Abstract
The purpose of this study was to evaluate the gene expression pattern of the caries-inhibiting effect of Cudrania tricuspidata (C. tricuspidata) extract on cariogenic bacteria Streptococcus mutans (S. mutans). We examined bacterial growth, tooth surface attachment, biofilm formation, acid production, free calcium release, and toxicity gene expression. The major components of the extract were investigated by UPLC-Q-TOF-MS analysis. Exposure to C. tricuspidata inhibited bacterial growth and attachment at concentrations of ≥15 μg/mL. Inhibition effects on biofilm formation, acid production, and free calcium release due to acid production were observed at concentrations ≥ 30 μg/mL. S. mutans virulence gene expression analysis showed that it inhibited the expression of gbpB and spaP, which mediate bacterial attachment to the tooth surface, and that of genes contributing to biofilm formation (gtfB, gtfC, and gtfD) and acid resistance (brpA and relA), and regulation (vicR). Analysis using UPLC–Q–TOF–MS/MS showed that the main component was phenylpropanoids. These results suggest that C. tricuspidata may inhibit the cariogenic properties associated with the expression of caries-related genes in S. mutans.
1. Introduction
Dental biofilm formation is important in the development of dental caries. It is formed through a multi-step process. The first step involves the formation of an acquired pellicle on the tooth surfaces. Subsequently, primary colonizers, such as Gram-positive streptococci (e.g., Streptococcus mutans [S. mutans], Streptococcus oralis, Streptococcus sanguinis, and Streptococcus mitis), as well as Neisseria species, adhere to the surface of the acquired pellicle. Dental biofilms progressively form through the proliferation of primary colonizing bacteria and the aggregation and adhesion of secondary colonizing microorganisms. Secondary colonizers mainly consist of Gram-negative bacteria such as Fusobacterium nucleatum, Prevotella intermedia, Actinomyces species, and Capnocytophaga species [1]. Among the microorganisms present in the dental biofilms, mutans streptococci related to dental caries include S. mutans. Once sucrose has been broken down into glucose and fructose, S. mutans releases glucosyltransferase (GTF), which allows glucose to form dental plaques. These plaques calcify and serve as a matrix for the fixation of the bacterium, while fructose induces demineralization in the teeth, which become susceptible to dental caries, as pH is lowered by lactic acids produced by S. mutans [2,3,4]. Virulence factors of S. mutans, which include bacterial adherence, extracellular polysaccharide formation, biofilm formation, glucose absorption and metabolism, acid tolerance, and regulation and involvement, contribute to disease development [5]. Genes related to adherence include spaP, which interacts with salivary agglutinin glycoprotein, and gbpB, which affects the tooth surface adherence of glucan, both of which affect S. mutans adherence to the tooth surface [6,7]. Glucan synthases GTFB, GTFC, and GTFD are produced by gtfB, gtfC, and gtfD and regulated by vicR [8,9]. In addition, brpA and relA stabilize biofilm formation and are involved in the glucose phosphotransferase system, which mediates acid tolerance [10,11,12]. Overall, this evidence suggests that suppressing the growth of S. mutans and associated virulence factors may help prevent the onset of dental caries.
Previous studies have shown that lotus leaf, dandelion, and prickly pear extracts inhibited the growth of S. mutans [13,14]. Some studies have reported the inhibition of S. mutans growth, acid production, adherence, and insoluble glucan synthesis, as mediated by Caesalpinia sappan and Aconitum koreanum extracts [15,16]. In addition, Diospyros malabarica stem extracts had an inhibitory effect on biofilm formation associated with S. mutans [17].
The Cudrania tricuspidata (C. tricuspidata) is a plant mainly found in East Asia, including Korea, China, and Japan, and has long been used as a folk remedy. In traditional Korean medicine, it has been used as a nutritional tonic, helping to improve insomnia, hangover, vision, and liver and kidney function. The Dictionary of Traditional Chinese Medicine describes it as effective in treating joint pain, jaundice, and swelling [18,19]. The physiological activity of C. tricuspidata is mediated by xanthones and flavonoids. Isolated prenylated xanthones show anti-obesity and anti-diabetes effects based on protein tyrosine phosphatase 1B (PTP1B) inhibition [20]. Cudratricusxanthone A demonstrates strong antiproliferative, antioxidant, and monoamine oxidase inhibitory effects [21]. The prenylated isoflavone skeleton has been reported to be key to antibacterial activity [22]. However, their inhibitory effect on dental caries has not been examined previously. Hence, this study examined the inhibitory effect of C. tricuspidata extracts on S. mutans growth, adherence, biofilm formation, organic acid production, free calcium release, and virulence gene expression and subsequently evaluated their potential to inhibit dental caries.
2. Results
2.1. UPLC-Q-TOF-MS Analysis Results of C. tricuspidata Extract
As a result of analyzing the ethanol extract of C. tricuspidata roots by UPLC Q-TOP, 48 major components were identified (Table 1). The isomer components analyzed were isoarundinin II, cudratricusxanthone L, gericudranin E, kuwanon E, cudraflavenone A, cudraxanthone B, mortatarin B, and mortatarin C (Table 1). The total ion mass spectrum of C. tricuspidata extract in negative ion mode is shown in Figure 1.

Table 1.
Compounds identified in Cudrania tricuspidata by UPLC-Q-TOF-MS negative ion mode.
