A Synergistic Approach Using Photoacoustic Spectroscopy and AI-Based Image Analysis for Post-Harvest Quality Assessment of Conference Pears
Abstract
1. Introduction
2. Results
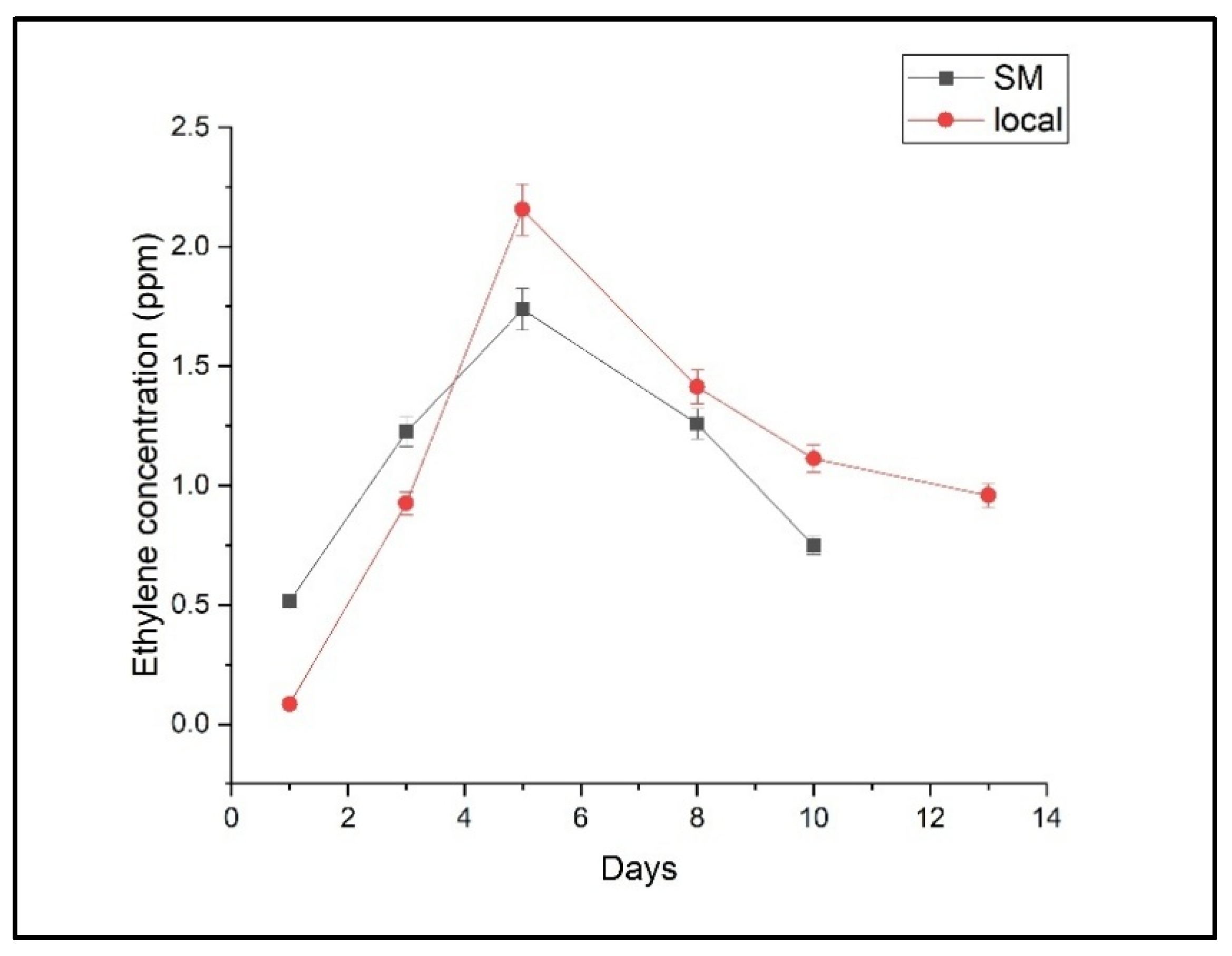
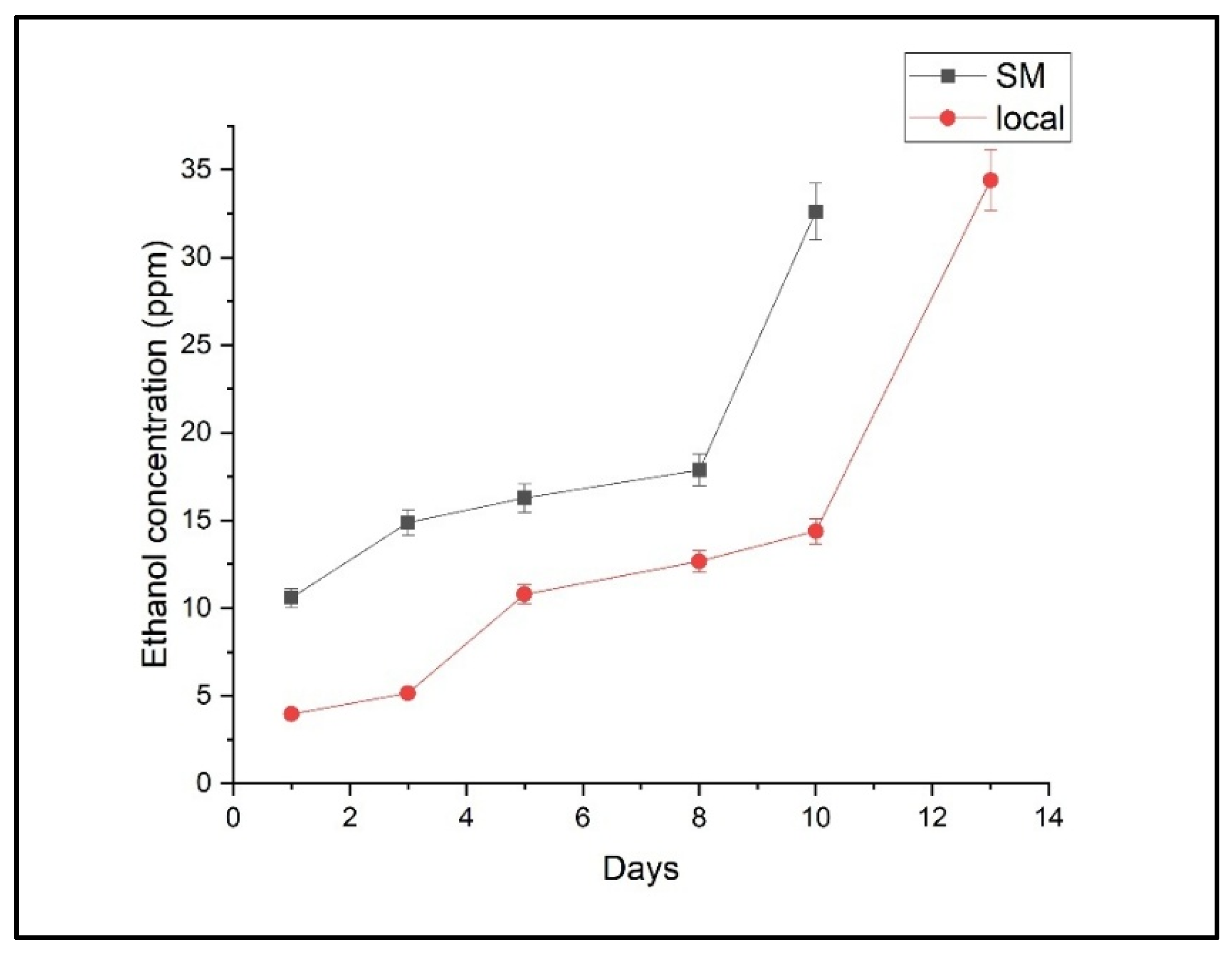
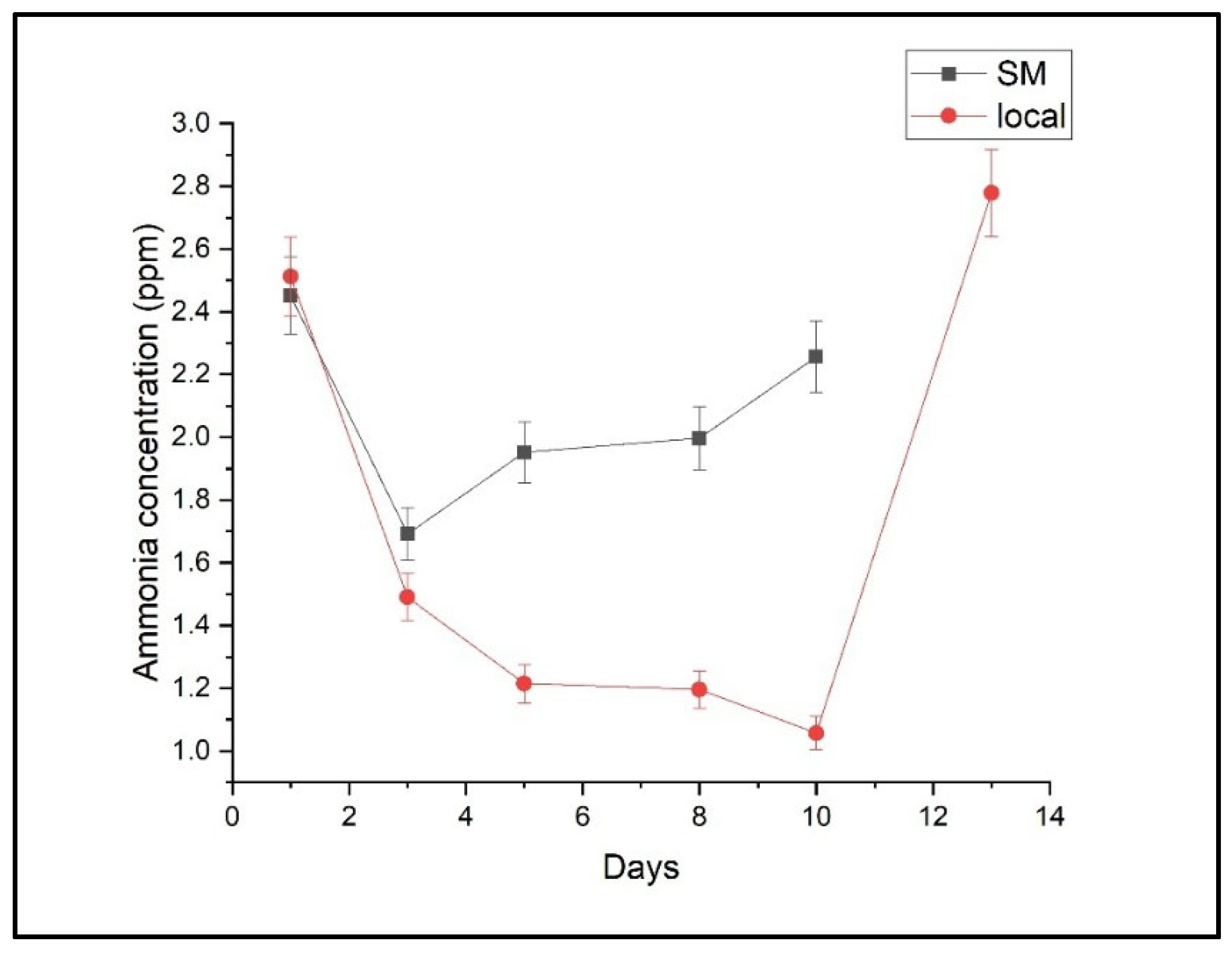
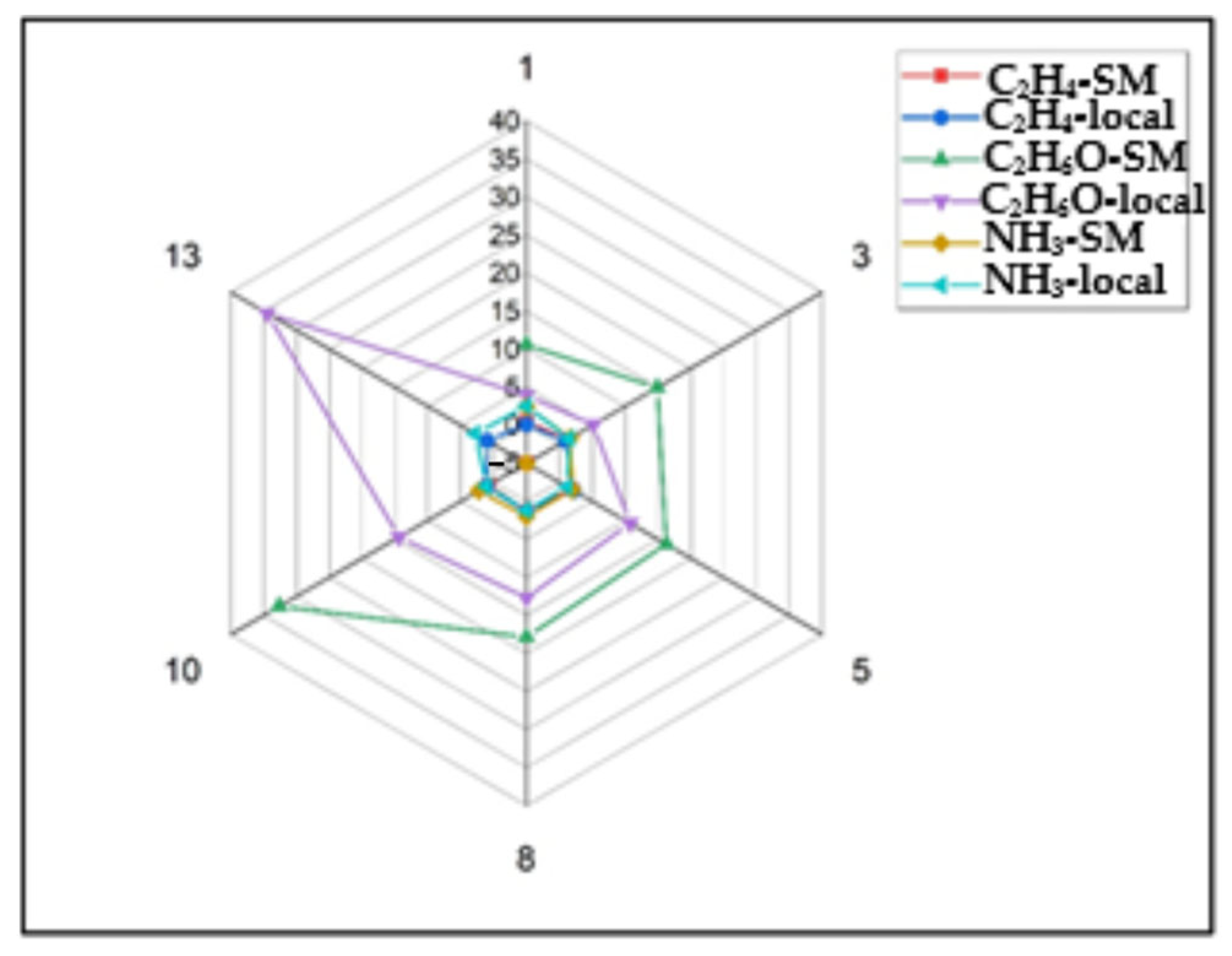
2.1. Ethylene, Ethanol, and Ammonia Emissions
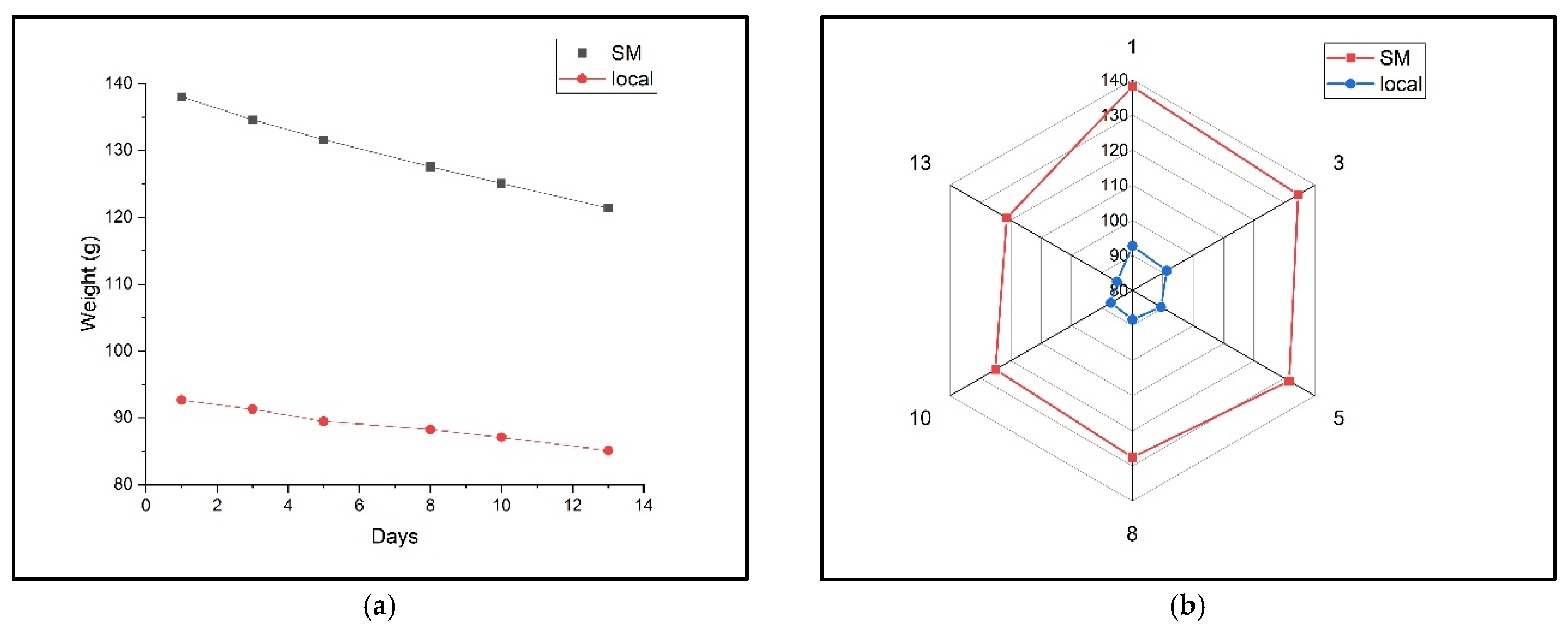
2.2. Comparative Analysis of Storage Effects
2.3. Statistical Analysis
- -
- is the partial eta squared, indicating the proportion of variance in the dependent variable explained by a given factor, after controlling for other sources of variance in the model;
- -
- is the degrees of freedom associated with the factor being analyzed (e.g., Batch, Day, or their interaction);
- -
- is the degrees of freedom associated with the error or residual term in the model;
- -
- F is the F-value from the ANOVA table.
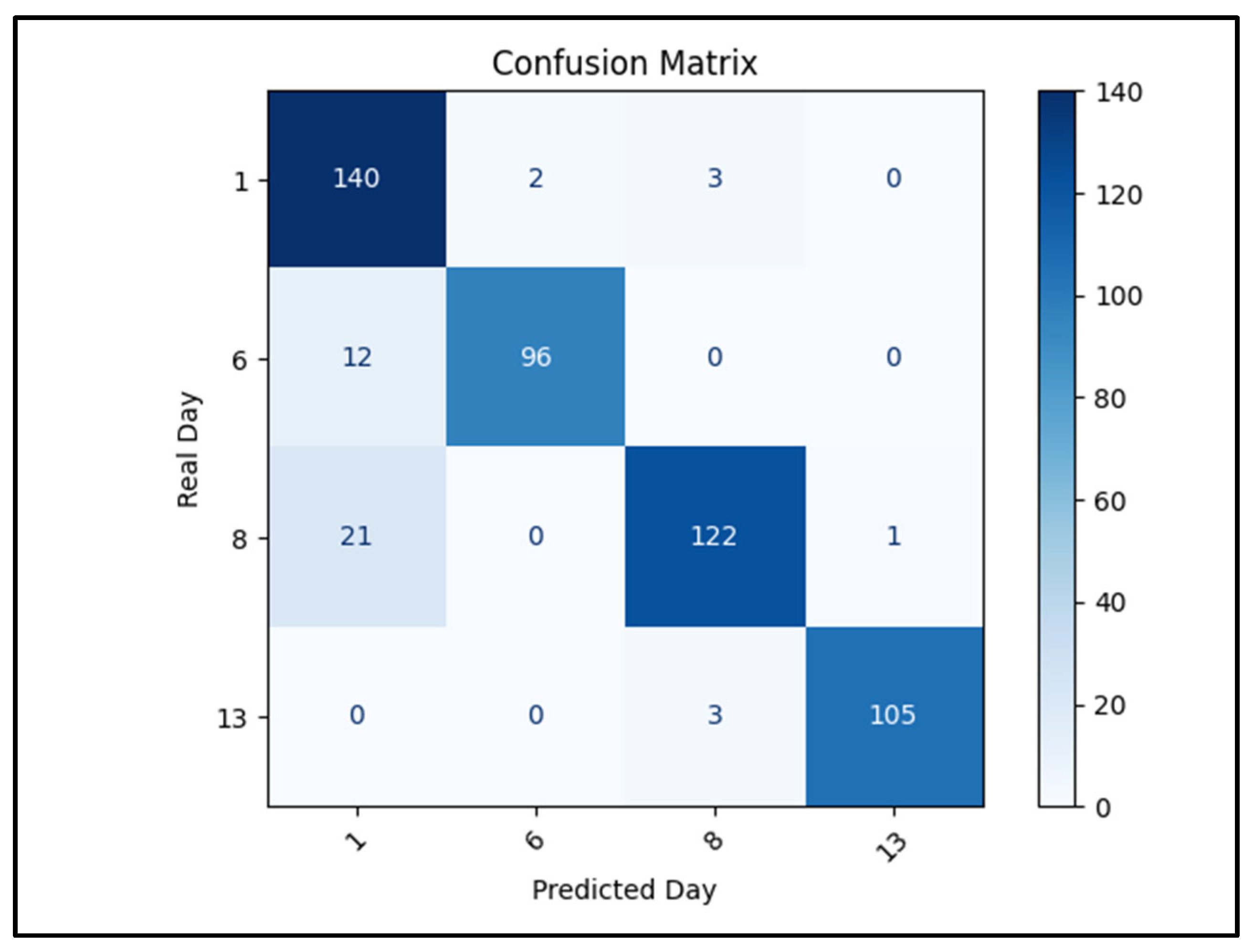
2.4. AI-Based Ripening Classification
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
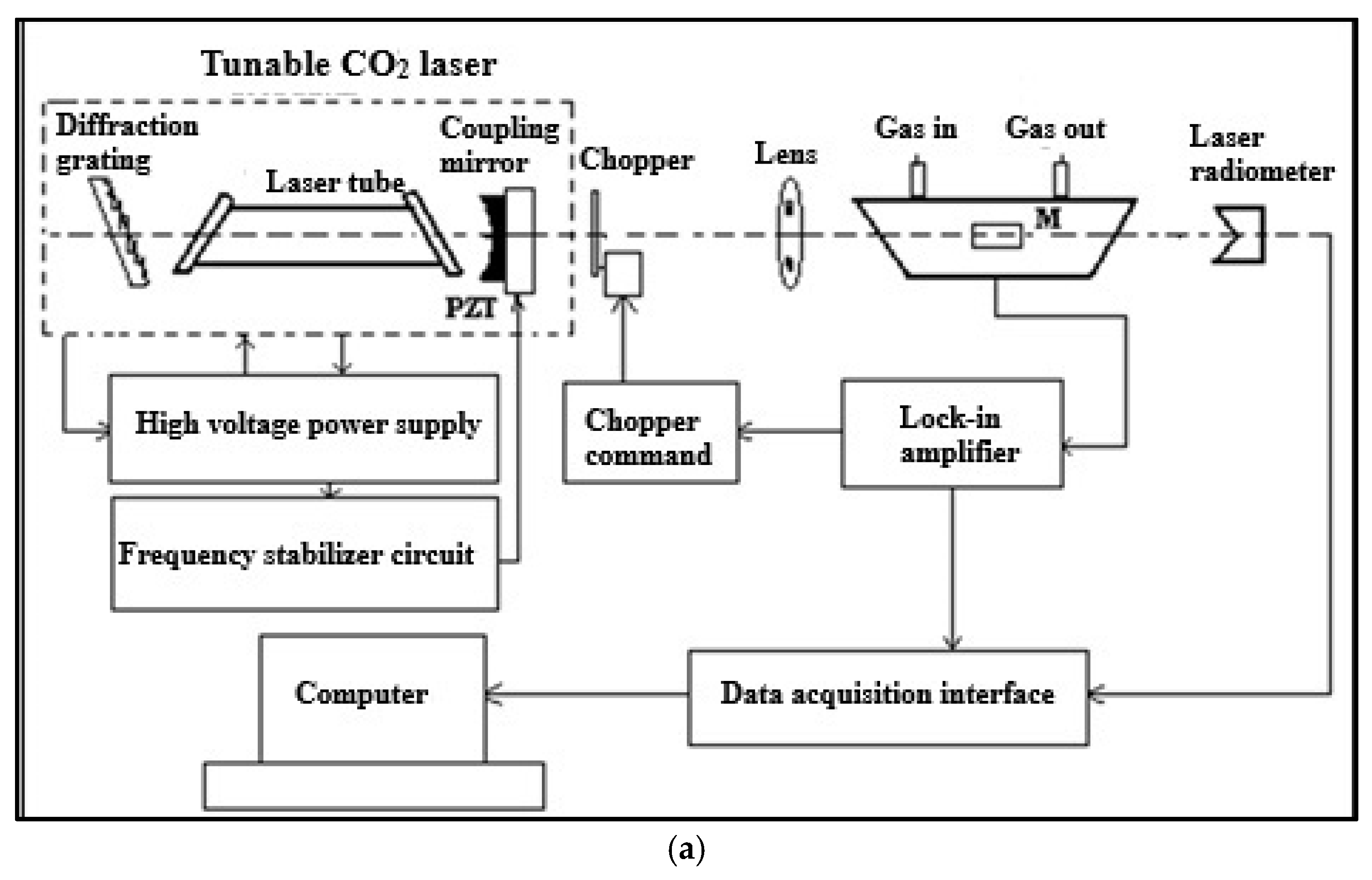
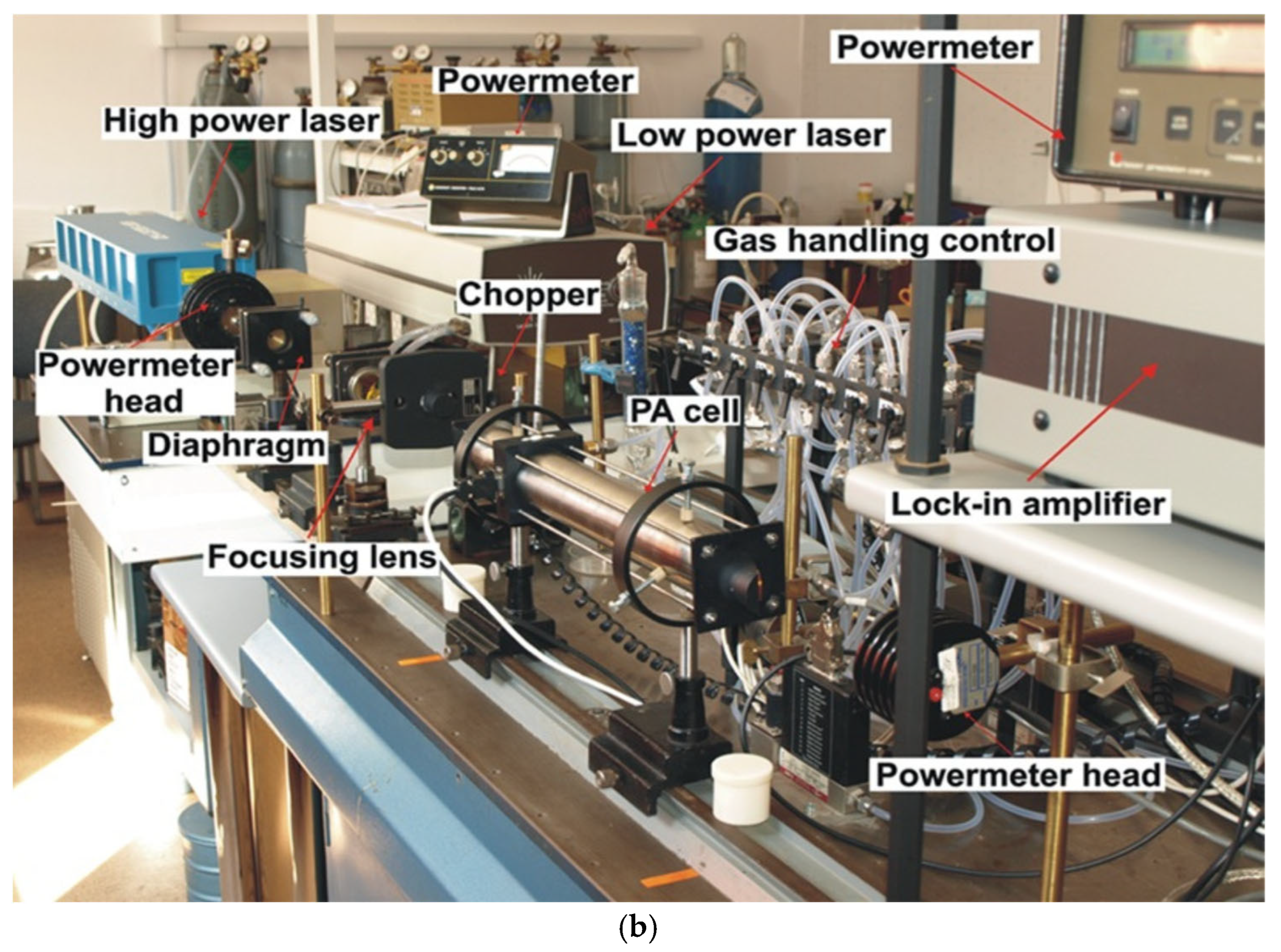
4.2. CO2LPAS Measurement System
4.3. Data Analysis
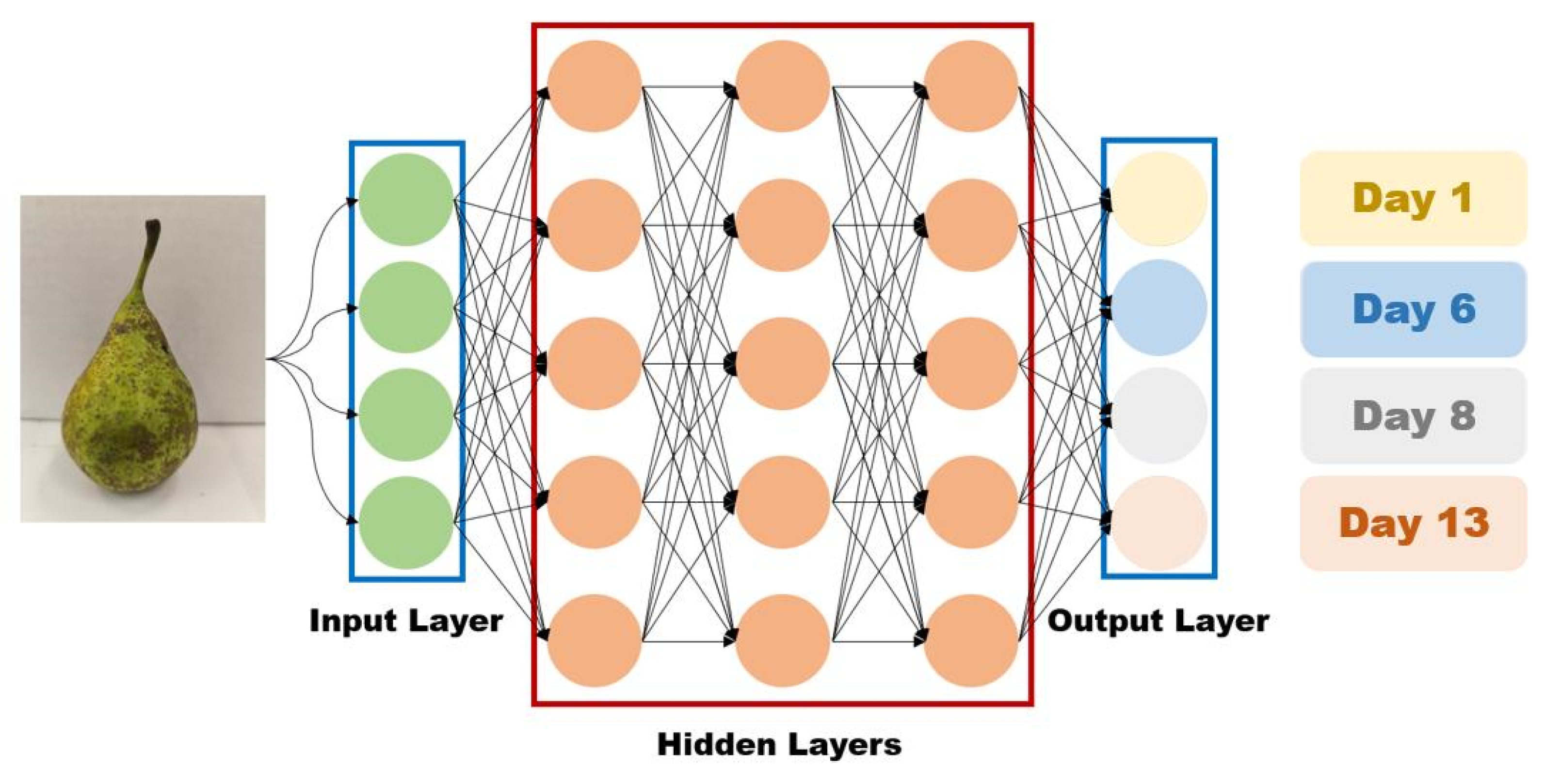
4.4. AI-Based Image Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gidado, M.J.; Gunny, A.A.N.; Gopinath, S.C.B.; Ali, A.; Wongs-Aree, C.; Salleh, N.H.M. Challenges of postharvest water loss in fruits: Mechanisms, influencing factors, and effective control strategies—A comprehensive review. J. Agric. Food Res. 2024, 17, 101249. [Google Scholar] [CrossRef]
- Distefano, M.; Mauro, R.P.; Page, D.; Giuffrida, F.; Bertin, N.; Leonardi, C. Aroma Volatiles in Tomato Fruits: The Role of Genetic, Preharvest and Postharvest Factors. Agronomy 2022, 12, 376. [Google Scholar] [CrossRef]
- Wijesinghe, D.R.; Zobair, M.A.; Esmaeelpour, M. A Review on Photoacoustic Spectroscopy Techniques for Gas Sensing. Sensors 2024, 24, 6577. [Google Scholar] [CrossRef] [PubMed]
- Boamfa, E.I.; Steeghs, M.M.L.; Cristescu, S.M.; Harren, F.J.M. Trace gas detection from fermentation processes in apples; an intercomparison study between proton-transfer-reaction mass spectrometry and laser photoacoustics. Int. J. Mass Spectrom. 2004, 239, 193–201. [Google Scholar] [CrossRef]
- Valenzuela, J.L. Advances in Postharvest Preservation and Quality of Fruits and Vegetables. Foods 2023, 12, 1830. [Google Scholar] [CrossRef]
- Paul, V.; Pandey, R. Role of internal atmosphere on fruit ripening and storability-a review. J. Food Sci. Technol. 2014, 51, 1223–1250. [Google Scholar] [CrossRef]
