Occurrence, Sources, and Prioritization of Per- and Polyfluoroalkyl Substances (PFASs) in Drinking Water from Yangtze River Delta, China: Focusing on Emerging PFASs
Abstract
1. Introduction
2. Results and Discussion
2.1. Concentrations and Compositions of PFASs in Drinking Water
2.2. Contamination Profile Analysis
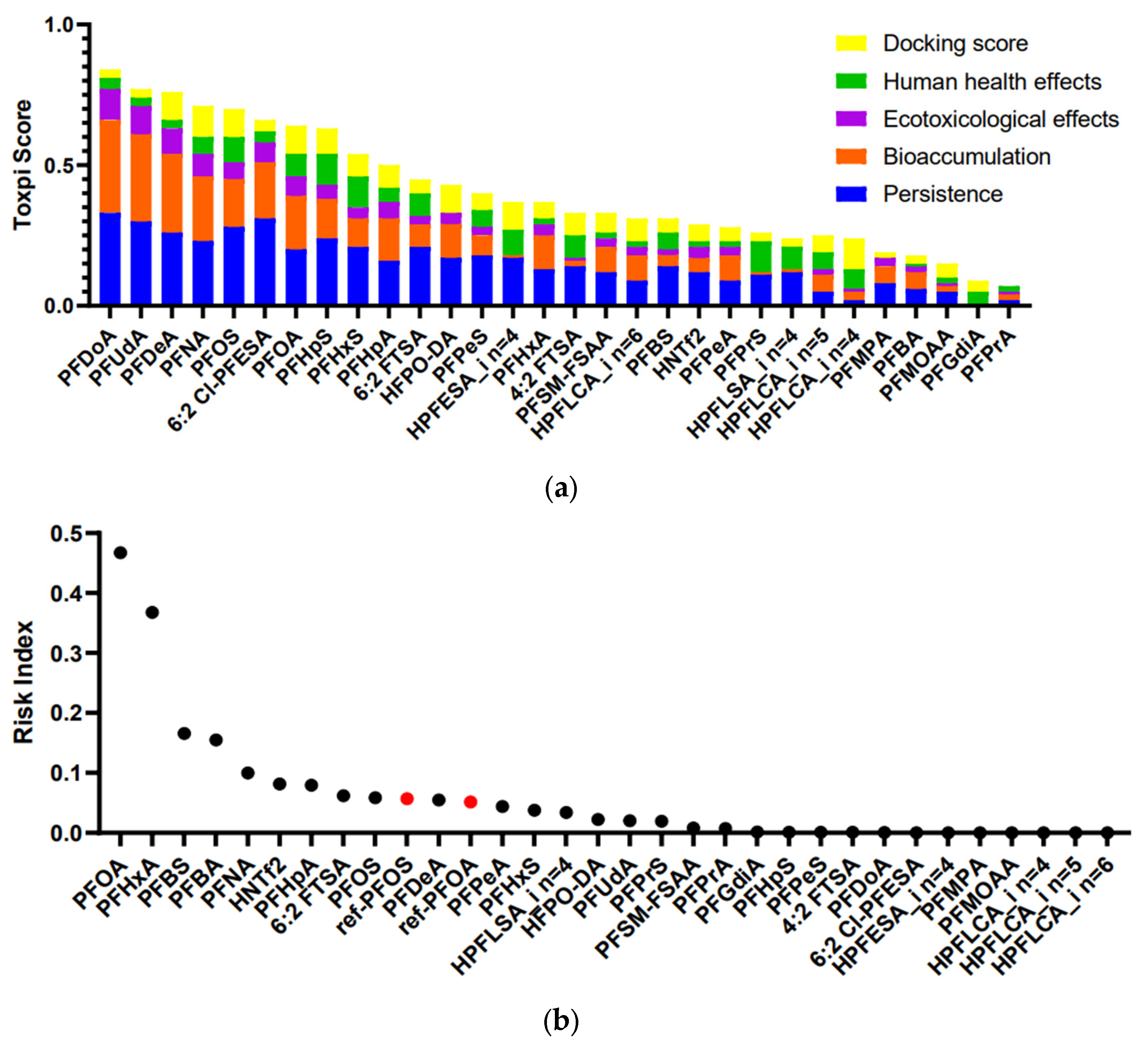
2.3. Prioritization and Risk Assessment of Identified PFASs
3. Materials and Methods
3.1. Chemicals and Standards
3.2. Sample Collections and Pretreatment
3.3. Instrumental Analysis
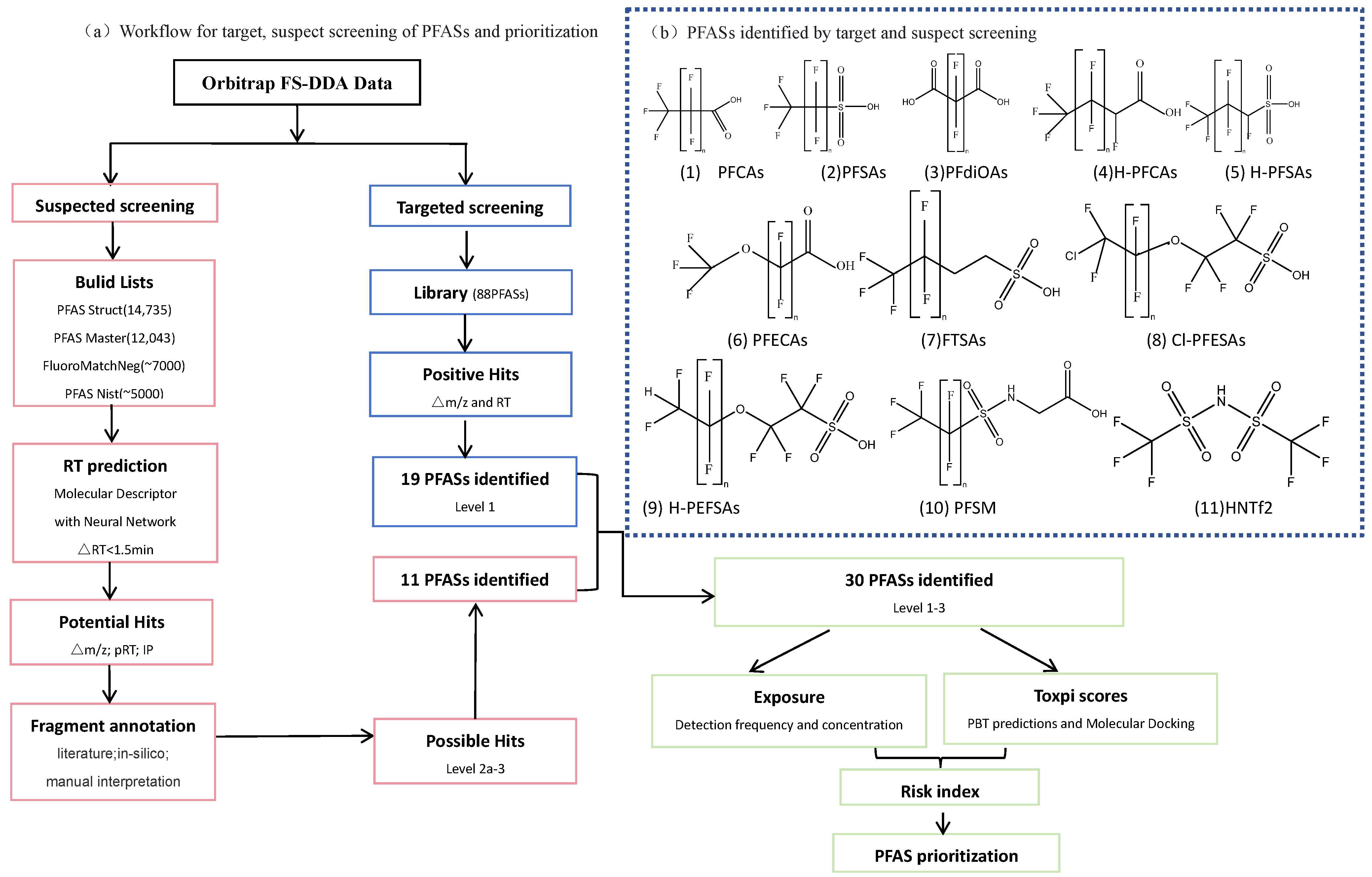
3.4. PFAS Screening Workflow
3.5. Semi-Quantification of Suspect PFASs
3.6. Quality Assurance/Quality Control (QA/QC)
3.7. Molecular Docking
3.8. Prioritization and Risk Ranking of Identified PFASs
3.9. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antonopoulou, M.; Spyrou, A.; Tzamaria, A.; Efthimiou, I.; Triantafyllidis, V. Current state of knowledge of environmental occurrence, toxic effects, and advanced treatment of PFOS and PFOA. Sci. Total Environ. 2024, 913, 169332. [Google Scholar] [CrossRef] [PubMed]
- Barreca, S.; Busetto, M.; Colzani, L.; Clerici, L.; Marchesi, V.; Tremolada, L.; Daverio, D.; Dellavedova, P. Hyphenated High Performance Liquid Chromatography–Tandem Mass Spectrometry Techniques for the Determination of Perfluorinated Alkylated Substances in Lombardia Region in Italy, Profile Levels and Assessment: One Year of Monitoring Activities During 2018. Separations 2020, 7, 17. [Google Scholar] [CrossRef]
- Panieri, E.; Baralic, K.; Djukic-Cosic, D.; Buha Djordjevic, A.; Saso, L. PFAS Molecules: A Major Concern for the Human Health and the Environment. Toxics 2022, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Evich, M.G.; Davis, M.J.; McCord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J.; et al. Per- and polyfluoroalkyl substances in the environment. Science 2022, 375, eabg9065. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Green, M.P.; Shearer, C.; Patrick, R.; Kabiri, S.; Rivers, N.; Nixon, B. The perils of poly- and perfluorinated chemicals on the reproductive health of humans, livestock, and wildlife. Reprod. Fertil. Dev. 2024, 36, RD24034. [Google Scholar] [CrossRef]
