Valorization Pathway for Grape Pruning and Pomace Waste from the Wine Industry: Energy and Non-Energy Applications
Abstract
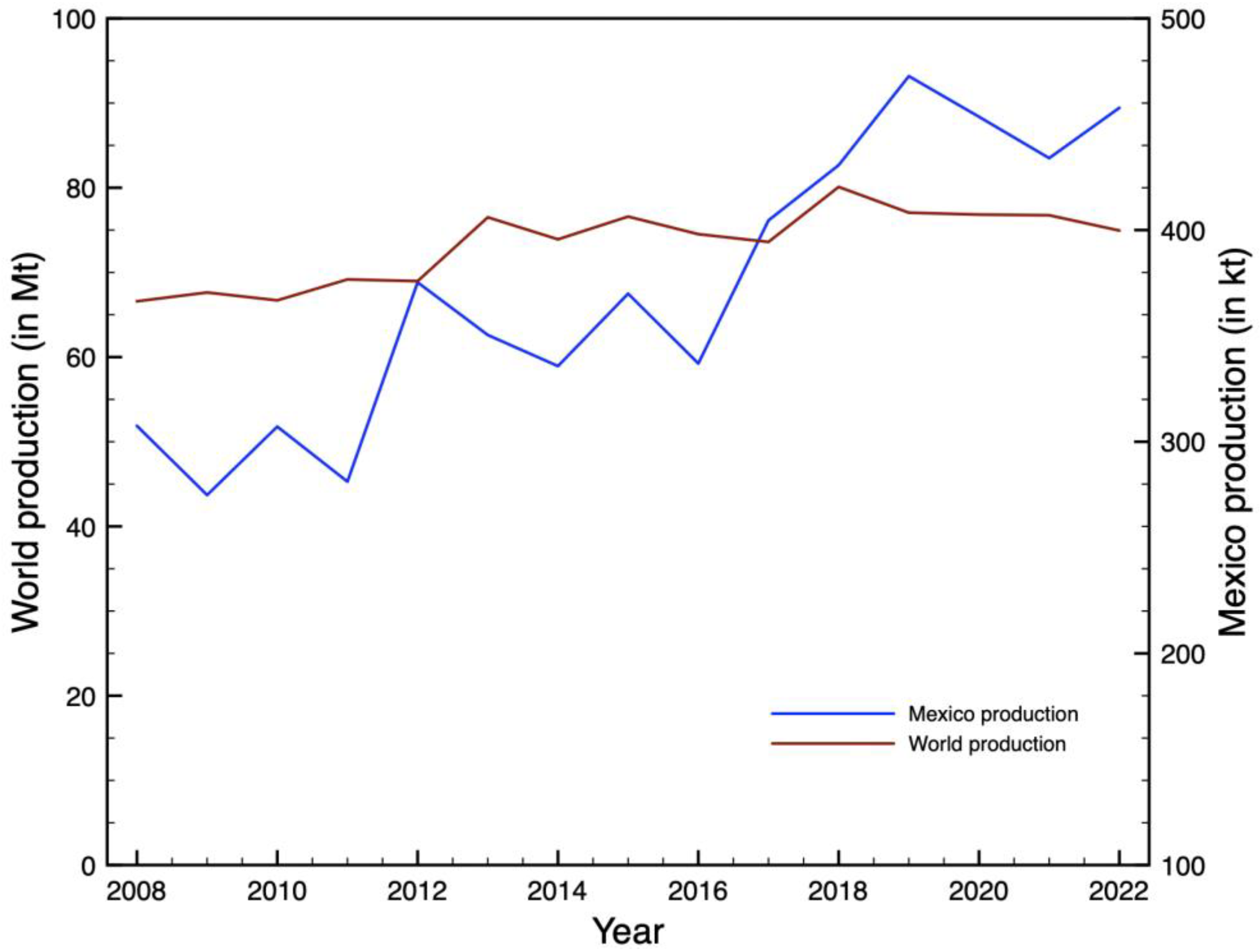
1. Introduction
2. Results and Discussions
2.1. Characterization Results of Grapevine Prunings and Grape Pomace
2.2. Thermogravimetric Analyses Results
2.3. Antioxidant Capacity Results
2.4. HPLC Profiles of Sunflower Oil and Grape Pomace Extract
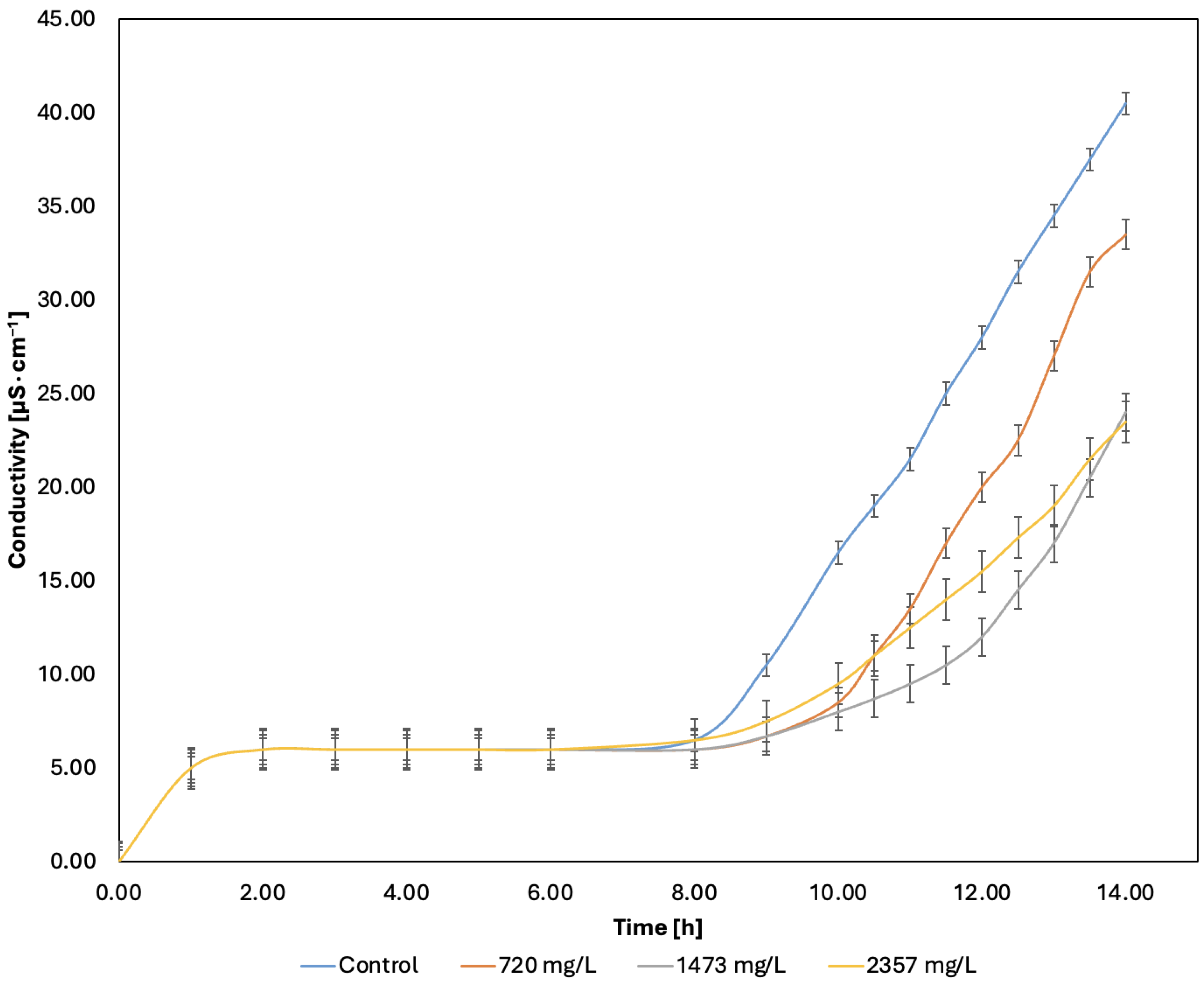
2.5. Oxidative Stability Index Results
3. Materials and Methods
3.1. Sample Collection and Preparation
3.2. Proximate Analysis
3.3. Chemical Composition and Higher Heating Value Analysis
3.4. Elemental, Thermogravimetric, Protein, and Fat Analyses
3.5. Extraction Elaboration
3.6. Antioxidant Capacity and Phytochemical Content
3.6.1. Total Phenolic Content (TPC)
3.6.2. Ferric Reducing Antioxidant Power (FRAP) Assay
3.6.3. ABTS Scavenging
3.6.4. Total Flavonoid Content (TFC)
3.6.5. ORAC Assay
3.6.6. DPPH Free Radical Scavenging
3.6.7. Total Anthocyanin (TA) Content
3.7. HPLC Profile of Grape Pomace Extract and Sunflower Oil
3.7.1. Phenolic Acids
3.7.2. Catechin and Epicatechin Determination
3.8. Oxidative Stability Index of Sunflower Oil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guerrero, R.F.; Biais, B.; Richard, T.; Puertas, B.; Waffo-Teguo, P.; Merillon, J.-M.; Cantos-Villar, E. Grapevine cane’s waste is a source of bioactive stilbenes. Ind. Crops Prod. 2016, 94, 884–892. [Google Scholar] [CrossRef]
- Ali, K.; Maltese, F.; Choi, Y.H.; Verpoorte, R. Metabolic constituents of grapevine and grape-derived products. Phytochem. Rev. 2010, 9, 357–378. [Google Scholar] [CrossRef]
- Fernández-Puratich, H.; Hernández, D.; Tenreiro, C. Analysis of energetic performance of vine biomass residues as an alternative fuel for Chilean wine industry. Renew. Energy 2015, 83, 1260–1267. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations, Crop Production, FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 2 October 2024).
