Abstract
Achillea nobilis and its subspecies (A. nobilis subsp. neilreichii and A. nobilis subsp. sipylea) have been traditionally used in various ethnomedical systems across Eurasia. However, comprehensive studies on their phytochemical composition and pharmacological properties are still insufficient. This review aims to provide a critical synthesis of current knowledge regarding the botanical characteristics, geographic distribution, traditional applications, chemical constituents, and pharmacological effects of A. nobilis A structured search was conducted using eight scientific platforms, including Scopus, PubMed, Web of Science, Google Scholar, Science.gov, ScienceDirect, JSTOR, and BASE. Keywords related to phytochemistry, pharmacology, and ethnomedicine were applied, and a total of 28,000 records were initially retrieved. After a multi-stage screening process based on inclusion and exclusion criteria, 167 peer-reviewed publications from 1952 to 2023 were selected for detailed evaluation. Findings reveal a diverse range of bioactive compounds, such as flavonoids, monoterpenes, sesquiterpenes, and sesquiterpene lactones, which demonstrate antioxidant, antimicrobial, anti-inflammatory, antinociceptive, antispasmodic, and anticonvulsant activities. Most studies have focused on aerial parts and water-based extracts, while the root chemistry and organ-specific metabolite profiles remain largely unexplored. This review highlights the therapeutic potential of A. nobilis and underscores the need for future studies using multi-omics and advanced analytical techniques to support its development in pharmaceutical and nutraceutical applications.
1. Introduction
The exploration of specific plant species with ethnomedicinal value has proven instrumental in identifying novel bioactive compounds for therapeutic use [1]. Achillea nobilis L. and its subspecies (A. nobilis subsp. sipylea, A. nobilis subsp. neilreichii, and A. nobilis subsp. nobilis) represent a group of aromatic and medicinal plants widely used in traditional medicine systems across Eurasia [2,3,4]. These taxa belong to the Asteraceae family and have been historically used in herbal decoctions, infusions, oils, and topical applications aimed at treating pain, inflammation, gastrointestinal discomfort, respiratory infections, and dermatological conditions [5,6,7,8,9,10]. Their longstanding therapeutic applications are rooted in folk medicine systems from Turkey, Iran, Bosnia and Herzegovina, and Kazakhstan, among others [11,12,13,14,15].
Despite their documented use in traditional medicine, comprehensive scientific analysis of their phytochemical composition and pharmacological properties remains fragmented [16,17]. Reports suggest that A. nobilis and its subspecies contain a diverse array of bioactive secondary metabolites, including flavonoids, flavonoid glycosides, monoterpenes, sesquiterpenes, sesquiterpene lactones, and terpenoid derivatives [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. These compounds have been linked to various biological activities, particularly antimicrobial, anti-inflammatory, antispasmodic, and neuroprotective effects. For example, flavonoids such as apigenin and luteolin glycosides exhibit strong antioxidant and anti-inflammatory properties, while volatile monoterpenes such as α-thujone, cineole, and camphor have shown antimicrobial activity (Figure 1) [21].
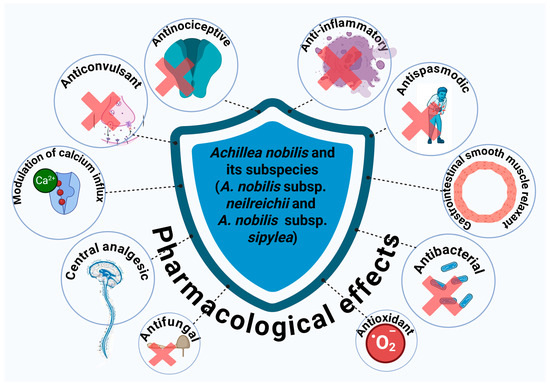
Figure 1.
Pharmacological effects of A. nobilis and its subspecies.
Phytochemical investigations from 1978 to 2020 have primarily focused on aerial parts of the plant and water-soluble extracts, often overlooking the phytochemical diversity of underground organs and lipid-soluble fractions. This creates a significant knowledge gap in understanding the complete metabolic profile of the species. Furthermore, studies suggest that the chemical profiles of different plant parts and subspecies vary significantly due to genetic and ecological factors, necessitating chemotaxonomic approaches and organ-specific metabolite profiling [27,28]. Seasonal variations have been reported to influence the concentration of essential oils and flavonoid content, with certain bioactive compounds peaking during flowering or specific phenological stages. Similarly, regional differences, including altitude, soil composition, and climatic conditions, can significantly modulate metabolite accumulation, leading to variability in pharmacological potency across populations [2,20,25]. However, these differences have not yet been fully characterized in a systematic manner.
Although several review articles have addressed the phytochemistry and pharmacological properties of the Achillea genus more broadly [4,35,36,37], none have provided a comprehensive, species-specific focus on Achillea nobilis and its subspecies. Existing reviews such as Barda et al. [4] and Saeidnia et al. [37] compile data primarily for A. millefolium and other well-studied species, while limited attention is given to the distinct phytochemical profiles and ethnomedicinal uses of A. nobilis taxa. Furthermore, no review has yet systematically explored the chemotaxonomic distinctions among A. nobilis subspecies or the variation in lipophilic and underground organ-derived metabolites. This review aims to fill this gap by offering a dedicated analysis of A. nobilis and its subspecies, integrating phytochemical, ethnopharmacological, and pharmacological data, and proposing future research directions using multi-omics and regional comparative approaches.
Recent advances in plant metabolomics, transcriptomics, and chemoinformatics offer the opportunity to resolve these limitations [29,30,31,32,33]. Future research should incorporate integrative multi-omics strategies to explore biosynthetic pathways, quantify metabolite variation across plant parts and environments, and establish pharmacological correlations with specific compounds. Additionally, understanding the influence of geographical and seasonal variability on phytochemical expression will enhance the reproducibility and quality control of derived herbal products. Given these gaps, a focused and comprehensive review is essential to consolidate current knowledge and guide future investigations.
This review aims to offer a thorough and cohesive summary of A. nobilis and its subspecies by consolidating existing information on their botanical traits, geographic distribution, traditional medicinal applications, phytochemical compositions, and pharmacological effects. The objective is to bridge traditional ethnobotanical insights with contemporary phytochemical and pharmacological evidence while identifying research gaps related to underexplored plant parts such as roots. Furthermore, the review proposes future research directions using advanced analytical and systems biology tools to support the safe and effective integration of A. nobilis into pharmaceutical and nutraceutical development.
2. Methods
A comprehensive and systematic literature review was conducted to identify relevant scientific publications pertaining to Achillea nobilis and its subspecies. The search aimed to capture a wide spectrum of studies addressing botanical, phytochemical, ethnomedicinal, and pharmacological aspects. Eight databases were strategically selected to ensure broad and high-quality coverage of the literature. These included bibliographic databases (Scopus, PubMed, Web of Science), academic search engines (Google Scholar, Science.gov), and full-text repositories (ScienceDirect, JSTOR, BASE), which collectively provide access to peer-reviewed journals, open-access materials, institutional repositories, and preprints.
To refine the search and extract relevant literature, targeted keywords were employed, including “A. nobilis”, “A. nobilis phytochemicals”, “A. nobilis compounds”, “A. nobilis pharmacological”, “A. nobilis traditional use”, and “A. nobilis ethnomedicine”. The search strategy incorporated Boolean operators and was applied uniformly across all platforms. The number of search results obtained per keyword from each platform is summarized in Table 1.

Table 1.
The number of searches for each keyword.
The literature review and data collection were conducted between August 2024 and March 2025. Scientific publications in English, Russian, Arabic, and Chinese were included in the review. The primary database searches and article screening were performed by Anastassiya Shevchenko and Akerke Amirkhanova. Other co-authors contributed by verifying the eligibility of the literature, extracting phytochemical and pharmacological data from selected manuscripts, and assisting with data synthesis and final categorization. The review process involved both gradual and parallel stages of search and screening to ensure coverage and accuracy. No conflicts arose during the literature review process.
We compared phytochemical composition data reported in selected studies to assess chemotypic variation across subspecies and plant organs. Specifically, we recorded identified metabolites, extraction solvents, analytical techniques (e.g., GC–MS, LC–MS/MS, NMR), and the corresponding plant parts and subspecies. Studies reporting distinct compound profiles from different subspecies (e.g., A. nobilis subsp. neilreichii vs. A. nobilis subsp. nobilis) or organs (e.g., aerial parts vs. flower heads) were reviewed comparatively to infer chemotypic differences. These patterns were synthesized to identify recurring subspecies-specific chemical signatures. The final synthesis was conducted by categorizing phytochemicals according to chemical class (e.g., flavonoids, terpenes, phenolic acids) and mapping them against plant parts and subspecies. This comparative approach allowed us to infer chemotypic variations and associate them with environmental or genetic factors, as reported by primary authors. Differences in extraction protocols and analytical techniques were also considered to contextualize the findings.
By applying the inclusion and exclusion criteria, papers that met the inclusion criteria were selected for further investigation and content assessment. The inclusion criteria encompassed peer-reviewed articles, biological/pharmacological studies, systematic reviews, and meta-analyses that specifically addressed A. nobilis, its phytochemical composition, pharmacological properties, and ecological significance. Conversely, the exclusion criteria involved omitting gray literature, extended abstracts, presentations, keynotes, book chapters, and inaccessible publications. Additionally, articles discussing Achillea species without specific information on A. nobilis were excluded, as they fell outside the scope of this review, which aims to define the status quo of medicinal and ethnobotanical significance (MES).
The general screening process and the flow of selecting relevant literature are illustrated in Figure 2. Initially, approximately 28,000 records were retrieved from seven platforms. After eliminating non-relevant works, including gray literature, extended abstracts, presentations, keynotes, book chapters, and inaccessible sources, the number of retained articles was reduced to 893 for further title screening. Subsequently, 385 articles met the eligibility criteria for abstract evaluation. Following abstract screening, only 293 articles were deemed relevant for a full-text review. Among them, 167 studies specifically assessed MES, and these articles were downloaded for further detailed screening. Ultimately, 167 publications satisfied all inclusion criteria and were selected for the final analysis (Figure 2). The final set of relevant publications was then used for further evaluation and synthesis.
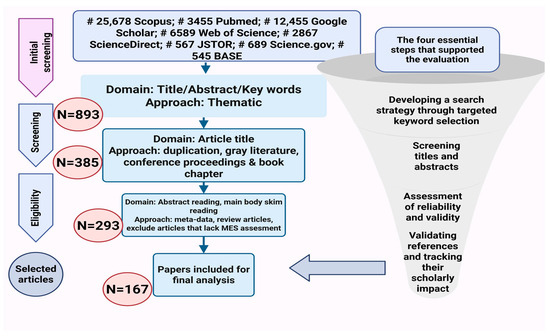
Figure 2.
Schematic representation of the database search process for identifying and selecting publications in a systematic review (MES: medicinal and ethnobotanical significance).
These terms facilitated the identification of studies related to the phytochemical composition, pharmacological potential, and ethnomedicinal applications of A. nobilis. Moreover, to enhance the accuracy of data retrieval, non-relevant materials were systematically excluded based on their lack of direct relevance to the study’s objectives. This methodological approach ensures the inclusion of high-quality and scientifically rigorous literature, thereby strengthening the reliability and validity of the findings. Additional studies were identified through manual screening of references in the selected articles. Furthermore, books containing high-quality taxonomic, ethnobotanical, and pharmacological information were also reviewed to ensure comprehensive coverage of A. nobilis. In addition to taxonomic and ethnobotanical aspects, particular attention was given to the pharmacological activities of the identified bioactive compounds. This approach provided a broader understanding of the therapeutic potential of A. nobilis, allowing for the identification of key bioactive constituents and their possible mechanisms of action. The literature reviewed spanned from 1952 to 2023.