- Wang, Q.; He, H.; Liu, C.; Wang, C.; Chen, B.; Wang, X.; Niu, Q.; Wang, K.; Zhu, W.; Qiao, Y.; et al. Insights of Freshness Phenotype Detection for Postharvest Fruit and Vegetables. Plant Phenomics 2025, 7, 100042. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Luo, D.; Ba, L. Advances in the Understanding of Postharvest Physiological Changes and the Storage and Preservation of Pitaya. Foods 2024, 13, 1307. [Google Scholar] [CrossRef]
- Frontini, A.; Negro, C.; Accogli, R.; Minonne, F.; Luvisi, A.; De Bellis, L. Valorization of a Local Italian Pear (Pyrus communis L. cv. ‘Petrucina’). Foods 2024, 13, 1528. [Google Scholar] [CrossRef]
- Híc, P.; Kožíšková, J.; Omastová, P.; Balík, J.; Goliáš, J.; Horák, M. Physiochemical Changes of European Pear cv. Conference and Asian Pear cv. Yali during Cold Storage. Horticulturae 2023, 9, 378. [Google Scholar] [CrossRef]
- Brandes, N.; Zude-Sasse, M. Respiratory patterns of European pear (Pyrus communis L. ‘Conference’) throughout pre- and post-harvest fruit development. Heliyon 2019, 5, e01160. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, T.; Salauddin, M.; Kirtonia, K.; Pati, S.; Rebezov, M.; Khayrullin, M.; Panasenko, S.; Tretyak, L.; Temerbayeva, M.; Kapustina, N.; et al. A Review on the Commonly Used Methods for Analysis of Physical Properties of Food Materials. Appl. Sci. 2022, 12, 2004. [Google Scholar] [CrossRef]
- Martínez, A.; Hernández, A.; Moraga, C.; Tejero, P.; Córdoba, M.G.; Martín, A. Detection of volatile organic compounds associated with mechanical damage in apple cv. ‘Golden Delicious’ by headspace solid-phase microextraction (HS-SPME) and GC-MS analysis. LWT 2022, 172, 11421330. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, W.; Zhu, N.; Mao, S.; Tu, K. Early detection and classification of pathogenic fungal disease in post-harvest strawberry fruit by electronic nose and gas chromatography-mass spectrometry. Food Res. Int. 2014, 62, 162–168. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, X.; Yan, Z.; Cheng, Y.; Li, H.; Xu, G. Monitoring Volatile Organic Compounds in Different Pear Cultivars during Storage Using HS-SPME with GC-MS. Foods 2022, 11, 3778. [Google Scholar] [CrossRef] [PubMed]
- Mahanti, N.K.; Shivashankar, S.; Chhetri, K.B.; Kumar, A.; Rao, B.B.; Aravind, J.; Swami, D.V. Enhancing food authentication through E-nose and E-tongue technologies: Current trends and future directions. Trends Food Sci. Technol. 2024, 150, 104574. [Google Scholar] [CrossRef]
- Rabehi, A.; Helal, H.; Zappa, D.; Comini, E. Advancements and Prospects of Electronic Nose in Various Applications: A Comprehensive Review. Appl. Sci. 2024, 14, 4506. [Google Scholar] [CrossRef]
- Kombo, O.K.; Ihsan, N.; Syahputra, T.S.; Hidayat, S.N.; Puspita, M.; Wahyono; Roto, R.; Triyana, K. Enhancing classification rate of electronic nose system and piecewise feature extraction method to classify black tea with superior quality. Sci. Afr. 2024, 24, e02153. [Google Scholar] [CrossRef]