- Sistema De Información Agroalimentaria Y Pesquera, Producción Agrícola, SIAP. Available online: https://www.gob.mx/siap/acciones-y-programas/produccion-agricola-33119 (accessed on 2 October 2024).
- Morandé, J.A.; Stockert, C.M.; Liles, G.C.; Williams, J.N.; Smart, D.R.; Viers, J.H. From berries to blocks: Carbon stock quantification of a California vineyard. Carbon Balance Manag. 2017, 12, 1–12. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Valls, J.; Agnolet, S.; Haas, F.; Struffi, I.; Ciesa, F.; Robatscher, P.; Oberhuber, M. Valorization of Lagrein grape pomace as a source of phenolic compounds: Analysis of the contents of anthocyanins, flavanols and antioxidant activity. Eur. Food Res. Technol. 2017, 243, 2211–2224. [Google Scholar] [CrossRef]
- Tournour, H.H.; Segundo, M.A.; Magalhaes, L.M.; Barreiros, L.; Queiroz, J.; Cunha, L.M. Valorization of grape pomace: Extraction of bioactive phenolics with antioxidant properties. Ind. Crops Prod. 2015, 74, 397–406. [Google Scholar] [CrossRef]
- Haas, I.C.d.S.; Toaldo, I.M.; Burin, V.M.; Bordignon-Luiz, M.T. Extraction optimization for polyphenolic profiling and bioactive enrichment of extractives of non-pomace residue from grape processing. Ind. Crops Prod. 2018, 112, 593–601. [Google Scholar] [CrossRef]
- Pintać, D.; Četojević-Simin, D.; Berežni, S.; Orčić, D.; Mimica-Dukić, N. Investigation of the chemical composition and biological activity of edible grapevine (Vitis vinifera L.) leaf varieties. Food Chem. 2019, 286, 686–695. [Google Scholar] [CrossRef]
- Kontaxi, N.-I.; Panoutsopoulou, E.; Ofrydopolou, A.; Tsoupras, A. Anti-Inflammatory Benefits of Grape Pomace and Tomato Bioactives as Ingredients in Sun Oils against UV Radiation for Skin Protection. Appl. Sci. 2024, 14, 6236. [Google Scholar] [CrossRef]
- García-Lomillo, J.; González-SanJosé, M.L. Applications of wine pomace in the food industry: Approaches and functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef]
- Hubner, A.; Sobreira, F.; Vetore, A.N.; de Pinto, C.A.S.O.; Dario, M.F.; Díaz, I.E.C.; Lourenço, F.R.; Rosado, C.; Baby, A.R.; Bacchi, E.M. The Synergistic Behavior of Antioxidant Phenolic Compounds Obtained from Winemaking Waste’s Valorization, Increased the Efficacy of a Sunscreen System. Antioxidants 2019, 8, 530. [Google Scholar] [CrossRef] [PubMed]
- Gómez, I.; Melero, B.; Jaime, I.; Muñiz, P. Effect of White Pomace Seasoning as a Natural Antioxidant for Chicken Products Packaged in Vacuum or Modified Atmosphere Conditions. Appl. Sci. 2024, 14, 6421. [Google Scholar] [CrossRef]
- Martínez-Antequera, F.P.; Simó-Mirabet, P.; de las Heras, V.; Romá, M.; Mancera, J.M.; Martos-Sitcha, J.A.; Moyano, F.J. Grape pomace in diets for European sea bass: Influence on oxidative status, intestinal microbiota, and fillet quality. Aquac. Int. 2024, 32, 7771–7788. [Google Scholar] [CrossRef]
- Klimek, K.E.; Kapłan, M.; Maj, G.; Buczyński, K. Evaluation of green residues management of selected grape varieties. J. Water Land Dev. 2024, 62, 68–75. [Google Scholar] [CrossRef]
- Madadian, E.; Rahimi, J.; Mohebbi, M.; Simakov, D.S.A. Grape pomace as an energy source for the food industry: A thermochemical and kinetic analysis. Food Bioprod. Process. 2022, 132, 177–187. [Google Scholar] [CrossRef]
