Iodine-Substituted Dithiocarbamic Flavanones—A Structure–Activity Relationship Study of Their Antioxidant Properties
Abstract
1. Introduction
2. Results
2.1. 3-Dithiocarbamic Flavanones
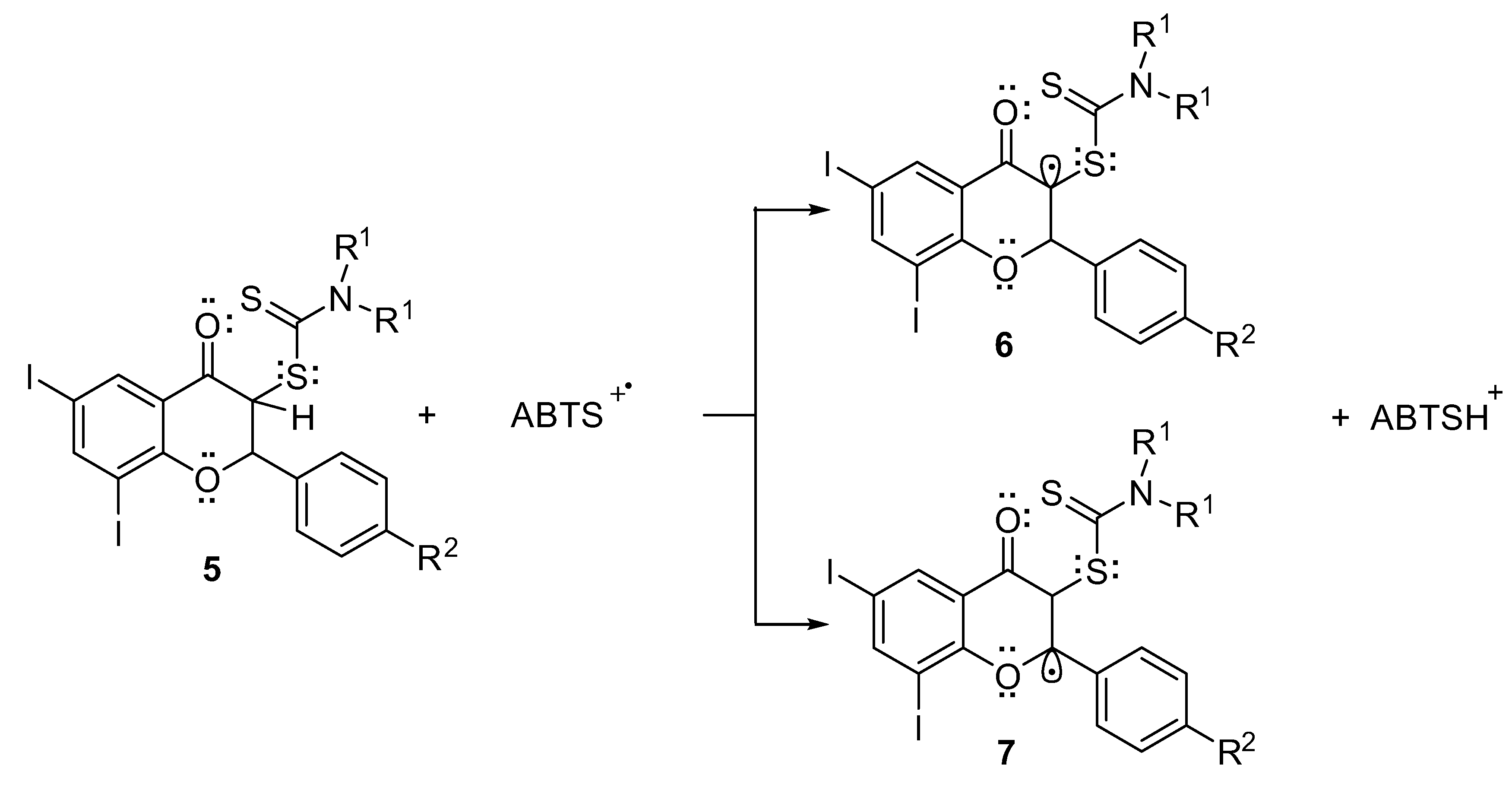
2.2. In Vitro Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. General Procedure for 1-(2-Hydroxy-3,5-diiodophenyl)-1-oxaethan-2-yl-dithiocarbamates 3
4.1.2. General Procedure for 6,8-Diiodo-2-(4-fluorophenyl)-4-oxochroman-3-yl-pyrrolidine-1-carbodithioate (5a)
4.1.3. 6,8-Diiodo-2-(4-bromophenyl)-4-oxochroman-3-yl-pyrrolidine-1-carbodithioate (5c)
4.1.4. 6,8-Diiodo-2-phenyl-4-oxochroman-3-yl-pyrrolidine-1-carbodithioate (5d)
4.1.5. 6,8-Diiodo-2-(4-fluorophenyl)-4-oxochroman-3-yl-piperidine-1-carbodithioate (5e)
4.1.6. 6,8-Diiodo-2-(4-bromophenyl)-4-oxochroman-3-yl-piperidine-1-carbodithioate (5g)
4.1.7. 6,8-Diiodo-2-phenyl-4-oxochroman-3-yl-piperidine-1-carbodithioate (5h)
4.1.8. 6,8-Diiodo-2-(4-fluorophenyl)-4-oxochroman-3-yl-morpholine-4-carbodithioate (5i)
4.1.9. 6,8-Diiodo-2-(4-bromophenyl)-4-oxochroman-3-yl-morpholine-4-carbodithioate (5k)
4.1.10. 6,8-Diiodo-2-phenyl-4-oxochroman-3-yl-morpholine-4-carbodithioate (5l)
4.2. In Vitro Antioxidant Activities
4.2.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Assay
4.2.2. 2,2′-Azino-bis(3-Ethylbenzothiazoline-6-sulfonic Acid) (ABTS) Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corcoran, M.P.; McKay, D.L.; Blumberg, J.B. Flavonoid basics: Chemistry, sources, mechanisms of action, and safety. J. Nutr. Gerontol. Geriatr. 2012, 31, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly) phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.; Feskens, E.J.; Kromhout, D.; Hollman, P.C.H.; Katan, M.B. Dietary antioxidant flavonoids and risk of coronary heart disease: The zutphen elderly study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.J.; Dwyer, J.T.; Jacques, P.F.; McCullough, M.L. Associations between flavonoids and cardiovascular disease incidence or mortality in European and US populations. Nutr. Rev. 2012, 70, 491–508. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; Hollman, P.C. Flavonoids and cardiovascular health: Which compounds, what mechanisms? Am. J. Clin. Nutr. 2008, 88, 12–13. [Google Scholar] [CrossRef]
