Lavandula pedunculata subsp. atlantica: A Multifunctional Essential Oil for Potentially Combating Microbial Infections and Inflammatory Processes
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of the Essential Oil (EO)
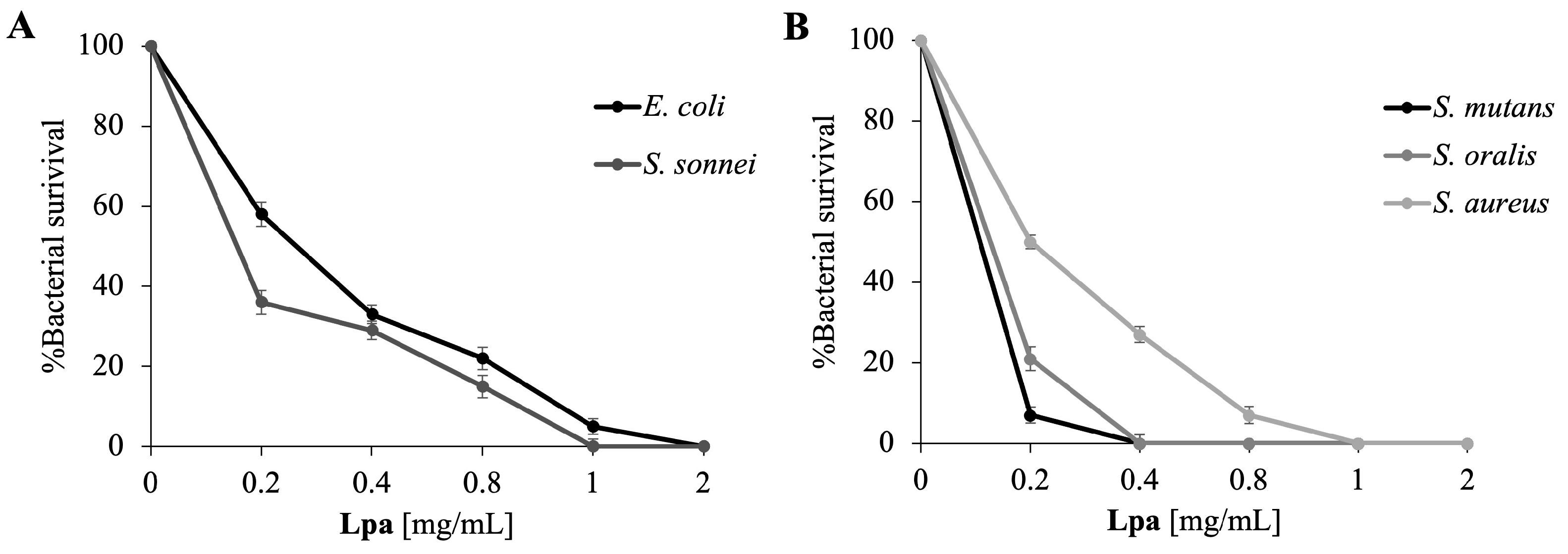
2.2. Antimicrobial Properties of Lpa
2.3. Lpa Target Determination in Bacteria
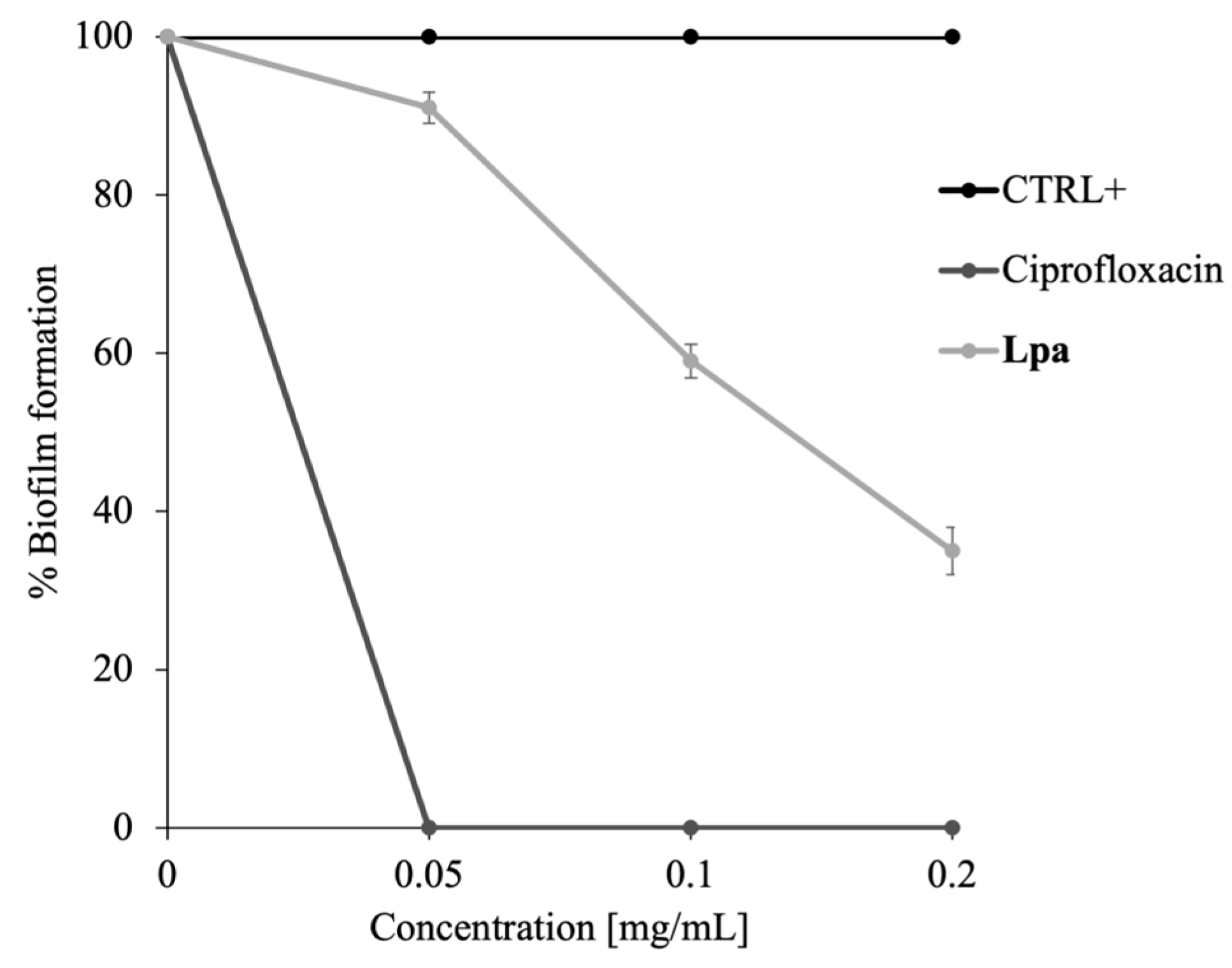
2.4. Antibiofilm Activity of Lpa
2.5. Antioxidant Activity of Lpa
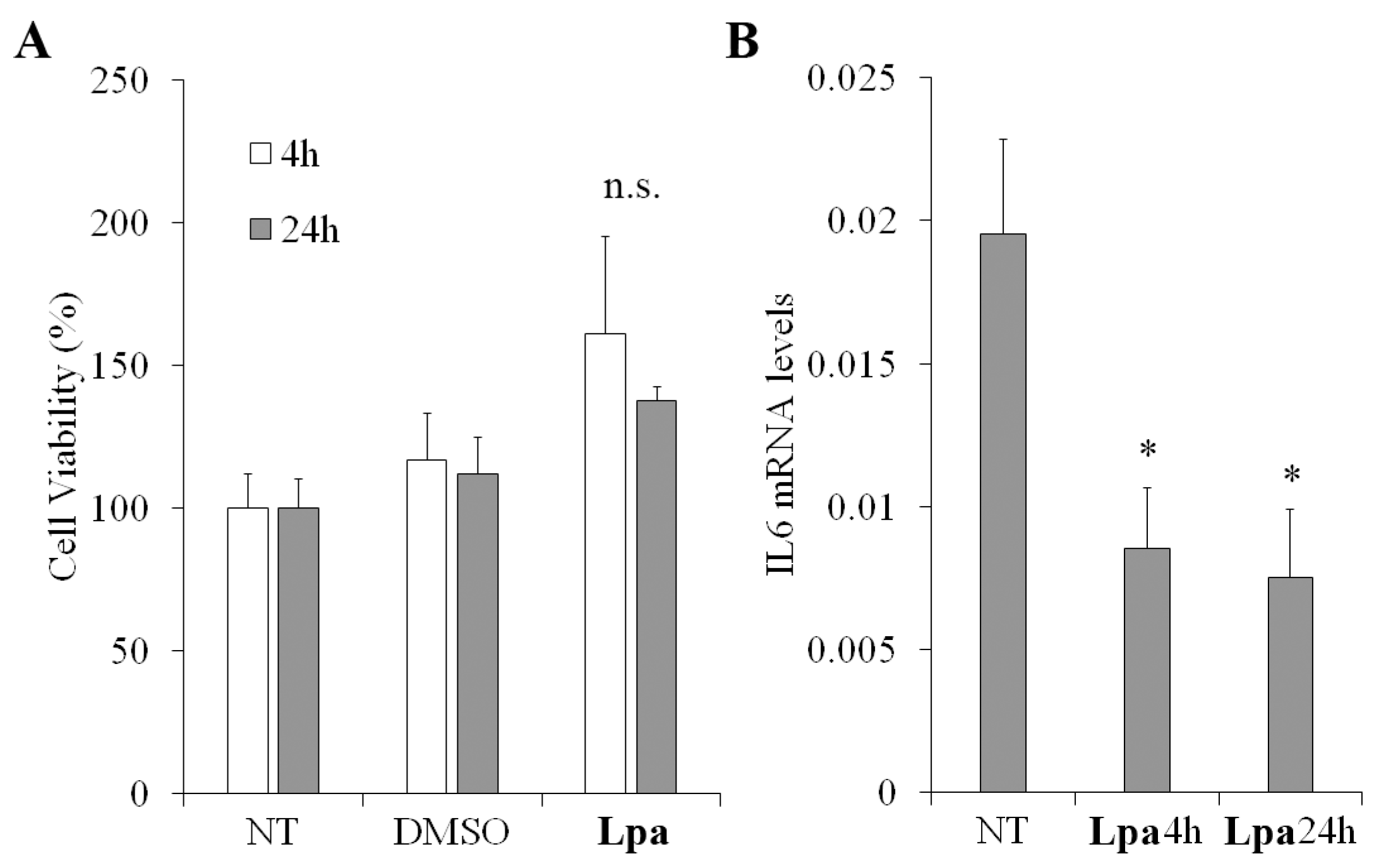
2.6. Cell Viability and Anti-Inflammatory Properties of Lpa
3. Materials and Methods
3.1. Plant Material
3.2. Isolation of Lpa
3.3. GC-MS Analysis
3.4. Bacterial Strains
3.5. Antimicrobial Assay
3.6. Determination of Minimal Inhibitory Concentration
3.7. Fluorescence Microscopy Experiments: DAPI/PI
3.8. Antibiofilm Tests
3.9. DPPH and ABTS Scavenging Capacity Assay
3.10. Cell Viability Assay
3.11. RNA Isolation and Real Time RT-qPCR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Statement
References
- Upson, T.; Andrews, S. The Genus Lavandula; Royal Botanic Gardens: Kew, UK, 2004. [Google Scholar]
- Héral, B.; Stierlin, E.; Fernandez, X.; Michel, T. Phytochemicals from the genus Lavandula: A review. Phytochem. Rev. 2021, 20, 751–771. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online. Available online: https://powo.science.kew.org/ (accessed on 22 January 2025).
- Bachiri, L.; Labazi, N.; Daoudi, A.; Ibijbijien, J.; Nassiri, L.; Echchegadda, G.; Mokhtari, F. Etude ethnobotanique de quelques lavandes marocaines spontanées. Int. J. Biol. Chem. Sci. 2015, 9, 1308–1318. [Google Scholar] [CrossRef]
- Najem, M.; Nassiri, L.; Ibijbijen, J. Vernacular names of plants between diversity and potential risks of confusion: Case of toxic plants used in medication in the central Middle Atlas, Morocco. J. Pharm. Pharmacogn. Res. 2021, 9, 222–250. [Google Scholar] [CrossRef]
- Ajjoun, M.; Kharchoufa, L.; Alami Merrouni, I.; Elachouri, M. Moroccan medicinal plants traditionally used for the treatment of skin diseases: From ethnobotany to clinical trials. J. Ethnopharmacol. 2022, 297, 115532. [Google Scholar] [CrossRef]
- Chaachouay, N.; Benkhnigue, O.; Fadli, M.; El Ibaoui, H.; El Ayadi, R.; Zidane, L. Ethnobotanical and ethnopharmacological study of medicinal and aromatic plants used in the treatment of respiratory system disorders in the Moroccan Rif. Ethnobot. Res. Appl. 2019, 18, 1–17. [Google Scholar] [CrossRef]
- Teixidor-Toneu, I.; Martin, G.J.; Ouhammou, A.; Puri, R.K.; Hawkins, J.A. An ethnomedicinal survey of a Tashelhit-speaking community in the High Atlas, Morocco. J. Ethnopharmacol. 2016, 188, 96–110. [Google Scholar] [CrossRef]