- Mitrayana; Apriyanto, D.K.; Satriawan, M. CO2 Laser Photoacoustic Spectrometer for Measuring Acetone in the Breath of Lung Cancer Patients. Biosensors 2020, 10, 55. [Google Scholar] [CrossRef]
- Harren, F.J.M.; Berkelmas, R.; Kuiper, K.; Hekkert, S.T.L.; Scheepers, P.; Dekhuijzen, R.; Hollander, P.; Parker, D.H. On-line laser photoacoustic detection of ethene in exhaled air as biomarker of ultraviolet radiation damage of the human skin. Appl. Phys. Lett. 1999, 74, 1761–1763. [Google Scholar] [CrossRef]
- Henderson, B.; Khodabakhsh, A.; Metsälä, M.; Ventrillard, I.; Schmidt, F.M.; Romanini, D.; Ritchie, G.A.D.; Hekkert, S.T.L.; Briot, R.; Risby, T.; et al. Laser spectroscopy for breath analysis: Towards clinical implementation. Appl. Phys. B 2018, 124, 161. [Google Scholar] [CrossRef]
- Teodoro, C.G.; Schramm, D.U.; Sthel, M.S.; Lima, G.R.; Rocha, M.V.; Tavares, J.R.; Vargas, H. CO2 laser photoacoustic detection of ethylene emitted by diesel engines used in urban public transports. Infrared Phys. Technol. 2010, 53, 151–155. [Google Scholar] [CrossRef]
- Bercu, V.; Duliu, O.G.; Manea, B.; Opris, V.; Emandi, A.; Persa, D.; Gebac, L.; Tuta, C.; Parnic, V.; Mirea, P.; et al. Electron paramagnetic resonance and discriminant analysis of white pigment used by Early Neolithic potters in the Eastern Romanian Plain. J. Archaeol. Sci. Rep. 2024, 60, 104810. [Google Scholar] [CrossRef]
- Gao, Y.; Jiao, L.; Jiao, F.; Dong, D. Non-intrusive prediction of fruit spoilage and storage time via detecting volatiles in sealed packaging using laser spectroscopy. LWT 2022, 155, 112930. [Google Scholar] [CrossRef]
- Kamilaris, A.; Prenafeta-Boldú, F.X. Deep learning in agriculture: A survey. Comput. Electron. Agric. 2018, 147, 70–90. [Google Scholar] [CrossRef]
- Shorten, C.; Khoshgoftaar, T.M. A survey on image data augmentation for deep learning. J. Big Data 2019, 6, 60. [Google Scholar] [CrossRef]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. ImageNet classification with deep convolutional neural networks. Adv. Neural Inf. Process. Syst. 2012, 25. [Google Scholar] [CrossRef]
- Liu, M.; Pirrello, J.; Chervin, C.; Roustan, J.P.; Bouzayen, M. Ethylene Control of Fruit Ripening: Revisiting the Complex Network of Transcriptional Regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef]
- Saeed, M.; Zhao, L.; Rashwan, A.K.; Osman, A.I.; Chen, Z.; Wang, G.; Zhou, C.; Tu, T.; Alabd, A.; Jiao, Y.; et al. Ethylene-Induced Postharvest Changes in Five Chinese Bayberry Cultivars Affecting the Fruit Ripening and Shelf Life. Horticulturae 2024, 10, 1144. [Google Scholar] [CrossRef]
- Khan, S.; Alvi, A.F.; Khan, N.A. Role of Ethylene in the Regulation of Plant Developmental Processes. Stresses 2024, 4, 28–53. [Google Scholar] [CrossRef]
