Abstract
Recently, research and development in the field of dye-sensitized solar cells has been actively advanced, as the technology constitutes a potential alternative to silicon-based photovoltaic devices. Modification of the molecular structure of the dye can enhance the adsorption on the TiO2 surface, improve the light absorption capacity, suppress the charge recombination, increase the electron injection rate, and thereby improve the overall performance of the solar cell. Carbazole and phenothiazine are rigid heterocyclic compounds containing nitrogen as a heteroatom with large π-conjugated skeletons. Phenothiazine differs from carbazole by the presence of sulfur as an additional electron-rich heteroatom. The inclusion of this heteroatom in the structure of the compounds can indeed improve the electron-donating properties, affect the conjugation, and thus affect the optical, electronic, and electrochemical properties of the chromophores as a whole. The difference in planarity when comparing carbazole with phenothiazine can be useful from several points of view. The planar structure of carbazole increases the degree of conjugation and the electron transfer capacity, which can increase the photocurrent of the cell. The nonplanar structure of phenothiazine helps to prevent π-stacking aggregation. This review comprehensively summarizes the progress in the field of synthesis of organic dyes for solar cells with an emphasis on the comparative analysis of two electron-donating moieties, carbazole and phenothiazine. In addition, the review describes in detail the relationship between the structure of the compounds (dyes), their properties, and the performance of solar cells.
1. Introduction
In recent years, there has been growing scientific interest in the development of new third-generation photovoltaic technologies such as dye-sensitized solar cells (DSSCs), organic solar cells (OSCs), and perovskite solar cells (PSCs), which are being developed as alternatives to silicon solar cells. Numerous studies in this area are related to the fact that these devices can compete with traditional silicon-based solar cells with their lower cost, ease of fabrication, and ability to be processed on flexible substrates [1]. However, despite the above advantages, all the mentioned solar cell technologies are not without their drawbacks. To date, OSCs have had difficulties due to the proper ordering of charge domains; PSCs suffer from their stability and sensitivity to moisture; and DSSCs require photosensitive materials with a broad absorption spectrum to demonstrate higher efficiency. In general, DSSCs have shown the best performance under practical outdoor conditions [2]. Dye-sensitized solar cells exhibit significant power generation performance throughout the day, even under low-intensity light, regardless of the incident angle of the light [3]. The photophysical characteristics of DSSCs are determined by the molecular structure of the dye. Therefore, they can be relatively easily tuned at the molecular level by varying the substituents and structural moieties. In addition, DSSCs are stable and efficient under harsh conditions such as low temperatures and low-light environments, even in the presence of moisture [4,5,6] Thus, DSSCs are still one of the most promising solar cell technologies, and research aimed at improving the efficiency of such solar cells is relevant today. Recent studies have shown that the best DSSCs based on metal-free organic dyes exhibited an energy conversion efficiency of 15.2% [7]. As is known, the efficiency of solar cells is determined by the molecular structure of the dye, which is a π-conjugated system with terminal electron-donating and electron-accepting fragments [8]. Many strategies have been considered in the literature to design highly efficient organic dyes depending on many factors such as the planarity and rigidity of donors and acceptors, bond type, conjugation length, and side chains [9,10,11,12,13]. Therefore, researchers face a significant challenge in designing electron donor moieties capable of highly efficient electron transfer and inhibition of molecular aggregation. In light of the previous considerations, phenothiazine (PTZ) and carbazole (CZ) are considered as effective options for donors (Figure 1) [14,15,16,17,18].
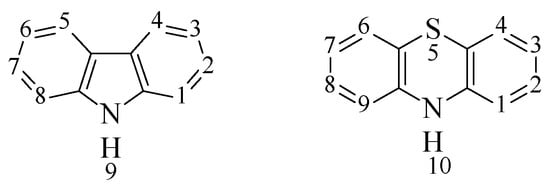
Figure 1.
The structural formulas of carbazole and phenothiazine.
The introduction of carbazole and phenothiazine fragments into the structure of organic dyes is justified by their chemical stability and resistance to environmental influences. Substitution of hydrogen atoms at the nitrogen atom occurs easily, which makes it possible to improve solubility and adjust optical and electrical properties by introducing substituents. Carbazole and phenothiazine can be substituted at the third and sixth or seventh atoms to form 3,6- or 3,7-disubstituted derivatives, respectively, which are used to bind to other aromatic fragments either directly or through various π-conjugated bridges. The advantage of carbazole is that new synthetic methods now allow obtaining compounds in which carbazole fragments are included in the structure of the compound because of the participation of different carbon atoms and the nitrogen atom of the carbazole cycle: 2C-7C, 1C-8C, 9N-2C, 9N-3C, which changes the optical and electrochemical properties of the resulting compound over a wide range [19,20,21]. In addition, the structure of carbazole contains a diphenyl fragment, which makes the molecule flat and rigid. This structure promotes more efficient intramolecular charge transfer, which leads to high mobility of charge carriers. Phenothiazine, because of the presence of two electron-saturated heteroatoms (nitrogen and sulfur), has stronger electron-donor properties than carbazole. In addition, the introduction of this fragment into the structure of the compound prevents the formation of excimers (dimers in the excited state) that arise as a result of the interaction of excited and unexcited molecules and lead to a decrease in the fluorescence quantum yield [22]. This is explained, first of all, by the nonplanar structure of the phenothiazine molecule, which has a butterfly conformation in which the angle between the two benzene rings is 27.36 (9) [23]. The difference in planarity when comparing CZ with PTZ can be useful from different points of view. The planar structure of CZ increases the degree of conjugation and the ability to transfer electrons, which can increase the photocurrent [24]. The nonplanar PTZ block (butterfly-like skeletons) can prevent π-stacking aggregation [25,26]. Meanwhile, the addition of long alkyl chains such as 2-ethylhexyl group to the nitrogen atoms in the structures plays a role in creating steric hindrance and then inhibiting the aggregation of molecules [27].
The introduction of a carbazole or phenothiazine moiety has different effects on the optical, physical, physicochemical, and electrochemical properties of the compounds, which in turn determines the nature and efficiency of photoelectronic devices. Below is a review demonstrating a comparative analysis of compounds containing electron-donating carbazole and phenothiazine moieties in their structure, used as materials for dye-sensitized solar cells.
In order to determine the relationship between the structure of dyes, their properties and the efficiency of the device, a description of the structure of DSSCs and their operating principle is presented below.
The cells consist of two electrodes and an iodine-containing electrolyte. One electrode usually consists of a mesoporous dye-saturated oxide semiconductor (TiO2/ZnO/NiO) deposited on a transparent conductive substrate. The other electrode is a conductive glass plate onto which a reduction catalyst is deposited—metallic platinum or graphite [28]. The advantages of using TiO2 for the production of solar cells, compared with other materials, are the chemical resistance, nontoxicity, and low cost of the oxide. It features significant photoactivity, as well as a pronounced dependence of electrical properties on the surface morphology and type of crystal lattice. The band gap for TiO2, which does not absorb visible light, is 3.2 eV [29]. Figure 2 shows the operating principle of the cell [30].
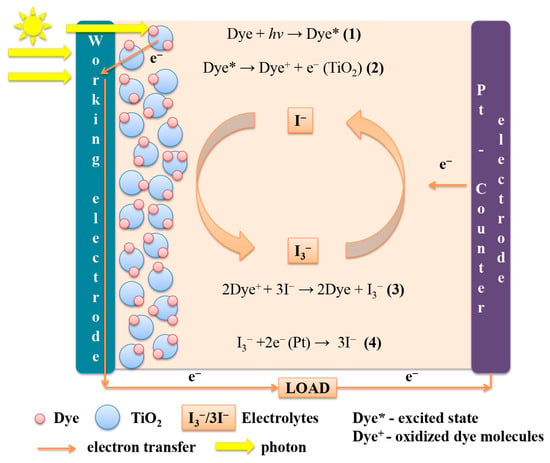
Figure 2.
Operating principle of dye-sensitized solar cells.
