Enhancing the Structural Stability and Diffusion Kinetics of a Tunnel-Phase Cathode by the Synergistic Effect of Cation-Anion Co-Doping for Advanced Sodium-Ion Batteries
Abstract
1. Introduction
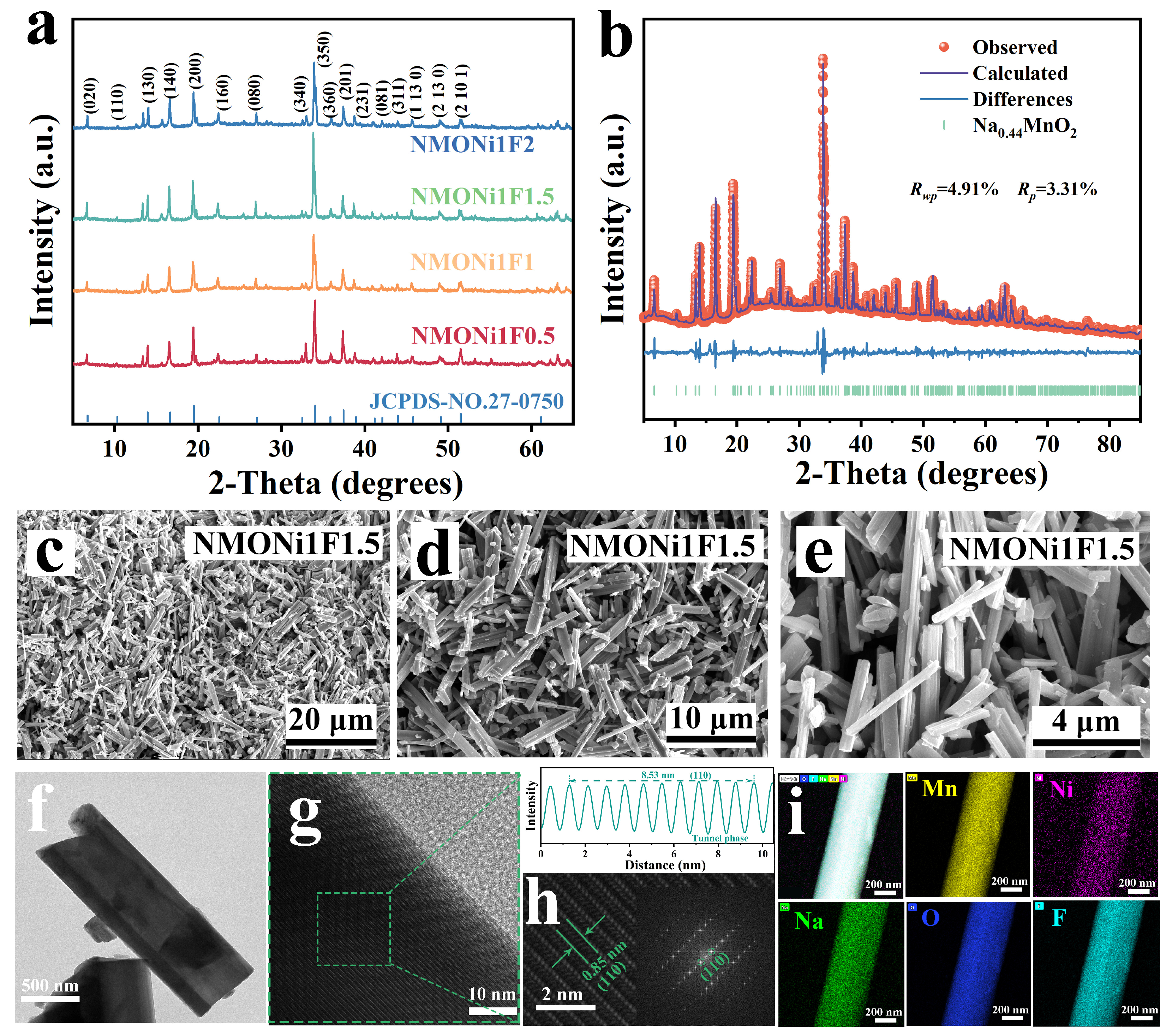
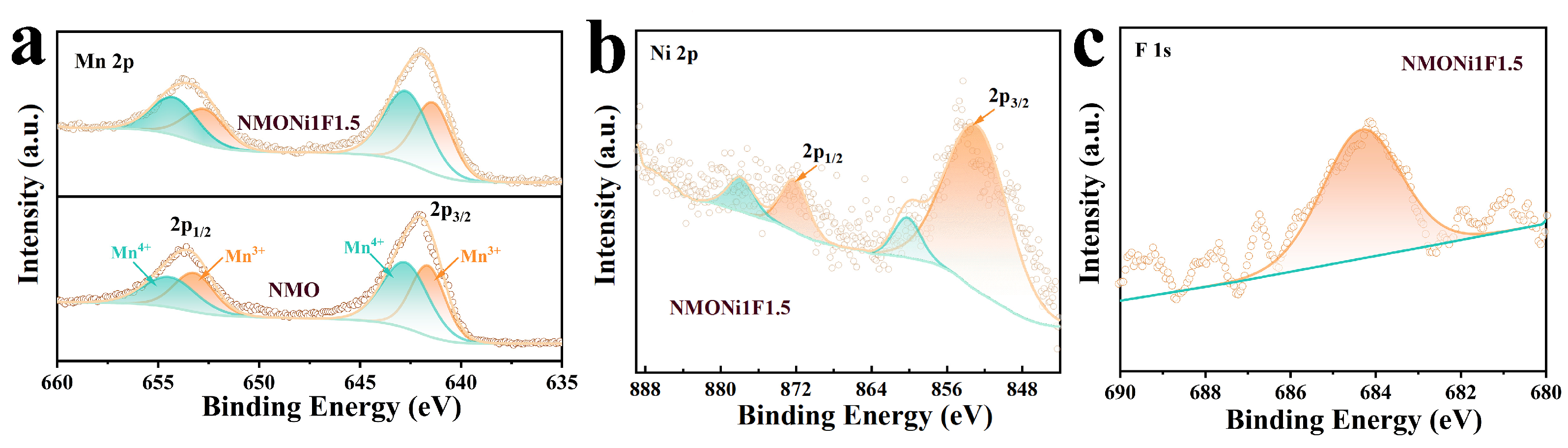
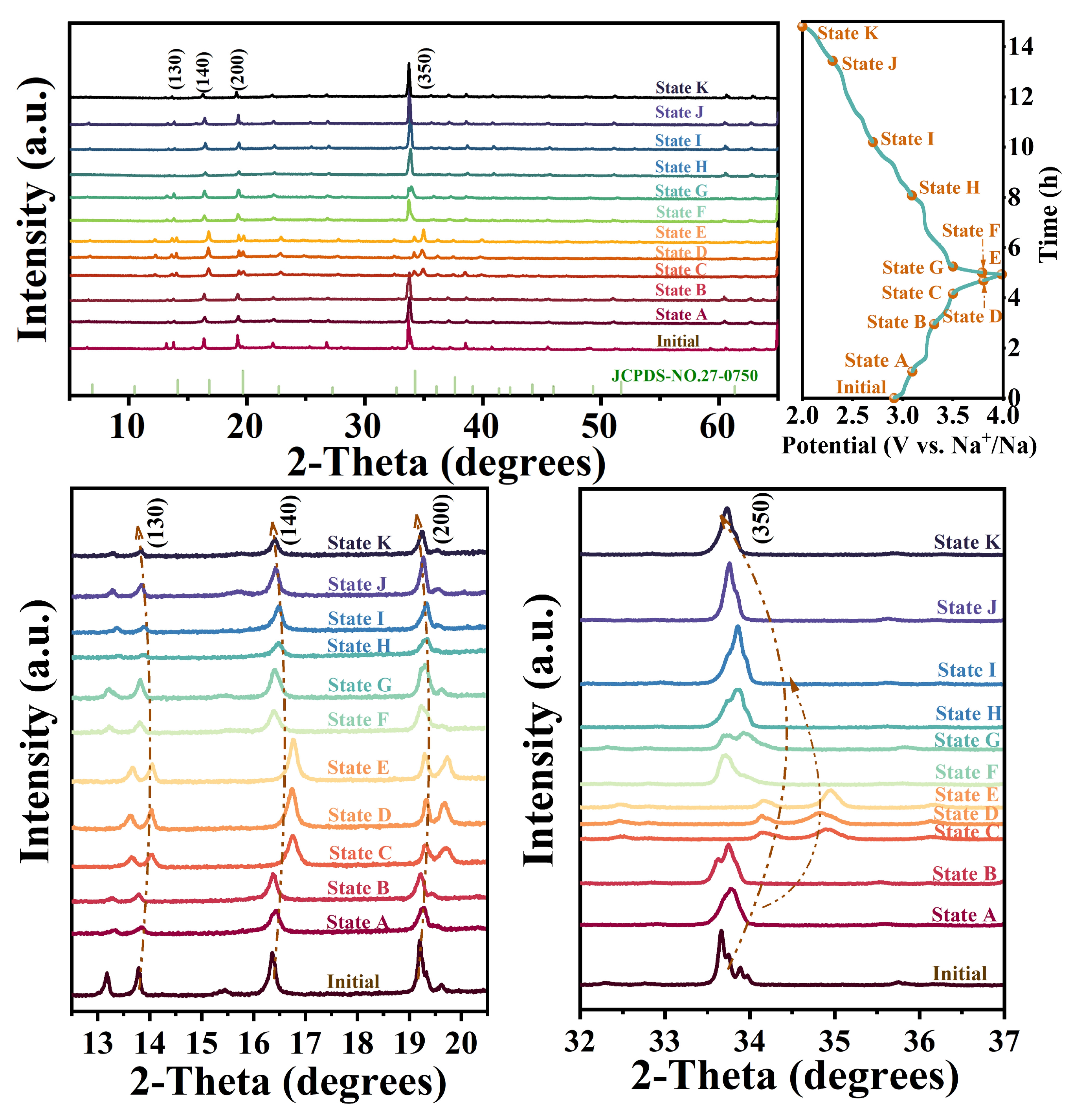
2. Results and Discussion
3. Experimental Section
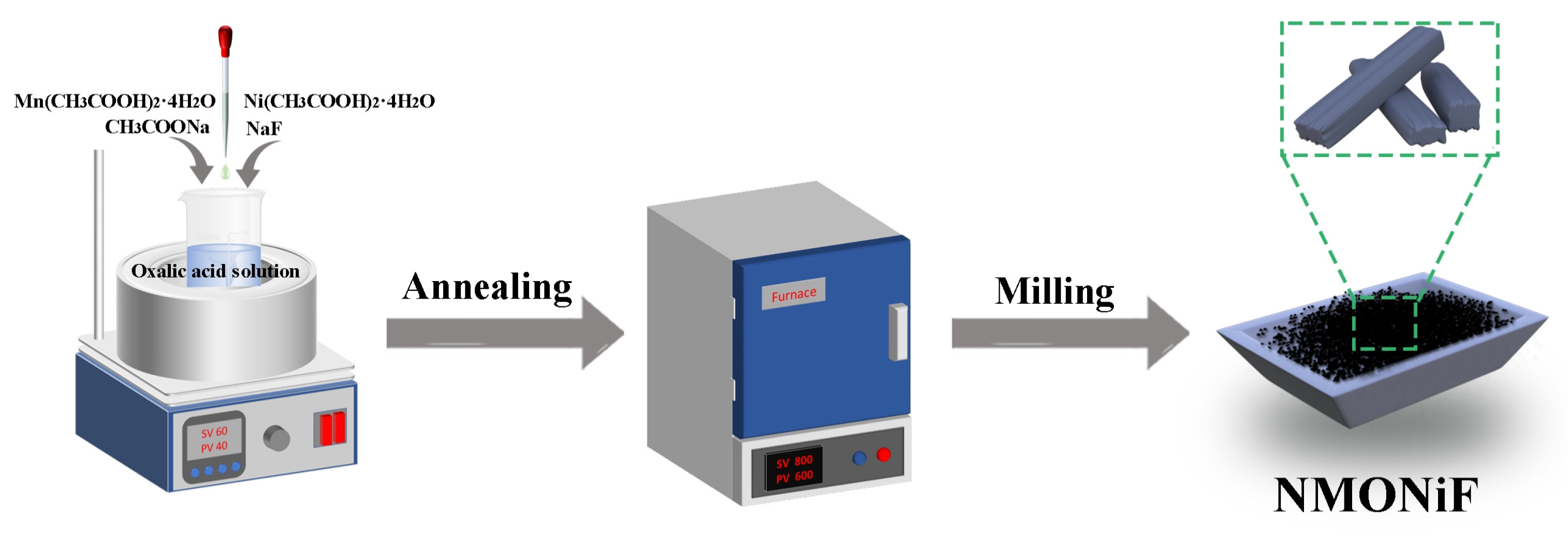
3.1. Materials Preparation
3.2. Materials Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ke, J.; Su, L. Advancing high-voltage cathodes for sodium-ion batteries: Challenges, material innovations and future directions. Energy Storage Mater. 2025, 76, 104133. [Google Scholar] [CrossRef]
- Zou, Z.; Mu, Y.; Han, M.; Chu, Y.; Liu, J.; Zheng, K.; Zhang, Q.; Song, M.; Jian, Q.; Wang, Y.; et al. Integrated polyanion-layered oxide cathodes enabling 100000 cycle life for sodium-ion batteries. Energy Environ. Sci. 2025, 18, 2216–2230. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.-C.; Zhou, H.; Guo, S. Wide-temperature-range sodium-metal batteries: From fundamentals and obstacles to optimization. Energy Environ. Sci. 2023, 16, 4759–4811. [Google Scholar] [CrossRef]
- Li, P.; Yuan, T.; Qiu, J.; Che, H.; Ma, Q.; Pang, Y.; Ma, Z.-F.; Zheng, S. A comprehensive review of layered transition metal oxide cathodes for sodium-ion batteries: The latest advancements and future perspectives. Mater. Sci. Eng. R Rep. 2025, 163, 100902. [Google Scholar] [CrossRef]
- Chen, X.; Lin, G.; Liu, P.; Sun, Z.; Si, Y.; Wang, Q.; Jiao, L. Synergetic enhancement of structural stability and kinetics of P’2-type layered cathode for sodium-ion batteries via cation–anion co-doping. Energy Storage Mater. 2024, 67, 103303. [Google Scholar] [CrossRef]
- Huang, J.; Gao, J.; Hong, N.; Zhang, B.; Wang, H.; Zhu, F.; Ni, L.; Zou, G.; Hou, H.; Chen, H.; et al. Dual-ion regulation of coordination chemistry for high-voltage stabilized P2-type cathode. Nano Energy 2024, 126, 109676. [Google Scholar] [CrossRef]