- Maxwell, D.L.; Oluwayiose, O.A.; Houle, E.; Roth, K.; Nowak, K.; Sawant, S.; Paskavitz, A.L.; Liu, W.; Gurdziel, K.; Petriello, M.C.; et al. Mixtures of per- and polyfluoroalkyl substances (PFAS) alter sperm methylation and long-term reprogramming of offspring liver and fat transcriptome. Environ. Int. 2024, 186, 108577. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- Li, K.; Gao, P.; Xiang, P.; Zhang, X.; Cui, X.; Ma, L.Q. Molecular mechanisms of PFOA-induced toxicity in animals and humans: Implications for health risks. Environ. Int. 2017, 99, 43–54. [Google Scholar] [CrossRef]
- Chen, X.; Lv, Z.; Yang, Y.; Yang, R.; Shan, G.; Zhu, L. Screening Novel Per- and Polyfluoroalkyl Substances in Human Blood Based on Nontarget Analysis and Underestimated Potential Health Risks. Environ. Sci. Technol. 2024, 58, 150–159. [Google Scholar] [CrossRef]
- Wang, X.; Yu, N.; Jiao, Z.; Li, L.; Yu, H.; Wei, S. Machine learning-enhanced molecular network reveals global exposure to hundreds of unknown PFAS. Sci. Adv. 2024, 10, eadn1039. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lyu, Y.; Chen, H.; Cai, L.; Li, J.; Cao, X.; Sun, W. Integration of target, suspect, and nontarget screening with risk modeling for per- and polyfluoroalkyl substances prioritization in surface waters. Water Res. 2023, 233, 119735. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Lin, Y.; Le, S.; Ji, J.; Chen, Y.; Wang, G.; Xiao, P.; Zhao, Y.; Lu, D. Suspect, Nontarget Screening, and Toxicity Prediction of Per- and Polyfluoroalkyl Substances in the Landfill Leachate. Environ. Sci. Technol. 2024, 58, 4737–4750. [Google Scholar] [CrossRef]
- Yu, N.; Wen, H.; Wang, X.; Yamazaki, E.; Taniyasu, S.; Yamashita, N.; Yu, H.; Wei, S. Nontarget Discovery of Per- and Polyfluoroalkyl Substances in Atmospheric Particulate Matter and Gaseous Phase Using Cryogenic Air Sampler. Environ. Sci. Technol. 2020, 54, 3103–3113. [Google Scholar] [CrossRef]
- Li, L.; Yu, N.; Wang, X.; Shi, W.; Liu, H.; Zhang, X.; Yang, L.; Pan, B.; Yu, H.; Wei, S. Comprehensive Exposure Studies of Per- and Polyfluoroalkyl Substances in the General Population: Target, Nontarget Screening, and Toxicity Prediction. Environ. Sci. Technol. 2022, 56, 14617–14626. [Google Scholar] [CrossRef]
- Pan, Y.; Wen, B.; Zhang, H.; Zhang, S. Comparison of 6:2 chlorinated polyfluorinated ether sulfonate (6:2 Cl-PFESA) and perfluorooctane sulfonate (PFOS) accumulation and toxicity in mung bean. Environ. Pollut. 2021, 287, 117332. [Google Scholar] [CrossRef]
- Lin, H.; Wu, H.; Liu, F.; Yang, H.; Shen, L.; Chen, J.; Zhang, X.; Zhong, Y.; Zhang, H.; Liu, Z. Assessing the hepatotoxicity of PFOA, PFOS, and 6:2 Cl-PFESA in black-spotted frogs (Rana nigromaculata) and elucidating potential association with gut microbiota. Environ. Pollut. 2022, 312, 120029. [Google Scholar] [CrossRef]
