Bactericidal Effect of Synthetic Phenylalkylamides Inspired by Gibbilimbol B Against Neisseria gonorrhoeae
Abstract
1. Introduction
2. Results and Discussion
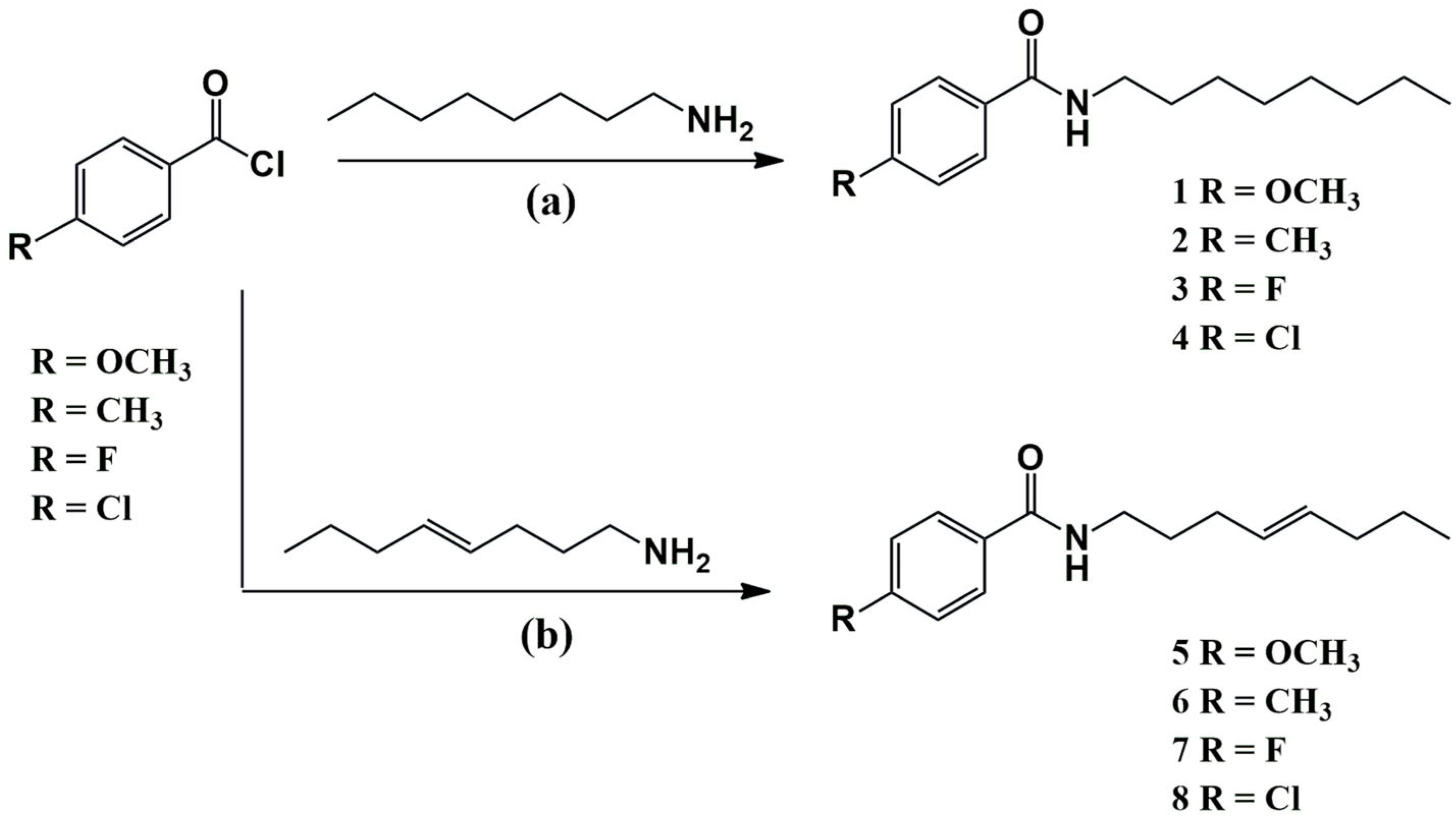
2.1. Chemistry
2.2. Biological Evaluation
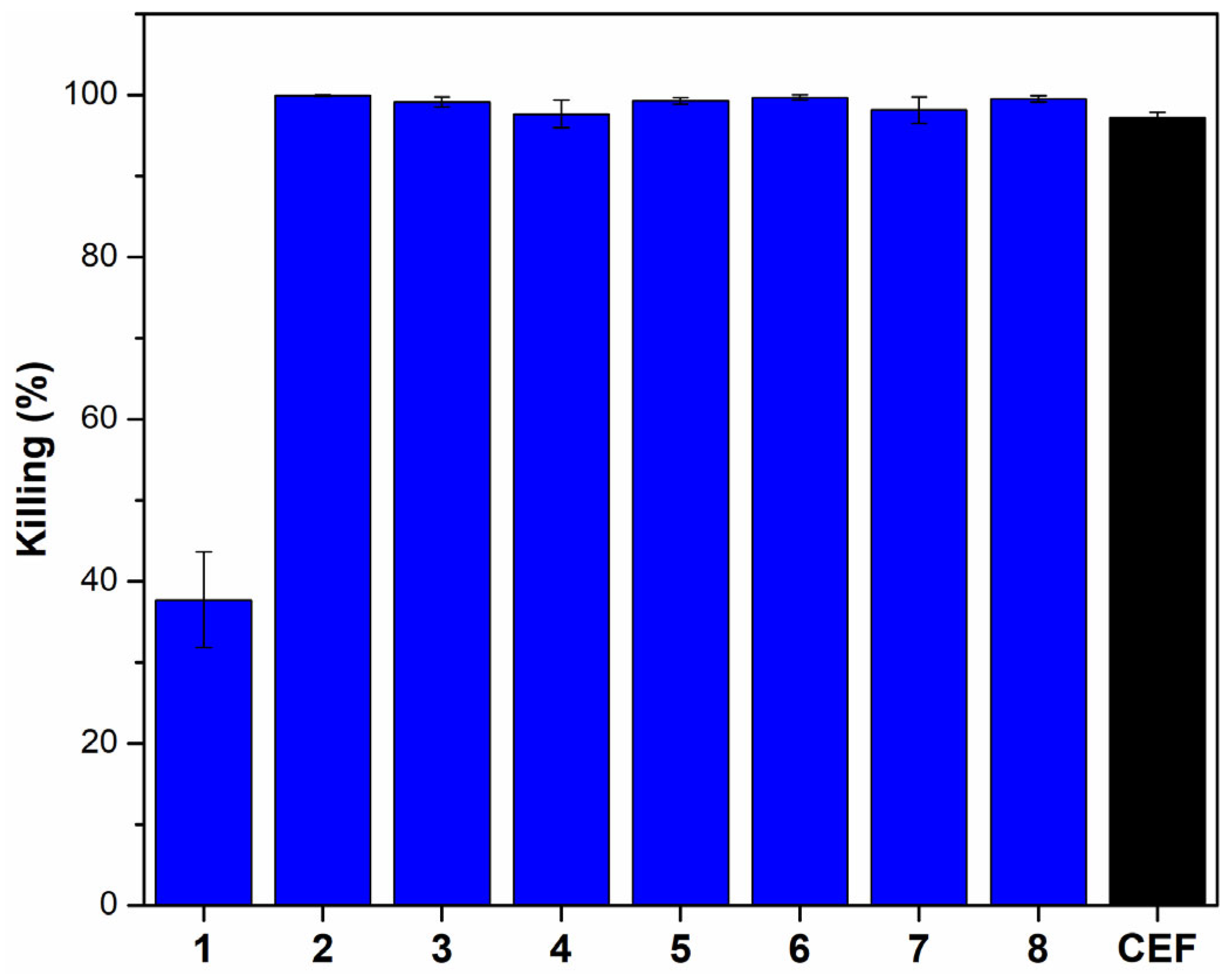
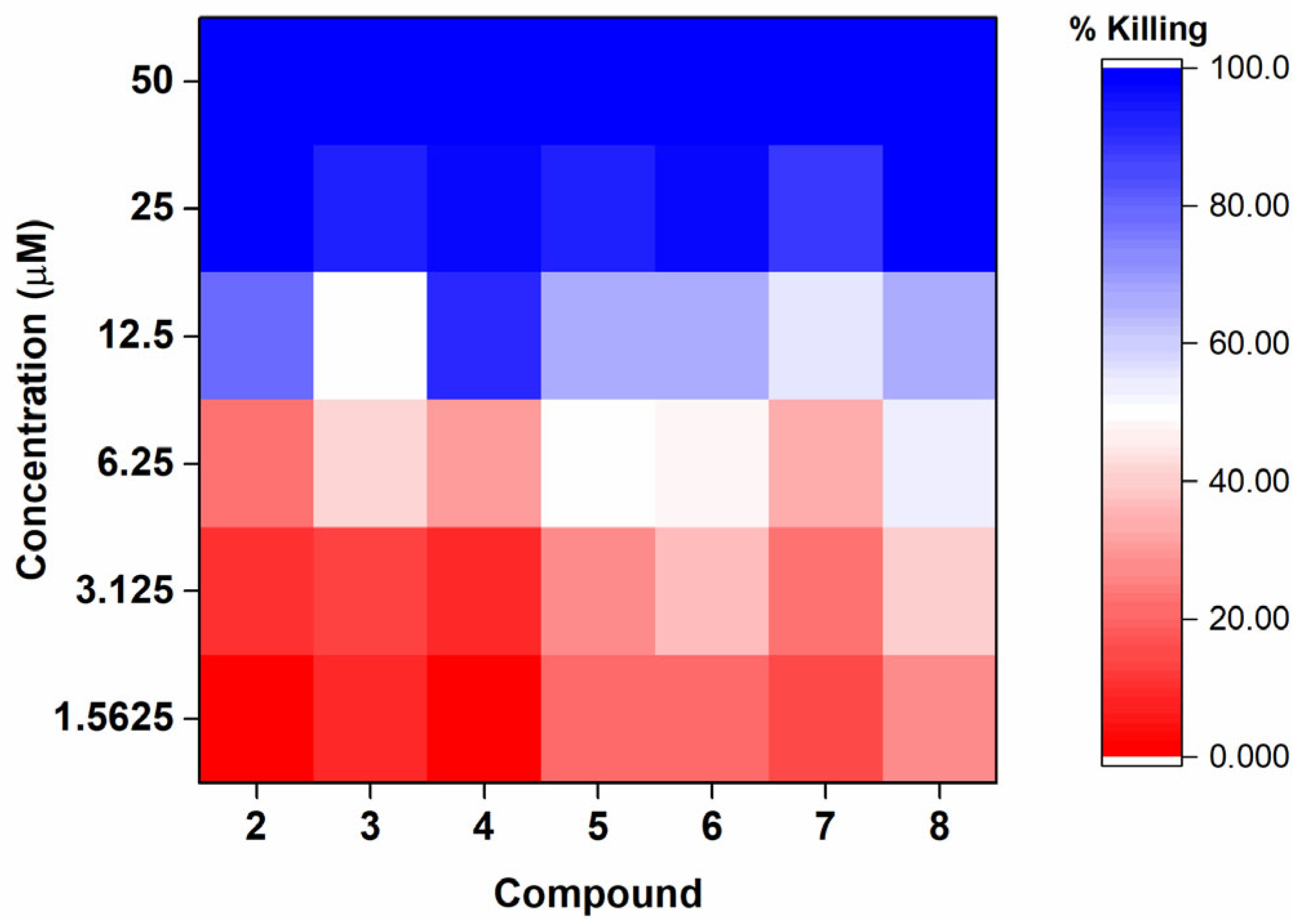
2.2.1. Assessment of Anti-Gonococcal Activity
2.2.2. Selected Compounds Tested Against Resistant N. gonorrhoeae
3. Materials and Methods
3.1. General Procedures
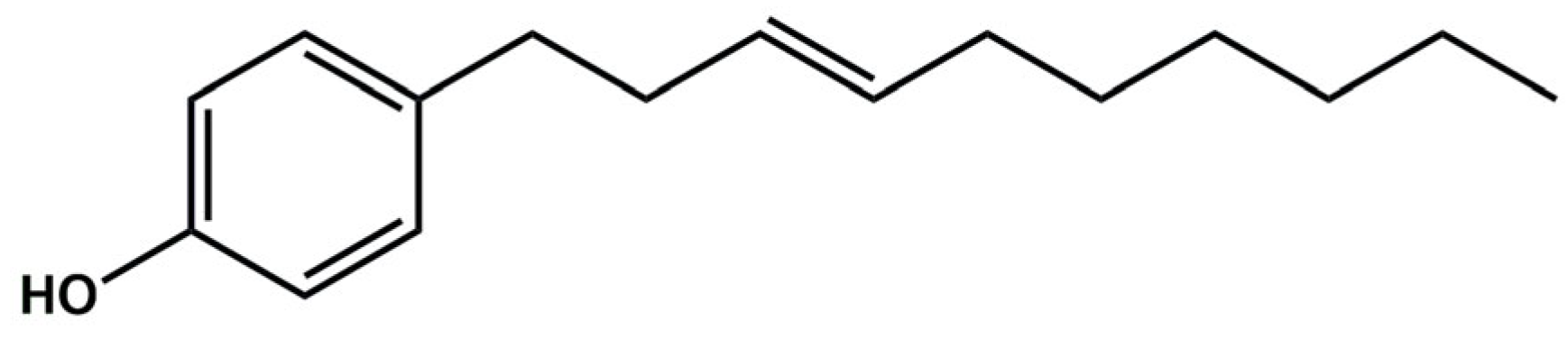
3.2. Isolation of Gibbilimbol B from Piper malacophyllum
3.3. Synthesis of Amides Inspired by Gibbilimbol B
3.3.1. Methoxy-N-Octylbenzamide (1)
3.3.2. Methyl-N-Octylbenzamide (2)
3.3.3. Fluoro-N-Octylbenzamide (3)
3.3.4. Chloro-N-Octylbenzamide (4)
3.3.5. (E)-4-Methoxy-N-(oct-4-en-1-yl)benzamide (5)
3.3.6. (E)-4-Methyl-N-(oct-4-en-1-yl)benzamide (6)
3.3.7. (E)-4-Fluoro-N-(oct-4-en-1-yl)benzamide (7)
3.3.8. (E)-4-Chloro-N-(oct-4-en-1-yl)benzamide (8)
3.4. Bactericidal Activity of Amides Towards Neisseria gonorrhoeae
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 19 February 2023).
- Raccagni, A.R.; Ranzenigo, M.; Bruzzesi, E.; Maci, C.; Castagna, A.; Nozza, S. Antimicrobial Resistance: The Future of Antibiotic Therapy. J. Clin. Med. 2023, 12, 7767. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.A.; Shafer, W.M.; Ram, S.; Jerse, A.E. Neisseria gonorrhoeae: Drug Resistance, Mouse Models, and Vaccine Development. Annu. Rev. Microbiol. 2017, 71, 665–686. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.W.; Sanderson, N.D.; Lord, E.; Regisford-Reimmer, N.; Chau, K.; Barker, L.; Morgan, M.; Newnham, R.; Golparian, D.; Unemo, M.; et al. Gonorrhoea Treatment Failure Caused by a Neisseria gonorrhoeae Strain with Combined Ceftriaxone and High-Level Azithromycin Resistance, England, February 2018. Eurosurveillance 2018, 23, 1800323. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the Sustainable Discovery and Development of New Antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the Role of Plant Natural Products in Antibiotic Drug Discovery. Chem. Rev. 2020, 121, 3495–3560. [Google Scholar] [CrossRef]
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural Products as Platforms to Overcome Antibiotic Resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Williams, D.; Perry, D.; Carraway, J.; Simpson, S.; Uwamariya, P.; Christian, O.E. Antigonococcal Activity of (+)-Medicarpin. ACS Omega 2021, 6, 15274–15278. [Google Scholar] [CrossRef]
- Yarlagadda, V.; Rao, V.N.; Kaur, M.; Guitor, A.K.; Wright, G.D. A Screen of Natural Product Extracts Identifies Moenomycin as a Potent Antigonococcal Agent. ACS Infect. Dis. 2021, 7, 1569–1577. [Google Scholar] [CrossRef]
- Fu, C.W.; Chiang, L.; Chao, C.H.; Huang, Y.L.; Chiou, S.F.; Wang, L.C.; Chang, H.W.; Chen, S.L.; Wang, H.C.; Yu, M.C.; et al. Nakamusines A−C, New 9-Methyladeninium Diterpenoid Alkaloids from a Formosan Marine Sponge Agelas nakamurai. Tetrahedron 2023, 149, 133745. [Google Scholar] [CrossRef]