The process of reviewing scientific articles to identify appropriate materials involves four key actions. First, selecting relevant keywords and developing a precise search strategy using Boolean operators ensures comprehensive database searches. Second, screening article titles and abstracts helps filter studies based on relevance and predefined inclusion/exclusion criteria. Third, full-text evaluation assesses methodological rigor, scientific credibility, and alignment with research objectives. Lastly, reference cross-checking and citation tracking enhance the review by identifying additional relevant studies through backward and forward citation analysis. This systematic approach ensures the selection of high-quality, scientifically relevant materials.
3. Distribution and Botanical Characterization
Based on botanical systematics, Achillea species belong to the Angiosperms, within the Eudicots clade, Campanulates order, Asteraceae family, Tubuliflorae subfamily, and Anthemideae tribe [37]. The Achillea genus comprises approximately 140 perennial herbaceous species worldwide [38]. These species are highly adaptable and thrive in diverse climates, particularly in semi-tropical, temperate, dry, and semi-arid regions [39]. They are commonly found in mountainous forests and steppes, exhibiting resilience in various soil conditions, including degraded lands and roadsides. Their adaptability is largely attributed to their ability to withstand moisture deficiency and direct sunlight, making them versatile in different environmental settings [37,40].
Among these species, A. nobilis is native to Europe, Asia, and parts of North Africa (Figure 3). Additionally, it has been reported to have been introduced into Great Britain, as well as several federal subjects of Russia, including Khabarovsk Krai, Primorye Krai, and Sakhalin. Moreover, its presence has been documented in the United States, specifically in Minnesota and Montana [41].
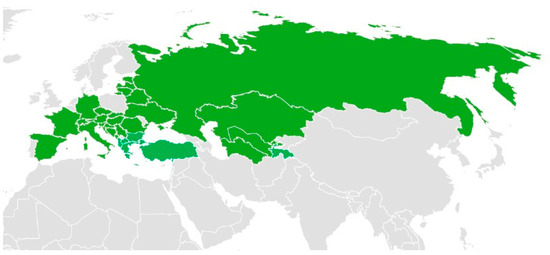
Figure 3.
The distribution of A. nobilis (Green indicates the native distribution) [41]. The map was created using Microsoft Excel based on publicly available distribution data.
A. nobilis, commonly known as “noble yarrow”, is a perennial herbaceous plant reaching heights of 15–70 cm, with a rootstock that lacks stolons. Its stems are terete, longitudinally striped, and covered with spreading pilose hairs. The leaves are woolly-pubescent, with basal leaves being 2–3-pinnatipartite, oblong-lanceolate (2–10 × 1–3 cm) with a dentate rachis, while median cauline leaves are ovate-oblong to broadly ovate (2–5 × 1–3 cm), with primary segments regularly 1-pinnatifid to 1-pinnatisect. The species produces 50–150 capitula in dense corymbose inflorescences (2–10 cm wide), each capitulum being broadly obovoid to oblong (3–3.5 × 2–3 mm), with phyllaries ranging from triangular-lanceolate and acute to oblong and obtuse, covered in either sparse hairs or dense woolly-shaggy indumentum. The ligules are pale yellow above and white below (0.8–1.5(–2) mm), with 10–25 disk flowers per capitulum. Flowering occurs from June to August [42].
4. Uses of A. nobilis in Ethnomedicine
Plants constitute the principal healthcare resource in numerous communities globally [43]. They additionally function as vital sources for the advancement of biomedicines, and species traditionally utilized have made substantial contributions to the formulation of biomedical pharmaceuticals [44]. Statistical evidence indicates that traditional remedies, including phytotherapeutic agents, represent the most significant and at times the sole means of therapeutics for nearly 80% of the global population [45]. For example, approximately 85% of the global populace utilizes herbal medicines for both disease prevention and treatment, with demand rising in both developed and developing nations [46,47]. It is estimated that around 500 million individuals in South Asian countries alone seek health security through plant-based resources [44].
A. nobilis widely recognized for its medicinal properties, is one of the most notable examples of a plant extensively utilized in ethnomedicine across diverse cultures, where it has been traditionally employed for its anti-inflammatory, wound healing, and antimicrobial effects (Table 2) [48,49,50,51,52]. For instance, A. nobilis has been traditionally used in Bosnia and Herzegovina for treating bedwetting in children, blood purification, and various skin conditions, including injuries, rashes, and psoriasis. Moreover, its flower tincture has been widely applied, as it is believed to effectively restore the skin to its normal condition. This longstanding use highlights the plant’s significance in ethnomedicine for dermatological and internal health applications [48].

Table 2.
Ethnomedicinal use of A. nobilis in different cultures.
The seminal text Shipagerlik Bayan (“Confessions of a Healer”), authored by the esteemed Kazakh medical practitioner Oteyboydak Tleukabyl during the 15th century AD, provides a comprehensive exposition of the medical paradigms pertaining to pharmacology, anatomy, pathology, immunology, and nutritional care within the framework of Traditional Kazakh Medicine (TKM). Each of these paradigms constitutes a systematic framework comprising six principal components, specifically, kengistik tugyr (space), turak tugyr (earth), suwik tugyr (coldness), ystyk tugyr (heat), zharyk tugyr (light), and karangy tugyr (darkness) [53,54,55]. The foundations of ancient Kazakh folk medicine are rooted in the empirical observations and practical knowledge accrued over numerous generations. Traditionally, the array of medicinal flora utilized by the Kazakh populace was categorized into four primary classifications—fortifying, refreshing, warming, and laxative plants [55,56]—which were adeptly employed to address a variety of ailments, including bronchitis, bronchial asthma, urethritis, chronic rheumatoid arthritis, gastric discomfort, hyperacidity, diarrhea, hemostatic issues, metrorrhagia, envenomations from snake bites, and neoplastic diseases [56,57,58]. Among the diverse Achillea species, A. nobilis has been particularly noted within Kazakh folk medicine for its therapeutic application in treating urethritis and bronchitis. Additionally, its aqueous tincture has been employed in dermatological treatments for psoriasis [52].
5. Phytochemistry
Studying the phytoconstituents of A. nobilis is essential, particularly due to their pharmacological significance. However, a comprehensive literature survey indicates that the majority of phytochemical studies on A. nobilis employing various techniques and methodologies, were conducted between 1978 and 2020 (Table 3) [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. This suggests a limited number of investigations dedicated to the plant’s chemical composition. Furthermore, an analysis of existing studies on A. nobilis and its subspecies (A. nobilis subsp. neilreichii and A. nobilis subsp. sipylea) reveals that most research has focused on water-based extracts. Additionally, studies have predominantly examined the aerial parts of the plant, with no reported investigations of the root composition. Consequently, A. nobilis and its subspecies exhibit a diverse array of phytochemicals, including phenolic compounds (flavonoids and flavonoid glycosides), terpenes (monoterpenes, oxygenated monoterpenes, sesquiterpenes), sesquiterpene lactones, guaianolides, and steroidal compounds (terpenoid derivatives).

Table 3.
Phytochemical composition studies of A. nobilis and its subspecies.
Based on the literature, several compounds have been isolated from Achillea nobilis and its subspecies, including apigenin 7-glycoside [18], luteolin 7-glucoside [18], apigenin 7-glucuronide [18], the guaianolide estafiatin [19], hanphyllin [19], anobin [19], 3,5-dihydroxy-6,7,8-trimethoxyflavone [19], anolide [21], tanaparthin-β-peroxide [22], 5-hydroxy-3,6,7,4′-tetramethoxyflavone [22], chrysartemin A [22], canin [22,23], and chrysartemin [23]. These compounds were obtained through phytochemical isolation and characterized using standard techniques such as nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS). In comparison, the remaining compounds discussed in this review, which include phenolic compounds, terpenes and their derivatives, and other biologically active constituents, were identified based on analytical profiling or reported in the literature without direct isolation from A. nobilis.
Flavonoids are one of the primary categories of phytochemicals found in Achillea nobilis L. and its subspecies. Initial research by Solomko et al. [18] employing chromatographic and spectroscopic methods uncovered flavonoid glycosides such as apigenin-7-glucoside, luteolin-7-glucoside, and apigenin-7-glucuronide, from the non-flowering shoots of A. nobilis Follow-up studies by Adekenov et al. [19] and Krenn et al. [24] built upon these discoveries by recognizing flavonoid subclasses like 3,5-dihydroxy-6,7,8-trimethoxyflavone and quercetin-3-o-methyl ether from flower heads and aerial parts, respectively. In a more recent study, Taşkın et al. [33] revealed the existence of phenolic compounds like dicaffeoylquinic acid and several glycosylated derivatives of apigenin and luteolin in A. nobilis subsp. neilreichii, affirming the continued importance of flavonoids and phenolics in the phytochemistry of this group.
Various studies of A. nobilis and its subspecies have consistently reported monoterpenes, oxygenated monoterpenes, and sesquiterpene lactones. Palić et al. [25] discovered oxygenated monoterpenes, including α-thujone (25.7%) and borneol (9.9%), while Karamanendres et al. [26] and Ghani et al. [27] reported elevated concentrations of α-thujone, 1,8-cineole, and fragranol via GC–MS examinations of essential oils. Demirci’s research [28] further highlighted the prevalence of fragranol (24%) and β-eudesmol (8%) in the aerial parts. Additional research (e.g., Azizi et al. [30], Rustaiyan et al. [31], Kazemizadeh et al. [32]) verified the extensive occurrence of monoterpenes like cineole, camphor, and spathulenol. The headspace examination conducted by Ozdemir [34] similarly identified camphor and α-thujone as key volatile compounds. These terpenes are linked to various pharmacological effects, such as antimicrobial and anti-inflammatory properties.
The chemical makeup of A. nobilis differs not just by plant section but also by subspecies. For example, A. nobilis subsp. neilreichii exhibited a diverse range of monoterpenes and sesquiterpenes (e.g., Demirci [28], Ozdemir [34]), while A. nobilis subsp. nobilis and A. nobilis subsp. neilreichii were studied mainly for flavonoids and flavonoid glycosides (Valant-Vetschera [20]). These results indicate the presence of chemotypic variations among subspecies, probably shaped by genetic and environmental influences. These differences hold significant consequences for choosing suitable subspecies for medicinal or industrial uses.
A diverse array of extraction methods and solvents has been used to obtain secondary metabolites from A. nobilis. Previous research utilized comprehensive aqueous extraction (Solomko et al. [18]) and chloroform-based techniques (Adekenov et al. [19]), while subsequent studies embraced more focused methods, such as hydro-distillation (e.g., Ghani et al. [27], Kazemizadeh et al. [32]), headspace solid-phase micro-extraction (Ozdemir [34]), and ethanol-ethyl acetate solvent combinations (Taşkın et al. [33]). Analytical methods evolved over the decades, transitioning from UV and IR spectroscopy to more sophisticated techniques such as GC–MS, LC–MS/MS, and NMR spectroscopy. This methodological advancement indicates a wider movement towards high-resolution, multi-faceted characterization of plant metabolites.
Newer research from 2010 and later shows a distinct emphasis on thorough chemical profiling through contemporary methods. For example, Azizi et al. [30] and Rustaiyan et al. [31] conducted GC–MS analyses on the aerial components, pinpointing significant monoterpenes and sesquiterpenes. Kazemizadeh et al. [32] enhanced this method by examining both leaves and flowers, whereas Taşkın et al. [33] utilized LC–MS/MS to detect phenolic components. Ozdemir’s latest study [34] utilized HS–SPME/GC–MS for identifying volatile compounds in A. nobilis subsp. neilreichii. These investigations highlight a transition towards accurate, quantitative analysis of intricate phytochemical mixtures, with increasing attention on essential oils and phenolics.