- Hong, J.; Du, K.; Jin, H.; Chen, Y.; Jiang, Y.; Zhang, W.; Chen, D.; Zheng, S.; Cao, L. Evidence of promoting effects of 6:2 Cl-PFESA on hepatocellular carcinoma proliferation in humans: An ideal alternative for PFOS in terms of environmental health? Environ. Int. 2024, 186, 108582. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Liu, J.; Zaya, G.; Wang, Y.; Xiang, Q.; Li, J.; Wu, Y. 6:2 Chlorinated Polyfluoroalkyl Ether Sulfonates Exert Stronger Thyroid Homeostasis Disruptive Effects in Newborns than Perfluorooctanesulfonate: Evidence Based on Bayesian Benchmark Dose Values from a Population Study. Environ. Sci. Technol. 2023, 57, 11489–11498. [Google Scholar] [CrossRef]
- Jane, L.E.L.; Yamada, M.; Ford, J.; Owens, G.; Prow, T.; Juhasz, A. Health-related toxicity of emerging per- and polyfluoroalkyl substances: Comparison to legacy PFOS and PFOA. Environ. Res. 2022, 212, 113431. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res. 2019, 177, 108648. [Google Scholar] [CrossRef] [PubMed]
- Per- and Polyfluoroalkyl Substances (PFAS) Final PFAS National Primary Drinking Water Regulation. Available online: https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas (accessed on 15 May 2025).
- Manojkumar, Y.; Pilli, S.; Rao, P.V.; Tyagi, R.D. Sources, occurrence and toxic effects of emerging per- and polyfluoroalkyl substances (PFAS). Neurotoxicol. Teratol. 2023, 97, 107174. [Google Scholar] [CrossRef] [PubMed]
- Jiao, E.; Larsson, P.; Wang, Q.; Zhu, Z.; Yin, D.; Kärrman, A.; van Hees, P.; Karlsson, P.; Qiu, Y.; Yeung, L.W.Y. Further Insight into Extractable (Organo)fluorine Mass Balance Analysis of Tap Water from Shanghai, China. Environ. Sci. Technol. 2023, 57, 14330–14339. [Google Scholar] [CrossRef]
- Munoz, G.; Liu, M.; Duy, S.V.; Liu, J.; Sauvé, S. Target and nontarget screening of PFAS in drinking water for a large-scale survey of urban and rural communities in Quebec, Canada. Water Res. 2023, 233, 119750. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Hu, L.-X.; Liu, T.; Zhao, J.-H.; Yang, Y.-Y.; Liu, Y.-S.; Ying, G.-G. Per- and polyfluoralkyl substances (PFAS) in drinking water system: Target and non-target screening and removal assessment. Environ. Int. 2022, 163, 107219. [Google Scholar] [CrossRef]
