Morphology-Engineered NiMo Alloy on Nickel Foam for Enhanced Hydrogen Evolution Reaction Performance
Abstract
1. Introduction
2. Results and Discussion
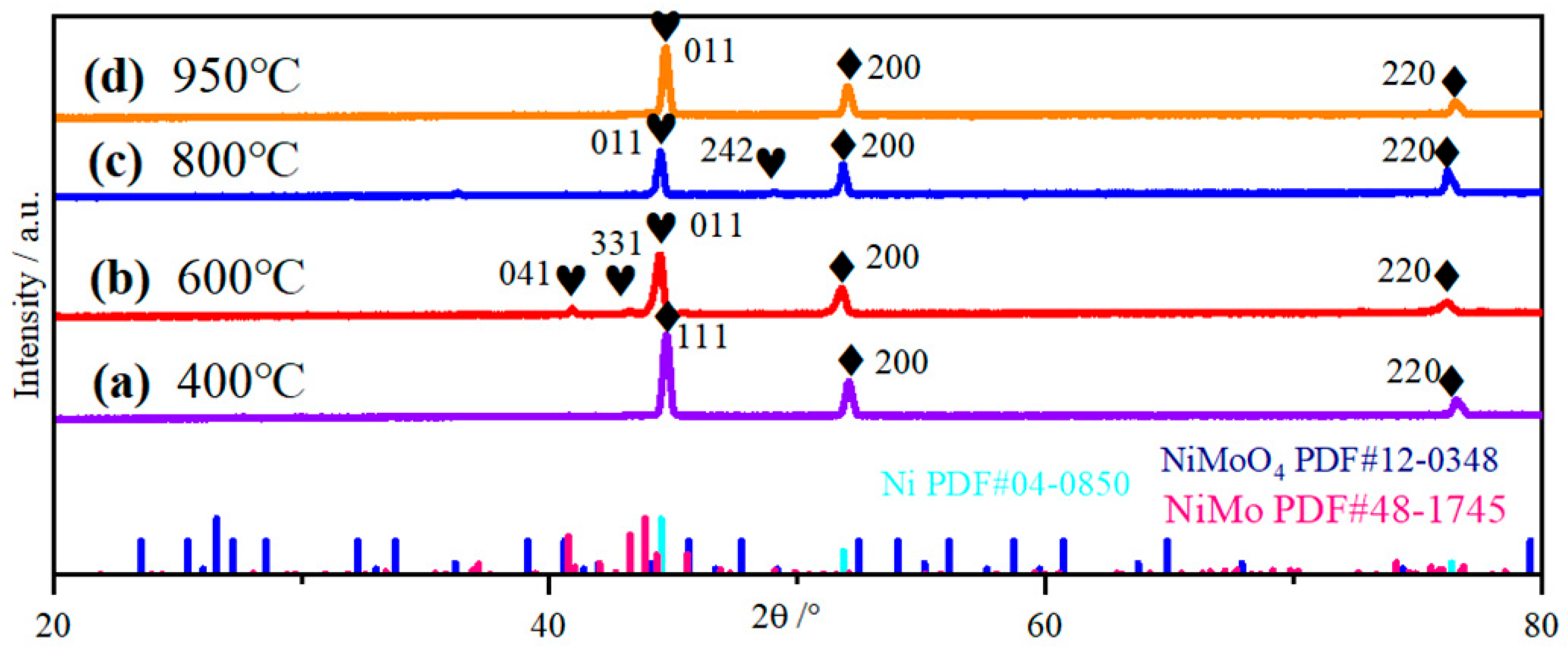
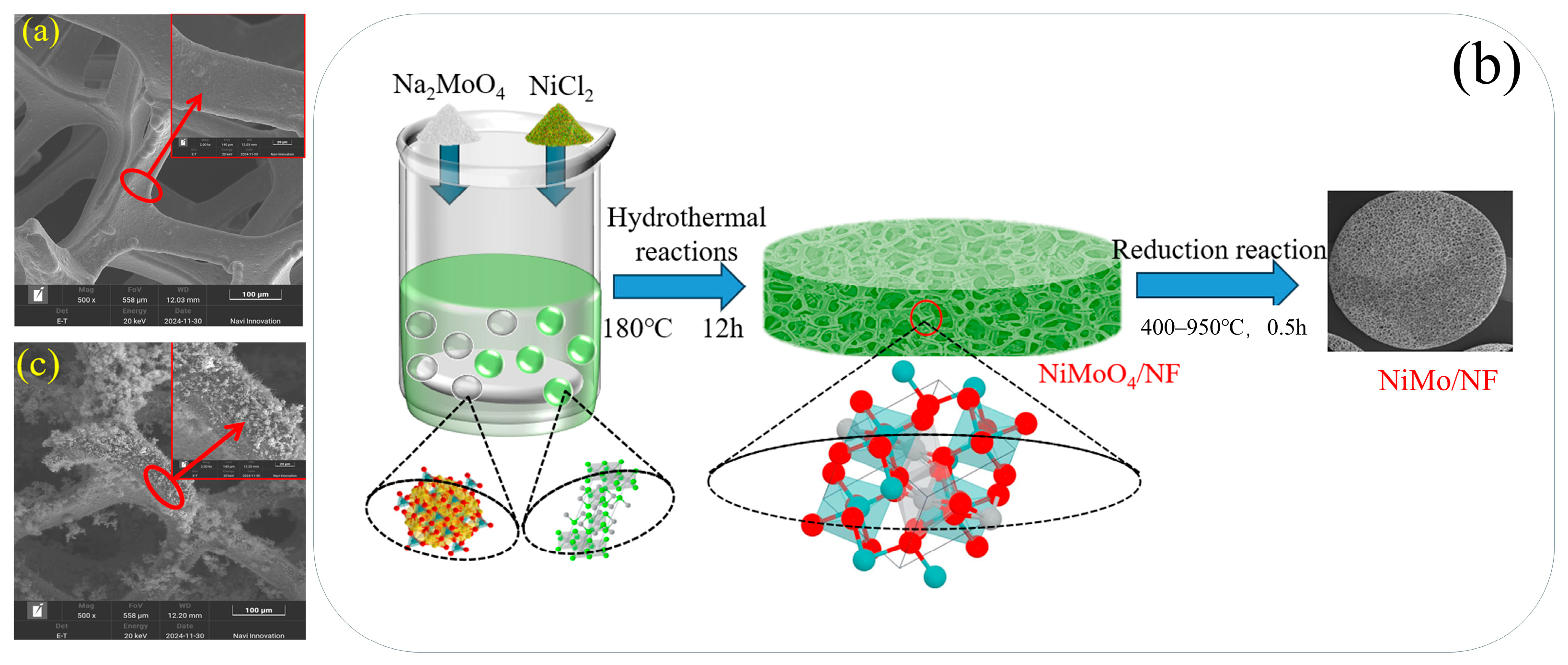
2.1. Structural Topography Analysis
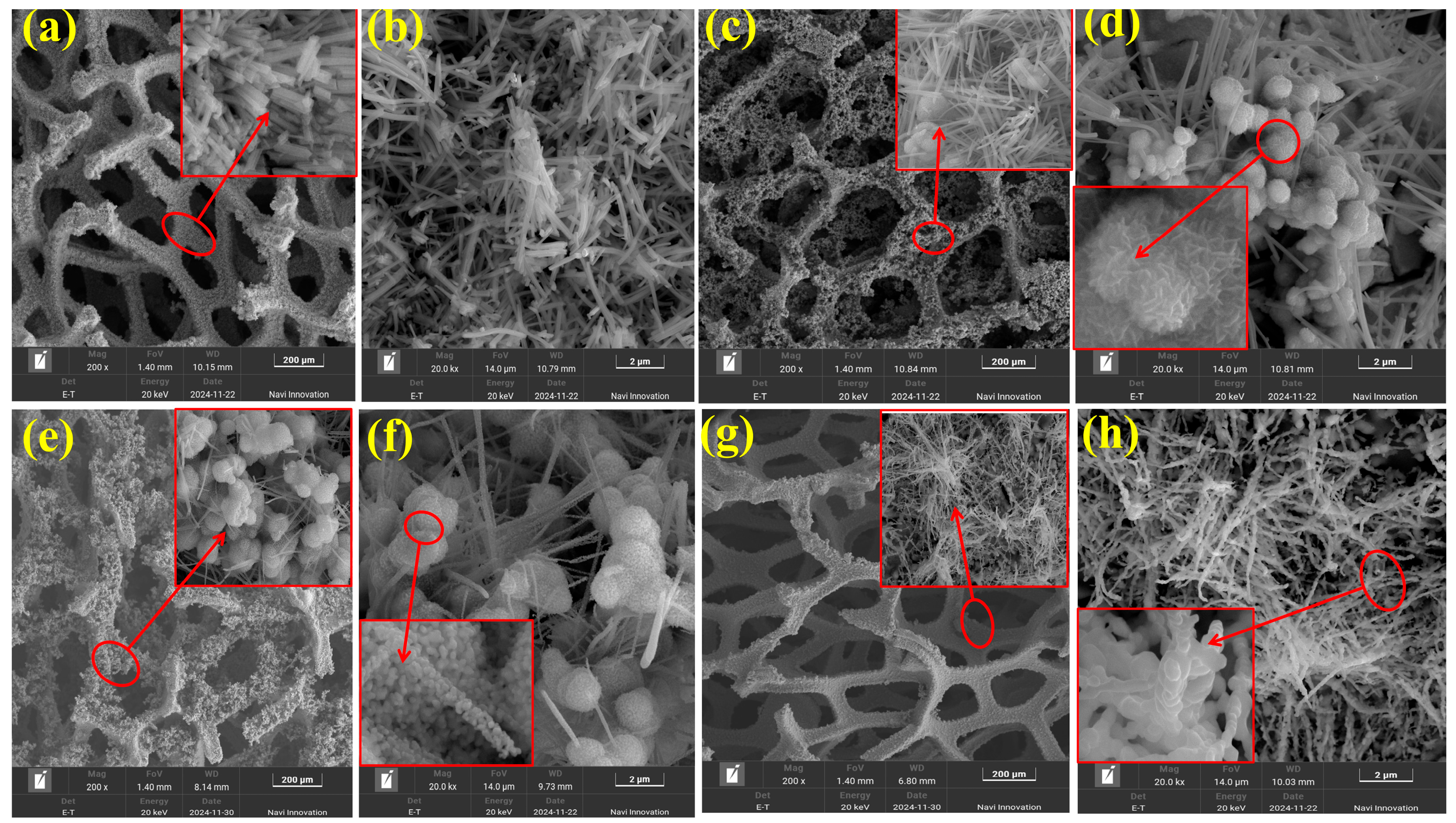
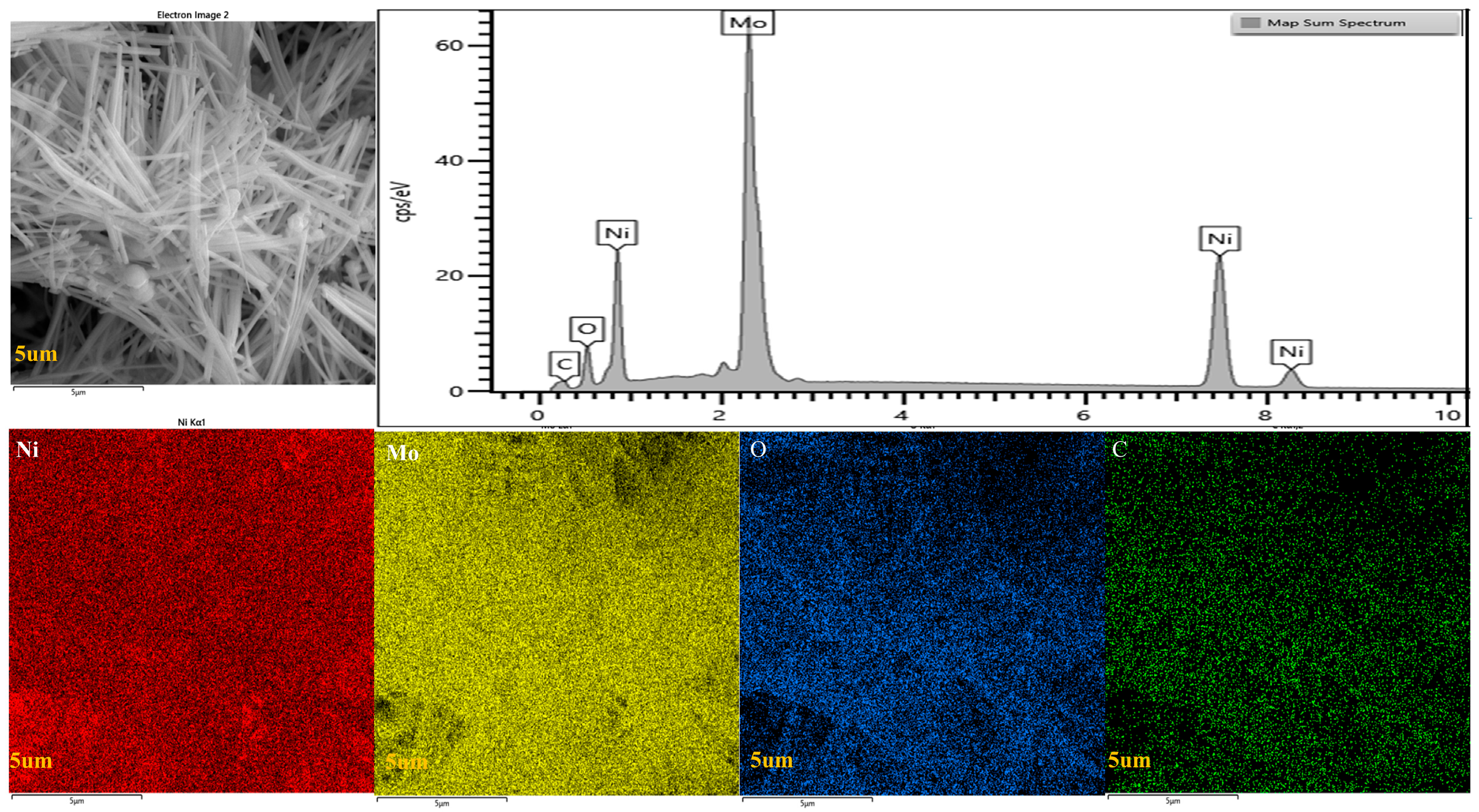
2.2. SEM Analysis
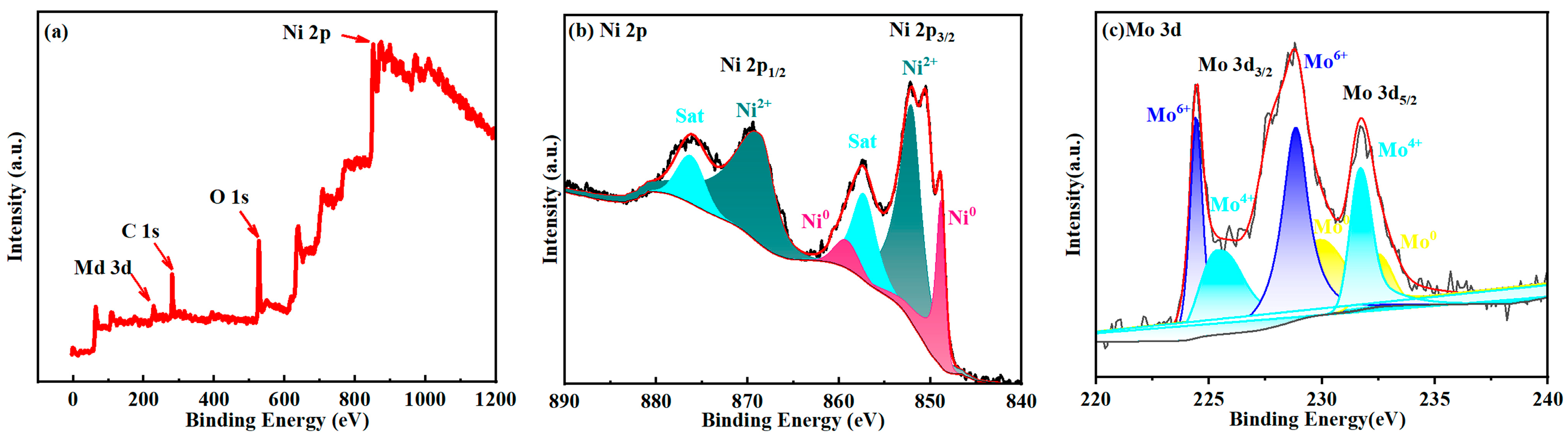
2.3. XPS Analysis
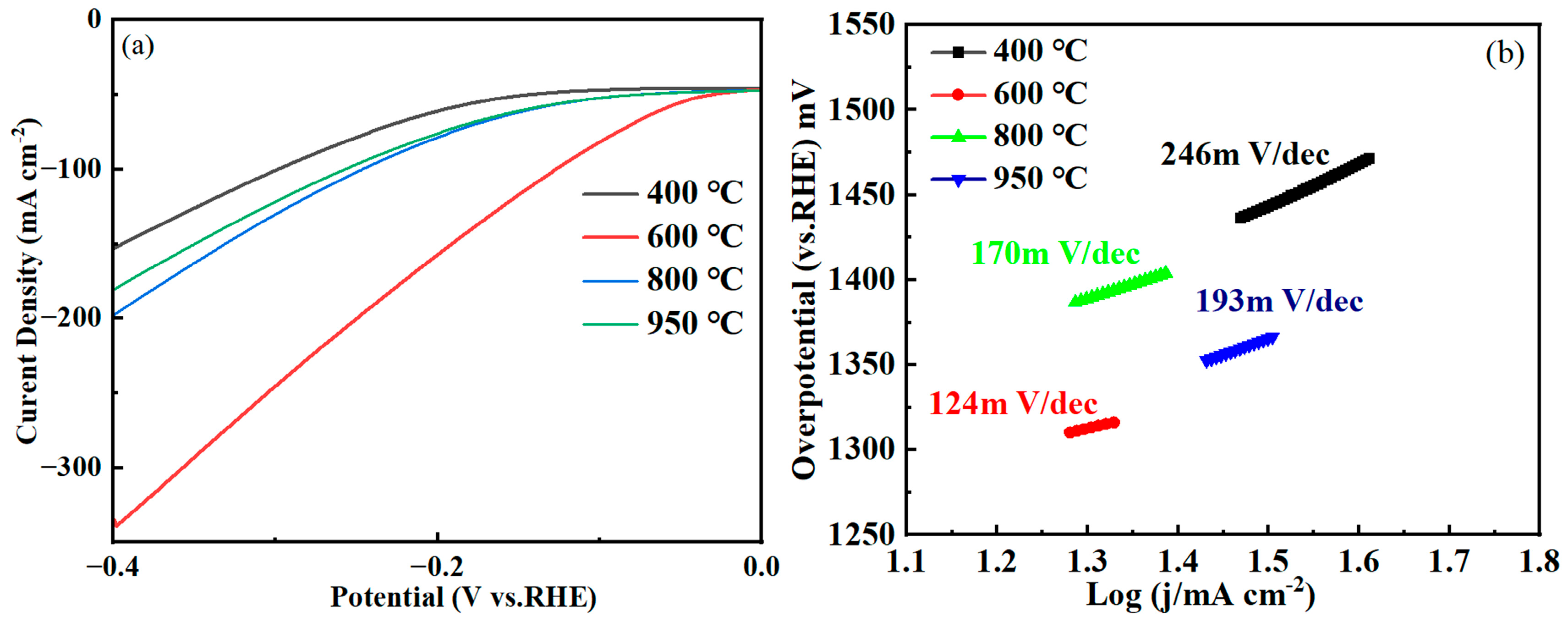
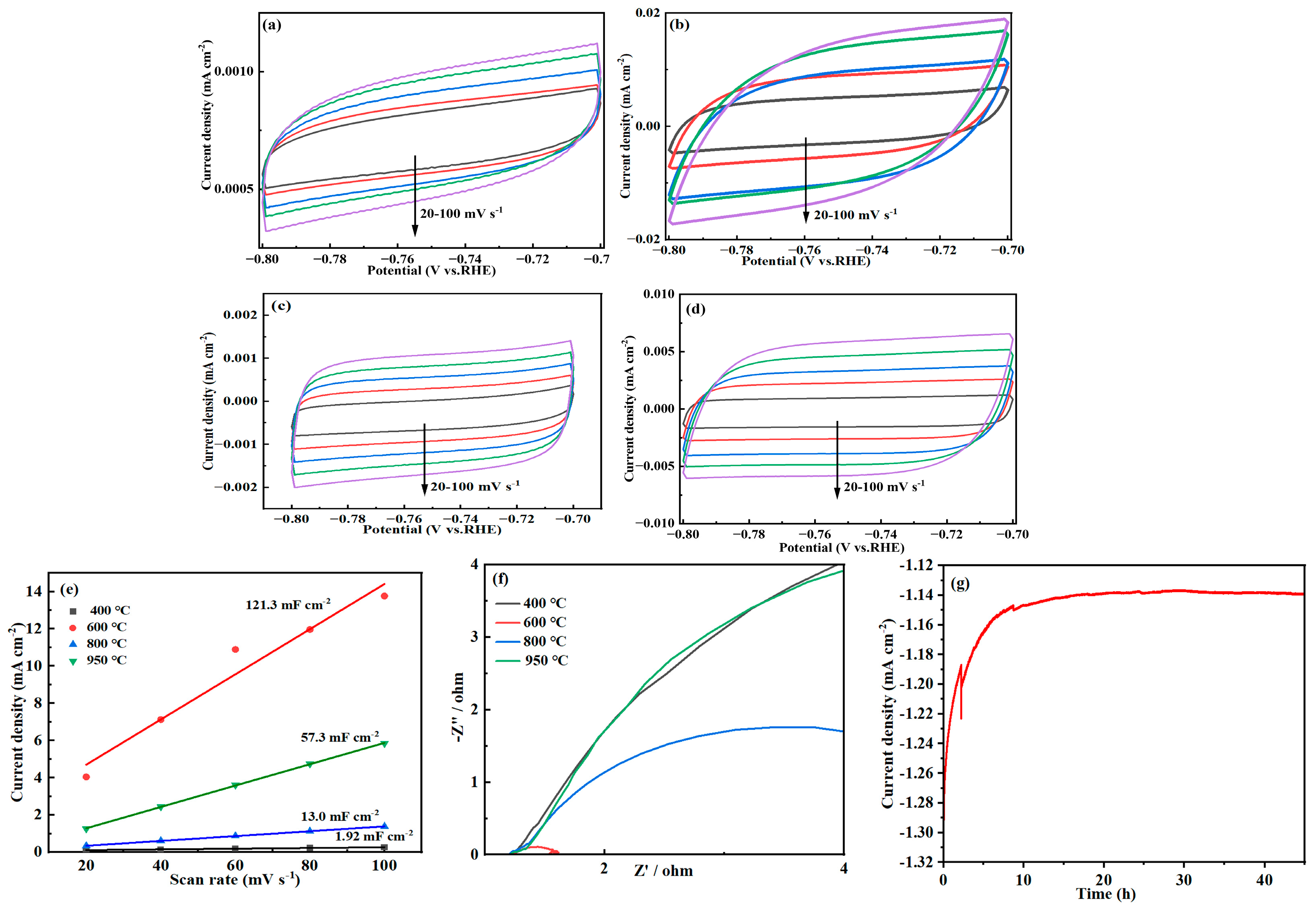
2.4. Electrochemical Performance Analysis
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sebbahi, S.; Assila, A.; Alaoui Belghiti, A.; Laasri, S.; Kaya, S.; Hlil, E.K.; Rachidi, S.; Hajjaji, A. A comprehensive review of recent advances in alkaline water electrolysis for hydrogen production. Int. J. Hydrogen Energy 2024, 82, 583–599. [Google Scholar] [CrossRef]
- Shaya, N.; Glöser-Chahoud, S.J.E. A Review of Life Cycle Assessment (LCA) Studies for Hydrogen Production Technologies through Water Electrolysis: Recent Advances. Energies 2024, 17, 3968. [Google Scholar] [CrossRef]
- Dash, S.; Singh, A.; Jose, S.; Elangovan, D.; Surapraraju, S.K.; Natarajan, S.K. Advances in green hydrogen production through alkaline water electrolysis: A comprehensive review. Int. J. Hydrogen Energy 2024, 83, 614–629. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, R.; Xu, Y.; Chen, W.; Hu, Z.; Xi, L.; Xie, Y.; Hou, H.; Liu, T.; Amine, K.; et al. Anion vacancies coupling with heterostructures enable advanced aerogel cathode for ultrafast aqueous zinc-ion storage. Adv. Mater. 2025, 37, 2419582. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Shao, Z.; Jung, W. Advanced electrocatalysts with unusual active sites for electrochemical water splitting. InfoMat 2024, 6, e12494. [Google Scholar] [CrossRef]
- Zhong, W.; Zhao, R.; Zhu, Y.; Xu, Y.; Chen, W.; Peng, C. Vacancy engineering on MnSe cathode enables high-rate and stable zinc-ion storage. Adv. Funct. Mater. 2025, 35, 2419720. [Google Scholar] [CrossRef]