Light absorption occurs because of a monolayer of dye chemically adsorbed on the surface of a semiconductor (e.g., TiO2) (1). After excitation by a light photon, the dye gives up an electron to the semiconductor (TiO2) (2), i.e., it passes into the conduction band of TiO2. The transition occurs very quickly and takes 10−15 s. In the semiconductor, the electron diffuses through the TiO2 film and reaches the glass electrode. The dye molecule is oxidized with the loss of an electron. The dye molecule is restored to its original state by obtaining an electron from the oxidation–reduction medium (electrolyte) (3). To complete the cycle, the electrolytic medium is neutralized by photoelectrons that reach the counter electrode through the external circuit (4). According to this principle, a dye-sensitized solar cell converts solar energy into electric current flowing through an external conductor [30,31]. To prevent liquid from leaking out during operation of the device, the cell is made hermetically sealed [31,32].
An analysis of the literature covering research in the field of dye-sensitized solar cells [28,33,34,35] allowed us to formulate a number of basic requirements for organic dyes, the fulfillment of which is necessary to achieve high efficiency of DSSCs.
- It is believed that the sensitizer for DSSCs should have a high molar absorption coefficient (ε), which characterizes how strongly a substance absorbs light at a given wavelength.
- To ensure a high electron transfer rate, efficient conjugation of the donor and acceptor groups is necessary, and it is also important that the LUMO energy of the dye molecule is higher than the LUMO energy of TiO2. This will allow the HOMO electrons of the dye molecule to move to the LUMO orbital of the semiconductor (TiO2) rather than the dye when absorbing light quanta. The LUMO energy level is defined as the difference between the oxidation potential (Eox) and the band gap (Egopt), while it is worth noting that for TiO2 this value is −0.5 V vs. NHE [36].
- The photosensitizer (dye) in the oxidized state should be easily reduced by the electrolyte used (pair I3−/I−), i.e., the oxidation potential of the dye should be greater than the oxidation potential of the electrolyte (Eox (I3−/I−) = 0.4 V).
- It is better for the dye to be covalently bound to the semiconductor surface; thus, the presence of “anchor” acid groups in its molecule is necessary.
- One of the factors causing a decrease in the energy conversion efficiency of many organic dyes in DSSCs is the formation of dye aggregates on the semiconductor surface. This affects light absorption via the internal filter effect. Therefore, to obtain optimal solar cell performance, it is necessary to avoid aggregation of organic dyes by structurally modifying the photosensitizer.
- Another important aspect when using organic dyes is their stability, which is usually lower than that of metal complexes. This is due to the formation of an excited triplet state and the formation of unstable radicals. The dye must be resistant to side photochemical and electrochemical processes and have noticeable thermal stability (withstand about 108 cycles of device operation).
The efficiency of using the approach described above is demonstrated below using examples of compounds containing carbazole and phenothiazine fragments that are structural analogues of each other.
2. D-π-A Type Organic Dyes
The D-π-A configuration is the most popular structure for organic dyes in DSSCs that facilitates charge separation and transfer. For example, compounds 1, 2 (Scheme 1) contain alkyl chains in their composition in order to increase the solubility of the compounds, as well as to prevent dye aggregation. The electron-withdrawing fragment of cyanoacrylic acid acts as an “anchor” group [37,38]. Compounds 1 and 2 differ from each other in the nature of the electron-donating fragment. The synthesis of D-A dyes 1, 2 is a sequential implementation of three reactions—N-alkylation, formylation, and the Knoevenagel reaction (Scheme 1).
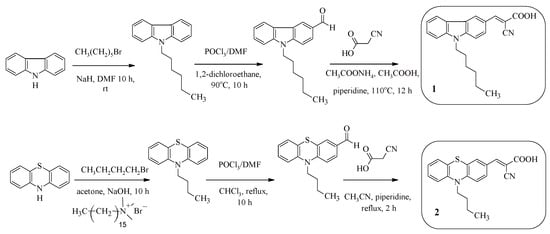
Scheme 1.
Synthesis of compounds 1 and 2.
The photophysical properties of compounds 1 and 2 are presented in Table 1. Compound 2, containing a phenothiazine fragment, absorbs in a longer-wavelength region of the spectrum than the carbazole-containing compound 1, which, in turn, leads to a decrease in the value of the band gap (1: Egopt = 2.88 eV, 2: Egopt = 2.37 eV). It was found that the wavelength of the absorption maximum for dyes 1 and 2 adsorbed on a titanium dioxide surface underwent either a bathochromic shift (1: 33 nm) or a hypsochromic shift (2: 27 nm) compared with the absorption peaks for compound solutions, which in turn could lead to the formation of aggregates on TiO2.

Table 1.
Photophysical properties of compounds 1, 2.
Based on the results of electrochemical oxidation of compounds by cyclic voltammetry, it was proven that such structures had a more positive HOMO level than the electrolyte (I3−/I−). A quantitative estimate of this parameter is the oxidation potential (1: Eox = 1.44 V, 2: Eox = 1.10 V, I3−/I−: Eox = 0.4 V). This fact indicates that the oxidized compounds were easily reduced by the redox couple of the electrolyte (I3−/I−), which indicated the effective regeneration of dyes. To determine the LUMO energy levels, cyclic voltammetry data and absorption spectra data were used, which allowed calculating the excited state oxidation potential (Eox − Egopt). The LUMO levels of compounds 1 and 2 showed more negative values than TiO2 (1: Eox − Egopt = −1.44 V, 2: Eox − Egopt = −1.27 V, TiO2: Eox − Egopt = −0.5 B), which contributed to the effective electron injection process.
Because of the narrow absorption range of the carbazole-containing dye, the dye-sensitized solar cell manufactured on its basis demonstrated low power conversion efficiency (1: η = 0.42%). At the same time, despite the short conjugation chain in dye 2, the presence of a phenothiazine fragment instead of a carbazole fragment contributed to a significant increase in this parameter (2: η = 5.5%).
The introduction of a 4-tert-butylphenyl fragment into the dye structure instead of alkyl chains is also one of the ways to provide steric hindrances in order to suppress intermolecular aggregation. Such an approach is presented in [39]. Dyes 11 and 12 contain in their structures 9-(4-tert-butylphenyl)-9H-carbazole or 10-(4-tert-butylphenyl)-10H-phenothiazine fragments connected via a thiophene–phenylene spacer to an electron-withdrawing fragment of cyanoacrylic acid (Scheme 2). A multistep approach was used to synthesize such compounds, including initial N-arylation of the corresponding secondary amine (carbazole or phenothiazine) in the presence of copper salts (Ullmann reaction) or catalyzed by palladium complexes (Buchwald–Hartwig reaction) (Scheme 2).
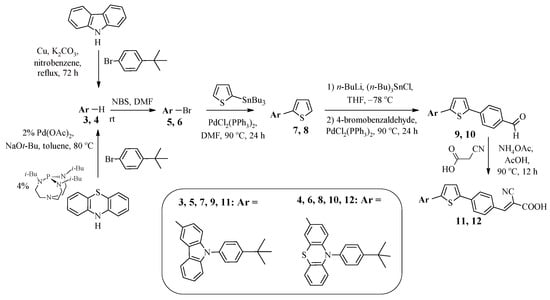
Scheme 2.
Synthesis of compounds 11 and 12.
Further bromination of 9-(4-tert-butylphenyl)-9H-carbazole 3 or 10-(4-tert-butylphenyl)-10H-phenothiazine 4 afforded products 5 and 6; subsequent chain extension was achieved by their initial reaction with 2-(tributylstannyl)thiophene under Stille reaction conditions to form 2-arylthiophenes 7 and 8 (Scheme 2). Afterwards, reaction with BuLi afforded the corresponding lithium derivatives, the reaction of which with tri(n-butyl)tin chloride afforded the corresponding organotin compounds. Their cross-coupling with 4-bromobenzaldehyde introduced an additional phenylene spacer into compounds 7 and 8. The final stage was the condensation of carbaldehydes 9 and 10 with cyanoacetic acid in the presence of catalytic amounts of ammonium acetate.
A study of the optical properties of compounds 11 and 12 revealed the presence of two absorption maxima corresponding to electron transitions in the electron-donor fragment (short-wave peak) and transitions characterizing intramolecular charge transfer (long-wave peak). It is worth noting that when replacing the 9-(4-tert-butylphenyl)-9H-carbazole fragment in compound 11 with 10-(4-tert-butylphenyl)-10H-phenothiazine, as in the previous example, a bathochromic shift of the wavelengths of the absorption maxima of the solutions of the compounds occurred, and the value of the molar extinction coefficient decreased (Table 2).

Table 2.