Grasping the chemical makeup of A. nobilis and its subspecies has important consequences for the pharmaceutical sciences. Flavonoids such as apigenin and luteolin, found in various studies, display strong antioxidant, anti-inflammatory, and anticancer effects [54]. Likewise, monoterpenes like α-thujone and 1,8-cineole exhibit antimicrobial and neuroprotective properties, positioning them as potential candidates for therapeutic advancement [56]. Additionally, phenolic acids and their derivatives might play a role in cardiovascular protection and metabolic regulation [57]. Therefore, thorough phytochemical analysis bolsters the creation of evidence-backed herbal treatments and increases the significance of traditional medicinal flora in contemporary healthcare.
Although significant advancements have been made, there are still numerous gaps in the chemical profiling of A. nobilis and its subspecies. Interestingly, the plant’s roots remain unexplored, and the majority of research has combined aerial components (leaves, stems, and flowers) without conducting separate assessments. This restricts our comprehension of the biosynthetic abilities specific to organs. Studies indicate that various plant components can generate unique metabolite profiles as a result of different metabolic pathways and ecological roles [58]. Thus, upcoming research should focus on the separate examination of specific organs, such as stems, roots, and flowers, to completely clarify the phytochemical variation of the species.
To thoroughly investigate the chemical profile of A. nobilis and its subspecies, future studies should employ a multi-omics strategy that combines metabolomics, transcriptomics, and chemotaxonomy. Sophisticated methods like UHPLC–HRMS, MALDI–TOF imaging, and molecular networking may reveal new bioactive compounds. Moreover, investigations of seasonal and geographical variations should be carried out to assess the stability of chemotypes in different environments. Partnerships with pharmacologists and bioinformaticians will be crucial for connecting phytochemicals to particular bioactivities. These integrative studies will enhance the phytochemical profile of A. nobilis and promote its thoughtful use in pharmaceuticals and nutraceuticals.
5.1. Phenolic Compounds
Phenolic compounds, including phenolic acids and flavonoids, are widely recognized for their antioxidant and anti-inflammatory characteristics, which may confer protective effects against malignancies, metabolic disorders such as diabetes, and neurodegenerative conditions [59,60,61]. Moreover, flavonoids, classified as a subclass of phenolic compounds, exhibit an extensive array of pharmacological properties, encompassing antioxidant, anti-inflammatory, anticancer, and cardioprotective effects [62]. Flavonol glycosides possess analogous attributes and are particularly investigated for their antioxidant and anti-inflammatory capabilities [63]. Furthermore, they also demonstrate anti-inflammatory, anticancer, and cardiovascular protective properties [64]. These compounds, which are plentiful in the natural environment, present a promising pathway for the innovation of novel therapeutic agents (Table 4, Compounds 1–23) [65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88].

Table 4.
Phenolic compounds found in A. nobilis and its subspecies.
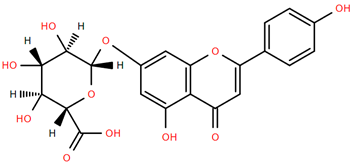
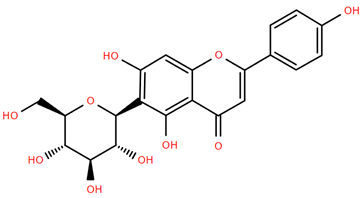
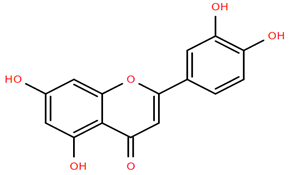
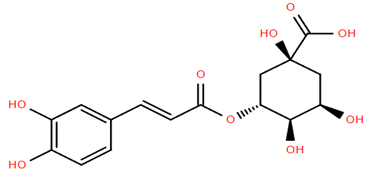
Flavonoid glucuronides such as apigenin-7-O-glucuronide (Compound 11) and luteolin-7-O-glucuronide (Compound 12) derived from A. nobilis demonstrate strong anti-inflammatory and neuroprotective properties. Compound 11 suppresses the production of nitric oxide and tumor necrosis factor-alpha in LPS-activated macrophages, suggesting significant immunomodulatory capabilities [65]. Compound 12 modulates microglial polarization and enhances endogenous antioxidant pathways, offering antidepressant, neuroprotective, and anti-inflammatory benefits [66]. It also displays antibacterial and anticancer effects by inhibiting reactive oxygen species and biofilm formation while modulating critical pathways such as MET/AKT/mTOR [67].
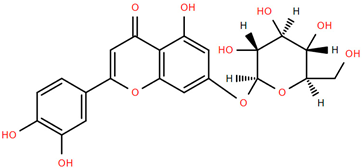
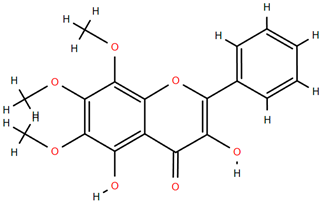
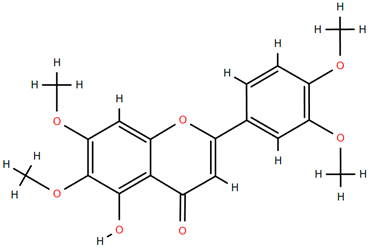
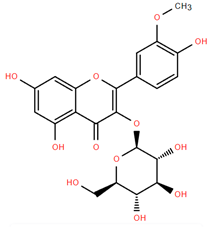
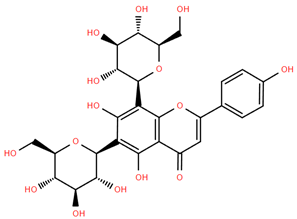
Methoxylated flavones, including 3,5-dihydroxy-6,7,8-trimethoxyflavone (Compound 13) and 5-hydroxy-3,6,7,4′-tetramethoxyflavone (Compound 18) from A. nobilis have shown enhanced anticancer effects, particularly when combined with 5-fluorouracil, resulting in reduced off-target toxicity in colon and pancreatic cancer treatments [71]. C-glycosylflavones such as vitexin (Compound 3, Figure 4), isovitexin (Compound 14), swertisin (Compound 15), orientin (Compound 1, Figure 4), and isoorientin (Compound 2, Figure 4), isolated from A. nobilis subsp. neilreichii, exhibit a wide range of pharmacological effects. These include antioxidant, anti-inflammatory, neuroprotective, cardioprotective, antidiabetic, anticancer, hepatoprotective, and anxiolytic activities [72,73,74,75]. Swertiajaponin (Compound 16), a C-methylated glycoside, has been implicated in the alleviation of metabolic syndromes such as hyperglycemia, hyperlipidemia, and insulin resistance [76], highlighting its therapeutic potential for metabolic disorders.
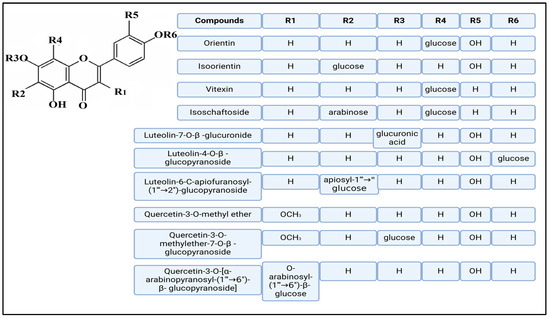
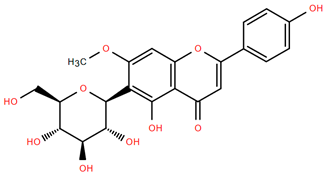
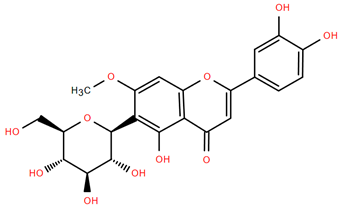
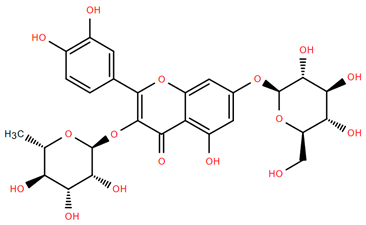
Figure 4.
Chemical structures of selected flavone and flavonol glycoside derivatives identified in Achillea nobilis L. and its subspecies [24]. Compound 1: orientin; Compound 2: isoorientin; Compound 3: vitexin; Compound 4: isoschaftoside; Compound 5: luteolin-7-O-β-glucuronide; Compound 6: luteolin-4-O-β-glucopyranoside; Compound 7: luteolin-6-C-apiofuranosyl-(1 → 2)-glucopyranoside; Compound 8: quercetin-3-O-methyl ether; Compound 9: quercetin-3-O-methylether-7-O-β-glucopyranoside; Compound 10: quercetin-3-O-[α-arabinopyranosyl-(1 → 6)-β-glucopyranoside].
Flavonoids like quercetin-3-O-rhamnoside-7-O-glucoside (Compound 17) and quercetin-3-O-methylether-7-O-β-glucopyranoside (Compound 9, Figure 4) demonstrate strong antioxidant activities. Quercetin-3-O-rhamnoside-7-O-glucoside (Compound 17) inhibits polyphenol oxidase, preventing enzymatic browning, and enhances food antioxidant properties [77]. Quercetin-3-O-methylether-7-O-β-glucopyranoside (Compound 9, Figure 4) shows protective effects against oxidative stress and in breast cancer models by inducing apoptosis and modulating PI3K/Akt and MAPK/Erk signaling pathways [82,83,84]. Luteolin (Compound 19) and isorhamnetin-3-O-glucoside (Compound 20) obtained from A. nobilis subsp. neilreichii also exhibit potent antioxidant, anti-inflammatory, and antiproliferative activities in endothelial and cancer cells, primarily through the STAT3 pathway and suppression of epithelial–mesenchymal transition (EMT) [85,86]. These compounds collectively enhance the plant’s medicinal potential through their multi-target bioactivities.
Among phenolic acids, dicaffeoylquinic acid (Compound 22) and chlorogenic acid (Compound 23) exhibit significant bioactivities. Compound 22 demonstrates antioxidant, anti-inflammatory, antimicrobial, cardioprotective, hepatoprotective, and antispasmodic properties, proving beneficial in the treatment of respiratory and metabolic disorders [87]. Compound 23, an ester of hydroxycinnamic acid, has a wide range of pharmacological effects, including antibacterial, antiviral, anti-obesity, antihypertensive, antipyretic, and CNS-stimulating activities [88]. It also regulates lipid and glucose metabolism, making it useful in managing cardiovascular disease, diabetes, and obesity.
Several compounds such as luteolin-4-O-β-glucopyranoside (Compound 6, Figure 4), luteolin-6-C-apiofuranosyl-(1”→2”)-glucopyranoside (Compound 7, Figure 4), apigenin-6,8-di-C-glucoside (Compound 21), and quercetin-3-O-[α-arabinopyranosyl-(1”→6”)-β-glucopyranoside] (Compound 10, Figure 4) currently lack comprehensive pharmacological data (Figure 4). However, due to their structural similarity to well-characterized flavonoids such as luteolin and quercetin, they are hypothesized to possess antioxidant, anti-inflammatory, and neuroprotective activities. Studies have shown that C- and O-glycosylation enhance flavone stability and bioactivity, improving their modulatory effects on oxidative stress and inflammatory pathways [65,66,85]. Moreover, glycosidic modifications may enhance solubility and absorption, suggesting favorable pharmacokinetic profiles for future therapeutic applications [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103].
5.2. Terpenes and Derivatives
A literature review revealed that A. nobilis and its subspecies, such as A. nobilis subsp. sipylea and A. nobilis subsp. neilreichii, contain a total of 157 terpene and terpene-derived compounds (Table 5). These substances are divided into various chemical categories: terpenes, terpenoids, sesquiterpenes, and derivatives of sesquiterpenoids. The chemical components were mainly extracted from different plant sections, including flower heads, aerial portions, and leaves, showcasing the plant’s abundant phytochemical diversity and the structural variety of its secondary metabolites. This wide range of terpenoid composition emphasizes the potential of A. nobilis and its subspecies as important sources of bioactive natural compounds.