- Weed, R.A.; Campbell, G.; Brown, L.; May, K.; Sargent, D.; Sutton, E.; Burdette, K.; Rider, W.; Baker, E.S.; Enders, J.R. Non-Targeted PFAS Suspect Screening and Quantification of Drinking Water Samples Collected through Community Engaged Research in North Carolina’s Cape Fear River Basin. Toxics 2024, 12, 403. [Google Scholar] [CrossRef]
- Zhang, S.; Chou, L.; Zhu, W.; Luo, W.; Zhang, C.; Qiu, J.; Li, M.; Tan, H.; Guo, J.; Wang, C.; et al. Identify organic contaminants of high-concern based on non-targeted toxicity testing and non-targeted LC-HRMS analysis in tap water and source water along the Yangtze River. Water Res. 2024, 253, 121303. [Google Scholar] [CrossRef]
- Reif, D.M.; Sypa, M.; Lock, E.F.; Wright, F.A.; Wilson, A.; Cathey, T.; Judson, R.R.; Rusyn, I. ToxPi GUI: An interactive visualization tool for transparent integration of data from diverse sources of evidence. Bioinformatics 2013, 29, 402–403. [Google Scholar] [CrossRef]
- Marvel, S.W.; To, K.; Grimm, F.A.; Wright, F.A.; Rusyn, I.; Reif, D.M. ToxPi Graphical User Interface 2.0: Dynamic exploration, visualization, and sharing of integrated data models. BMC Bioinform. 2018, 19, 80. [Google Scholar] [CrossRef]
- Fleming, J.F.; House, J.S.; Chappel, J.R.; Motsinger-Reif, A.A.; Reif, D.M. Guided optimization of ToxPi model weights using a Semi-Automated approach. Comput Toxicol. 2024, 29, 100294. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Yin, Y.; Zhao, L.; Zhou, R.; Cui, Y.; Wang, Y.; Wang, P.; Li, X. Organophosphate esters in milk across thirteen countries from 2020 to 2023: Concentrations, sources, temporal trends and ToxPi priority to humans. J. Hazard. Mater. 2024, 473, 134632. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Fan, J.; Guo, C.; Chen, M.; Lv, J.; Sun, W.; Xi, B.; Xu, J. Comprehensive investigation and risk assessment of organic contaminants in Yellow River Estuary using suspect and nontarget screening strategies. Environ. Int. 2023, 173, 107843. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Huang, K.; Han, B.; Li, R.; Shen, Y.; Zhuang, Z.; Wang, Z.; Wang, L.; Zhou, Y.; et al. Human Biomonitoring of Environmental Chemicals among Elderly in Wuhan, China: Prioritizing Risks Using EPA’s ToxCast Database. Environ. Sci. Technol. 2024, 58, 10001–10014. [Google Scholar] [CrossRef]
- Zhao, F.; Li, L.; Chen, Y.; Huang, Y.; Keerthisinghe, T.P.; Chow, A.; Dong, T.; Jia, S.; Xing, S.; Warth, B.; et al. Risk-Based Chemical Ranking and Generating a Prioritized Human Exposome Database. Environ. Health Perspect. 2021, 129, 47014. [Google Scholar] [CrossRef]