- Neves, J.M.; Matos, C.; Moutinho, C.; Queiroz, G.; Gomes, L.R. Ethnopharmacological notes about ancient uses of medicinal plants in Trás-os-Montes (northern of Portugal). J. Ethnopharmacol. 2009, 124, 270–283. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Goméz-García, R.; Machado, M.; Nunes, C.; Ribeiro, S.; Nunes, J.; Oliveira, A.L.S.; Pintado, M. Lavandula pedunculata polyphenol-rich extracts obtained by conventional, MAE and UAE methods: Exploring the bioactive potential and safety for use a medicine plant as food and nutraceutical ingredient. Foods 2023, 12, 4462. [Google Scholar] [CrossRef]
- Ferreira, A.; Proença, C.; Serralheiro, M.L.M.; Araújo, M.E.M. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J. Ethnopharmacol. 2006, 108, 31–37. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Almeida, C.; Nogueira, J.M.F.; Romano, A. Metabolic profile and biological activities of Lavandula pedunculata subsp. lusitanica (Chaytor) Franco: Studies on the essential oil and polar extracts. Food Chem. 2013, 141, 2501–2506. [Google Scholar] [CrossRef]
- Bouazama, S.; Harhar, H.; Costa, J.; Desjobert, J.M.; Talbaoui, A.; Tabyaoui, M. Chemical composition and antibacterial activity of the essential oils of Lavandula pedunculata and Lavandula dentata. J. Mat. Environ. Sci. 2017, 8, 2154–2160. [Google Scholar]
- Chroho, M.; El Karkouri, J.; Hadi, N.; Elmoumen, B.; Zair, T.; Bouissane, L. Chemical composition, antibacterial and antioxidant activities of the essential oil of Lavandula pedunculata from Khenifra Morocco. IOP Conf. Ser. Earth Environ. Sci. 2022, 1090, 012022. [Google Scholar] [CrossRef]
- Nafis, A.; Ouedrhiri, W.; Iriti, M.; Mezrioui, N.; Marraiki, N.; Elgorban, A.M.; Syed, A.; Hassani, L. Chemical composition and synergistic effect of three Moroccan lavender EOs with ciprofloxacin against foodborne bacteria: A promising approach to modulate antimicrobial resistance. Lett. Appli. Microbiol. 2021, 72, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Radi, F.Z.; Zekri, N.; Drioiche, A.; Zerkani, H.; Boutakiout, A.; Bouzoubaa, A.; Zair, T. Volatile and non-volatile chemical compounds and biological power of the genus Lavandula: Case of two Moroccan Lavenders Lavandula angustifolia Mill. (cultivated lavender) and Lavandula pedunculata (Mill.) Cav. (spontaneous lavender). Egypt. J. Chem. 2022, 65, 273–294. [Google Scholar] [CrossRef]
- Domingues, J.; Goulão, M.; Delgado, F.; Gonçalves, J.C.; Gonçalves, J.; Pintado, C.S. Essential oils of two Portuguese endemic species of Lavandula as a source of antifungal and antibacterial agents. Processes 2023, 11, 1165. [Google Scholar] [CrossRef]