- Zuo, W.; Innocenti, A.; Zarrabeitia, M.; Bresser, D.; Yang, Y.; Passerini, S. Layered oxide cathodes for sodium-ion batteries: Storage mechanism, electrochemistry, and techno-economics. Acc. Chem. Res. 2023, 56, 284–296. [Google Scholar] [CrossRef]
- Tan, X.; Zeng, J.; Sun, L.; Peng, C.; Li, Z.; Zou, S.; Shi, Q.; Wang, H.; Liu, J. Current issues and corresponding optimizing strategies of layered oxide cathodes for sodium-ion batteries. InfoMat 2025, e12636. [Google Scholar] [CrossRef]
- Dang, Y.; Wu, Y.; Xu, Z.; Zheng, R.; Wang, Z.; Lin, X.; Liu, Y.; Liu, S.; Zhang, L.; Wang, D. Lithium and niobium dual-mediated P2-layered cathode for low-temperature and ultralong lifespan sodium-ion batteries. Energy Storage Mater. 2025, 76, 104152. [Google Scholar] [CrossRef]
- Chen, D.; He, B.; Jiang, S.; Wang, X.; Song, J.; Chen, H.; Xiao, D.; Zhao, Q.; Meng, Y.; Wang, Y. Enhancing the structural stability and strength of P2-type layered oxide sodium ion battery cathodes by Zn/F dual-site doping. Chem. Eng. J. 2025, 510, 161676. [Google Scholar] [CrossRef]
- Sauvage, F.; Laffont, L.; Tarascon, J.M.; Baudrin, E. Study of the insertion/deinsertion mechanism of sodium into Na0.44MnO2. Inorg. Chem. 2007, 46, 3289–3294. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Luo, S.; Cai, K.; Yan, S.; Wang, Q.; Zhang, Y.; Liu, X. Research progress of tunnel-type sodium manganese oxide cathodes for SIBs. Chin. Chem. Lett. 2022, 33, 2316–2326. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, A.; Chen, Z.; Cao, Y. Research progress of tunnel-structural Na0.44MnO2 cathode for sodium-ion batteries: A mini review. Electrochem. Commun. 2021, 122, 106897. [Google Scholar] [CrossRef]
- Hua, Z.; Jian, Y.; Wang, J.; Lin, Y.; Zhou, W.; Jiang, H.; Shen, Y.; Wu, X.; Xiang, Y. Lattice regulation strategy for constructing high-rate performance Na0.44Mn0.895Ti0.1Mg0.005O2 sodium-ion batteries cathode materials. J. Solid. State Chem. 2024, 329, 124415. [Google Scholar]
