Flavonoids Identified in Terminalia spp. Inhibit Gastrointestinal Pathogens and Potentiate Conventional Antibiotics via Efflux Pump Inhibition
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Activity of Flavonoids
2.2. Fractional Inhibitory Concentration
2.3. Isobologram Analysis
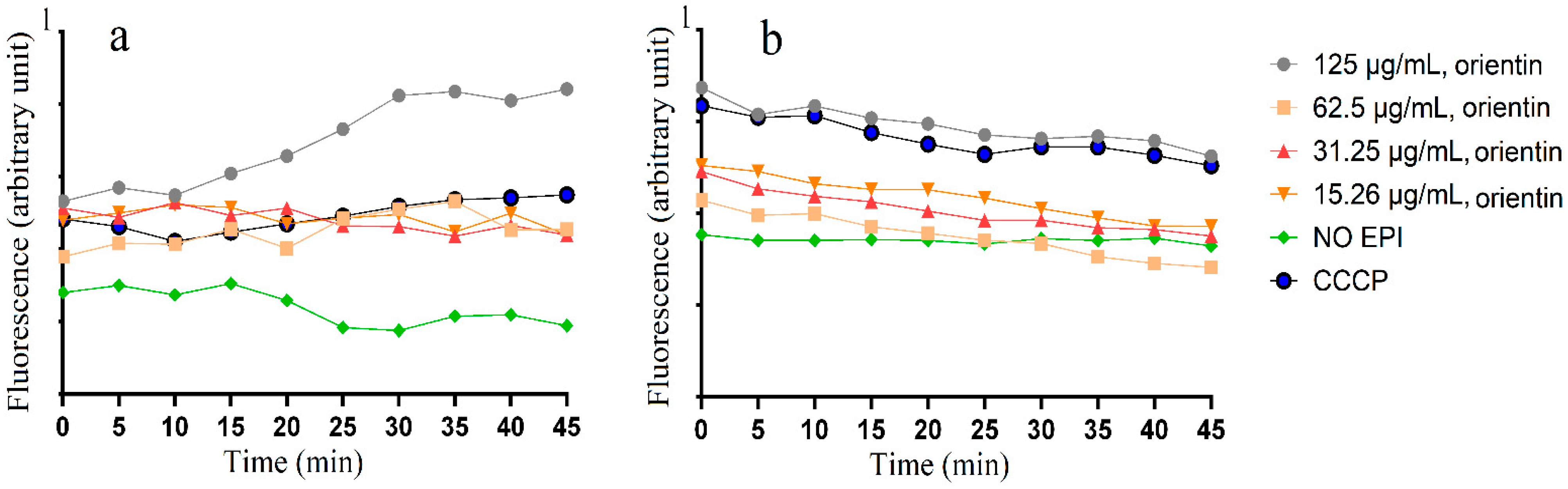
2.4. Accumulation and Efflux of Ethifium Bromide (EtBr)
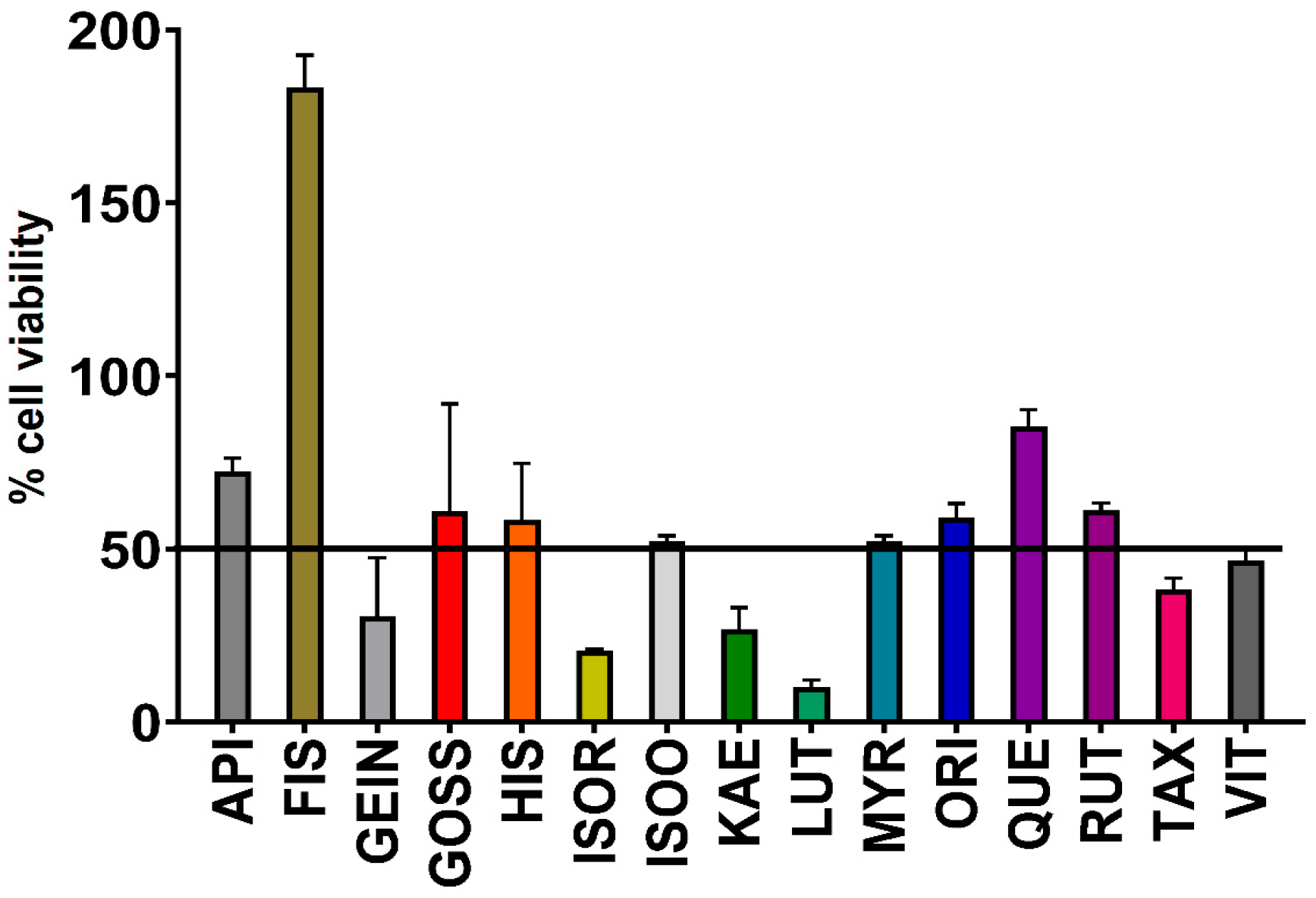
2.5. Assessment of Toxicity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Bacterial Strains
4.3. Bacterial Growth Conditions
4.4. Antibacterial Susceptibility Assay
4.5. Analysis of Flavonoids: Antibiotic Optimal Ratios
4.6. Ethidium Bromide (EtBr) Accumulation Assay
4.7. EtBr Efflux Assay
4.8. Toxicity Studies
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmes, A.H.; Moore, L.S.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. London: The Review on Antimicrobial Resistance. 2016. Available online: http://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 28 April 2025).
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Wallace, M.J.; Fishbein, S.R.S.; Dantas, G. Antimicrobial resistance in enteric bacteria: Current state and next-generation solutions. Gut Microbes 2020, 12, 1799654. [Google Scholar] [CrossRef] [PubMed]
- Shane, A.L.; Mody, R.K.; Crump, J.A.; Tarr, P.I.; Steiner, T.S.; Kotloff, K.; Langley, J.M.; Wanke, C.; Warren, C.A.; Cheng, A.C.; et al. 2017 Infectious diseases society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin. Infect. Dis. 2017, 65, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the global burden of disease study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef]
- CDC. National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS): Human Isolates Surveillance Report for 2015 (Final Report); Atlanta (GA). 2018. Available online: https://www.cdc.gov/narms/reports/annual-human-isolates-report-2015.html (accessed on 28 April 2025).