- Vică, M.L.; Glevitzky, I.; Glevitzky, M.; Siserman, C.V.; Matei, H.V.; Teodoru, C.A. Antibacterial Activity of Propolis Extracts from the Central Region of Romania against Neisseria gonorrhoeae. Antibiotics 2021, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.; Mesquita, J.T.; Tempone, A.G.; Lago, J.H.G.; Guimarães, E.F.; Kato, M.J. Leishmanicidal Activity of an Alkenylphenol from Piper malacophyllum Is Related to Plasma Membrane Disruption. Exp. Parasitol. 2012, 132, 383–387. [Google Scholar] [CrossRef]
- Muñoz, D.; Brucoli, M.; Zecchini, S.; Sandoval-Hernandez, A.; Arboleda, G.; Lopez-Vallejo, F.; Delgado, W.; Giovarelli, M.; Coazzoli, M.; Catalani, E.; et al. XIAP as a Target of New Small Organic Natural Molecules Inducing Human Cancer Cell Death. Cancers 2019, 11, 1336. [Google Scholar] [CrossRef]
- Muñoz, D.R.; Sandoval-hernandez, A.G.; Delgado, W.A.; Gonzalo, H.; Cuca, L.E. In Vitro Anticancer Screening of Colombian Plants from Piper Genus (Piperaceae). J. Pharm. Phytother. 2018, 10, 174–181. [Google Scholar] [CrossRef]
- Guzman, J.D.; Gupta, A.; Evangelopoulos, D.; Basavannacharya, C.; Pabon, L.C.; Plazas, E.A.; Muñoz, D.R.; Delgado, W.A.; Cuca, L.E.; Ribon, W.; et al. Anti-Tubercular Screening of Natural Products from Colombian Plants: 3-Methoxynordomesticine, an Inhibitor of MurE Ligase of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2010, 65, 2101–2107. [Google Scholar] [CrossRef]
- Orjala, J.; Mian, P.; Rali, T.; Sticher, O. Gibbilimbols A-D, Cytotoxic and Antibacterial Alkenylphenols from Piper gibbilimbum. J. Nat. Prod. 1998, 61, 939–941. [Google Scholar] [CrossRef]
- Vyvyan, J.R.; Holst, C.L.; Johnson, A.J.; Schwenk, C.M. Total Synthesis of Gibbilimbols A-D. J. Org. Chem. 2002, 67, 2263–2265. [Google Scholar] [CrossRef]