- Tüysüz, H. Alkaline Water Electrolysis for Green Hydrogen Production. Acc. Chem. Res. 2024, 57, 558–567. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Lin, L.; Zhang, S. Analysis of Hydrogen Production Potential Based on Resources Situation in China. E3S Web Conf. 2019, 118, 03021. [Google Scholar] [CrossRef]
- Ham, K.; Bae, S.; Lee, J. Classification and technical target of water electrolysis for hydrogen production. J. Energy Chem. 2024, 95, 554–576. [Google Scholar] [CrossRef]
- Guo, Y.; Li, G.; Zhou, J.; Liu, Y. Comparison between hydrogen production by alkaline water electrolysis and hydrogen production by PEM electrolysis. IOP Conf. Ser. Earth Environ. Sci. 2019, 371, 042022. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Hui, Y.; Wang, M.; Guo, S.; Akhtar, S.; Bhattacharya, S.; Dai, B.; Yu, J. Comprehensive review of development and applications of hydrogen energy technologies in China for carbon neutrality: Technology advances and challenges. Energy Convers. Manag. 2024, 315, 118776. [Google Scholar] [CrossRef]
- Yu, J.; Le, T.A.; Tran, N.Q.; Lee, H. Frontispiece: Earth-Abundant Transition-Metal-Based Bifunctional Electrocatalysts for Overall Water Splitting in Alkaline Media. Chem. A Eur. J. 2020, 26, 6423–6436. [Google Scholar] [CrossRef]
- Kempler, P.A.; Coridan, R.H.; Luo, L. Gas Evolution in Water Electrolysis. Chem. Rev. 2024, 124, 10964–11007. [Google Scholar] [CrossRef]
- Lei, Q.; Wang, B.; Wang, P.; Liu, S. Hydrogen generation with acid/alkaline amphoteric water electrolysis. J. Energy Chem. 2019, 38, 162–169. [Google Scholar] [CrossRef]
- Bayro-Kaiser, V.; Nelson, N. Microalgal hydrogen production: Prospects of an essential technology for a clean and sustainable energy economy. Photosynth. Res. 2017, 133, 49–62. [Google Scholar] [CrossRef]