- Landete, J.M. Updated knowledge about polyphenols: Functions, bioavailability, metabolism, and health. Crit. Rev. Food Sci. Nutr. 2012, 52, 936–948. [Google Scholar] [CrossRef]
- Birsa, M.L.; Sarbu, L.G. Hydroxy Chalcones and Analogs with Chemopreventive Properties. Int. J. Mol. Sci. 2023, 24, 10667. [Google Scholar] [CrossRef] [PubMed]
- Birsa, M.L.; Sarbu, L.G. Health Benefits of Key Constituents in Cichorium intybus L. Nutrients 2023, 15, 1322. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013, 78, A18–A25. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Zhang, D.; Du, M.; Wei, Y.; Wang, C.; Shen, L. A review on the structure-activity relationship of dietary flavonoids for protecting vascular endothelial function: Current understanding and future issues. J. Food Biochem. 2018, 42, e12557. [Google Scholar] [CrossRef]
- Chang, H.; Lei, L.; Zhou, Y.; Ye, F.; Zhao, G. Dietary flavonoids and the risk of colorectal cancer: An updated meta-analysis of epidemiological studies. Nutrients 2018, 10, 950. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Yeo, J. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef]
- Williams, C.A.; Grayer, R.J. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004, 21, 539–573. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: An application of the phenol-explorer database. J. Agric. Food Chem. 2010, 58, 4959–4969. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Mulligan, A.A.; Lentjes, M.A.; Luben, R.N.; Spencer, J.P.; Schroeter, H.; Khaw, K.; Kuhnle, G.G. Flavonoid intake in european adults (18 to 64 years). PLoS ONE 2015, 10, e0128132. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Croft, K.D.; Ward, N.; Considine, M.J.; Hodgson, J.M. Dietary flavonoids and nitrate: Effects on nitric oxide and vascular function. Nutr. Rev. 2015, 73, 216–235. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Hwang, S.L.; Shih, P.H.; Yen, G.C. Neuroprotective Effects of Citrus Flavonoids. J. Agric. Food Chem. 2012, 60, 877–885. [Google Scholar] [CrossRef]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral Properties of Flavonoids and Delivery Strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C. Plant polyphenols: Free radical scavengers or chain-breaking antioxidants? Biochem. Soc. Symp. 1995, 61, 103–116. [Google Scholar] [CrossRef]