- Sun, R.; Wu, M.; Tang, L.; Li, J.; Qian, Z.; Han, T.; Xu, G. Perfluorinated compounds in surface waters of Shanghai, China: Source analysis and risk assessment. Ecotoxicol. Environ. Saf. 2018, 149, 88–95. [Google Scholar] [CrossRef]
- Chai, J.F.; Lei, P.-H.; Xia, X.-Y.; Xu, G.; Wang, D.-J.; Sun, R.; Gu, J.-Z.; Tang, L. Pollution patterns and characteristics of perfluorinated compounds in surface water adjacent potential industrial emission categories of Shanghai, China. Ecotoxicol. Environ. Saf. 2017, 145, 659–664. [Google Scholar] [CrossRef]
- Kim, Y.; Pike, K.A.; Gray, R.; Sprankle, J.W.; Faust, J.A.; Edmiston, P.L. Non-targeted identification and semi-quantitation of emerging per- and polyfluoroalkyl substances (PFAS) in US rainwater. Environ. Sci. Process Impacts 2023, 25, 1771–1787. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, S.; Wang, B.; Chen, X.; Lu, G. Comparison of developmental toxicity induced by PFOA, HFPO-DA, and HFPO-TA in zebrafish embryos. Chemosphere 2023, 311, 136999. [Google Scholar] [CrossRef]
- Li, C.H.; Ren, X.M.; Guo, L.H. Adipogenic Activity of Oligomeric Hexafluoropropylene Oxide (Perfluorooctanoic Acid Alternative) through Peroxisome Proliferator-Activated Receptor gamma Pathway. Environ. Sci. Technol. 2019, 53, 3287–3295. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, Z.; Chen, M. Immunotoxicity and Transcriptome Analyses of Zebrafish (Danio rerio) Embryos Exposed to 6:2 FTSA. Toxics 2023, 11, 459. [Google Scholar] [CrossRef]
- Sheng, N.; Zhou, X.; Zheng, F.; Pan, Y.; Guo, X.; Guo, Y.; Sun, Y.; Dai, J. Comparative hepatotoxicity of 6:2 fluorotelomer carboxylic acid and 6:2 fluorotelomer sulfonic acid, two fluorinated alternatives to long-chain perfluoroalkyl acids, on adult male mice. Arch. Toxicol. 2017, 91, 2909–2919. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Kong, Y.; Yuan, Q.; Wei, Z.; Gu, J.; Ji, C.; Jin, H.; Zhao, M. The toxic mechanism of 6:2 Cl-PFESA in adolescent male rats: Endocrine disorders and liver inflammation regulated by the gut microbiota-gut-testis/liver axis. J. Hazard. Mater. 2023, 459, 132155. [Google Scholar] [CrossRef]
- Wang, X.; Yu, N.; Qian, Y.; Shi, W.; Zhang, X.; Geng, J.; Yu, H.; Wei, S. Non-target and suspect screening of per- and polyfluoroalkyl substances in Chinese municipal wastewater treatment plants. Water Res. 2020, 183, 115989. [Google Scholar] [CrossRef]