Photophysical properties of compounds 11 and 12.
It is interesting that the wavelength of the absorption maximum of the CZ-containing compound 11 adsorbed on the titanium dioxide surface was slightly shifted to the short-wave region of the spectrum (10 nm) compared with the absorption peak for the compound solution. This is explained, first of all, by the fact that when the carboxyl group is bound to TiO2, its electron-acceptor properties decrease. In the case of compound 12, containing a phenothiazine fragment in its structure, this difference reached 38 nm, which could lead to dye aggregation. In order to determine the efficiency of the solar cell, the HOMO and LUMO energies of dyes 11 and 12 were calculated. The results are presented in Table 2. The compounds demonstrated a sufficiently high LUMO level compared with TiO2 and a sufficiently low HOMO level compared with the ion pair of the electrolyte (I3−/I−). Thus, such structures provide efficient dye regeneration as well as an efficient electron injection process during the conversion of solar energy into electrical energy. Because compound 11, containing a 9-(4-tert-butylphenyl)-9H-carbazole fragment in its structure, had the lowest HOMO level, a device made on its basis, according to the authors of [39], would demonstrate the most efficient charge regeneration.
Comparative analysis of the main characteristics of DSSCs showed that a device based on carbazole-containing dye were characterized by higher short-circuit current, higher open-circuit voltage, and higher field factor (11: Jsc = 14.63 mA cm2, Voc = 0.685 V, FF = 0.67) than a device based on a phenothiazine analogue (12: Jsc = 14.12 mA cm2, Voc = 0.68 V, FF = 0.64). As a result, the overall conversion efficiency of the former was better than that of the latter (6.70% versus 6.32%). The obtained values were quite close to the value of the well-known ruthenium complex N-719 (6.56%).
It was found that the use of condensed heterocyclic systems as a π-spacer has a number of advantages over their linearly linked analogs. Namely, such structures enhance light absorption and lead to the suppression of charge recombination [40]. This explains the interest in the synthesis of dyes 16 and 17, containing 4,4-didodecyl-4H-cyclopenta [2,1-b:3,4-b’]dithiophene as a spacer (Scheme 3). The starting carbaldehyde 13 was obtained from 4H-cyclopenta [2,1-b:3,4-b’]dithiophene by sequentially carrying out alkylation, formylation, and bromination reactions.
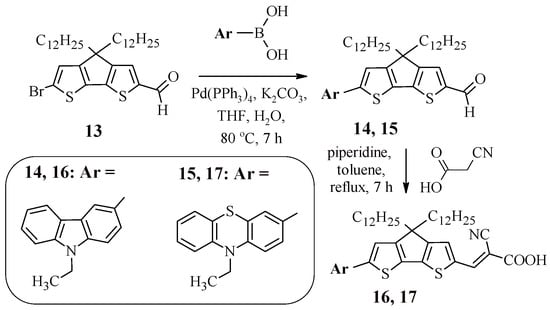
Scheme 3.
Synthesis of compounds 16, 17.
To introduce carbazole or phenothiazine fragments into the structure of the target product, the Suzuki reaction was used, the products of which (14, 15) upon interaction with cyanoacetic acid led to the production of dyes 16 and 17. Table 3 presents the results of studies of the optical and electrochemical properties of compounds 16 and 17. The presence of a condensed heteroaromatic system in the structure of compounds 16 and 17 led to the leveling of the electron-donor properties of carbazole and phenothiazine, since both dyes exhibit absorption at the same wavelength, while, as in the case of compounds 1, 2, 11, and 12, compound 16, containing a carbazole fragment, had the highest value of the molar extinction coefficient.

Table 3.
Photophysical properties of compounds 16 and 17.
Experimentally calculated values of HOMO and LUMO energies proved the efficiency of using such dyes in DSSCs (16: η = 7.5%, 17: η = 7.0%). Devices based on dyes 16 and 17 showed comparable PCEs with similar Jsc and Voc values (16: Jsc = 15.2 mA cm2, Voc = 0.691 V, 17: Jsc = 12.9 mA cm2, Voc = 0.774 V). This proves that for this type of structure, there is no major influence of the donor group on the PCE values of the devices.
3. D-A-π-A-Type Organic Dyes
In D-A-π-A-type compounds, an additional electron-withdrawing fragment allows for a significant facilitation of charge transfer from the donor to the acceptor located on the periphery of the molecule, as well as helping to tune the absorption region and HOMO and LUMO levels. In addition, compounds of this type have high photostability [41]. Scheme 4 shows the synthesis of compounds 18 and 19, the carbazole or phenothiazine fragments of which were linked to a cyanoacrylic acid fragment via an additional benzothiadiazole bridge [42,43]. It is worth noting that such structures can be obtained by two different methods. The first method involves carrying out four reactions—the Stille reaction, formylation, the Suzuki reaction, and the Knoevenagel reaction (Method A). The second method reduces the number of steps to three and involves a sequence of two Suzuki reactions, the final product of which, when reacted with cyanoacetic acid in chloroform with a catalytic amount of piperidine, leads to the formation of compounds 18 and 19 (Method B).
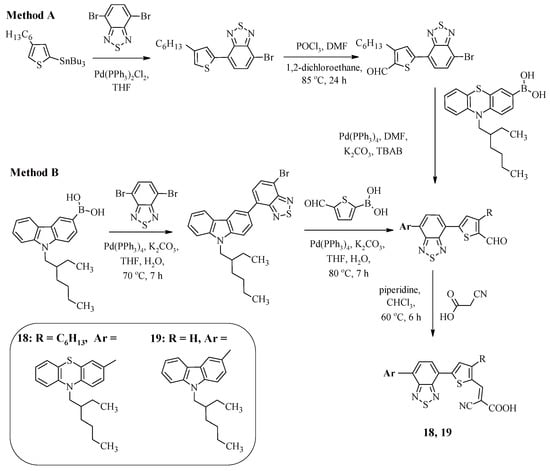
Scheme 4.
Synthesis of compounds 18 and 19.
To clarify the question of how the introduction of an electron-withdrawing fragment as a π-spacer into the structure of dyes affects their photophysical properties, it is interesting to compare the properties of compound 18 with the characteristics of its analogue, compound 20 [41] (Figure 3).
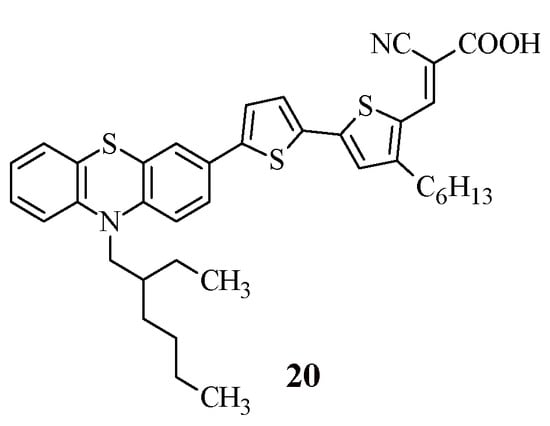
Figure 3.
Structural formulas of compound 20.
The replacement of the thiophene ring in compound 20 with a benzothiadiazole ring (compound 18) led to a slight shift in the wavelength of the absorption maximum to the long-wave region of the spectrum (18: λmaxabs = 507 nm, 20: λmaxabs = 494 nm), which allowed expanding the spectral range of absorption. It is worth noting that the HOMO and LUMO energy values did not undergo significant changes (Table 4), but the experimentally obtained values of the power conversion efficiency differed by almost two times (18: η = 2.60%, 20: η = 5.00%). It is known that the power conversion efficiency (η) is determined by the short-circuit current (Jsc), open-circuit voltage (Voc), and fill factor (FF). For DSSCs based on dyes 18 and 20, a significant difference was observed in the short-circuit current (18: Jsc = 5.78 mA cm2, 20: Jsc = 10.35 mA cm2). The better Jsc of dye 20 can be attributed to its broader absorption profile and higher molar extinction coefficient. The introduction of a carbazole fragment into the dye structure instead of a phenothiazine fragment for this type of structure makes it possible to obtain a cell with a higher value of power conversion efficiency 19: Jsc = 3.80 mA cm2.

Table 4.
Photophysical properties of compounds 18–20.