- Varela, M.T.; Dias, R.Z.; Martins, L.F.; Ferreira, D.D.; Tempone, A.G.; Ueno, A.K.; Lago, J.H.G.; Fernandes, J.P.S. Gibbilimbol Analogues as Antiparasitic Agents—Synthesis and Biological Activity against Trypanosoma cruzi and Leishmania (L.) Infantum. Bioorg. Med. Chem. Lett. 2016, 26, 1180–1183. [Google Scholar] [CrossRef]
- Leão, L.P.M.O.; de B. Vieira, N.; Oliveira, P.P.S.; Chagas-Paula, D.A.; Soares, M.G.; Souza, T.B.; Baldim, J.L.; Costa-Silva, T.A.; Tempone, A.G.; Dias, D.F.; et al. Structure-Activity Relationship Study of Antitrypanosomal Analogues of Gibbilimbol B Using Multivariate Analysis and Computation-Aided Drug Design. Bioorg. Med. Chem. Lett. 2023, 83, 129190. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, F.N.; Amaral, M.; Romanelli, M.M.; de Castro Levatti, E.V.; Ramos, F.F.; Leão, L.P.M.O.; Chagas-Paula, D.A.; Soares, M.G.; Ferreira Dias, D.F.; Aranha, C.M.S.Q.; et al. Toward New Therapeutics for Visceral Leishmaniasis: Efficacy and Mechanism of Action of Amides Inspired by Gibbilimbol, B. ACS Omega 2024, 9, 44385–44395. [Google Scholar] [CrossRef]
- Lucío, M.I.; Kyriazi, M.E.; Hamilton, J.; Batista, D.; Sheppard, A.; Sams-Dodd, E.; Humbert, M.V.; Hussain, I.; Christodoulides, M.; Kanaras, A.G.; et al. Bactericidal Effect of 5-Mercapto-2-Nitrobenzoic Acid-Coated Silver Nanoclusters against Multidrug-Resistant Neisseria gonorrhoeae. ACS Appl. Mater. Interfaces 2020, 12, 27994–28003. [Google Scholar] [CrossRef]
- Santana, B.d.M.; Armentano, G.M.; Ferreira, D.A.S.; de Freitas, C.S.; Carneiro-Ramos, M.S.; Seabra, A.B.; Christodoulides, M. In Vitro Bactericidal Activity of Biogenic Copper Oxide Nanoparticles for Neisseria gonorrhoeae with Enhanced Compatibility for Human Cells. ACS Appl. Mater. Interfaces 2024, 16, 21633–21642. [Google Scholar] [CrossRef]
