Qualitative Analysis of Nitrogen and Sulfur Compounds in Vacuum Gas Oils via Matrix-Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry
Abstract
1. Introduction
2. Experimental
2.1. VGO Feeds and Their HDS Reaction
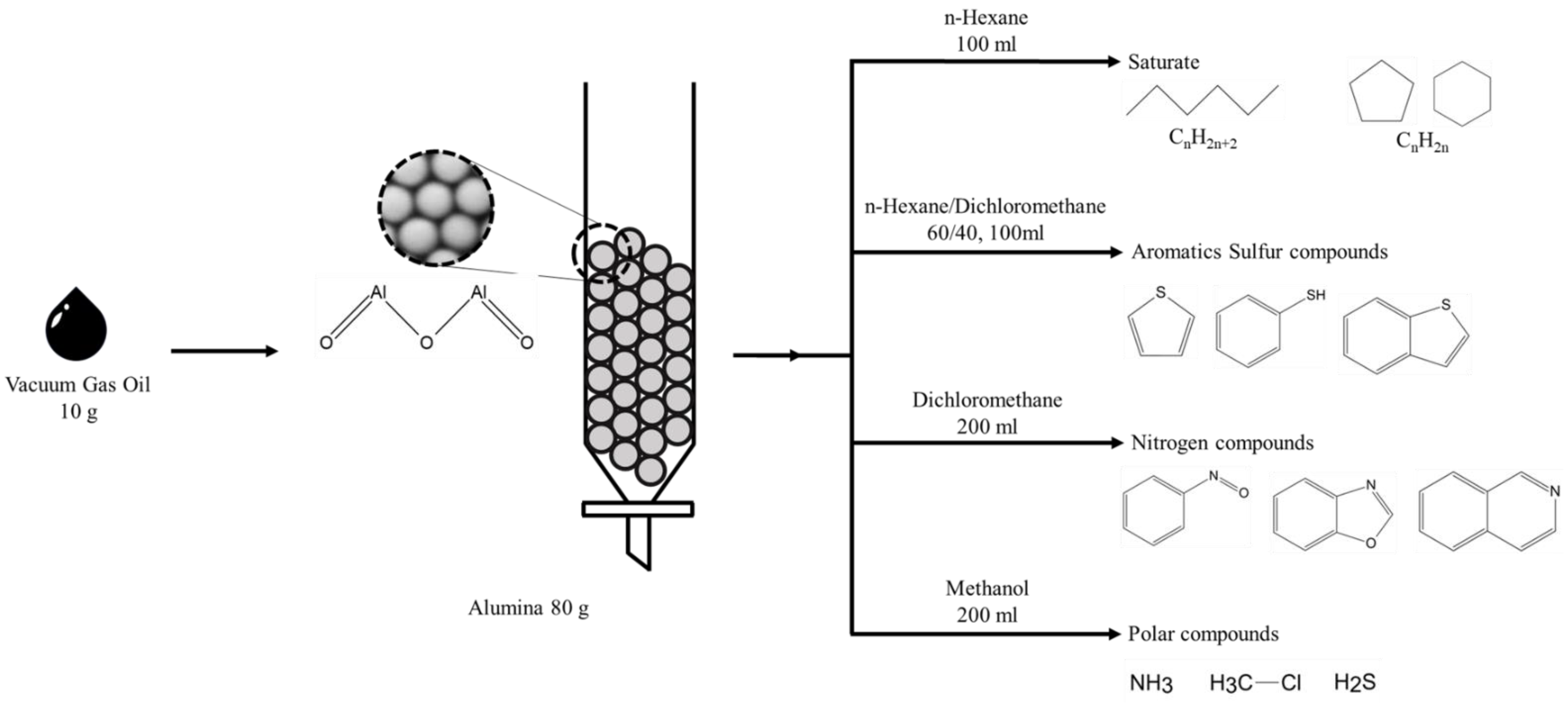
2.2. Solvent Fractionation
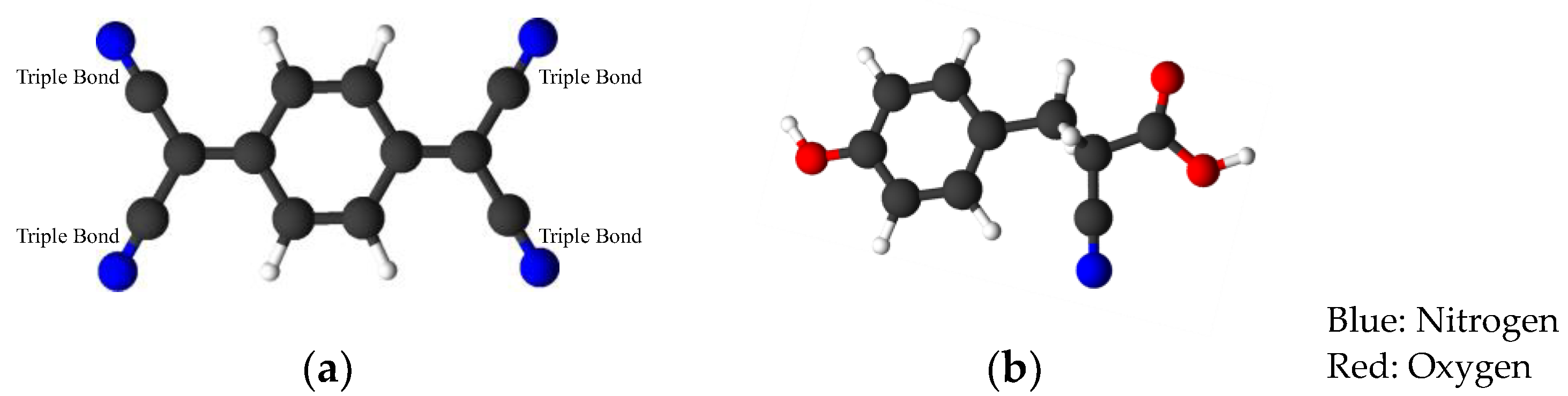
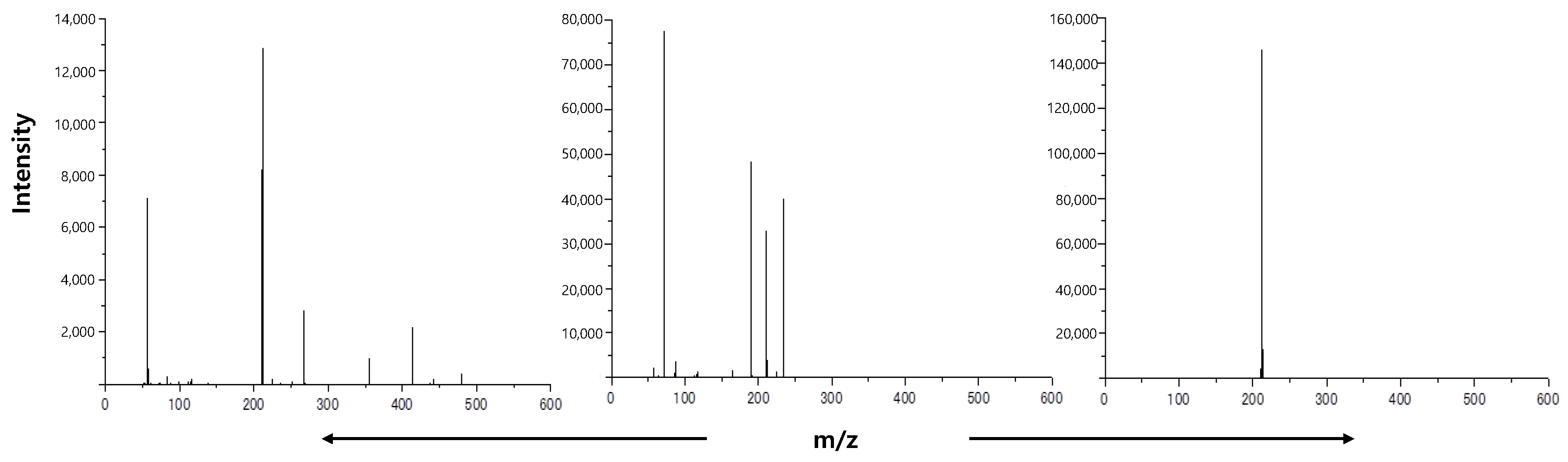
2.3. Sample Preparation for MALDI-TOF-MS
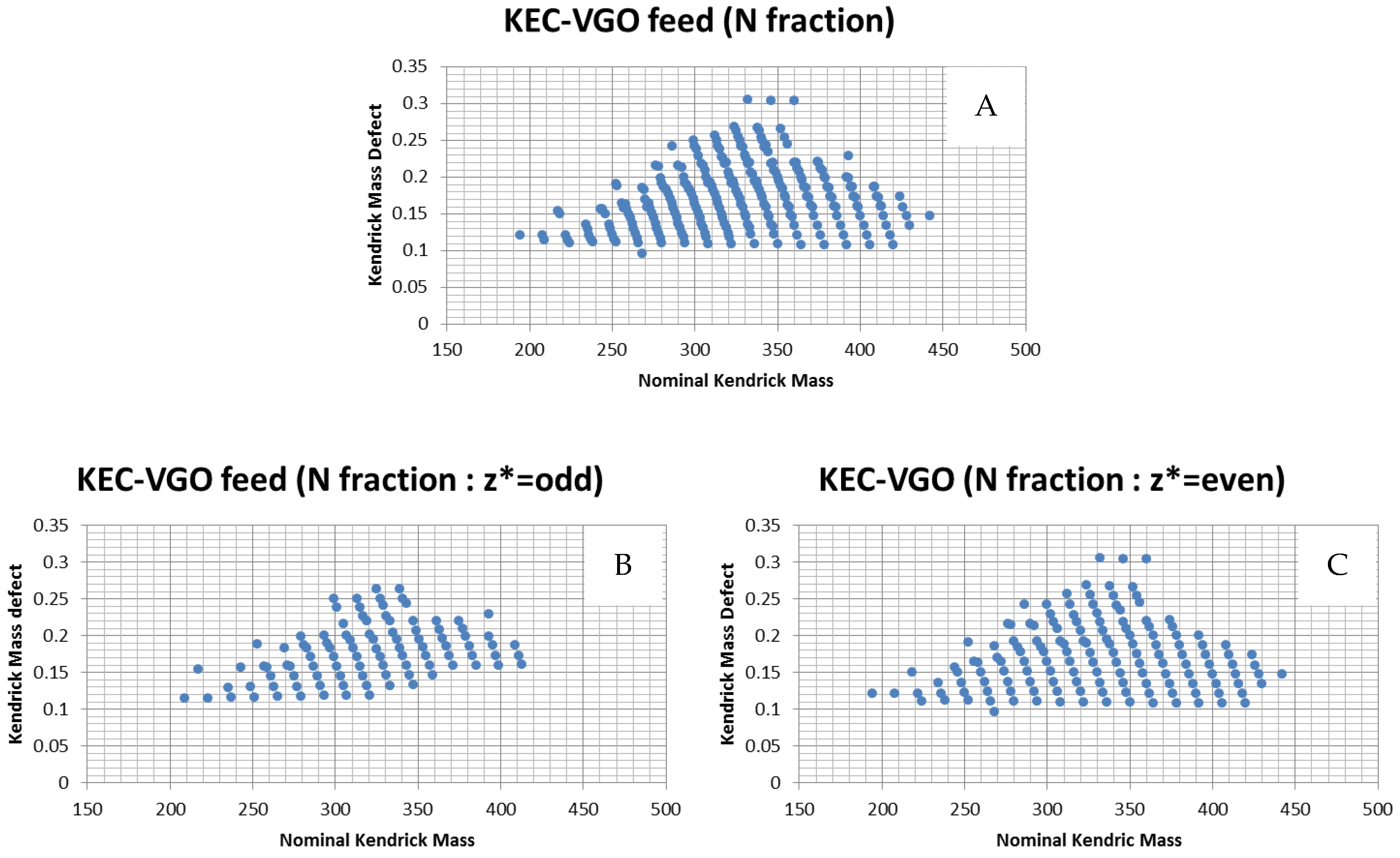
2.4. Kendrick Plot and Nominal Mass Series (z*)
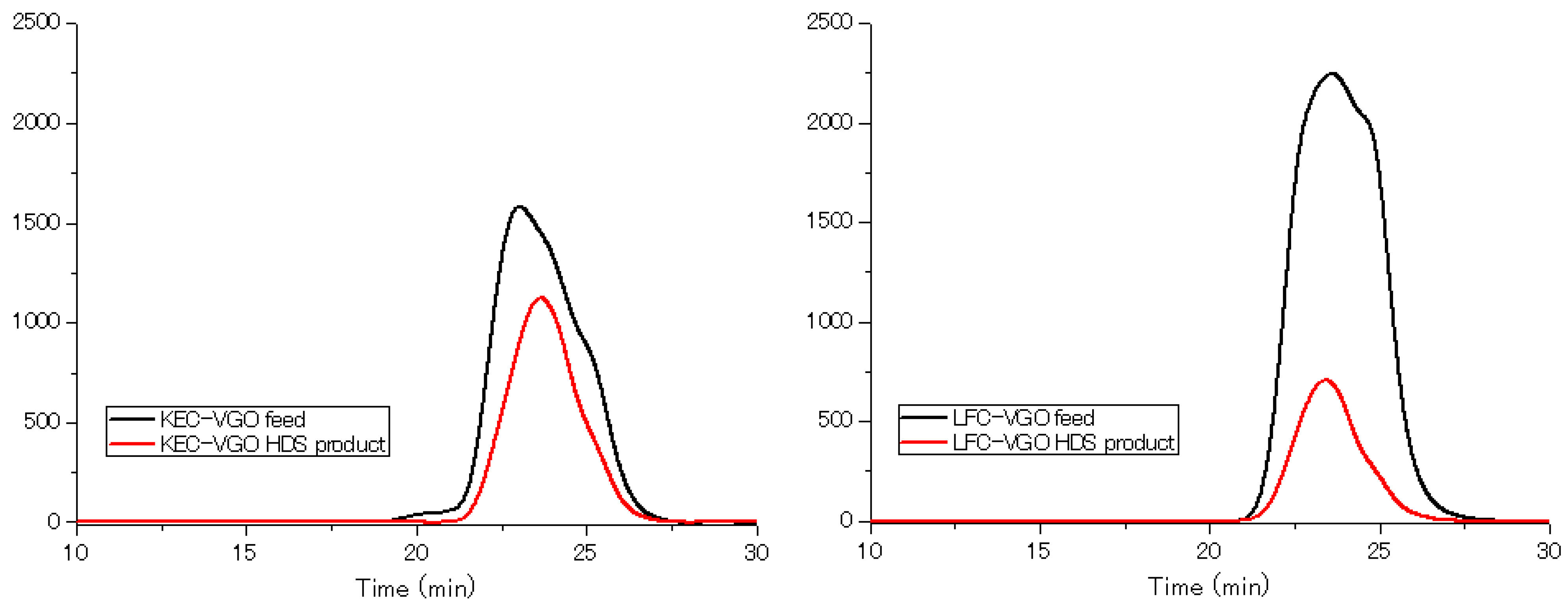
2.5. GPC-UV Measurement
3. Results and Discussions
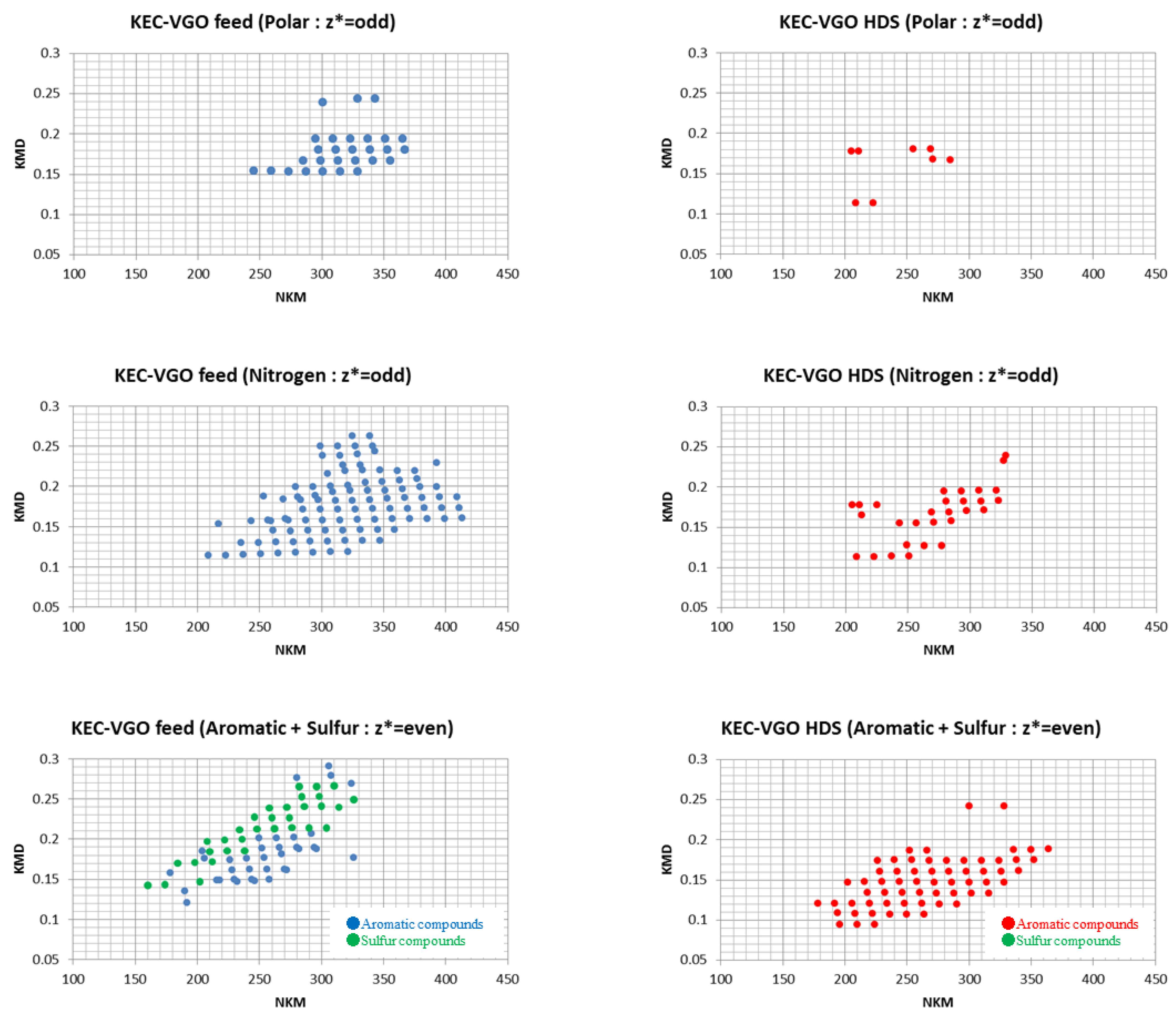
3.1. Classification of Compounds in KEC-VGO Feed and HDS Products
3.1.1. Polar and Nitrogen Fraction in KEC-VGO Feed and HDS Products
3.1.2. Aromatic and Sulfur Fraction in KEC-VGO Feed and HDS Products
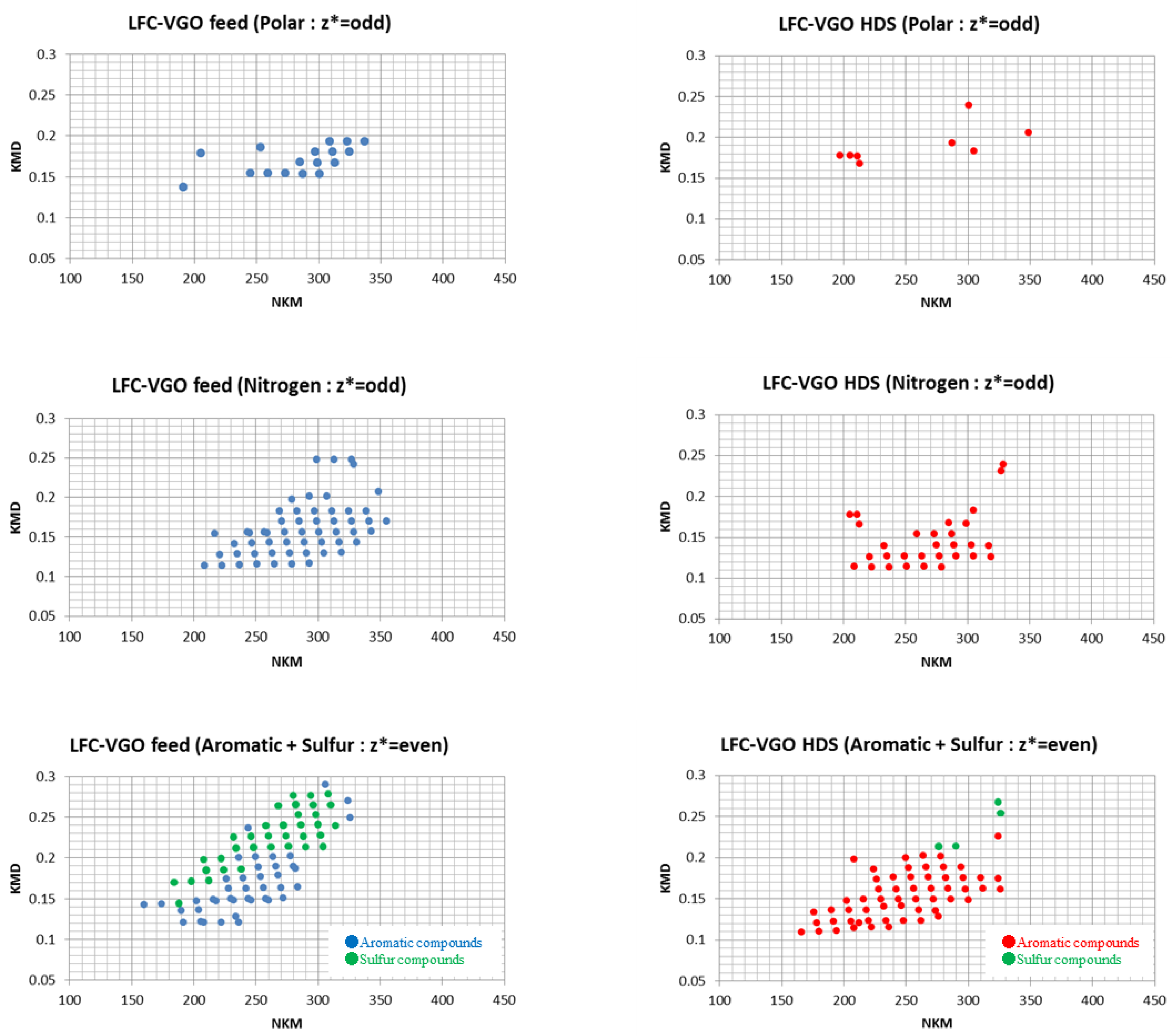
3.2. Classification of Compounds in LFC-VGO Feed and HDS Products
3.2.1. Polar and Nitrogen Fraction in LFC-VGO Feed and HDS Products
3.2.2. Aromatic and Sulfur Fractions in LFC-VGO Feed and HDS Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, G.C.; Rodgers, R.P.; Marshall, A.G. Identification of hydrotreatment-resistant heteroatomic species in a crude oil distillation cut by electrospray ionization FT-ICR mass spectrometry. Fuel 2006, 85, 2071–2080. [Google Scholar] [CrossRef]
- Stanford Lateefah, A.; Kim, S.; Rodgers Ryan, P.; Marshall Alan, G. Characterization of Compositional Changes in Vacuum Gas Oil Distillation Cuts by Electrospray Ionization Fourier Transform−Ion Cyclotron Resonance (FT−ICR) Mass Spectrometry. Energy Fuels 2006, 20, 1664–1673. [Google Scholar] [CrossRef]
- Frątczak, J.; de Paz Carmona, H.; Tišler, Z.; Hidalgo Herrador, J.; Gholami, Z. Hydrocracking of Heavy Fischer–Tropsch Wax Distillation Residues and Its Blends with Vacuum Gas Oil Using Phonolite-Based Catalysts. Molecules 2021, 26, 7172. [Google Scholar] [CrossRef] [PubMed]
