Fenbendazole Exhibits Antitumor Activity Against Cervical Cancer Through Dual Targeting of Cancer Cells and Cancer Stem Cells: Evidence from In Vitro and In Vivo Models
Abstract
1. Introduction
2. Results
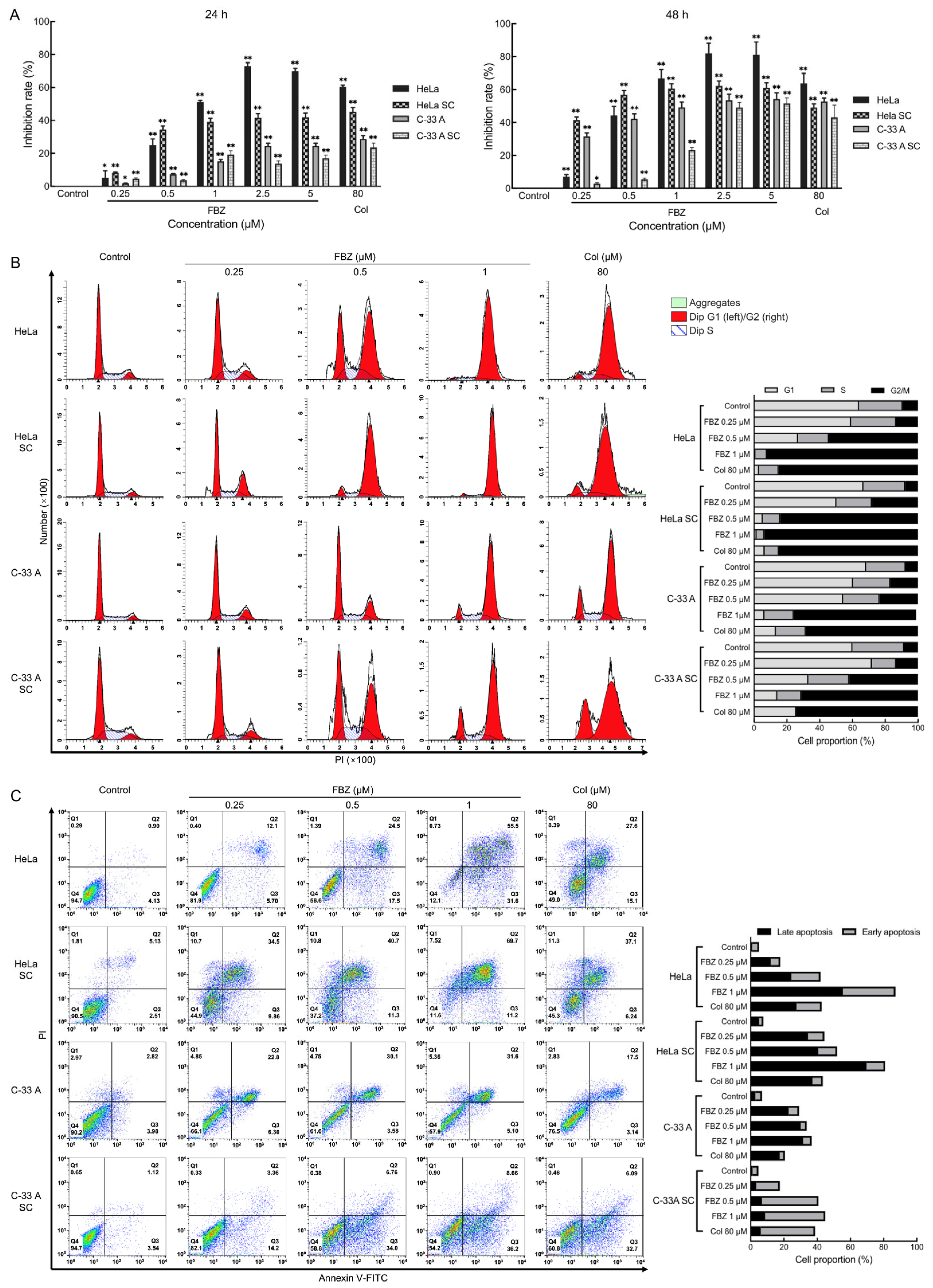
2.1. The Effect of FBZ on Tumor Cell Proliferation
2.2. Isolation and Identification of CCSCs
2.3. The Effect of FBZ on Proliferation, Cell Cycle, and Apoptosis of CCCs and CCSCs
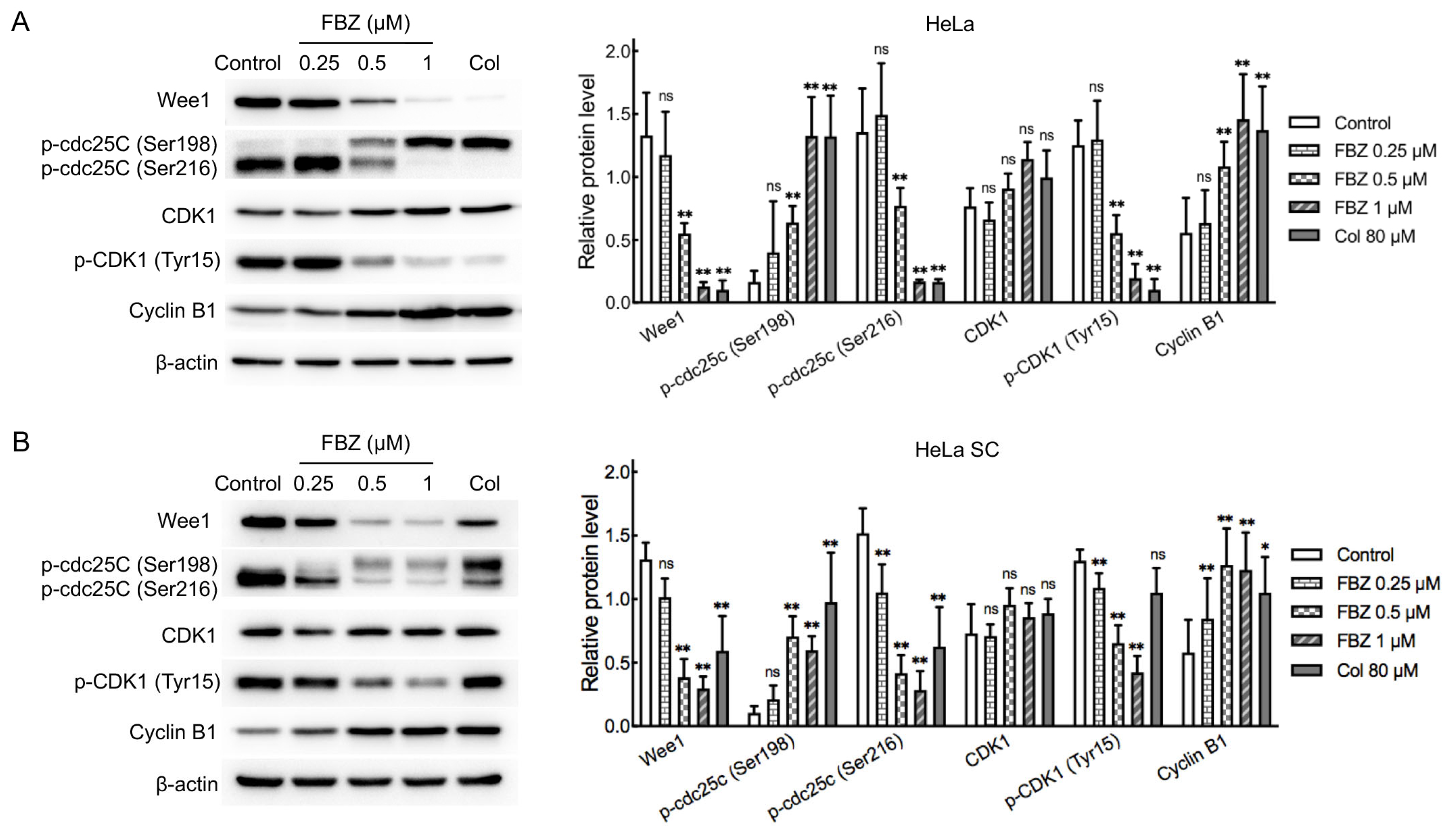
2.4. Mechanistic Investigation of FBZ Antitumor Activity
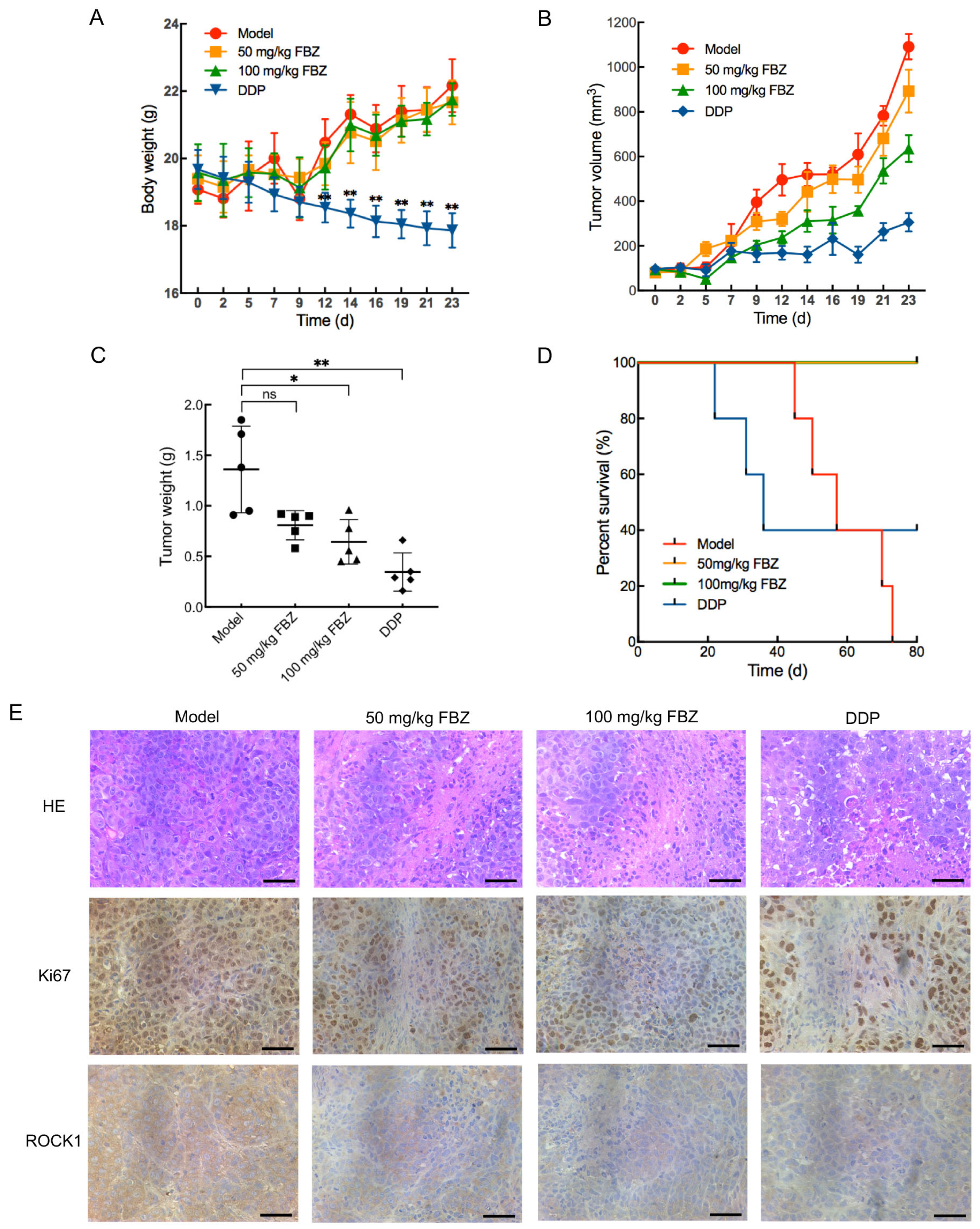
2.5. The Effect of FBZ on Cervical Cancer Xenografts
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Lines
4.3. Cell Proliferation Assay
4.4. Isolation of CD133+CD44+ Cells from CCCs
4.5. Flow Cytometry Detection of Cell Surface Molecules
4.6. Colony Formation Assay
4.7. Western Blot Analysis
4.8. Cell Cycle and Apoptosis Analysis
4.9. In Vivo Xenograft Tumor Experiments
4.10. HE and Immunohistochemistry Staining
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | absorbance |
| bFGF | basic fibroblast growth factor |
| CCC | cervical cancer cell |
| CCSC | cervical cancer stem cell |
| CDK1 | cyclin-dependent kinase 1 |
| CMC | sodium carboxymethyl cellulose |
| Col | colchicine |
| CSC | cancer stem cell |
| DDP | cisplatin |
| DMSO | dimethyl sulfoxide |
| EGF | epidermal growth factor |
| FBS | fetal bovine serum |
| FBZ | fenbendazole |
| FITC | fluorescein isothiocyanate |
| HE | Hematoxylin-Eosin |
| IC50 | half-maximal inhibitory concentration |
| MTS | 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium |
| PI | propidium iodide |
| ROCK1 | Rho-associated coiled-coil containing protein kinase |
| SC | stem cell |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, M.; Giubbi, C.; Saderi, L.; Musumeci, R.; Perdoni, F.; Leone, B.E.; Fruscio, R.; Landoni, F.; Piana, A.; Sotgiu, G.; et al. Evaluation of Human Papilloma Virus (HPV) Genotyping and Viral Load Determination as Diagnostic Biomarkers of Cervical Cancer Risk. Int. J. Mol. Sci. 2023, 24, 1320. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rustum, N.R.; Yashar, C.M.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Crispens, M.A.; et al. NCCN Guidelines® Insights: Cervical Cancer, Version 1.2024. J. Natl. Compr. Cancer Netw. 2023, 21, 1224–1233. [Google Scholar] [CrossRef]