- Wang, Y.W.; Nambeesan, S.U. Ethylene promotes fruit ripening initiation by downregulating photosynthesis, enhancing abscisic acid and suppressing jasmonic acid in blueberry (Vaccinium ashei). BMC Plant Biol. 2024, 24, 418. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.L.P.; Pham, T.T.; Syium, Z.H.; Zsom-Muha, V.; Baranyai, L.; Zsom, T.; Hitka, G. Delay of 1-MCP Treatment on Post-Harvest Quality of ‘Bosc Kobak’ Pear. Horticulturae 2022, 8, 89. [Google Scholar] [CrossRef]
- Alonso-Salinas, R.; López-Miranda, S.; Pérez-López, A.J.; Acosta-Motos, J.R. Strategies to Delay Ethylene-Mediated Ripening in Climacteric Fruits: Implications for Shelf Life Extension and Postharvest Quality. Horticulturae 2024, 10, 840. [Google Scholar] [CrossRef]
- Ziv, C.; Fallik, E. Postharvest Storage Techniques and Quality Evaluation of Fruits and Vegetables for Reducing Food Loss. Agronomy 2021, 11, 1133. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Y.; Einhorn, T.C. Postharvest physiology, storage quality and physiological disorders of ‘Gem’ pear (Pyrus communis L.) treated with 1-methylcyclopropene. Sci. Hortic. 2018, 240, 631–637. [Google Scholar] [CrossRef]
- Bai, J.; Yin, X.; Whitaker, B.D.; Deschuytter, K.; Chen, P.M. Combination of 1-methylcyclopene and ethoxyquin to control superficial scald of ‘Anjou’ pears. HortTechnology 2009, 19, 521–525. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y. The combination of ethoxyquin, 1-methylcyclopropene and ethylene treatments controls superficial scald of ‘d’Anjou’ pears with recovery of ripening capacity after long-term controlled atmosphere storage. Postharvest Biol. Technol. 2017, 127, 53–59. [Google Scholar] [CrossRef]
- Mahajan, P.V.; Caleb, O.J.; Singh, Z.; Watkins, C.B.; Geyer, M. Postharvest treatments of fresh produce. Philos. Trans. A Math. Phys. Eng. Sci. 2014, 372, 20130309. [Google Scholar] [CrossRef]
- Asrey, R.; Sharma, S.; Barman, K.; Prajapati, U.; Negi, N.; Meena, N.K. Biological and postharvest interventions to manage the ethylene in fruit: A review. Sustain. Food Technol. 2023, 1, 8. [Google Scholar] [CrossRef]
- Ito, Y.; Nishizawa-Yokoi, A.; Endo, M.; Mikami, M.; Toki, S. CRISPR/Cas9-mediated mutagenesis of the RIN locus that regulates tomato fruit ripening. Biochem. Biophys. Res. Commun. 2015, 467, 76–82. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, K.; Liu, S. Evaluation of 1-methylcyclopropene (1-MCP) and low temperature conditioning (LTC) to control browning of Huangguan pears. Sci. Hortic. 2020, 259, 108738. [Google Scholar] [CrossRef]
- Schaller, G.E.; Binder, B.M. Inhibitors of ethylene biosynthesis and signaling. In Methods in Molecular Biology, 2nd ed.; Humana Press Inc.: New York, NY, USA, 2017; pp. 223–235. [Google Scholar]
- Onik, J.C.; Wai, S.C.; Li, A.; Lin, Q.; Sun, Q.; Wang, Z.; Duan, Y. Melatonin treatment reduces ethylene production and maintains fruit quality in apple during postharvest storage. Food Chem. 2021, 337, 127753. [Google Scholar] [CrossRef] [PubMed]
