Preparation and Electrochemical Characteristics of the Co-Doped Li7La3Zr2O12 Solid Electrolyte with Fe3+ and Bi3+
Abstract
1. Introduction
2. Results and Discussion
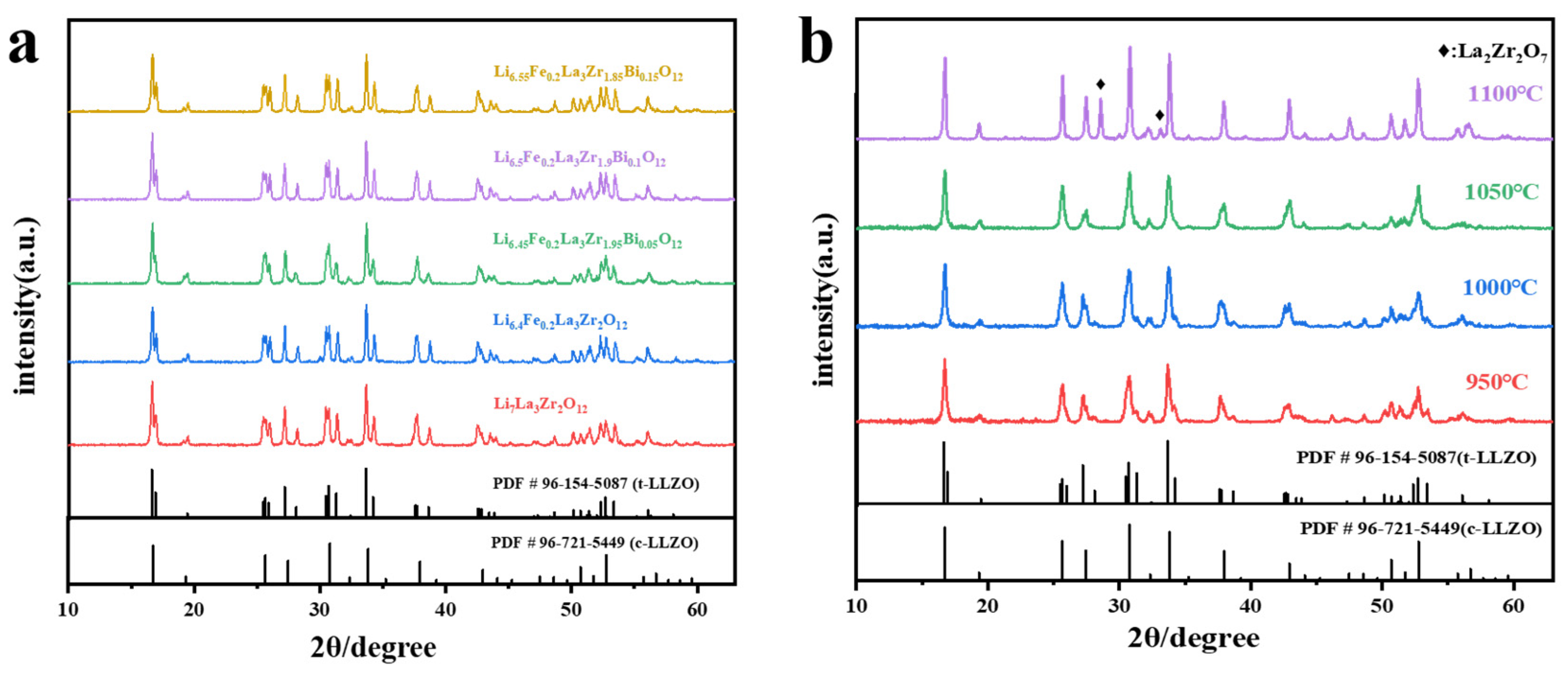
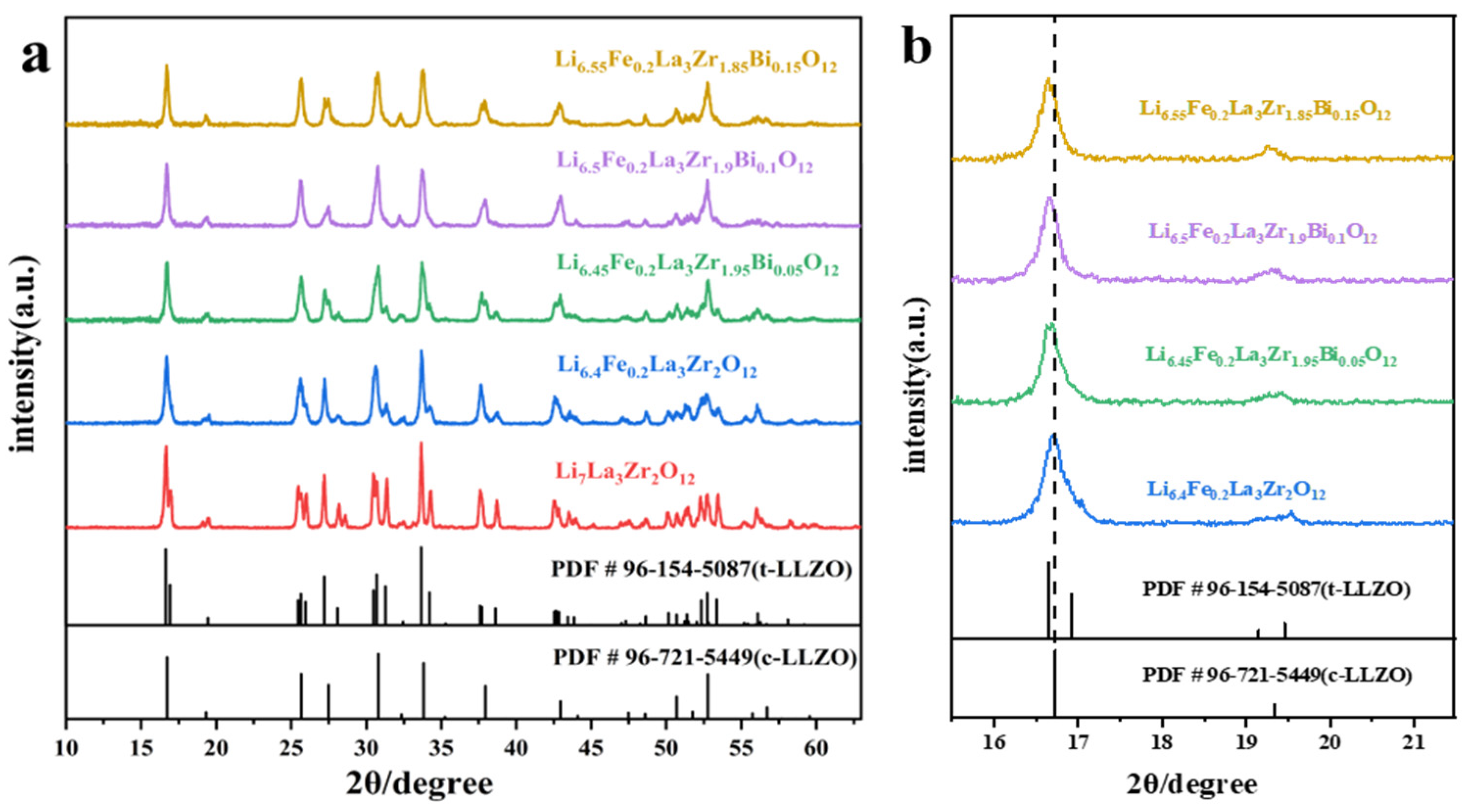
2.1. Phase Analysis
2.2. Analysis of the Valence of Fe and Bi
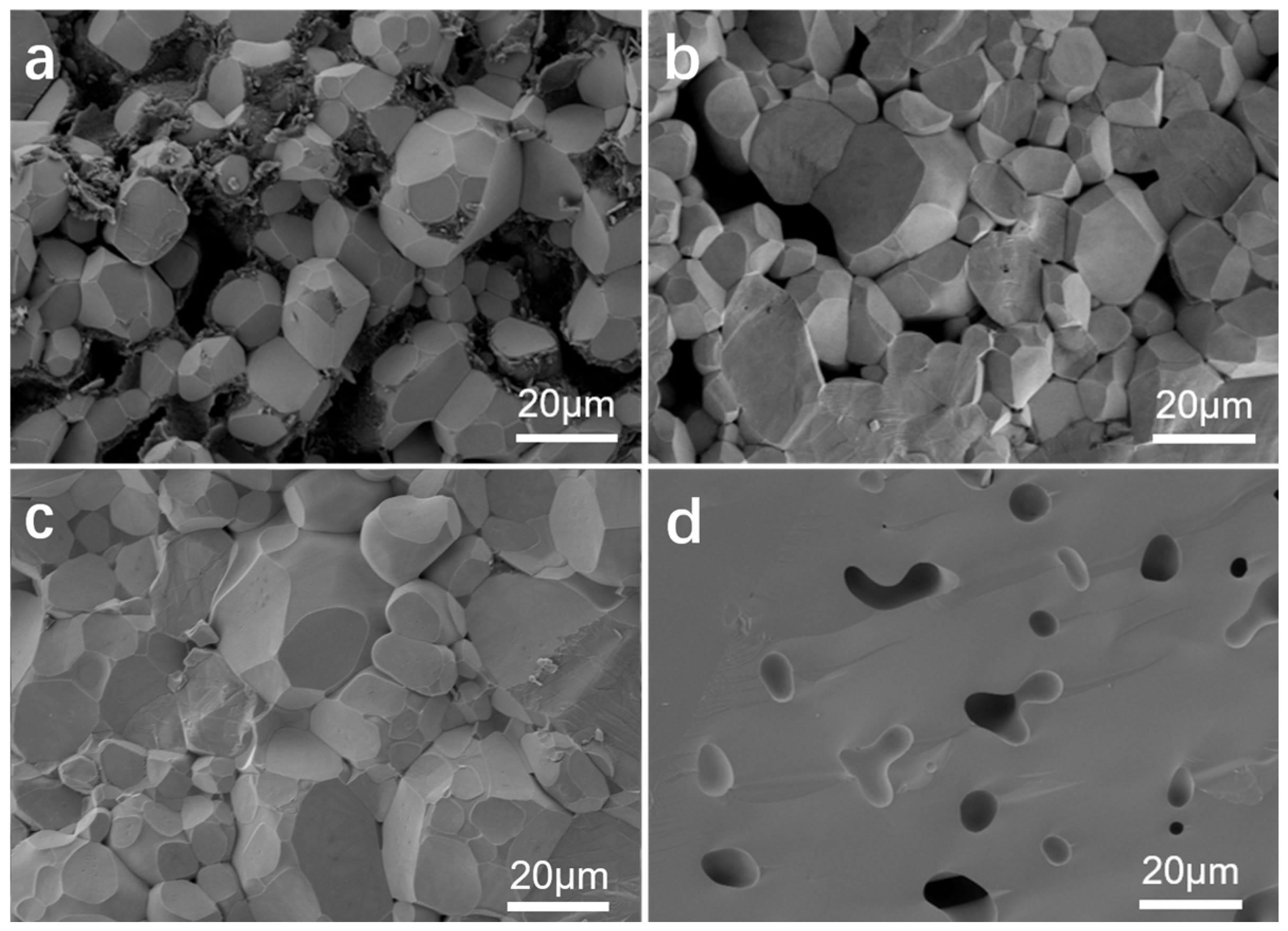
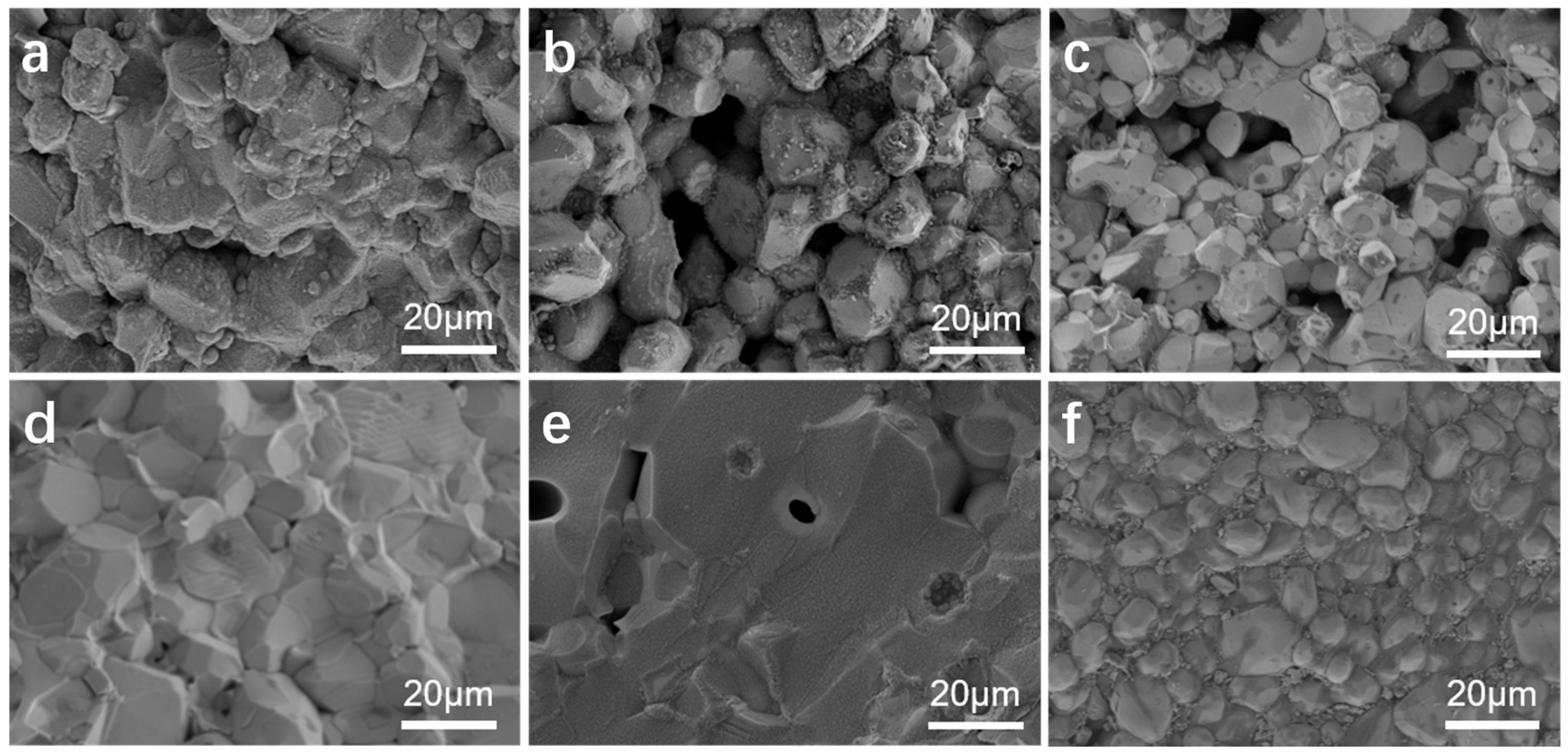
2.3. Microscopic Morphology Analysis
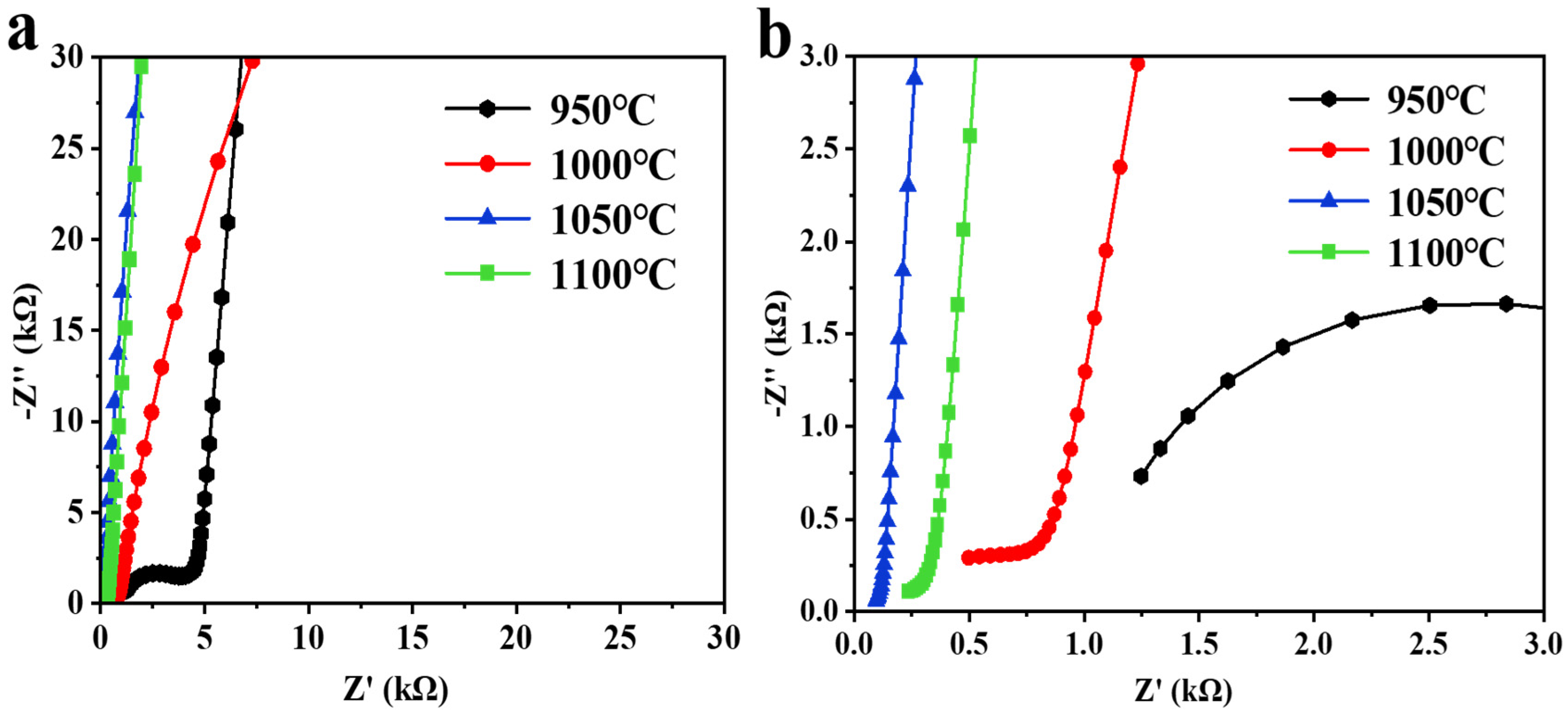
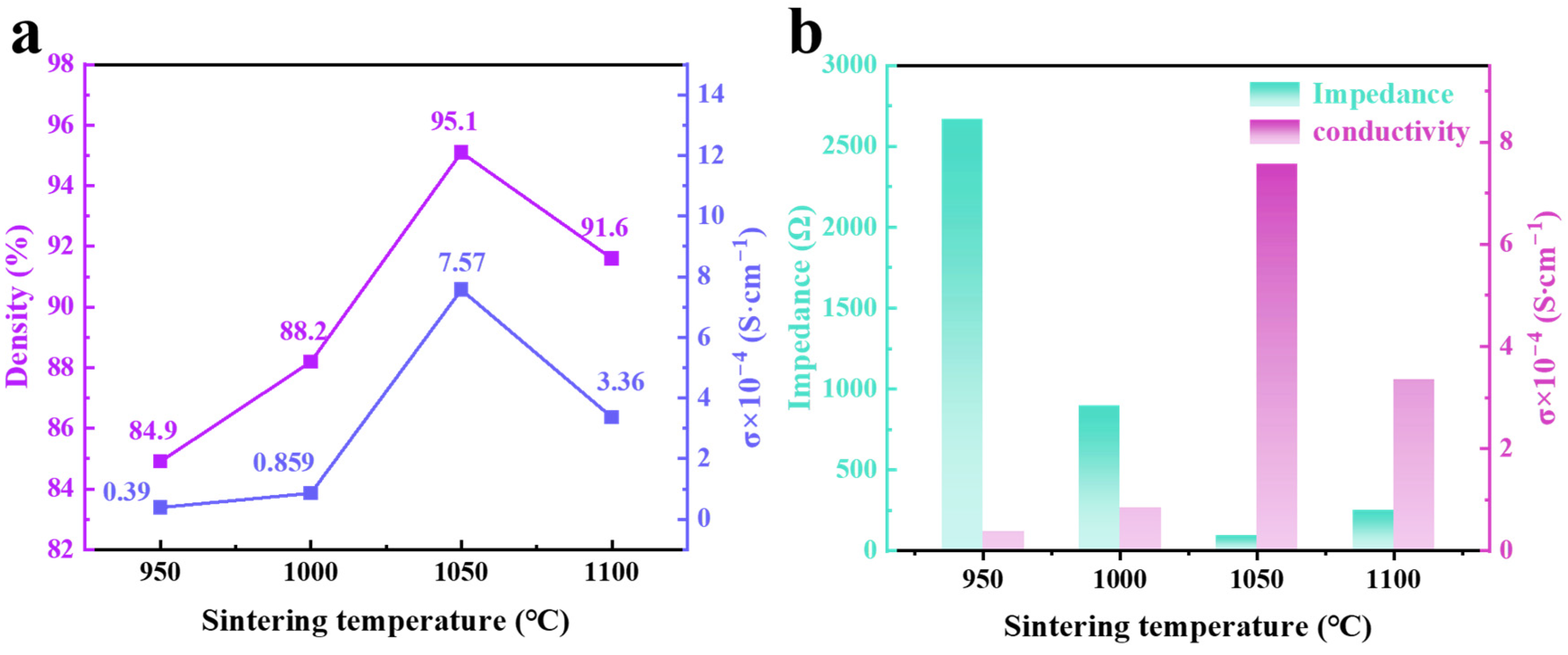
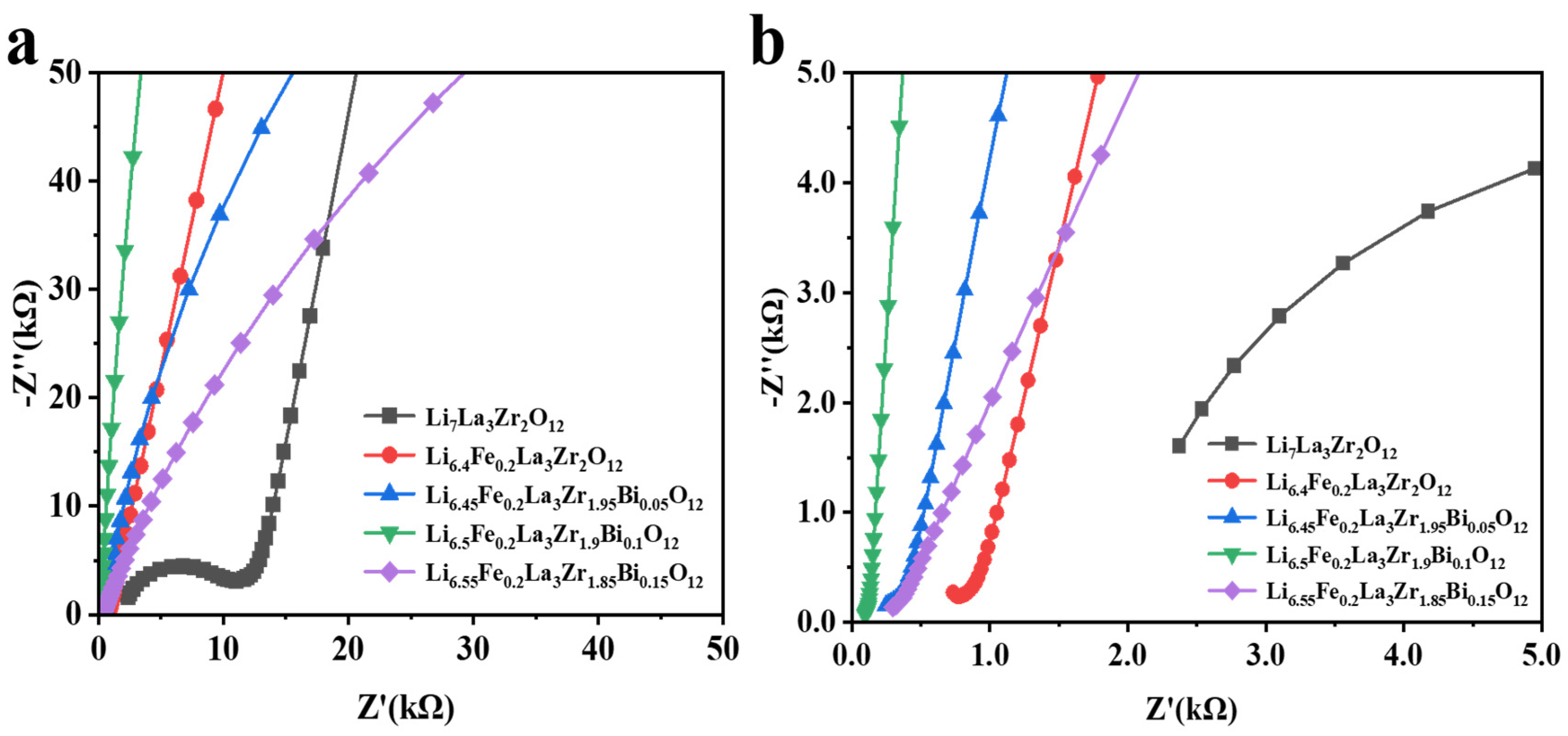
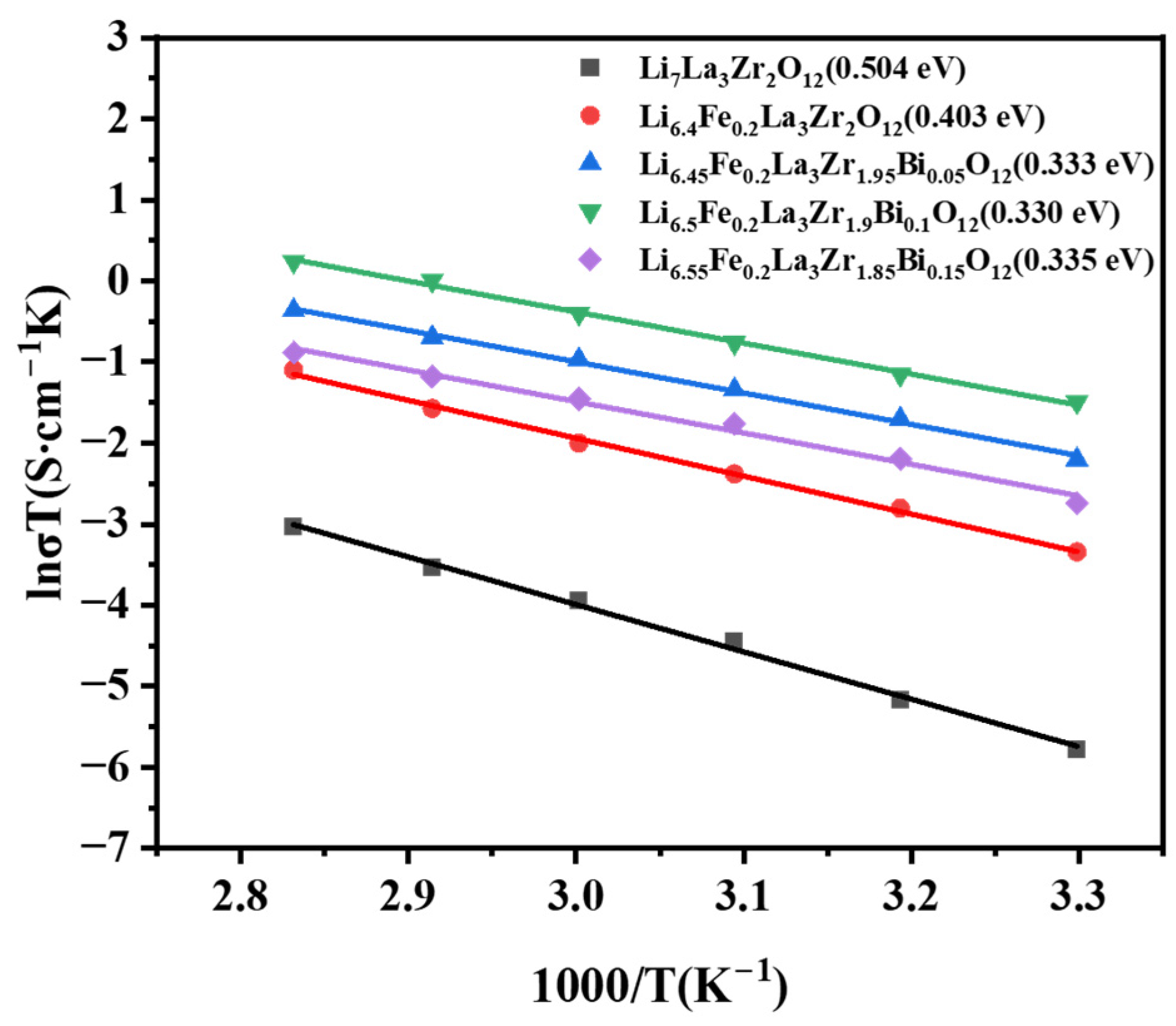
2.4. Ion Conductivity Analysis
3. Materials and Methods
3.1. Material Synthesis
3.2. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; He, W.; Ding, L.-X.; Wang, S.; Wang, H. Enhancing interfacial contact in all solid state batteries with a cathode-supported solid electrolyte membrane framework. Energy Environ. Sci. 2019, 12, 938–944. [Google Scholar] [CrossRef]
- Zhang, L.; Zhuang, Q.; Zheng, R.; Wang, Z.; Sun, H.; Arandiyan, H.; Wang, Y.; Liu, Y.; Shao, Z. Recent advances of Li7La3Zr2O12-based solid-state lithium batteries towards high energy density. Energy Storage Mater. 2022, 49, 299–338. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Cui, Y.; Da, H.; Cai, Y.; Zhang, S. Constructing interfacial gradient layers and enhancing lithium salt dissolution kinetics for high-rate solid-state batteries. Nano Energy 2022, 102, 107716. [Google Scholar] [CrossRef]
- Albertus, P.; Anandan, V.; Ban, C.; Balsara, N.; Belharouak, I.; Buettner-Garrett, J.; Chen, Z.; Daniel, C.; Doeff, M.; Dudney, N.J.; et al. Challenges for and Pathways toward Li-Metal-Based All-Solid-State Batteries. ACS Energy Lett. 2021, 6, 1399–1404. [Google Scholar] [CrossRef]
- Sun, H.; Kang, S.; Cui, L. Prospects of LLZO type solid electrolyte: From material design to battery application. Chem. Eng. J. 2023, 454, 140375. [Google Scholar] [CrossRef]