- Marques, M.P.; Neves, B.G.; Varela, C.; Zuzarte, M.; Gonçalves, A.C.; Dias, M.I.; Amaral, J.S.; Barros, L.; Magalhães, M.; Cabral, C. Essential oils from Côa Valley Lamiaceae Species: Cytotoxicity and antiproliferative effect on glioblastoma cells. Pharmaceutics 2023, 15, 341. [Google Scholar] [CrossRef]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Dinis, A.M.; Canhoto, J.M.; Salgueiro, L.R. Chemical composition and antifungal activity of the essential oils of Lavandula pedunculata (Miller) Cav. Chem. Biodiv. 2009, 6, 1283–1292. [Google Scholar] [CrossRef]
- Laghzaoui, E.M.; Kasrati, A.; Abbad, A.; Leach, D.; Spooner-Hart, R.; El Mouden, E.H. Acaricidal properties of essential oils from Moroccan plants against immature ticks of Hyalomma aegyptium (Linnaeus, 1758); an external parasite of the spur-thighed tortoise (Testudo graeca). Int. J. Acarol. 2018, 44, 315–321. [Google Scholar] [CrossRef]
- Soulaimani, B.; Abbad, I.; Amssayef, A.; Varoni, E.M.; Iriti, M.; Mezrioui, N.E.; Hassani, L.; Abbad, A. Comparative evaluation of the essential oil chemical variability and antimicrobial activity between Moroccan Lavender species. J. Biol. Regul. Homeost. Agents 2023, 37, 5621–5631. [Google Scholar]
- Sayout, A.; Ouarhach, A.; Rabie, R.; Dilagui, I.; Soraa, N.; Romane, A. Evaluation of antibacterial activity of Lavandula pedunculata subsp. atlantica (Braun-Blanq.) Romo essential oil and selected terpenoids against resistant bacteria strains–structure–activity relationships. Chem. Biodiv. 2020, 17, e1900496. [Google Scholar] [CrossRef]
- Zrira, S.; Benjilali, B. The constituents of the oils of Lavandula stoechas L. ssp. atlantica Br.-Bl. and L. stoechas ssp. stoechas from Morocco. J. Essent. Oil Res. 2003, 15, 68–69. [Google Scholar] [CrossRef]
- Matos, F.; Miguel, M.G.; Duarte, J.; Venâncio, F.; Moiteiro, C.; Correia, A.I.D.; Figueiredo, A.C.; Barroso, J.; Pedro, L. Antioxidant capacity of the essential oils from Lavandula luisieri, L. stoechas subsp. lusitanica, L. stoechas subsp. lusitanica x L. luisieri and L. viridis grown in Algarve (Portugal). J. Essent. Oil Res. 2009, 21, 327–336. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Deborah, A.; Hogan, D.A.; Mylonakis, E. Medically important bacterial–fungal interactions. Nature Rev. Microbiol. 2010, 8, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Soro, N.K.; Majdouli, K.; Khabbal, Y.; Zair, T. Chemical composition and antibacterial activity of Lavandula Species L. dentata L., L. pedunculata Mill. and Lavandula abrialis essential oils from Morocco against food-borne and nosocomial pathogens. Int. J. Innov. Appl. Stud. 2014, 7, 774–781. [Google Scholar]