- Strano, M.C.; Altieri, G.; Allegra, M.; Di Renzo, G.C.; Paterna, G.; Matera, A.; Genovese, F. Postharvest Technologies of Fresh Citrus Fruit: Advances and Recent Developments for the Loss Reduction during Handling and Storage. Horticulturae 2022, 8, 612. [Google Scholar] [CrossRef]
- Ali, M.; Batool, S.; Khalid, N.; Manzoor, M.F.; Zhao, X.; Li, X.; Li, F.; Xinhua, Z.; Aadil, R.M. Alcoholic Off-Flavor Disorders in Fresh Fruits. J. Food Biochem. 2023, 2023, 3959653. [Google Scholar] [CrossRef]
- Pesis, E. The role of the anaerobic metabolites, acetaldehyde and ethanol, in fruit ripening, enhancement of fruit quality and fruit deterioration. Postharvest Biol. Technol. 2005, 37, 1–19. [Google Scholar] [CrossRef]
- Belay, Z.A.; Caleb, O.J.; Mahajan, P.V.; Opara, U.L. Response of pomegranate arils (cv. Wonderful) to low oxygen stress under active modified atmosphere condition. J. Sci. Food Agric. 2019, 99, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Amodio, M.L.; Colelli, G.; Cantwell, M. Ammonia accumulation in plant tissues: A potentially useful indicator of postharvest physiological stress. Acta Hortic. 2018, 1194, 1511–1518. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Farhan, M.; Sathish, M.; Kiran, R.; Mushtaq, A.; Baazeem, A.; Hasnain, A.; Hakim, F.; Naqvi, S.A.H.; Mubeen, M.; Iftikhar, Y.; et al. Plant Nitrogen Metabolism: Balancing Resilience to Nutritional Stress and Abiotic Challenges. Phyton-Int. J. Exp. Bot. 2024, 93, 581–609. [Google Scholar] [CrossRef]
- Adeniyi, A.; Bello, I.; Mukaila, T.; Sarker, N.C.; Hammed, A. Trends in Biological Ammonia Production. BioTech 2023, 12, 41. [Google Scholar] [CrossRef]
- Wu, X.; Cai, W.; Zhu, P.; Peng, Z.; Zheng, T.; Li, D.; Li, J.; Zhou, G.; Du, G.; Zhang, J. Profiling the role of microorganisms in quality improvement of the aged flue-cured tobacco. BMC Microbiol. 2022, 22, 197. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, S.M.; Schouten, R.E.; Harren, F.J.M. On-line monitoring of fruit ripening by laser photoacoustic spectroscopy. Appl. Phys. B 2008, 91, 565–570. [Google Scholar]
- Patel, N.G.; Dhale, D.A.; Rathod, M.C. Analysis of Pesticide Residue in Fruit Using the QuEChERS Method Coupled With LC-MS/MS Detection. Curr. Agri. Res. J. 2024, 12. [Google Scholar] [CrossRef]
- Frenkel, C.; Klein, I.; Dilley, D.R. Protein synthesis in relation to ripening of pome fruits. Plant Physiol. 1968, 43, 1146–1153. [Google Scholar] [CrossRef]
- Lee, B.-R.; Baek, Y.-J.; Wi, S.G.; Yang, U.; Lee, S.-H. Comparative Analysis of Protein Patterns and Fruit Size in Three Asian Pears with Different Fruit Maturity Periods. Hortic. Sci. Technol. 2021, 39, 1–9. [Google Scholar] [CrossRef]