- Kubo, I.; Nihei, K.I.; Tsujimoto, K. Antibacterial Action of Anacardic Acids against Methicillin Resistant Staphylococcus aureus (MRSA). J. Agric. Food Chem. 2003, 51, 7624–7628. [Google Scholar] [CrossRef]
- Seidel, V.; Taylor, P.W. In Vitro Activity of Extracts and Constituents of Pelagonium against Rapidly Growing Mycobacteria. Int. J. Antimicrob. Agents 2004, 23, 613–619. [Google Scholar] [CrossRef]
- Mcgaw, L.J.; Jäger, A.K.; Van Staden, J. Antibacterial Effects of Fatty Acids and Related Compounds from Plants. S. Afr. J. Bot. 2002, 68, 417–423. [Google Scholar] [CrossRef]
- Uppu, D.S.S.M.; Bhowmik, M.; Samaddar, S.; Haldar, J. Cyclization and Unsaturation Rather than Isomerisation of Side Chains Govern the Selective Antibacterial Activity of Cationic-Amphiphilic Polymers. Chem. Commun. 2016, 52, 4644–4647. [Google Scholar] [CrossRef]
- Wickramasingha, W.G.D.; Jayasinghe, S.; Karunaratne, D.N.; Ekanayake, E.W.M.A.; Liyanapathirana, V.; Senadeera, S.P.D.; Karunaratne, V. Design and Synthesis of Novel Derivatives of 6β-Hydroxy Betunolic Acid as Antibacterial Agents. Tetrahedron 2022, 128, 133125. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial Fatty Acids: An Update of Possible Mechanisms of Action and Implications in the Development of the next-Generation of Antibacterial Agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef] [PubMed]