- Inga, M.; Betalleluz-Pallardel, I.; Puma-Isuiza, G.; Cumpa-Arias, L.; Osorio, C.; Valdez-Arana, J.C.; Vargas-De-La-Cruz, C. Chemical analysis and bioactive compounds from agrifood by-products of Peruvian crops. Front. Sustain. Food Syst. 2024, 8, 1341895. [Google Scholar] [CrossRef]
- Luz, B.R.T.; da Silva, C.N.; Hercos, G.d.F.d.L.; Ribeiro, B.D.; Egea, M.B.; Lemes, A.C. Innovative Craft Beers Added with Purple Grape Pomace: Exploring Technological, Sensory, and Bioactive Characteristics. Beverages 2024, 10, 80. [Google Scholar] [CrossRef]
- Timón, M.L.; Andrés, A.I.; Petrón, M.J. Antioxidant activity of aqueous extracts obtained from by-products of grape, olive, tomato, lemon, red pepper and pomegranate. Foods 2024, 13, 1802. [Google Scholar] [CrossRef]
- Kabakcı, S.B.; Cevik, B.K.; Ates, E.B.; Borand, M.N. Increasing the energy density of high-moisture wastes of beverage industry by hydrothermal pretreatment: Comparison of thermochemical conversion behaviors. Int. J. Energy Res. 2022, 46, 20369–20385. [Google Scholar] [CrossRef]
- Oliveira, M.; Teixeira, B.M.M.; Toste, R.; Borges, A.D.S. Transforming wine by-products into energy: Evaluating grape pomace and distillation stillage for biomass pellet production. Appl. Sci. 2024, 14, 7313. [Google Scholar] [CrossRef]
- Čabalová, I.; Krilek, J.; Kačík, F.; Lagaña, R.; Jurczyková, T. Valorization of Wood-Based Waste from Grapevine. Forests 2023, 14, 442. [Google Scholar] [CrossRef]
- Bergrath, J.; Zeppetzauer, F.; Rumpf, J.; Kamm, B.; Putz, R.; Kling, H.-W.; Schulze, M. Mechanochemical tailoring of lignin structure: Influence of different particle sizes in the organosolv process. Macromol. Biosci. 2024, 24, 2400090. [Google Scholar] [CrossRef]
- Mangione, R.; Simões, R.; Pereira, H.; Catarino, S.; Ricardo-da-Silva, J.; Miranda, I.; Ferreira-Dias, S. Potential use of grape stems and pomaces from two red grapevine cultivars as source of oligosaccharides. Processes 2022, 10, 1896. [Google Scholar] [CrossRef]
- Baldán, Y.; Riveros, M.; Fabani, M.P.; Rodríguez, R. Grape pomace powder valorization: A novel ingredient to improve the nutritional quality of gluten-free muffins. Biomass Conv. Bioref. 2021, 13, 9997–10009. [Google Scholar] [CrossRef]
- Šelo, G.; Planinic, M.; Tišma, M.; Martinovic, J.; Perkovic, G.; Bucic-Kojic, A. Bioconversion of grape pomace with Rhizopus oryzae under solid-state conditions: Changes in the chemical composition and profile of phenolic compounds. Microorganisms 2023, 11, 956. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion of Biomass. Energy Sources Part A Recovery Util. Environ. Eff. 2007, 29, 549–561. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Nobre, C.; Rodrigues, R.M.; Genisheva, Z.; Botelho, C.; Teixeira, J.A. Extraction of phenolic compounds from grape pomace using ohmic heating: Chemical composition, bioactivity and bioaccessibility. Food Chem. 2024, 436, 137780. [Google Scholar] [CrossRef]
- Madkour, M.; Abdel-Fattah, S.A.; Ali, S.I.; Ali, N.G.M.; Shourrap, M.; Hosny, M.; Elolimy, A.A. Impact of in ovo feeding of grape pomace extract on the growth performance, antioxidant status, and immune response of hatched broilers. Poult. Sci. 2024, 103, 103914. [Google Scholar] [CrossRef]