Table 5.
Terpenes and derivatives found in A. nobilis and its subspecies.
A significant variety of terpenoids from A. nobilis display marked anti-inflammatory and antioxidant properties. For example, artemetin and caryophyllene oxide exhibit significant anti-inflammatory effects by blocking proinflammatory enzymes and mediators, which helps lower oxidative stress in multiple inflammatory models [105,112]. Carvacrol and thymol are crucial in managing inflammation by inhibiting NF-κB and MAPK pathways, and they have demonstrated effectiveness in decreasing oxidative damage, positioning them as promising options for treating inflammatory diseases [115,117]. Borneol exhibits synergistic anti-inflammatory properties and improves the bioavailability of concurrently administered anti-inflammatory agents [119]. These substances play a major role in the conventional application of A. nobilis for addressing inflammation-related ailments.
Multiple terpenes found in A. nobilis and its subspecies have demonstrated anticancer properties. Significantly, β-caryophyllene and caryophyllene oxide promote apoptosis and hinder cancer cell proliferation by affecting various signaling pathways, such as the PI3K/Akt axis [109,112]. Additionally, thymol and borneol have shown cytotoxic properties against several cancer cell lines, such as liver and breast cancer, with proof indicating their capability to induce cell cycle arrest and apoptosis [117,119]. Camphor, while mainly recognized for its antimicrobial properties, has also demonstrated potential cytostatic effects in cancer studies [120]. These results emphasize the therapeutic potential of A. nobilis terpenes in cancer prevention and therapy.
A significant amount of evidence backs the antimicrobial and antiviral properties of A. nobilis terpenes. Camphor, borneol, thymol, and carvacrol have shown significant antimicrobial properties against Gram-positive and Gram-negative bacteria, along with fungi [115,117,119]. Thymol and carvacrol specifically display membrane-disrupting characteristics that hinder microbial survival and biofilm development. Furthermore, sabinene and β-pinene have demonstrated antiviral properties by disrupting viral replication processes, aligning with the application of essential oils in traditional medicine for addressing respiratory and viral illnesses [113,114]. These terpenoids could function as useful substitutes for synthetic antimicrobial substances.
Certain terpene compounds derived from A. nobilis are noted to exert positive effects on the central nervous system. Thymol has been demonstrated to have anxiolytic and neuroprotective properties by influencing GABAergic neurotransmission and diminishing oxidative damage to neurons [117]. Borneol has shown notable neuroprotective effects, in part because it can traverse the blood–brain barrier and improve drug transport to the brain [119]. In a similar manner, β-caryophyllene engages with cannabinoid receptors and demonstrates anxiolytic, antidepressant, and neuroprotective effects [109]. These results indicate a possible function for A. nobilis terpenoids in the treatment of neurodegenerative diseases and mental health therapies.
Besides the primary therapeutic areas mentioned earlier, various terpenoids exhibit a range of bioactivities. For instance, thujone and sabinene have shown insecticidal and antifeedant properties that are beneficial in pest control [122]. Myrtenol and camphene show gastroprotective and antispasmodic properties, which could be helpful for gastrointestinal issues [110,121]. Additionally, α-pinene and β-pinene exhibit bronchodilatory and mucolytic properties, aiding their application in respiratory disorders [113]. While certain compounds like aristolone and germacrene D remain inadequately researched, their structural resemblance to active terpenes implies probable antioxidant and anti-inflammatory functions. These adaptable pharmacological characteristics endorse the multifunctional capabilities of A. nobilis terpenes in clinical and ethnopharmacological uses.
5.3. Other Biologically Active Compounds
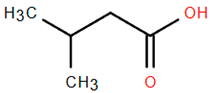
Other biologically active compounds identified in A. nobilis and its subspecies include simple hydrocarbons, fatty alcohols, fatty acids, heterocyclic compounds, and simple ketones, all of which contribute to the plant’s diverse pharmacological potential (Table 6). Among these, 1-octadecanol (Compound 26) stands out due to its antibacterial, anti-inflammatory, emollient, and mucosal-protective characteristics, which support its use in vaginal drug delivery systems and various mucosal therapeutic formulations [159,160]. Hexadecanoic acid (Compound 27), commonly referred to as palmitic acid, has been shown to promote the growth of bone marrow mesenchymal stem cells and displays various pharmacological properties, such as antimicrobial, antioxidant, anti-inflammatory, anticancer, hypocholesterolemic, and skin-protective effects. This establishes it as a significant bioactive lipid for therapeutic advancements [161]. Likewise, dillapiol (Compound 29) has demonstrated antimicrobial and antifungal properties, indicating its relevance in the development of antimicrobial medications and natural preservation methods [162]. Additional compounds extracted from A. nobilis also show specific pharmacological effects. For instance, isovaleric acid (Compound 30) has been reported to improve ovariectomy-induced osteoporosis by suppressing osteoclast differentiation, suggesting its potential use in managing bone health after menopause [163]. This supports the inclusion of isovaleric acid among clinically significant bioactives for bone metabolism and osteoporosis-related conditions.

Table 6.
Other biologically active compounds found in A. nobilis and its subspecies.
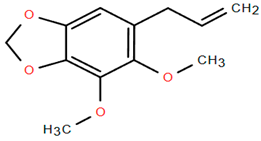
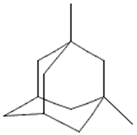
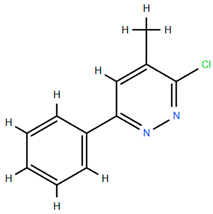
On the other hand, several compounds found in A. nobilis such as tricosane (Compound 24), hexadecanol (Compound 25), indipone (Compound 28), adamantane-1,3-dimethyl (Compound 31), 3-chloro-4-tert-butyl-6-phenylpyridazin (Compound 32), and pentadecanone (Compound 33), currently do not have documented pharmacological activity according to the existing literature. Although these compounds have not been extensively studied, their structural features and chemical classifications suggest potential biological activity. For example, alkanes such as tricosane and ketones like pentadecanone frequently occur in essential oils recognized for their insect-repellent or antimicrobial properties, while pyridazine derivatives are being explored for their anti-inflammatory and anticancer effects. Therefore, future bioactivity-oriented studies are needed to determine the medicinal relevance of these lesser-known compounds.
6. Pharmacological Effects Studies on A. nobilis and Its Subspecies
Achillea nobilis L. and its subspecies (A. nobilis subsp. neilreichii and A. nobilis subsp. sipylea) have garnered growing scientific interest owing to their extensive array of bioactive compounds and varied pharmacological characteristics, establishing them as a potential natural shield against a broad range of pathological issues, as demonstrated by their various pharmacological effects (Figure 4). Notably, the ethanolic extract of A. nobilis demonstrated significant anticonvulsant potential in preclinical models. In a comparative study using maximal electroshock (MES), pentylenetetrazole (PTZ), and strychnine nitrate (STN)-induced seizure models in rats, A. nobilis extract exhibited dose-dependent anticonvulsant effects, particularly at 200 and 300 mg/kg doses. Specifically, in the MES and PTZ-induced convulsion tests, the extract delayed the onset of seizures and reduced seizure duration, indicating central nervous system depressant activity. Moreover, at a dose of 300 mg/kg, A. nobilis provided 60% protection against STN-induced seizures, suggesting its potential role in modulating glycine-mediated neurotransmission. However, lower doses (100 and 200 mg/kg) did not show significant protection in the STN model, highlighting a possible threshold for efficacy. Consequently, these findings support the potential of A. nobilis as a natural source of anticonvulsant agents and warrant further investigation into its active constituents and mechanisms of action [164].
Another study revealed that the ethanol extract of A. nobilis subsp. neilreichii has noteworthy antinociceptive and anti-inflammatory effects, highlighting its possible therapeutic importance in managing pain and inflammation. The extract produced a significant antinociceptive response during the later phase of the formalin test at doses of 100, 200, and 400 mg/kg (i.p.), demonstrating its efficacy in alleviating inflammatory pain. Furthermore, it demonstrated anti-inflammatory effects in the carrageenan-induced paw edema model, especially at 100 and 200 mg/kg, indicating the suppression of acute inflammatory reactions. Nonetheless, no notable antinociceptive effect was noted in the tail-flick test, suggesting restricted effectiveness in altering spinal reflexes to thermal stimuli. Importantly, the administration of 400 mg/kg lengthened the latency on the hot-plate test at 60 and 90 min, indicating possible central analgesic effects without impairing sensory motor function. Additionally, the extract showed a significant safety margin, exhibiting an intraperitoneal LD50 value of 4456 mg/kg in mice. As a result, these results emphasize the anti-inflammatory and mild analgesic effects of A. nobilis subsp. neilreichii, endorsing its continued investigation in pain management studies [165].
Additionally, the research examined the antispasmodic properties of the total extract from A. nobilis subsp. sipylea on isolated rat duodenum, offering pharmacological support for its historical use in treating gastrointestinal spasms. The extract was assessed via a range of experiments that included cumulative dose–response curves for acetylcholine (ACh) and calcium chloride (CaCl2), conducted both without and with established spasmolytic agents like atropine, papaverine, and verapamil. The findings showed that the extract had a concentration-dependent inhibitory effect on contractions induced by ACh and CaCl2, noticeably decreasing the maximum contractile responses of the duodenal tissue. Significantly, this effect resembled that of papaverine and verapamil, but was different from atropine, indicating a mechanism of action that does not depend on muscarinic receptor antagonism and is more likely related to direct relaxation of smooth muscle or modulation of calcium channels. Additionally, the extract reduced K+-induced contractions, highlighting its muscle-relaxing properties. As a result, these results suggest that A. nobilis subsp. sipylea has significant antispasmodic properties, probably through the disruption of calcium influx and smooth muscle contractions, and may offer potential therapeutic benefits for gastrointestinal issues marked by hypermotility or spasms [166].
The protective benefits of Achillea species indigenous to Turkey, such as A. nobilis subspecies, have been studied regarding oxidative stress caused by hydrogen peroxide (H2O2) in human erythrocytes and leucocytes. This research assessed the antioxidant capacity of infusions made from 15 Achillea species, which are commonly used in traditional Turkish medicine, by measuring their effect on vital antioxidant enzymes—catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx)—along with lipid peroxidation (LPO) and glutathione (GSH) concentrations. The findings revealed that all Achillea infusions provided considerable protective benefits against oxidative damage induced by H2O2 by boosting the function of antioxidant defense systems in both erythrocytes and leucocytes. Significantly, A. falcata was the most potent in improving CAT, SOD, and GPx activities in red blood cells. Among the infusions tested, A. crithmifolia and A. nobilis subsp. neilreichii showed the greatest stimulation of CAT activity in leukocytes, while A. millefolium subsp. pannonica was the most effective for SOD, and A. teretifolia for GPx. Moreover, A. nobilis subsp. sipylea and A. setacea exhibited the most significant protective effects on LPO levels in leucocytes, while A. millefolium subsp. pannonica and A. falcata showed the least LPO levels in erythrocytes. As a result, these results indicate that Achillea species, especially the A. nobilis subspecies, serve as a valuable source of natural antioxidants that may be useful in the prevention and management of diseases linked to oxidative stress [167].