- Luna, N.; Zelong, L.; Songbai, T. Identification and Characterization if Sulfur Compounds in Straight- Run Diesel Using Comprehensive Two-Dimensional GC Coupled with TOF/MS. China Pet. Process. Petrochem. Technol. 2014, 16, 10–18. [Google Scholar]
- Ishihara, A.; Dumeignil, F.; Lee, J.; Mitsuhashi, K.; Qian, E.W.; Kabe, T. Hydrodesulfurization of sulfur-containing polyaromatic compounds in light gas oil using noble metal catalyst. Appl. Catal. A: Gen. 2005, 289, 163–173. [Google Scholar] [CrossRef]
- Azizi, N.; Ali, S.A.; Alhooshani, K.; Kim, T.; Lee, Y.; Park, J.I.; Miyawaki, K.; Yoon, S.H.; Mochida, I. Hydrotreating of light cycle oil over NiMo and CoMo catalysts with different supports. Fuel Process. Technol. 2013, 109, 172–178. [Google Scholar] [CrossRef]
- Amrani, A.; Deev, A.; Sessions, A.; Tang, Y.; Adkins, J.F.; Hill, R.J.; Moldowan, M.; Wel, Z. The sulfur-isotopic compositions of benzothiophenes and dibenzothiophenes as a proxy for thermochemical sulfate reduction. Geochim. Cosmochim. Acta 2012, 84, 152–164. [Google Scholar] [CrossRef]
- Schade, T.; Andersson, J.T. Speciation of Alkylated Divenzothiophenes in a Deeply Desulfurized Diesel Fuel. Energy Fuel 2006, 20, 1614–1620. [Google Scholar] [CrossRef]
- Schade, T.; Andersson, J.T. Speciation of alkylated dibenzothiophenes through correlation of structure and gas chromatographic retention indexes. J. Chromatogr. A 2006, 1117, 206–213. [Google Scholar] [CrossRef]
- Hughey, C.A.; Hendrickson, C.L.; Rodgers, R.P.; Marshall, A.G. Elemental Composition Analysis of Processed and Unprocessed Diesel Fuel by Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuel 2001, 15, 1186–1193. [Google Scholar] [CrossRef]
- Schaub, T.M.; Rodgers, R.P.; Marshall, A.G. Speciation of Aromatic Compounds in Petroleum Refinery Streams by Continuous by Flow Field Desorption Ionization FT-ICR Mass Spectrometry. Energy Fuel 2005, 19, 1566–1573. [Google Scholar] [CrossRef]
