Quantitative Analysis of Isoflavones from Fabaceae Species and Their Chemopreventive Potential on Breast Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Optimization
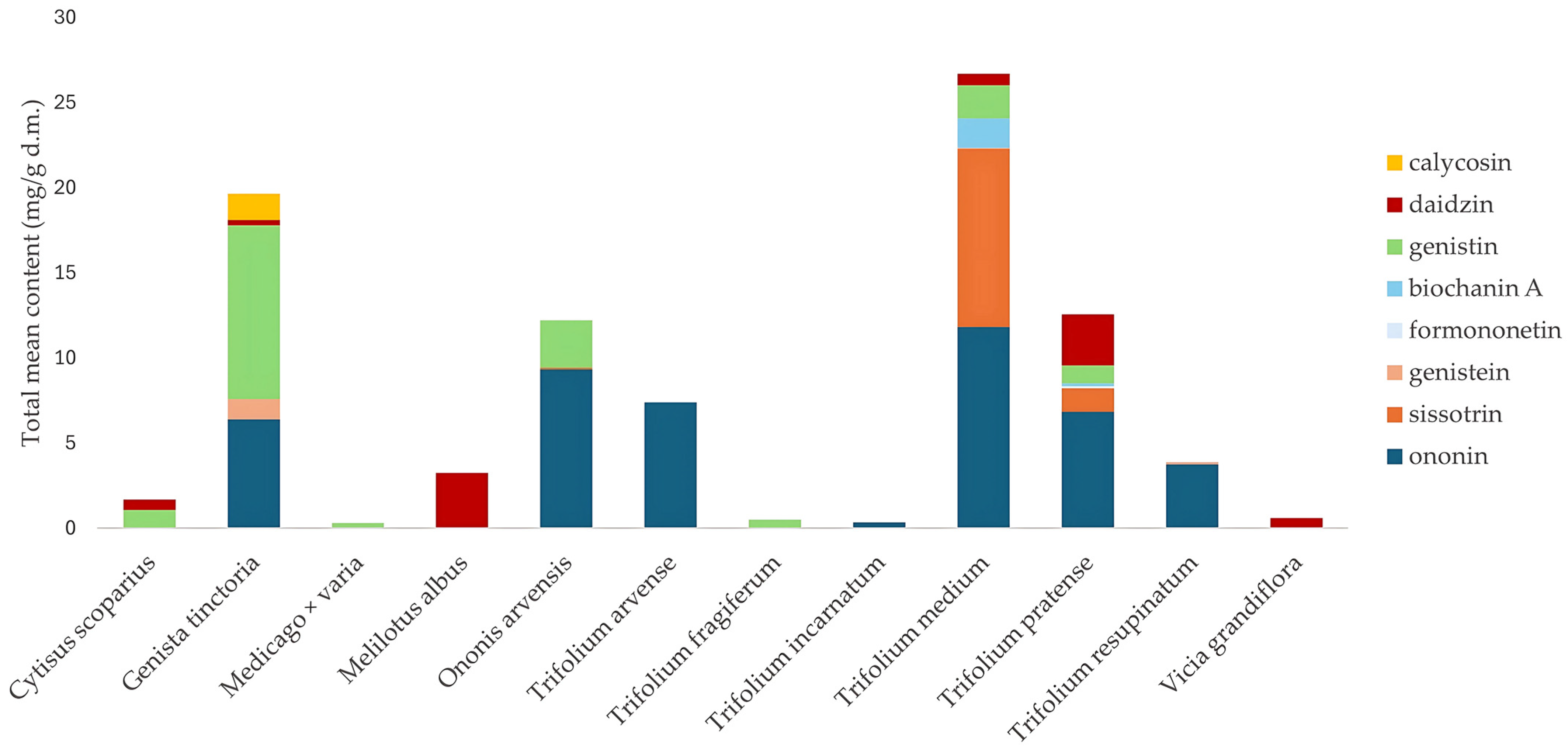
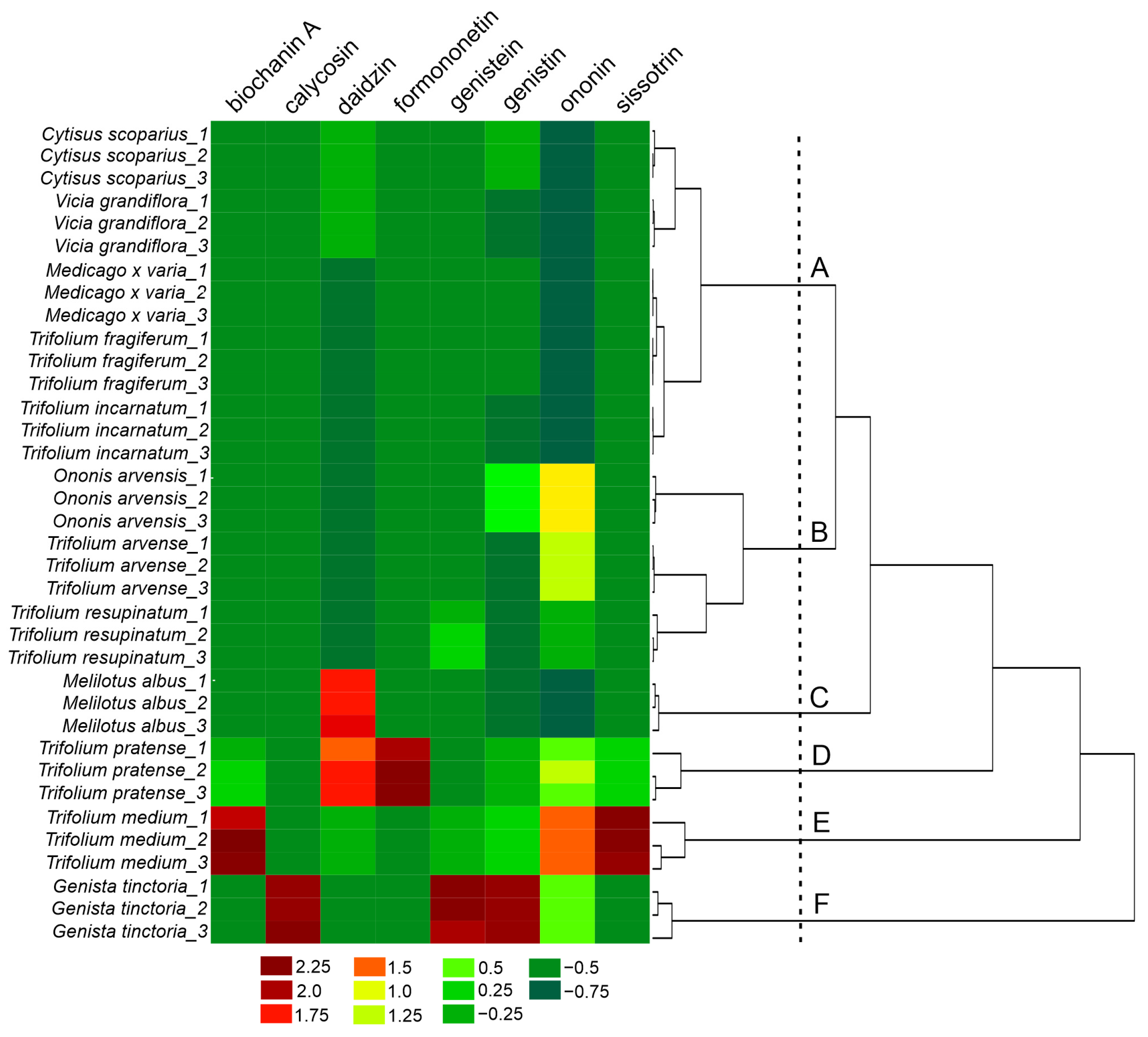
2.2. Qualitative and Quantitative Analysis of Fabaceae Plants
2.3. Assessment of Chemopreventive Properties of the Extracts
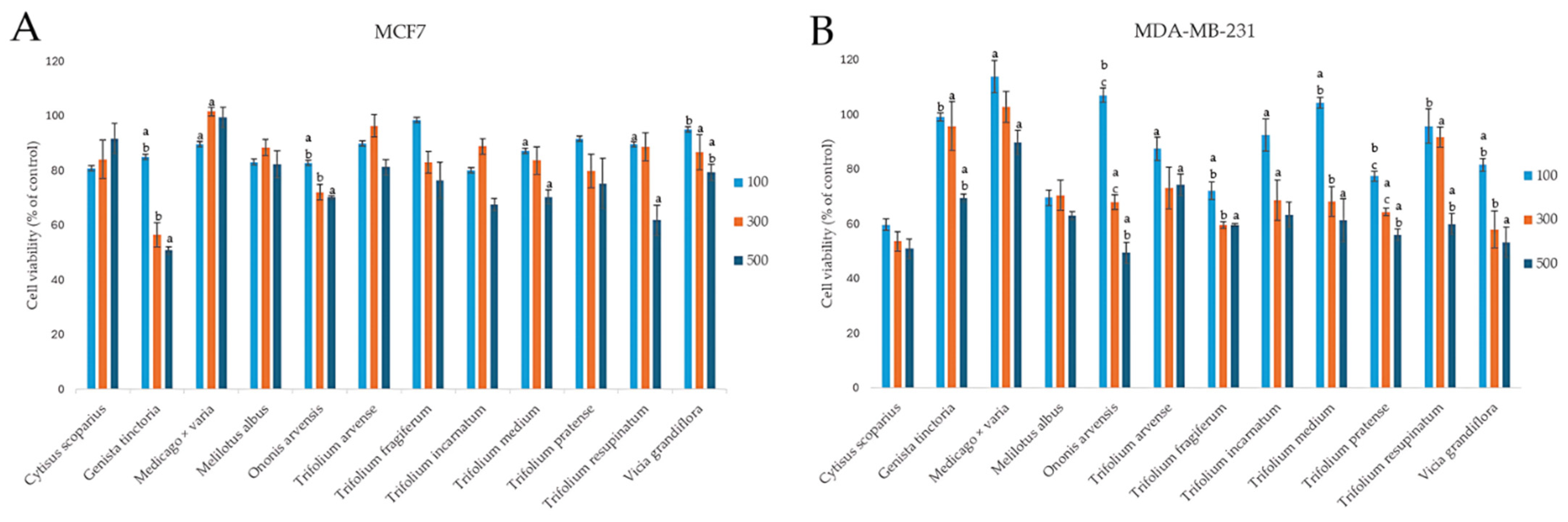
2.3.1. Effect on the Viability of Cancer Cells
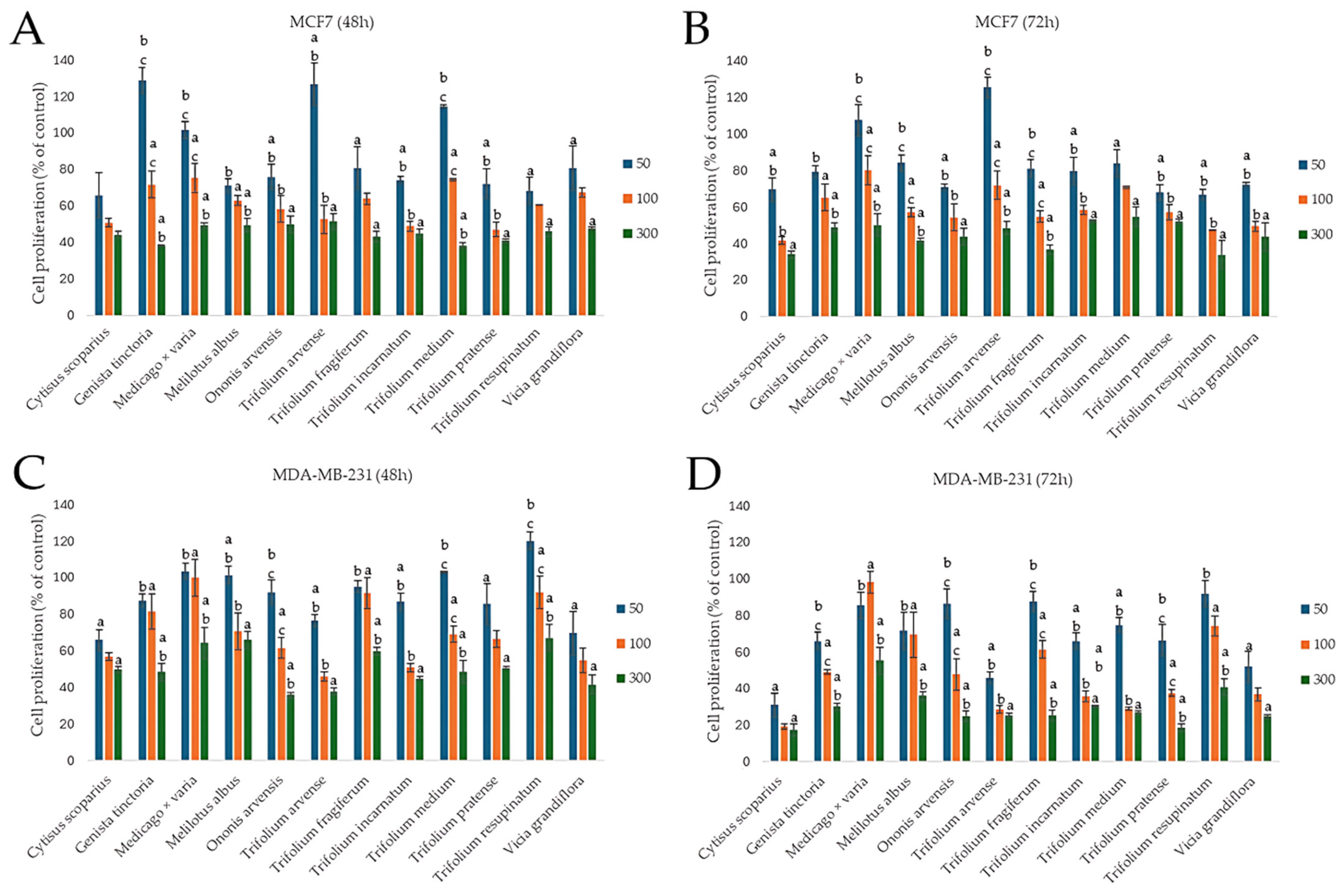
2.3.2. Effect on the Proliferation of Cancer Cells
2.3.3. Antioxidant Potential
2.4. Safety Assessment
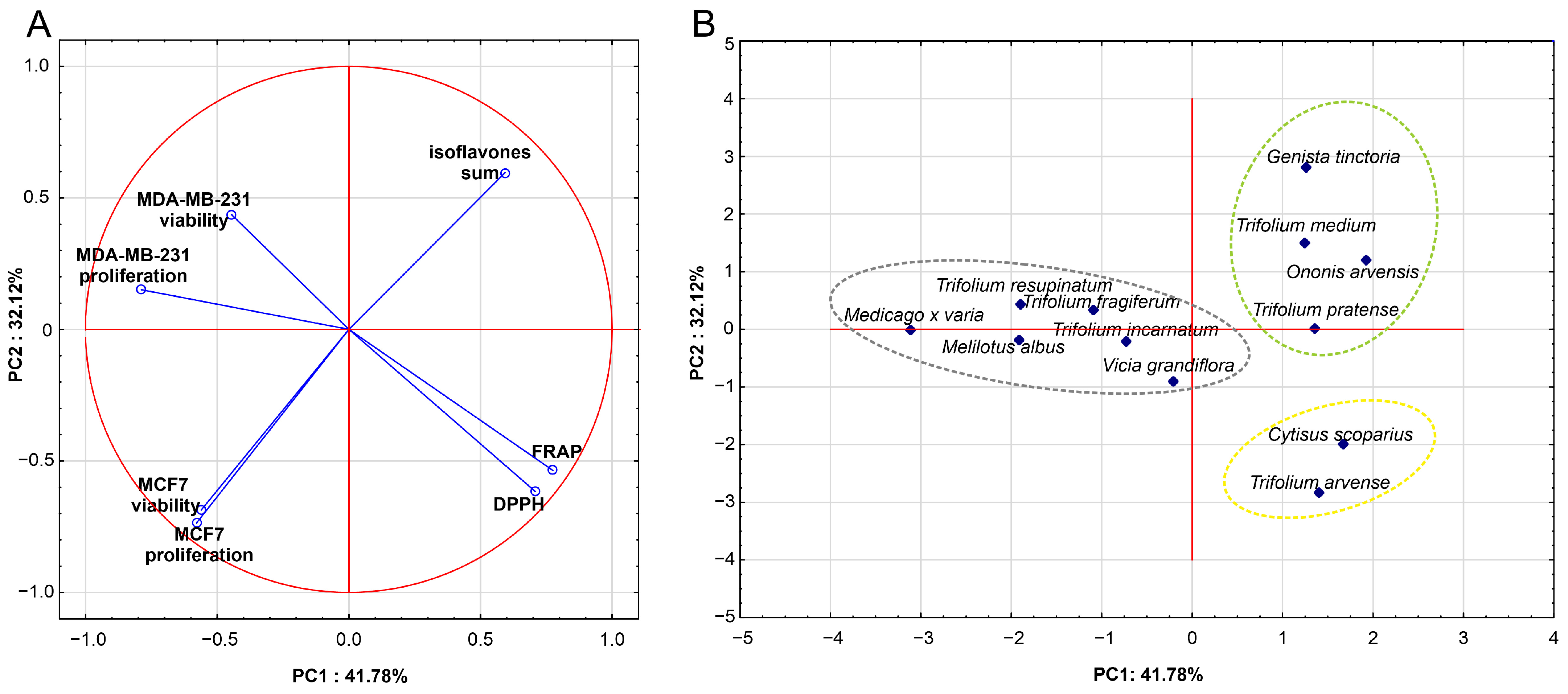
2.5. Influence of Isoflavone Content on the Results of In Vitro Studies
3. Materials and Methods
3.1. Chemical and Solutions
3.2. Extraction Optimization—Design of the Experiment
3.3. Plant Material and Sample Preparation for Optimization Procedure
3.4. HPLC Analysis Conditions
3.5. Plant Material for Quantitative Analysis
3.6. Sample Preparation and Extraction for Quantitative Analysis
3.7. Determination of Antioxidant Activity
3.8. Determination of Cytotoxic and Antiproliferative Activity
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grela, E.R.; Kiczorowska, B.; Samolińska, W.; Matras, J.; Kiczorowski, P.; Rybiński, W.; Hanczakowska, E. Chemical composition of leguminous seeds: Part I—Content of basic nutrients, amino acids, phytochemical compounds, and antioxidant activity. Eur. Food Res. Technol. 2017, 243, 1385–1395. [Google Scholar] [CrossRef]
- Carvalho, A.A.; dos Santos, L.R.; de Freitas, J.S.; Chaves, M.H. Isoflavonoids of the tribe Dalbergieae: A chemosystematic contribution to the subfamily Papilionoideae. Quim. Nova 2020, 43, 1294–1311. [Google Scholar]
- Bijak, M.; Połać, I.; Borowiecka, M.; Nowak, P.; Stetkiewicz, T.; Pertyński, T. Izoflawony jako alternatywa dla terapii hormonalnej wieku menopauzalnego. Przegląd Menopauzalny 2010, 6, 402–406. [Google Scholar]
- Nilsson, S.; Mäkelä, S.; Treuter, E.; Tujague, M.; Thomsen, J.; Andersson, G.; Enmark, E.; Pettersson, K.; Warner, M.; Gustafsson, J.A. Mechanisms of estrogen action. Physiol. Rev. 2001, 81, 1535–1565. [Google Scholar] [CrossRef]
- Hajirahimkhan, A.; Simmler, C.; Yuan, Y.; Anderson, J.R.; Chen, S.N.; Nikolić, D.; Dietz, B.M.; Pauli, G.F.; van Breemen, R.B.; Bolton, J.L. Evaluation of estrogenic activity of licorice species in comparison with hops used in botanicals for menopausal symptoms. PLoS ONE 2013, 8, e67947. [Google Scholar] [CrossRef]
- Basu, P.; Maier, C. Phytoestrogens and breast cancer: In vitro anticancer activities of isoflavones, lignans, coumestans, stilbenes and their analogs and derivatives. Biomed. Pharmacother. 2018, 107, 1648–1666. [Google Scholar] [CrossRef]