- Ranjbar, R.; Farahani, A. Shigella: Antibiotic resistance mechanisms and new horizons for treatment. Infect. Drug Resist. 2019, 12, 3137–3167. [Google Scholar] [CrossRef]
- Gal-Mor, O.; Boyle, E.C.; Grassl, G.A. Same species, different diseases: How and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front. Microbiol. 2014, 5, 391. [Google Scholar] [CrossRef]
- Cohen, E.; Davidovich, M.; Rokney, A.; Valinsky, L.; Rahav, G.; Gal-Mor, O. Emergence of new variants of antibiotic resistance genomic islands among multidrug-resistant Salmonella enterica in poultry. Environ. Microbiol. 2020, 22, 413–432. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar]
- McGaw, L.J.; Rabe, T.; Sparg, S.G.; Jager, A.K.; Eloff, J.N.; van Staden, J. An investigation on the biological activity of Combretum species. J. Ethnopharmacol. 2001, 75, 45–50. [Google Scholar] [CrossRef]
- Cock, I.E. The medicinal properties and phytochemistry of plants of the genus Terminalia (Combretaceae). Inflammopharmacology 2015, 23, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Zai, M.J.; Cheesman, M.J.; Cock, I.E. Terminalia petiolaris A.Cunn ex Benth. extracts have antibacterial activity and potentiate conventional antibiotics against beta-lactam-drug-resistant bacteria. Antibiotics 2023, 12, 1643. [Google Scholar] [CrossRef] [PubMed]
- Zai, M.J.; Cheesman, M.J.; Cock, I.E. Selected Australian Terminalia species extracts inhibit beta-lactam drug-resistant bacteria growth and potentiate the activity of conventional antibiotics: Bioactivities and phytochemistry. Microorganisms 2024, 12, 498. [Google Scholar] [CrossRef]
- Zai, M.J.; Cheesman, M.J.; Cock, I.E. Phytochemical evaluation of Terminalia canescens DC. Radlk. extracts with antibacterial and antibiotic potentiation activities against selected beta-lactam drug-resistant bacteria. Molecules 2024, 29, 1385. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- McGaw, L.J.; Elgorashi, E.E.; Eloff, J.N. Cytotoxicity of African medicinal plants against normal animal and human cells. In Toxicological Survey of African Medicinal Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 181–233. [Google Scholar]
- Zai, M.J.Y.; Cheesman, M.J.; Cock, I.E. Antimicrobial activity of selected native Australian Terminalia spp. against gastrointestinal pathogens and potentiation of selected antibiotics. Pharmacol. Res.-Nat. Prod. 2025, 6, 100158. [Google Scholar] [CrossRef]
- Osonga, F.J.; Akgul, A.; Miller, R.M.; Eshun, G.B.; Yazgan, I.; Akgul, A.; Sadik, O.A. Antimicrobial activity of a new class of phosphorylated and modified flavonoids. ACS Omega 2019, 4, 12865–12871. [Google Scholar] [CrossRef]
- van Vuuren, S.; Viljoen, A. Plant-based antimicrobial studies—Methods and approaches to study the interaction between natural products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef]