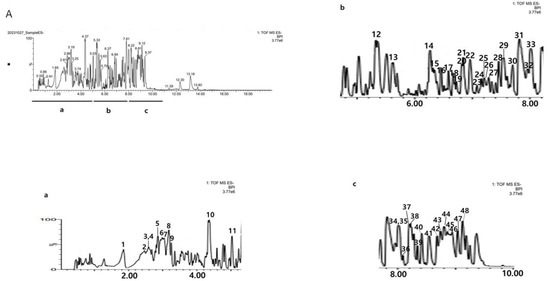
Figure 1.
The base peak chromatograms (BPC) of C. tricuspidata monitored by UPLC-Q-TOF/MS in negative ion mode. The numbers in (A) are RT (min). (a) The BPC of RT from 0 to 5 min. (b) The BPC of RT from 5 to 8 min. (c) The BPC of RT from 8 to 10 min. The numbers in (a–c) are the peak No. in Table 1.
2.2. Inhibitory Effect on S. mutans Growth
The inhibitory effect of ethanol extracts of C. tricuspidata on S. mutans growth was examined at concentrations of 15, 30, 45, and 60 μg/mL, which showed 13%, 50%, 87%, and 96% inhibition, respectively, compared to the growth rate of 100% in the control group that was not treated with the extracts. Chlorhexidine (CHX), which was used as the positive control, showed 99% inhibition of growth at a concentration of 0.05% (Figure 2).
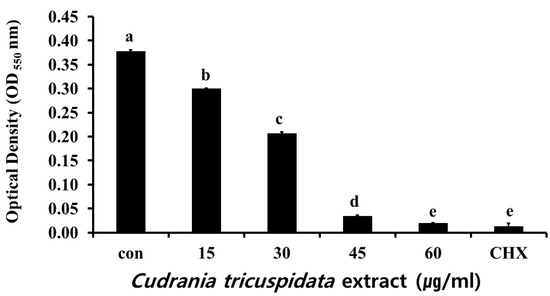
Figure 2.
Inhibitory effect of C. tricuspidata extract on the growth of S. mutans. A degree of inhibition was observed at concentrations of 15, 30, 45, and 60 μg/mL of C. tricuspidata extract; 0.05% CHX was used as a positive control. Significance differences among the groups are indicated by different letters (p ≤ 0.05).
2.3. Inhibitory Effect on S. mutans Biofilm Formation
The inhibitory effect of C. tricuspidata extracts on S. mutans biofilm formation was examined. The dish and artificial teeth observed under the scanning electron microscope showed that higher concentrations of the extracts resulted in a higher inhibition rate of S. mutans biofilm formation compared to that observed in the negative control group (Figure 3, Figure 4 and Figure 5). In particular, the inhibitory effect of the extracts on S. mutans biofilm formation was the highest at concentrations ≥45 μg/mL (Figure 3). The effect of the extracts on the expression of gtfB, gtfC, and gtfD, which affect S. mutans biofilm formation, and vicR, which regulates these genes, was measured via real-time PCR. The expression of gtfB was inhibited at ≥60 μg/mL. The expression of gtfC and gtfD was inhibited by the extract in a concentration-dependent manner at ≥15 μg/mL and ≥30 μg/mL, respectively, while the extract concentrations ≥30 μg/mL inhibited the expression of vicR (Figure 6).
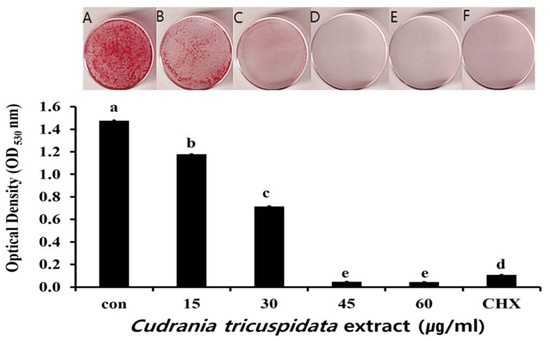
Figure 3.
Inhibitory effect of C. tricuspidata extract on biofilm formation in S. mutans. Safranin staining of S. mutans biofilms after treatment with C. tricuspidata extract (A) control, (B) 15 μg/mL, (C) 30 μg/mL, (D) 45 μg/mL, (E) 60 μg/mL, and (F) positive control (0.05% CHX). Significance differences among the groups are indicated by different letters (p ≤ 0.05).
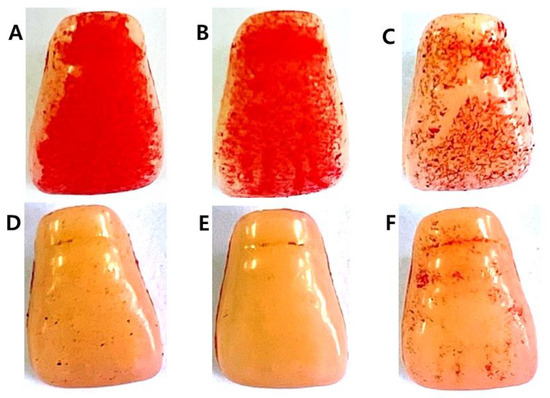
Figure 4.
Inhibitory effect of C. tricuspidata extract on S. mutans biofilm formation on the surface of a resin-based tooth. (A) Control, (B) 15 μg/mL, (C) 30 μg/mL, (D) 45 μg/mL, (E) 60 μg/mL, and (F) positive control (0.05% CHX).