Recently, dyes 21 and 22 were synthesized, containing carbazole or phenothiazine as an electron-donor fragment as well as benzothiadiazole as an auxiliary acceptor, benzene as a π-bridge, and cyanoacrylic acid as an anchor group [44] (Scheme 5).
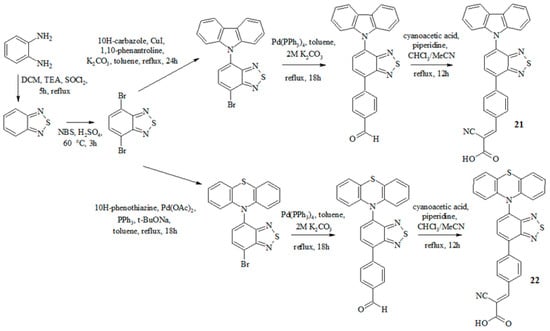
Scheme 5.
Synthesis of compounds 21 and 22.
The synthesis of these dyes is shown in Scheme 5 and included the initial preparation of 4,7-dibromobenzothiadiazole. The Buchwald–Hartwig reaction was used to introduce the phenothiazine fragment into the structure, and the Ullmann coupling reaction was used for the carbazole fragment. Further implementation of the palladium-catalyzed cross-coupling reaction of the obtained halides with 4-formylphenylboronic acid allowed us to obtain structures containing terminal aldehyde groups. The final stage of the synthesis of dyes 21 and 22 was the Knoevenagel reaction. The study of optical and electrochemical properties is presented in Table 5.

Table 5.
Photophysical properties of compounds 21 and 22.
The synthesized dyes were characterized by a bathochromic shift of the absorption wavelength maxima in comparison with the compounds that did not contain a benzothiadiazole fragment [45]. It is worth noting that compound 22, containing a phenothiazine fragment, had a longer-wavelength absorption region than the carbazole-containing compound 21, but 22 was characterized by a lower value of the molar absorption coefficient. The HOMO level values for dyes 21 (1.10 V) and 22 (1.04 V) were higher than the corresponding value for the electrolyte (0.4 V for the redox pair I3−/I−), which ensured dye regeneration. At the same time, the HOMO level for the dye containing a phenothiazine fragment was less positive than that of the carbazole-containing dye, which was explained by the stronger electron-donor ability of the phenothiazine unit. On the other hand, the LUMO level values for dyes 21 (–1.39 V) and 22 (–1.16 V) were more negative than the conduction band of TiO2 (−0.5 V versus NHE), which ensured efficient electron injection into TiO2. The authors of the article tested the synthesized dyes in a DSSC. It was found that a cell based on a carbazole-containing dye was characterized by a higher value of energy conversion efficiency.
The DSSC based on carbazole dye 21 showed the highest PCE of 3.51% compared with the cell based on phenothiazine dye 22 (η = 1.76%). This was due to the relatively higher Jsc and Voc values for the cell based on dye 21 (21: Jsc = 7.86 mA cm2, Voc = 0.687 V; 22: Jsc = 4.20 mA cm2, Voc = 0.666 V). The better Jsc of compound 21 could be attributed to its broader absorption profile and higher molar extinction coefficient. Compared with dye 22, the higher Voc of dye 21 may have been due to the lower HOMO energy level, indicating a higher driving force for dye regeneration. Thus, more electrons could be accumulated in TiO2, indicating better suppression of charge recombination between TiO2 and electrolyte.
Compounds 23 and 24 contained dithieno [3′,2′:3,4;2»,3»:5,6]benzo [1,2-c]furazan as a π-spacer [46]. The advantage of introducing such an electron-withdrawing fragment is its rigid planar structure, which leads to efficient intramolecular charge transfer from the donor to the acceptor and allows reducing the reorganization energy of the dye molecule during photoexcitation. The synthetic approach to obtaining the compounds is presented in Scheme 6. It uses a combination of the Stille reaction, formylation, bromination, and, in the final stage, condensation.
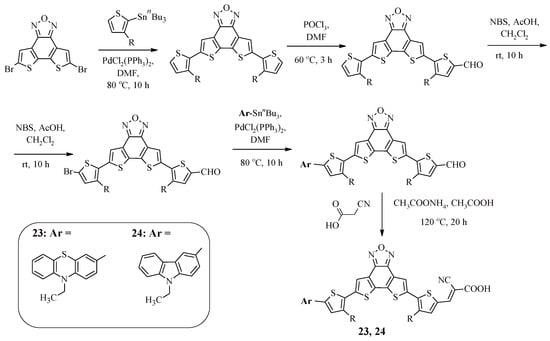
Scheme 6.
Synthesis of compounds 23 and 24.
As in the case of dyes 16 and 17 containing a fused heterocyclic spacer, compounds 23 and 24 demonstrated absorption in the same region, but their distinctive feature was the molar extinction coefficient, which reached a higher value in the case of compounds of the D-A-π-A type (23, 24) in the presence of a phenothiazine fragment in the structure (Table 6). The results of electrochemical oxidation for dyes 23 and 24 confirmed the pattern characteristic of compounds 1, 2, 11, 12, and 16–19, namely, structures containing a carbazole fragment have a higher oxidation potential value and a deeper HOMO and LUMO level than FTZ-containing compounds, which is directly related to the power conversion efficiency (23: η = 1.42%, 24: η = 5.98%). In addition, the low performance of the dye 23 cell was likely due to the fact that dye 23 is less effective in blocking electrolytes from approaching the TiO2 surface, resulting in a lower open circuit voltage (Voc). The dye 23 cell also had slower electron injection.

Table 6.
Photophysical properties of compounds 23 and 24.
Recently, D-D-π-A-π-A quinoxaline dyes 25 and 26 were synthesized, differing from each other in the type of electron-donor fragment (Figure 4) [47].
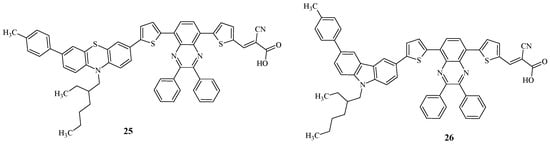
Figure 4.
Structural formulas of compounds 25 and 26.
Quinoxaline is a coplanar and rigid ring with strong electron-withdrawing ability due to two symmetric unsaturated nitrogen atoms in the pyrazine ring. In addition, the presence of the imine moiety increases the π-conjugation in the dye structure. It has been shown that the introduction of 2,3-diphenylquinoxaline into the dye structure reduces charge recombination by inhibiting intermolecular aggregation due to two separate phenyl rings attached to the quinoxaline block [48]. The multistep synthesis of dyes 25 and 26 is shown in Scheme 7.
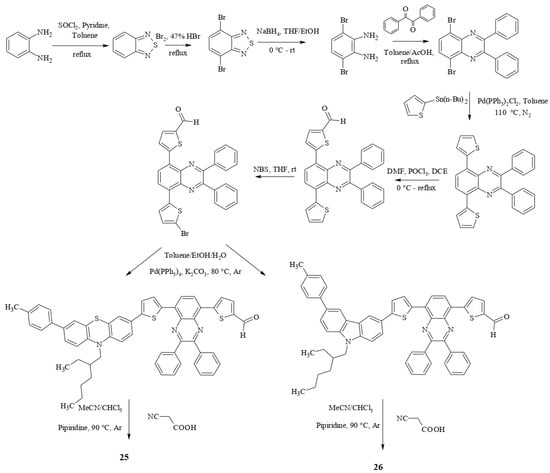
Scheme 7.
Synthesis of compounds 25 and 26.
The results of a study on the optical and electrochemical properties of the dyes are presented in Table 7. The absorption spectra of the dyes demonstrated absorption bands in the region of 240–440 nm, which corresponded to the π-π*-electron transition due to the presence of aromatic fragments in the structure. Also in the low-energy region, peaks were observed at 440–660 nm, characterizing the intramolecular charge transfer between donor and acceptor fragments. The dyes were characterized by high values of the molar extinction coefficient (Table 7). It is important that these values were greater than those of the standard ruthenium dyes N719 and N3 (14,000 and 13,900 M− 1 cm−1, respectively). This fact proves that the presented dyes collected light significantly better than typical organometallic dyes.

Table 7.
Photophysical properties of compounds 25 and 26.