- Neira-Ospina, E.; Rojas, J.; Santa-González, G.A.; Gil, M.A.G. Phenolic compounds in grapes (genus Vitis): A review of their antioxidant activity, antiproliferative capacity, and cytotoxic effect on colorectal cancer. J. Appl. Pharm. Sci. 2024, 14, 71–89. [Google Scholar] [CrossRef]
- Poblete, J.; Quispe-Fuentes, I.; Aranda, M.; Vega-Gálvez, A. Application of Vacuum and Convective Drying Processes for the Valorization of Pisco Grape Pomace to Enhance the Retention of its Bioactive Compounds. Waste Biomass Valorization 2024, 15, 3093–3107. [Google Scholar] [CrossRef]
- Lizárraga-Chaidez, M.; Abadía-García, L.; Mendoza-Sánchez, M.; Huerta-Manzanilla, E.L.; Mendoza-Sánchez, M. Optimization of the green extraction process of antioxidants derived from grape pomace. Sustain. Chem. Pharm. 2024, 37, 101396. [Google Scholar] [CrossRef]
- Tuhanioglu, A.; Sumanjot, K.; De Barros, G.L.; Ahmadzadeh, S.; Threlfall, R.; Ubeyitogullari, A. Optimizing ethanol–water cosolvent systems for green supercritical carbon dioxide extraction of muscadine grape pomace polyphenols. ACS Omega 2025, 10, 4860–4869. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Domínguez-Perles, R.; Medina, S. Winery by-products as sources of bioactive tryptophan, serotonin, and melatonin: Contributions to the antioxidant power. Foods 2023, 12, 1571. [Google Scholar] [CrossRef]
- Ongkowijoyo, P.; Luna-Vital, D.A.; De Mejia, E.G. Extraction Techniques and Analysis of Anthocyanins from Food Sources by Mass Spectrometry: An Update. Food Chem. 2018, 250, 113–126. [Google Scholar] [CrossRef]
- Choi, Y.M.; Shin, M.J.; Yoon, H.; Lee, S.; Yi, J.; Wang, X.; Desta, K.T. Nutritional qualities, metabolite contents, and antioxidant capacities of Yardlong Beans (Vigna unguiculata subsp. sesquipedalis) of different pod and seed colors. Antioxidants 2024, 13, 1134. [Google Scholar] [CrossRef]
- Córdova, A.; Catalán, A.; Carrasco, V.; Farias, F.O.; Trentin, J.; López, J.; Salazar, F.; Mussagy, C.U. Sustainable assessment of ultrasound-assisted extraction of anthocyanins with bio-based solvents for upgrading grape pomace Cabernet Sauvignon derived from a winemaking process. Ultrason. Sonochem. 2025, 112, 107201. [Google Scholar] [CrossRef]
- Fernández-Pérez, R.; Ayuso, S.; Moreta, C.; Saiz-Abajo, M.-J.; Gastón-Lorente, M.; Ruiz-Larrea, F.; Tenorio, C. Chemical Profile and Antibacterial Activity of Vitis vinifera L. cv Graciano Pomace Extracts Obtained by Green Supercritical CO2 Extraction Method Against Multidrug-Resistant Escherichia coli Strains. Foods 2025, 14, 17. [Google Scholar] [CrossRef]
- Ferrara, A.; D’Auria, D.; Barile, D.; Baller, M.I.; Nitride, C.; Mamone, G.; Ferranti, P. The valorization of grape pomace from Montepulciano winemaking: A new source of functional ingredients for sustainable food industry. Food Res. Int. 2025, 200, 115443. [Google Scholar] [CrossRef]
- Giosuè, A.; Siano, F.; Di Stasio, L.; Picariello, G.; Medoro, C.; Cianciabella, M.; Giacco, R.; Predieri, S.; Vasca, E.; Vaccaro, O.; et al. Turning Wastes into Resources: Red Grape Pomace-Enriched Biscuits with Potential Health-Promoting Properties. Foods 2024, 13, 2195. [Google Scholar] [CrossRef]