- Tabatabaei, F.S.; Saeedian, A.; Azimi, A.; Kolahdouzan, K.; Esmati, E.; Maddah Safaei, A. Evaluation of Survival Rate and Associated Factors in Patients with Cervical Cancer: A Retrospective Cohort Study. J. Res. Health Sci. 2022, 22, e00552. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.G.; Bundy, B.N.; Watkins, E.B.; Thigpen, J.T.; Deppe, G.; Maiman, M.A.; Clarke-Pearson, D.L.; Insalaco, S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 1999, 340, 1144–1153. [Google Scholar] [CrossRef]
- Odiase, O.; Noah-Vermillion, L.; Simone, B.A.; Aridgides, P.D. The Incorporation of Immunotherapy and Targeted Therapy Into Chemoradiation for Cervical Cancer: A Focused Review. Front. Oncol. 2021, 11, 663749. [Google Scholar] [CrossRef]
- Wright, J.D.; Matsuo, K.; Huang, Y.; Tergas, A.I.; Hou, J.Y.; Khoury-Collado, F.; St Clair, C.M.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L. Prognostic Performance of the 2018 International Federation of Gynecology and Obstetrics Cervical Cancer Staging Guidelines. Obstet. Gynecol. 2019, 134, 49–57. [Google Scholar] [CrossRef]
- Yoshida, K.; Kajiyama, H.; Utsumi, F.; Niimi, K.; Sakata, J.; Suzuki, S.; Shibata, K.; Kikkawa, F. A post-recurrence survival-predicting indicator for cervical cancer from the analysis of 165 patients who developed recurrence. Mol. Clin. Oncol. 2018, 8, 281–285. [Google Scholar]
- Yang, J.; Cai, H.; Xiao, Z.X.; Wang, H.; Yang, P. Effect of radiotherapy on the survival of cervical cancer patients: An analysis based on SEER database. Medicine 2019, 98, e16421. [Google Scholar] [CrossRef]
- Ayob, A.Z.; Ramasamy, T.S. Cancer stem cells as key drivers of tumour progression. J. Biomed. Sci. 2018, 25, 20. [Google Scholar] [CrossRef]
