A. Characterisation of microplastics is key for reliable data interpretation. Chemosphere 2023, 331, 138691. [Google Scholar] [CrossRef]
- Fu, Q.; Tan, X.; Ye, S.; Ma, L.; Gu, Y.; Zhang, P.; Chen, Q.; Yang, Y.; Tang, Y. Mechanism analysis of heavy metal lead captured by natural-aged microplastics. Chemosphere 2021, 270, 128624. [Google Scholar] [CrossRef]
- Thakur, B.; Singh, J.; Singh, J.; Angmo, D.; Vig, A.P. Identification and characterization of extracted microplastics from agricultural soil near industrial area: FTIR and X-ray diffraction method. Environ. Qual. Manag. 2023, 33, 173–181. [Google Scholar] [CrossRef]
- Zhang, S.; Han, B.; Sun, Y.; Wang, F. Microplastics influence the adsorption and desorption characteristics of Cd in an agricultural soil. J. Hazard. Mater. 2020, 388, 121775. [Google Scholar] [CrossRef]
- Fabris, H.J.; Knauss, W.G. 5—Synthetic Polymer Adhesives. In Comprehensive Polymer Science and Supplements; Allen, G., Bevington, J.C., Eds.; Pergamon: Amsterdam, The Netherlands, 1989; pp. 131–177. [Google Scholar]
- Sorasan, C.; Ortega-Ojeda, F.E.; Rodríguez, A.; Rosal, R. Modelling the Photodegradation of Marine Microplastics by Means of Infrared Spectrometry and Chemometric Techniques. Microplastics 2022, 1, 198–210. [Google Scholar] [CrossRef]
- Sandt, C.; Waeytens, J.; Deniset-Besseau, A.; Nielsen-Leroux, C.; Rejasse, A. Use and misuse of FTIR spectroscopy for studying the bio-oxidation of plastics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 258, 119841. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B. Effect of Diatomite on the Thermal Degradation Behavior of Polypropylene and Formation of Graphene Products. Polymers 2022, 14, 3764. [Google Scholar] [CrossRef]
- Dimassi, S.N.; Hahladakis, J.N.; Daly Yahia, M.N.; Ahmad, M.I.; Sayadi, S.; Al-Ghouti, M.A. Insights into the degradation mechanism of PET and PP under marine conditions using FTIR. J. Hazard. Mater. 2023, 447, 130796. [Google Scholar] [CrossRef]
- Jeyakumar, D.; Chirsteen, J.; Doble, M. Synergistic effects of pretreatment and blending on fungi mediated biodegradation of polypropylenes. Bioresour. Technol. 2013, 148, 78–85. [Google Scholar] [CrossRef]
- Kowalczuk, D.; Pitucha, M. Application of FTIR Method for the Assessment of Immobilization of Active Substances in the Matrix of Biomedical Materials. Materials 2019, 12, 2972. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Allendorf, M.E. Use of FTIR for rapid authentication and detection of adulteration of food. Annu. Rev. Food Sci. Technol. 2011, 2, 467–483. [Google Scholar] [CrossRef]
- Mayerhofer, T.G.; Pahlow, S.; Popp, J. The Bouguer-Beer-Lambert Law: Shining Light on the Obscure. Chemphyschem 2020, 21, 2029–2046. [Google Scholar] [CrossRef]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 2016, 145, 265–268. [Google Scholar] [CrossRef]
- Ruud, J.B.P.; Relou, E.; Sijtsma, E.L.E.; Undas, A.K. Evaluation of Nanoparticle Tracking Analysis (NTA) for the Measurement of Nanoplastics in Drinking Water. Research Square 2024, preprint. [Google Scholar] [CrossRef]
- Arkatkar, A.; Arutchelvi, J.; Bhaduri, S.; Uppara, P.V.; Doble, M. Degradation of unpretreated and thermally pretreated polypropylene by soil consortia. Int. Biodeterior. Biodegrad. 2009, 63, 106–111. [Google Scholar] [CrossRef]