The essential oils extracted from A. nobilis subsp. sipylea and A. nobilis subsp. neilreichii show different levels of antibacterial and antifungal effects against various clinically significant microorganisms, with A. nobilis subsp. sipylea displaying the strongest antimicrobial capability. This subspecies exhibited significant antifungal properties against Candida albicans, resulting in an inhibition zone of 41 mm, and also demonstrated considerable antibacterial effects against Escherichia coli, Staphylococcus aureus, and Salmonella typhimurium, with inhibition zones varying from 12 to 15 mm. Conversely, essential oils obtained from A. nobilis subsp. neilreichii, gathered from various geographic sites, showed diminished antimicrobial properties; however, there was still some activity, especially against Gram-positive bacteria like Staphylococcus epidermidis and S. aureus. None of the essential oils evaluated were successful against Pseudomonas aeruginosa, renowned for its significant inherent resistance [27]. While the antimicrobial effectiveness of these essential oils was typically less than that of standard antibiotics like amoxicillin and sulbactam/ampicillin, the findings still indicate significant biological activity [141,164]. These results back the possible application of A. nobilis, especially subsp. sipylea, as a hopeful natural source of antimicrobial compounds, with regional differences in chemical makeup probably explaining variations in effectiveness [26,27]. Another study assessed the antibacterial properties of various extracts from A. nobilis subsp. neilreichii against S. aureus ATCC 25923, emphasizing how the extraction method and solvent type influence antimicrobial effectiveness. The Soxhlet extracts, especially those derived from ethyl acetate and chloroform, exhibited the strongest activity, showing minimum inhibitory concentrations (MICs) of 3.1 µg/mL and 3.87 µg/mL, respectively. Conversely, extracts obtained via ultrasonic bath with chloroform and maceration with n-hexane, chloroform, or ethanol exhibited lesser effects, resulting in MIC values surpassing 5 µg/mL and exceeding 6.25 µg/mL in certain instances. These findings indicate that Soxhlet extraction using semi-polar solvents improves the extraction of antibacterial compounds from the plant material. Furthermore, while the plant extracts were not as effective as the standard antibiotic meropenem (MIC, 0.012 µg/mL), they still suggest that A. nobilis subsp. neilreichii could be a valuable source of natural antibacterial compounds, especially when suitable extraction techniques are utilized [33].
While the cited pharmacological studies on A. nobilis and its subspecies provide valuable insights into their biological activities, several limitations should be acknowledged. Most experiments have been conducted using in vivo rodent models or basic in vitro assays, with limited mechanistic investigations to identify specific molecular targets. Additionally, many studies rely on crude extracts rather than purified compounds, which makes it difficult to determine which constituents are responsible for the observed effects. The sample sizes and control designs are sometimes insufficiently described, and variations in extraction methods may lead to inconsistent results across studies.
Importantly, a comprehensive literature review using multiple scientific databases (as detailed in the methodology section) found no published human clinical studies investigating A. nobilis or its subspecies. This significant gap indicates that the species is underexplored in clinical research, and highlights the need for rigorous human trials to validate its safety, efficacy, and therapeutic relevance. Furthermore, comprehensive toxicity and pharmacokinetic evaluations are lacking, and most studies do not assess long-term safety. These limitations underscore the need for standardized experimental protocols and advanced preclinical and clinical research to support the integration of A. nobilis and its bioactive components into evidence-based pharmaceutical and nutraceutical applications.
7. Conclusions
This review highlights the ethnomedicinal importance of A. nobilis and its subspecies (A. nobilis subsp. neilreichii and A. nobilis subsp. sipylea), which have been esteemed for ages in conventional medical practices. These plants have been extensively used to address a range of issues, such as inflammation, pain, infections, spasms, and convulsions. Their application in decoctions, oils, and infusions showcases the vibrant cultural history related to their therapeutic uses. Although there is extensive traditional usage, scientific investigation into their pharmacological mechanisms and bioactive components is still incomplete.
Existing phytochemical investigations show that A. nobilis comprises a variety of compounds, particularly flavonoids, flavonoid glycosides, monoterpenes, sesquiterpenes, sesquiterpene lactones, and terpenoid derivatives. These substances are associated with noteworthy pharmacological impacts, including antioxidant, antibacterial, antifungal, anti-inflammatory, antinociceptive, antispasmodic, central analgesic, and anticonvulsant effects. The existence of these bioactive compounds not only confirms traditional applications but also establishes the plant as a strong contender for drug development. Nonetheless, the majority of research has focused on water-based extracts and above-ground portions, with minimal emphasis on the chemistry specific to roots or variations within organs.
As indicated in the summarized pharmacological profile, A. nobilis and its varieties display a wide range of bioactivities. However, these effects differ based on subspecies, part of the plant, and extraction technique. Significant chemotypic variation is impacted by environmental and genetic influences, necessitating accurate chemotaxonomic and organ-specific evaluations. Incorporating multi-omics strategies, including metabolomics, transcriptomics, and sophisticated analytical methods (e.g., UHPLC–HRMS, MALDI–TOF), will be crucial for thoroughly charting the metabolite profile of this species and linking it to pharmacological results.
In summary, this review emphasizes the medical potential and overlooked research prospects related to A. nobilis and its subspecies. Future research should focus on examining underexplored plant components such as roots, assess seasonal and geographical impacts on phytochemical variations, and create solid pharmacological connections through interdisciplinary teamwork. Such initiatives will yield important understanding for the evidence-driven use of these plants in contemporary pharmacology and nutraceutical development.
Author Contributions
Conceptualization, A.A. (Akerke Amirkhanova), A.A. (Aiman Аkhelova), A.S., S.N., A.M., A.K., R.D., K.R. and Y.I.; methodology, L.S.; software, M.K. and A.T.; data curation, G.S., A.K. and A.A. (Akerke Amirkhanova); writing—original draft preparation, A.S., A.A. (Akerke Amirkhanova), A.A. (Aiman Аkhelova), A.S., S.N., A.M. and M.K.; writing—review and editing, A.S., R.D., K.R., Y.I., A.T., A.K. and A.A. (Akerke Amirkhanova); supervision, A.S. and A.A. (Akerke Amirkhanova). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abilkassymova, A.; Turgumbayeva, A.; Sarsenova, L.; Tastambek, K.; Altynbay, N.; Ziyaeva, G.; Blatov, R.; Altynbayeva, G.; Bekesheva, K.; Abdieva, G.; et al. Exploring Four Atraphaxis Species: Traditional Medicinal Uses, Phytochemistry, and Pharmacological Activities. Molecules 2024, 29, 910. [Google Scholar] [CrossRef] [PubMed]
- Shynykul, Z. Comparative Evaluation of Chemical Carbon Acid Extract of the Ordinary Harmala (Peganum harmala) in Central Asia Region. Asian J. Plant Sci. 2022, 21, 574–581. [Google Scholar] [CrossRef]
- Amirkhanova, S.A.; Ustenova, G.O.; Krauze, M.B.; Pobłocka, L.O.; Shynykul, S.Z. Thin-Layer Chromatography Analysis of Extract Oxytropis glabra Lam. DC. Int. Multidiscip. Sci. GeoConf. SGEM 2018, 18, 775–782. [Google Scholar]
- Barda, C.; Grafakou, M.-E.; Tomou, E.-M.; Skaltsa, H. Phytochemistry and Evidence-Based Traditional Uses of the Genus Achillea L.: An Update (2011–2021). Sci. Pharm. 2021, 89, 50. [Google Scholar] [CrossRef]
- Khalid, T.; Chang, C.W.; Ross, S.A.; Naseer, F.; Qadeer, A.; Chen, C.C.; Rafey, H.A. Traditional Uses, Botanical Description, Phytochemistry, and Pharmacological Activities of Phytolacca acinosa: A Review. Front. Pharmacol. 2025, 15, 1480034. [Google Scholar] [CrossRef]
- Maiyo, Z.C.; Njeru, S.N.; Toroitich, F.J.; Indieka, S.A.; Obonyo, M.A. Ethnobotanical Study of Medicinal Plants Used by the People of Mosop, Nandi County in Kenya. Front. Pharmacol. 2024, 14, 1328903. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, C. Emerging applications of metabolomics in studying chemopreventive phytochemicals. AAPS J. 2013, 15, 941–950. [Google Scholar] [CrossRef]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Okigbo, R.N.; Anuagasi, C.L.; Amadi, J.E. Advances in selected medicinal and aromatic plants indigenous to Africa. J. Med. Plants Res. 2009, 3, 86–95. [Google Scholar]
- Gakuya, D.W.; Okumu, M.O.; Kiama, S.G.; Mbaria, J.M.; Gathumbi, P.K.; Mathiu, P.M.; Nguta, J.M. Traditional Medicine in Kenya: Past and Current Status, Challenges, and the Way Forward. Sci. Afr. 2020, 8, e00360. [Google Scholar] [CrossRef]
- Si, X.T.; Zhang, M.L.; Shi, Q.W.; Kiyota, H. Chemical Constituents of the Plants in the Genus Achillea. Chem. Biodivers. 2006, 3, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Nickavar, B.; Kamalinejad, M.; Haj-Yahya, M.; Shafaghi, B. Comparison of the Free Radical Scavenging Activity of Six Iranian Achillea Species. Pharm. Biol. 2006, 44, 208–212. [Google Scholar] [CrossRef]
- Kishkentayeva, A.S.; Mantler, S.N.; Zhakanov, M.M.; Adekenov, S.M. Biologically Active Substances from Achillea nobilis L. Vestn. Karagand. Univ. Ser. Khim. 2020, 4, 52–59. (In Russian) [Google Scholar]
- Karaalp, C.; Yurtman, A.N.; Karabay Yavasoglu, N.U. Evaluation of Antimicrobial Properties of Achillea L. Flower Head Extracts. Pharm. Biol. 2009, 47, 86–91. [Google Scholar] [CrossRef]
- Bulut, G.; Tuzlacı, E. Folk Medicinal Plants of Bayramiç (Çanakkale-Turkey). J. Fac. Pharm. Istanb. Univ. 2009, 40, 87–99. [Google Scholar]
- Karunamoorthi, K.; Husen, E. Knowledge and self-reported practice of the local inhabitants on traditional insect repellent plants in Western Hararghe zone, Ethiopia. J. Ethnopharmacol. 2012, 141, 212–219. [Google Scholar] [CrossRef]
- Solomko, O.V.; Dzhumyrko, S.F.; Kompantsev, V.A. Flavonoids of Achillea nobilis. Chem. Nat. Compd. 1978, 14, 224. [Google Scholar] [CrossRef]
- Adekenov, S.M.; Mukhametzhanov, M.N.; Kagarlitskii, A.D.; Turmukhambetov, A.Z. A chemical investigation of Achillea nobilis. Chem. Nat. Compd. 1984, 20, 568–571. [Google Scholar] [CrossRef]
- Valant-Vetschera, K.M. Flavonoid glycoside accumulation trends of Achillea nobilis L. and related species. Biochem. Syst. Ecol. 1988, 15, 45–52. [Google Scholar] [CrossRef]
- Trudybekov, K.M.; Turmukhambetov, A.Z.; Adekenov, S.M.; Struchkov, Y.T. Anolide—A new guaianolide from Achillea nobilis. Chem. Nat. Compd. 1994, 30, 460–463. [Google Scholar] [CrossRef]
- Kastner, U.; Breuer, J.; Glasl, S.; Baumann, A.; Robien, W.; Jurenitsch, J.; Kubelka, W. Guaianolide-Endoperoxide and Monoterpene-Hydroperoxides from Achillea nobilis. Planta Medica 1995, 61, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Turmukhambetov, A.Z.; Buketova, G.K.; Gafurov, N.M.; Adekenov, S.M. Chrysartemin a and canin from Achillea nobilis. Chem. Nat. Compd. 1999, 35, 102. [Google Scholar] [CrossRef]
- Krenn, L.; Miron, A.; Pemp, E.; Petr, U.; Kopp, B. Flavonoids from Achillea nobilis L. Z. Naturforsch. C J. Biosci. 2003, 58, 11–16. [Google Scholar] [CrossRef]
- Palić, R.; Stojanović, G.; Nasković, T.; Ranelović, N. Composition and Antibacterial Activity of Achillea crithmifolia and Achillea nobilis Essential Oils. J. Essent. Oil Res. 2003, 15, 434–437. [Google Scholar] [CrossRef]
- Karamenderes, C.; Karabay Yavasoglu, N.U.; Zeybek, U. Composition and antimicrobial activity of the essential oils of Achillea nobilis L. subsp. sipylea and subsp. neilreichii. Chem. Nat. Compd. 2007, 43, 632–634. [Google Scholar] [CrossRef]
- Ghani, A.; Azizi, M.; Hassanzadeh-Khayyat, M.; Pahlavanpour, A.A. Essential Oil Composition of Achillea eriophora, A. nobilis, A. biebersteinii and A. wilhelmsii from Iran. J. Essent. Oil Bear. Plants 2008, 11, 460–467. [Google Scholar] [CrossRef]
- Demirci, F.; Guven, K.; Demirci, B.; Dadalioglu, I.; Baser, K.H.C. Characterization and biological activity of Achillea teretifolia Willd. and A. nobilis L. subsp. neilreichii (Kerner) Formanek essential oils. Turk. J. Biol. 2009, 33, 129–136. [Google Scholar]
- Serkerov, S.V.; Mustafaeva, S.J. Detection of acetyleucanbin in Achillea nobilis. Chem. Nat. Compd. 2010, 46, 666. [Google Scholar] [CrossRef]
- Azizi, M.; Chizzola, R.; Ghani, A.; Oroojalian, F. Composition at different development stages of the essential oil of four Achillea species grown in Iran. Nat. Prod. Commun. 2010, 5, 1934578X1000500224. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Masoudi, S.; Ezatpour, L.; Danaii, E.; Taherkhani, M.; Aghajani, Z. Composition of the essential oils of Anthemis hyalina DC., Achillea nobilis L., and Cichorium intybus L.—Three Asteraceae herbs growing wild in Iran. J. Essent. Oil Bear. Plants 2011, 14, 472–480. [Google Scholar] [CrossRef]
- Kazemizadeh, Z.; Moradi, A.; Yousefi, M. Volatile constituents from leaf and flower of Achillea nobilis L. subsp. neilreichii from North of Iran. J. Med. Plants 2011, 10, 156–162. [Google Scholar]
- Taşkın, D.; Taşkın, T.; Rayaman, E. Phenolic composition and biological properties of Achillea nobilis L. subsp. neilreichii (Kerner) Formanek. Ind. Crops Prod. 2018, 111, 555–562. [Google Scholar] [CrossRef]
- Ozdemir, F.A. Potential effects of essential oil compositions on antibacterial activities of Achillea nobilis L. subsp. neilreichii. J. Essent. Oil Bear. Plants 2019, 22, 574–580. [Google Scholar] [CrossRef]
- Jangjoo, M.; Joshaghani, A.; Tahernejadgatabi, F. The Role of Achillea millefolium in Traditional Medicine: A Review of Its Use in Different Cultures. J. Multidiscip. Care 2023, 12, 152–156. [Google Scholar] [CrossRef]
- Stevens, P.F. Angiosperm Phylogeny Website. Version 14, July 2017 [and More or Less Continuously Updated Since]. Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 17 April 2025).