- Arnold, C.R.; Mangesius, J.; Skvortsova, II; Ganswindt, U. The Role of Cancer Stem Cells in Radiation Resistance. Front. Oncol. 2020, 10, 164. [Google Scholar] [CrossRef]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.G.; Lee, S.H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer Stem Cells (CSCs) in Drug Resistance and their Therapeutic Implications in Cancer Treatment. Stem Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Hoque, M.O. Targeting Cancer Stem Cells: A Strategy for Effective Eradication of Cancer. Cancers 2019, 11, 732. [Google Scholar] [CrossRef]
- Kuşoğlu, A.; Biray Avcı, Ç. Cancer stem cells: A brief review of the current status. Gene 2019, 681, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Saw, P.E.; Liu, Q.; Wong, P.P.; Song, E. Cancer stem cell mimicry for immune evasion and therapeutic resistance. Cell Stem Cell 2024, 31, 1101–1112. [Google Scholar] [CrossRef]
- Walcher, L.; Kistenmacher, A.K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer Stem Cells-Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Won, M.; Kim, J.H.; Jung, E.; Min, K.; Jangili, P.; Kim, J.S. Cancer stem cell-targeted bio-imaging and chemotherapeutic perspective. Chem. Soc. Rev. 2020, 49, 7856–7878. [Google Scholar] [CrossRef]
- Prasetyanti, P.R.; Medema, J.P. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol. Cancer 2017, 16, 41. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, M.; Huang, H.; Jin, W.L. Drug repurposing for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 92. [Google Scholar] [CrossRef]
- Paul, S.; Ghosh, S.; Kumar, S. Tumor glycolysis, an essential sweet tooth of tumor cells. Semin. Cancer Biol. 2022, 86 Pt. 3, 1216–1230. [Google Scholar] [CrossRef]
- Narasimhan, P.B.; Akabas, L.; Tariq, S.; Huda, N.; Bennuru, S.; Sabzevari, H.; Hofmeister, R.; Nutman, T.B.; Tolouei Semnani, R. Similarities and differences between helminth parasites and cancer cell lines in shaping human monocytes: Insights into parallel mechanisms of immune evasion. PLoS Negl. Trop. Dis. 2018, 12, e0006404. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Outschoorn, U.E.; Pestell, R.G.; Howell, A.; Tykocinski, M.L.; Nagajyothi, F.; Machado, F.S.; Tanowitz, H.B.; Sotgia, F.; Lisanti, M.P. Energy transfer in “parasitic” cancer metabolism: Mitochondria are the powerhouse and Achilles’ heel of tumor cells. Cell Cycle 2011, 10, 4208–4216. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.; Ganguly, S. Structure activity relationship (SAR) study of benzimidazole scaffold for different biological activities: A mini-review. Eur. J. Med. Chem. 2015, 97, 419–443. [Google Scholar] [CrossRef]
- Bansal, Y.; Silakari, O. The therapeutic journey of benzimidazoles: A review. Bioorg Med. Chem. 2012, 20, 6208–6236. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.J. Modes of action of anthelmintic drugs. Vet. J. 1997, 154, 11–34. [Google Scholar] [CrossRef]
- Dawson, P.J.; Gutteridge, W.E.; Gull, K. A comparison of the interaction of anthelmintic benzimidazoles with tubulin isolated from mammalian tissue and the parasitic nematode Ascaridia galli. Biochem. Pharmacol. 1984, 33, 1069–1074. [Google Scholar] [CrossRef]
- An, G.; Murry, D.J.; Gajurel, K.; Bach, T.; Deye, G.; Stebounova, L.V.; Codd, E.E.; Horton, J.; Gonzalez, A.E.; Garcia, H.H.; et al. Pharmacokinetics, Safety, and Tolerability of Oxfendazole in Healthy Volunteers: A Randomized, Placebo-Controlled First-in-Human Single-Dose Escalation Study. Antimicrob. Agents Chemother. 2019, 63, e02255-18. [Google Scholar] [CrossRef]
- Dogra, N.; Mukhopadhyay, T. Impairment of the ubiquitin-proteasome pathway by methyl N-(6-phenylsulfanyl-1H-benzimidazol-2-yl)carbamate leads to a potent cytotoxic effect in tumor cells: A novel antiproliferative agent with a potential therapeutic implication. J. Biol. Chem. 2012, 287, 30625–30640. [Google Scholar] [CrossRef]
- Duan, Q.; Liu, Y.; Rockwell, S. Fenbendazole as a potential anticancer drug. Anticancer. Res. 2013, 33, 355–362. [Google Scholar]
- Mrkvová, Z.; Uldrijan, S.; Pombinho, A.; Bartůněk, P.; Slaninová, I. Benzimidazoles Downregulate Mdm2 and MdmX and Activate p53 in MdmX Overexpressing Tumor Cells. Molecules 2019, 24, 2152. [Google Scholar] [CrossRef]
