Bridged Mesoporous Oxo-Phosphonates: A General Strategy Toward Functional, Hybrid Materials
Abstract
1. Introduction
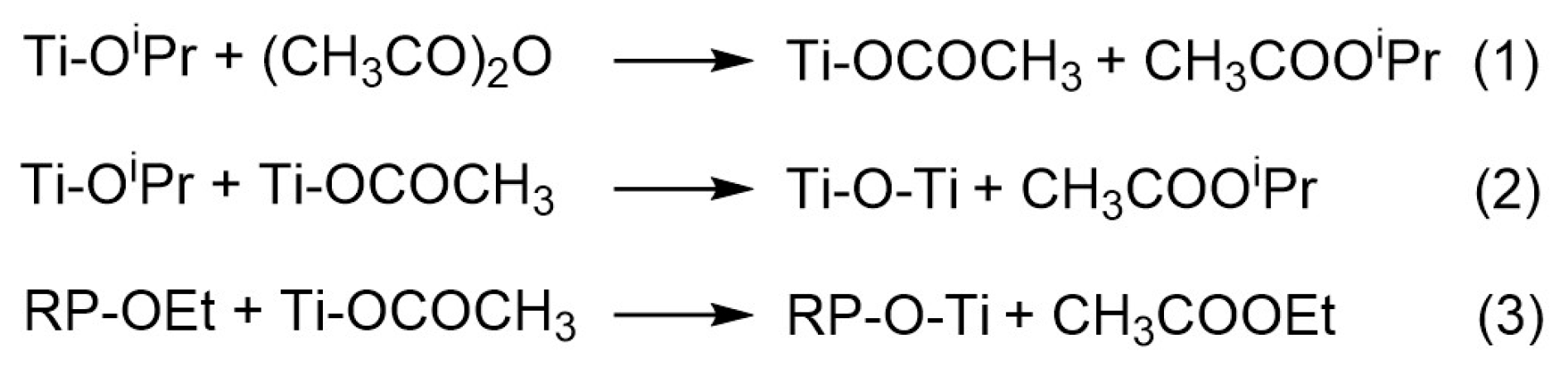
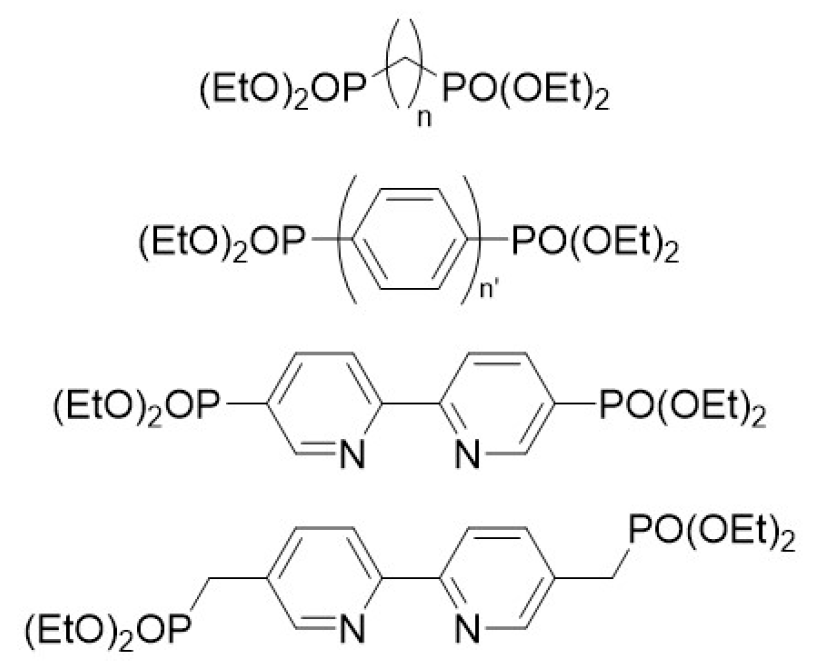
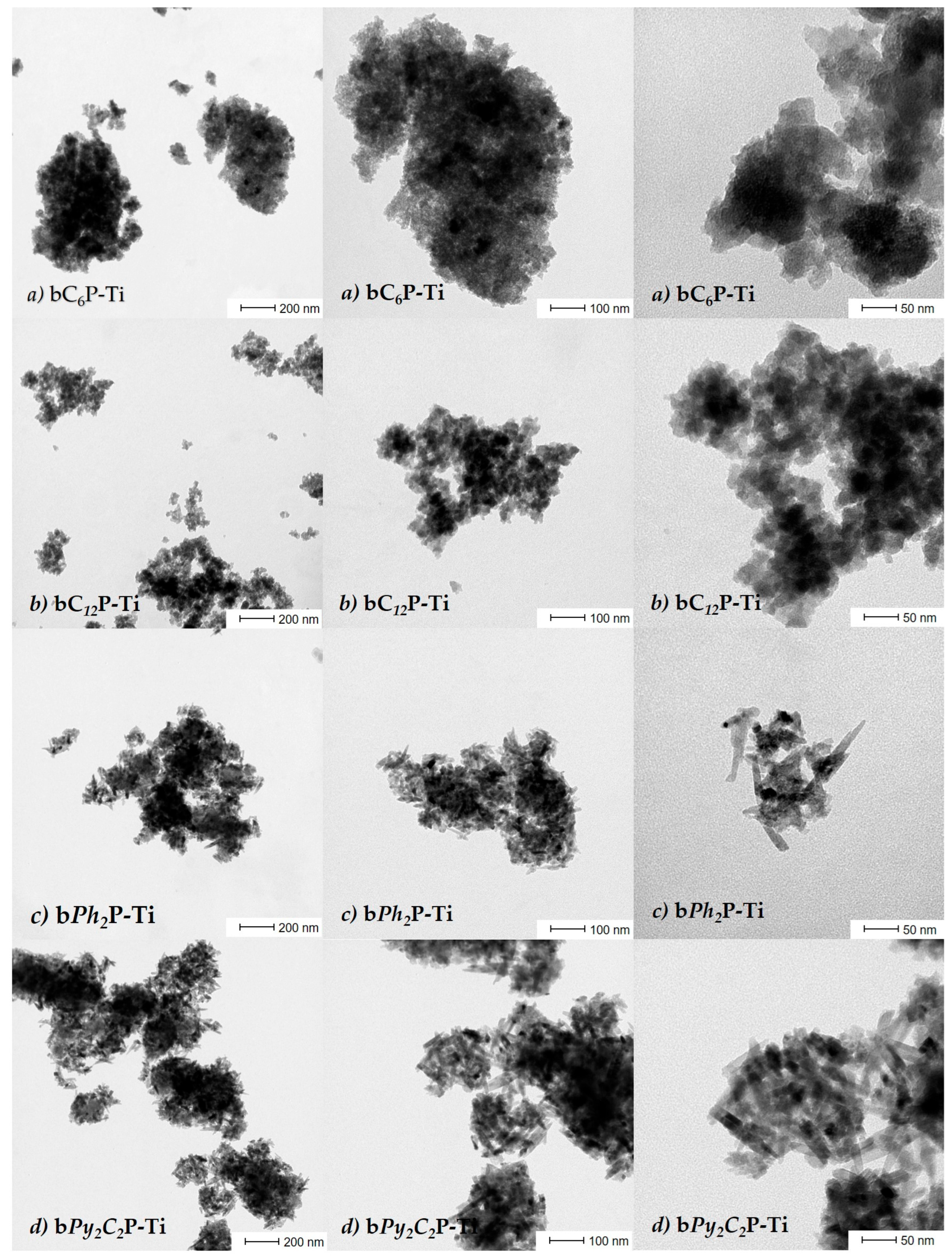
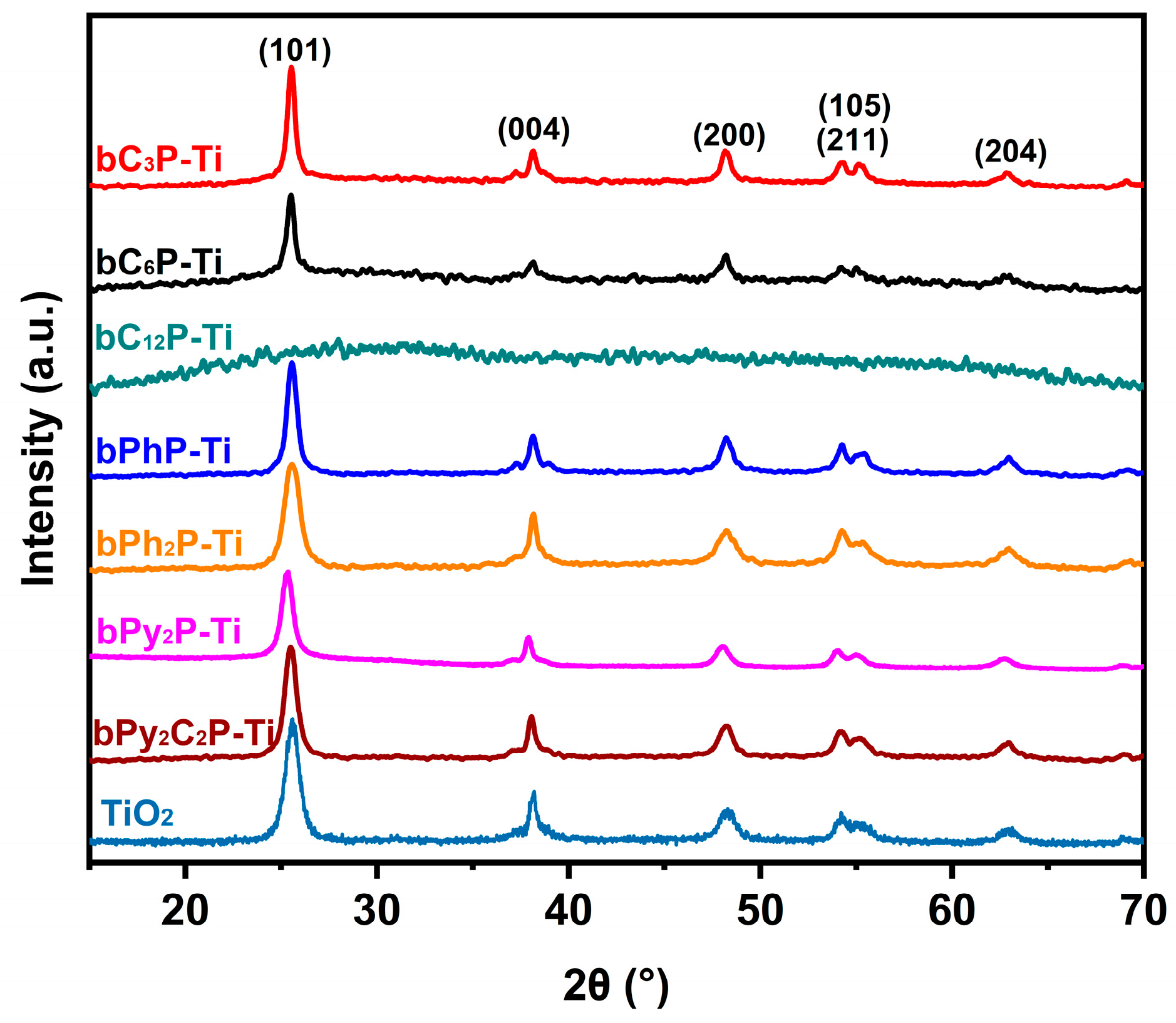
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Hybrid Materials
3.2. Characterization Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faustini, M.; Nicole, L.; Ruiz-Hitzky, E.; Sanchez, C. History of Organic–Inorganic Hybrid Materials: Prehistory, Art, Science, and Advanced Applications. Adv. Funct. Mater. 2018, 28, 1704158. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of Hybrid Organic–Inorganic Nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-Based Mesoporous Organic–Inorganic Hybrid Materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef]
- Mizoshita, N.; Tani, T.; Inagaki, S. Syntheses, Properties and Applications of Periodic Mesoporous Organosilicas Prepared from Bridged Organosilane Precursors. Chem. Soc. Rev. 2011, 40, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Santha Moorthy, M.; Ha, C.-S. Periodic Mesoporous Organosilicas for Advanced Applications. NPG Asia Mater. 2014, 6, e96. [Google Scholar] [CrossRef]
