Research Advances in COx Hydrogenation to Valuable Hydrocarbons over Carbon-Supported Fe-Based Catalysts
Abstract
1. Introduction
2. CO Hydrogenation
2.1. Carbon Nanotubes
2.2. Mesoporous Carbon
2.3. Graphene
2.4. Activated Carbon
2.5. Carbon Spheres
2.6. Biomass Derived
2.7. Derivation of MOFs
3. CO2 Hydrogenation Reaction
3.1. Carbon Nanotubes
3.2. Mesoporous Carbon
3.3. Graphene
3.4. Activated Carbon
3.5. Biomass Derivation
3.6. MOFs Derivation
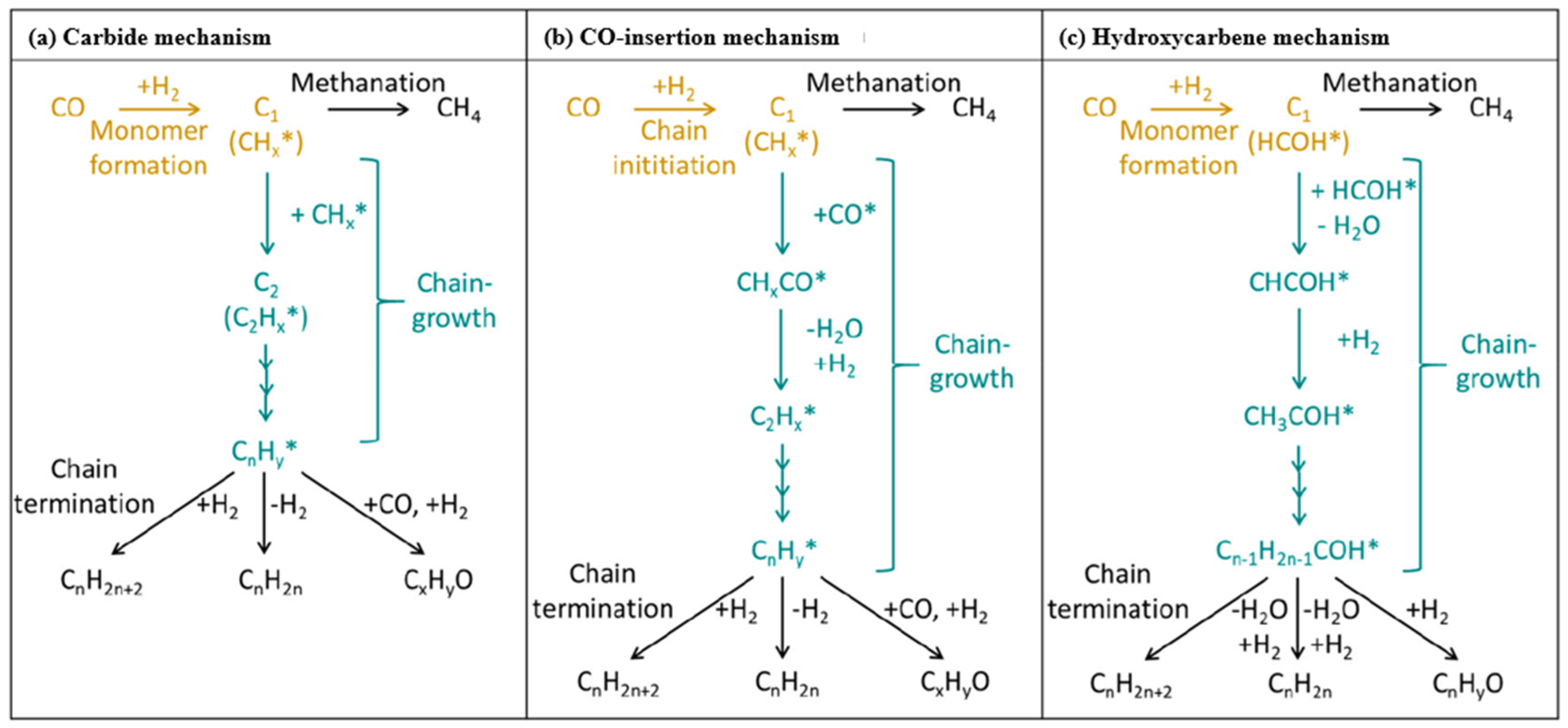
4. Summary and Challenges
Funding
Conflicts of Interest
References
- Ipadeola, A.K.; Chitt, M.; Abdelgawad, A.; Eid, K.; Abdullah, A.M. Graphene-based catalysts for carbon monoxide oxidation: Experimental and theoretical insights. Int. J. Hydrog. Energy 2023, 48, 17434–17467. [Google Scholar] [CrossRef]
- Ipadeola, A.K.; Gamal, A.; Abdullah, A.M.; Haruna, A.B.; Ozoemena, K.I.; Eid, K. Pd nanocrystals encapsulated in MOF-derived Ni/N-doped hollow carbon nanosheets for efficient thermal CO oxidation: Unveiling the effect of porosity. Catal. Sci. Technol. 2023, 13, 4873–4882. [Google Scholar] [CrossRef]
- Rommens, K.T.; Saeys, M. Molecular Views on Fischer-Tropsch Synthesis. Chem. Rev. 2023, 123, 5798–5858. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Zhang, C.; Gao, P.; Wang, H.; Li, X.P.; Zhong, L.S.; Wei, W.; Sun, Y.H. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. Catal. Sci. Technol. 2017, 7, 4580–4598. [Google Scholar] [CrossRef]
- Mihet, M.; Dan, M.; Lazar, M.D. CO2 Hydrogenation Catalyzed by Graphene-Based Materials. Molecules 2022, 27, 3367. [Google Scholar] [CrossRef]
- Puga, A.V. On the nature of active phases and sites in CO and CO2 hydrogenation catalysts. Catal. Sci. Technol. 2018, 8, 5681–5707. [Google Scholar] [CrossRef]
- Visconti, C.G.; Martinelli, M.; Falbo, L.; Fratalocchi, L.; Lietti, L. CO2 hydrogenation to hydrocarbons over Co and Fe-based Fischer-Tropsch catalysts. Catal. Today 2016, 277, 161–170. [Google Scholar] [CrossRef]
- Arizapana, K.; Schossig, J.; Wildy, M.; Weber, D.; Gandotra, A.; Jayaraman, S.; Wei, W.Y.; Xu, K.; Yu, L.; Mugweru, A.M.; et al. Harnessing the Synergy of Fe and Co with Carbon Nanofibers for Enhanced CO2 Hydrogenation Performance. ACS Sustain. Chem. Eng. 2024, 12, 1868–1883. [Google Scholar] [CrossRef]
- Oschatz, M.; Lamme, W.S.; Xie, J.X.; Dugulan, A.I.; de Jong, K.P. Ordered Mesoporous Materials as Supports for Stable Iron Catalysts in the Fischer-Tropsch Synthesis of Lower Olefins. ChemCatChem 2016, 8, 2846–2852. [Google Scholar] [CrossRef]
- Liu, J.X.; Wang, P.; Xu, W.; Hensen, E.J.M. Particle Size and Crystal Phase Effects in Fischer-Tropsch Catalysts. Engineering 2017, 3, 467–476. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, G.H.; Li, W.H.; Zhang, X.B.; Ding, F.S.; Song, C.S.; Guo, X.W. Deconvolution of the Particle Size Effect on CO2 Hydrogenation over Iron-Based Catalysts. ACS Catal. 2020, 10, 7424–7433. [Google Scholar] [CrossRef]
- Yahyazadeh, A.; Borugadda, V.B.; Dalai, A.K.; Zhang, L.F. Optimization of olefins’ yield in Fischer-Tropsch synthesis using carbon nanotubes supported iron catalyst with potassium and molybdenum promoters. Appl. Catal. A-Gen. 2022, 643, 118759. [Google Scholar] [CrossRef]