- Xiaobo, C.; Yibin, L.; Jin, W.; Honghong, S.; Chaohe, Y.; Chunyi, L. Characterization of nitrogen compounds in coker gas oil by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry and Fourier transform infrared spectroscopy. Appl. Petrochem. Res. 2014, 4, 417–422. [Google Scholar] [CrossRef][Green Version]
- Rodgers, R.P.; Hendrickson, C.L.; Emmett, M.R.; Marshall, A.G. Reading Chemical Fine Print: Resolution and Identification of 3000 Nitrogen-Containing Aromatic Compounds from a Single Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrum of Heavy Petroleum Crude Oil. Energy Fuel 2001, 15, 492–498. [Google Scholar]
- Zhu, X.; Shi, Q.; Zhang, Y.; Pan, N.; Xu, C.; Chung, K.H.; Zhao, S. Characterization of Nitrogen Compounds in Coker Heavy Gas Oil and Its Subfractions by Liquid Chromatographic Separation Followed by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuel 2011, 25, 281–287. [Google Scholar] [CrossRef]
- Shi, Q.; Xu, C.; Zhao, S.; Chung, K.H.; Zhang, Y.; Gao, W. Characterization of Basic Nitrogen in Coker Gas Oils by Positive-Ion Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuel 2010, 24, 563–569. [Google Scholar] [CrossRef]
- Chen, X.; Shen, B.; Sun, J.; Wang, C.; Shan, H.; Yang, C.; Li, C. Characterization and Comparison of Nitrogen Compounds in Hydrotreated and Untreated Shale Oil by Electrospray Ionization (ESI) Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FT-ICR MS). Energy Fuel 2012, 26, 1707–1714. [Google Scholar] [CrossRef]
- Li, Z.K.; Wang, G.; Shi, Q.; Xu, C.M.; Gao, J.S. Retardation Effect of Basic Nitrogen Compounds on Hydrocarbons Catalytic Cracking in Coker Gas Oil and Their Structural Identification. Ind. Eng. Chem. Res. 2011, 50, 4123–4132. [Google Scholar] [CrossRef]
- Shi, Q.; Zhao, S.; Xu, Z.; Chung, K.H.; Zhang, Y.; Xu, C. Distribution of Acids and Neutral Nitrogen Compounds in a Chinese Crude Oil and Its Fractions: Characterized by Negative-Ion Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy & Fuel. 2010, 24, 4005–4011. [Google Scholar]