- Ju, H.; Badwal, S.; Giddey, S. A comprehensive review of carbon and hydrocarbon assisted water electrolysis for hydrogen production. Appl. Energy 2018, 231, 502–533. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Miller, E.L.; Randolph, K.; Peterson, D.; Rustagi, N.; Vickers, J.W.; Lyubovsky, M.; Cierpik-Gold, K.; Byham, S. (Invited) Addressing Fundamental Materials Challenges in Electrochemical Water Splitting Technologies for Sustainable Hydrogen Production. ECS Meet. Abstr. 2018, MA2018-02, 1595. [Google Scholar] [CrossRef]
- Bonifacio, R.M.; Mena, M.G. Activity of electrodeposited rhodium in acidic and basic water electrolysis. Int. J. Hydrogen Energy 2024, 52, 364–377. [Google Scholar] [CrossRef]
- Ahn, S.H.; Choi, I.; Park, H.-Y.; Hwang, S.J.; Yoo, S.J.; Cho, E.; Kim, H.-J.; Henkensmeier, D.; Nam, S.W.; Kim, S.-K.; et al. Effect of morphology of electrodeposited Ni catalysts on the behavior of bubbles generated during the oxygen evolution reaction in alkaline water electrolysis. Chem. Commun. 2013, 49, 9323–9325. [Google Scholar] [CrossRef]
- Hu, B.; Xu, L.; Li, Y.; Sun, F.; Wang, Z.; Yang, M.; Zhang, Y.; Kong, W.; Shen, B.; Wang, X.; et al. Biochar and Fe2+ mediation in hydrogen production by water electrolysis: Effects of physicochemical properties of biochars. Energy 2024, 297, 131275. [Google Scholar] [CrossRef]
- Yadav, V.; Dileep, N.P.; Nair, N.; Kumar Behura, P.; Shaijumon, M.M. Hydrogen production via alkaline seawater electrolysis using iron-doped nickel diselenide as an efficient bifunctional electrocatalyst. Mater. Today Chem. 2024, 40, 102276. [Google Scholar] [CrossRef]
- Wu, W.; Wu, X.-Y.; Wang, S.-S.; Lu, C.-Z. Highly efficient hydrogen evolution from water electrolysis using nanocrystalline transition metal phosphide catalysts. RSC Adv. 2018, 8, 39291–39295. [Google Scholar] [CrossRef]
- Pein, M.; Neumann, N.C.; Venstrom, L.J.; Vieten, J.; Roeb, M.; Sattler, C. Two-step thermochemical electrolysis: An approach for green hydrogen production. Int. J. Hydrogen Energy 2021, 46, 24909–24918. [Google Scholar] [CrossRef]