- Dad, M.; Lancee, R.J.; van Vuuren, M.J.; van de Loosdrecht, J.; Niemantsverdriet, J.W.H.; Fredriksson, H.O.A. SiO2-supported Fe & FeMn colloids-Fischer-Tropsch synthesis on 3D model catalysts. Appl. Catal. A-Gen. 2017, 537, 83–92. [Google Scholar] [CrossRef]
- Abrokwah, R.Y.; Rahman, M.M.; Deshmane, V.G.; Kuila, D. Effect of titania support on Fischer-Tropsch synthesis using cobalt, iron, and ruthenium catalysts in silicon-microchannel microreactor. Mol. Catal. 2019, 478, 110566. [Google Scholar] [CrossRef]
- Ali, S.; Zabidi, N.A.M.; Al-Marri, M.J.; Khader, M.M. Effect of the support on physicochemical properties and catalytic performance of cobalt based nano-catalysts in Fischer-Tropsch reaction. Mater. Today Commun. 2017, 10, 67–71. [Google Scholar] [CrossRef]
- Munirathinam, R.; Minh, D.P.; Nzihou, A. Effect of the Support and Its Surface Modifications in Cobalt-Based Fischer-Tropsch Synthesis. Ind. Eng. Chem. Res. 2018, 57, 16137–16161. [Google Scholar] [CrossRef]
- Dlamini, M.W.; Phaahlamohlaka, T.N.; Kumi, D.O.; Forbes, R.; Jewell, L.L.; Coville, N.J. Post doped nitrogen-decorated hollow carbon spheres as a support for Co Fischer-Tropsch catalysts. Catal. Today 2020, 342, 99–110. [Google Scholar] [CrossRef]
- Asalieva, E.; Sineva, L.; Sinichkina, S.; Solomonik, I.; Gryaznov, K.; Pushina, E.; Kulchakovskaya, E.; Gorshkov, A.; Kulnitskiy, B.; Ovsyannikov, D.; et al. Exfoliated graphite as a heat-conductive frame for a new pelletized Fischer-Tropsch synthesis catalyst. Appl. Catal. A-Gen. 2020, 601, 117639. [Google Scholar] [CrossRef]
- Galvis, H.M.T.; Bitter, J.H.; Khare, C.B.; Ruitenbeek, M.; Dugulan, A.I.; de Jong, K.P. Supported Iron Nanoparticles as Catalysts for Sustainable Production of Lower Olefins. Science 2012, 335, 835–838. [Google Scholar] [CrossRef]
- Guo, L.S.; Zhang, P.P.; Cui, Y.; Liu, G.B.; Wu, J.H.; Yang, G.H.; Yoneyama, Y.; Tsubaki, N. One-Pot Hydrothermal Synthesis of Nitrogen Functionalized Carbonaceous Material Catalysts with Embedded Iron Nanoparticles for CO2 Hydrogenation. ACS Sustain. Chem. Eng. 2019, 7, 8331–8339. [Google Scholar] [CrossRef]
- Cheng, K.; Ordomsky, V.V.; Virginie, M.; Legras, B.; Chernavskii, P.A.; Kazak, V.O.; Cordier, C.; Paul, S.; Wang, Y.; Khodakov, A.Y. Support effects in high temperature Fischer-Tropsch synthesis on iron catalysts. Appl. Catal. A-Gen. 2014, 488, 66–77. [Google Scholar] [CrossRef]
- Sun, B.; Xu, K.; Nguyen, L.; Qiao, M.H.; Tao, F. Preparation and Catalysis of Carbon-Supported Iron Catalysts for Fischer-Tropsch Synthesis. ChemCatChem 2012, 4, 1498–1511. [Google Scholar] [CrossRef]
- Xiong, H.F.; Jewell, L.L.; Coville, N.J. Shaped Carbons As Supports for the Catalytic Conversion of Syngas to Clean Fuels. ACS Catal. 2015, 5, 2640–2658. [Google Scholar] [CrossRef]
- Xiong, H.; Moyo, M.; Motchelaho, M.A.; Tetana, Z.N.; Dube, S.M.A.; Jewell, L.L.; Coville, N.J. Fischer–Tropsch synthesis: Iron catalysts supported on N-doped carbon spheres prepared by chemical vapor deposition and hydrothermal approaches. J. Catal. 2014, 311, 80–87. [Google Scholar] [CrossRef]