- Xiao, J.; Li, X.; Tang, K.; Wang, D.; Long, M.; Gao, H.; Chen, W.; Liu, C.; Liu, H.; Wang, G. Recent progress of emerging cathode materials for sodium ion batteries. Mater. Chem. Front. 2021, 5, 3735–3764. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Jamil, S.; Xie, J.; Liu, W.; Xia, J.; Nie, S.; Wang, X. Al2O3 coated Na0.44MnO2 as high-voltage cathode for sodium ion batteries. Appl. Surf. Sci. 2019, 494, 1156–1165. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, M.; Dong, W.; Cai, T.; Gao, Y.; Bi, H.; Huang, F. Multifunctional Na2TiO3 coating-enabled high-voltage and capacitive-like sodium-ion storage of Na0.44MnO2. ACS Appl. Mater. Interfaces 2023, 15, 40469–40477. [Google Scholar] [CrossRef]
- Yu, C.J.; Pak, Y.C.; Kim, C.H.; Kim, J.S.; Ri, K.C.; Ri, K.H.; Choe, S.H.; Cottenier, S. Structural and electrochemical trends in mixed manganese oxides NaxM0.44Mn0.56O2 (M = Mn, Fe, Co, Ni) for sodium-ion battery cathode. J. Power Sources 2021, 511, 230395. [Google Scholar] [CrossRef]
- Chae, M.S.; Elias, Y.; Aurbach, D. Tunnel-type sodium manganese oxide cathodes for sodium-ion batteries. ChemElectroChem 2021, 8, 798–811. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.F.; Li, H.W.; Li, J.Y.; Wang, J.Q.; Hu, H.Y.; Su, Y.; Jian, Z.C.; Yao, H.R.; Chen, S.Q.; et al. Insights into layered–tunnel dynamic structural evolution based on local coordination chemistry regulation for high-energy-density and long-cycle-life sodium-ion oxide cathodes. InfoMat 2023, 5, e12475. [Google Scholar] [CrossRef]
- Jian, Z.-C.; Liu, Y.-F.; Zhu, Y.-F.; Li, J.-Y.; Hu, H.-Y.; Wang, J.; Kong, L.-Y.; Jia, X.-B.; Liu, H.-X.; Guo, J.-X.; et al. Solid-state synthesis of low-cost and high-energy-density sodium layered-tunnel oxide cathodes: Dynamic structural evolution, Na+/vacancy disordering, and prominent moisture stability. Nano Energy 2024, 125, 109528. [Google Scholar] [CrossRef]
- Shi, W.-J.; Li, H.-X.; Zhang, D.; Du, F.-H.; Zhang, Y.-H.; Wang, Z.-Y.; Zhang, X.-H.; Zhang, P.-F. Insights into unrevealing the effects of the monovalent cation substituted tunnel-type cathode for high-performance sodium-ion batteries. Chem. Eng. J. 2023, 477, 146976. [Google Scholar] [CrossRef]