- Hulme, A.C. Studies in the Nitrogen Metabolism of Apple Fruits: The Climacteric Rise in Respiration in Relation to Changes in the Equilibrium Between Protein Synthesis and Breakdown. J. Exp. Bot. 1954, 5, 159–172. [Google Scholar] [CrossRef]
- Martin, D.; Lewis, T.L.; Cern, Y. Nitrogen Metabolism During Storage in Relation to the Breakdown of Apples. I. Changes in Protein Nitrogen Level in Relation to Incidence. Aust. J. Agric. Res. 1967, 18, 271–288. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, L.; Zhang, L.; Kang, R.; Yu, Z.; Liu, Y.; Wu, J.; Zhang, S. Dynamic Changes in Proteins during Apple (Malus x domestica) Fruit Ripening and Storage. Hortic. Res. 2014, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhao, Y.; Yang, J.; Jiang, Y.; Lu, F.; Jia, Y.; Yang, B. Metabolomic analyses of banana during postharvest senescence by 1H-high resolution-NMR. Food Chem. 2017, 218, 406–412. [Google Scholar] [CrossRef]
- Alsmairat, N.; Engelgau, P.; Beaudry, R. Changes in Free Amino Acid Content in the Flesh and Peel of ‘Cavendish’ Banana Fruit as Related to Branched-Chain Ester Production, Ripening, and Senescence. J. Am. Soc. Hortic. Sci. 2018, 143, 370–380. [Google Scholar] [CrossRef]
- Yun, Z.; Li, T.; Gao, H.; Zhu, H.; Gupta, V.K.; Jiang, Y.; Duan, X. Integrated Transcriptomic, Proteomic, and Metabolomics Analysis Reveals Peel Ripening of Harvested Banana under Natural Condition. Biomolecules 2019, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Liu, P.; He, W.; Bi, F.; Hu, C.; Deng, G.; Dou, T.; Yang, Q.; Li, C.; Yi, G.; et al. Ripening-Stage Variations in Small Metabolites across Six Banana Cultivars: A Metabolomic Perspective. Foods 2025, 478, 143658. [Google Scholar] [CrossRef]
- Paul, V.; Pandey, R.; Srivastava, G.C. The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene-An overview. J. Food Sci. Technol. 2012, 49, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wills, R.B.H.; McGlasson, W.B.; Graham, D.; Joyce, D.C. Physiology and biochemistry. In Postharvest: An Introduction to Physiologyand Handling of Fruits, Vegetables and Ornamentals, 5th ed.; UNSW Press: Sydney, Australia; CAB International: Oxfordshire, UK, 2007; p. 29. [Google Scholar]
- Tipu, M.M.H.; Sherif, S.M. Ethylene and its crosstalk with hormonal pathways in fruit ripening: Mechanisms, modulation, and commercial exploitation. Front. Plant Sci. 2024, 15, 1475496. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wu, H.; Dong, L.; Li, B.; Ma, W.; Zhang, L.; Yin, W.; Xiao, L.; Jia, S.; Tittel, F.K. ppb-Level SO2 Photoacoustic Sensors with a Suppressed Absorption-Desorption Effect by Using a 7.41 μm External-Cavity Quantum Cascade Laser. ACS Sens. 2020, 5, 549–556. [Google Scholar] [CrossRef]
- Chen, B.; Li, H.; Zhao, X.; Gao, M.; Cheng, K.; Shao, X.; Wu, H.; Dong, L.; Yin, X. Trace Photoacoustic SO2 Gas Sensor in SF6 Utilizing a 266 nm UV Laser and an Acousto-Optic Power Stabilizer. Opt. Express 2023, 31, 6974–6981. [Google Scholar] [CrossRef]