- Jonson, R.A.; Battaglia, V.S.; Tucker, M.C. Lithium Batteries with Small-Molecule Quinone Cathode Enabled by Lithium Garnet Separators. ACS Appl. Energy Mater. 2023, 6, 745–752. [Google Scholar] [CrossRef]
- Teng, Y.; Liu, H.; Wang, Q.; He, Y.; Hua, Y.; Li, C.; Bai, J. In-doped Li7La3Zr2O12 nanofibers enhances electrochemical properties and conductivity of PEO-based composite electrolyte in all-solid-state lithium battery. J. Energy Storage 2024, 76, 109784. [Google Scholar] [CrossRef]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, E.D.; Mo, Y.; et al. Garnet-Type Solid-State Electrolytes: Materials, Interfaces, and Batteries. Chem. Rev. 2020, 120, 4257–4300. [Google Scholar] [CrossRef]
- Neises, J.; Scheld, W.S.; Seok, A.-R.; Lobe, S.; Finsterbusch, M.; Uhlenbruck, S.; Schmechel, R.; Benson, N. Study of thermal material properties for Ta- and Al-substituted Li7La3Zr2O12 (LLZO) solid-state electrolyte in dependency of temperature and grain size. J. Mater. Chem. A 2022, 10, 12177–12186. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, J.; Khalid, B.; Zijian, Z.; Wen, X.; Geng, C.; Huang, Y.; Tian, G. Sintering analysis of garnet-type ceramic as oxide solid electrolytes for rapid Li+ migration. J. Eur. Ceram. Soc. 2022, 42, 7063–7071. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem.-Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef]
- Lu, F.-F.; Tian, H.-K. Dopant-induced modulation of lithium-ion conductivity in cubic garnet solid electrolytes: A first-principles study. Phys. Chem. Chem. Phys. 2023, 25, 18973–18982. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, J.; Qiang, W.; Huang, B. Synthesis of mixed ionic and electronic conducting garnet with doping of transition elements (Fe, Co, Ni). Ceram. Int. 2020, 46, 3731–3737. [Google Scholar] [CrossRef]