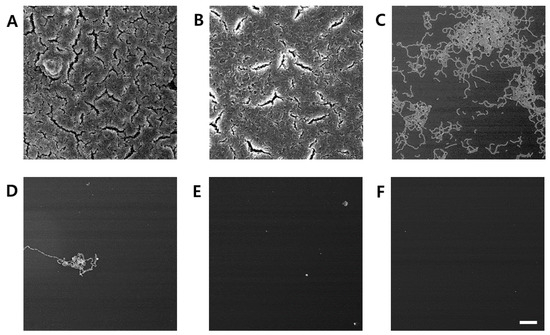
Figure 5.
Inhibitory effect of C. tricuspidata extract on S. mutans biofilm formation observed using scanning electron microscopy. (A) Control, (B) 15 μg/mL, (C) 30 μg/mL, (D) 45 μg/mL, (E) 60 μg/mL, and (F) positive control (0.05% CHX) bar = 100 μm.
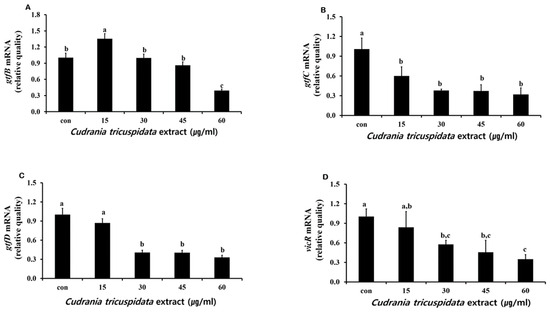
Figure 6.
Real-time PCR analysis of multiple genes encoding biofilm formation-associated virulence factors. After culturing S. mutans with various concentrations of C. tricuspidata extract, real-time PCR analysis of gtfB (A), gtfC (B), gtfD (C), and vicR (D) was performed. Each value is expressed as mean ± standard deviation. Significance differences among the groups are indicated by different letters (p ≤ 0.05).
2.4. Inhibitory Effect on S. mutans Adherence
We analyzed the inhibitory effect of the extracts on saliva-coated hydroxyapatite (S-HA) bead adherence measurement. C. tricuspidata extracts at concentrations of 15, 30, 45, and 60 μg/mL showed 40%, 60%, 82%, and 97% inhibition of adherence, respectively, compared to 0% inhibition observed in the negative control group (Figure 7). The expression of gbpB and spaP involved in mediating S. mutans adherence was observed in real-time PCR. The expression of gbpB and spaP was inhibited at concentrations ≥15 μg/mL and ≥30 μg/mL, respectively (Figure 8).
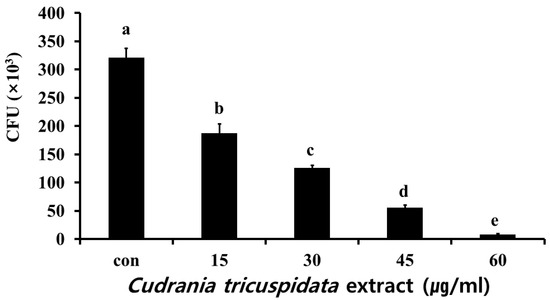
Figure 7.
Inhibitory effect of C. tricuspidata extract on the adherence of S. mutans. Colony forming units (CFU) of S. mutans observed after treatment of 30 mg of S-HA beads by various concentrations of C. tricuspidata extract. Significance differences among the groups are indicated by different letters (p ≤ 0.05).
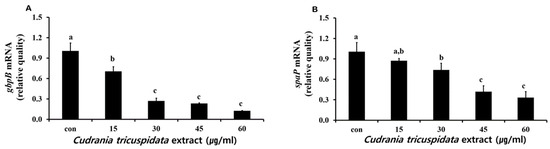
Figure 8.
Real-time PCR analysis of the genes (gbpB and spaP) encoding adherence virulence factors. Real-time PCR analysis of gbpB (A) and spaP (B) was performed by culturing S. mutans with various concentrations of C. tricuspidata extract. Each value is expressed as mean ± standard deviation. Significance differences among the groups are indicated by different letters (p ≤ 0.05).
2.5. Inhibitory Effect on Organic Acid and Free Calcium Production by S. mutans
S. mutans were inoculated with different concentrations of the extracts to analyze the inhibitory effect of C. tricuspidata extracts on organic acid production. The pH was measured before incubation and after 24 h of incubation. A pH of 7.33 ± 0.04 in the negative control group and that of 5.39 ± 0.10 was observed after 24 h of incubation. At a concentration of 15 μg/mL, the extract showed a pH of 5.32 ± 0.012, which was comparable to that in the control group. The concentrations of 30 μg/mL, 45 μg/mL, and 60 μg/mL yielded pH values of 6.26 ± 0.09, 7.13 ± 0.04, and 7.17 ± 0.07, respectively. At concentrations ≥30 μg/mL, the extract inhibited organic acid production (Table 2).

Table 2.
pH changes in S. mutans cultures incubated with different concentrations of C. tricuspidata extract.
We measured free calcium levels after a reaction in which hydroxyapatite/tricalcium phosphate (HA/TCP) was dissolved by organic acid released by S. mutans. We observed 24.6 μM of free calcium in the negative control group. At each concentration, the extract inhibited free calcium release (Figure 9). The expression levels of relA and brpA, which contribute to S. mutans acid tolerance, were examined by real-time PCR. The results showed that the expression of relA and brpA was inhibited at concentrations ≥15 μg/mL (Figure 10).
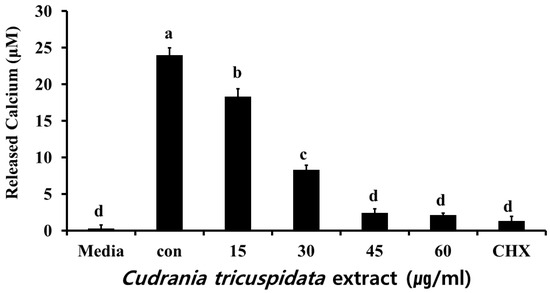
Figure 9.