For the presented type of structures, dye 26, including a carbazole fragment, demonstrated a bathochromic shift of the absorption band, characterizing intramolecular charge transfer, in comparison with the phenothiazine-containing dye. The authors believed that this was due to the increased planarity of the carbazole fragment compared with the phenothiazine, which has a nonplanar butterfly shape. Parallel alignment of two benzene rings in the carbazole fragment provided smooth conjugation and, therefore, higher absorption.
Study of the electrochemical properties of the synthesized dyes showed that they were characterized by lower HOMO energies compared with the redox electrolyte (I3−/I−) (Table 7). This fact indicates that oxidative regeneration of the dye is possible through I− in the DSSC electrolyte. On the other hand, the calculated LUMO values for the dyes were higher than that for TiO2, indicating possible electron injection from excited dyes into TiO2. As a result, all the dyes had sufficient fundamentality for their use as sensitizers in DSSCs.
4. Organic Dyes with a Star-Shaped Structure
Several approaches to dye design that prevent intermolecular aggregation of dye molecules during solar cell operation have been previously presented, namely the introduction of long alkyl chains or a 4-tert-butylphenyl fragment, as well as the use of a fused heteroaromatic system as a π-spacer. Dyes with a star-shaped structure are also of interest from the point of view of charge recombination suppression. For example, compounds 27 and 28 contain a central triphenylamine core, on the periphery of which there are carbazole or phenothiazine fragments, as well as a cyanoacrylic acid fragment [49]. The Ullmann reaction was used to synthesize these structures, the starting compound for which was 4-[bis(4-iodophenyl)amino]benzaldehyde, obtained in two stages from triphenylamine (Scheme 8).
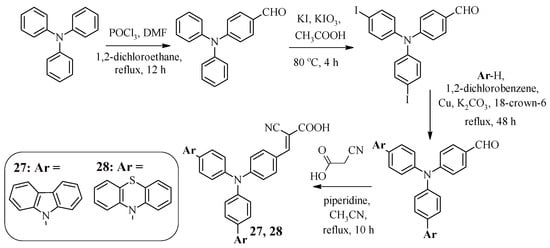
Scheme 8.
Synthesis of compounds 27 and 28.
Table 8 presents the results of the analysis of the optical properties of dyes 27 and 28. Both compounds had one intense absorption band in the visible region of the spectrum. Moreover, for the phenothiazine-containing compound 28, as in the case of compound 23, a higher value of the molar absorption coefficient was characteristic. The absorption spectra for solutions of compounds 27 and 28 in acetonitrile and the absorption spectra of compounds 27 and 28 adsorbed on the surface of TiO2 did not show a significant shift in the absorption wavelength, which indicates the prevention of aggregation of dyes on the titanium dioxide film (Table 8).

Table 8.
Photophysical properties of compounds 27 and 28.
The experimentally obtained values of HOMO energies of compounds 27 and 28 (Table 8) proved that the oxidized dyes formed as a result of the corresponding injection of electrons into the conduction band of TiO2 accepting electrons from the electrolyte (I3−/I−), which led to their reduction. At the same time, the more negative value of the LUMO energy of the dyes (Table 8) compared with the LUMO energy of TiO2 (−0.5 V vs. NHE) indicates that the process of electron injection from the dye molecule in the excited state into the conduction band of titanium dioxide was energetically allowed. It was found that dyes 27 and 28 did not exhibit significant differences in photophysical properties. However, the FTZ-containing compound 28 was characterized by a higher value of power conversion efficiency (27: η = 3.26%, 28: η = 4.54%), which was most likely due to its higher absorption capacity.
Study of the incident monochromatic photon-to-current conversion efficiency (IPCE) showed that the solar cells based on dyes 27 and 28 in the range of 400–500 nm showed high IPCE above 44%. Compared with dye 27, the cell based on phenothiazine dye 28 gave a higher IPCE value, which implies that the dye would show a relatively large photocurrent in DSSCs. This fact may be related to the higher molar extinction coefficient for dye 28. On the other hand, the IPCE values of 27 and 28 obviously decreased above 500 nm in the long-wavelength region, which could be attributed to the decrease in light collection for the dyes. These results are consistent with the absorption spectra in solution and on TiO2 films of the dyes.
Along with triphenylamine, the introduction of which into the structure of the molecule allows expanding the absorption region and reducing the tendency of aggregation on the TiO2 surface, triphenylethylene is of interest. For example, compounds 39–42 have a star-shaped structure, the central core of which is triphenylethylene connected to terminal carbazole or phenothiazine fragments as well as to the electron-withdrawing fragment of cyanoacrylic acid through electron-donating N-substituted carbazole or phenothiazine bridges (Scheme 9) [50]. The starting compound for the synthesis of these structures was bis(4-fluorophenyl)ketone, which interacted with carbazole or phenothiazine; the products then entered into the Wadsworth–Emmons reaction, which led to the formation of the central triphenylethylene core (31, 32) (Scheme 9). The presence of the bromine atom in compounds 31 and 32 made it possible to obtain the corresponding boric acids 33 and 34 from them, from which carbaldehydes 35–38 were then synthesized under Suzuki reaction conditions. The final stage was the Knoevenagel reaction, which led to the target dyes 39–42.
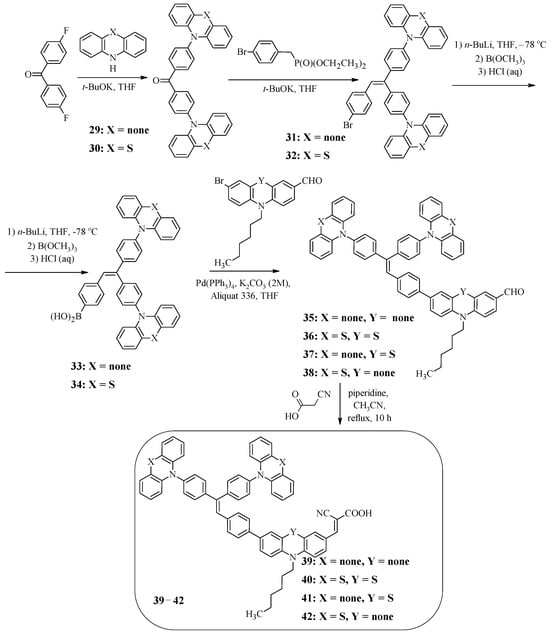
Scheme 9.
Synthesis of compounds 39–42.
The spectra of dyes 39 and 41 had several absorption peaks in the ultraviolet region (39: λmaxabs = 238, 293, 341 nm, 41: λmaxabs = 238, 293, 329, 341 nm), as well as one peak in the visible light region (39: λmaxabs = 412 nm, 41: λmaxabs = 462 nm), and the long-wave absorption maximum of compound 41 was shifted to the red region of the spectrum (50 nm) (Table 9). The absorption spectra of dyes 40 and 42, containing phenothiazine fragments at the periphery of the molecule, in dichloromethane contained three absorption peaks (40: λmaxabs = 258, 326, 466 nm, 42: λmaxabs = 258, 335, 412 nm). It is also worth noting that for compound 40, a bathochromic shift of the absorption maximum wavelength was observed compared with compound 42, which was associated with the presence in its structure of a stronger electron-donating phenothiazine fragment, which was part of the π-spacer.

Table 9.
Photophysical properties of compounds 39–42.
In the absorption spectra of dyes adsorbed on the surface of titanium dioxide, the absorption peaks were significantly shifted to the red region of the spectrum compared with the absorption wavelengths for solutions of compounds. Dyes 39–42 had a fairly deep HOMO level, about −5.0 eV, compared with the I3−/I− ion pair (EHOMO = −4.60 eV) [51], which indicated effective regeneration of the dye. It was found that the FTZ-containing compounds 40 and 41 had more negative values for their LUMO levels, which may have indicated their higher efficiency in terms of using dyes in solar cells, as evidenced by the experimentally obtained values of power conversion efficiency (39: η = 2.14%, 40: η = 6.55%, 41: η = 5.51%, 42: η = 2.69%). In this case, the replacement of 9-hexyl-9H-carbazole in dye 39 with nonplanar 10-hexyl-10H-phenothiazine to construct the structure of dye 41 resulted in an improvement in the DSSC performance. In this case, further modification of the structure of compound 41, namely the introduction of a more twisted triphenylethylenephenothiazine block instead of the triphenylethylenecarbazole one, allowed achieving a further increase in the DSSC performance based on dye 40. Comparative analysis of dyes 39–42 showed that the more electron-rich and twisted nonplanar phenothiazine could be considered more favorable than planar carbazole in terms of its use in dye-sensitized solar cells. The nonplanar structure contributes to the slowing down of charge recombination and a reduction in dye aggregation, which leads to a higher Voc.