- Han, S.; Wang, Z.; Ma, Y.; Miao, Y.; Wang, X.; Wang, Y.; Wang, Y. Fast ion-conducting high-entropy garnet solid-state electrolytes with excellent air stability. J. Adv. Ceram. 2023, 12, 1201–1213. [Google Scholar] [CrossRef]
- Gonzalez Puente, P.M.; Song, S.; Cao, S.; Rannalter, L.Z.; Pan, Z.; Xiang, X.; Shen, Q.; Chen, F. Garnet-type solid electrolyte: Advances of ionic transport performance and its application in all-solid-state batteries. J. Adv. Ceram. 2021, 10, 933–972. [Google Scholar] [CrossRef]
- Dhivya, L.; Karthik, K.; Ramakumar, S.; Murugan, R. Facile synthesis of high lithium ion conductive cubic phase lithium garnets for electrochemical energy storage devices. RSC Adv. 2015, 5, 96042–96051. [Google Scholar] [CrossRef]
- Kotobuki, M.; Yan, B.; Lu, L.; Hanc, E.; Molenda, J. Study on stabilization of cubic Li7La3Zr2O12 by Ge substitution in various atmospheres. Funct. Mater. Lett. 2017, 9, 1642005. [Google Scholar] [CrossRef]
- Schwab, C.; Häuschen, G.; Mann, M.; Roitzheim, C.; Guillon, O.; Fattakhova-Rohlfing, D.; Finsterbusch, M. Towards economic processing of high performance garnets—Case study on zero Li excess Ga-substituted LLZO. J. Mater. Chem. A 2023, 11, 5670–5680. [Google Scholar] [CrossRef]
- Li, J.; Luo, H.; Liu, K.; Zhang, J.; Zhai, H.; Su, X.; Wu, J.; Tang, X.; Tan, G. Excellent Stability of Ga-Doped Garnet Electrolyte against Li Metal Anode via Eliminating LiGaO2 Precipitates for Advanced All-Solid-State Batteries. ACS Appl. Mater. Interfaces 2023, 15, 7165–7174. [Google Scholar] [CrossRef]
- Geiger, C.A.; Alekseev, E.; Lazic, B.; Fisch, M.; Armbruster, T.; Langner, R.; Fechtelkord, M.; Kim, N.; Pettke, T.; Weppner, W. Crystal Chemistry and Stability of “Li7La3Zr2O12” Garnet: A Fast Lithium-Ion Conductor. Inorg. Chem. 2010, 50, 1089–1097. [Google Scholar] [CrossRef]