- Urata, C.; Yamada, H.; Wakabayashi, R.; Aoyama, Y.; Hirosawa, S.; Arai, S.; Takeoka, S.; Yamauchi, Y.; Kuroda, K. Aqueous Colloidal Mesoporous Nanoparticles with Ethenylene-Bridged Silsesquioxane Frameworks. J. Am. Chem. Soc. 2011, 133, 8102–8105. [Google Scholar] [CrossRef]
- Horike, S.; Shimomura, S.; Kitagawa, S. Soft Porous Crystals. Nat. Chem. 2009, 1, 695–704. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Machac, P.; Styskalik, A.; Moravec, Z.; Pinkas, J. Non-Hydrolytic Sol-Gel Synthesis of Zirconium Phosphonates with Controlled Mesoporosity. Microporous Mesoporous Mater. 2023, 362, 112787. [Google Scholar] [CrossRef]
- Popov, K.; Oshchepkov, M.; Tkachenko, S.; Sergienko, V.; Oshchepkov, A. Bisphosphonates: Synthesis, Structures, Properties, Medical and Industrial Applications. J. Mol. Liq. 2022, 351, 118619. [Google Scholar] [CrossRef]
- Pawlak, B.; Marchal, W.; Ramesha, B.M.; Joos, B.; Calvi, L.; D’Haen, J.; Ruttens, B.; Hardy, A.; Meynen, V.; Adriaensens, P.; et al. Hybrid Porous Titania Phosphonate Networks with Different Bridging Functionalities: Synthesis, Characterization, and Evaluation as Efficient Solvent Separation Materials. Microporous Mesoporous Mater. 2022, 341, 112080. [Google Scholar] [CrossRef]
- Machac, P.; Alauzun, J.; Styskalik, A.; Debecker, D.; Mutin, P.H.; Pinkas, J. Synthesis of High Surface Area Aluminophosphate and -Phosphonate Xerogels by Non-Hydrolytic Sol-Gel Reactions. Microporous Mesoporous Mater. 2021, 311, 110682. [Google Scholar] [CrossRef]