- Vimala, P.P.; Mathew, L. Biodegradation of Polyethylene Using Bacillus Subtilis. Procedia Technol. 2016, 24, 232–239. [Google Scholar] [CrossRef]
- Urbanek, A.K.; Rymowicz, W.; Strzelecki, M.C.; Kociuba, W.; Franczak, L.; Mironczuk, A.M. Isolation and characterization of Arctic microorganisms decomposing bioplastics. AMB Express 2017, 7, 148. [Google Scholar] [CrossRef]
- Taghavi, N.; Udugama, I.A.; Zhuang, W.Q.; Baroutian, S. Challenges in biodegradation of non-degradable thermoplastic waste: From environmental impact to operational readiness. Biotechnol. Adv. 2021, 49, 107731. [Google Scholar] [CrossRef]
- Hou, L.; Xi, J.; Liu, J.; Wang, P.; Xu, T.; Liu, T.; Qu, W.; Lin, Y.B. Biodegradability of polyethylene mulching film by two Pseudomonas bacteria and their potential degradation mechanism. Chemosphere 2022, 286 Pt 3, 131758. [Google Scholar] [CrossRef]
- Xiang, P.; Zhang, Y.; Zhang, T.; Wu, Q.; Zhao, C.; Li, Q. A novel bacterial combination for efficient degradation of polystyrene microplastics. J. Hazard. Mater. 2023, 458, 131856. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, C.G. Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere 2019, 222, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Tiso, T.; Bertling, J.; O’Connor, K.; Blank, L.M.; Bornscheuer, U.T. Possibilities and limitations of biotechnological plastic degradation and recycling. Nat. Catal. 2020, 3, 867–871. [Google Scholar] [CrossRef]
- Urbanek, A.K.; Strzelecki, M.C.; Mironczuk, A.M. The potential of cold-adapted microorganisms for biodegradation of bioplastics. Waste Manag. 2021, 119, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Charnock, C. A simple and novel method for the production of polyethylene terephthalate containing agar plates for the growth and detection of bacteria able to hydrolyze this plastic. J. Microbiol. Methods 2021, 185, 106222. [Google Scholar] [CrossRef]
- Chiou, C.-H.; Hsieh, S.-J. Empirical study and prediction of contact angle and surface free energy of commonly used plastics with pillar-like structure. Surf. Interface Anal. 2015, 47, 45–55. [Google Scholar] [CrossRef]
- Benke, A.; Sonnenberg, J.; Oelschlägel, K.; Schneider, M.; Lux, M.; Potthoff, A. Wettability after Artificial and Natural Weathering of Polyethylene Terephthalate. Environments 2022, 9, 134. [Google Scholar] [CrossRef]
- Shafea, L.; Felde, V.J.M.N.L.; Woche, S.K.; Bachmann, J.; Peth, S. Microplastics effects on wettability, pore sizes and saturated hydraulic conductivity of a loess topsoil. Geoderma 2023, 437, 116566. [Google Scholar] [CrossRef]
- Sorolla-Rosario, D.; Llorca-Porcel, J.; Pérez-Martínez, M.; Lozano-Castelló, D.; Bueno-López, A. Study of microplastics with semicrystalline and amorphous structure identification by TGA and DSC. J. Environ. Chem. Eng. 2022, 10, 106886. [Google Scholar] [CrossRef]
- Majewsky, M.; Bitter, H.; Eiche, E.; Horn, H. Determination of microplastic polyethylene (PE) and polypropylene (PP) in environmental samples using thermal analysis (TGA-DSC). Sci. Total Environ. 2016, 568, 507–511. [Google Scholar] [CrossRef]