- Saeidnia, S.; Gohari, A.R.; Mokhber-Dezfuli, N.; Kiuchi, F. A Review on Phytochemistry and Medicinal Properties of the Genus Achillea. DARU J. Pharm. Sci. 2011, 19, 173–186. [Google Scholar]
- Oberprieler, C.; Himmelreich, S.; Vogt, R. A new subtribal classification of the tribe Anthemideae (Compositae). Willdenowia 2007, 37, 89–114. [Google Scholar] [CrossRef]
- Rechinger, K.H. Flora Iranica. Compositae II. Anthemideae; Akademische Druck- und Verlagsanstalt: Graz, Austria, 1965; Volume 158. [Google Scholar]
- Khatun, M.A.; Reza, M.A.; Rahman, M.S. Achillea Species: A Comprehensive Review on Its Ethnopharmacology, Phytochemistry, and Pharmacological Properties. Pharmacogn. Rev. 2016, 10, 148–156. [Google Scholar] [CrossRef]
- USDA; NRCS. The PLANTS Database; National Plant Data Team: Greensboro, NC, USA. Available online: http://plants.usda.gov (accessed on 17 April 2025).
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Apigenin: A Promising Molecule for Cancer Prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Habtemariam, S.; Daglia, M.; Nabavi, S.M. Apigenin and Breast Cancers: From Chemistry to Medicine. Anticancer Agents Med. Chem. 2015, 15, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Ninfali, P.; Angelino, D. Nutritional and Biological Value of Vegetables. In Functional Foods; Springer: New York, NY, USA, 2014; pp. 329–338. [Google Scholar]
- Seelinger, G.; Merfort, I.; Schempp, C.M. Anti-Oxidant, Anti-Inflammatory and Anti-Allergic Activities of Luteolin. Planta Med. 2008, 74, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Tsai, Y.J.; Tsay, J.S.; Lin, J.K. Factors Affecting the Levels of Tea Polyphenols and Caffeine in Tea Leaves. J. Agric. Food Chem. 2003, 51, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-Inflammatory Effects of Luteolin: A Review of in Vitro, in Vivo, and in Silico Studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Nabavi, S.M.; Eslami, S.; Moghaddam, A.H. In Vivo Protective Effects of Luteolin Against Sodium Fluoride-Induced Oxidative Stress in Rat Heart. Food Chem. Toxicol. 2012, 50, 3562–3566. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 2015, 5, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.; Towers, G.H.N.; Mitchell, J.C. Biological activities of sesquiterpene lactones. Phytochemistry 1976, 15, 1573–1580. [Google Scholar] [CrossRef]
- Ferreira, D.; Slade, D.; Marais, J.P. Flavonoids and Related Compounds: Bioavailability and Function. In Natural Products as Source of Molecules with Therapeutic Potential; Springer: Cham, Switzerland, 2018; pp. 411–448. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biological, physiological, and molecular differences in the production of secondary metabolites in plant organs. Plants 2022, 11, 1234. [Google Scholar] [CrossRef]
- Liang, J.-L.; Jin, X.-K.; Deng, X.-C.; Huang, Q.-X.; Zhang, S.-M.; Chen, W.-H.; Zhang, X.-Z. Targeting activation of cGAS-STING signaling pathway by engineered biomaterials for enhancing cancer immunotherapy. Mater. Today 2024, 78, 251–296. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Vari, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Cazarolli, L.H.; Zanatta, L.; Alberton, E.H.; Figueiredo, M.S.R.B.; Folador, P.; Damazio, R.G.; Pizzolatti, M.G.; Silva, F.R.M.B. Flavonoids: Prospective Drug Candidates. Mini Rev. Med. Chem. 2008, 8, 1429–1440. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. ScientificWorldJournal 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Zhang, S.; Li, L.; Chen, W.; Xu, S.; Feng, X.; Zhang, L. Natural products: The role and mechanism in low-density lipoprotein oxidation and atherosclerosis. Phytother. Res. 2021, 35, 2945–2967. [Google Scholar] [CrossRef]
- Isla, M.I.; Anesini, C.; Ferraro, G.E.; Bongiovanni, G.A.; Coussio, J.D. Apigenin-7-glucuronide from Urera aurantiaca: Anti-inflammatory Activity in LPS-Stimulated Macrophages. Evid.-Based Complement. Altern. Med. 2011, 2011, 537393. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Wang, J.; Xu, Z.; Wang, W.; Luo, Y.; Li, D. Cynaroside Alleviates Depression by Inhibiting M1 Polarization of Microglia and Ferroptosis via IRF1/SLC7A11/GPX4 Pathway. J. Ethnopharmacol. 2023, 316, 116347. [Google Scholar] [CrossRef]
- Zangeneh, M.M.; Fazeli-Nasab, B.; Zangeneh, A.; Moradi, R.; Amirian, H.; Kord, M. Biological and Pharmacological Properties of Cynaroside: A Comprehensive Review. Biomed. Pharmacother. 2022, 153, 113427. [Google Scholar] [CrossRef]
- Han, J.H.; Lee, E.J.; Park, W.; Choi, J.G.; Ha, K.T.; Chung, H.S. Cosmosiin Induces Apoptosis in Colorectal Cancer by Inhibiting PD-L1 Expression and Inducing ROS. Antioxidants 2023, 12, 2131. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Yin, H.; Yan, R.; Wang, Z.; Deng, J.; Li, G.; Pan, Y. The Anti-Tumor Effects of Cosmosiin through Regulating AhR/CYP1A1-PPARγ in Breast Cancer. FASEB J. 2024, 38, e70002. [Google Scholar] [CrossRef]
- Min, Z.; Tang, Y.; Hu, X.T.; Zhu, B.L.; Ma, Y.L.; Zha, J.S.; Deng, X.J.; Yan, Z.; Chen, G.J. Cosmosiin Increases ADAM10 Expression via Mechanisms Involving 5′UTR and PI3K Signaling. Front. Mol. Neurosci. 2018, 11, 198. [Google Scholar] [CrossRef]
- Cartwright, B.M.; Corso, J.N.; Lightner, J.; Whitted, C.; Torrenegra, R.D.; Krishnan, K.; Palau, V.E. Achyrocline B (3,5-Dihydroxy-6,7,8-Trimethoxyflavone) Synergizes with 5-Fluorouracil Allowing for Dose Reduction and Reduced Off-Target Toxicity in the Treatment of Colonic and Pancreatic Cancers. Biomed. Pharmacother. 2023, 167, 115546. [Google Scholar] [CrossRef]
- Mustapha, M.; Mat Taib, C.N. Beneficial Role of Vitexin in Parkinson’s Disease. Malays. J. Med. Sci. 2023, 30, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, Z.; Wang, H.; Jiang, M.; Lu, H.; Wei, Y.; Hu, Y.; Mo, L.; Liu, Y.; Zhou, C.; et al. Isovitexin Targets SIRT3 to Prevent Steroid-Induced Osteonecrosis of the Femoral Head by Modulating Mitophagy-Mediated Ferroptosis. Bone Res. 2025, 13, 18. [Google Scholar] [CrossRef]
- Bhardwaj, G.; Vakani, M.; Srivastava, A.; Patel, D.; Pappachan, A.; Murumkar, P.; Shah, H.; Shah, R.; Gupta, S. Swertisin, a Novel SGLT2 Inhibitor, with Improved Glucose Homeostasis for Effective Diabetes Therapy. Arch. Biochem. Biophys. 2021, 710, 108995. [Google Scholar] [CrossRef]
- Chlebda, E.; Magdalan, J.; Merwid-Ląd, A.; Trocha, M.; Kopacz, M.; Kuźniar, A.; Nowak, D.; Szeląg, A. Influence of water-soluble flavonoids, quercetin-5′-sulfonic acid sodium salt and morin-5′-sulfonic acid sodium salt, on antioxidant parameters in the subacute cadmium intoxication mouse model. Exp. Toxicol. Pathol. 2010, 62, 105–108. [Google Scholar] [CrossRef]
- Ziqubu, K.; Dludla, P.V.; Joubert, E.; Muller, C.J.F.; Louw, J.; Tiano, L.; Nkambule, B.B.; Kappo, A.P.; Mazibuko-Mbeje, S.E. Isoorientin: A Dietary Flavone with the Potential to Ameliorate Diverse Metabolic Complications. Pharmacol. Res. 2020, 158, 104867. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.M.; Lee, B.; Cho, W.K.; Lee, B.S.; Kim, C.Y.; Ma, J.Y. Swertiajaponin as an Anti-Browning and Antioxidant Flavonoid. Food Chem. 2018, 252, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ding, H.; Zhong, L.; Xin, W.; Yi, X.; Fang, L. Spectrum-Effect Relationship-Based Strategy Combined with Molecular Docking to Explore Bioactive Flavonoids from Sceptridium ternatum. Molecules 2022, 27, 5698. [Google Scholar] [CrossRef] [PubMed]
- Papapetrou, P.; Dimitriadis, K.; Galani, V.; Zoi, V.; Giannakopoulou, M.; Papathanasopoulou, V.A.; Sioka, C.; Tsekeris, P.; Kyritsis, A.P.; Lazari, D.; et al. Antitumor Activity of 5-Hydroxy-3′,4′,6,7-Tetramethoxyflavone in Glioblastoma Cell Lines and Its Antagonism with Radiotherapy. Biomol. Concepts 2024, 15, 1. [Google Scholar] [CrossRef]
- Su, Y.; Kang, Y.; Yi, J.; Lin, Q.; Zhang, C.; Lin, Z.; Yan, Z.; Qu, J.; Liu, J. Isoschaftoside Reverses Nonalcoholic Fatty Liver Disease via Activating Autophagy In Vivo and In Vitro. Evid.-Based Complement. Altern. Med. 2022, 2022, 2122563. [Google Scholar] [CrossRef]
- Guan, S.; Sun, L.; Wang, X.; Huang, X.; Luo, T. Isoschaftoside Inhibits Lipopolysaccharide-Induced Inflammation in Microglia through Regulation of HIF-1α-Mediated Metabolic Reprogramming. Evid.-Based Complement. Altern. Med. 2022, 2022, 5227335. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, Y.; Zhao, J.; Li, H.; Yin, X.; Wang, Y.; Xie, Y.; Chen, X.; Lu, J.; Dong, Z.; et al. Quercetin-3-Methyl Ether Inhibits Esophageal Carcinogenesis by Targeting the AKT/mTOR/p70S6K and MAPK Pathways. Mol. Carcinog. 2018, 57, 1540–1552. [Google Scholar] [CrossRef]
- Li, J.; Zhu, F.; Lubet, R.A.; De Luca, A.; Grubbs, C.; Ericson, M.E.; D’Alessio, A.; Normanno, N.; Dong, Z.; Bode, A.M. Quercetin-3-Methyl Ether Inhibits Lapatinib-Sensitive and -Resistant Breast Cancer Cell Growth by Inducing G2/M Arrest and Apoptosis. Mol. Carcinog. 2013, 52, 134–143. [Google Scholar] [CrossRef]