Effect of C. tricuspidata extract on calcium release by S. mutans. The degree of inhibition of calcium release was observed in the presence of C. tricuspidata extract at concentrations of 15, 30, 45, and 60 µg/mL, and CHX (0.05%) was used as a positive control. Significance differences among the groups are indicated by different letters (p ≤ 0.05).
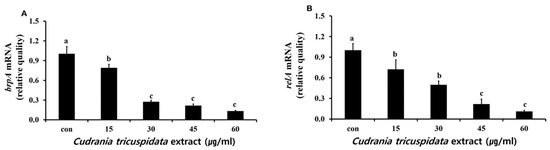
Figure 10.
Real-time PCR analysis of brpA (A) and relA (B) genes encoding acid resistance-associated virulence factors in S. mutans cultured and treated with various concentrations of C. tricuspidata extract. Each value is expressed as mean ± standard deviation. Significance differences among the groups are indicated by different letters (p ≤ 0.05).
3. Discussion
Dental caries are common and associated with the interaction of bacteria in the dental plaque, as well as food and saliva components in the mouth. S. mutans is a major bacterium linked to the onset of dental caries. Treatments with CHX and fluoride can be used to prevent dental caries; however, they may lead to tooth discoloration, dental calculus formation, oral mucosal peeling, and the development of resistant bacterial strains. Remedies based on natural substances have been proposed to address these limitations [23].
The major components of the ethanol extract of C. tricuspidata analyzed by UPLC–Q–TOF–MS/MS were phenylpropanoids (14.19%), flavonoids (9.83%), and xanthones (6.54%). C. tricuspidata leaf and root bark extracts, obtained with methanol using a disk diffusion test, showed an antibacterial effect against S. mutans [24], and the component analysis results were similar to our analysis [20]. Isoarundinin II, which was first isolated from Arundina bambusifolia, belongs to phenylpropanoids and has been reported to exhibit anti-inflammatory, anticancer, and other inhibitory activities, including the inhibitory effect of NO production [25] and potent DPPH free radical scavenging effect [26]. Cudraatricusxanthone L has been reported to have antineuro–inflammatory effects [27]. The anticancer and antioxidant effects of flavanone gericudranin E [28,29], the antibacterial effect of kuwanone E [30], and the inhibitory effect of cudraflavenone A on pathogenic bacteria and biofilm formation [31] have been reported.
This study analyzed the inhibitory effect of C. tricuspidata extracts on dental caries induced by S. mutans. We observed the effects of the extract on bacterial growth, adherence, organic acid production, free calcium release, and biofilm formation and confirmed the expression patterns of virulence genes related to the development of dental caries by S. mutans via real-time PCR. At concentrations of 15, 30, 45, and 60 μg/mL, C. tricuspidata extracts showed 13%, 50%, 87%, and 96% inhibition of bacterial growth, respectively, compared to that observed in the control group. Compared to the growth inhibition of 31%, 95%, 98%, and 99.6% observed at concentrations of 125, 250, 500, and 1000 μg/mL of methanol extracts of Caesalpinia sappan, respectively [16], the inhibitory effect of C. tricuspidata extracts was more potent. For tooth surface adherence, S. mutans releases three types of GTF enzymes. GTF-S produces soluble glucan, while GTF-I and GTF-SI produce insoluble glucan. Three types of gtf genes are involved in the production of these enzymes: gtfB, gtfC, and gtfD produce GTF-I, GTF-SI, and GTF-S, respectively. Glucan is an insoluble polysaccharide synthesized by the aforementioned enzymes. Insoluble glucan turns into an underlying substance for the formation of dental plaque and helps S. mutans to adhere to the tooth surface [9,32]. C. tricuspidata extracts inhibited S. mutans tooth surface adherence (up to 40–97%) in a concentration-dependent manner. These effects were more potent than those of 16, 31, 63, and 125 μg/mL of Aconitum koreanum ethanol extracts with adherence inhibition of 5%, 26%, 50%, and 54%, respectively [15]. The key proteins of the surface protein antigen P (spaP), glucosyltransferase B (gtfB), and glucan-binding protein B (gbpB) are produced by S. mutans and are essential mechanisms for biofilm formation. spaP interacts with salivary α-galactosides and plays a key role in the early stages of biofilm development [33]. However, the expression of C. tricuspidata extracts was suppressed at a lower concentration (≥45 μg/mL) than other genes (gtfC, gtfD, gbpB, brpA, and relA). The reason is presumed to be due to the effect of planktonic culture conditions, not the presence of saliva in the oral cavity. After the initial biofilm action of spaP, S mutans adhere to the tooth surface via the gtfB gene, facilitating the synthesis of extracellular glucans. This binding plays a key role in plaque formation. Refs. [9,34] Unlike gtfB, gbpB binds to existing glucans formed by gtfB, enhancing the initial binding of S mutans to the tooth surface. Therefore, key genes such as gtfB, gbpB, and spaP can be considered indicators for the development of new drugs aimed at treating dental caries [33]. The low expression of spaP and gtfB compared to other genes (gtfC, gtfD, gbpB) by Cudrania japonica is thought to be due to the influence of planktonic culture. However, the excellent inhibition of biofilm formation by C. tricuspidata is thought to be due to the interaction of gtfC, gtfD, and gbpB genes. Furthermore, S. mutans metabolizes glucose, produces organic acids, decreases pH in the oral cavity owing to acid production, and shows biofilm formation [35,36]. C. tricuspidata extracts at concentrations of ≥30 μg/mL infused above the dental caries threshold of pH 5.5 inhibited organic acid production by S. mutans. The expression of brpA and relA involved in acid tolerance declined in a concentration-dependent manner. Furthermore, with decreased organic acid production, the inhibitory effect of the extracts on free calcium released by HA/TCP likely contributed to the reduction in the occurrence of dental caries. The inhibitory effects of C. tricuspidata extracts on S. mutans growth, adherence, biofilm, and acid production were stronger than those of propolis extracts, which are considered beneficial for oral health [37]. In addition, while propolis extracts did not inhibit the expression of gtfB, gtfC, and gtfD involved in biofilm formation, C. tricuspidata extracts inhibited the expression of these genes, thereby decreasing dental plaque formation, and helping reduce the risk of dental caries [37].