- Bast, A.; Haenen, G.R. Ten misconceptions about antioxidants. Trends Pharmacol. Sci. 2013, 34, 430–436. [Google Scholar] [CrossRef]
- Umeno, A.; Biju, V.; Yoshida, Y. In vivo ROS production and use of oxidative stress-derived biomarkers to detect the onset of diseases such as alzheimer’s disease, parkinson’s disease, and diabetes. Free Radic. Res. 2017, 51, 413–427. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Fraga, C.G.; Galleano, M. Linking biomarkers of oxidative stress and disease with flavonoid consumption: From experimental models to humans. Redox Biol. 2021, 42, 101914. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Barrajón-Catalán, E.; Herranz-López, M.; Joven, J.; Segura-Carretero, A.; Alonso-Villaverde, C.; Menéndez, J.A.; Micol, V. Molecular promiscuity of plant polyphenols in the management of age-related diseases: Far beyond their antioxidant properties. Adv. Exp. Med. Biol. 2014, 824, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Proenca, C.; Rocha, S.; Lima, J.L.; Carvalho, F.; Fernandes, E.; Freitas, M. Immunomodulatory effects of flavonoids in the prophylaxis and treatment of inflammatory bowel diseases: A comprehensive review. Curr. Med. Chem. 2018, 25, 3374–3412. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Asp. Med. 2018, 61, 41–49. [Google Scholar] [CrossRef]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of neuroprotection by quercetin: Counteracting oxidative stress and more. Oxid. Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo? Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Franco, R.; Navarro, G.; Martínez-Pinilla, E. Hormetic and mitochondria-related mechanisms of antioxidant action of phytochemicals. Antioxidants 2019, 8, 373. [Google Scholar] [CrossRef]
- Hrelia, S.; Angeloni, C. New mechanisms of action of natural antioxidants in health and disease. Antioxidants 2020, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- Bahrin, L.G.; Apostu, M.O.; Birsa, L.M.; Stefan, M. The antibacterial properties of sulfur containing flavonoids. Bioorg. Med. Chem. Lett. 2014, 24, 2315–2318. [Google Scholar] [CrossRef] [PubMed]