- Liberatore, M.T.; Dilucia, F.; Rutigliano, M.; Viscecchia, R.; Spano, G.; Capozzi, T.; Bimbo, F.; Di Luccia, A.; la Gatta, B. Polyphenolic characterization, nutritional and microbiological assessment of newly formulated semolina fresh pasta fortified with grape pomace. Food Chem. 2025, 463, 141531. [Google Scholar] [CrossRef]
- Stanek-Wandzel, N.; Krzyszowska, A.; Zarębska, M.; Gębura, K.; Wasilewski, T.; Hordyjewicz-Baran, Z.; Tomaka, M. Evaluation of Cellulase, Pectinase, and Hemicellulase Effectiveness in Extraction of Phenolic Compounds from Grape Pomace. Int. J. Mol. Sci. 2024, 25, 13538. [Google Scholar] [CrossRef]
- Vlassa, M.; Filip, M.; Tăranu, I.; Marin, D.; Dragomir, C. Evaluation of some bioactive nutraceutical compounds in agro-industrial waste used as animal feed additives. Stud. Univ. Babes-Bolyai Chem. 2024, 69, 107–120. [Google Scholar] [CrossRef]
- Daneshniya, M.; Ebrahimi, P.; Mihaylova, D.; Alam, M.K.; Lante, A. From extraction to the synergistic effects of spent red and white grape pomaces on the oil’s oxidative stability. Food Sci. Appl. Biotechnol. 2025, 8, 89–98. [Google Scholar] [CrossRef]
- Gámez, N.; Noriega, J.; Leyva, L.; Ortega, J.; Bringas, L.; García, H.; Medina, L. Antioxidant activity comparison of thompson grape pomace extract, rosemary and tocopherols in soybean oil. J. Food Process. Preserv. 2009, 33, 110–120. [Google Scholar] [CrossRef]
- Poiana, M.-A.; Moigradean, D.; Dumbrava, D.-G.; Radulov, I.; Raba, D.N.; Rivis, A. Exploring the Potential of Grape Pomace Extract to Inhibit Thermo-Oxidative Degradation of Sunflower Oil: From Routine Tests to ATR-FTIR Spectroscopy. Foods 2022, 11, 3674. [Google Scholar] [CrossRef]
- ASTM E871-82; Standard Test Method for Moisture Analysis of Particulate Wood Fuels. ASTM: West Conshohocken, PA, USA, 2006.
- ASTM E872-82; Standard Test Method for Volatile Matter in the Analysis of Particulate Wood Fuels. ASTM: West Conshohocken, PA, USA, 2006.
- ASTM E830-87; Standard Test Method for Ash in the Analysis Sample of Refuse Derived Fuel. ASTM: West Conshohocken, PA, USA, 2004.
- TAPPI 264 cm-07; Preparation of Wood for Chemical Analysis. TAPPI: Peachtree Corners, GA, USA, 2007.
- Coronado, M.A.; Montero, G.; Montes, D.G.; Valdez-Salas, B.; Ayala, J.R.; García, C.; Carrillo, M.; León, J.A. Physicochemical Characterization and SEM-EDX Analysis of Brewer’s Spent Grain from the Craft Brewery Industry. Sustainability 2020, 12, 7744. [Google Scholar] [CrossRef]
- TAPPI T 207 cm–99; Water Solubility of Wood and Pulp. TAPPI: Peachtree Corners, GA, USA, 1999.
- ASTM D1106-96; Standard Test Method for Acid-Insoluble Lignin in Wood. ASTM: West Conshohocken, PA, USA, 2007.
- ASTM D1104-56; Method of Test for Holocellulose in Wood. ASTM: West Conshohocken, PA, USA, 1978.
- ASTM D1103-60; Method of Test for Alpha-Cellulose in Wood. ASTM: West Conshohocken, PA, USA, 1977.
- ASTM E711; International Standard Test Method for Gross Calorific Value of Refuse-Derived Fuel by the Bomb Calorimeter. ASTM: West Conshohocken, PA, USA, 2004.