- Mirza, S.; Sharma, G.; Parshad, R.; Gupta, S.D.; Pandya, P.; Ralhan, R. Expression of DNA methyltransferases in breast cancer patients and to analyze the effect of natural compounds on DNA methyltransferases and associated proteins. J. Breast Cancer 2013, 16, 23–31. [Google Scholar] [CrossRef]
- Adjakly, M.; Bosviel, R.; Rabiau, N.; Boiteux, J.P.; Bignon, Y.J.; Guy, L.; Bernard-Gallon, D. DNA methylation and soy phytoestrogens: Quantitative study in DU-145 and PC-3 human prostate cancer cell lines. Epigenomics 2011, 3, 795–803. [Google Scholar] [CrossRef]
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef]
- Dietz, B.M.; Hajirahimkhan, A.; Dunlap, T.L.; Bolton, J.L. Botanicals and Their Bioactive Phytochemicals for Women’s Health. Pharmacol. Rev. 2016, 68, 1026–1073. [Google Scholar] [CrossRef]
- Yasuda, M.T.; Sakakibara, H.; Shimoi, K. Estrogen- and stress-induced DNA damage in breast cancer and chemoprevention with dietary flavonoid. Genes Environ. 2017, 39, 10. [Google Scholar] [CrossRef]
- Tran, T.N.T.; Chew, K.W.; Bui, X.V.; Nguyen, T.D.P.; Le, T.T.A.; Truong, T.M.H.; Show, P.L. Optimization of isoflavones extraction from soybeans using full factorial design. J. Food Process. Preserv. 2019, 43, e14078. [Google Scholar] [CrossRef]
- Sentkowska, A.; Biesaga, M.; Pyrzynska, K. Application of hydrophilic interaction liquid chromatography for the quantification of flavonoids in Genista tinctoria extract. J. Anal. Methods Chem. 2016, 2016, 3789348. [Google Scholar] [CrossRef]
- Gampe, N.; Nagy, E.; Kursinszki, L.; Béni, S. Quantitative determination of isoflavonoids in Ononis species by UPLC-UV-DAD. Phytochem. Anal. 2021, 32, 474–481. [Google Scholar] [CrossRef]
- Paździora, W.; Paśko, P.; Grabowska, K.; Galanty, A. Can isoflavone-rich legume plants be useful in the chemoprevention of hormone-dependent cancers?—A systematic review. Int. J. Mol. Sci. 2024, 25, 7389. [Google Scholar] [CrossRef]
- Batista, D.; Falé, P.L.; Serralheiro, M.L.; Araújo, M.E.; Madeira, P.J.A.; Borges, C.; Martins, A.; Rauter, A.P. New in vitro studies on the bioprofile of Genista tenera antihyperglycemic extract. Nat. Prod. Bioprospect. 2015, 5, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Erdemoglu, N.; Tosun, F.; Eroglu, Y. LC-MS analysis of daidzein in the Turkish Genista species. Chem. Nat. Compd. 2006, 42, 517–519. [Google Scholar] [CrossRef]
- Zgórka, G. Ultrasound-assisted solid-phase extraction coupled with photodiode-array and fluorescence detection for chemotaxonomy of isoflavone phytoestrogens in Trifolium L. (Clover) species. J. Sep. Sci. 2009, 32, 965–972. [Google Scholar] [CrossRef]