- Chen, H.C.; Sun, F.G.; Wang, J.T.; Li, W.C.; Qiao, W.M.; Ling, L.C.; Long, D.H. Nitrogen Doping Effects on the Physical and Chemical Properties of Mesoporous Carbons. J. Phys. Chem. C 2013, 117, 8318–8328. [Google Scholar] [CrossRef]
- Liu, G.; Chen, Q.; Oyunkhand, E.; Ding, S.; Yamane, N.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Nitrogen-rich mesoporous carbon supported iron catalyst with superior activity for Fischer-Tropsch synthesis. Carbon 2018, 130, 304–314. [Google Scholar] [CrossRef]
- Deng, C.; Xu, L.J.; Hu, K.H.; Chen, X.X.; Gao, R.X.; Zhang, L.Y.; Wang, L.; Zhang, C.D. Research Advances on Nitrogen-Doped Carbon Materials in COx Hydrogenation. Atmosphere 2023, 14, 1510. [Google Scholar] [CrossRef]
- Evdokimenko, N.D.; Kapustin, G.I.; Tkachenko, O.P.; Kalmykov, K.B.; Kustov, A.L. Zn Doping Effect on the Performance of Fe-Based Catalysts for the Hydrogenation of CO2 to Light Hydrocarbons. Molecules 2022, 27, 1065. [Google Scholar] [CrossRef]
- Amin, M.; Usman, M.; Kella, T.; Khan, W.U.; Khan, I.A.; Lee, K.H. Issues and challenges of Fischer-Tropsch synthesis catalysts. Front. Chem. 2024, 12, 1462503. [Google Scholar] [CrossRef]
- van Deelen, T.W.; Mejía, C.H.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Wezendonk, T.A.; Sun, X.; Dugulan, A.I.; van Hoof, A.J.F.; Hensen, E.J.M.; Kapteijn, F.; Gascon, J. Controlled formation of iron carbides and their performance in Fischer-Tropsch synthesis. J. Catal. 2018, 362, 106–117. [Google Scholar] [CrossRef]
- Dry, M.E. The Fischer-Tropsch process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Liu, R.J.; Xu, Y.; Qiao, Y.; Li, Z.H.; Ma, X.B. Factors influencing the Fischer-Tropsch synthesis performance of iron-based catalyst: Iron oxide dispersion, distribution and reducibility. Fuel Process. Technol. 2015, 139, 25–32. [Google Scholar] [CrossRef]
- Valero-Romero, M.J.; Rodríguez-Cano, M.A.; Palomo, J.; Rodríguez-Mirasol, J.; Cordero, T. Carbon-Based Materials as Catalyst Supports for Fischer-Tropsch Synthesis: A Review. Front. Mater. 2021, 7, 617432. [Google Scholar] [CrossRef]
- Wan, H.J.; Wu, B.S.; Xiang, H.W.; Li, Y.W. Fischer-Tropsch Synthesis: Influence of Support Incorporation Manner on Metal Dispersion, Metal-Support Interaction, and Activities of Iron Catalysts. ACS Catal. 2012, 2, 1877–1883. [Google Scholar] [CrossRef]
- Zhang, G.J.; Qu, J.W.; Du, Y.N.; Guo, F.B.; Zhao, H.X.; Zhang, Y.F.; Xu, Y. Hydrogen production from CO2 reforming of methane over high pressure H2O2 modified different semi-cokes. J. Ind. Eng. Chem. 2014, 20, 2948–2957. [Google Scholar] [CrossRef]
- De Jong, K.P.; Geus, J.W. Carbon nanofibers: Catalytic synthesis and applications. Catal. Rev.-Sci. Eng. 2000, 42, 481–510. [Google Scholar] [CrossRef]
- Moussa, S.O.; Panchakarla, L.S.; Ho, M.Q.; El-Shall, M.S. Graphene-Supported, Iron-Based Nanoparticles for Catalytic Production of Liquid Hydrocarbons from Synthesis Gas: The Role of the Graphene Support in Comparison with Carbon Nanotubes. ACS Catal. 2014, 4, 535–545. [Google Scholar] [CrossRef]
- Serp, P.; Corrias, M.; Kalck, P. Carbon nanotubes and nanofibers in catalysis. Appl. Catal. A-Gen. 2003, 253, 337–358. [Google Scholar] [CrossRef]