Overall, C. tricuspidata extracts showed an inhibitory effect on S. mutans growth, adherence, organic acid production, free calcium release, biofilm formation, and virulence gene expression and are expected to help inhibit the onset of dental caries. These effects may be partly attributed to the major components of C. tricuspidata, namely phenylpropanoids, xanthones, and flavonoids, but further analysis of the components is required.
4. Materials and Methods
4.1. Material Extraction
C. tricuspidata roots were provided by the Jinan Kujibong Farm and identified by Dr. Seung Il Jeong at the Jeonju Agrobio-materials Institute (Jeonju, Republic of Korea). A reference specimen (no: 2-06-22) was stored in the Herbarium of the Department of Oral Biochemistry, School of Dentistry, at Wonkwang University. A total of 1500 mL of 95% ethanol was added to 100 g of C. tricuspidata, which was then extracted at room temperature for 72 h. The extracts were filtered, concentrated, dried, and refrigerated (−70 °C). They were thawed for every experiment and diluted with dimethyl sulfoxide to obtain different concentrations before use.
4.2. Bacterial Culture
The strain used in this experiment was inoculated with S. mutans ATCC 25175 at a concentration of 1 × 108 CFU/mL in brain heart infusion (BHI, Difco, Sparks, MD, USA) broth, and after adding C. tricuspidata extracts by concentration (15, 30, 45, and 60 μg/mL), was then incubated at 37 °C for 24 h. Then, the optical density was measured with a 550 nm spectrophotometer. Moreover, 0.05% chlorhexidine (CHX) was used as a positive control. The experiment was repeated three times.
4.3. Biofilm Formation Assay
Different concentrations (15, 30, 45, and 60 μg/mL) of C. tricuspidata extracts were added to the BHI liquid medium containing 1% sucrose, and the medium was inoculated with 5 × 105 CFU/mL of S. mutans. Artificial teeth (Endura, Shofu Inc., Kyoto, Japan) were placed in a 24-well plate, followed by incubation at 37 °C for 24 h. In addition, 35 mm dish cultures were cultured under the same strains and culture conditions as the artificial teeth. After incubation, the medium was removed, and the artificial teeth and dishes were washed with purified water, stained with 1% safranin for 30 s, washed again, and dried. The bound safranin at the bottom of dishes was released by 30% acetic acid, and the optical density of the solution was determined at 530 nm. The artificial teeth were photographed. The extracts were not added to the control group [38,39].
4.4. Scanning Electron Microscopic Analysis of S. mutans Biofilm Formation
The BHI liquid medium containing 1% sucrose was dispensed into a 35 mm dish. C. tricuspidata extracts (15, 30, 45, and 60 μg/mL) were added, and the bacterial solution was inoculated at the concentration of 5 × 105 CFU/mL and incubated at 37 °C for 24 h. The incubated solution was removed, followed by cleaning with purified water. The solution was fixed after reaction with 2.5% glutaraldehyde (0.1 M sodium cacodylate buffer, pH 7.2) at 4 °C for 24 h. It was dehydrated with 70%, 80%, 95%, and 100% of ethanol, lyophilized, coated with gold, and imaged using the scanning electron microscope (SEM, Hitachi S-4800, Tokyo, Japan).
4.5. Bacterial Adherence
To analyze the effect of C. tricuspidata on bacterial adherence, we used the method reported by Liljemark et al. [40]. Hydroxyapatite beads (diameter 80 μm, Bio-Rad, Hercules, CA, USA) were coated with purified human saliva. The beads (S-HA) were then mixed with various concentrations (15, 30, 45, and 60 μg/mL) of C. tricuspidata in the bacterial suspension (1 × 107 CFU/mL). After mixing gently, incubation was performed at 37 °C for 90 min. Then, the process of centrifuging at 10,000 rpm for 1 min and washing the S-HA with 1× PBS was repeated twice to remove non-attached bacteria and transferred to a new tube containing potassium phosphate buffer. S. mutans adhered to S-HA was dispersed at 50 W for 30 s using a sonicator (Fisher Scientific, Springfield, NJ, USA), and the supernatant was inoculated into the MSA plate medium (supplemented with 3.2 mg/mL of bacitracin). Colony-forming units were calculated after 48 h of incubation
4.6. Analysis of Organic Acid Production and Calcium Release by S. mutans
4.6.1. Analysis of Organic Acid Production
The effect of C. tricuspidata (15, 30, 45, and 60 μg/mL) on acid production by S. mutans was evaluated as previously reported [13]. Acid production was measured in the medium before and after bacterial incubation using a pH meter (METTLER TOLEDO 320 pH meter, Shanghai, China). The extracts were not added to the control group.