- Mohamed Abdoul-Latif, F.; Ainane, A.; Houmed Aboubaker, I.; Mohamed, J.; Ainane, T. Exploring the potent anticancer activity of essential oils and their bioactive compounds: Mechanisms and prospects for future cancer therapy. Pharmaceuticals 2023, 16, 1086. [Google Scholar] [CrossRef] [PubMed]
- Castagliuolo, G.; Di Napoli, M.; Vaglica, A.; Badalamenti, N.; Antonini, D.; Varcamonti, M.; Bruno, M.; Zanfardino, A.; Bazan, G. Thymus richardii subsp. nitidus (Guss.) Jalas essential oil: An ally against oral pathogens and mouth health. Molecules 2023, 28, 4803. [Google Scholar] [CrossRef]
- Castagliuolo, G.; Dell’Annunziata, F.; Pio, S.; Di Napoli, M.; Troiano, A.; Antonini, D.; Badalamenti, N.; Bruno, M.; Ilardi, V.; Folliero, V.; et al. Spectroscopic characterization and biological effects of 1-oxo-bisabolone-rich Pulicaria burchardii Hutch. subsp. burchardii essential oil against viruses, bacteria, and spore germination. Plants 2024, 14, 68. [Google Scholar] [CrossRef]
- Reichling, J. Anti-biofilm and virulence factor-reducing activities of essential oils and oil components as a possible option for bacterial infection control. Planta Med. 2020, 86, 520–537. [Google Scholar] [CrossRef]
- Boutahiri, S.; Eto, B.; Bouhrim, M.; Mechchate, H.; Saleh, A.; Al Kamaly, O.; Drioiche, A.; Remok, F.; Samaillie, J.; Neut, C.; et al. Lavandula pedunculata (Mill.) Cav. aqueous extract antibacterial activity improved by the addition of Salvia rosmarinus Spenn., Salvia lavandulifolia Vahl and Origanum compactum Benth. Life 2022, 12, 328. [Google Scholar] [CrossRef]
- Walasek-Janusz, M.; Grzegorczyk, A.; Zalewski, D.; Malm, A.; Gajcy, S.; Gruszecki, R. Variation in the antimicrobial activity of essential oils from cultivars of Lavandula angustifolia and L. × intermedia. Agronomy 2022, 12, 2955. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Cardile, V.; Arnold, N.A.; Senatore, F. Comparative Phytochemical profile and antiproliferative activity on human melanoma cells of essential oils of three Lebanese Salvia species. Ind. Crops Prod. 2016, 83, 492–499. [Google Scholar] [CrossRef]
- Oyedeji, A.O.; Ekundayo, O.; Olawore, O.N.; Adeniyi, B.A.; Koenig, W.A. Antimicrobial activity of the essential oils of five Eucalyptus species growing in Nigeria. Fitoterapia 1999, 70, 526–528. [Google Scholar] [CrossRef]
- Aldoghaim, F.S.; Flematti, G.R.; Hammer, K.A. Antimicrobial activity of several cineole-rich western Australian Eucalyptus essential oils. Microorganisms 2018, 6, 122. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Bersani, C.; Comi, G. Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceae and Compositae. Int. J. Food Microbiol. 2001, 67, 187–195. [Google Scholar] [CrossRef]
- Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Maresca, V.; Basile, A.; Bruno, M.; Varcamonti, M.; Zanfardino, A. Antimicrobial, antibiofilm, and antioxidant properties of essential oil of Foeniculum vulgare Mill. leaves. Plants 2022, 11, 3573. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, E.; Cheng, Y.; Mahmood, T.; Ge, F.; Zhou, K.; Bao, M.; Lv, L.; Li, L.; Yi, J.; et al. Is combined medication with natural medicine a promising therapy for bacterial biofilm infection? Biomed. Pharmacother. 2020, 128, 110184. [Google Scholar] [CrossRef]
- Kavanaugh, N.L.; Ribbeck, K. Selected antimicrobial essential oils eradicate Pseudomonas spp. and Staphylococcus aureus Biofilms. Appl. Environ. Microbiol. 2012, 78, 4057–4061. [Google Scholar] [CrossRef]
- Lu, C.; Li, H.; Li, C.; Chen, B.; Shen, Y. Chemical composition and radical scavenging activity of Amygdalus pedunculata Pall leaves’ essential oil. Food Chem. Toxicol. 2018, 119, 368–374. [Google Scholar] [CrossRef]
- Wojtunik, K.A.; Ciesla, L.M.; Waksmundzka-Hajnos, M. Model studies on the antioxidant activity of common terpenoid constituents of essential oils by means of the 2,2-diphenyl-1-picrylhydrazyl method. J. Agric. Food Chem. 2014, 62, 9088–9094. [Google Scholar] [CrossRef]
- Luo, Q.; Tian, Z.; Zheng, T.; Xu, S.; Ma, Y.; Zou, S.; Zuo, Z. Terpenoid composition and antioxidant activity of extracts from four chemotypes of Cinnamomum camphora and their main antioxidant agents. Biofuels Bioprod. Bioref. 2022, 16, 510–522. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [PubMed]
- Seol, G.H.; Kim, K.Y. Eucalyptol and Its Role in Chronic Diseases. In Drug Discovery from Mother Nature; Gupta, S.C., Prasad, S., Aggarwal, B.B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 929, pp. 389–398. ISBN 9783319413419. [Google Scholar]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 7, 225. [Google Scholar] [CrossRef]
- Ben Hassine, D.; Abderrabba, M.; Yvon, Y.; Lebrihi, A.; Mathieu, F.; Couderc, F.; Bouajila, J. Chemical composition and in vitro evaluation of the antioxidant and antimicrobial activities of Eucalyptus gillii essential oil and extracts. Molecules 2012, 17, 9540–9558. [Google Scholar] [CrossRef]
- Vieira, A.I.; Guerreiro, A.; Antunes, M.D.; Miguel, M.D.G.; Faleiro, M.L. Edible coatings enriched with essential oils on apples impair the survival of bacterial pathogens through a simulated gastrointestinal system. Foods 2019, 8, 57. [Google Scholar] [CrossRef]