- Bahrin, L.G.; Sarbu, L.G.; Hopf, H.; Jones, P.G.; Babii, C.; Stefan, M.; Birsa, M.L. The influence of halogen substituents on the biological properties of sulfur-containing flavonoids. Bioorg. Med. Chem. 2016, 24, 3166–3173. [Google Scholar] [CrossRef] [PubMed]
- Bahrin, L.G.; Hopf, H.; Jones, P.G.; Sarbu, L.G.; Babii, C.; Mihai, A.C.; Stefan, M.; Birsa, M.L. Antibacterial structure–activity relationship studies of several tricyclic sulfur-containing flavonoids. Beilstein J. Org. Chem. 2016, 12, 1065–1071. [Google Scholar] [CrossRef]
- Babii, C.; Mihalache, G.; Bahrin, L.G.; Neagu, A.N.; Gostin, I.; Mihai, C.T.; Sarbu, L.G.; Birsa, L.M.; Stefan, M. A novel synthetic flavonoid with potent antibacterial properties: In vitro activity and proposed mode of action. PLoS ONE 2018, 13, e0194898. [Google Scholar] [CrossRef]
- Sarbu, L.G.; Shova, S.; Peptanariu, D.; Sandu, I.A.; Birsa, L.M.; Bahrin, L.G. The Cytotoxic Properties of Some Tricyclic 1,3-Dithiolium Flavonoids. Molecules 2019, 24, 2459. [Google Scholar] [CrossRef] [PubMed]
- Bahrin, L.G.; Asaftei, I.V.; Sandu, I.G.; Sarbu, L.G. Synthesis of (4-Methylpiperazin-1-yl)carbodithioates and of their 1,3-Dithiolium Derivatives. Rev. Chim. 2014, 65, 1046–1048. [Google Scholar]
- Sarbu, L.G.; Lungu, N.C.; Balan, A.; Bahrin, L.G. Synthesis of Sulfur Containing Piperazine Derivatives with Potential Biological Activities. Rev. Chim. 2014, 65, 1135–1137. [Google Scholar]
- Sandulache, A.; Cascaval, A.; Toniutti, N.; Giumanini, A.G. New flavones by a novel synthetic route. Tetrahedron 1997, 53, 9813–9822. [Google Scholar] [CrossRef]
- Bahrin, L.G.; Jones, P.G.; Hopf, H.; Earar, K.; Birsa, M.L. Synthesis of new iodine containing 1,3-dithiol-2-ylium salts. Rev. Chim. 2016, 67, 61–63. [Google Scholar]
- Seliger, H.; Happ, E.; Cascaval, A.; Birsa, M.L.; Nicolaescu, T.; Poinescu, I.; Cojocariu, C. Synthesis and characterization of new photostabilizers from 2,4-dihydroxybenzophenone. Eur. Polym. J. 1999, 35, 827–833. [Google Scholar] [CrossRef]