4.6.2. Analysis of Calcium Release
After C. tricuspidata (15, 30, 45, and 60 μg/mL) extract was added to the BHI liquid medium containing HA/TCP, bacteria were inoculated at 1 × 108 CFU/mL, incubated at 37 °C for 24 h, and centrifuged at 1000 rpm for 10 min. The supernatant was extracted for analysis. Then, 100 μL of supernatant was carefully dispensed into a 96-well plate, and the free calcium level was measured in accordance with the protocol of the calcium assay kit (QuantiChrom™ Calcium Assay Kit [DICA-500], BioAssay system, Hayward, CA, USA). The absorbance was measured using an ELISA reader (Molecular Devices Co, San Jose, CA, USA) at 610 nm.
4.7. Real-Time Polymerase Chain Reaction (PCR) Analysis of S. mutans Virulence Genes
To evaluate the effect of C. tricuspidata (15, 30, 45, and 60 μg/mL) on gene expression of S. mutans, real-time PCR analysis was conducted. Total RNA was isolated from S. mutans, treated with different concentrations of the extract, and cDNA was synthesized. The StepOnePlus Real-Time PCR System with QPCR SYBR Green Mixes (Applied Biosystem, Foster City, CA, USA) was used for amplification. The expression level of 16S rRNA was used as the control. Primer pairs were reported in a previous study [41] (Table 3). The biological roles of each gene are in Table 4 [5].

Table 3.
Oligonucleotide primers used in this study.

Table 4.
The functions of virulence genes in Streptococcus mutans *.
4.8. UPLC-Q-TOF-MS Analysis
The aqueous extract of the MSJZD sample was analyzed on a Waters ACQUITY UPLC I-Class PLUS system (Waters Corporation, Milford, MA, USA), equipped with a Waters UPLC BEH C18 column (100 mm × 2.1 mm, 1.7 µm particle size), at a column temperature of 40 °C. The mobile phase consisted of acetonitrile (A) and water (B), each containing 0.1% formic acid. The elution procedure was as follows: 99–99% B at 0–1 min; 99–50% B at 1–15 min; 50–40% B at 15–17 min; 40–1% B at 17–18 min; 1% B at 18–21 min. The flow rate was 0.3 mL/min, and the injection volume was 2 μL.
The mass spectrometric data were collected using a time-of-flight analyzer with TurboIonSpray (AB Sciex, Singapore) ion source in negative ion mode. The specific conditions were as follows: nebulizing gas (N2): 55 psi; drying gas (N2): 45 psi; curtain gas: 35 psi; source temperature: 600 °C; ions apart voltage floating: 5500 V/−4500 V; TOF MS scan m/z range: 50–1500 Da; TOF-MS/MS scan m/z range: 25–1000 Da; TOF MS scan accumulation time: 0.25 s/spectra; product ion scan accumulation time: 0.035 s/spectra. Secondary mass spectrometry was obtained by information-dependent acquisition and high sensitivity mode. Declustering potential was ±60 V; collision energy was 35 ± 15 eV. IDA setup was as follows: Exclude isotopes within 4 Da; candidate ions to monitor per cycle was 12. The data were processed using SCIEX OS software (ver. 3.0) with multiple confidence criteria, including quality accuracy, retention time, isotopes, and matching use of compound libraries. In the current study, the TCM MS/MS Library in the SCIEX OS software was employed to identify the major constituents in MSJZD according to the first-order accurate mass number, isotope distribution ratio, and MS/MS of the constituents.
4.9. Statistical Analysis
The experiment was repeated three times, and the results were expressed as the mean and standard deviation (SD) using the statistical program SPSS (ver. 12.0). After analysis of ANOVA, a post hoc test was performed using Tukey HSD. p ≤ 0.05 was considered statistically significant.
5. Conclusions
This study demonstrated the anti-cariogenic effects of C. tricuspidata ethanol extracts against S. mutans. Additionally, some effects on gene expression related to biofilm formation of S. mutans were observed. The components of the C. tricuspidata root ethanol extract included phenylpropanoids, flavonoids, and xanthan; these compounds may account for the anti-cariogenic effect of S. mutans. These results suggest that C. tricuspidata may inhibit the cariogenic effects associated with the expression of caries-related genes in S. mutans.