5. Organic Dyes of the A-D-A Type
The use of D-π-A configuration in organic dyes has some disadvantages; namely, the short length of π-conjugation contributes to a narrow absorption band. Although an increase in the length of π-conjugation expands the absorption region, this structure is not very stable when irradiated with high-energy photons. In addition, such a structure can cause aggregation and charge recombination, which can lead to a decrease in photoelectric characteristics.
Therefore, new configurations of organic dyes are currently being developed, such as A-D-A-type structures. Recently, Murali et al. synthesized two organic sensitizers with the A-D-A-D-A configuration, in which the donor was carbazole or phenothiazine, the auxiliary acceptor was benzothiadiazole, and the terminal group was cyanoacrylic acid (Figure 5) [52]. The presence of two terminal electron-acceptor fragments in the dye structure facilitated efficient electron injection.
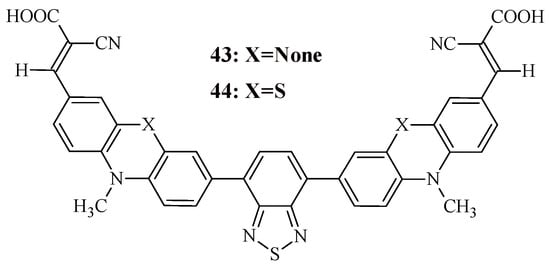
Figure 5.
Structural formulas of compounds 43 and 44.
The synthesis of the dyes consisted of the following reactions: N-alkylation, the Miyaura borylation reaction, the Suzuki cross-coupling reaction, the Vilsmeier–Haack reaction, and the Knoevenagel reaction (Scheme 10).
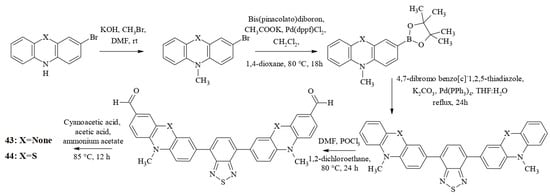
Scheme 10.
Synthesis of compounds 43 and 44.
The optical and electrochemical properties of organic dyes 43 and 44 are presented in Table 10. Both organic dyes exhibited two main absorption bands in the range of 340–410 nm and 420–540 nm. It is known that a wider absorption range contributes to better DSSC performance, since it allows collecting more photons. The observed red shift in the absorption maxima of dye 44 compared with those of dye 43 may have been due to the stronger electron-donating ability of the phenothiazine unit.

Table 10.
Photophysical properties of compounds 43 and 44.
It is worth noting that for both organic dyes, the molar extinction coefficient values for the absorption maxima in the range of 420–540 nm, which characterize intramolecular charge transfer, were higher than those of the ruthenium dye N719 [53]. It has been proven that high molar absorption coefficients of organic dyes allow obtaining a thinner nanocrystalline film on their basis, which leads to better device performance. In addition, they promote the diffusion of electrolyte in the film and reduce the possibility of recombination of light-induced charges during transport [54,55].
In the absorption spectra of the dyes adsorbed on the transparent TiO2 thin film (2.5 mm), the absorption maxima of dyes 43 and 44 showed red shifts of about 18 and 12 nm, respectively, compared with their dissolved state. The observed red shifts of the absorption maxima and broadening of the absorption spectra for both dyes were possibly due to the formation of J-aggregates on the thin films.
To evaluate the possibility of electron transfer from the excited organic dye molecule to the conduction band of TiO2 and from the redox couple in the electrolyte to the oxidized dye molecule, cyclic voltammetry measurements were performed for both dyes. The results are presented in Table 10.
It was found that for organic dyes 43 and 44, the ground state oxidation potential (Eox) was observed at 0.91 and 0.62 V, respectively, which was significantly higher than the iodide/triiodide oxidation–reduction potential (0.4 V). In addition, it is worth noting that the phenothiazine moiety had a significant effect on the oxidation potential of the dye, which led to a lower Eox value for 44 compared with 43. The calculated excited state oxidation potentials for the dyes were more negative (43: Eox* = 1.45 V, 44: Eox* = 1.56 V) than the conduction band edge energy level of TiO2 (0.5 V). This fact indicates that electrons from the excited dye molecules can be effectively injected into the conduction band of TiO2. In general, the energy levels of dyes 43 and 44 were in good agreement with the requirements for efficient electron transfer in DSSC.
Solar cells prepared based on dyes 43 and 44 exhibited power conversion efficiencies of 4.35% and 6.87%, respectively. Moreover, the PCE values of the cells did not even show significant changes after aging at room temperature for 7 days. The presence of a phenothiazine moiety in compound 44 resulted in an improved Jsc value compared with compound 43 (43: Jsc = 7.51 mA cm2, 44: Jsc = 13.1 mA cm2). The observed high Jsc value of the dye-44-based cell can be attributed to its stronger ability to collect power in the longer wavelength region. On the other hand, the dye-44-based device exhibited a comparatively lower Voc value than dye 43 due to its higher oxidation potential value (43: Voc = 0.762 V, 44: Voc = 0.728 V). Overall, it is evident that the high short circuit density value of 44 resulted in a higher efficiency of 6.87%. Furthermore, the observed high performance of the phenothiazine dye-based device may have been due to its lower tendency to aggregate.
It is worth noting that rhodanine-3-acetic acid can be used as an “anchor” electron-withdrawing group in dyes instead of cyanoacrylic acid. For example, compounds 45 and 46 (Scheme 11), which are A-D-A-type structures, contain terminal rhodanine-3-acetic acid fragments linked to an N-ethylcarbazole or N-ethylphenathiazine fragment via a methylene bridge [56].
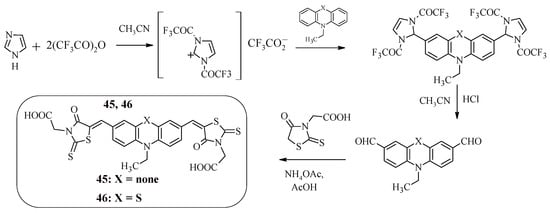
Scheme 11.
Synthesis of compounds 45 and 46.
An alternative approach to the Vilsmeier–Haack reaction, in which the starting dicarbaldehydes were obtained in two stages, was used to synthesize these structures. Initially, the reaction product of imidazole with trifluoroacetic anhydride interacted with N-substituted carbazole or phenothiazine to form intermediate compounds containing trifluoroacetyl groups, the further hydrolysis of which led to the production of dicarbaldehydes. The final stage of the synthesis of compounds 45 and 46 was the Knoevenagel reaction.
A comparative analysis of the photophysical characteristics of compounds 45 and 46 is presented in Table 11. As expected, the presence of a phenothiazine fragment in the structure of compound 46 shifted the absorption band to the long-wavelength region of the spectrum compared with the CZ-containing compound 45, which, in turn, led to a decrease in the value of the band gap (45: Egopt = 2.56 eV, 46: Egopt = 2.18 eV).

Table 11.
Photophysical properties of compounds 45 and 46.
Compound 45 was characterized by a more positive HOMO level, which indicated effective dye regeneration, but compound 46 had an effective electron injection process due to a deeper LUMO level. A study of the volt–ampere characteristics of solar cells found that the FTZ-containing compound 46 had the highest power conversion efficiency (45: η = 2.81%, 46: η = 4.91%). The improved efficiency exhibited by the dye-based sensitizer 46 was due to the stronger electron-donating ability of the phenothiazine block in transferring electrons from the phenothiazine block to the rhodanine-3-acetic acid group and the possible vectorized photon-induced charge transfer of the phenothiazine block toward the electrodes. According to the authors of the paper, this characteristic could not only suppress the interaction between molecules, leading to energetic quenching of excited states, but suppress I3− ions in the electrolyte on the TiO2 surface, which favored a higher Voc.