- Cho, Y.; Kim, Y.H.; Kim, S. Planar Limit-Assisted Structural Interpretation of Saturates Aromatics Resins Asphaltenes Fractionated Crude Oil Compounds Observed by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Anal. Chem. 2011, 83, 6068–6073. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, Q.; Li, A.; Chung, K.H.; Zhao, S.; Xu, C. Partitioning of Crude Oil Acidic Compounds into Subfractions by Extrography and Identification of Isoprenoidyl Phenols and Tocopherols. Energy Fuel 2011, 25, 5083–5089. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, L.; Zhou, Y.; Wei, Q.; Chung, K.H.; Zhao, S.; Xu, C.; Shi, Q. Transformation of Nitrogen Compounds in Deasphalted Oil Hydrotreating: Characterized by Electrospray Ionization Fourier Transform-Ion Cyclotron Resonance Mass Spectrometry. Energy Fuel 2013, 27, 2952–2959. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Wu, B.; Mao, X. Characterization of Acidic Compounds in Vacuum Gas Oils and Their Dewaxed Oils by Fourier Transform-Ion Cyclotron Resonance Mass Spectrometry. Energy Fuel 2012, 26, 5646–5654. [Google Scholar] [CrossRef]
- Zhang, J.; Shan, H.; Chen, X.; Liu, W.; Yang, C. Synergistic Process for High Nitrogen Content Feedstocks Catalytic Cracking: A Case Study of Controlling the Reactions of Nitrogen Compounds in Situ. Ind. Eng. Chem. Res. 2014, 53, 5718–5727. [Google Scholar] [CrossRef]
- Fu, J.; Klein, G.C.; Smith, D.F.; Kim, S.; Rodgers, R.P.; Hendrickson, C.L.; Marshall, A.G. Comprehensive Compositional Analysis of Hydrotreated and Untreated Nitrogen-Concentrated Fractions from Syncrude Oil by Electron Ionization, Field Desorption Ionization, and Electrospray Ionization Ultrahigh-Resolution FT-ICR Mass Spectrometry. Energy Fuel 2006, 20, 1235–1241. [Google Scholar] [CrossRef]