- Alzate-Arbeláez, A.F.; Dorta, E.; López-Alarcón, C.; Cortés, F.B.; Rojano, B.A. Immobilization of Andean berry (Vaccinium meridionale) polyphenols on nanocellulose isolated from banana residues: A natural food additive with antioxidant properties. Food Chem. 2019, 294, 503–517. [Google Scholar] [CrossRef]
- Rosales, D.S.; Alzate, A.A.F.; Rojano, B. Antioxidant capacity, bioactive compounds in coffee pulp and implementation in the production of infusions. Acta Sci. Pol. Technol. Aliment. 2019, 18, 235–248. [Google Scholar] [CrossRef]
- Corrales-Bernal, A.; Vergara, A.I.; Rojano, B.; Yahia, E.; Maldonado, M.E. Características nutricionales y antioxidantes de la uchuva colombiana (Physalys peruviana L.) en tres estadios de su maduración. Arch. Latinoam. Nutr. 2015, 65, 254–262. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Kelebek, H.; Selli, S.; Canbas, A.; Cabaroglu, T. HPLC determination of organic acids, sugars, phenolic compositions and antioxidant capacity of orange juice and orange wine made from a Turkish cv. Kozan. Microchem. J. 2019, 91, 187–192. [Google Scholar] [CrossRef]
- Zapata-Vahos, I.C.; Villacorta, V.; Maldonado, M.E.; Castro, D.; Rojano, B. Antioxidant and cytotoxic activity of black and green tea from Vaccinium meridionale Swartz leaves. J. Med. Plants Res. 2015, 9, 445–453. [Google Scholar] [CrossRef]
- Alzate, A.F.A.; Cogollo, P.A.; Rojano, B. Composition, antioxidant activity, thermal and oxidative stability of Lecythis tuyrana oil. J. Food Nutr. Res. 2018, 57, 87–97. [Google Scholar]
- American Oils Chemist Society. Oils Chemist Society. Oil stability index. In Sampling and Analysis of Commercial Fats and Oils AOCS Official Method Cd 12b-92, 6th ed.; AOCS Press: Champaign, IL, USA, 1997; pp. 1–2. [Google Scholar]

| Parameter | Grapevine Prunings | Grape Pomace | ||||
|---|---|---|---|---|---|---|
| Value | Other Authors | Reference | Value | Other Authors | Reference | |
| Humidity (%) | 9.14 ± 0.14 | 9.24 | [17] | 63.2 ± 0.22 | 59.81–72.73 | [19,20,21] |
| Volatile material (%) | 73.05 ± 0.18 | 66.29 | [17] | 79.64 ± 0.12 | 62.07–70.6 | [18,22,23] |
| Ashes (%) | 3.70 ± 0.69 | 12.36 | [17] | 7.36 ± 0.26 | 0.65–8.3 | [18,22,23] |
| Fixed carbon (%) | 23.25 ± 0.62 | 12.12 | [17] | 13.34 ± 0.27 | 25.6–30.95 | [19,20,21] |
| Water extractables (%) | 11.46 ± 0.54 | 14.2 | [24] | 11.29 ± 0.49 | 16.65 | [18] |
| Solvent extractables (%) | 19.72 ± 0.34 | - | - | 13.22 ± 0.62 | 1.63 | [18] |
| Lignocellulose (%) | 17.66 ± 1.07 | 41.10–90.00 | [25,26] | 36.94 ± 1.18 | 46.70–63.00 | [25,26,27] |
| Holocellulose (%) | 78.06 ± 0.71 | - | - | 58.70 ± 0.83 | 21.45–45.00 | [18,28] |
| Cellulose (%) | 48.68 ± 0.056 | - | - | 16.76 ± 0.44 | 12.9–19.00 | [18,28] |
| Hemicellulose (%) | 29.41 ± 0.86 | - | - | 41.93 ± 0.72 | 8.55–26.00 | [18,28] |
| HHV (MJ/kg) | 17.02 ± 0.26 | 14.36 | [17] | 20.74 ± 0.33 | 18.76–19.67 | [18,22,23] |
| Protein (%/g d.w.) | 5.51 ± 0.19 | - | - | 30.48 ± 0.38 | 1.30–10.04 | [19,20,27] |
| Fat content (%) | - | - | - | 4.485 ± 0.11 | 3.67 | [19] |
| C (%) | 43.07 | 39.88–46.20 | [17,18,19,20,21,22,23,24] | 49.32 | 49.10–53.71 | [18,22,23] |
| H (%) | 5.31 | 7.16 | [17,18,19,20,21,22,23,24] | 6.07 | 5.14–6.28 | [18,22,23] |
| O (%) | 40.93 | 0.16 | [17,18,19,20,21,22,23,24] | - | 33.33–38.46 | [18,22,23] |
| N (%) | 0.78 | 0.60–1.86 | [17,18,19,20,21,22,23,24] | 3.54 | 1.17–2.94 | [18,22,23] |
| S (%) | - | 38.83 | [17,18,19,20,21,22,23,24] | 0.55 | 0.04–1.15 | [18,22,23] |
| Analysis | Current Work | Other Authors | |||||
|---|---|---|---|---|---|---|---|