Author Contributions
Conceptualization, Y.-O.Y.; Data curation, Y.-O.Y. and Y.-H.K.; Formal analysis, E.-S.K. and Y.-H.K.; Investigation, E.-S.K. and J.-E.J.; Methodology, E.-S.K. and J.-E.J.; Resources, E.-S.K. and J.-E.J.; Writing—original draft, E.-S.K.; Writing—review and editing, Y.-O.Y. All authors will be informed about each step of manuscript processing including submission, revision, revision reminder, etc., via emails from our system or assigned Assistant Editor. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
This paper was supported by Wonkwang University in 2024.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Seneviratne, C.J.; Zhang, C.F.; Samaranayake, L.P. Dental plaque biofilm in oral health and disease. Chin. J. Dent. Res. 2011, 14, 87–94. [Google Scholar] [PubMed]
- de Soet, J.J.; van Loveren, C.; van Lammens, A.J.; Pavicić, M.J.; Homburg, C.H.; ten Cate, J.M.; de Graaff, J. Differences in cariogenicity between fresh isolates of Streptococcus sobrinus and Streptococcus mutans. Caries Res. 1991, 25, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- van Houte, J. Role of micro-organisms in caries etiology. J. Dent. Res. 1994, 73, 672–681. [Google Scholar] [CrossRef]
- You, Y.O. Virulence genes of Streptococcus mutans and dental caries. Int. J. Oral. Biol. 2019, 44, 31–36. [Google Scholar] [CrossRef]
- Shemesh, M.; Tam, A.; Steinberg, D. Expression of biofilm-associated genes of Streptococcus mutans in response to glucose and sucrose. J. Med. Microbiol. 2007, 56, 1528–1535. [Google Scholar] [CrossRef]
- Yang, J.; Deng, D.; Brandt, B.W.; Nazmi, K.; Wu, Y.; Crielaard, W.; Ligtenberg, A.J.M. Diversity of SpaP in genetic and salivary agglutinin mediated adherence among Streptococcus mutans strains. Sci. Rep. 2019, 9, 9943. [Google Scholar] [CrossRef]
- Burne, R.A. Oral streptococci… products of their environment. J. Dent. Res. 1998, 77, 445–452. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Q.; Wang, Y.; Wu, H.; Zou, J. Molecular mechanisms of inhibiting glucosyltransferases forbiofilm formation in Streptococcus mutans. Int. J. Oral Sci. 2021, 13, 30. [Google Scholar] [CrossRef]
- Lemos, J.A.C.; Brown, T.A., Jr.; Burne, R.A. Effects of RelA on key virulence properties of planktonic and biofilm populations of Streptococcus mutans. Infect. Immun. 2004, 72, 1431–1440. [Google Scholar] [CrossRef]
- Steinberg, D.; Moreinos, D.; Featherstone, J.; Shemesh, M.; Feuerstein, O. Genetic and physiological effects of noncoherent visible light combined with hydrogen peroxide on Streptococcus mutans in biofilm. Antimicrob. Agents Chemother. 2008, 52, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, X.D.; Wu, C.D. The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrob. Agents Chemother. 2011, 55, 1229–1236. [Google Scholar] [CrossRef]
- Chi, B.R.; Jo, D.Y.; Cha, S.Y.; Chi, M.J.; Hye-won Jeong, H.W.; Kang, K.H. The effect on growth inhibition of S. mutans by lotus leaf and Dandelion extracts. J. Korea Acad.-Ind. Coop. Soc. 2011, 12, 5773–5778. [Google Scholar] [CrossRef]
- Jung, E.J.; Hong, S.J.; Choi, J.I.; Jeong, S.S.; Oh, H.N.; Lee, H.J.; Choi, C.H. In vitro growth inhibition of Streptococcus mutans by extract of prickly pear (Opuntia ficus-indica var. saboten Makino). J. Korea Acad. Oral Health 2010, 34, 28–35. [Google Scholar]
- Kang, S.Y.; An, S.Y.; Lee, M.W.; Kwon, S.K.; Lee, D.H.; Jeon, B.H.; You, Y.O.; Lee, M.W. Effects of Aconitum Koreanum extract on the growth, acid production, adhesion and insoluble glucan synthesis of Streptococcus mutans. J. Physiol. Pathol. Korean Med. 2015, 29, 27–32. [Google Scholar] [CrossRef]
- You, Y.O.; Yu, H.H.; Kim, Y.J.; You, M.S.; Seo, S.J.; Lee, L.; Lee, H.S. Effects of Caesalpinia sappan extracts on the growth, acid production, adhesion, and insoluble glucan synthesis of Streptococcus mutans. J. Korean Acad. Dent. Health 2003, 27, 277–288. [Google Scholar]
- Kim, H.S.; Lee, S.W.; Sydara, K.; Cho, S.J. Antibacterial and antibiofilm activities of Diospyros malabarica stem extract against Streptococcus mutans. J. Life Sci. 2019, 29, 90–96. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, C.T.; Do, M.Y.; Rang, M.J. Physiological activities of Cudrania tricuspidata extracts (Part I). J. Korea Acad.-Ind. Coop. Soc. 2013, 14, 3907–3915. [Google Scholar] [CrossRef]
- Kim, J.W.; Cho, N.M.; Kim, E.M.; Park, K.S.; Kang, Y.W.; Nam, J.H.; Nam, M.S.; Kim, K.K. Cudrania tricuspidata leaf extracts and its components, chlorogenic acid, kaempferol, and quercetin, increase claudin 1 expression in human keratinocytes, enhancing intercellular tight junction capacity. Appl. Biol. Chem. 2020, 32, 260–274. [Google Scholar] [CrossRef]
- Quang, T.H.; Ngan, N.T.; Yoon, C.S.; Cho, K.H.; Kang, D.G.; Lee, H.S.; Kim, Y.C.; Oh, H. Protein tyrosine phosphatase 1B inhibitors from the roots of Cudrania tricuspidata. Molecules 2015, 20, 11173–11183. [Google Scholar] [CrossRef]