- Gutiérrez-Venegas, G.; Gómez-Mora, J.A.; Meraz-Rodríguez, M.A.; Flores-Sánchez, M.A.; Ortiz-Miranda, L.F. Effect of flavonoids on antimicrobial activity of microorganisms present in dental plaque. Heliyon 2019, 5, e03013. [Google Scholar] [CrossRef]
- Pandey, A.; Shashank Kumar, S.K. Perspective on plant products as antimicrobials agents: A review. Pharmacologia 2013, 4, 469–480. [Google Scholar] [CrossRef]
- Buchmann, D.; Schultze, N.; Borchardt, J.; Böttcher, I.; Schaufler, K.; Guenther, S. Synergistic antimicrobial activities of epigallocatechin gallate, myricetin, daidzein, gallic acid, epicatechin, 3-hydroxy-6-methoxyflavone and genistein combined with antibiotics against ESKAPE pathogens. J. Appl. Microbiol. 2022, 132, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Solmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Zeng, F.; Wang, M.; Guo, S.; Tang, Z.; Itagaki, K.; Lin, Y.; Shen, X.; Cao, Y.; Duan, J.A.; et al. Antimicrobial activities of lavandulylated flavonoids in Sophora flavences against methicillin-resistant Staphylococcus aureus via membrane disruption. J. Adv. Res. 2024, 57, 197–212. [Google Scholar] [CrossRef]
- Wu, M.; Brown, A.C. Applications of catechins in the treatment of bacterial infections. Pathogens 2021, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Du, Y.; Gao, D. Licochalcone A: A review of its pharmacology activities and molecular mechanisms. Front. Pharmacol. 2024, 15, 1453426. [Google Scholar] [CrossRef]
- Krawczyk, S.J.; Leśniczak-Staszak, M.; Gowin, E.; Szaflarski, W. Mechanistic insights into clinically relevant ribosome-targeting antibiotics. Biomolecules 2024, 14, 1263. [Google Scholar] [CrossRef]
- Wood, E.; Schulenburg, H.; Rosenstiel, P.; Bergmiller, T.; Ankrett, D.; Gudelj, I.; Beardmore, R. Ribosome-binding antibiotics increase bacterial longevity and growth efficiency. Proc. Natl. Acad. Sci. USA 2023, 120, e2221507120. [Google Scholar] [CrossRef]
- Lin, R.D.; Chin, Y.P.; Hou, W.C.; Lee, M.H. The effects of antibiotics combined with natural polyphenols against clinical methicillin-resistant Staphylococcus aureus (MRSA). Planta Med. 2008, 74, 840–846. [Google Scholar] [CrossRef]
- Miklasinska-Majdanik, M.; Kepa, M.; Wojtyczka, R.D.; Idzik, D.; Wasik, T.J. Phenolic compounds diminish antibiotic resistance of Staphylococcus aureus clinical strains. Int. J. Env. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef]
- Viveiros, M.; Martins, A.; Paixao, L.; Rodrigues, L.; Martins, M.; Couto, I.; Fähnrich, E.; Kern, W.V.; Amaral, L. Demonstration of intrinsic efflux activity of Escherichia coli K-12 AG100 by an automated ethidium bromide method. Int. J. Antimicrob. Agents 2008, 31, 458–462. [Google Scholar] [CrossRef]
- Banerjee, A.; Majumder, P.; Sanyal, S.; Singh, J.; Jana, K.; Das, C.; Dasgupta, D. The DNA intercalators ethidium bromide and propidium iodide also bind to core histones. FEBS Open Bio 2014, 4, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Kosmidis, C.; Seo, S.M.; Kaatz, G.W. Ethidium bromide MIC screening for enhanced efflux pump gene expression or efflux activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 2010, 54, 5070–5073. [Google Scholar] [CrossRef] [PubMed]