- Huang, J.; Yin, L.; Dong, L.; Quan, H.; Chen, R.; Hua, S.; Ma, J.; Guo, D.; Fu, X. Quality evaluation for Radix Astragali based on fingerprint, indicative components selection and QAMS. Biomed. Chromatogr. 2018, 32, e4343. [Google Scholar] [CrossRef]
- Hanganu, D.; Vlase, L.; Olah, N.K. Phytochemical Analysis of isoflavons from some Fabaceae species extracts. Not. Bot. Hort. Agrobot. Cluj 2010, 38, 57–60. [Google Scholar]
- Maciejewska-Turska, M.; Zgórka, G. In-depth phytochemical and biological studies on potential AChE inhibitors in red and zigzag clover dry extracts using reversed–phase liquid chromatography (RP-LC) coupled with photodiode array (PDA) and electron spray ionization-quadrupole/time of flight-mass spectrometric (ESI-QToF/MS-MS) detection and thin-layer chromatography-bioautography. Food Chem. 2022, 375, 131846. [Google Scholar] [PubMed]
- Luo, Z.; Xu, Y.; Qiu, L.; Lv, S.; Zeng, C.; Tan, A.; Ou, D.; Song, X.; Yang, J. Optimization of ultrasound-assisted extraction based on response surface methodology using HPLC-DAD for the analysis of red clover (Trifolium pratense L.) isoflavones and its anti-inflammatory activities on LPS-induced 3D4/2 cell. Front. Vet. Sci. 2023, 10, 1279178. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.C.; Coqueiro, A.; Sultan, A.R.; Lemmens, N.; Kim, H.K.; Verpoorte, R.; van Wamel, W.J.B.; Simões, M.; Choi, Y.H. Looking to nature for a new concept in antimicrobial treatments: Isoflavonoids from Cytisus striatus as antibiotic adjuvants against MRSA. Sci. Rep. 2017, 7, 3777. [Google Scholar] [CrossRef]
- Wyse, J.M.; Latif, S.; Gurusinghe, S.; Berntsen, E.D.; Weston, L.A.; Stephen, C.P. Characterization of Phytoestrogens in Medicago sativa L. and Grazing Beef Cattle. Metabolites 2021, 11, 550. [Google Scholar] [CrossRef]
- Ilhan, M.; Ali, Z.; Khan, I.A.; Küpeli Akkol, E. A new isoflavane-4-ol derivative from Melilotus officinalis (L.) Pall. Nat. Prod. Res. 2018, 33, 1856–1861. [Google Scholar] [CrossRef]
- Zgórka, G.; Maciejewska-Turska, M.; Makuch-Kocka, A.; Plech, T. In vitro evaluation of the antioxidant activity and chemopreventive potential in human breast cancer cell lines of the standardized extract obtained from the aerial parts of zigzag clover (Trifolium medium L.). Pharmaceuticals 2022, 15, 699. [Google Scholar] [CrossRef]
- Butkutė, B.; Padarauskas, A.; Cesevičienė, J.; Taujenis, L.; Norkevičienė, E. Phytochemical composition of temperate perennial legumes. Crop Pasture Sci. 2022, 69, 1020–1030. [Google Scholar] [CrossRef]