- Liang, X.; Kim, H.; Jung, H.G.; Sun, Y.K. Lithium-substituted tunnel/spinel heterostructured cathode material for high-performance sodium-ion batteries. Adv. Funct. Mater. 2020, 31, 2008569. [Google Scholar] [CrossRef]
- Li, X.-L.; Bao, J.; Li, Y.-F.; Chen, D.; Ma, C.; Qiu, Q.-Q.; Yue, X.-Y.; Wang, Q.-C.; Zhou, Y.-N. Boosting reversibility of Mn-based tunnel-structured cathode materials for sodium-ion batteries by magnesium substitution. Adv. Sci. 2021, 8, 2004448. [Google Scholar] [CrossRef]
- Zhong, W.; Huang, Q.; Zheng, F.; Deng, Q.; Pan, Q.; Liu, Y.; Li, Y.; Li, Y.; Hu, J.; Yang, C.; et al. Structural insight into the abnormal capacity of a Co-substituted tunnel-type Na0.44MnO2 cathode for sodium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 47548–47555. [Google Scholar] [CrossRef]
- Liu, Z.M.; Feng, X.T.; Zhao, H.J.; Han, X.Q.; Ye, Z.X.; Yao, Z.C.; Zhang, D. High-performance B-doped Na0.44MnO2 cathode materials for sodium-ion batteries. ACS Omega 2025, 10, 10023–10033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiang, Y.; Liu, B.; Li, G.; Dun, C.; Huang, H.; Zou, Q.; Xiong, L.; Wu, X. Fe doping mechanism of Na0.44MnO2 tunnel phase cathode electrode in sodium-ion batteries. J. Colloid Interf. Sci. 2024, 661, 389–400. [Google Scholar] [CrossRef]
- Liu, H.; Kong, L.; Wang, H.; Li, J.; Wang, J.; Zhu, Y.; Li, H.; Jian, Z.; Jia, X.; Su, Y.; et al. Reviving sodium tunnel oxide cathodes based on structural modulation and sodium compensation strategy toward practical sodium-ion cylindrical battery. Adv. Mater. 2024, 36, e2407994. [Google Scholar] [CrossRef]
- Shi, W.-J.; Zhang, D.; Meng, X.-M.; Bao, C.-X.; Xu, S.-D.; Chen, L.; Wang, X.-M.; Liu, S.-B.; Wu, Y.-C. Low-strain reticular sodium manganese oxide as an ultrastable cathode for sodium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 14174–14184. [Google Scholar] [CrossRef]
- Liu, H.; Feng, R.; Hussain, F.; Liu, Y.; Wang, L.; Fan, Q.; Ni, M.; Qiu, C.; Sun, M.; Wang, J.; et al. Ultrafast and highly efficient sodium ion storage in manganese-based tunnel-structured cathode. Adv. Funct. Mater. 2024, 34, 2404442. [Google Scholar] [CrossRef]