- Hugheya, C.A.; Rodgersa, R.P.; Marshalla, A.G.; Qianc, K.; Robbinsc, W.K. Identification of acidic NSO compounds in crude oils of different geochemical origins by negative ion electrospray Fourier transform ion cyclotron resonance mass spectrometry. Org. Geochem. 2002, 33, 743–759. [Google Scholar] [CrossRef]
- Dutriez, T.; Courtiade, M.; Ponthus, J.; Thiébaut, D.; Dulot, H.; Hennion, M.C. Complementarity of Fourier Transform Ion Cyclotron Resonance Mass Spectrometry and high temperature comprehensive two-dimensional gas chromatography for the characterization of resin fractions from vacuum gas oils. Fuel 2012, 96, 108–119. [Google Scholar] [CrossRef]
- Wiwel, P.; Hinnemann, B.; Vivas, A.H.; Zeuthen, P.; Petersen, B.O.; Duus, J. Ø. Characterization and Identification of the most Refractory Nitrogen Compounds in Hydroprocessed Vacuum Gas Oil. Ind. Eng. Chem. Res. 2010, 49, 3184–3193. [Google Scholar] [CrossRef]
- Thomson, J.S.; Green, J.B.; McWilliams, T.B. Determination of sulfides and thiols in petroleum distillates using solid-phase extraction and derivatization with pentafluorobenzoyl chloride. Energy Fuels 1997, 11, 909–914. [Google Scholar] [CrossRef]
- de Peinder, P.; Visser, T.; Wagemans, R.; Blomberg, J.; Chaabani, H.; Soulimani, F.; Weckhuysen, B.M. Sulfur Speciation of Crude Oils by Partial Least Squares Regression Modeling of Their Infrared Spectra. Energy Fuels 2010, 24, 557–562. [Google Scholar] [CrossRef]
- Dijkmans, T.; Van Geem, K.M.; Djokic, M.R.; Marin, G.B. Combined Comprehensive Two-Dimensional Gas Chromatography Analysis of Polyaromatic Hydrocarbons /Polyaromatic Sulfur-Con-taining Hydrocarbons (PAH/PASH) in Complex Matrices. Ind. Eng. Chem. Res. 2014, 53, 15436–15446. [Google Scholar] [CrossRef]
- Melville, J. Matrix-Assisted Laser Desorption/Ionization(MALDI). UC Berkeley Coll. Chem. Instrum. Methods Anal. Chem. 2014, 1–16. [Google Scholar]
- Ibarra, A.; Veloso, A.; Bilbao, J.; Arendes, J.M.; Castano, P. Dual coke deactivation pathways during the catalytic cracking of rawbio-oil and vacuum gasoil in FCC conditions. Appl. Catal. B Environ. 2016, 182, 336–346. [Google Scholar] [CrossRef]