- Shah, A.M.; Modi, K.H.; Pataniya, P.M.; Joseph, K.S.; Dabhi, S.; Bhadu, G.R.; Sumesh, C.K. Self-Supported Mn-Ni3Se2 Electrocatalysts for Water and Urea Electrolysis for Energy-Saving Hydrogen Production. ACS Appl. Mater. Interfaces 2024, 16, 11440–11452. [Google Scholar] [CrossRef]
- Wang, N.; Otor, H.O.; Rivera-Castro, G.; Hicks, J.C. Plasma Catalysis for Hydrogen Production: A Bright Future for Decarbonization. ACS Catal. 2024, 14, 6749–6798. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, F.; Zaffora, A.; Pupillo, D.; Seminara, B.; Pärnamäe, R.; Tedesco, M.; Santamaria, M. Optimized base metals electrodeposition on Ni perforated plate type electrodes for high-performance alkaline water electrolysis. Int. J. Hydrogen Energy 2024, 70, 548–556. [Google Scholar] [CrossRef]
- Yao, H.; Wang, S.; Cao, Y.; Chen, R.; Lu, Z.; Hu, J.; Xie, J.; Hao, A. High-performance bifunctional electrocatalysts of CoFe-LDH/NiCo2O4 heterostructure supported on nickel foam for effective overall water splitting. J. Alloys Compd. 2022, 926, 166846. [Google Scholar] [CrossRef]
- Deng, B.; Zhou, L.; Jiang, Z.; Jiang, Z.-J. High catalytic performance of nickel foam supported Co2P-Ni2P for overall water splitting and its structural evolutions during hydrogen/oxygen evolution reactions in alkaline solutions. J. Catal. 2019, 373, 81–92. [Google Scholar] [CrossRef]
- Chen, M.-T.; Duan, J.-J.; Feng, J.-J.; Mei, L.-P.; Jiao, Y.; Zhang, L.; Wang, A.-J. Iron, rhodium-codoped Ni2P nanosheets arrays supported on nickel foam as an efficient bifunctional electrocatalyst for overall water splitting. J. Colloid Interface Sci. 2022, 605, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lin, T.; Luo, X.; Yan, Z.; Wu, J.; Wang, J.; Shen, Y. Mechanistic study on nickel-molybdenum based electrocatalysts for the hydrogen evolution reaction. J. Catal. 2020, 388, 122–129. [Google Scholar] [CrossRef]