- Ding, Q.; Zheng, W.; Zhao, A.; Zhao, Y.; Chen, K.; Zhou, X.; Zhang, H.; Li, Q.; Ai, X.; Yang, H.; et al. W-doping induced efficient tunnel-to-layered structure transformation of Na0.44Mn1-xWxO2: Phase evolution, sodium-storage properties, and moisture stability. Adv. Energy Mater. 2023, 13, 2203802. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Q.Q.; Yu, J.; Guo, J.X.; Mo, N.K.; Li, H.W.; Su, Y.; Zhao, S.; Zhu, Y.F.; Chu, H.; et al. Constructing layered/tunnel interlocking oxide cathodes for sodium-ion batteries based on breaking Mn3+/Mn4+ equilibrium in Na0.44MnO2 via trace Mo doping. Compos. Part. B Eng. 2024, 284, 111664. [Google Scholar] [CrossRef]
- Wang, Q.C.; Qiu, Q.Q.; Xiao, N.; Fu, Z.W.; Wu, X.J.; Yang, X.Q.; Zhou, Y.N. Tunnel-structured Na0.66Mn0.66Ti0.34O2-xFx (x < 0.1) cathode for high performance sodium-ion batteries. Energy Storage Mater. 2018, 15, 1–7. [Google Scholar]
- Liu, K.; Tan, S.; Moon, J.; Jafta, C.J.; Li, C.; Kobayashi, T.; Lyu, H.; Bridges, C.A.; Men, S.; Guo, W.; et al. Insights into the enhanced cycle and rate performances of the F-substituted P2-type oxide cathodes for sodium-ion batteries. Adv. Energy Mater. 2020, 10, 2000135. [Google Scholar] [CrossRef]
- Hu, H.; He, H.C.; Xie, R.K.; Cheng, C.; Yan, T.; Chen, C.; Sun, D.; Chan, T.S.; Wu, J.; Zhang, L. Achieving reversible Mn2+/Mn4+ double redox couple through anionic substitution in a P2-type layered oxide cathode. Nano Energy 2022, 99, 107390. [Google Scholar] [CrossRef]
- Kong, F.; Liang, C.; Longo, R.C.; Yeon, D.-H.; Zheng, Y.; Park, J.-H.; Doo, S.-G.; Cho, K. Conflicting roles of anion doping on the electrochemical performance of Li-ion battery cathode materials. Chem. Mater. 2016, 28, 6942–6952. [Google Scholar] [CrossRef]