- Yin, X.; Dong, L.; Wu, H.; Gao, M.; Zhang, L.; Zhang, X.; Liu, L.; Shao, X.; Tittel, F.K. Compact QEPAS Humidity Sensor in SF6 Buffer Gas for High-Voltage Gas Power Systems. Photoacoustics 2021, 25, 100319. [Google Scholar] [CrossRef]
- Dumitras, D.C.; Dutu, D.C.; Matei, C.; Magureanu, A.M.; Petrus, M.; Popa, C. Laser photoacoustic spectroscopy: Principles, instrumentation, and characterization. J. Optoelectron. Adv. Mater. 2007, 21, 3655–3701. [Google Scholar]
- Dumitras, D.C.; Petrus, M.; Bratu, A.-M.; Popa, C. Applications of Near Infrared Photoacoustic Spectroscopy for Analysis of Human Respiration: A Review. Molecules 2020, 25, 1728. [Google Scholar] [CrossRef]
- Bratu, A.M.; Popa, C.; Bojan, M.; Logofatu, P.C.; Petrus, M. Non-destructive methods for fruit quality evaluation. Sci. Rep. 2021, 11, 7782. [Google Scholar] [CrossRef]
- Dumitras, D.C.; Dutu, D.C.; Matei, C.; Cernat, R.; Banita, S.; Patachia, M.; Bratu, A.M.; Petrus, M.; Popa, C. Evaluation of Ammonia Absorption Coefficients by Photoacoustic Spectroscopy for Detection of Ammonia Levels in Human Breath. Laser Phys. 2011, 21, 745–749. [Google Scholar] [CrossRef]
- Bratu, A.M.; Popa, C.; Petrus, M. Monitoring of Post-Harvest Maturation Processes Inside Stored Fruit Using Photoacoustic Gas Sensing Spectroscopy. Materials 2020, 13, 2694. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.; Bratu, A.M.; Petrus, M.; Bacalum, M. The Analysis of Lead Phytotoxicity in Seeds Using CO2 Laser Photoacoustic Spectroscopy. Molecules 2020, 25, 1637. [Google Scholar] [CrossRef] [PubMed]
- Bratu, A.M.; Popa, C.; Matei, C.; Banita, S.; Dutu, D.C.A.; Dumitras, D.C. Removal of interfering gases in breath biomarker measurements. J. Optoelectron. Adv. Mater. 2011, 13, 1045–1050. [Google Scholar]
- Petrus, M.; Popa, C.; Bratu, A.-M. Determination of Ozone Concentration Levels in Urban Environments Using a Laser Spectroscopy System. Environments 2024, 11, 9. [Google Scholar] [CrossRef]
- Fukushima, K. Neocognitron: A self-organizing neural network model for a mechanism of pattern recognition unaffected by shift in position. Biol. Cybern. 1980, 36, 193–202. [Google Scholar] [CrossRef]
- Nair, V.; Hinton, G.E. Rectified linear units improve restricted boltzmann machines. In Proceedings of the 27th International Conference on Machine Learning (ICML-10) 2010, Haifa, Israel, 21–24 June 2010. [Google Scholar]
- LeCun, Y.; Bottou, L.; Bengio, Y.; Hner, P. Gradient-based learning applied to document recognition. Proc. IEEE 1998, 86, 2278–2324. [Google Scholar] [CrossRef]
- Rosenblatt, F. The perceptron: A probabilistic model for information storage and organization in the brain. Psychol. Rev. 1958, 65, 386. [Google Scholar] [CrossRef]