- Panda, S.K.; Andersson, J.T.; Schrader, W. Characterization of supercomplex crude oil mixtures: What is really in there? Angew. Chem. Int. Ed. 2009, 48, 1788–1791. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Hirai, T.; Komasawa, I. Photochemical Desulfurization and Denitrogenation Prodcess for Vacuum Gas Oil Using an Organic Two-Phase Extraction System. Ind. Eng. Chem. Res. 2001, 40, 293–303. [Google Scholar] [CrossRef]
- Yang, J.; Nakabayashi, K.; Miyawaki, J.; Yoon, S.-H. Preparation of isotropic pitch-based carbon fiber using hyper coal through co-carbonation with ethylene bottom oil. J. Ind. Eng. Chem. 2016, 34, 397–404. [Google Scholar] [CrossRef]
- Kendrick, E. A Mass Scale Based on CH2 = 14.0000 for High Resolution Mass Spectrometry of Organic Compounds. Anal. Chem. 1963, 35, 2146–2154. [Google Scholar] [CrossRef]
| Name | Structure | External | Internal |
|---|---|---|---|
| Carbazole |  Blue: Nitrogen | O | |
| Anthracene |  | O | O |
| 4,6-dimethyldibenzothiophene (4,6-DMDBT) |  Yellow: Sulfur | O | O |
| Pyrene |  | O | |
| benzonaphthothiophene |  Yellow: Sulfur | O | |
| Perylene | 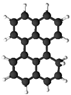 | O | O |
| coronene | 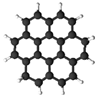 | O | O |
| benzocarbazole |  Blue: Nitrogen | O | |
| Dibenzo[g,p]chrysene (DBC) | 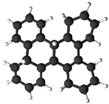 | O | O |
| Dibenzo[a,l]pentacene (DBP) |  | O | |
| Polyethylene glycol (PEG) | 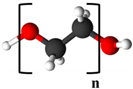 Red: Oxygen | O | O |
| Fraction and Volume | Anthracene | 4,6-DMDBT | Benzocarbazole | Perylene | Coronene | DBC | PEG | |
|---|---|---|---|---|---|---|---|---|
| KEC-VGO feed | Polar 10 μL | - | - | - | - | 1 μL (1 mg/mL) | - | - |
| Nitrogen 10 μL | - | - | 10 μL (1 mg/mL) | 1 μL (1 mg/mL) | - | 1 μL (1 mg/mL) | 3 μL (10 mg/mL) | |