- Cui, T.; Li, X.; Si, Y.; Fu, Y. Synergetic anion-cation co-doping in Na0.44MnO2 boosting a high-stability and improved-kinetics cathode for sodium ion battery. Energy Storage Mater. 2024, 65, 103161. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, C.; Liu, J.; Yang, L.; Liu, J.; Zhu, M. Phase tuning of P2/O3-type layered oxide cathode for sodium ion batteries via a simple Li/F co-doping route. Chem. Eng. J. 2022, 431, 134273. [Google Scholar] [CrossRef]
- Chae, M.S.; Kim, H.J.; Lyoo, J.; Attias, R.; Gofer, Y.; Hong, S.-T.; Aurbach, D. Anomalous sodium storage behavior in Al/F dual-doped P2-type sodium manganese oxide cathode for sodium-ion batteries. Adv. Energy Mater. 2020, 10, 2002205. [Google Scholar] [CrossRef]
- Zhang, D.; Shi, W.-J.; Yan, Y.-W.; Xu, S.-D.; Chen, L.; Wang, X.-M.; Liu, S.-B. Fast and scalable synthesis of durable Na0.44MnO2 cathode material via an oxalate precursor method for Na-ion batteries. Electrochim. Acta 2017, 258, 1035–1043. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, H.; Yang, C.; Lui, Z.; Lin, Z.; Lei, Y.; Zhang, S.; Li, S.; Zhang, S. Promoting threshold voltage of P2-Na0.67Ni0.33Mn0.67O2 with Cu2+ cation doping toward high-stability cathode for sodium-ion battery. J. Colloid Interface Sci. 2024, 659, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Kalyoncuoglu, B.; Ozgul, M.; Altundag, S.; Harfouche, M.; Oz, E.; Avci, S.; Ji, X.; Altin, S.; Ates, M.N. Unveiling the outstanding full-cell performance of P2-type Na0.67Mn0.44Ni0.06Fe0.43Ti0.07O2 cathode active material for Na-ion batteries. J. Power Sources 2024, 591, 233775. [Google Scholar] [CrossRef]
- Li, S.; Zhou, W.; Liu, F.; Guan, C.; Gao, X.; Zhang, Y.; Jin, R.; Lai, Y.; Zhang, Z. Mitigating long range Jahn-Teller ordering to stabilize Mn redox reaction in biphasic layered sodium oxide. Adv. Energy Mater. 2024, 15, 2403955. [Google Scholar] [CrossRef]
- Huang, J.; Li, L.; Ma, Z.; Wang, X.; Luo, Z. Co-operative interaction of multiple ions for P2-type sodium-ion battery cathodes at high-voltage cyclability. ACS Appl. Energy Mater. 2025, 8, 99–107. [Google Scholar] [CrossRef]
- Oz, E.; Altin, S.; Avci, S. Investigation of physical and electrochemical properties of Ni-doped tunnel/P2 hybrid Na0.44MnO2 cathode material for sodium-ion batteries. J. Solid State Chem. 2023, 318, 123741. [Google Scholar] [CrossRef]