- Tiwana, G.; Cock, I.E.; White, A.; Cheesman, M.J. Use of specific combinations of the triphala plant component extracts to potentiate the inhibition of gastrointestinal bacterial growth. J. Ethnopharmacol. 2020, 260, 112937. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.; Wagner, D.; Viveiros, M.; Sampaio, D.; Couto, I.; Vavra, M.; Kern, W.V.; Amaral, L. Thioridazine and chlorpromazine inhibition of ethidium bromide efflux in Mycobacterium avium and Mycobacterium smegmatis. J. Antimicrob. Chemother. 2008, 61, 1076–1082. [Google Scholar] [CrossRef]


| C. freundii µg/mL (µM) | S. flexneri µg/mL (µM) | S. sonnei µg/mL (µM) | A. hydrophilia µg/mL (µM) | A. faecalis µg/mL (µM) | S. typhimurium µg/mL (µM) | B. cereus µg/mL (µM) | |
|---|---|---|---|---|---|---|---|
| Apigenin | - | - | - | - | - | - | - |
| Fisetin | 250 (873) | 250 (873) | - | 125 (436) | 250 (873) | 250 (873) | - |
| Genistein | - | - | - | - | - | - | - |
| Gossypetin | - | - | - | - | - | - | - |
| Hispidulin | 125 (416) | 250 (832) | 250 (832) | 250 (832) | 125 (416) | 250 (832) | 250 (832) |
| Isoorientin | 125 (278) | 125 (278) | 125 (278) | 125 (278) | 62.5 (140) | 125 (278) | 62.5 (140) |
| Isorhamnetin | - | - | - | - | - | - | - |
| Kaempferol | - | - | - | - | - | - | - |
| Luteolin | - | - | - | - | - | - | |
| Myricetin | - | - | - | - | - | - | - |
| Orientin | 125 (278) | 125 (278) | 125 (278) | 125 (278) | 62.5 (140) | 125 (278) | 62.5 (140) |
| Quercetin | - | - | - | - | - | - | - |
| Rutin | 250 (409) | 125 (204) | 250 (409) | 125 (204) | 125 (204) | 250 (409) | 250 (409) |
| Taxifolin | - | - | - | - | - | - | - |
| Vitexin | 250 (578) | 250 (578) | 250 (578) | 250 (578) | 250 (578) | 250 (578) | 250 (578) |
| Positive control | |||||||
| Tetracycline | 0.63 (1.40) | 0.63 (1.40) | 0.63 (1.40) | 0.63 (1.40) | - | 1.25 (2.81) | 0.63 (1.40) |
| Chloramphenicol | - | - | - | - | - | - | - |
| Ciprofloxacin | 1.25 (3.77) | 0.63 (1.88) | 0.63 (1.88) | 0.63 (1.88) | 2.5 (7.55) | 1.25 (3.77) | 0.63 (1.88) |
| Gentamicin | 0.31 (0.65) | 0.31 (0.65) | 0.31 (0.65) | 0.07 (0.16) | 0.31 (0.65) | 0.63 (1.30) | 0.31 (0.65) |
| Erythromycin | 0.31 (0.42) | 0.31 (0.42) | 0.63 (0.84) | 0.31 (0.42) | - | 0.63 (0.84) | 0.31 (0.42) |
| CCCP | 7.81 (38) | 7.81 (38) | 15.62 (76) | 3.90 (19) | 15.62 (76) | 7.81 (38) | 3.90 (19) |
| EtBr | 15.61 (40) | 15.61 (40) | 31.25 (80) | 7.81 (19) | 31.25 (80) | 15.61 (40) | 7.81 (19) |
| Negative control | - | - | - | - | - | - | - |
| Tetracycline | Chloramphenicol | Ciprofloxacin | Gentamicin | Erythromycin | ||
|---|---|---|---|---|---|---|
| A.faecalis | Isoorientin | - | - | 1.25 | 3 | - |
| Orientin | 2 | - | 2 | 0.63 | 0.63 | |