- Ajayan, P.M. Nanotubes from carbon. Chem. Rev. 1999, 99, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Fan, Z.L.; Pan, X.L.; Bao, X.H. Effect of confinement in carbon nanotubes on the activity of Fischer-Tropsch iron catalyst. J. Am. Chem. Soc. 2008, 130, 9414–9419. [Google Scholar] [CrossRef]
- Guczi, L.; Stefler, G.; Geszti, O.; Koppány, Z.; Kónya, Z.; Molnár, É.; Urbán, M.; Kiricsi, I. CO hydrogenation over cobalt and iron catalysts supported over multiwall carbon nanotubes: Effect of preparation. J. Catal. 2006, 244, 24–32. [Google Scholar] [CrossRef]
- Xiong, H.F.; Motchelaho, M.A.M.; Moyo, M.; Jewell, L.L.; Coville, N.J. Cobalt catalysts supported on a micro-coil carbon in Fischer-Tropsch synthesis: A comparison with CNTs and CNFs. Catal. Today 2013, 214, 50–60. [Google Scholar] [CrossRef]
- Xiong, H.F.; Motchelaho, M.A.M.; Moyo, M.; Jewell, L.L.; Coville, N.J. Correlating the preparation and performance of cobalt catalysts supported on carbon nanotubes and carbon spheres in the Fischer-Tropsch synthesis. J. Catal. 2011, 278, 26–40. [Google Scholar] [CrossRef]
- Duan, X.; Wang, D.; Qian, G.; Walmsley, J.C.; Holmen, A.; Chen, D.; Zhou, X. Fabrication of K-promoted iron/carbon nanotubes composite catalysts for the Fischer–Tropsch synthesis of lower olefins. J. Energy Chem. 2016, 25, 311–317. [Google Scholar] [CrossRef]
- Kundu, S.; Wang, Y.M.; Xia, W.; Muhler, M. Thermal Stability and Reducibility of Oxygen-Containing Functional Groups on Multiwalled Carbon Nanotube Surfaces: A Quantitative High-Resolution XPS and TPD/TPR Study. J. Phys. Chem. C 2008, 112, 16869–16878. [Google Scholar] [CrossRef]
- Yahyazadeh, A.; Borugadda, V.B.; Dalai, A.K.; Zhang, L.F. Optimization of carbon nanotube growth via response surface methodology for Fischer-Tropsch synthesis over Fe/CNT catalyst. Catal. Today 2022, 404, 117–131. [Google Scholar] [CrossRef]
- Fang, Y.; Cao, J.; Zhang, X.; Cao, Y.; Song, N.; Qian, G.; Zhou, X.; Duan, X. Crucial roles of support modification and promoter introduction in Fe/CNT catalyzed syngas conversion to lower olefins. Catal. Today 2021, 368, 126–132. [Google Scholar] [CrossRef]
- Li, Z.; Liu, R.; Xu, Y.; Ma, X. Enhanced Fischer–Tropsch synthesis performance of iron-based catalysts supported on nitric acid treated N-doped CNTs. Appl. Surf. Sci. 2015, 347, 643–650. [Google Scholar] [CrossRef]
- Chew, L.M.; Kangvansura, P.; Ruland, H.; Schulte, H.J.; Somsen, C.; Xia, W.; Eggeler, G.; Worayingyong, A.; Muhler, M. Effect of nitrogen doping on the reducibility, activity and selectivity of carbon nanotube-supported iron catalysts applied in CO2 hydrogenation. Appl. Catal. A Gen. 2014, 482, 163–170. [Google Scholar] [CrossRef]
- Fu, T.J.; Lv, J.; Li, Z.H. Effect of Carbon Porosity and Cobalt Particle Size on the Catalytic Performance of Carbon Supported Cobalt Fischer-Tropsch Catalysts. Ind. Eng. Chem. Res. 2014, 53, 1342–1350. [Google Scholar] [CrossRef]
- Xiong, H.; Motchelaho, M.A.; Moyo, M.; Jewell, L.L.; Coville, N.J. Fischer–Tropsch synthesis: Iron-based catalysts supported on nitrogen-doped carbon nanotubes synthesized by post-doping. Appl. Catal. A Gen. 2014, 482, 377–386. [Google Scholar] [CrossRef]