- Sivanantham, A.; Shanmugam, S. Nickel selenide supported on nickel foam as an efficient and durable non-precious electrocatalyst for the alkaline water electrolysis. Appl. Catal. B Environ. 2017, 203, 485–493. [Google Scholar] [CrossRef]
- Deng, B.L.; Guo, L.P.; Lu, Y.; Rong, H.B.; Chen, D.C. Sulfur–nitrogen co-doped graphene supported cobalt–nickel sulfide rGO@SN-CoNi2S4 as highly efficient bifunctional catalysts for hydrogen/oxygen evolution reactions. Rare Met. 2022, 41, 911–920. [Google Scholar] [CrossRef]
- Zhao, X.; Che, M.; Gong, Y. Synthesis and performance study of Fe2WO6@Ni3S2-WS2/NF for efficient electrolytic water to hydrogen. Sustain. Mater. Technol. 2025, 43, e01302. [Google Scholar] [CrossRef]
- Nady, H.; El-Rabiei, M.M.; Samy, M.; Deyab, M.A.; Abd El-Hafez, G.M. Novel Ni–Cr-based alloys as hydrogen fuel sources through alkaline water electrolytes. Int. J. Hydrogen Energy 2021, 46, 34749–34766. [Google Scholar] [CrossRef]
- Ren, T.; Chen, Q.; Tang, C.; Chen, J.; Huang, X.; Feng, G.; Xie, H.; Bao, F.; Guo, W. Facile electrodeposition of Iron-doped NiMo alloys as bifunctional electrocatalysts for alkaline overall water splitting. Fuel 2025, 381, 133302. [Google Scholar] [CrossRef]
- Wang, Y.; He, F.; Ren, Y.; Lin, Y.; Zhong, M.; Su, B.; Lei, Z. Straightforward preparation of nickel selenide nanosheets supported on nickel foam as a highly efficient electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 25631–25637. [Google Scholar] [CrossRef]
- Xiao, R.; Wang, F.; Luo, L.; Yao, X.; Huang, Y.; Wang, Z.; Balogun, M.S. Efficient Self-Powered Overall Water Splitting by Ni4Mo/MoO2 Heterogeneous Nanorods Trifunctional Electrocatalysts. Small Methods 2023, 7, 2201659. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Martínez, J.I.; García-Lastra, J.M.; Abad, E.; Koper, M.T.M. Oxygen reduction and evolution at single-metal active sites: Comparison between functionalized graphitic materials and protoporphyrins. Surf. Sci. 2013, 607, 47–53. [Google Scholar] [CrossRef]