6. Conclusions
Carbazole and phenothiazine are important structural fragments in the creation of materials for dye-sensitized solar cells. This is explained, first of all, by the fact that compounds containing CZ- or FTZ-fragments have high thermal and morphological stability. In addition, conjugated systems containing electron-donating carbazole and phenothiazine fragments in combination with the electron-accepting fragment of cyanoacrylic acid have a sufficiently deep HOMO level compared with the I3−/I− ion pair and are characterized by a more negative LUMO energy value than that of TiO2. The first fact proves that oxidized dyes formed as a result of the corresponding electron injection into the conduction band of TiO2 accept electrons from the electrolyte (I3−/I−), which leads to their reduction. The second fact indicates that the process of electron injection from the dye molecule in an excited state into the conduction band of titanium dioxide is energetically allowed. As a result, the use of such structures as materials for dye-sensitized solar cells will allow obtaining devices with high power conversion efficiency.
Author Contributions
Conceptualisation, D.S.; writing—original draft preparation, D.S.; writing—review and editing, D.S.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out within the framework of State Assignment (State Registration No. 124022200026-7).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author under reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Giannouli, M. Current Status of Emerging PV Technologies: A Comparative Study of Dye-Sensitized, Organic, and Perovskite Solar Cells. Int. J. Photoenergy 2021, 2021, 6692858. [Google Scholar] [CrossRef]
- Agarwal, R.; Vyas, Y.; Chundawat, P.; Ameta, C. Outdoor performance and stability assessment of dye-sensitized solar cells (DSSCs). In Solar Radiation-Measurement, Modeling and Forecasting Techniques for Photovoltaic Solar Energy Applications; Aghaei, M., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Saeed, M.A.; Yoo, K.; Kang, H.C.; Shim, J.W.; Lee, J.J. Recent developments in dye-sensitized photovoltaic cells under ambient illumination. Dye. Pigment. 2021, 194, 109626. [Google Scholar] [CrossRef]
- Khir, H.; Pandey, A.K.; Saidur, R.; Ahmad, M.S.; Abd Rahim, N.; Dewika, M.; Samykano, M. Recent advancements and challenges in flexible low temperature dye sensitised solar cells. Sustain. Energy Technol. Assess. 2022, 53, 102745. [Google Scholar]
- Yeoh, M.E.; Chan, K.Y. A review on semitransparent solar cells for real-life applications based on dye-sensitized technology. IEEE J. Photovolt. 2021, 11, 354–361. [Google Scholar] [CrossRef]
- Avilés-Betanzos, R.; Oskam, G.; Pourjafari, D. Low-temperature fabrication of flexible dye-sensitized solar cells: Influence of electrolyte solution on performance under solar and indoor illumination. Energies 2023, 16, 5617. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, D.; Suo, J.; Cao, Y.; Eickemeyer, F.T.; Vlachopoulos, N.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. Hydroxamic acid pre-adsorption raises the efficiency of cosensitized solar cells. Nature 2023, 613, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.Y.-Y.; Wu, F.-L.; Chang, C.-H.; Chou, H.-H.; Chuang, T.-M.; Chu, T.-C. Y-shaped metal-free D-[π]-(A)2 sensitizers for high-performance dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 3092–3101. [Google Scholar]
- Wang, Y.; Zheng, Z.; Li, T.; Robertson, N.; Xiang, H.; Wu, W.; Hua, J.; Zhu, W.H.; Tian, H. D-A-π-A motif quinoxaline-based bensitizers with high molar extinction coefficient for quasiolid-state dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2016, 8, 31016–31024. [Google Scholar] [CrossRef]
- Peddapuram, A.; Cheema, H.; McNamara, L.; Zhang, Y.; Hammer, N.; Delcamp, J. Quinoxaline-based dual donor, dual acceptor organic dyes for dye-sensitized solar cells. Appl. Sci. 2018, 8, 1421. [Google Scholar] [CrossRef]
- Shi, J.; Chai, Z.; Su, J.; Chen, J.; Tang, R.; Fan, K.; Zhang, L.; Han, H.; Qin, J.; Peng, T.; et al. New sensitizers bearing quinoxaline moieties as an auxiliary acceptor for dye-sensitized solar cells. Dye. Pigment. 2013, 98, 405–413. [Google Scholar] [CrossRef]
- Pei, K.; Wu, Y.; Li, H.; Geng, Z.; Tian, H.; Zhu, W.H. Cosensitization of D-A-π-A quinoxaline organic dye: Efficiently filling the absorption valley with high photovoltaic efficiency. ACS Appl. Mater. Interfaces 2015, 7, 5296–5304. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Jia, X.; Wang, Z.S.; Zhou, G. X-shaped organic dyes with a quinoxaline bridge for use in dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 9697–9706. [Google Scholar] [CrossRef]
- Munir, R.; Zahoor, A.F.; Anjum, M.N.; Nazeer, U.; Haq, A.U.; Mansha, A.; Chaudhry, A.R.; Irfan, A. Synthesis And Photovoltaic Performance of Carbazole (Donor) Based Photosensitizers in Dye-Sensitized Solar Cells (DSSC): A Review. Top. Curr. Chem. 2025, 383, 1–54. [Google Scholar] [CrossRef]
- Devadiga, D.; Selvakumar, M.; Shetty, P.; Sridhar Santosh, M.; Chandrabose, R.S.; Karazhanov, S. Recent developments in metal-free organic sensitizers derived from carbazole, triphenylamine, and phenothiazine for dye-sensitized solar cells. Int. J. Energy Res. 2021, 45, 6584–6643. [Google Scholar] [CrossRef]
- Luo, J.S.; Wan, Z.Q.; Jia, C.Y. Recent advances in phenothiazine-based dyes for dye-sensitized solar cells. Chin. Chem. Lett. 2016, 27, 1304–1318. [Google Scholar] [CrossRef]
- Gangadhar, P.S.; Reddy, G.; Prasanthkumar, S.; Giribabu, L. Phenothiazine functional materials for organic optoelectronic applications. Phys. Chem. Chem. Phys. 2021, 23, 14969–14996. [Google Scholar] [CrossRef]
- Buene, A.F.; Almenningen, D.M. Phenothiazine and phenoxazine sensitizers for dye-sensitized solar cells—An investigative review of two complete dye classes. J. Mater. Chem. C 2021, 9, 11974–11994. [Google Scholar] [CrossRef]
- Michinobu, T.; Osako, H.; Shigehara, K. Synthesis and properties of 1,8-carbazole-based conjugated copolymers. Polymers 2010, 2, 159–173. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, Y.S.; Shin, W.S.; Moon, S.J.; Park, T. Carbazole-based copolymers: Effects of conjugation breaks and steric hindrance. Macromolecules 2011, 44, 1909–1919. [Google Scholar] [CrossRef]
- Vacareanu, L.; Catargiu, A.M.; Grigoras, M. Spectroelectrochemical characterization of isomeric conjugated polymers containing 2, 7-and 3, 6-carbazole linked by vinylene and ethynylene segments. High Perform. Polym. 2015, 27, 476–485. [Google Scholar] [CrossRef]
- Wu, W.; Yang, J.; Hua, J.; Tang, J.; Zhang, L.; Long, Y.; Tian, H. Efficient and stable dye-sensitized solar cells based on phenothiazine sensitizers with thiophene units. J. Mater. Chem. 2010, 20, 1772–1779. [Google Scholar] [CrossRef]
- Mahalakshmi, V.; Gouthaman, S.; Sugunalakshmi, M.; Bargavi, S.; Lakshmi, S. Crystal structure of 10-ethyl-7-(9-ethyl-9Hcarbazol-3-yl)-10H-phenothiazine-3-carbaldehyde. Acta Cryst. 2017, E73, 726–728. [Google Scholar]
- Unny, D.; Sivanadanam, J.; Mandal, S.; Aidhen, I.S.; Ramanujam, K. Effect of flexible, rigid planar and non-planar donors on the performance of dye-sensitized solar cells. J. Electrochem. Soc. 2018, 165, H845–H860. [Google Scholar] [CrossRef]