- Chew, L.M.; Xia, W.; Düdder, H.; Weide, P.; Ruland, H.; Muhler, M. On the role of the stability of functional groups in multi-walled carbon nanotubes applied as support in iron-based high-temperature Fischer-Tropsch synthesis. Catal. Today 2016, 270, 85–92. [Google Scholar] [CrossRef]
- Wu, D.C.; Fu, R.W.; Dresselhaus, M.S.; Dresselhaus, G. Fabrication and nano-structure control of carbon aerogels via a microemulsion-templated sol-gel polymerization method. Carbon 2006, 44, 675–681. [Google Scholar] [CrossRef]
- Enterría, M.; Figueiredo, J.L. Nanostructured mesoporous carbons: Tuning texture and surface chemistry. Carbon 2016, 108, 79–102. [Google Scholar] [CrossRef]
- Li, W.; Yue, Q.; Deng, Y.H.; Zhao, D.Y. Ordered Mesoporous Materials Based on Interfacial Assembly and Engineering. Adv. Mater. 2013, 25, 5129–5152. [Google Scholar] [CrossRef]
- Liang, C.D.; Li, Z.J.; Dai, S. Mesoporous carbon materials: Synthesis and modification. Angew. Chem.-Int. Ed. 2008, 47, 3696–3717. [Google Scholar] [CrossRef] [PubMed]
- Long, D.H.; Zhang, J.; Yang, J.H.; Hu, Z.J.; Cheng, G.; Liu, X.M.; Zhang, R.; Zhan, L.; Qiao, W.M.; Ling, L.C. Chemical state of nitrogen in carbon aerogels issued from phenol-melamine-formaldehyde gels. Carbon 2008, 46, 1259–1262. [Google Scholar] [CrossRef]
- Lee, J.S.; Joo, S.H.; Ryoo, R. Synthesis of mesoporous silicas of controlled pore wall thickness and their replication to ordered nanoporous carbons with various pore diameters. J. Am. Chem. Soc. 2002, 124, 1156–1157. [Google Scholar] [CrossRef]
- Oschatz, M.; Hofmann, J.P.; van Deelen, T.W.; Lamme, W.S.; Krans, N.A.; Hensen, E.J.M.; de Jong, K.P. Effects of the Functionalization of the Ordered Mesoporous Carbon Support Surface on Iron Catalysts for the Fischer–Tropsch Synthesis of Lower Olefins. ChemCatChem 2017, 9, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.X.; Webley, P.A.; Zhao, D.Y. Comprehensive Study of Pore Evolution, Mesostructural Stability, and Simultaneous Surface Functionalization of Ordered Mesoporous Carbon (FDU-15) by Wet Oxidation as a Promising Adsorbent. Langmuir 2010, 26, 10277–10286. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.P.; Dou, Y.Q.; Zhao, D.Y.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Carbon Materials for Chemical Capacitive Energy Storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Sun, B.; Qiao, M.; Wei, J.; Yue, Q.; Wang, C.; Deng, Y.; Kaliaguine, S.; Zhao, D. A General Chelate-Assisted Co-Assembly to Metallic Nanoparticles-Incorporated Ordered Mesoporous Carbon Catalysts for Fischer–Tropsch Synthesis. J. Am. Chem. Soc. 2012, 134, 17653–17660. [Google Scholar] [CrossRef]
- Chen, X.Q.; Deng, D.H.; Pan, X.L.; Hu, Y.F.; Bao, X.H. N-doped graphene as an electron donor of iron catalysts for CO hydrogenation to light olefins. Chem. Commun. 2015, 51, 217–220. [Google Scholar] [CrossRef]
- Hwang, S.-M.; Zhang, C.; Han, S.J.; Park, H.-G.; Kim, Y.T.; Yang, S.; Jun, K.-W.; Kim, S.K. Mesoporous carbon as an effective support for Fe catalyst for CO2 hydrogenation to liquid hydrocarbons. J. CO2 Util. 2020, 37, 65–73. [Google Scholar] [CrossRef]