- Ahn, C.; Lee, J.; Park, M.; Kim, J.; Yang, J.; Yoo, Y.; Jeung, E. Cytostatic effects of plant essential oils on human skin and lung cells. Exp. Ther. Med. 2020, 19, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- Pérez, G.S.; Zavala, S.M.; Arias, G.L.; Ramos, L.M. Anti-inflammatory activity of some essential oils. J. Essent. Oil Res. 2011, 23, 38–44. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, L.; Wang, S.; Gao, Y.; Jin, F. Molecular mechanism of the anti-inflammatory effects of plant essential oils: A systematic review. J. Ethnopharmacol. 2023, 301, 115829. [Google Scholar] [CrossRef]
- Egbuta, M.A.; McIntosh, S.; Waters, D.L.E.; Vancov, T.; Liu, L. In vitro anti-inflammatory activity of essential oil and β-bisabolol derived from cotton gin trash. Molecules 2022, 27, 526. [Google Scholar] [CrossRef]
- Bhatt, D.; Singh, S.; Singh, M.K.; Maurya, A.K.; Chauhan, A.; Padalia, R.C.; Verma, R.V.; Bawankule, D.U.U. Acyclic monoterpenoid-rich essential oil of Cymbopogon distans mitigates skin inflammation: A chemico-pharmacological study. Inflammopharmacology 2024, 32, 509–521. [Google Scholar] [CrossRef]
- European Pharmacopoeia 10.3. Monograph: Determination of Essential Oils in Herbal Drugs; EDQM: Strasbourg, France, 2019. [Google Scholar]
- Castagliuolo, G.; Di Napoli, M.; Zangmo, T.; Szpunar, J.; Ronga, L.; Zanfardino, A.; Varcamonti, M.; Tesauro, D. Antimicrobial activity and mode of action of n-heterocyclic carbene silver(i) complexes. Molecules 2024, 30, 76. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Cicio, A.; Bruno, M.; Piacente, S.; Maresca, V.; Cianciullo, P.; Capasso, L.; et al. The chemical composition of the aerial parts of Stachys spreitzenhoferi (Lamiaceae) growing in Kythira Island (Greece), and their antioxidant, antimicrobial, and antiproliferative properties. Phytochemistry 2022, 203, 113373. [Google Scholar] [CrossRef] [PubMed]



| Taxa | Origin | Compounds | MH | OM | SH | OS | O | Ref. |
|---|---|---|---|---|---|---|---|---|
| L. pedunculata | Morocco, Tafraout | camphor (53.1), eucalyptol (6.5), camphene (6.1), α-pinene (2.0) | 11.7 | 72.7 | 0.5 | 2.1 | - | [13] |
| L. pedunculata | Morocco, Khenifra | camphor (44.2), 1-epi-cubenol (8.6), fenchone (7.5), camphene (6.4), α-pinene (4.9) | 16.0 | 64.1 | 1.6 | 12.4 | - | [14] |
| L. pedunculata | Morocco, Khenifra | camphor (47.5), fenchone (27.1), 1-epi-cubenol (2.8), verbenone (2.4), borneol (2.0) | 0.7 | 87.3 | 0.3 | 4.4 | 1.4 | [15] |
| L. pedunculata | Morocco, Azrou | camphor (41.1), fenchone (15.8), 1,10-diepi-cubenol (7.5), borneol (5.7), camphene (5.8), α-pinene (2.1) | 10.1 | 72.7 | 0.8 | 16.4 | - | [16] |
| L. pedunculata | Portugal, Serra da Malcata | fenchone (50.5), camphor (30.0), α-pinene (7.0), limonene (2.1) | 11.4 | 85.5 | 0.3 | 1.5 | - | [17] |
| L. pedunculata | Portugal, Coa Valley | camphor (39.0), α-pinene (6.9), bornyl acetate (5.9), fenchone (5.4), camphene (4.0), endo-borneol (2.4) | 14.7 | 73.0 | 0.8 | 7.3 | - | [18] |