- Matsui, M.; Takahashi, K.; Sakamoto, K.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Imanishi, N. Phase stability of a garnet-type lithium ion conductor Li7La3Zr2O12. Dalton Trans. 2014, 43, 1019–1024. [Google Scholar] [CrossRef]
- Dhivya, L.; Murugan, R. Effect of Simultaneous Substitution of Y and Ta on the Stabilization of Cubic Phase, Microstructure, and Li+ Conductivity of Li7La3Zr2O12 Lithium Garnet. ACS Appl. Mater. Interfaces 2014, 6, 17606–17615. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, K.; Nakata, Y.; Matsui, M.; Uechi, I.; Takeda, Y.; Yamamoto, O.; Imanishi, N. Stability of Nb-Doped Cubic Li7La3Zr2O12 with Lithium Metal. J. Electrochem. Soc. 2013, 160, A1690–A1693. [Google Scholar] [CrossRef]
- Song, S.; Yan, B.; Zheng, F.; Duong, H.M.; Lu, L. Crystal structure, migration mechanism and electrochemical performance of Cr-stabilized garnet. Solid State Ion. 2014, 268, 135–139. [Google Scholar] [CrossRef]
- Huang, M.; Shoji, M.; Shen, Y.; Nan, C.-W.; Munakata, H.; Kanamura, K. Preparation and electrochemical properties of Zr-site substituted Li7La3(Zr2−xMx)O12 (M = Ta, Nb) solid electrolytes. J. Power Sources 2014, 261, 206–211. [Google Scholar] [CrossRef]
- Im, C.; Park, D.; Kim, H.; Lee, J. Al-incorporation into Li7La3Zr2O12 solid electrolyte keeping stabilized cubic phase for all-solid-state Li batteries. J. Energy Chem. 2018, 27, 1501–1508. [Google Scholar] [CrossRef]
- Schwanz, D.K.; Villa, A.; Balasubramanian, M.; Helfrecht, B.; Marinero, E.E. Bi aliovalent substitution in Li7La3Zr2O12 garnets: Structural and ionic conductivity effects. AIP Adv. 2020, 10, 035204. [Google Scholar] [CrossRef]