- Birsa, M.L. Synthesis of some new substituted flavanones and related 4-chromanones by a novel synthetic method. Synth. Commun. 2002, 32, 115–118. [Google Scholar] [CrossRef]
- Birsa, M.L.; Sarbu, L.G. A Structure–Activity Relationship Study on the Antioxidant Properties of Dithiocarbamic Flavanones. Antioxidants 2024, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Takayanagi, T.; Harada, K.; Makino, K.; Ishikawa, F. Antioxidative activity of anthocyanins from purple sweet potato Ipomoera batatas cultivar Ayamurasaki. Biosci. Biotechnol. Biochem. 2005, 69, 979–988. [Google Scholar] [CrossRef]
- Ricci, D.; Fraternale, D.; Giamperi, L.; Bucchini, A.; Epifano, F.; Burini, G.; Curini, M. Chemical composition, antimicrobial and antioxidant activity of the essential oil of Teucrium marum (Lamiaceae). J. Ethnopharmacol. 2005, 98, 195–200. [Google Scholar] [CrossRef]
- Mimica-Dukic, N.; Bozin, B.; Sokovic, M.; Simin, N. Antimicrobial and antioxidant activities of Melissa officinalis L. (Lamiaceae) essential oil. J. Agric. Food Chem. 2004, 52, 2485–2489. [Google Scholar] [CrossRef]
- Birsa, M.L.; Sarbu, L.G. Novel dithiocarbamic flavanones with antioxidant properties—A structure activity relationship study. Int. J. Mol. Sci. 2024, 25, 13698. [Google Scholar] [CrossRef]
- Birsa, M.L. A new approach to preparation of 1,3-dithiolium salts. Synth. Commun. 2001, 31, 1271–1275. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Gursoy, N.; Sarikurkcu, C.; Cengiz, M.; Halil Solak, M. Antioxidant activities, metal contents, total phenolics and flavonoids of seven Morchella species. Food Chem. Toxicol. 2009, 47, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Arts, M.J.T.J.; Haenen, G.R.M.M.; Voss, H.-P.; Bast, A. Antioxidant capacity of reaction products limits the applicability of the Trolox Equivalent Antioxidant Capacity (TEAC) assay. Food Chem. Toxicol. 2004, 42, 45–49. [Google Scholar] [CrossRef] [PubMed]

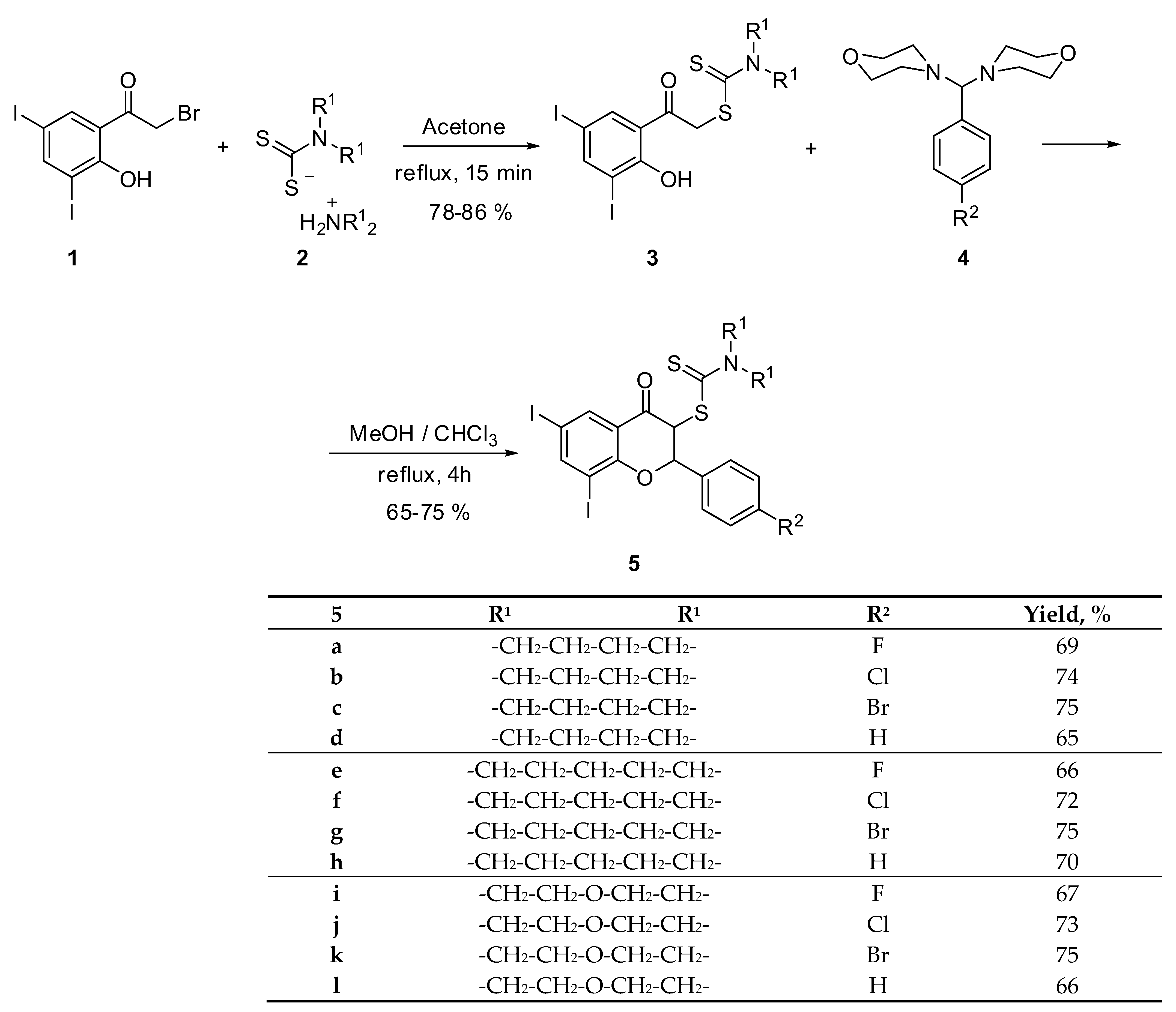

| Flavanones 5 | a | b | c | d | E | f | g | h | i | j | k | l |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3JH2–H3syn (Hz) | 3.8 | 3.5 | 2.5 | 4.0 | 2.6 | 2.9 | 3.5 | 1.6 | 1.3 | 2.5 | 2.9 | 2.2 |