- Jeon, S.M.; Lee, D.S.; Jeong, G.S. Cudraticusxanthone A isolated from the roots of Cudrania tricuspidata inhibits metastasis and induces apoptosis in breast cancer cells. J. Ethnopharmacol. 2016, 194, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.W.; Jo, Y.H.; Choi, J.S.; Lee, M.K.; Lee, K.Y.; Kang, S.Y. Antibacterial activities of prenylated isoflavones from Maclura tricuspidata against fish pathogenic Streptococcus: Their structure-activity relationships and extraction optimization. Molecules 2021, 26, 7451. [Google Scholar] [CrossRef] [PubMed]
- Katsura, H.; Tsukiyama, R.I.; Suzuki, A.; Kobayashi, M. In vitro antimicrobial activities of bakuchiol against oral microorganisms. Antimicrob. Agents Chemother. 2001, 45, 3009–3013. [Google Scholar] [CrossRef]
- Choi, S.R.; You, D.H.; Kim, J.Y.; Park, C.B.; Kim, D.H.; Ryu, J.; Choi, D.G.; Park, H.M. Antimicrobial Activity of Methanol Extracts from Cudrania tricuspidata Bureau according to the Parts Harvested and Time. Korean J. Med. Crop. Sci. 2009, 17, 335–340. [Google Scholar]
- Geske, L.; Baier, J.; Joelle, C.; Boulos, J.C.; Efferth, T.; Opatz, T. Xylochemical synthesis and biological evaluation of the orchidaceous natural products isoarundinin I, bleochrin F, blestanol K, and pleionol. J. Nat. Prod. 2023, 86, 131–137. [Google Scholar] [CrossRef]
- Li, W.; Fu, J.R.; Zheng, L.J.; Ni, L.; Liu, J.Q.; Zhai, J.W.; Zhou, Z.; Wu, S.S. Two new bibenzyls from Pleione grandiflora (Rolfe) Rolfe and their antioxidant activity. Nat. Prod. Res. 2023, 37, 2486–2492. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.C.; Quang, T.H.; Oh, H.; Kim, Y.C. Cudratricusxanthone L suppresses lipopolysaccharideinduced activation of BV2 and primary rat microglial cells by inhibiting JNK, p38 MAPK, and NF-κB signaling. Preprints 2018, 2018080197. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, J.; Park, S.H.; Woo, E.R.; Kim, A.R.; Lee, S.K.; Kim, Y.S.; Kim, J.O.; Hong, J.H.; Lee, C.J. Effects of Morus alba L. and natural products including morusin on in vivo secretion and in vitro production of airway MUC5AC mucin. Tuberc. Respir. Dis. 2014, 77, 65–72. [Google Scholar] [CrossRef]
- Park, J.H.; Park, K.L.; Ho, H.S.; Kim, H.D.; Pyo, M.Y. Synthesis and antitumor activity of novel gericudranin E derivatives. J. Pharm. Soc. Korea 1999, 43, 559–565. [Google Scholar]
- Lee, B.W.; Kang, N.S.; Park, K.H. Isolation of antibacterial prenylated flavonoids from Cudrania tricuspidata. Appl. Biol. Chem. 2004, 47, 270–273. [Google Scholar]
- Dong, H.B.; Liao, L.; Yu, P.; Long, B.; Che, Y.; Lu, L.; Xu, B. Total syntheses and antibacterial evaluations of cudraflavones A-C and related Flavones. Bioorg Chem. 2023, 140, 106764. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Terao, Y.; Hoshino, T.; Kawabata, S.; Ooshima, T.; Sobue, S.; Kimura, S.; Hamada, S. Molecular analyses of glucosyltransferase genes among strains of Streptococcus mutans. FEMS Microbiol. Lett. 1998, 161, 331–336. [Google Scholar] [CrossRef]
- Dua, Y.; Li, G.; Li, X.; Jian, X.; Wang, X.; Xie, Y.; Li, Z.; Zhang, Z. Dietary Immunoglobulin Y by Targeting Both GbpB and GtfB Enhances the Anticaries Effect in Rats. Int. Dent. J. 2024, 74, 1298–1305. [Google Scholar] [CrossRef]
- Krzysciak, W.; Jurczak, A.; Koscielniak, D.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Frostell, G. Dental plaque pH in relation to intake of carbohydrate products. Acta Odontol. Scand. 1969, 27, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Köhler, B.; Birkhed, D.; Olsson, S. Acid production by human strains of Streptococcus mutans and Streptococcus sobrinus. Caries Res. 1995, 29, 402–406. [Google Scholar] [CrossRef]
- Park, B.I.; Jung, Y.W.; Kim, Y.H.; Lee, S.M.; Kwon, L.S.; Kim, K.J.; You, Y.O. Effect of the ethanol extract of propolis on formation of Streptococcus mutans biofilm. Int. J. Oral Biol. 2016, 41, 253–262. [Google Scholar] [CrossRef]
- O’Neill, E.; Hilary, H.; James, P.O. Carriage of both the fnbA and fnbB genes and growth at 37 °C promote FnBP mediated biofilm development in meticillin-resistant Staphylococcus aureus clinical isolates. J. Med. Microbiol. 2009, 58, 399–402. [Google Scholar] [CrossRef]
- Kwang, H.L.; Kim, B.S.; Keum, K.S.; Yu, H.H.; Kim, Y.H.; Chang, B.S.; Ra, J.Y.; Moon, H.D.; Seo, B.R.; Choi, N.Y.; et al. Essential oil of Curcuma longa inhibits Streptococcus mutans biofilm formation. J. Food Sci. 2011, 76, H226–H230. [Google Scholar] [CrossRef]
- Liljemark, W.F.; Bloomquist, C.G.; Germaine, G.R. Effect of bacterial aggregation on the adherence of oral streptococci to hydroxyapatite. Infect. Immun. 1981, 31, 935–941. [Google Scholar] [CrossRef]
- Jeong, S.I.; Kim, B.S.; Keum, K.S.; Lee, K.H.; Kang, S.Y.; Park, B.I.; Lee, Y.R.; You, Y.O. Kaurenoic acid from Aralia continentalis inhibits biofilm formation of Streptococcus mutans. Evid. Based Complement. Altern. Med. 2013, 2013, 160592. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).