- Villa, A.; Verduzco, J.C.; Libera, J.A.; Marinero, E.E. Ionic conductivity optimization of composite polymer electrolytes through filler particle chemical modification. Ionics 2021, 27, 2483–2493. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, L.; Elkedim, O.; Xie, Y.; Luo, Y.; Chen, Q.; Zhang, Y.; Chen, Y. Sr2+ and Mo6+ co-doped Li7La3Zr2O12 with superior ionic conductivity. J. Alloys Compd. 2022, 891, 161906. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Wang, X.; Liu, G.; Yu, W.; Dong, X.; Wang, J. The synergistic effects of dual substitution of Bi and Ce on ionic conductivity of Li7La3Zr2O12 solid electrolyte. Ceram. Int. 2024, 50, 6472–6480. [Google Scholar] [CrossRef]
- Kobi, S.; Amardeep; Vyas, A.; Bhargava, P.; Mukhopadhyay, A. Al and Mg Co-Doping Towards Development of Air-Stable and Li-Ion Conducting Li-La-Zirconate Based Solid Electrolyte Exhibiting Low Electrode/Electrolyte Interfacial Resistance. J. Electrochem. Soc. 2020, 167, 120519. [Google Scholar] [CrossRef]
- Liu, X.-Z.; Ding, L.; Liu, Y.-Z.; Xiong, L.-P.; Chen, J.; Luo, X.-L. Room-temperature ionic conductivity of Ba, Y, Al co-doped Li7La3Zr2O12 solid electrolyte after sintering. Rare Metals 2020, 40, 2301–2306. [Google Scholar] [CrossRef]
- Hong, S.; Song, S.H.; Cho, M.; Kim, S.; Yu, S.H.; Lee, D.; Kim, H. Structural and Chemical Compatibilities of Li1−xNi0.5Co0.2Mn0.3O2 Cathode Material with Garnet-Type Solid Electrolyte for All-Solid-State Batteries. Small 2021, 17, 2103306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Paggiaro, G.; Okur, F.; Huwiler, J.; Cancellieri, C.; Jeurgens, L.P.H.; Chernyshov, D.; van Beek, W.; Kovalenko, M.V.; Kravchyk, K.V. On High-Temperature Thermal Cleaning of Li7La3Zr2O12 Solid-State Electrolytes. ACS Appl. Energy Mater. 2023, 6, 6972–6980. [Google Scholar] [CrossRef]
- Geng, H.; Chen, K.; Yi, D.; Mei, A.; Huang, M.; Lin, Y.; Nan, C. Formation Mechanism of Garnet-Like Li7La3Zr2O12 Powder Prepared by Solid State Reaction. Rare Met. Mater. Eng. 2016, 45, 612–616. [Google Scholar] [CrossRef]