- Park, D.; Lee, J.H.; Yoon, S.P. Anti-cancer effects of fenbendazole on 5-fluorouracil-resistant colorectal cancer cells. Korean J. Physiol. Pharmacol. 2022, 26, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Park, D. Fenbendazole Suppresses Growth and Induces Apoptosis of Actively Growing H4IIE Hepatocellular Carcinoma Cells via p21-Mediated Cell-Cycle Arrest. Biol. Pharm. Bull. 2022, 45, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Pan, J.; Ou, F.; Wang, W.; Hu, H.; Chen, L.; Zeng, S.; Zeng, K.; Yu, L. Fenbendazole and its synthetic analog interfere with HeLa cells’ proliferation and energy metabolism via inducing oxidative stress and modulating MEK3/6-p38-MAPK pathway. Chem. Biol. Interact. 2022, 361, 109983. [Google Scholar] [CrossRef] [PubMed]
- KalantarMotamedi, Y.; Ejeian, F.; Sabouhi, F.; Bahmani, L.; Nejati, A.S.; Bhagwat, A.M.; Ahadi, A.M.; Tafreshi, A.P.; Nasr-Esfahani, M.H.; Bender, A. Transcriptional drug repositioning and cheminformatics approach for differentiation therapy of leukaemia cells. Sci. Rep. 2021, 11, 12537. [Google Scholar] [CrossRef]
- Javed, S.; Sharma, B.K.; Sood, S.; Sharma, S.; Bagga, R.; Bhattacharyya, S.; Rayat, C.S.; Dhaliwal, L.; Srinivasan, R. Significance of CD133 positive cells in four novel HPV-16 positive cervical cancer-derived cell lines and biopsies of invasive cervical cancer. BMC Cancer 2018, 18, 357. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.J.; Bian, L.; Fang, Z.H.; Zhang, Q.Y.; Cheng, J.X. CD44+/CD24+ cervical cancer cells resist radiotherapy and exhibit properties of cancer stem cells. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1745–1754. [Google Scholar]
- Abdel-Hamid, N.M.; Fathy, M.; Koike, C.; Yoshida, T.; Okabe, M.; Zho, K.; Abouzied, M.; Nikaido, T. Identification of Chemo and Radio-Resistant Sub-Population of Stem Cells in Human Cervical Cancer HeLa Cells. Cancer Invest. 2021, 39, 661–674. [Google Scholar] [CrossRef]