- Yu, J.; Wang, P.; Ni, F.; Cizdziel, J.; Wu, D.; Zhao, Q.; Zhou, Y. Characterization of microplastics in environment by thermal gravimetric analysis coupled with Fourier transform infrared spectroscopy. Mar. Pollut. Bull. 2019, 145, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.; Moghadam, T.T.; Ranjbar, B. Differential scanning calorimetry techniques: Applications in biology and nanoscience. J. Biomol. Tech. 2010, 21, 167–193. [Google Scholar]
- Rodriguez Chialanza, M.; Sierra, I.; Perez Parada, A.; Fornaro, L. Identification and quantitation of semi-crystalline microplastics using image analysis and differential scanning calorimetry. Environ. Sci. Pollut. Res. Int. 2018, 25, 16767–16775. [Google Scholar] [CrossRef]
- Shabaka, S.H.; Ghobashy, M.; Marey, R.S. Identification of marine microplastics in Eastern Harbor, Mediterranean Coast of Egypt, using differential scanning calorimetry. Mar. Pollut. Bull. 2019, 142, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Bitter, H.; Lackner, S. Fast and easy quantification of semi-crystalline microplastics in exemplary environmental matrices by differential scanning calorimetry (DSC). Chem. Eng. J. 2021, 423, 129941. [Google Scholar] [CrossRef]
- Dehaut, A.; Cassone, A.L.; Frere, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Riviere, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Santos, L.; Insa, S.; Arxe, M.; Buttiglieri, G.; Rodriguez-Mozaz, S.; Barcelo, D. Analysis of microplastics in the environment: Identification and quantification of trace levels of common types of plastic polymers using pyrolysis-GC/MS. MethodsX 2023, 10, 102143. [Google Scholar] [CrossRef] [PubMed]
- Dierkes, G.; Lauschke, T.; Becher, S.; Schumacher, H.; Foldi, C.; Ternes, T. Quantification of microplastics in environmental samples via pressurized liquid extraction and pyrolysis-gas chromatography. Anal. Bioanal. Chem. 2019, 411, 6959–6968. [Google Scholar] [CrossRef]
- Morioka, T.; Tanaka, S.; Kohama-Inoue, A.; Watanabe, A. The quantification of the airborne plastic particles of 0.43–11 μm: Procedure development and application to atmospheric environment. Chemosphere 2024, 351, 141131. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Himber, C.; Boricaud, B.; Kazour, M.; Amara, R.; Cassone, A.L.; Laurentie, M.; Paul-Pont, I.; Soudant, P.; Dehaut, A.; et al. Optimization, performance, and application of a pyrolysis-GC/MS method for the identification of microplastics. Anal. Bioanal. Chem. 2018, 410, 6663–6676. [Google Scholar] [CrossRef] [PubMed]
- Swetha Sri, R.; Bhavya, S.K.; Mounika, C. A review on comparative study of HPLC and UPLC. Res. J. Pharm. Technol. 2020, 13, 1570–1574. [Google Scholar] [CrossRef]
- Kosiorowska, K.E.; Moreno, A.D.; Iglesias, R.; Leluk, K.; Mironczuk, A.M. Production of PETase by engineered Yarrowia lipolytica for efficient poly(ethylene terephthalate) biodegradation. Sci. Total Environ. 2022, 846, 157358. [Google Scholar] [CrossRef] [PubMed]
- Atiq, N.; Garba, A.; Ali, M.I.; Andleeb, S. Isolation and identification of polystyrene biodegrading bacteria from soil Study of Pyocyanin induced pathogenicity of Pseudomonas aeruginosa. Artic. Afr. J. Microbiol. Res. 2010, 53, 570. [Google Scholar]
- Gu, Y.; Ma, J.; Zhu, Y.; Xu, P. Refactoring Ehrlich Pathway for High-Yield 2-Phenylethanol Production in Yarrowia lipolytica. ACS Synth. Biol. 2020, 9, 623–633. [Google Scholar] [CrossRef]