| L. pedunculata | Portugal, Coimbra | fenchone (45.5), camphor (8.7), α-pinene (8.0), eucalyptol (5.1), bornyl acetate (3.5), α-cadinol (2.5) | 15.6 | 72.1 | 1.1 | 2.7 | - | [19] |
| L. pedunculata | Portugal, Bragança | eucalyptol (34.3), camphor (9.9), β-pinene (9.0), fenchone (7.6), linalool (3.8), borneol (3.4), α-cadinol (3.1), cis-verbenol (2.8), α-pinene (2.5) | 15.3 | 72.4 | 2.0 | 3.7 | - | [19] |
| L. pedunculata | Portugal, Guarda | camphor (34.0), eucalyptol (25.1), fenchone (6.2), camphene (6.1), α-pinene (3.8), trans-verbenol (2.0) | 15.6 | 75.1 | 0.6 | 0.3 | - | [19] |
| L. pedunculata ssp. atlantica | Morocco, Touflihte | camphor (30.8), α-pinene (14.8), camphene (14.6), 1,10-di-epi- cubenol (11.9), fenchone (7.5), selina-3.7(11)-diene (2.9), linalool (2.8), limonene (2.1) | 34.4 | 44.6 | 5.5 | 11.9 | - | [20] |
| L. pedunculata ssp. atlantica | Morocco, Touflihte | camphor (41.5), fenchone (16.8), camphene (11.5), epi-cubenol (2.9), α-pinene (2.5), eucalyptol (2.3), endo-borneol (2.0) | 15.8 | 67.5 | 3.0 | 4.5 | 5.8 | [21] |
| L. pedunculata ssp. atlantica | Morocco, Tazakka | camphor (50.4), fenchone (14.1), camphene (5.6), α-pinene (2.4), borneol (2.3) | 10.7 | 78.0 | 0.5 | 4.4 | - | [22] |
| L. pedunculata ssp. atlantica | Morocco, Oulmès | camphor (39.2), fenchone (9.2), camphene (6.7), α-pinene (6.5), borneol (2.5), linalool (2.5), α-selinene (2.2), δ-cadinene (2.2) | 16.0 | 55.7 | 8.0 | 9.0 | - | [23] |
| L. pedunculata ssp. lusitanica | Portugal, Algarve | camphor (40.6), fenchone (38.0), α-fenchol (2.6), linalool (2.0) | 0.1 | 90.6 | 1.6 | 2.0 | 0.6 | [12] |
| L. pedunculata ssp. lusitanica | Portugal, Faro | fenchone (41.9), camphor (34.6), α-pinene (2.8), linalool (2.7) | 5.7 | 86.8 | 0.5 | 0.3 | 0.2 | [24] |
| No. | Compounds a | LRI b | LRI c | Area (%) d |
|---|---|---|---|---|
| 1 | Tricyclene | 924 | 926 | 0.7 |
| 2 | α-Pinene | 935 | 938 | 7.4 |
| 3 | Camphene | 947 | 947 | 10.9 |
| 4 | β-Pinene | 972 | 975 | 0.6 |
| 5 | 1-Octen-3-ol | 979 | 979 | 0.6 |
| 6 | β-Myrcene | 990 | 988 | 0.4 |
| 7 | α-Terpinene | 1011 | 1015 | 0.2 |
| 8 | p-Cymene | 1019 | 1021 | 1.0 |
| 9 | Eucalyptol | 1025 | 1031 | 8.5 |
| 10 | β-cis-Ocimene | 1035 | 1032 | 0.6 |
| 11 | β-trans-Ocimene | 1042 | 1045 | 0.2 |
| 12 | γ-Terpinene | 1054 | 1056 | 0.3 |
| 13 | trans-Linalool oxide | 1069 | 1072 | 0.3 |
| 14 | Fenchone | 1080 | 1078 | 10.6 |
| 15 | β-Linalool | 1099 | 1103 | 3.1 |
| 16 | Fenchol | 1110 | 1110 | 0.5 |
| 17 | 1,7,7-Trimethylbicyclo[2.2.1]hept-5-en-2-ol | 1118 | 1115 | 0.5 |
| 18 | Camphor | 1136 | 1139 | 27.8 |
| 19 | Pinocarvone | 1156 | 1154 | 0.2 |
| 20 | Borneol | 1157 | 1157 | 1.5 |