- Zhang, N.; Long, X.; Wang, Z.; Yu, P.; Han, F.; Fu, J.; Ren, G.; Wu, Y.; Zheng, S.; Huang, W.; et al. Mechanism Study on the Interfacial Stability of a Lithium Garnet-Type Oxide Electrolyte against Cathode Materials. ACS Appl. Energy Mater. 2018, 1, 5968–5976. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Y.; Gao, X.; Lv, C.; Luo, D.; Xiang, X. High-efficiency and low-cost preparation of solid electrolytes Li7La3Zr2O12 based on molten salt method. J. Alloys Compd. 2021, 881, 160620. [Google Scholar] [CrossRef]
- Xiang, X.; Liu, Y.; Chen, F.; Yang, W.; Yang, J.; Ma, X.; Chen, D.; Su, K.; Shen, Q.; Zhang, L. Crystal structure and lithium ionic transport behavior of Li site doped Li7La3Zr2O12. J. Eur. Ceram. Soc. 2020, 40, 3065–3071. [Google Scholar] [CrossRef]
- Rettenwander, D.; Geiger, C.A.; Amthauer, G. Synthesis and Crystal Chemistry of the Fast Li-Ion Conductor Li7La3Zr2O12 Doped with Fe. Inorg. Chem. 2013, 52, 8005–8009. [Google Scholar] [CrossRef]
- Kotobuki, M.; Munakata, H.; Kanamura, K.; Sato, Y.; Yoshida, T. Compatibility of Li7La3Zr2O12 Solid Electrolyte to All-Solid-State Battery Using Li Metal Anode. J. Electrochem. Soc. 2010, 157, A1076–A1079. [Google Scholar] [CrossRef]
- Kumazaki, S.; Iriyama, Y.; Kim, K.-H.; Murugan, R.; Tanabe, K.; Yamamoto, K.; Hirayama, T.; Ogumi, Z. High lithium ion conductive Li7La3Zr2O12 by inclusion of both Al and Si. Electrochem. Commun. 2011, 13, 509–512. [Google Scholar] [CrossRef]
- Guo, S.; Li, Y.; Zhang, H.; Cao, Z.; Wang, L.; Li, G. The effect of Y doping on the bottleneck size, microstructure and ionic conductivity of Li6.4Fe0.2La3-xYxZr2O12 solid electrolytes. Ceram. Int. 2024, 50, 559–565. [Google Scholar] [CrossRef]