- Moskalik, M.Y.; Astakhova, V.V. Triflamides and Triflimides: Synthesis and Applications. Molecules 2022, 27, 5201. [Google Scholar] [CrossRef]
- Pan, Z.; Miao, W.; Wang, C.; Tu, W.; Jin, C.; Jin, Y. 6:2 Cl-PFESA has the potential to cause liver damage and induce lipid metabolism disorders in female mice through the action of PPAR-gamma. Environ. Pollut. 2021, 287, 117329. [Google Scholar] [CrossRef]
- Charbonnet, J.A.; McDonough, C.A.; Xiao, F.; Schwichtenberg, T.; Cao, D.; Kaserzon, S.; Thomas, K.V.; Dewapriya, P.; Place, B.J.; Schymanski, E.L.; et al. Communicating Confidence of Per- and Polyfluoroalkyl Substance Identification via High-Resolution Mass Spectrometry. Environ. Sci. Technol. Lett. 2022, 9, 473–481. [Google Scholar] [CrossRef]
- Kowalska, D.; Sosnowska, A.; Bulawska, N.; Stępnik, M.; Besselink, H.; Behnisch, P.; Puzyn, T. How the Structure of Per- and Polyfluoroalkyl Substances (PFAS) Influences Their Binding Potency to the Peroxisome Proliferator-Activated and Thyroid Hormone Receptors-An In Silico Screening Study. Molecules 2023, 28, 479. [Google Scholar] [CrossRef]
- Li, L.; Guo, Y.; Ma, S.; Wen, H.; Li, Y.; Qiao, J. Association between exposure to per- and perfluoroalkyl substances (PFAS) and reproductive hormones in human: A systematic review and meta-analysis. Environ. Res. 2024, 241, 117553. [Google Scholar] [CrossRef]
- De Toni, L.; Di Nisio, A.; Rocca, M.S.; Pedrucci, F.; Garolla, A.; Dall’Acqua, S.; Guidolin, D.; Ferlin, A.; Foresta, C. Comparative Evaluation of the Effects of Legacy and New Generation Perfluoralkyl Substances (PFAS) on Thyroid Cells In Vitro. Front. Endocrinol. 2022, 13, 915096. [Google Scholar] [CrossRef]
- Benninghoff, A.D.; Bisson, W.H.; Koch, D.C.; Ehresman, D.J.; Kolluri, S.K.; Williams, D.E. Estrogen-like activity of perfluoroalkyl acids in vivo and interaction with human and rainbow trout estrogen receptors in vitro. Toxicol. Sci. 2011, 120, 42–58. [Google Scholar] [CrossRef]
- Tachachartvanich, P.; Singam, E.R.A.; Durkin, K.A.; Furlow, J.D.; Smith, M.T.; La Merrill, M.A. In Vitro characterization of the endocrine disrupting effects of per- and poly-fluoroalkyl substances (PFASs) on the human androgen receptor. J. Hazard. Mater. 2022, 429, 128243. [Google Scholar] [CrossRef] [PubMed]


| Category | Structure/Proposed Structure | Acronym | N | CL | DF | Max (ng L-1) |
|---|---|---|---|---|---|---|