| Aromatics + Sulfur 10 μL (10 mg) | - | - | 1 μL (1 mg/mL) | - | - | 1 μL (1 mg/mL) | 1 μL (10 mg/mL) | |
| KEC-VGO HDS products | Polar 1000 μL | - | 100 μL (1 mg/mL) | - | - | 0.3 μL (1 mg/mL) | - | |
| Nitrogen 10 μL | - | 5 μL (1 mg/mL) | - | - | - | 1 μL (1 mg/mL) | 1 μL (10 mg/mL) | |
| Aromatics + Sulfur 100 μL (100 mg) | 1 μL (1 mg/mL) | - | - | - | 1 μL (1 mg/mL) | 1 μL (10 mg/mL) | ||
| LFC-VGO feed | Polar 10 μL | - | - | - | 1 μL (1 mg/mL) | - | - | - |
| Nitrogen 10 μL | - | - | 10 μL (1 mg/mL) | - | - | 3 μL (1 mg/mL) | 3 μL (10 mg/mL) | |
| Aromatics + Sulfur 10 μL (10 mg/mL) | 1 μL (1 mg/mL) | - | - | - | - | 1 μL (1 mg/mL) | 1 μL (10 mg/mL) | |
| LFC-VGO HDS products | Polar 1000 μL | - | 100 μL (1 mg/mL) | - | - | 0.3 μL (1 mg/mL) | - | - |
| Nitrogen 10 μL | - | 10 μL (1 mg/mL) | - | - | - | 1 μL (1 mg/mL) | - | |
| Aromatics + Sulfur 100 μL (10 mg/mL) | - | 50 μL (10 mg/mL) | - | - | 0.5 μL (1 mg/mL) | 0.1 μL (10 mg/mL) | - |
| KEC-VGO | ||||
|---|---|---|---|---|
| Fraction | Formula (DBE) | Expected Structure | Feed | HDS Products |
| Polar | CnH2n-25SN (14) | - | O | |
| CnH2n-27N (15) | Dibenzocarbazole | O | ||
| CnH2n-25N (14) | Tetrahydrodibenzoacridine | O | O | |
| CnH2n-23N (13) | Benzoacridine | O | O | |
| CnH2n-21N (12) | Benzocarbazole | O | ||
| CnH2n-15N (9) | Carbazole | O | ||
| Nitrogen | CnH2n-25SN (14) | - | O | |
| CnH2n-23SN (13) | - | O | ||
| CnH2n-21SN (12) | - | O | ||
| CnH2n-19SN (11) | - | O | ||
| CnH2n-27N (15) | Dibenzocarbazole | O | ||
| CnH2n-25N (14) | Tetrahydrodibenzoacridine | O | ||
| CnH2n-23N (13) | Benzoacridine | O | ||
| CnH2n-21N (12) | Benzocarbazole | O | ||
| CnH2n-19N (11) | Tetrahydrobenzoacridine | O | ||
| CnH2n-17N (10) | Acridine | O | O | |
| CnH2n-15N (9) | Carbazole | O | O | |
| Aromatic + Sulfur | CnH2n-30S (15) | Cholanthrenthiophene | O | |
| CnH2n-28S (14) | Chrysenothiophene | O | ||
| CnH2n-26S (13) | Pyrenothiophene | O | ||
| CnH2n-24S (12) | Naphthenephenanthrenothiophene | O | ||