- Gao, Z.-W.; Liu, J.-Y.; Chen, X.-M.; Zheng, X.-L.; Mao, J.; Liu, H.; Ma, T.; Li, L.; Wang, W.-C.; Du, X.-W. Engineering NiO/NiFe LDH Intersection to Bypass Scaling Relationship for Oxygen Evolution Reaction via Dynamic Tridimensional Adsorption of Intermediates. Adv. Mater. 2019, 31, 1804769. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Bai, J.; Lau, W.-M. Efficient Self-Supported Bifunctional NiMo Alloy Electrocatalysts for Water Splitting in Alkaline Environment. ChemistrySelect 2022, 7, e202200468. [Google Scholar] [CrossRef]
- Du, W.; Shi, Y.; Zhou, W.; Yu, Y.; Zhang, B. Unveiling the In Situ Dissolution and Polymerization of Mo in Ni4Mo Alloy for Promoting the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2021, 60, 7051–7055. [Google Scholar] [CrossRef]
- Sun, S.; Diao, P.; Feng, C.; Ungureanu, E.-M.; Tang, Y.; Hu, B.; Hu, Q. Nickel-foam-supported β-Ni(OH)2 as a green anodic catalyst for energy efficient electrooxidative degradation of azo-dye wastewater. RSC Adv. 2018, 8, 19776–19785. [Google Scholar] [CrossRef]
- Chen, N.; Mo, Q.; He, L.; Huang, X.; Yang, L.; Zeng, J.; Gao, Q. Heterostructured MoC-MoP/N-doped carbon nanofibers as efficient electrocatalysts for hydrogen evolution reaction. Electrochim. Acta 2019, 299, 708–716. [Google Scholar] [CrossRef]
- Tang, J.; Guan, D.; Xu, H.; Zhao, L.; Arshad, U.; Fang, Z.; Zhu, T.; Kim, M.; Pao, C.-W.; Hu, Z.; et al. Undoped ruthenium oxide as a stable catalyst for the acidic oxygen evolution reaction. Nat. Commun. 2025, 16, 801. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pan, Y.; Zhong, Y.; Ge, L.; Jiang, S.P.; Shao, Z. From scheelite BaMoO4 to perovskite BaMoO3: Enhanced electrocatalysis toward the hydrogen evolution in alkaline media. Compos. Part B Eng. 2020, 198, 108214. [Google Scholar] [CrossRef]