- Camelia Nuţă, D.; Chifiriuc, M.C.; Drăghici, C.; Limban, C.; Vasile Missir, A.; Moruşciag, L. Synthesis, Characterization and Antimicrobial Activity Evaluation of New Agents from Benzamides Class. Farmacia 2013, 61, 966–974. [Google Scholar]
- Farhan, N.; Rageh Al-Maleki, A.; Ataei, S.; Muhamad Sarih, N.; Yahya, R. Synthesis, DFT Study, Theoretical and Experimental Spectroscopy of Fatty Amides Based on Extra-Virgin Olive Oil and Their Antibacterial Activity. Bioorg. Chem. 2023, 135, 106511. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.; Tilford, H.; Freitas, C.S.; Ferreira, D.A.S.; Ng, J.; Rucinski, G.; Watkins, J.; Pemberton, R.; Abramyan, T.M.; Contreras, S.C.; et al. Antimicrobial Activity of Compounds Identified by Artificial Intelligence Discovery Engine Targeting Enzymes Involved in Neisseria gonorrhoeae Peptidoglycan Metabolism. Biol. Res. 2024, 57, 62. [Google Scholar] [CrossRef]
- Unemo, M.; Lahra, M.M.; Escher, M.; Eremin, S.; Cole, M.J.; Galarza, P.; Ndowa, F.; Martin, I.; Dillon, J.A.R.; Galas, M.; et al. WHO Global Antimicrobial Resistance Surveillance for Neisseria gonorrhoeae 2017–18: A Retrospective Observational Study. Lancet Microbe 2021, 2, e627–e636. [Google Scholar] [CrossRef]
- Ward, M.E.; Watt, P.J.; Glynn, A.A. Gonococci in Urethral Exudates Possess a Virulence Factor Lost on Subculture. Nature 1970, 227, 382–384. [Google Scholar] [CrossRef]
| Compound | MBC50 (µM) | MBC90 (µM) |
|---|---|---|
| 1 | NA | NA |
| 2 | 12.5 | 25.0 (12.5, 25.0) |
| 3 | 12.5 | 25.0 |
| 4 | 12.5 | 12.5 |
| 5 | 12.5 (6.25, 12.5) | 25.0 |
| 6 | 12.5 (6.25, 12.5) | 25.0 |
| 7 | 12.5 (6.25, 25) | 25.0 |
| 8 | 6.25 (6.25, 12.5) | 25.0 |
| Gibbilimbol B | 25.0 | 50.0 |
| Ceftriaxone | 2.0 | 10.0 |