- Hloucalová, P.; Skládanka, J.; Horký, P.; Klejdus, B.; Pelikán, J.; Knotová, D. Determination of phytoestrogen content in fresh-cut legume forage. Animals 2016, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Visnevschi-Necrasov, T.; Faria, M.A.; Cunha, S.C.; Harris, J.; Meimberg, H.W.E.; Curto, M.A.C.; Graça Pereira, M.; Oliveira, M.B.P.P.; Nunes, E. Isoflavone synthase (IFS) gene phylogeny in Trifolium species associated with plant isoflavone contents. Plant Syst. Evol. 2013, 299, 357–367. [Google Scholar] [CrossRef]
- Vetter, J. Isoflavones in different parts of common Trifolium species. J. Agric. Food Chem. 1995, 45, 106–108. [Google Scholar] [CrossRef]
- Benedec, D.; Vlase, L.; Oniga, I.; Toiu, A.; Tamas, M.; Brindusa, T. Isoflavonoids from Glycyrrhiza sp. and Ononis spinosa. Farmacia 2012, 60, 615–620. [Google Scholar]
- Vlase, L.; Popa, D.S.; Tero-Vescan, A.; Olah, N. New liquid chromatography: Mass spectrometry assay for natural phytoestrogens from vegetable extracts. Acta Chromatogr. 2011, 23, 509–520. [Google Scholar] [CrossRef]
- Rigano, D.; Cardile, V.; Formisano, C.; Maldini, M.T.; Piacente, S.; Bevelacqua, Y.; Russo, A.; Senatore, F. Genista sessilifolia DC. and Genista tinctoria L. inhibit UV light and nitric oxide-induced DNA damage and human melanoma cell growth. Chem. Biol. Interact. 2009, 180, 211–219. [Google Scholar] [CrossRef]
- Cunha, S.C.; Faria, M.A.; Sousa, T.; Nunes, E. Isoflavone determination in spontaneous legumes identified by DNA barcodes. Food Chem. 2012, 134, 2262–2267. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, P.B.; Duke, J.A.; Brielmann, H.; Boik, J.; Hoyt, J.E. A comparative survey of leguminous plants as sources of the isoflavones, genistein and daidzein: Implications for human nutrition and health. J. Altern. Complement. Med. 1997, 3, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Šibul, F.; Orčić, D.; Vasić, M.; Anačkov, G.; Nađpal, J.; Savić, A.; Mimica-Dukić, N. Phenolic profile, antioxidant and anti-inflammatory potential of herb and root extracts of seven selected legumes. Crops Prod. 2016, 83, 641–653. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Grever, M.R.; Schepartz, S.A.; Chabner, B.A. The National Cancer Institute: Cancer drug discovery and development program. Semin. Oncol. 1992, 19, 622–638. [Google Scholar]
- Booth, N.L.; Overk, C.R.; Yao, P.; Totura, S.; Deng, Y.; Hedayat, A.S.; Bolton, J.L.; Pauli, G.F.; Farnsworth, N.R. Seasonal variation of red clover (Trifolium pratense L., Fabaceae) isoflavones and estrogenic activity. J. Agric. Food Chem. 2006, 54, 1277–1282. [Google Scholar] [CrossRef]
- Talib, W.H.; Mahasneh, A.M. Antiproliferative Activity of Plant Extracts Used against Cancer in Traditional Medicine. Sci. Pharm. 2010, 78, 33–46. [Google Scholar] [CrossRef]