| PFCAs | 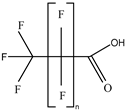 | PFPrA | n 3 | 2b | 63.27% | 6.45 |
| PFBA | 4 | 1 | 95.92% | 44.83 | ||
| PFPeA | 5 | 1 | 87.76% | 8.75 | ||
| PFHxA | 6 | 1 | 100.00% | 48.92 | ||
| PFHpA | 7 | 1 | 91.84% | 8.42 | ||
| PFOA | 8 | 1 | 97.96% | 37.22 | ||
| PFNA | 9 | 1 | 89.80% | 7.59 | ||
| PFDeA | 10 | 1 | 79.59% | 4.40 | ||
| PFUdA | 11 | 1 | 57.14% | 2.25 | ||
| PFDoA | 12 | 1 | 51.02% | 0.09 | ||
| PFSAs | 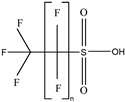 | PFPrS | n 3 | 2b | 69.39% | 5.32 |
| PFBS | 4 | 1 | 100.00% | 26.77 | ||
| PFPeS | 5 | 1 | 79.59% | 0.16 | ||
| PFHxS | 6 | 1 | 91.84% | 3.73 | ||
| PFHpS | 7 | 1 | 59.18% | 0.14 | ||
| PFOS | 8 | 1 | 67.35% | 6.10 | ||
| PFdiOAs | 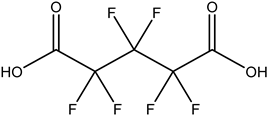 | PFGdiA | n 5 | 1 | 46.94% | 1.51 |
| H-PFCAs | 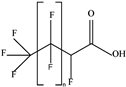 | HPFLCA_i n = 4 | n 4 | 3a | 87.76% | 0.03 |
| HPFLCA_i n = 5 | 5 | 3a | 77.55% | 0.01 | ||
| HPFLCA_i n = 6 | 6 | 3a | 18.37% | 0.00 | ||
| H-PFSAs | 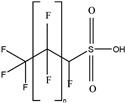 | HPFLSA_i n = 4 | n 4 | 2a | 87.76% | 7.42 |
| PFECAs | 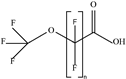 | PFMOAA | n 3 | 2a | 10.20% | 0.43 |
| PFMPA | 4 | 2a | 4.08% | 0.81 | ||
| HFPO-DA | 6 | 1 | 79.59% | 3.20 | ||
| FTSAs | 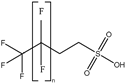 | 4:2 FTSA | n 6 | 1 | 20.41% | 0.75 |
| 6:2 FTSA | 8 | 1 | 83.67% | 8.17 | ||
| Cl-PFESAs |  | 6:2 Cl-PFESA | n 8 | 1 | 10.20% | 0.18 |
| H-PEFSAs |  | HPFESA_i n = 4 | n 4 | 2a | 12.24% | 0.24 |
| PFSM |  | PFSM-FSAA | n 6 | 2b | 24.49% | 5.11 |
| HNTf2 |  | HNTf2 | n 2 | 2b | 87.76% | 15.02 |
| Criteria | Attributes | Unit | Scaling | Weight | Software | Model | ||
|---|---|---|---|---|---|---|---|---|
| Persistence | Biowin1 | unitless | -x | 1/9 | EPI Suite v4.11 | BIOWIN v4.10 | ||
| Biowin3 | unitless | -x | 1/9 | BIOWIN v4.10 | ||||
| Biowin5 | unitless | -x | 1/9 | BIOWIN v4.10 | ||||
| Bioaccumulation | BAF | L/kg | log 10(x) | 1/6 | EPI Suite v4.11 | BCFBAF v3.01 | ||
| Log Kow | unitless | x | 1/6 | KOWWIN v1.68 | ||||
| Toxicity | Ecotoxicological effects | Fish LC50 (96 h) | mg L−1 | −log 10(x) | 1/27 | ECOSAR v2.0 | ECOSAR v2.0 | |
| Daphnid LC50 (48 h) | mg L−1 | −log 10(x) | 1/27 | ECOSAR v2.0 | ||||
| Green Algae EC50 (96 h) | mg L−1 | −log 10(x) | 1/27 | ECOSAR v2.0 | ||||
| Human health effects | Carcinogenicity | unitless | x | 1/45 | VEGA v1.2.0 | CAESAR v2.1.10 | ||
| ISS v 1.0.3 | ||||||||
| IRFMN-ISSCAN-CGX v1.0.1 | ||||||||