- Srivastava, N.; Hinton, G.; Krizhevsky, A.; Sutskever, I.; Salakhutdinov, R. Dropout: A simple way to prevent neural networks from overfitting. J. Mach. Learn. Res. 2014, 15, 1929–1958. [Google Scholar]
- Kingma, D.P.; Ba, J. Adam: A method for stochastic optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar]
- Rumelhart, D.E.; Hinton, G.E.; Williams, R.J. Learning representations by back-propagating errors. Nature 1986, 323, 533–536. [Google Scholar] [CrossRef]
- Mihai, D.; Gebac, L. Image Dataset and Code for Visual Classification of Pear Ripening via Convolutional Neural Networks. Zenodo 2025. [Google Scholar] [CrossRef]
| Pair | r-Value | p-Value |
|---|---|---|
| C2H4 vs. C2H6O | 0.82 | <0.001 |
| C2H4 vs. NH3 | 0.65 | 0.003 |
| C2H6O vs. NH3 | 0.71 | <0.001 |
| Factor | F-Value | p-Value | Conclusion (α = 0.05) |
|---|---|---|---|
| Batch | 15.2 | <0.001 | Reject H0 |
| Day | 28.7 | <0.001 | Reject H0 |
| Batch × Day | 3.1 | 0.02 | Interaction |
| Factor | F-Value | p-Value | Conclusion |
|---|---|---|---|
| Batch | 25.41 | <0.001 | Significant |
| Day | 38.76 | <0.001 | Significant |
| Interaction | 7.92 | 0.002 | Significant |
| Factor | F-Value | p-Value | Conclusion | Partial η2 |
|---|---|---|---|---|
| Batch | 1.84 | 0.18 | Not significant | 0.071 |
| Day | 6.29 | 0.001 | Significant | 0.440 |
| Batch × Day | 0.97 | 0.41 | Not significant | 0.108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrus, M.; Popa, C.; Bratu, A.M.; Bercu, V.; Gebac, L.; Mihai, D.-M.; Butcaru, A.-C.; Stanica, F.; Gogot, R. A Synergistic Approach Using Photoacoustic Spectroscopy and AI-Based Image Analysis for Post-Harvest Quality Assessment of Conference Pears. Molecules 2025, 30, 2431. https://doi.org/10.3390/molecules30112431
Petrus M, Popa C, Bratu AM, Bercu V, Gebac L, Mihai D-M, Butcaru A-C, Stanica F, Gogot R. A Synergistic Approach Using Photoacoustic Spectroscopy and AI-Based Image Analysis for Post-Harvest Quality Assessment of Conference Pears. Molecules. 2025; 30(11):2431. https://doi.org/10.3390/molecules30112431
Chicago/Turabian StylePetrus, Mioara, Cristina Popa, Ana Maria Bratu, Vasile Bercu, Leonard Gebac, Delia-Mihaela Mihai, Ana-Cornelia Butcaru, Florin Stanica, and Ruxandra Gogot. 2025. "A Synergistic Approach Using Photoacoustic Spectroscopy and AI-Based Image Analysis for Post-Harvest Quality Assessment of Conference Pears" Molecules 30, no. 11: 2431. https://doi.org/10.3390/molecules30112431
APA StylePetrus, M., Popa, C., Bratu, A. M., Bercu, V., Gebac, L., Mihai, D.-M., Butcaru, A.-C., Stanica, F., & Gogot, R. (2025). A Synergistic Approach Using Photoacoustic Spectroscopy and AI-Based Image Analysis for Post-Harvest Quality Assessment of Conference Pears. Molecules, 30(11), 2431. https://doi.org/10.3390/molecules30112431



.jpg)

.jpg)