- Asadi-Samani, M.; Rafieian-Kopaei, M.; Lorigooini, Z.; Shirzad, H. A screening of anti-breast cancer effects and antioxidant activity of twenty medicinal plants gathered from Chaharmahal va Bakhtyari province, Iran. J. Pharm. Pharmacogn. Res. 2019, 7, 213–222. [Google Scholar] [CrossRef]
- Abu-Dahab, R.; Afifi, F. Antiproliferative activity of selected medicinal plants of Jordan against a breast adenocarcinoma cell line (MCF7). Sci. Pharm. 2007, 75, 121–136. [Google Scholar] [CrossRef]
- Al-Zereini, W.A. Ononis natrix and Salvia verbenaca: Two Jordanian Medicinal Plants with Cytotoxic and Antibacterial Activities. J. Herbs Spices Med. Plants 2012, 8, 417–423. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Carreira-Casais, A.; Pereira, E.; Dias, M.I.; Pereira, C.; Calhelha, R.C.; Stojkovic, D.; Sokovic, M.; Simal-Gandara, J.; Prieto, M.A.; et al. From Tradition to Health: Chemical and Bioactive Characterization of Five Traditional Plants. Molecules 2022, 27, 6495. [Google Scholar] [CrossRef]
- Martinez-Montemayor, M.M.; Otero-Franqui, E.; Martinez, J.; De La Mota-Peynado, A.; Cubano, L.A.; Dharmawardhane, S.F. Individual and combined soy isoflavones exert differential effects on metastatic cancer cells. J. Nutr. Biochem. 2010, 21, 729–739. [Google Scholar]
- Bouziane, A.; Bakchiche, B.; Dias, M.I.; Barros, L.; Ferreira, I.C.F.R.; Al Salamat, H.A.; Bardaweel, S.K. Phenolic Compounds and Bioactivity of Cytisus villosus Pourr. Molecules 2018, 23, 1994. [Google Scholar] [CrossRef]
- Bilušić, T.; Čulić, V.Č.; Zorić, Z.; Čošić, Z.; Vujić, L.; Šola, I. Biological Activities of Selected Medicinal and Edible Plants Aqueous Infusions. Appl. Sci. 2025, 15, 3254. [Google Scholar] [CrossRef]
- Stojković, D.; Drakulić, D.; Gašić, U.; Zengin, G.; Stevanović, M.; Rajčevićd, N.; Soković, M. Ononis spinosa L., an edible and medicinal plant: UHPLC-LTQ-Orbitrap/MS chemical profiling and biological activities of the herbal extract. Food Funct. 2020, 11, 7138–7151. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Nakayama, A.; Alladin, K.P.; Igbokwe, O.; White, J.D. Systematic Review: Generating Evidence-Based Guidelines on the Concurrent Use of Dietary Antioxidants and Chemotherapy or Radiotherapy. Cancer Investig. 2011, 29, 655–667. [Google Scholar] [CrossRef]
- Zouhri, A.; El Menyiy, N.; El-mernissi, Y.; Bouddine, T.; El-mernissi, R.; Amhamdi, H.; Elharrak, A.; Salamatullah, A.M.; Nafidi, H.A.; Khallouki, F.; et al. Mineral composition, principal polyphenolic components, and evaluation of the anti-inflammatory, analgesic, and antioxidant properties of Cytisus villosus Pourr leaf extracts. Open Chem. 2023, 21, 20220338. [Google Scholar] [CrossRef]