| A.hydrophilia | Isoorientin | 3 | - | 3 | 2.125 | 0.625 |
| Orientin | - | - | 1.25 | 3 | - | |
| B.cereus | Isoorientin | 4 | - | 4 | 0.63 | 0.63 |
| Orientin | 4 | - | 4 | 0.63 | 0.63 | |
| C. freundii | Isoorientin | 1.50 | - | 1 | 0.63 | 0.63 |
| Orientin | 3 | - | 2 | 0.63 | 0.63 | |
| S. flexneri | Isoorientin | 3 | - | 3 | 0.63 | 0.63 |
| Orientin | 3 | - | 3 | 0.63 | 0.63 | |
| S. sonnei | Isoorientin | 3 | - | 3 | 2.13 | 0.63 |
| Orientin | 2.50 | - | 2.50 | 0.50 | 0.25 | |
| S. typhimurium | Isoorientin | 2 | - | 2 | 0.63 | 0.63 |
| Orientin | 4 | - | 4 | 0.63 | 0.63 |
| Cat# | Flavonoid | Formula | Molecular Weight (g/mol) | Purity | Manufacturer |
|---|---|---|---|---|---|
| A12135 | Vitexin | C21H20O10 | 432.4 | >98% | Adooq Bioscience, Irving, TX, USA |
| 18647 | Taxifolin | C15H12O7 | 304.3 | ≥98% | Cayman Chemical, Ann Arbor, MI, USA |
| A10815 | Rutin | C27H30O16 | 610.5 | >98% | Adooq Bioscience |
| A10766 | Quercetin | C15H10O7 | 302.2 | >98% | Adooq Bioscience |
| A12096 | Orientin | C21H20O11 | 448.38 | >98% | Adooq Bioscience |
| A10615 | Myricetin | C15H10O8 | 318.2 | >98% | Adooq Bioscience |
| A10541 | Luteolin | C15H10O6 | 286.2 | >98% | Adooq Bioscience |
| A10495 | Kaempferol | C15H10O6 | 286.2 | >98% | Adooq Bioscience |
| 16496 | Isorhamnetin | C16H12O7 | 316.3 | ≥98% | Cayman Chemical |
| 26862 | Isoorientin | C21H20O11 | 448.4 | ≥95% | Cayman Chemical |
| A13945 | Hispidulin | C16H12O6 | 300.26 | >98% | Adooq Bioscience |
| G-500 | Gossypetin | C15H10O8 | 318.24 | >93% | Indofine Chemical, Hillsborough, NC, USA |
| 10005167 | Genistein | C15H10O5 | 270.2 | ≥98% | Cayman Chemical |
| A10388 | Fisetin | C15H10O6 | 286.2 | >98% | Adooq Bioscience |
| 10010275 | Apigenin | C15H10O5 | 270.2 | ≥98% | Cayman Chemical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zai, M.J.; Cheesman, M.J.; Cock, I.E. Flavonoids Identified in Terminalia spp. Inhibit Gastrointestinal Pathogens and Potentiate Conventional Antibiotics via Efflux Pump Inhibition. Molecules 2025, 30, 2300. https://doi.org/10.3390/molecules30112300
Zai MJ, Cheesman MJ, Cock IE. Flavonoids Identified in Terminalia spp. Inhibit Gastrointestinal Pathogens and Potentiate Conventional Antibiotics via Efflux Pump Inhibition. Molecules. 2025; 30(11):2300. https://doi.org/10.3390/molecules30112300
Chicago/Turabian StyleZai, Muhammad Jawad, Matthew James Cheesman, and Ian Edwin Cock. 2025. "Flavonoids Identified in Terminalia spp. Inhibit Gastrointestinal Pathogens and Potentiate Conventional Antibiotics via Efflux Pump Inhibition" Molecules 30, no. 11: 2300. https://doi.org/10.3390/molecules30112300
APA StyleZai, M. J., Cheesman, M. J., & Cock, I. E. (2025). Flavonoids Identified in Terminalia spp. Inhibit Gastrointestinal Pathogens and Potentiate Conventional Antibiotics via Efflux Pump Inhibition. Molecules, 30(11), 2300. https://doi.org/10.3390/molecules30112300