| CnH2n-30 (16) | Dicyclopentapyrene | O | ||
| CnH2n-20S (11) | Benzonaphthothiophene | O | ||
| CnH2n-28 (15) | Perylene | O | ||
| CnH2n-18S (10) | Acenaphthenothiophene | O | ||
| CnH2n-26 (14) | Tetrahydropicene | O | O | |
| CnH2n-16S (9) | Dibenzothiophene | O | ||
| CnH2n-24 (13) | Chrysene | O | ||
| CnH2n-22 (12) | Pyrene | O | O | |
| CnH2n-12S (7) | Tetrahydrodibenzothiphene | O | ||
| CnH2n-20 (11) | Tetrahydrobenzoanthracene | O | ||
| CnH2n-18 (10) | Anthracene | O | O | |
| CnH2n-16 (9) | Octahydronaphthacene | O | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, M.; Lee, J.; Yi, H.; Lee, G.-H.; Kim, Y.-J.; Kim, G.-H.; Oh, K.; Yoon, S.-H.; Nakabayashi, K.; Park, J.-I. Qualitative Analysis of Nitrogen and Sulfur Compounds in Vacuum Gas Oils via Matrix-Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry. Molecules 2024, 29, 2508. https://doi.org/10.3390/molecules29112508
Ueda M, Lee J, Yi H, Lee G-H, Kim Y-J, Kim G-H, Oh K, Yoon S-H, Nakabayashi K, Park J-I. Qualitative Analysis of Nitrogen and Sulfur Compounds in Vacuum Gas Oils via Matrix-Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry. Molecules. 2024; 29(11):2508. https://doi.org/10.3390/molecules29112508
Chicago/Turabian StyleUeda, Morio, Jongbeom Lee, Hyeonseok Yi, Gang-Ho Lee, Yu-Jin Kim, Geon-Hee Kim, Kyeongseok Oh, Seong-Ho Yoon, Koji Nakabayashi, and Joo-Il Park. 2024. "Qualitative Analysis of Nitrogen and Sulfur Compounds in Vacuum Gas Oils via Matrix-Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry" Molecules 29, no. 11: 2508. https://doi.org/10.3390/molecules29112508
APA StyleUeda, M., Lee, J., Yi, H., Lee, G.-H., Kim, Y.-J., Kim, G.-H., Oh, K., Yoon, S.-H., Nakabayashi, K., & Park, J.-I. (2024). Qualitative Analysis of Nitrogen and Sulfur Compounds in Vacuum Gas Oils via Matrix-Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry. Molecules, 29(11), 2508. https://doi.org/10.3390/molecules29112508