- Mishra, R.; Patel, A.; Tiwari, A.; Samriddhi; Singh, S.P.; Yadav, V.; Tiwari, R.K.; Singh, R.K. Enhanced electrochemical performance of Mg-doped P2–Na0.7Ni0.3Mn0.6Fe0.1O2 cobalt-free cathode materials for sodium-ion batteries. ACS Appl. Energy Mater. 2024, 7, 6736–6745. [Google Scholar] [CrossRef]
- Pamidi, V.; Naranjo, C.; Fuchs, S.; Stein, H.; Diemant, T.; Li, Y.; Biskupek, J.; Kaiser, U.; Dinda, S.; Reupert, A.; et al. Single-crystal P2–Na0.67Mn0.67Ni0.33O2 cathode material with improved cycling stability for sodium-ion batteries. ACS Appl. Mater. Interfaces 2024, 16, 25953–25965. [Google Scholar] [CrossRef]
- Qin, W.; Liu, Y.; Liu, J.; Yang, Z.; Liu, Q. Boosting the ionic transport and structural stability of Zn-doped O3-type NaNi1/3Mn1/3Fe1/3O2 cathode material for half/full sodium-ion batteries. Electrochim. Acta 2022, 418, 140357. [Google Scholar] [CrossRef]
- Pronin, I.A.; Averin, I.A.; Karmanov, A.A.; Yakushova, N.D.; Komolov, A.S.; Lazneva, E.F.; Sychev, M.M.; Moshnikov, V.A.; Korotcenkov, G. Control over the surface properties of zinc oxide powders via combining mechanical, electron beam, and thermal processing. Nanomaterials 2022, 12, 1924. [Google Scholar] [CrossRef]
- Pronin, I.A.; Filippov, I.A.; Komolov, A.S.; Dubov, E.A.; Karmanov, A.A.; Yakushova, N.D.; Korotcenkov, G. Photocatalytic degradation of paracetamol on ZnO powders: Investigating the effect grain size. Vacuum 2025, 238, 114340. [Google Scholar] [CrossRef]
- Shi, W.-J.; Yan, Y.-W.; Chi, C.; Ma, X.-T.; Zhang, D.; Xu, S.-D.; Chen, L.; Wang, X.-M.; Liu, S.-B. Fluorine anion doped Na0.44MnO2 with layer-tunnel hybrid structure as advanced cathode for sodium ion batteries. J. Power Sources 2019, 427, 129–137. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Dar, J.A.; Joshi, A.; Paperni, A.; Taragin, S.; Maddegalla, A.; Gautam, G.S.; Mukherjee, A.; Noked, M. Unveiling the structural integrity of tunnel-type Na0.44MnO2 cathode for sodium ion battery. J. Mater. Chem. A 2024, 12, 25109–25116. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Ben, L.; Lyu, Y.; Song, N.; Yang, Z.; Li, Y.; Mu, L.; Yang, H.-T.; Gu, L.; et al. Fe-based tunnel-type Na0.61[Mn0.27Fe0.34Ti0.39]O2 designed by a new strategy as a cathode material for sodium-ion batteries. Adv. Energy Mater. 2015, 5, 1501156. [Google Scholar] [CrossRef]
- Shi, W.-J.; Zheng, Y.-M.; Meng, X.-M.; Liu, S.-B.; Xu, S.-D.; Chen, L.; Wang, X.-M.; Zhang, D. Designing sodium manganese oxide with 4 d-cation Zr doping as a high-rate-performance cathode for sodium-ion batterie. ChemElectroChem 2020, 7, 2545–2552. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; Duan, X.; Xiao, Z.; Fan, X.; Zhang, H.; Wang, Y.; Liu, L.; Zhang, P.; Li, H. Enhancing the Structural Stability and Diffusion Kinetics of a Tunnel-Phase Cathode by the Synergistic Effect of Cation-Anion Co-Doping for Advanced Sodium-Ion Batteries. Molecules 2025, 30, 2299. https://doi.org/10.3390/molecules30112299
Shi W, Duan X, Xiao Z, Fan X, Zhang H, Wang Y, Liu L, Zhang P, Li H. Enhancing the Structural Stability and Diffusion Kinetics of a Tunnel-Phase Cathode by the Synergistic Effect of Cation-Anion Co-Doping for Advanced Sodium-Ion Batteries. Molecules. 2025; 30(11):2299. https://doi.org/10.3390/molecules30112299
Chicago/Turabian StyleShi, Wenjing, Xuezeng Duan, Zihan Xiao, Xiaofei Fan, Hao Zhang, Yan Wang, Lingyang Liu, Pengfang Zhang, and Hengxiang Li. 2025. "Enhancing the Structural Stability and Diffusion Kinetics of a Tunnel-Phase Cathode by the Synergistic Effect of Cation-Anion Co-Doping for Advanced Sodium-Ion Batteries" Molecules 30, no. 11: 2299. https://doi.org/10.3390/molecules30112299
APA StyleShi, W., Duan, X., Xiao, Z., Fan, X., Zhang, H., Wang, Y., Liu, L., Zhang, P., & Li, H. (2025). Enhancing the Structural Stability and Diffusion Kinetics of a Tunnel-Phase Cathode by the Synergistic Effect of Cation-Anion Co-Doping for Advanced Sodium-Ion Batteries. Molecules, 30(11), 2299. https://doi.org/10.3390/molecules30112299