- Štampar, M.; Breznik, B.; Filipič, M.; Žegura, B. Characterization of in vitro 3D cell model developed from human hepatocellular carcinoma (HepG2) Cell Line. Cells 2020, 9, 2557. [Google Scholar] [CrossRef]
- Tahboub, R.; Arafah, B.M. Sex steroids and the thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 769–780. [Google Scholar] [CrossRef]
- Galanty, A.; Paśko, P.; Podolak, I.; Zagrodzki, P. Optimization of usnic acid extraction conditions using fractional factorial design. Lichenologist 2020, 52, 397–401. [Google Scholar] [CrossRef]
- Galanty, A.; Niepsuj, M.; Grudzińska, M.; Zagrodzki, P.; Podolak, I.; Paśko, P. In the search for novel, isoflavone-rich functional foods—Comparative studies of four clover species sprouts and their chemopreventive potential for breast and prostate cancer. Pharmaceuticals 2022, 15, 806. [Google Scholar] [CrossRef]
- Paśko, P.; Galanty, A.; Zagrodzki, P.; Żmudzki, P.; Bieniek, U.; Prochownik, E.; Domínguez-Álvarez, E.; Bierła, K.; Łobiński, R.; Szpunar, J.; et al. Varied effect of fortification of kale sprouts with novel organic selenium compounds on the synthesis of sulphur and phenolic compounds in relation to cytotoxic, antioxidant and anti-inflammatory activity. Microchem. J. 2022, 179, 107509. [Google Scholar] [CrossRef]
- Grudzińska, M.; Paśko, P.; Wróbel-Biedrawa, D.; Podolak, I.; Galanty, A. Antimelanoma Potential of Cladonia mitis Acetone Extracts—Comparative in Vitro Studies in Relation to Usnic Acid Content. Chem. Biodivers. 2022, 19, e202200408. [Google Scholar] [CrossRef]
- Galanty, A.; Koczurkiewicz, P.; Wnuk, D.; Paw, M.; Karnas, E.; Podolak, I.; Węgrzyn, M.; Borusiewicz, M.; Madeja, Z.; Czyż, J.; et al. Usnic acid and atranorin exert selective cytostatic and anti-invasive effects on human prostate and melanoma cancer cells. Toxicol. Vitr. 2017, 40, 161–169. [Google Scholar] [CrossRef]

| Isoflavones Sum Content (mg/g d.m.), Mean ± SD | |||
|---|---|---|---|
| Set Number | Content | Set Number | Content |
| 1 UAE | 3.62 ± 0.01 b | 1 HRE | 4.53 ± 0.10 b |
| 2 UAE | 7.40 ± 0.13 a | 2 HRE | 10.09 ± 0.22 b |
| 3 UAE | 5.13 ± 0.2 b | 3 HRE | 7.67 ± 0.04 b |
| 4 UAE | 7.96 ± 0.19 a | 4 HRE | 12.56 ± 0.67 a |
| 5 UAE | 5.76 ± 0.03 b | 5 HRE | 8.35 ± 0.11 b |
| 6 UAE | 3.63 ± 0.11 b | 6 HRE | 5.54 ± 0.22 b |
| 7 UAE | 4.89 ± 0.02 b | 7 HRE | 8.75 ± 0.17 b |
| 8 UAE | 4.51 ± 0.08 b | 8 HRE | 5.67 ± 0.23 b |
| 9 UAE | 7.53 ± 0.14 a | 9 HRE | 13.27 ± 0.30 a |
| Isoflavone | Trifolium medium | Trifolium pratense |
|---|---|---|
| biochanin A | 1.69 ± 0.23 a | 0.17 ± 0.02 b |
| daidzin | 0.67 ± 0.03 a | 3.00 ± 0.05 b |
| formononetin | n.d. | 0.13 ± 0.02 |
| genistein | 0.07 ± 0.01 | n.d. |
| genistin | 1.97 ± 0.05 a | 1.05 ± 0.05 b |
| ononin | 11.84 ± 0.19 a | 6.85 ± 0.52 b |