- Cheng, Y.; Fan, Y.Q.; Pei, Y.; Qiao, M.H. Graphene-supported metal/metal oxide nanohybrids: Synthesis and applications in heterogeneous catalysis. Catal. Sci. Technol. 2015, 5, 3903–3916. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Sood, A.K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene: The New Two-Dimensional Nanomaterial. Angew. Chem.-Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Su, C.Y.; Lu, A.Y.; Xu, Y.P.; Chen, F.R.; Khlobystov, A.N.; Li, L.J. High-Quality Thin Graphene Films from Fast Electrochemical Exfoliation. ACS Nano 2011, 5, 2332–2339. [Google Scholar] [CrossRef]
- Shang, N.G.; Papakonstantinou, P.; McMullan, M.; Chu, M.; Stamboulis, A.; Potenza, A.; Dhesi, S.S.; Marchetto, H. Catalyst-Free Efficient Growth, Orientation and Biosensing Properties of Multilayer Graphene Nanoflake Films with Sharp Edge Planes. Adv. Funct. Mater. 2008, 18, 3506–3514. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Todd, A.D.; Bielawski, C.W. Harnessing the chemistry of graphene oxide. Chem. Soc. Rev. 2014, 43, 5288–5301. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.L.; Ang, P.K.; Yang, J.X. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar] [CrossRef]
- Zhang, X.L.; Lu, Z.S.; Xu, G.L.; Wang, T.X.; Ma, D.W.; Yang, Z.X.; Yang, L. Single Pt atom stabilized on nitrogen doped graphene: CO oxidation readily occurs via the tri-molecular Eley-Rideal mechanism. Phys. Chem. Chem. Phys. 2015, 17, 20006–20013. [Google Scholar] [CrossRef] [PubMed]
- Fampiou, I.; Ramasubramaniam, A. Binding of Pt Nanoclusters to Point Defects in Graphene: Adsorption, Morphology, and Electronic Structure. J. Phys. Chem. C 2012, 116, 6543–6555. [Google Scholar] [CrossRef]
- Okamoto, Y. Density-functional calculations of icosahedral M13 (M = Pt and Au) clusters on graphene sheets and flakes. Chem. Phys. Lett. 2006, 420, 382–386. [Google Scholar] [CrossRef]
- Wang, X.R.; Li, X.L.; Zhang, L.; Yoon, Y.; Weber, P.K.; Wang, H.L.; Guo, J.; Dai, H.J. N-Doping of Graphene Through Electrothermal Reactions with Ammonia. Science 2009, 324, 768–771. [Google Scholar] [CrossRef]
- Huang, S.F.; Terakura, K.; Ozaki, T.; Ikeda, T.; Boero, M.; Oshima, M.; Ozaki, J.; Miyata, S. First-principles calculation of the electronic properties of graphene clusters doped with nitrogen and boron: Analysis of catalytic activity for the oxygen reduction reaction. Phys. Rev. B 2009, 80, 235410. [Google Scholar] [CrossRef]
- Xu, L.Y.; Wang, Q.X.; Xu, Y.D.; Huang, J.S. Promotion effect of K2O and MnO additives on the selective production of light alkenes via syngas over Fe/silicalite-2 catalysts. Catal. Lett. 1995, 31, 253–266. [Google Scholar]
- Zhang, J.L.; Fan, S.B.; Zhao, T.S.; Li, W.H.; Sun, Y.H. Carbon modified Fe-Mn-K catalyst for the synthesis of light olefins from CO hydrogenation. React. Kinet. Mech. Catal. 2011, 102, 437–445. [Google Scholar] [CrossRef]
- Hassan, H.M.A.; Abdelsayed, V.; Khder, A.; AbouZeid, K.M.; Terner, J.; El-Shall, M.S.; Al-Resayes, S.I.; El-Azhary, A.A. Microwave synthesis of graphene sheets supporting metal nanocrystals in aqueous and organic media. J. Mater. Chem. 2009, 19, 3832–3837. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, C.; Si, Z.; Ma, L.; Chen, L.; Liu, Q.; Zhang, Q.; Huang, H. Fischer-Tropsch synthesis to light olefins over iron-based catalysts supported on KMnO4 modified activated carbon by a facile method. Appl. Catal. A Gen. 2017, 541, 50–59. [Google Scholar] [CrossRef]