| 21 | α-Phellandren-8-ol | 1159 | 1159 | 0.8 |
| 22 | 4-Terpineol | 1169 | 1172 | 1.0 |
| 23 | 4-Methylacetophenone | 1173 | 1175 | 0.1 |
| 24 | p-Cymen-8-ol | 1177 | 1179 | 0.2 |
| 25 | α-Terpineol | 1183 | 1180 | 0.5 |
| 26 | Myrtenal | 1185 | 1188 | 0.4 |
| 27 | Myrtenol | 1189 | 1191 | 2.1 |
| 28 | Verbenone | 1197 | 1201 | 0.3 |
| 29 | cis-Carveol | 1210 | 1214 | 0.2 |
| 30 | Fenchyl acetate | 1212 | 1217 | 0.3 |
| 31 | Carvone | 1233 | 1231 | 0.4 |
| 32 | Bornyl acetate | 1278 | 1283 | 6.7 |
| 33 | Myrtenyl acetate | 1317 | 1316 | 2.8 |
| 34 | α-Cubebene | 1342 | 1345 | t |
| 35 | Eugenol | 1347 | 1348 | 0.1 |
| 36 | Neryl acetate | 1367 | 1362 | 0.1 |
| 37 | Caryophyllene | 1409 | 1413 | 0.2 |
| 38 | δ-Cadinene | 1514 | 1519 | 0.6 |
| 39 | Viridiflorol | 1579 | 1580 | 0.6 |
| 40 | Di-epi-1,10-cubenol | 1589 | 1587 | 0.5 |
| 41 | δ-Cadinol | 1628 | 1626 | 0.4 |
| 42 | τ-Cadinol | 1640 | 1640 | 0.5 |
| Monoterpene Hydrocarbons | 22.3 | |||
| Oxygenated Monoterpenes | 68.9 | |||
| Sesquiterpene Hydrocarbons | 0.8 | |||
| Oxygenated Sesquiterpenes | 2.0 | |||
| Others | 0.7 | |||
| Total | 94.7 |
| Strains | MIC [mg/mL] |
|---|---|
| E. coli | 2 |
| S. sonnei | 1 |
| S. aureus | 1 |
| S. mutans | 0.2 |
| S. oralis | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castagliuolo, G.; Badalamenti, N.; Ilardi, V.; Fontana, G.; Antonini, D.; Varcamonti, M.; Bruno, M.; Zanfardino, A. Lavandula pedunculata subsp. atlantica: A Multifunctional Essential Oil for Potentially Combating Microbial Infections and Inflammatory Processes. Molecules 2025, 30, 2267. https://doi.org/10.3390/molecules30112267
Castagliuolo G, Badalamenti N, Ilardi V, Fontana G, Antonini D, Varcamonti M, Bruno M, Zanfardino A. Lavandula pedunculata subsp. atlantica: A Multifunctional Essential Oil for Potentially Combating Microbial Infections and Inflammatory Processes. Molecules. 2025; 30(11):2267. https://doi.org/10.3390/molecules30112267
Chicago/Turabian StyleCastagliuolo, Giusy, Natale Badalamenti, Vincenzo Ilardi, Gianfranco Fontana, Dario Antonini, Mario Varcamonti, Maurizio Bruno, and Anna Zanfardino. 2025. "Lavandula pedunculata subsp. atlantica: A Multifunctional Essential Oil for Potentially Combating Microbial Infections and Inflammatory Processes" Molecules 30, no. 11: 2267. https://doi.org/10.3390/molecules30112267
APA StyleCastagliuolo, G., Badalamenti, N., Ilardi, V., Fontana, G., Antonini, D., Varcamonti, M., Bruno, M., & Zanfardino, A. (2025). Lavandula pedunculata subsp. atlantica: A Multifunctional Essential Oil for Potentially Combating Microbial Infections and Inflammatory Processes. Molecules, 30(11), 2267. https://doi.org/10.3390/molecules30112267