| δ H2, H3 (ppm) | 5.99, 6.25 | 5.89, 6.41 | 5.89, 6.39 | 6.04, 6.29 | 5.98, 6.32 | 6.01, 6.33 | 5.98, 6.32 | 6.00, 6.38 | 5.95, 6.40 | 5.95, 6.42 | 5.95, 6.40 | 5.99, 6.41 |
| 3JH2–H3anti (Hz) | 10.1 | 9.8 | 10.5 | 6.8 | 10.6 | 8.9 | 8.9 | 9.7 | 10.3 | 9.1 | 10.3 | 9.5 |
| δ H2, H3 (ppm) | 5.78, 5.99 | 5.82, 6.25 | 5.82, 6.21 | 5.52, 6.06 | 5.88, 6.23 | 5.78, 5.99 | 5.77, 5.98 | 5.86, 6.22 | 5.88, 6.24 | 5.88, 6.23 | 5.88, 6.23 | 5.87, 6.24 |
| syn: anti ratio | 28:72 | 35:65 | 48:52 | 20:80 | 42:58 | 40:60 | 67:33 | 43:57 | 47:53 | 33:67 | 46:54 | 45:55 |
| R1 | R1 | Compound | DPPH | ABTS+• |
|---|---|---|---|---|
| -CH2-CH2-CH2-CH2- | 5a | 277.00 ± 0.2 | 91.35 ± 0.5 | |
| 5b | 273.84 ± 0.3 | 98.39 ± 0.4 | ||
| 5c | 264.37 ± 0.5 | 79.85 ± 0.3 | ||
| 5d | 291.84 ± 0.4 | 58.69 ± 0.4 | ||
| -CH2-CH2-CH2-CH2-CH2- | 5e | 233.86 ± 0.4 | 123.51 ± 0.4 | |
| 5f | 268.22 ± 0.2 | 71.80 ± 0.3 | ||
| 5g | 219.35 ± 0.3 | 44.85 ± 0.2 | ||
| 5h | 248.69 ± 0.3 | 87.53 ± 0.4 | ||
| -CH2-CH2-O-CH2-CH2- | 5i | 173.54 ± 0.1 | 121.67 ± 0.3 | |
| 5j | 158.27 ± 0.3 | 119.21 ± 0.3 | ||
| 5k | 174.55 ± 0.2 | 89.36 ± 0.2 | ||
| 5l | 145.87 ± 0.4 | 94.83 ± 0.4 | ||
| Ascorbic acid | 184.94 ± 0.2 | 147.47 ± 0.2 | ||
| BHT | 297.76 ± 0.2 | 148.29 ± 0.3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birsa, M.L.; Sarbu, L.G. Iodine-Substituted Dithiocarbamic Flavanones—A Structure–Activity Relationship Study of Their Antioxidant Properties. Molecules 2025, 30, 2280. https://doi.org/10.3390/molecules30112280
Birsa ML, Sarbu LG. Iodine-Substituted Dithiocarbamic Flavanones—A Structure–Activity Relationship Study of Their Antioxidant Properties. Molecules. 2025; 30(11):2280. https://doi.org/10.3390/molecules30112280
Chicago/Turabian StyleBirsa, Mihail Lucian, and Laura Gabriela Sarbu. 2025. "Iodine-Substituted Dithiocarbamic Flavanones—A Structure–Activity Relationship Study of Their Antioxidant Properties" Molecules 30, no. 11: 2280. https://doi.org/10.3390/molecules30112280
APA StyleBirsa, M. L., & Sarbu, L. G. (2025). Iodine-Substituted Dithiocarbamic Flavanones—A Structure–Activity Relationship Study of Their Antioxidant Properties. Molecules, 30(11), 2280. https://doi.org/10.3390/molecules30112280