- Tan, H.; Pan, C.; Wang, G.; Wu, Y.; Zhang, Y.; Zou, Y.; Yu, G.; Zhang, M. Phenoxazine-based organic dyes with different chromophores for dye-sensitized solar cells. Org. Electron. 2013, 14, 2795–2801. [Google Scholar] [CrossRef]
- Wong, W.Y.; Li, L.; Wang, L. Synthesis and characterization of platinum(II) polymetallaynes functionalized with phenoxazine-based spacer. A comparison with the phenothiazine congener. J. Organomet. Chem. 2019, 897, 192–199. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.E.; Hu, J.; Hong, Y. Novel metal-free organic dyes containing linear planar 11,12-dihydroindolo[2,3-a]carbazole donor for dye-sensitized solar cells: Effects of π spacer and alkyl chain. Dye. Pigment. 2019, 164, 213–221. [Google Scholar] [CrossRef]
- Gratzel, M. Recent advances in sensitized mesoscopic solar cells. Acc. Chem. Res. 2009, 42, 1788–1798. [Google Scholar] [CrossRef]
- Parashchuk, D.Y.; Kokorin, A.I. Modern photoelectric and photochemical methods of solar energy conversion. Russ. Chem. J. 2008, 52, 107–117. [Google Scholar]
- Kalaignan, G.P.; Kang, Y.S. A review on mass transport in dye-sensitized nanocrystalline solar cells. J. Photochem. Photobiol. C 2006, 7, 17–22. [Google Scholar] [CrossRef]
- Ryan, M. Progress in Ruthenium Complexes for Dye Sensitised Solar Cells. Platin. Met. Rev. 2009, 53, 216–218. [Google Scholar] [CrossRef]
- Goncalves, L.M.; Bermudes, V.d.Z.; Ribeiro, H.A.; Mendes, A.M. Dye-sensitized solar cells: A safe bet for the future. Energy Environ. Sci. 2008, 1, 655–667. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Garche, J.; Dyer, C.K.; Moseley, P.T.; Ogumi, Z.; Rand, D.A.; Scrosati, B. (Eds.) Encyclopedia of Electrochemical Power Sources; Elsevier Science: Amsterdam, The Netherlands, 2009; 4538p. [Google Scholar]
- Grätzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Grӓtzel, M. Light-induced redox reactions in nanocrystalline systems. Chem. Rev. 1995, 95, 49–68. [Google Scholar] [CrossRef]
- Kim, M.S.; Yang, H.S.; Jung, D.Y.; Han, Y.S.; Kim, J.H. Effects of the number of chromophores and the bulkiness of a nonconjugated spacer in a dye molecule on the performance of dye-sensitized solar cells. Colloids Surf. A Physicochem. Eng. Asp. 2013, 420, 22–29. [Google Scholar] [CrossRef]
- Tian, H.; Yang, X.; Chen, R.; Pan, Y.; Li, L.; Hagfeldt, A.; Sun, L. Phenothiazine derivatives for efficient organic dye-sensitized solar cells. Chem. Commun. 2007, 36, 3741–3743. [Google Scholar] [CrossRef]
- Chang, Y.J.; Chou, P.-T.; Lin, S.-Y.; Watanabe, M.; Liu, Z.-Q.; Lin, J.-L.; Chen, K.-Y.; Sun, S.-S.; Liu, C.-Y.; Chow, T.J. High-Performance Organic Materials for Dye-Sensitized Solar Cells: Triarylene-Linked Dyads with a 4-tert-Butylphenylamine Donor. Chem. Asian J. 2012, 7, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, J.; Cai, N.; Zhang, M.; Wang, P. Synchronously Reduced Surface States, Charge Recombination, and Light Absorption Length for High-Performance Organic Dye-Sensitized Solar Cells. J. Phys. Chem. B 2010, 114, 4461–4464. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, W. Organic sensitizers from D–p–A to D–A–p–A: Effect of the internal electron-withdrawing units on molecular absorption, energy levels and photovoltaic performances. Chem. Soc. Rev. 2013, 42, 2039–2058. [Google Scholar] [CrossRef]
- Liu, X.; Long, J.; Wang, G.; Pei, Y.; Zhao, B.; Tan, S. Effect of structural modification on the performances of phenothiazine-dye sensitized solar cells. Dye. Pigm. 2015, 121, 118–127. [Google Scholar] [CrossRef]
- Keerthi, A.; Sriramulu, D.; Liu, Y.; Thuang, C.; Timothy, Y.; Wang, Q.; Valiyaveettil, S. Architectural influence of carbazole pushepullepull dyes on dye sensitized solar cells. Dye. Pigm. 2013, 99, 787–797. [Google Scholar] [CrossRef]
- Arslan, B.S. Effect of electron donor groups on the performance of benzothiadiazole dyes with simple structures for dye-sensitized solar cells. J. Photochem. Photobiol. A 2024, 449, 115392. [Google Scholar] [CrossRef]
- Wan, Z.; Jia, C.; Zhou, L.; Huo, W.; Yao, X.; Shi, Y. Influence of different arylamine electron donors in organic sensitizers for dye-sensitized solar cells. Dye. Pigm. 2012, 95, 41–46. [Google Scholar] [CrossRef]
- Ni, J.-S.; You, J.-H.; Hung, W.-I.; Kao, W.-S.; Chou, H.-H.; Lin, J.T. Organic Dyes Incorporating the Dithieno[3′,2′:3,4;2″,3″:5,6]benzo[1,2-c]furazan Moiety for Dye Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 22612–22621. [Google Scholar] [CrossRef]
- Al-Marhabi, A.R.; El-Shishtawy, R.M.; Bouzzine, S.M.; Hamidi, M.; Al-Ghamdi, H.A.; Al-Footy, K.O. DD-π-A-π-A-based quinoxaline dyes incorporating phenothiazine, phenoxazine and carbazole as electron donors: Synthesis, photophysical, electrochemical, and computational investigation. J. Photochem. Photobiol. A 2023, 436, 114389. [Google Scholar] [CrossRef]
- Zając, D.; Przybylski, D.; Sołoducho, J. Synthesis and theoretical investigation using DFT of 2,3-diphenylquinoxaline derivatives for electronic and photovoltaic effects. J. Electron. Mater. 2021, 50, 5226–5234. [Google Scholar] [CrossRef]
- Wan, Z.; Jia, C.; Duan, Y.; Zhou, L.; Zhang, J.; Lin, Y.; Shi, Y. Influence of the antennas in starburst triphenylamine-based organic dyesensitized solar cells: Phenothiazine versus carbazole. RSC Adv. 2012, 2, 4507–4514. [Google Scholar] [CrossRef]
- Chen, C.; Liao, J.-Y.; Chi, Z.; Xu, B.; Zhang, X.; Kuang, D.-B.; Zhang, Y.; Liu, S.; Xu, J. Metal-free organic dyes derived from triphenylethylene for dye-sensitized solar cells: Tuning of the performance by phenothiazine and carbazole. J. Mater. Chem. 2012, 22, 8994–9005. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, Y.; Li, R.; Shi, D.; Wenger, S.; Zakeeruddin, S.M.; Grӓtzel, M.; Wang, P. Employ a bisthienothiophene linker to construct an organic chromophore for efficient and stable dye-sensitized solar cells. Energy Environ. Sci. 2009, 2, 92–95. [Google Scholar] [CrossRef]
- Murali, M.G.; Wang, X.; Wang, Q.; Valiyaveettil, S. New banana shaped A-D-π-D-A type organic dyes containing two anchoring groups for high performance dye-sensitized solar cells. Dye. Pigm. 2016, 134, 375–381. [Google Scholar] [CrossRef]
- Ardo, S.; Meyer, G.J. Photodriven heterogeneous charge transfer with transitionmetal compounds anchored to TiO2 semiconductor surfaces. Chem. Soc. Rev. 2009, 38, 115–164. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wu, W.; Hua, J.; Li, J.; Li, X.; Tian, H. Starburst triphenylamine-based cyanine dye for efficient quasi-solid-state dye-sensitized solar cells. Energy Environ. Sci. 2009, 2, 982–990. [Google Scholar] [CrossRef]
- Khazraji, A.C.; Hotchandani, S.; Das, S.; Kamat, P.V. Controlling dye (Merocyanine540) aggregation on nanostructured TiO2 films. An organized assembly approach for enhancing the efficiency of photosensitization. J. Phys. Chem. B 1999, 103, 4693–4700. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Tsao, M.-H.; Su, S.-G.; Wang, H.P.; Lin, Y.-C.; Chen, F.-L.; Chang, C.-W.; Sun, I.-W. Synthesis, Characterization and Photovoltaic Properties of Di-Anchoring Organic Dyes. J. Braz. Chem. Soc. 2011, 22, 780–789. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).