| IRFMN-Antares v1.0.1 | ||||||||
| Developmental toxicity | unitless | x | 1/45 | VEGA v1.2.0 | CAESAR v2.1.8 | |||
| VEGA v1.2.0 | PG v1.1.2 | |||||||
| T.E.S.T. v5.1.1 | T.E.S.T. v5.1.1 | |||||||
| QSAR Toolbox v 4.5 | DART | |||||||
| Mutagenicity | unitless | x | 1/45 | VEGA v1.2.0 | Consensus v1.0.4 | |||
| CORAL v1.0.1 | ||||||||
| IRFMN-VERMEER v1.0.1 | ||||||||
| IRFMN v1.0.2 | ||||||||
| Skin sensitization | unitless | x | 1/45 | VEGA v1.2.0 | CAESAR v2.1.7 | |||
| IRFMN-JRC v1.0.1 | ||||||||
| NCSTOX v1.0.1 | ||||||||
| TOXTREE v1.0.0 | ||||||||
| Oral rat LD50 | mg L−1 | −log 10(x) | 1/45 | T.E.S.T. v5.1.1 | T.E.S.T. v5.1.1 | |||
| Molecular docking | Endocrine disrupting effect | ERa | unitless | x | 1/45 | Biovia Discovery Studio 2021 | Biovia Discovery Studio 2021 | |
| ERb | unitless | x | 1/45 | |||||
| AR | unitless | x | 1/45 | |||||
| PPARa | unitless | x | 1/45 | |||||
| TRa | unitless | x | 1/45 | |||||
| TRb | unitless | x | 1/45 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Z.; Feng, C.; Chen, Y.; Lin, Y.; Liang, Z.; Qian, H.; Zhou, J.; Ma, J.; Jin, Y.; Lu, D.; et al. Occurrence, Sources, and Prioritization of Per- and Polyfluoroalkyl Substances (PFASs) in Drinking Water from Yangtze River Delta, China: Focusing on Emerging PFASs. Molecules 2025, 30, 2313. https://doi.org/10.3390/molecules30112313
Qian Z, Feng C, Chen Y, Lin Y, Liang Z, Qian H, Zhou J, Ma J, Jin Y, Lu D, et al. Occurrence, Sources, and Prioritization of Per- and Polyfluoroalkyl Substances (PFASs) in Drinking Water from Yangtze River Delta, China: Focusing on Emerging PFASs. Molecules. 2025; 30(11):2313. https://doi.org/10.3390/molecules30112313
Chicago/Turabian StyleQian, Zixin, Chao Feng, Yuhang Chen, Yuanjie Lin, Ziwei Liang, Hailei Qian, Jingxian Zhou, Jinjing Ma, Yue Jin, Dasheng Lu, and et al. 2025. "Occurrence, Sources, and Prioritization of Per- and Polyfluoroalkyl Substances (PFASs) in Drinking Water from Yangtze River Delta, China: Focusing on Emerging PFASs" Molecules 30, no. 11: 2313. https://doi.org/10.3390/molecules30112313
APA StyleQian, Z., Feng, C., Chen, Y., Lin, Y., Liang, Z., Qian, H., Zhou, J., Ma, J., Jin, Y., Lu, D., Wang, G., Xiao, P., & Zhou, Z. (2025). Occurrence, Sources, and Prioritization of Per- and Polyfluoroalkyl Substances (PFASs) in Drinking Water from Yangtze River Delta, China: Focusing on Emerging PFASs. Molecules, 30(11), 2313. https://doi.org/10.3390/molecules30112313