| sissotrin | 10.45 ± 0.35 a | 1.35 ± 0.02 b |
| Species | DPPH (μM TEAC/g d.m.) | FRAP (μM/Fe2+/g d.m.) |
|---|---|---|
| Cytisus scoparius | 178.1 ± 5.9 a | 232.2 ± 3.7 a |
| Genista tinctoria | 67.5 ± 2.3 b | 112.7 ± 1.7 b |
| Medicago x varia | 24.0 ± 0.7 b | 61.2 ± 3.6 b |
| Melilotus albus | 36.3 ± 4.6 b | 77.0 ± 9.9 b |
| Ononis arvensis | 73.3 ± 42.5 b | 143.2 ± 4.3 b |
| Trifolium arvense | 182.4 ± 9.3 a | 248.8 ± 8.6 a |
| Trifolium fragiferum | 30.4 ± 5.1 b | 72.5 ± 7.5 b |
| Trifolium incarnatum | 43.7 ± 6.1 b | 80.2 ± 9.1 b |
| Trifolium medium | 66.2 ± 5.2 b | 137.4 ± 7.7 b |
| Trifolium pratense | 103. ± 26.6 b | 189.6 ± 32.4 b |
| Trifolium resupinatum | 41.9 ± 4.7 b | 93.3 ± 9.6 b |
| Vicia grandiflora | 59.2 ± 5.0 b | 110.0 ± 6.0 b |
| Eigenvalues (Cumulative) | % Total Variance (Cumulative (%)) | Variables | Factor Loadings PC1 | Factor Loadings PC2 |
|---|---|---|---|---|
| 2.92 | 41.78% | FRAP | 0.775 | −0.536 |
| 2.25 | 32.13% | DPPH | 0.707 | −0.616 |
| (5.17) | (73.91%) | MCF7 viability | −0.561 | −0.687 |
| MDA-MB-231 viability | −0.446 | 0.437 | ||
| MCF7 proliferation | −0.579 | −0.736 | ||
| MDA-MB-231 proliferation | −0.789 | 0.151 | ||
| Isoflavones sum | 0.594 | 0.596 |
| Time of Extraction (HRE, UAE: min) | Solvent Concentration (%) | Ratio of Plant Material to Solvent Volume | ||||
|---|---|---|---|---|---|---|
| Set Number | Parameter Value | Code | Parameter Value | Code | Parameter Value | Code |
| 1 | HRE: 30 UAE: 10 | −1 | 50 | −1 | 1:25 | −1 |
| 2 | HRE: 30 UAE: 10 | −1 | 75 | 0 | 1:125 | 1 |
| 3 | HRE: 30 UAE: 10 | −1 | 100 | 1 | 1:50 | 0 |
| 4 | HRE: 60 UAE: 20 | 0 | 50 | −1 | 1:125 | 1 |
| 5 | HRE: 60 UAE: 20 | 0 | 75 | 0 | 1:50 | 0 |
| 6 | HRE: 60 UAE: 20 | 0 | 100 | 1 | 1:25 | −1 |
| 7 | HRE: 120 UAE: 30 | 1 | 50 | −1 | 1:50 | 0 |
| 8 | HRE: 120 UAE: 30 | 1 | 75 | 0 | 1:25 | −1 |
| 9 | HRE: 120 UAE: 30 | 1 | 100 | 1 | 1:125 | 1 |
| Species | Localization | Geographical Coordinates | Voucher Specimens |
|---|---|---|---|
| Anthyllis vulneraria | Kraków | 50.06′ N, 19.92′ E | KFg/2024/Av |
| Astragalus cicer | Kraków | 50.08′ N, 20.03′ E | KFg/2024/Ac |
| Coronilla varia | Olkusz | 50.31′ N, 19.57′ E | KFg/2024/Cv |
| Cytisus scoparius | Węgrzce Wielkie | 50.02′ N, 20.12′ E | KFg/2024/Cs |
| Genista tinctoria | Kraków | 50.03′ N, 19.91′ E | KFg/2024/Gt |
| Lathyrus latifolius | Wieliczka | 49.99′ N, 20.04′ E | KFg/2024/Lal |
| Lathyrus odoratus | Gdów | 49.90′ N, 20.19′ E | KFg/2024/Lo |
| Lathyrus pratensis | Kraków | 50.06′ N, 19.92′ E | KFg/2024/Lpr |
| Lotus corniculatus | Olkusz | 50.31′ N, 19.57′ E | KFg/2024/Lc |
| Lupinus polyphyllus | Olkusz | 50.30′ N, 19.57′ E | KFg/2024/Lpo |
| Medicago falcata | Kraków | 50.09′ N, 19.90′ E | KFg/2024/Mf |