- Bao, S.-S.; Qin, M.-F.; Zheng, L.-M. Metal Phosphonates Incorporating Metalloligands: Assembly, Structures and Properties. Chem. Commun. 2020, 56, 12090–12108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal–Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef]
- Lim, D.-W.; Kitagawa, H. Proton Transport in Metal-Organic Frameworks. Chem. Rev. 2020, 120, 8416–8467. [Google Scholar] [CrossRef]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, Thermal and Mechanical Stabilities of Metal–Organic Frameworks. Nat. Rev. Mater. 2016, 1, 15018. [Google Scholar] [CrossRef]
- Schmitt Pauly, C.; Genix, A.-C.; Alauzun, J.G.; Guerrero, G.; Appavou, M.-S.; Pérez, J.; Oberdisse, J.; Mutin, P.H. Simultaneous Phase Transfer and Surface Modification of TiO2 Nanoparticles Using Alkylphosphonic Acids: Optimization and Structure of the Organosols. Langmuir 2015, 31, 10966–10974. [Google Scholar] [CrossRef]
- Mutin, P.H.; Guerrero, G.; Vioux, A. Hybrid Materials from Organophosphorus Coupling Molecules. J. Mater. Chem. 2005, 15, 3761–3768. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Ma, T.-Y.; Liu, Y.-L.; Ren, T.-Z.; Yuan, Z.-Y. Metal Phosphonate Hybrid Materials: From Densely Layered to Hierarchically Nanoporous Structures. Inorg. Chem. Front. 2014, 1, 360–383. [Google Scholar] [CrossRef]