- Tseng, H.L.; Li, C.J.; Huang, L.H.; Chen, C.Y.; Tsai, C.H.; Lin, C.N.; Hsu, H.Y. Quercetin 3-O-Methyl Ether Protects FL83B Cells from Copper Induced Oxidative Stress through the PI3K/Akt and MAPK/Erk Pathway. Toxicol. Appl. Pharmacol. 2012, 264, 104–113. [Google Scholar] [CrossRef]
- De Stefano, A.; Caporali, S.; Di Daniele, N.; Rovella, V.; Cardillo, C.; Schinzari, F.; Minieri, M.; Pieri, M.; Candi, E.; Bernardini, S.; et al. Anti-Inflammatory and Proliferative Properties of Luteolin-7-O-Glucoside. Int. J. Mol. Sci. 2021, 22, 1321. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a Flavonoid, as an Anticancer Agent: A Review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, J.Y.; Ma, Y.K.; Park, K.Y.; Lee, S.H.; Ham, K.S.; Moon, J.H. Dicaffeoylquinic Acid Derivatives and Flavonoid Glucosides from Glasswort (Salicornia herbacea L.) and Their Antioxidative Activity. Food Chem. 2011, 125, 55–62. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, X.; Wu, L.; Shen, T.; Ji, L.; Zhao, X.; Si, C.L.; Jiang, Y.; Wang, G. Apigenin-7-O-β-D-glucuronide Inhibits LPS-Induced Inflammation through the Inactivation of AP-1 and MAPK Signaling Pathways in RAW 264.7 Macrophages and Protects Mice against Endotoxin Shock. Food Funct. 2016, 7, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Song, Y.S. Luteolin and Luteolin-7-O-Glucoside Inhibit Lipopolysaccharide-Induced Inflammatory Responses through Modulation of NF-κB/AP-1/PI3K-Akt Signaling Cascades in RAW 264.7 Cells. Nutr. Res. Pract. 2013, 7, 423–429. [Google Scholar] [CrossRef]
- Chen, S.-M.; Wang, M.-H.; Chang, K.-C.; Fang, C.-H.; Lin, Y.-W.; Tseng, H.-C. Vitexin Mitigates Haloperidol-Induced Orofacial Dyskinesia in Rats through Activation of the Nrf2 Pathway. Int. J. Mol. Sci. 2024, 25, 10206. [Google Scholar] [CrossRef]
- Rahman, A.; Al-Reza, S.; Kang, S. Antifungal activity of essential oil and extracts of Piper chaba Hunter against phytopathogenic fungi. J. Am. Oil Chem. Soc. 2011, 88, 573–579. [Google Scholar] [CrossRef]
- Kozłowska, A. Clinical Insights into Non-Alcoholic Fatty Liver Disease and the Therapeutic Potential of Flavonoids: An Update. Nutrients 2025, 17, 956. [Google Scholar] [CrossRef]
- Simionatto, E.; Porto, C.; Dalcol, I.I.; da Silva, U.F.; Morel, A.F. Essential oil from Zanthoxylum hyemale. Planta Med. 2005, 71, 759–763. [Google Scholar] [CrossRef]
- Koudou, J.; Abena, A.A.; Ngaissona, P.; Bessière, J.M. Chemical composition and pharmacological activity of essential oil of Canarium schweinfurthii. Fitoterapia 2005, 76, 700–703. [Google Scholar] [CrossRef]
- Wu, J.Y.; Chen, Y.J.; Bai, L.; Liu, Y.X.; Fu, X.Q.; Zhu, P.L.; Li, J.K.; Chou, J.Y.; Yin, C.L.; Wang, Y.P.; et al. Chrysoeriol Ameliorates TPA-Induced Acute Skin Inflammation in Mice and Inhibits NF-κB and STAT3 Pathways. Phytomedicine 2020, 68, 153173. [Google Scholar] [CrossRef] [PubMed]
- Orav, A. Identification of terpenes by gas chromatography-mass spectrometry. In Current Practice of Gas Chromatography-Mass Spectrometry; Marcel Dekker, Inc.: New York, NY, USA, 2001; pp. 483–494. [Google Scholar]
- Gharras, H. Tricin as an anticancer agent: Potential and mechanisms. Int. J. Mol. Sci. 2019, 20, 3966. [Google Scholar]
- Cheng, A.-X.; Lou, Y.-G.; Mao, Y.-B.; Lu, S.; Wang, L.-J.; Chen, X.-Y. Plant terpenoids: Biosynthesis and ecological functions. J. Integr. Plant Biol. 2007, 49, 179–186. [Google Scholar] [CrossRef]
- Pontin, M.; Bottini, R.; Burba, J.L.; Piccoli, P. Allium sativum produces terpenes with fungistatic properties in response to infection with Sclerotium cepivorum. Phytochemistry 2015, 115, 152–160. [Google Scholar] [CrossRef]
- Solá, R.J.; Griebenow, K. Effects of Glycosylation on the Stability of Protein Pharmaceuticals. J. Pharm. Sci. 2009, 98, 1223–1245. [Google Scholar] [CrossRef]
- Hollman, P.C. Bioavailability of Flavonoids. Eur. J. Clin. Nutr. 1997, 51 (Suppl. S1), S66–S69. [Google Scholar] [PubMed]
- Hu, L.; Luo, Y.; Yang, J.; Cheng, C. Botanical Flavonoids: Efficacy, Absorption, Metabolism and Advanced Pharmaceutical Technology for Improving Bioavailability. Molecules 2025, 30, 1184. [Google Scholar] [CrossRef]
- Ahn, J.H.; Song, E.J.; Jung, D.H.; Kim, Y.J.; Seo, I.S.; Park, S.C.; Jung, Y.S.; Cho, E.S.; Mo, S.H.; Hong, J.J.; et al. The Sesquiterpene Lactone Estafiatin Exerts Anti-Inflammatory Effects on Macrophages and Protects Mice from Sepsis Induced by LPS and Cecal Ligation Puncture. Phytomedicine 2022, 99, 153934. [Google Scholar] [CrossRef]
- Ryu, Y.; Lee, D.; Jung, S.H.; Lee, K.J.; Jin, H.; Kim, S.J.; Lee, H.M.; Kim, B.; Won, K.J. Sabinene Prevents Skeletal Muscle Atrophy by Inhibiting the MAPK-MuRF-1 Pathway in Rats. Int. J. Mol. Sci. 2019, 20, 4955. [Google Scholar] [CrossRef]
- Sousa, L.G.V.; Castro, J.; Cavaleiro, C.; Salgueiro, L.; Tomás, M.; Palmeira-Oliveira, R.; Martinez-Oliveira, J.; Cerca, N. Synergistic Effects of Carvacrol, α-Terpinene, γ-Terpinene, ρ-Cymene and Linalool against Gardnerella Species. Sci. Rep. 2022, 12, 4417. [Google Scholar] [CrossRef]
- Cai, Z.M.; Peng, J.Q.; Chen, Y.; Tao, L.; Zhang, Y.Y.; Fu, L.Y.; Long, Q.D.; Shen, X.C. 1,8-Cineole: A Review of Source, Biological Activities, and Application. J. Asian Nat. Prod. Res. 2021, 23, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.S.; Kovaleva, K.S.; Yarovaya, O.I.; Bormotov, N.I.; Shishkina, L.N.; Serova, O.A.; Sergeev, A.A.; Agafonov, A.P.; Maksuytov, R.A.; Salakhutdinov, N.F. (+)-Camphor and (−)-Borneol Derivatives as Potential Anti-Orthopoxvirus Agents. Arch. Pharm. 2021, 354, e2100038. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Júnior, F.; Medeiros, D.; Nóbrega, J.; Silva, D.; Martins, E.; Barbosa-Filho, J.; et al. Terpinen-4-ol as an Antibacterial and Antibiofilm Agent against Staphylococcus aureus. Int. J. Mol. Sci. 2020, 21, 4531. [Google Scholar] [CrossRef]
- Jin, J.S.; Chou, J.M.; Tsai, W.C.; Chen, Y.C.; Chen, Y.; Ong, J.R.; Tsai, Y.L. Effectively α-Terpineol Suppresses Glioblastoma Aggressive Behavior and Downregulates KDELC2 Expression. Phytomedicine 2024, 127, 155471. [Google Scholar] [CrossRef]
- Dragomanova, S.; Andonova, V.; Volcho, K.; Salakhutdinov, N.; Kalfin, R.; Tancheva, L. Therapeutic Potential of Myrtenal and Its Derivatives—A Review. Life 2023, 13, 2086. [Google Scholar] [CrossRef]
- Mrabti, H.N.; Jaouadi, I.; Zeouk, I.; Ghchime, R.; El Menyiy, N.; Omari, N.E.; Balahbib, A.; Al-Mijalli, S.H.; Abdallah, E.M.; El-Shazly, M.; et al. Biological and Pharmacological Properties of Myrtenol: A Review. Curr. Pharm. Des. 2023, 29, 407–414. [Google Scholar] [CrossRef]
- Duraisamy, K.; Leelavinothan, P.; Ellappan, P.; Balaji, T.D.S.; Rajagopal, P.; Jayaraman, S.; Babu, S. Cuminaldehyde Ameliorates Hyperglycemia in Diabetic Mice. Front. Biosci. 2022, 14, 24. [Google Scholar] [CrossRef]
- Pina, L.T.S.; Serafini, M.R.; Oliveira, M.A.; Sampaio, L.A.; Guimarães, J.O.; Guimarães, A.G. Carvone and Its Pharmacological Activities: A Systematic Review. Phytochemistry 2022, 196, 113080. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.J.; Sun, Y.L.; Ruan, X.F. Bornyl Acetate: A Promising Agent in Phytomedicine for Inflammation and Immune Modulation. Phytomedicine 2023, 114, 154781. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.D.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, Thyme, and Other Plant Sources: Health and Potential Uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef] [PubMed]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-Caryophyllene and β-Caryophyllene Oxide—Natural Compounds of Anticancer and Analgesic Properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.M.; Zhao, G.D.; Zhao, J.J. δ-Cadinene Inhibits the Growth of Ovarian Cancer Cells via Caspase-Dependent Apoptosis and Cell Cycle Arrest. Int. J. Clin. Exp. Pathol. 2015, 8, 6046–6056. [Google Scholar] [PubMed]
- Akiel, M.A.; Alshehri, O.Y.; Aljihani, S.A.; Almuaysib, A.; Bader, A.; Al-Asmari, A.I.; Alamri, H.S.; Alrfaei, B.M.; Halwani, M.A. Viridiflorol Induces Anti-Neoplastic Effects on Breast, Lung, and Brain Cancer Cells through Apoptosis. Saudi J. Biol. Sci. 2022, 29, 816–821. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, J.; Smolensky, D.; Lee, S.H. Potential Benefits of Patchouli Alcohol in Prevention of Human Diseases: A Mechanistic Review. Int. Immunopharmacol. 2020, 89, 107056. [Google Scholar] [CrossRef]
- Abe, M.; Asada, N.; Kimura, M.; Fukui, C.; Yamada, D.; Wang, Z.; Miyake, M.; Takarada, T.; Ono, M.; Aoe, M.; et al. Antitumor Activity of α-Pinene in T-Cell Tumors. Cancer Sci. 2024, 115, 1317–1332. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, A.D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene—What Are the Potential Health Benefits of This Flavouring and Aroma Agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-Limonene: A Multifunctional Compound with Potent Therapeutic Effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef]