| Medicago lupulina | Wieliczka | 49.98′ N, 20.04′ E | KFg/2024/Ml |
| Medicago x varia | Kraków | 50.06′ N, 19.95′ E | KFg/2024/Mv |
| Melilotus albus | Kraków | 50.01′ N, 20.00′ E | KFg/2024/Ma |
| Melilotus officinalis | Kraków | 50.06′ N, 19.95′ E | KFg/2024/Mo |
| Onobrychis viciifolia | Kraków | 50.04′ N, 19.96′ E | KFg/2024/Ov |
| Ononis arvense | Kraków | 50.03′ N, 19.91′ E | KFg/2024/Oa |
| Trifolium arvense | Gdów | 49.90′ N, 20.18′ E | KFg/2024/Ta |
| Trifolium campestre | Wieliczka | 49.98′ N, 20.03′ E | KFg/2024/Tc |
| Trifolium fragiferum | Kraków | 50.02′ N, 19.97′ E | KFg/2024/Tf |
| Trifolium hybridum | Kraków | 50.04′ N, 19.96′ E | KFg/2024/Th |
| Trifolium incarnatum | Łazany | 49.95′ N, 20.16′ E | KFg/2024/Ti |
| Trifolium medium | Kraków | 50.03′ N, 19.90′ E | KFg/2024/Tme |
| Trifolium montanum | Kraków | 50.04′ N, 19.91′ E | KFg/2024/Tmo |
| Trifolium pratense | Kraków | 50.01′ N, 20.00′ E | KFg/2024/Tp |
| Trifolium repens | Kraków | 50.01′ N, 20.00′ E | KFg/2024/Trep |
| Trifolium resupinatum | Gdów | 49.90′ N, 20.19′ E | KFg/2024/Tres |
| Vicia angustifolia | Kraków | 50.08′ N, 19.92′ E | KFg/2024/Va |
| Vicia grandiflora | Kraków | 50.07′ N, 19.87′ E | KFg/2024/Vg |
| Vicia hirsuta | Kraków | 50.06′ N, 19.91′ E | KFg/2024/Vh |
| Vicia sepium | Kraków | 50.07′ N, 19.87′ E | KFg/2024/Vs |
| Vicia villosa | Kokotów | 50.01′ N, 20.07′ E | KFg/2024/Vv |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paździora, W.; Grabowska, K.; Zagrodzki, P.; Paśko, P.; Prochownik, E.; Podolak, I.; Galanty, A. Quantitative Analysis of Isoflavones from Fabaceae Species and Their Chemopreventive Potential on Breast Cancer Cells. Molecules 2025, 30, 2379. https://doi.org/10.3390/molecules30112379
Paździora W, Grabowska K, Zagrodzki P, Paśko P, Prochownik E, Podolak I, Galanty A. Quantitative Analysis of Isoflavones from Fabaceae Species and Their Chemopreventive Potential on Breast Cancer Cells. Molecules. 2025; 30(11):2379. https://doi.org/10.3390/molecules30112379
Chicago/Turabian StylePaździora, Wojciech, Karolina Grabowska, Paweł Zagrodzki, Paweł Paśko, Ewelina Prochownik, Irma Podolak, and Agnieszka Galanty. 2025. "Quantitative Analysis of Isoflavones from Fabaceae Species and Their Chemopreventive Potential on Breast Cancer Cells" Molecules 30, no. 11: 2379. https://doi.org/10.3390/molecules30112379
APA StylePaździora, W., Grabowska, K., Zagrodzki, P., Paśko, P., Prochownik, E., Podolak, I., & Galanty, A. (2025). Quantitative Analysis of Isoflavones from Fabaceae Species and Their Chemopreventive Potential on Breast Cancer Cells. Molecules, 30(11), 2379. https://doi.org/10.3390/molecules30112379