- Xu, Y.; Ye, H.; Dong, H.; Zeng, X.; Yang, J.; Xiao, G.; Bai, W.; Wu, J.; He, Q.; Xian, Y. Determination of 2-amino-1-methyl-6-phenylimidazole [4, 5-b] pyridine (PhIP) and its precursors and possible intermediates in a chemical model system and roast pork. LWT 2023, 177, 114581. [Google Scholar] [CrossRef]
- Yang, S.S.; Ding, M.Q.; He, L.; Zhang, C.H.; Li, Q.X.; Xing, D.F.; Cao, G.L.; Zhao, L.; Ding, J.; Ren, N.Q.; et al. Biodegradation of polypropylene by yellow mealworms (Tenebrio molitor) and superworms (Zophobas atratus) via gut-microbe-dependent depolymerization. Sci. Total Environ. 2021, 756, 144087. [Google Scholar] [CrossRef]
- Ekvall, M.T.; Gimskog, I.; Hua, J.; Kelpsiene, E.; Lundqvist, M.; Cedervall, T. Size fractionation of high-density polyethylene breakdown nanoplastics reveals different toxic response in Daphnia magna. Sci. Rep. 2022, 12, 3109. [Google Scholar] [CrossRef]
- Tomoda, B.T.; Yassue-Cordeiro, P.H.; Ernesto, J.V.; Lopes, P.S.; Péres, L.O.; da Silva, C.F.; de Moraes, M.A. Chapter 3—Characterization of biopolymer membranes and films: Physicochemical, mechanical, barrier, and biological properties. In Biopolymer Membranes and Films; de Moraes, M.A., da Silva, C.F., Vieira, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 67–95. [Google Scholar]
- Sun, X.; Chen, B.; Li, Q.; Liu, N.; Xia, B.; Zhu, L.; Qu, K. Toxicities of polystyrene nano- and microplastics toward marine bacterium Halomonas alkaliphila. Sci. Total Environ. 2018, 642, 1378–1385. [Google Scholar] [CrossRef]
- Moraz, A.; Breider, F. Detection and Quantification of Nonlabeled Polystyrene Nanoparticles Using a Fluorescent Molecular Rotor. Anal. Chem. 2021, 93, 14976–14984. [Google Scholar] [CrossRef]
- Maddison, C.; Sathish, C.I.; Lakshmi, D.; Wayne, O.; Palanisami, T. An advanced analytical approach to assess the long-term degradation of microplastics in the marine environment. Npj Mater. Degrad. 2023, 7, 59. [Google Scholar] [CrossRef]
- Wu, Q.; Tao, H.; Wong, M.H. Feeding and metabolism effects of three common microplastics on Tenebrio molitor L. Environ. Geochem. Health 2019, 41, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.; Gupta, P. A TG-FTIR investigation on the co-pyrolysis of the waste HDPE, PP, PS and PET under high heating conditions. J. Energy Inst. 2020, 93, 1020–1035. [Google Scholar] [CrossRef]
- Alzuhairi, M. Chemical Recycling of Polyethylene Terephthalate (PET) as Additive for Asphalt. ZANCO J. Pure Appl. Sci. 2016, 28, s675–s679. [Google Scholar]
- Afonso, E.; Martínez-Gómez, A.; Huerta, A.; Tiemblo, P.; García, N. Facile Preparation of Hydrophobic PET Surfaces by Solvent Induced Crystallization. Coatings 2022, 12, 137. [Google Scholar] [CrossRef]
- Ojha, N.; Pradhan, N.; Singh, S.; Barla, A.; Shrivastava, A.; Khatua, P.; Rai, V.; Bose, S. Evaluation of HDPE and LDPE degradation by fungus, implemented by statistical optimization. Sci. Rep. 2017, 7, 39515. [Google Scholar] [CrossRef]
- Jiao, L.; Xiao, H.; Wang, Q.; Sun, J. Thermal degradation characteristics of rigid polyurethane foam and the volatile products analysis with TG-FTIR-MS. Polym. Degrad. Stab. 2013, 98, 2687–2696. [Google Scholar] [CrossRef]