- Gagnon, K.J.; Perry, H.P.; Clearfield, A. Conventional and Unconventional Metal–Organic Frameworks Based on Phosphonate Ligands: MOFs and UMOFs. Chem. Rev. 2012, 112, 1034–1054. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Ren, T.-Z.; Yuan, Z.-Y. Insights into Mesoporous Metal Phosphonate Hybrid Materials for Catalysis. Catal. Sci. Technol. 2015, 5, 4258–4279. [Google Scholar] [CrossRef]
- Luca, V.; Tejada, J.J.; Vega, D.; Arrachart, G.; Rey, C. Zirconium(IV)-Benzene Phosphonate Coordination Polymers: Lanthanide and Actinide Extraction and Thermal Properties. Inorg. Chem. 2016, 55, 7928–7943. [Google Scholar] [CrossRef] [PubMed]
- Styskalik, A.; Skoda, D.; Moravec, Z.; Babiak, M.; Barnes, C.E.; Pinkas, J. Control of Micro/Mesoporosity in Non-Hydrolytic Hybrid Silicophosphate Xerogels. J. Mater. Chem. A 2015, 3, 7477–7487. [Google Scholar] [CrossRef]
- Vasylyev, M.; Neumann, R. Preparation, Characterizaton, and Catalytic Aerobic Oxidation by a Vanadium Phosphonate Mesoporous Material Constructed from a Dendritic Tetraphosphonate. Chem. Mater. 2006, 18, 2781–2783. [Google Scholar] [CrossRef]
- Kimura, T. A New Family of Nonsiliceous Porous Hybrids from Bisphosphonates. J. Nanosci. Nanotechnol. 2013, 13, 2461–2470. [Google Scholar] [CrossRef]
- Ma, T.-Y.; Li, H.; Tang, A.-N.; Yuan, Z.-Y. Ordered, Mesoporous Metal Phosphonate Materials with Microporous Crystalline Walls for Selective Separation Techniques. Small 2011, 7, 1827–1837. [Google Scholar] [CrossRef]