| N. gonorrhoeae Strains (AR Bank no) | Compounds | |||||
|---|---|---|---|---|---|---|
| 5 | 6 | 8 | ||||
| 6.25 µM | 25 µM | 6.25 µM | 25 µM | 6.25 µM | 25 µM | |
| 173 | 57.0 ± 14.1% | 90.5 ± 7.7% | 51.3 ± 3.0% | 94.1 ± 5.9% | 76.4 ± 14.3% | 96.9 ± 2.9% |
| 174 | 44.6 ± 3.0% | 85.9 ± 0.5% | 44.7 ± 8.2% | 93.2 ± 0.1% | 65.0 ± 13.9% | 88.9 ± 2.7% |
| 187 | 57.9 ± 9.1% | 80.6 ± 6.0% | 53.8 ± 1.1% | 88.6 ± 5.8% | 72.8 ± 3.5% | 85.9 ± 3.7% |
| 200 | 47.3 ± 2.2% | 84.7 ± 4.6% | 70.2 ± 16.5% | 83.9 ± 7.5% | 72.2 ± 15.2% | 87.0 ± 1.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, L.V.F.; Tempone, A.G.; Christodoulides, M.; Lago, J.H.G. Bactericidal Effect of Synthetic Phenylalkylamides Inspired by Gibbilimbol B Against Neisseria gonorrhoeae. Molecules 2025, 30, 2406. https://doi.org/10.3390/molecules30112406
Oliveira LVF, Tempone AG, Christodoulides M, Lago JHG. Bactericidal Effect of Synthetic Phenylalkylamides Inspired by Gibbilimbol B Against Neisseria gonorrhoeae. Molecules. 2025; 30(11):2406. https://doi.org/10.3390/molecules30112406
Chicago/Turabian StyleOliveira, Larissa V. F., Andre G. Tempone, Myron Christodoulides, and Joao Henrique G. Lago. 2025. "Bactericidal Effect of Synthetic Phenylalkylamides Inspired by Gibbilimbol B Against Neisseria gonorrhoeae" Molecules 30, no. 11: 2406. https://doi.org/10.3390/molecules30112406
APA StyleOliveira, L. V. F., Tempone, A. G., Christodoulides, M., & Lago, J. H. G. (2025). Bactericidal Effect of Synthetic Phenylalkylamides Inspired by Gibbilimbol B Against Neisseria gonorrhoeae. Molecules, 30(11), 2406. https://doi.org/10.3390/molecules30112406