- Gamerith, C.; Acero, E.H.; Pellis, A.; Ortner, A.; Vielnascher, R.; Luschnig, D.; Zartl, B.; Haernvall, K.; Zitzenbacher, S.; Strohmeier, G.; et al. Improving enzymatic polyurethane hydrolysis by tuning enzyme sorption. Polym. Degrad. Stab. 2016, 132, 69–77. [Google Scholar] [CrossRef]
- Yaseen, A.A.; Yousif, E.; Al-Tikrity, E.T.B.; El-Hiti, G.A.; Kariuki, B.M.; Ahmed, D.S.; Bufaroosha, M. FTIR, Weight, and Surface Morphology of Poly(vinyl chloride) Doped with Tin Complexes Containing Aromatic and Heterocyclic Moieties. Polymers 2021, 13, 3264. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, R.; Wang, X.; He, J.; Wang, J. Pyrolysis and Combustion of Polyvinyl Chloride (PVC) Sheath for New and Aged Cables via Thermogravimetric Analysis-Fourier Transform Infrared (TG-FTIR) and Calorimeter. Materials 2018, 11, 1997. [Google Scholar] [CrossRef]
- Karkanorachaki, K.; Tsiota, P.; Dasenakis, G.; Syranidou, E.; Kalogerakis, N. Nanoplastic Generation from Secondary PE Microplastics: Microorganism-Induced Fragmentation. Microplastics 2022, 1, 85–101. [Google Scholar] [CrossRef]
- Kosiorowska, K.; Mironczuk, A. Enhanced poly (ethylene terephthalate) degradation by modified Yarrowia lipolytica strains. FEBS OPEN Bio 2022, 12, 51. [Google Scholar] [CrossRef]
- Hu, X.; Thumarat, U.; Zhang, X.; Tang, M.; Kawai, F. Diversity of polyester-degrading bacteria in compost and molecular analysis of a thermoactive esterase from Thermobifida alba AHK119. Appl. Microbiol. Biotechnol. 2010, 87, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Tiso, T.; Narancic, T.; Wei, R.; Pollet, E.; Beagan, N.; Schröder, K.; Honak, A.; Jiang, M.; Kenny, S.T.; Wierckx, N.; et al. Towards bio-upcycling of polyethylene terephthalate. Metab. Eng. 2021, 66, 167–178. [Google Scholar] [CrossRef]
- Furukawa, M.; Kawakami, N.; Tomizawa, A.; Miyamoto, K. Efficient Degradation of Poly(ethylene terephthalate) with Thermobifida fusca Cutinase Exhibiting Improved Catalytic Activity Generated using Mutagenesis and Additive-based Approaches. Sci. Rep. 2019, 9, 16038. [Google Scholar] [CrossRef]
- Gu, J.D. Biodegradability of plastics: The issues, recent advances, and future perspectives. Env. Sci. Pollut. Res. Int. 2021, 28, 1278–1282. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przygoda-Kuś, P.; Kosiorowska, K.E.; Urbanek, A.K.; Mirończuk, A.M. Current Approaches to Microplastics Detection and Plastic Biodegradation. Molecules 2025, 30, 2462. https://doi.org/10.3390/molecules30112462
Przygoda-Kuś P, Kosiorowska KE, Urbanek AK, Mirończuk AM. Current Approaches to Microplastics Detection and Plastic Biodegradation. Molecules. 2025; 30(11):2462. https://doi.org/10.3390/molecules30112462
Chicago/Turabian StylePrzygoda-Kuś, Paula, Katarzyna E. Kosiorowska, Aneta K. Urbanek, and Aleksandra M. Mirończuk. 2025. "Current Approaches to Microplastics Detection and Plastic Biodegradation" Molecules 30, no. 11: 2462. https://doi.org/10.3390/molecules30112462
APA StylePrzygoda-Kuś, P., Kosiorowska, K. E., Urbanek, A. K., & Mirończuk, A. M. (2025). Current Approaches to Microplastics Detection and Plastic Biodegradation. Molecules, 30(11), 2462. https://doi.org/10.3390/molecules30112462