- McKone, J.R.; Sadtler, B.F.; Werlang, C.A.; Lewis, N.S.; Gray, H.B. Ni–Mo Nanopowders for Efficient Electrochemical Hydrogen Evolution. ACS Catal. 2013, 3, 166–169. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, B.; Xu, K.; Xia, X.; Zhang, Z.; Rawat, R.S.; Fan, H.J. Prereduction of Metal Oxides via Carbon Plasma Treatment for Efficient and Stable Electrocatalytic Hydrogen Evolution. Small 2018, 14, 1800340. [Google Scholar] [CrossRef]
- Fang, M.; Gao, W.; Dong, G.; Xia, Z.; Yip, S.; Qin, Y.; Qu, Y.; Ho, J.C. Hierarchical NiMo-based 3D electrocatalysts for highly-efficient hydrogen evolution in alkaline conditions. Nano Energy 2016, 27, 247–254. [Google Scholar] [CrossRef]
- He, Y.; Zhang, M.; Shi, J.-J.; Cen, Y.-L.; Wu, M. Improvement of Visible-Light Photocatalytic Efficiency in a Novel InSe/Zr2CO2 Heterostructure for Overall Water Splitting. J. Phys. Chem. C 2019, 123, 12781–12790. [Google Scholar] [CrossRef]
- Chen, G.-F.; Ma, T.Y.; Liu, Z.-Q.; Li, N.; Su, Y.-Z.; Davey, K.; Qiao, S.-Z. Efficient and Stable Bifunctional Electrocatalysts Ni/NiM (M = P, S) for Overall Water Splitting. Adv. Funct. Mater. 2016, 26, 3314–3323. [Google Scholar] [CrossRef]
- Xing, Z.; Liu, Q.; Asiri, A.M.; Sun, X. Closely Interconnected Network of Molybdenum Phosphide Nanoparticles: A Highly Efficient Electrocatalyst for Generating Hydrogen from Water. Adv. Mater. 2014, 26, 5702–5707. [Google Scholar] [CrossRef] [PubMed]
- Allam, M.; Benaicha, M.; Dakhouche, A. Electrodeposition and characterization of NiMoW alloy as electrode material for hydrogen evolution in alkaline water electrolysis. Int. J. Hydrogen Energy 2018, 43, 3394–3405. [Google Scholar] [CrossRef]
- Dharmaraj, K.; Hanna, R.; Lauermann, I.; Bagacki, R.; Xi, F.; Kemppainen, E.; Schlatmann, R.; Calnan, S. Electrodeposited Porous Nickel–Copper as a Non-Noble Metal Catalyst for Urea-Assisted Anion Exchange Membrane Electrolysis for Hydrogen Production. ACS Sustain. Chem. Eng. 2024, 12, 9908–9921. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, C.; Chen, H.; Zhai, Y.; Li, X.; Li, J.; Zhang, R.; Mao, Y.; Shan, T.; Zheng, K.; et al. Carbon-supported nickel–molybdenum alloy/tungsten carbide nanoparticles as efficient bifunctional catalysts for hydrogen evolution and oxidation reactions in alkaline media. Int. J. Hydrogen Energy 2025, 114, 229–238. [Google Scholar] [CrossRef]