- Zhong, Y.; Cao, C.; Tadé, M.O.; Shao, Z. Ionically and Electronically Conductive Phases in a Composite Anode for High-Rate and Stable Lithium Stripping and Plating for Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2022, 14, 38786–38794. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Cao, C.; Zhao, L.; Tadé, M.O.; Shao, Z. Optimization of two-dimensional solid-state electrolyte–anode interface by integrating zinc into composite anode with dual-conductive phases. Green Carbon 2024, 2, 94–100. [Google Scholar] [CrossRef]
- Tenhaeff, W.E.; Rangasamy, E.; Wang, Y.; Sokolov, A.P.; Wolfenstine, J.; Sakamoto, J.; Dudney, N.J. Resolving the Grain Boundary and Lattice Impedance of Hot-Pressed Li7La3Zr2O12 Garnet Electrolytes. ChemElectroChem 2013, 1, 375–378. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Rangasamy, E.; Allen, J.L.; Sakamoto, J. High conductivity of dense tetragonal Li7La3Zr2O12. J. Power Sources 2012, 208, 193–196. [Google Scholar] [CrossRef]
- Burbano, M.; Carlier, D.; Boucher, F.; Morgan, B.J.; Salanne, M. Sparse Cyclic Excitations Explain the Low Ionic Conductivity of Stoichiometric Li7La3Zr2O12. Phys. Rev. Lett. 2016, 116, 135901. [Google Scholar] [CrossRef]
- Meier, K.; Laino, T.; Curioni, A. Solid-State Electrolytes: Revealing the Mechanisms of Li-Ion Conduction in Tetragonal and Cubic LLZO by First-Principles Calculations. J. Phys. Chem. C 2014, 118, 6668–6679. [Google Scholar] [CrossRef]
- Buschmann, H.; Dölle, J.; Berendts, S.; Kuhn, A.; Bottke, P.; Wilkening, M.; Heitjans, P.; Senyshyn, A.; Ehrenberg, H.; Lotnyk, A.; et al. Structure and dynamics of the fast lithium ion conductor “Li7La3Zr2O12”. Phys. Chem. Chem. Phys. 2011, 13, 19378–19392. [Google Scholar] [CrossRef]
- Eggestad, K.; Selbach, S.M.; Williamson, B.A.D. Doping implications of Li solid state electrolyte Li7La3Zr2O12. J. Mater. Chem. A 2024, 12, 15666–15675. [Google Scholar] [CrossRef]