| Ex1 | Ex2 | Ex3 | Ex4 | Ex5 | Range | Reference | |
| DPPH (μmol Trolox/100 g sample) | 8699.17 ± 745.56 | 9180.13 ± 46.46 | 1762.06 ± 129.17 | 1278.96 ± 64.15 | 1704.41 ± 16.78 | 3355–6000 | [20,30,31] |
| FRAP (mg ascorbic acid/100 g sample) | 2003.45 ± 88.79 | 1551.33 ± 79.06 | 474.6 ± 23.33 | 553.92 ± 42.44 | 2179.19 ± 36.51 | 402–649 | [20,30,32] |
| TPC (mg gallic acid/100 g sample) | 1261.42 ± 12.48 | 1220.28 ± 26.71 | 350.98 ± 17.99 | 470.3 ± 17.13 | 1668.10 ± 4.76 | 386.62–8700 | [20,30,31,32] |
| TFC (mg Eq. catechin/100 g sample) | 589.07 ± 31.78 | 545.07 ± 28.45 | 210.49 ± 8.90 | 289.63 ± 8.63 | 1330.39 ± 43.92 | 742–2632 | [30,31,33] |
| ABTS (μmol Trolox/100 g sample) | 31,636.15 ± 2000.97 | 42,813.45 ± 2433.87 | 12,026.03 ± 541.57 | 6093.58 ± 409.10 | 48,271.31 ± 1544.47 | 3176.32–2573.80 | [20,34,35] |
| ORAC (μmol Trolox/100 g sample) | 28,724.52 ± 2016.07 | 34,554.21 ± 1987.52 | 8565.85 ± 608.34 | 13,329.37 ± 23.14 | 53,694.93 ± 1524.28 | 3641–76,854.80 | [32,33,36] |
| Anthocyanins (mg cyanidin 3-glucoside/100 g sample) | 7.04 ± 1.79 | 7.5 ± 0.29 | 4.67 ± 0.76 | 6.063 ± 0.19 | 12.61 ± 1.59 | 136.83–837 | [20,30,31] |
| PK | RT | Area Pct | Library ID | Ref | CAS | Type |
|---|---|---|---|---|---|---|
| 1 | 17.7982 | 6.5918 | Hexadecanoic acid, methyl ester | 115,367 | 000112-39-0 | Saturated |
| 2 | 20.5202 | 44.5955 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 116,129 | 000112-63-0 | Omega-6 |
| 3 | 20.6523 | 28.4879 | 7-Octadecenoic acid, methyl ester | 13,049 | 057396-98-2 | Omega-6 |
| 4 | 21.0399 | 6.2836 | Octadecanoic acid, methyl ester | 28,371 | 000112-61-8 | Omega-9 |
| 5 | 23.9116 | 0.4666 | Eicosanoic acid, methyl ester | 115,427 | 001120-28-1 | Omega-9 |
| 6 | 26.5455 | 1.5713 | Docosanoic acid, methyl ester | 115,474 | 000929-77-1 | Omega-9 |
| 7 | 29.4348 | 0.776 | Eicosanoic acid, methyl ester | 140,310 | 001120-28-1 | Omega-9 |
| 8 | 33.1874 | 1.8384 | 1-Docosene | 129,889 | 001599-67-3 | Omega-6 |
| 9 | 38.9837 | 2.8077 | 2-(2-Bromoethyl)cyclohexanone | 60,261 | 1000195-45-9 | Omega-9 |
| 10 | 46.7708 | 5.1199 | 2-Dodecylcyclobutanone | 83,998 | 035493-46-0 | Omega-9 |
| 11 | 53.0603 | 1.4612 | Carbonic acid, methyl tridecyl ester | 97,434 | 1000314-62-4 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala, J.R.; Rojano, B.A.; Coronado, M.A.; Alzate-Arbeláez, A.F.; Sagaste, C.A.; Vélez, A.D.; Montes, D.G. Valorization Pathway for Grape Pruning and Pomace Waste from the Wine Industry: Energy and Non-Energy Applications. Molecules 2025, 30, 2332. https://doi.org/10.3390/molecules30112332
Ayala JR, Rojano BA, Coronado MA, Alzate-Arbeláez AF, Sagaste CA, Vélez AD, Montes DG. Valorization Pathway for Grape Pruning and Pomace Waste from the Wine Industry: Energy and Non-Energy Applications. Molecules. 2025; 30(11):2332. https://doi.org/10.3390/molecules30112332
Chicago/Turabian StyleAyala, José R., Benjamín A. Rojano, Marcos A. Coronado, Andrés Felipe Alzate-Arbeláez, Carlos A. Sagaste, Angie D. Vélez, and Daniela G. Montes. 2025. "Valorization Pathway for Grape Pruning and Pomace Waste from the Wine Industry: Energy and Non-Energy Applications" Molecules 30, no. 11: 2332. https://doi.org/10.3390/molecules30112332
APA StyleAyala, J. R., Rojano, B. A., Coronado, M. A., Alzate-Arbeláez, A. F., Sagaste, C. A., Vélez, A. D., & Montes, D. G. (2025). Valorization Pathway for Grape Pruning and Pomace Waste from the Wine Industry: Energy and Non-Energy Applications. Molecules, 30(11), 2332. https://doi.org/10.3390/molecules30112332