- Veliscek-Carolan, J.; Rawal, A.; Luca, V.; Hanley, T.L. Zirconium Phosphonate Sorbents with Tunable Structure and Function. Microporous Mesoporous Mater. 2017, 252, 90–104. [Google Scholar] [CrossRef]
- Li, H.; Sun, Y.; Yuan, Z.-Y.; Zhu, Y.-P.; Ma, T.-Y. Titanium Phosphonate Based Metal–Organic Frameworks with Hierarchical Porosity for Enhanced Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2018, 57, 3222–3227. [Google Scholar] [CrossRef]
- Dines, M.B.; Cooksey, R.E.; Griffith, P.C.; Lane, R.H. Mixed-Component Layered Tetravalent Metal Phosphonates/Phosphates as Precursors for Microporous Materials. Inorg. Chem. 1983, 22, 1003–1004. [Google Scholar] [CrossRef]
- Wang, Y.; Alauzun, J.G.; Mutin, P.H. Water-Stable, Nonsiliceous Hybrid Materials with Tunable Porosity and Functionality: Bridged Titania-Bisphosphonates. Chem. Mater. 2020, 32, 2910–2918. [Google Scholar] [CrossRef]
- Van Velthoven, N.; Wang, Y.; Van Hees, H.; Henrion, M.; Bugaev, A.L.; Gracy, G.; Amro, K.; Soldatov, A.V.; Alauzun, J.G.; Mutin, P.H.; et al. Heterogeneous Single-Site Catalysts for C–H Activation Reactions: Pd(II)-Loaded S,O-Functionalized Metal Oxide-Bisphosphonates. ACS Appl. Mater. Interfaces 2020, 12, 47457–47466. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Pérez-Ramírez, J. Pore Size Determination in Modified Micro- and Mesoporous Materials. Pitfalls and Limitations in Gas Adsorption Data Analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Wang, Y.; Mutin, P.H.; Alauzun, J.G. One-Step Nonhydrolytic Sol–Gel Synthesis of Mesoporous TiO2 Phosphonate Hybrid Materials. Beilstein J. Nanotechnol. 2019, 10, 356–362. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, S.; Louvain, N.; Alauzun, J.G.; Mutin, P.H. Acetic Anhydride as an Oxygen Donor in the Non-Hydrolytic Sol-Gel Synthesis of Mesoporous TiO2 with High Electrochemical Lithium Storage Performances. Chem. Weinh. Bergstr. Ger. 2019, 25, 4767–4774. [Google Scholar] [CrossRef]
- Guerrero, G.; Mutin, P.H.; Vioux, A. Anchoring of Phosphonate and Phosphinate Coupling Molecules on Titania Particles. Chem. Mater. 2001, 13, 4367–4373. [Google Scholar] [CrossRef]
- Guerrero, G.; Mutin, P.H.; Vioux, A. Mixed Nonhydrolytic/Hydrolytic Sol−Gel Routes to Novel Metal Oxide/Phosphonate Hybrids. Chem. Mater. 2000, 12, 1268–1272. [Google Scholar] [CrossRef]


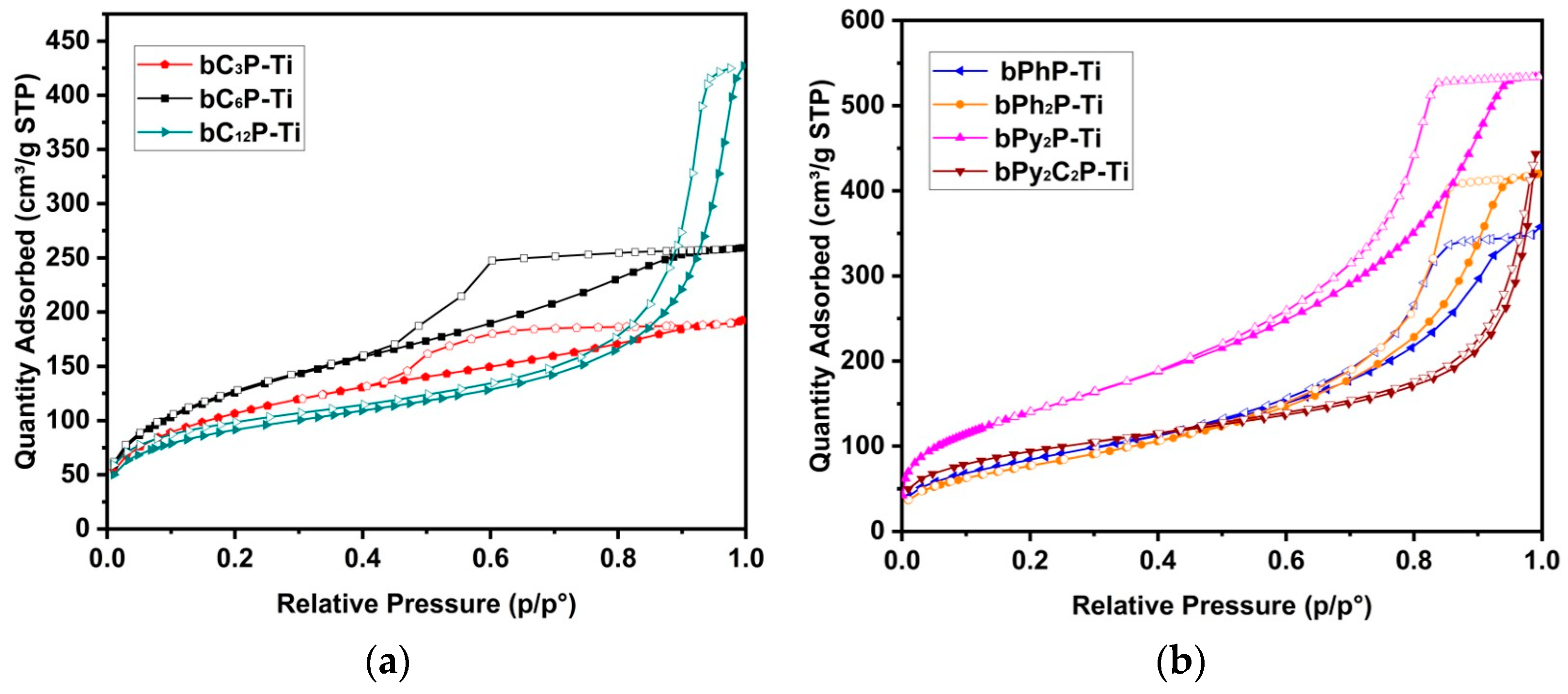
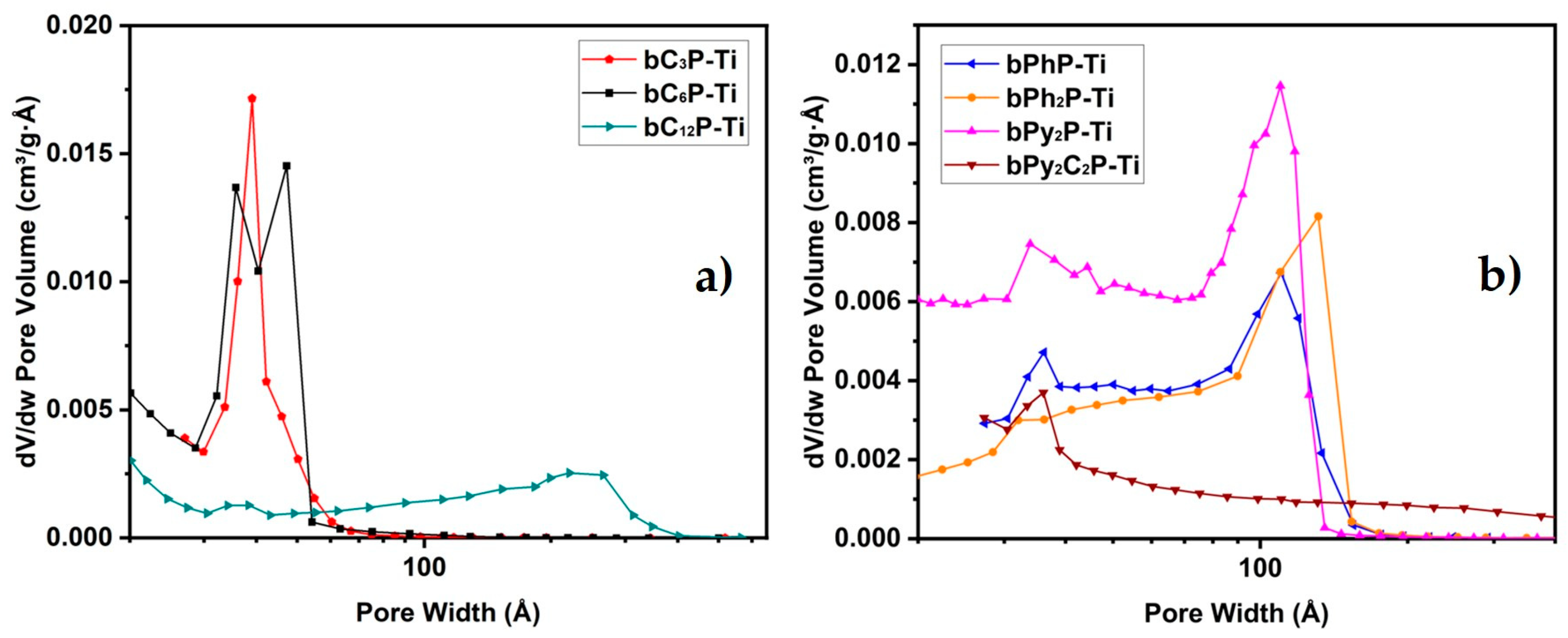

| Sample | SBET a (m2 g−1) | Vp b (cm3 g−1) | Vm c (cm3 g−1) | Dp d (nm) BJH | Dp e (nm) | CBET f | dcryst g (nm) | Crosslinking Rate (%) |
|---|---|---|---|---|---|---|---|---|
| bC3P-Ti | 390 | 0.30 | <0.01 | 4.0 | 4.0 | 72 | 14.6 | 96 |
| bC6P-Ti | 470 | 0.40 | <0.01 | 2.7 | 4.0 | 56 | 11.7 | 89 |