- Brugge, R.H.; Hekselman, A.K.O.; Cavallaro, A.; Pesci, F.M.; Chater, R.J.; Kilner, J.A.; Aguadero, A. Garnet Electrolytes for Solid State Batteries: Visualization of Moisture-Induced Chemical Degradation and Revealing Its Impact on the Li-Ion Dynamics. Chem. Mater. 2018, 30, 3704–3713. [Google Scholar] [CrossRef]
- Jin, Y.; McGinn, P.J. Li7La3Zr2O12 electrolyte stability in air and fabrication of a Li/Li7La3Zr2O12/Cu0.1V2O5 solid-state battery. J. Power Sources 2013, 239, 326–331. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, J.; Wang, Q.; Pan, X.; Wang, J.; Guan, W.; Yang, J. Synthesis and characterization of Li7La3Zr2O12 via a novel solid-liquid route. Solid State Ion. 2020, 345, 115179. [Google Scholar] [CrossRef]



| Element | Signal Type | Wt % Sigma | At % |
|---|---|---|---|
| O | EDS | 0.13 | 70.15 |
| Mg | EDS | 0.04 | 0.11 |
| Fe | EDS | 0.12 | 1.12 |
| Zr | EDS | 0.19 | 10.81 |
| La | EDS | 0.24 | 17.26 |
| Bi | EDS | 0.19 | 0.55 |
| Total content | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, J.; Duan, X.; Xue, K.; An, S. Preparation and Electrochemical Characteristics of the Co-Doped Li7La3Zr2O12 Solid Electrolyte with Fe3+ and Bi3+. Molecules 2025, 30, 2028. https://doi.org/10.3390/molecules30092028
Qu J, Duan X, Xue K, An S. Preparation and Electrochemical Characteristics of the Co-Doped Li7La3Zr2O12 Solid Electrolyte with Fe3+ and Bi3+. Molecules. 2025; 30(9):2028. https://doi.org/10.3390/molecules30092028
Chicago/Turabian StyleQu, Jialu, Xingyu Duan, Ke Xue, and Shengli An. 2025. "Preparation and Electrochemical Characteristics of the Co-Doped Li7La3Zr2O12 Solid Electrolyte with Fe3+ and Bi3+" Molecules 30, no. 9: 2028. https://doi.org/10.3390/molecules30092028
APA StyleQu, J., Duan, X., Xue, K., & An, S. (2025). Preparation and Electrochemical Characteristics of the Co-Doped Li7La3Zr2O12 Solid Electrolyte with Fe3+ and Bi3+. Molecules, 30(9), 2028. https://doi.org/10.3390/molecules30092028