- Ma, W.; Kugler, E.L.; Dadyburjor, D.B. Potassium Effects on Activated-Carbon-Supported Iron Catalysts for Fischer−Tropsch Synthesis. Energy Fuels 2007, 21, 1832–1842. [Google Scholar] [CrossRef]
- Arakawa, H.; Bell, A.T. Effects of potassium promotion on the activity and selectivity of iron Fischer-Tropsch catalysts. Ind. Eng. Chem. Process Des. Dev. 2002, 22, 97–103. [Google Scholar] [CrossRef]
- Campos, A.; Lohitharn, N.; Roy, A.; Lotero, E.; Goodwin, J.G.; Spivey, J.J. An activity and XANES study of Mn-promoted, Fe-based Fischer-Tropsch catalysts. Appl. Catal. A-Gen. 2010, 375, 12–16. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Kang, J.C.; Wang, Y. Development of Novel Catalysts for Fischer-Tropsch Synthesis: Tuning the Product Selectivity. ChemCatChem 2010, 2, 1030–1058. [Google Scholar] [CrossRef]
- Tian, Z.P.; Wang, C.G.; Si, Z.; Wen, C.Y.; Xu, Y.; Lv, W.; Chen, L.G.; Zhang, X.H.; Ma, L.L. Enhancement of Light Olefins Selectivity Over N-Doped Fischer-Tropsch Synthesis Catalyst Supported on Activated Carbon Pretreated with KMnO4. Catalysts 2019, 9, 505. [Google Scholar] [CrossRef]
- Asami, K.; Komiyama, K.; Yoshida, K.; Miyahara, H. Synthesis of Lower Olefins from Synthesis Gas over Active Carbon-Supported Iron Catalyst. Catal. Today 2018, 303, 117–122. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chem.-A Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef]
- Sun, X.M.; Li, Y.D. Ga2O3 and GaN semiconductor hollow spheres. Angew. Chem.-Int. Ed. 2004, 43, 3827–3831. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Sun, B.; Pei, Y.; Xie, S.; Yan, S.; Qiao, M.; Fan, K.; Zhang, X.; Zong, B. FexOy@C Spheres as an Excellent Catalyst for Fischer−Tropsch Synthesis. J. Am. Chem. Soc. 2009, 132, 935–937. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Moyo, M.; Motchelaho, M.A.M.; Jewell, L.L.; Coville, N.J. Fischer–Tropsch synthesis over model iron catalysts supported on carbon spheres: The effect of iron precursor, support pretreatment, catalyst preparation method and promoters. Appl. Catal. A Gen. 2010, 388, 168–178. [Google Scholar] [CrossRef]
- Teng, X.S.; Huang, S.Y.; Wang, J.; Wang, H.Y.; Zhao, Q.; Yuan, Y.; Ma, X.B. Fabrication of Fe2C Embedded in Hollow Carbon Spheres: A High-Performance and Stable Catalyst for Fischer-Tropsch Synthesis. ChemCatChem 2018, 10, 3883–3891. [Google Scholar] [CrossRef]
- Bai, J.; Qin, C.; Xu, Y.; Du, Y.; Ma, G.; Ding, M. Biosugarcane-based carbon support for high-performance iron-based Fischer-Tropsch synthesis. iScience 2021, 24, 102715. [Google Scholar] [CrossRef]
- Bai, J.; Qin, C.; Xu, Y.; Xu, D.; Ding, M. Preparation of Nitrogen Doped Biochar-Based Iron Catalyst for Enhancing Gasoline-Range Hydrocarbons Production. ACS Appl. Mater. Interfaces 2022, 14, 45516–45525. [Google Scholar] [CrossRef]
- Amin, M.; Munir, S.; Iqbal, N.; Wabaidur, S.; Iqbal, A. The Conversion of Waste Biomass into Carbon-Supported Iron Catalyst for Syngas to Clean Liquid Fuel Production. Catalysts 2022, 12, 1234. [Google Scholar] [CrossRef]