| bC12P-Ti | 330 | 0.66 | 0.09 | 5.4 | 25 | 134 | amorphous | 89 |
| bPhP-Ti | 315 | 0.54 | <0.01 | 9.6 | 13 | 54 | 12.8 | 96 |
| bPh2P-Ti | 290 | 0.65 | <0.01 | 7.1 | 13 | 47 | 8.5 | 96 |
| bPy2P-Ti | 520 | 0.93 | 0.06 | 6.5 | 10 | 46 | 8.9 | 86 |
| bPy2C2P-Ti | 345 | 0.69 | 0.05 | 10.9 | - | 79 | 10.1 | 97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gioan, E.; Su, Z.; Wang, Y.; Rodriguez, J.; Bouchmella, K.; Alauzun, J.G. Bridged Mesoporous Oxo-Phosphonates: A General Strategy Toward Functional, Hybrid Materials. Molecules 2025, 30, 2459. https://doi.org/10.3390/molecules30112459
Gioan E, Su Z, Wang Y, Rodriguez J, Bouchmella K, Alauzun JG. Bridged Mesoporous Oxo-Phosphonates: A General Strategy Toward Functional, Hybrid Materials. Molecules. 2025; 30(11):2459. https://doi.org/10.3390/molecules30112459
Chicago/Turabian StyleGioan, Elodie, Zijie Su, Yanhui Wang, Jeremy Rodriguez, Karim Bouchmella, and Johan G. Alauzun. 2025. "Bridged Mesoporous Oxo-Phosphonates: A General Strategy Toward Functional, Hybrid Materials" Molecules 30, no. 11: 2459. https://doi.org/10.3390/molecules30112459
APA StyleGioan, E., Su, Z., Wang, Y., Rodriguez, J., Bouchmella, K., & Alauzun, J. G. (2025). Bridged Mesoporous Oxo-Phosphonates: A General Strategy Toward Functional, Hybrid Materials. Molecules, 30(11), 2459. https://doi.org/10.3390/molecules30112459