- de Gooijer, M.C.; van den Top, A.; Bockaj, I.; Beijnen, J.H.; Würdinger, T.; van Tellingen, O. The G2 checkpoint-a node-based molecular switch. FEBS Open Bio 2017, 7, 439–455. [Google Scholar] [CrossRef]
- Toyoshima-Morimoto, F.; Taniguchi, E.; Nishida, E. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep. 2002, 3, 341–348. [Google Scholar] [CrossRef]
- Peng, C.Y.; Graves, P.R.; Thoma, R.S.; Wu, Z.; Shaw, A.S.; Piwnica-Worms, H. Mitotic and G2 checkpoint control: Regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 1997, 277, 1501–1505. [Google Scholar] [CrossRef]
- Leung, T.H.; Tang, H.W.; Siu, M.K.; Chan, D.W.; Chan, K.K.; Cheung, A.N.; Ngan, H.Y. CD71(+) Population Enriched by HPV-E6 Protein Promotes Cancer Aggressiveness and Radioresistance in Cervical Cancer Cells. Mol. Cancer Res. 2019, 17, 1867–1880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Wang, Y.S.; Zhou, T.; Yu, X.W.; Wei, Z.T.; Li, Y.L. Isolation and characterization of cancer stem cells from cervical cancer HeLa cells. Cytotechnology 2012, 64, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, T.; Yadav, J.; Janjua, D.; Chaudhary, A.; Joshi, U.; Senrung, A.; Chhokar, A.; Aggarwal, N.; Bharti, A.C. Targeting Cervical Cancer Stem Cells by Phytochemicals. Curr. Med. Chem. 2024, 31, 5222–5254. [Google Scholar] [CrossRef] [PubMed]
- Maity, A.; McKenna, W.G.; Muschel, R.J. Evidence for post-transcriptional regulation of cyclin B1 mRNA in the cell cycle and following irradiation in HeLa cells. Embo J. 1995, 14, 603–609. [Google Scholar] [CrossRef]
- Jang, W.Y.; Lee, J.Y.; Lee, S.T.; Jun, D.Y.; Kim, Y.H. Inhibition of JNK2 and JNK3 by JNK inhibitor IX induces prometaphase arrest-dependent apoptotic cell death in human Jurkat T cells. Biochem. Biophys. Res. Commun. 2014, 452, 845–851. [Google Scholar] [CrossRef]
- Chen, H.; Shan, J.; Chen, D.; Wang, R.; Qi, W.; Wang, H.; Ke, Y.; Liu, W.; Zeng, X. CtIP promotes G2/M arrest in etoposide-treated HCT116 cells in a p53-independent manner. J. Cell Physiol. 2019, 234, 11871–11881. [Google Scholar] [CrossRef]