- Guo, C.; Jiao, Y.; Zheng, Y.; Luo, J.; Davey, K.; Qiao, S.-Z. Intermediate Modulation on Noble Metal Hybridized to 2D Metal-Organic Framework for Accelerated Water Electrocatalysis. Chem 2019, 5, 2429–2441. [Google Scholar] [CrossRef]
- Liang, Z.; Ahn, H.; Bard, A. A Study of the Mechanism of the Hydrogen Evolution Reaction on Nickel by Surface Interrogation Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2017, 139, 4854–4858. [Google Scholar] [CrossRef]
- Jiang, L.; Pan, Y.; Zhang, J.; Chen, X.; Ye, X.; Li, Z.; Li, C.; Sun, Q. Mo propellant boosting the activity of Ni-P for efficient urea-assisted water electrolysis of hydrogen evolution. J. Colloid Interface Sci. 2022, 622, 192–201. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Cao, Y.; Gao, Z.; Ding, H.; Xu, H.; Liu, B.; Liu, F.; Zhu, Y. Morphology-Engineered NiMo Alloy on Nickel Foam for Enhanced Hydrogen Evolution Reaction Performance. Molecules 2025, 30, 2396. https://doi.org/10.3390/molecules30112396
Ding Y, Cao Y, Gao Z, Ding H, Xu H, Liu B, Liu F, Zhu Y. Morphology-Engineered NiMo Alloy on Nickel Foam for Enhanced Hydrogen Evolution Reaction Performance. Molecules. 2025; 30(11):2396. https://doi.org/10.3390/molecules30112396
Chicago/Turabian StyleDing, Yanhong, Yong Cao, Zhichao Gao, Hanzhou Ding, Haifeng Xu, Bin Liu, Fusheng Liu, and Yirong Zhu. 2025. "Morphology-Engineered NiMo Alloy on Nickel Foam for Enhanced Hydrogen Evolution Reaction Performance" Molecules 30, no. 11: 2396. https://doi.org/10.3390/molecules30112396
APA StyleDing, Y., Cao, Y., Gao, Z., Ding, H., Xu, H., Liu, B., Liu, F., & Zhu, Y. (2025). Morphology-Engineered NiMo Alloy on Nickel Foam for Enhanced Hydrogen Evolution Reaction Performance. Molecules, 30(11), 2396. https://doi.org/10.3390/molecules30112396