- Peana, A.T.; D’Aquila, P.S.; Panin, F.; Serra, G.; Pippia, P.; Moretti, M.D. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine 2002, 9, 721–726. [Google Scholar] [CrossRef]
- Zunino, M.P.; Zygadlo, J.A. Effect of monoterpenes on lipid peroxidation and liver damage in mice. Planta Med. 2004, 70, 1038–1040. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Mulyaningsih, S.; Sporer, F.; Reichling, J.; Wink, M. Antibacterial activity of essential oils from Eucalyptus and other Myrtaceae against multidrug-resistant Acinetobacter baumannii. Phytomedicine 2011, 18, 173–179. [Google Scholar] [CrossRef]
- Höferl, M.; Krist, S.; Buchbauer, G. Chirality influences the effects of linalool on physiological parameters of stress. Planta Med. 2006, 72, 1188–1192. [Google Scholar] [CrossRef]
- Takaki, I.; Bersani-Amado, L.E.; Vendruscolo, A.; Sartoretto, S.M.; Diniz, S.P.S.; Bersani-Amado, C.A.; Cuman, R.K.N. Anti-inflammatory and antinociceptive effects of eugenol essential oil in experimental animal models. Braz. J. Pharm. Sci. 2008, 44, 109–117. [Google Scholar]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Viljoen, A.M. A review of the application and pharmacological properties of α- and β-chrysanthenyl derivatives. Molecules 2010, 15, 5408–5433. [Google Scholar] [CrossRef]
- Ghelardini, C.; Galeotti, N.; Salvatore, G.; Mazzanti, G. Local anesthetic activity of essential oils. Planta Med. 2001, 67, 564–566. [Google Scholar] [CrossRef]
- Chen, W.; Vermaak, I.; Viljoen, A.M. Camphor—A fumigant during the Black Death and a coveted fragrant wood in ancient Egypt and Babylon—A review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef]
- Ambrož, M.; Boušová, I.; Skarka, A.; Hanušová, V.; Králová, V.; Matoušková, P.; Szotáková, B.; Skálová, L. The influence of sesquiterpenes from Myrica rubra on the antiproliferative and pro-oxidative effects of doxorubicin and its accumulation in cancer cells. Molecules 2015, 20, 15343–15358. [Google Scholar] [CrossRef]
- de Almeida, L.F.R.; Frei, F.; Mancini, E.; De Martino, L.; De Feo, V. Phytotoxic activities of essential oils. Molecules 2010, 15, 4309–4323. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Song, H.S.; Ukeda, H.; Sawamura, M. Radical-scavenging activities of citrus essential oils and their components: Detection using ESR. Biosci. Biotechnol. Biochem. 2000, 64, 205–208. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wang, C.; Zhang, Y.; Tang, H. Anti-inflammatory effects of Angelica pubescens Maxim. extract and its component, bornyl angelate. J. Ethnopharmacol. 2012, 143, 1001–1006. [Google Scholar] [CrossRef]
- Höferl, M.; Buchbauer, G.; Jirovetz, L. Anxiolytic and anticonvulsant effects of oxygenated monoterpenes in linalool-rich essential oils. Phytomedicine 2006, 13, 688–693. [Google Scholar] [CrossRef]
- Park, I.K.; Lee, S.G.; Shin, S.C.; Park, J.D.; Ahn, Y.J. Larvicidal activity of isoborneol and related compounds against Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2002, 39, 880–885. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahmad, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Ocimum sanctum essential oil and its active principles exert their antifungal activity by disrupting ergosterol biosynthesis and membrane integrity. Res. Microbiol. 2010, 161, 816–823. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, J.P.B.; Jorge, R.F.; Leite, M.F.; Furtado, N.A.J.C.; Bastos, J.K.; da Silva Filho, A.A.; Queiroga, C.L.; de Magalhães, P.M.; Soares, A.E.E. Seasonal variation of the (E)-nerolidol and other volatile compounds within ten different cultivated populations of Baccharis dracunculifolia D.C. (Asteraceae). J. Essent. Oil Res. 2009, 21, 308–314. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. An overview of natural antimicrobials role in food. J. Agric. Sci. 2017, 9, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antimicrobial activity and chemical composition of essential oil from Camellia oleifera seed cake. Molecules 2021, 26, 3128. [Google Scholar] [CrossRef]
- Jeong, J.B.; Choi, J.; Lou, Z.; Jiang, X.; Lee, S.H. α-Copaene inhibits LPS-induced nitric oxide and prostaglandin E2 production by suppressing the NF-κB pathway in RAW 264.7 cells. Food Chem. Toxicol. 2013, 62, 655–660. [Google Scholar] [CrossRef]
- Gazizova, A.; Datkhayev, U.; Amirkhanova, A.; Ustenova, G.; Kozhanova, K.; Ikhsanov, Y.; Berdgaleyeva, A. Phytochemical Profiling of Mentha asiatica Boriss. Leaf Extracts: Antioxidant and Antibacterial Activities. ES Food Agrofor. 2025, 19, 1355. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Zhang, C.; Wang, J.; He, X. γ-Curcumene inhibits cancer cell proliferation and induces apoptosis through mitochondrial pathway. Biomed. Pharmacother. 2019, 109, 757–764. [Google Scholar] [CrossRef]
- Wei, A.; Shibamoto, T. Antioxidant/lipoxygenase inhibitory activities and chemical compositions of selected essential oils. J. Agric. Food Chem. 2007, 55, 1737–1742. [Google Scholar] [CrossRef]
- Zunino, M.P.; Turina, A.V.; Zygadlo, J.A.; Perillo, M.A. Stereoselective effects of farnesene isomers on the lipid phase behavior of cellular membranes. Biochim. Biophys. Acta 2010, 1798, 2341–2348. [Google Scholar] [CrossRef]
- Fidan, H.; Stefanova, G.; Stoyanova, A.; Zheljazkov, V.D. Chemical composition and antimicrobial activity of essential oils from different parts of Tanacetum balsamita L. Ind. Crops Prod. 2019, 131, 130–136. [Google Scholar] [CrossRef]
- Braga, P.C.; Culici, M.; Alfieri, M.; Dal Sasso, M. Antioxidant potential of thymol determined by chemiluminescence inhibition in human neutrophils and cell-free systems. Pharmacology 2006, 77, 130–135. [Google Scholar] [CrossRef]
- Chan, W.-K.; Tan, L.T.-H.; Chan, K.-G.; Lee, L.-H.; Goh, B.-H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef]
- D’Agostino, M.; Tesse, N.; Frigerio, G.; Bertoli, A.; Pistelli, L. Antifungal and antioxidant activities of essential oils from leaves and inflorescences of Hedychium species. Planta Med. 2014, 80, 1225–1231. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Caputo, L.; De Martino, L.; Grulova, D.; Sedlák, V.; Camele, I. Efficacy of essential oils from Mediterranean plants against Xanthomonas campestris and Clavibacter michiganensis. Molecules 2019, 24, 900. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Zhang, Y.; Yu, H.; Zhang, Y. α-Cadinol as a potent antifungal and anti-inflammatory agent from Cryptomeria fortunei. Ind. Crops Prod. 2015, 63, 155–159. [Google Scholar]
- Togashi, N.; Shiraishi, A.; Nishizaka, M.; Matsuoka, K.; Endo, K.; Hamashima, H.; Inoue, Y. Antibacterial Activity of Long-Chain Fatty Alcohols against Staphylococcus aureus. Molecules 2007, 12, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, E.-Y.; Hillman, P.F.; Ko, J.; Yang, I.; Nam, S.-J. Chemical Structure and Biological Activities of Secondary Metabolites from Salicornia europaea L. Molecules 2021, 26, 2252. [Google Scholar] [CrossRef]
- Chen, D.F.; Li, X.; Xu, Z.; Liu, X.; Du, S.H.; Li, H.; Zhou, J.H.; Zeng, H.P.; Hua, Z.C. Hexadecanoic acid from Buzhong Yiqi decoction induced proliferation of bone marrow mesenchymal stem cells. J. Med. Food 2010, 13, 967–970. [Google Scholar] [CrossRef]
- Liu, S.Q.; Scott, I.M.; Pelletier, Y.; Kramp, K.; Durst, T.; Sims, S.R.; Arnason, J.T. Dillapiol: A pyrethrum synergist for control of the Colorado potato beetle. J. Econ. Entomol. 2014, 107, 797–805. [Google Scholar] [CrossRef]
- Cho, K.M.; Kim, Y.S.; Lee, M.; Lee, H.Y.; Bae, Y.S. Isovaleric acid ameliorates ovariectomy-induced osteoporosis by inhibiting osteoclast differentiation. J. Cell. Mol. Med. 2021, 25, 4287–4297. [Google Scholar] [CrossRef]
- Soliman, G.A.; Abood, S.H.; Hassen, H.F.; El-Kased, R.F. The Potential Anticonvulsant Activity of the Ethanolic Extracts of Achillea nobilis and Momordica charantia in Rats. J. Pharm. Pharmacogn. Res. 2016, 4, 107–114. [Google Scholar] [CrossRef]
- Karabay-Yavaşoğlu, N.Ü.; Karamenderes, C.; Baykan, S.; Apaydın, S. Antinociceptive and Anti-Inflammatory Activities and Acute Toxicity of Achillea nobilis subsp. neilreichii Extract in Mice and Rats. Pharm. Biol. 2007, 45, 162–168. [Google Scholar]
- Karamenderes, C.; Apaydın, S. Antispasmodic Effect of Achillea nobilis L. subsp. sipylea (O. Schwarz) Bässler on the Rat Isolated Duodenum. J. Ethnopharmacol. 2003, 84, 175–179. [Google Scholar]
- Konyalıoğlu, S.; Karamenderes, C. The Protective Effects of Achillea L. Species Native in Turkey against H2O2-Induced Oxidative Damage in Human Erythrocytes and Leucocytes. J. Ethnopharmacol. 2005, 102, 221–227. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).