- Kang, S.H.; Bak, D.H.; Chung, B.Y.; Bai, H.W. Centipedegrass extract enhances radiosensitivity in melanoma cells by inducing G2/M cell cycle phase arrest. Mol. Biol. Rep. 2021, 48, 1081–1091. [Google Scholar] [CrossRef]
- Son, D.S.; Lee, E.S.; Adunyah, S.E. The Antitumor Potentials of Benzimidazole Anthelmintics as Repurposing Drugs. Immune Netw. 2020, 20, e29. [Google Scholar] [CrossRef]
- Nguyen, J.; Nguyen, T.Q.; Han, B.O.; Hoang, B.X. Oral Fenbendazole for Cancer Therapy in Humans and Animals. Anticancer. Res. 2024, 44, 3725–3735. [Google Scholar] [CrossRef]
- Adham Foumani, E.; Irani, S.; Shokoohinia, Y.; Mostafaie, A. Colchicine of Colchicum autumnale, A Traditional Anti-Inflammatory Medicine, Induces Apoptosis by Activation of Apoptotic Genes and Proteins Expression in Human Breast (MCF-7) and Mouse Breast (4T1) Cell Lines. Cell J. 2022, 24, 647–656. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, X.; Wang, Y.; Chen, Y.; Duan, J.; Gao, X.; Cong, Z. Fenbendazole Exhibits Antitumor Activity Against Cervical Cancer Through Dual Targeting of Cancer Cells and Cancer Stem Cells: Evidence from In Vitro and In Vivo Models. Molecules 2025, 30, 2377. https://doi.org/10.3390/molecules30112377
Lei X, Wang Y, Chen Y, Duan J, Gao X, Cong Z. Fenbendazole Exhibits Antitumor Activity Against Cervical Cancer Through Dual Targeting of Cancer Cells and Cancer Stem Cells: Evidence from In Vitro and In Vivo Models. Molecules. 2025; 30(11):2377. https://doi.org/10.3390/molecules30112377
Chicago/Turabian StyleLei, Xi, Yi Wang, Yuanyuan Chen, Jinyue Duan, Xin Gao, and Zhongyi Cong. 2025. "Fenbendazole Exhibits Antitumor Activity Against Cervical Cancer Through Dual Targeting of Cancer Cells and Cancer Stem Cells: Evidence from In Vitro and In Vivo Models" Molecules 30, no. 11: 2377. https://doi.org/10.3390/molecules30112377
APA StyleLei, X., Wang, Y., Chen, Y., Duan, J., Gao, X., & Cong, Z. (2025). Fenbendazole Exhibits Antitumor Activity Against Cervical Cancer Through Dual Targeting of Cancer Cells and Cancer Stem Cells: Evidence from In Vitro and In Vivo Models. Molecules, 30(11), 2377. https://doi.org/10.3390/molecules30112377