- Wu, T.; Lin, J.; Cheng, Y.; Tian, J.; Wang, S.; Xie, S.; Pei, Y.; Yan, S.; Qiao, M.; Xu, H.; et al. Porous Graphene-Confined Fe–K as Highly Efficient Catalyst for CO2 Direct Hydrogenation to Light Olefins. ACS Appl. Mater. Interfaces 2018, 10, 23439–23443. [Google Scholar] [CrossRef]
- Chernyak, S.; Burtsev, A.; Maksimov, S.; Kupreenko, S.; Maslakov, K.; Savilov, S. Structural evolution, stability, deactivation and regeneration of Fischer-Tropsch cobalt-based catalysts supported on carbon nanotubes. Appl. Catal. A-Gen. 2020, 603, 13. [Google Scholar] [CrossRef]
- Lin, B.Y.; Guo, Y.J.; Lin, J.D.; Ni, J.; Lin, J.X.; Jiang, L.L.; Wang, Y. Deactivation study of carbon-supported ruthenium catalyst with potassium promoter. Appl. Catal. A-Gen. 2017, 541, 1–7. [Google Scholar] [CrossRef]
- Chakraborty, R.; Vilya, K.; Pradhan, M.; Nayak, A.K. Recent advancement of biomass-derived porous carbon based materials for energy and environmental remediation applications. J. Mater. Chem. A 2022, 10, 6965–7005. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, K.; Xing, Y.; Li, C.; Gao, X.; Ma, Q.; Zhao, T.-s.; Zhang, J. Preparation of Fe-based catalysts from waste biomass as a carbon carrier and its catalytic performance in CO2 hydrogenation. New J. Chem. 2024, 48, 9920–9930. [Google Scholar] [CrossRef]
- Li, S.Z.; Meitzner, G.D.; Iglesia, E. Structure and site evolution of iron oxide catalyst precursors during the Fischer-Tropsch synthesis. J. Phys. Chem. B 2001, 105, 5743–5750. [Google Scholar] [CrossRef]
- de Smit, E.; Beale, A.M.; Nikitenko, S.; Weckhuysen, B.M. Local and long range order in promoted iron-based Fischer-Tropsch catalysts: A combined in situ X-ray absorption spectroscopy/wide angle X-ray. J. Catal. 2009, 262, 244–256. [Google Scholar] [CrossRef]
- Herranz, T.; Rojas, S.; Pérez-Alonso, F.J.; Ojeda, M.; Terreros, P.; Fierro, J.L.G. Genesis of iron carbides and their role in the synthesis of hydrocarbons from synthesis gas. J. Catal. 2006, 243, 199–211. [Google Scholar] [CrossRef]
- Lyu, S.; Wang, L.; Li, Z.; Yin, S.K.; Chen, J.; Zhang, Y.H.; Li, J.L.; Wang, Y. Stabilization of ε-iron carbide as high-temperature catalyst under realistic Fischer-Tropsch synthesis conditions. Nat. Commun. 2020, 11, 6219. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Sun, B.; Lin, J.; Wen, W.; Pei, Y.; Yan, S.R.; Qiao, M.H.; Zhang, X.X.; Zong, B.N. ε-Iron carbide as a low-temperature Fischer-Tropsch synthesis catalyst. Nat. Commun. 2014, 5, 5783. [Google Scholar] [CrossRef]
- Zhao, Q.; Liang, H.T.; Huang, S.Y.; Han, X.X.; Wang, H.Y.; Wang, J.; Wang, Y.; Ma, X.B. Tunable Fe3O4 nanoparticles assembled porous microspheres as catalysts for Fischer-Tropsch synthesis to lower olefins. Catal. Today 2021, 368, 133–139. [Google Scholar] [CrossRef]
- Shetty, A.; Molahalli, V.; Sharma, A.; Hegde, G. Biomass-Derived Carbon Materials in Heterogeneous Catalysis: A Step towards Sustainable Future. Catalysts 2023, 13, 20. [Google Scholar] [CrossRef]
- Stepacheva, A.A.; Markova, M.E.; Lugovoy, Y.V.; Kosivtsov, Y.Y.; Matveeva, V.G.; Sulman, M.G. Plant-Biomass-Derived Carbon Materials as Catalyst Support, A Brief Review. Catalysts 2023, 13, 655. [Google Scholar] [CrossRef]
- Otun, K.O.; Liu, X.; Hildebrandt, D. Metal-organic framework (MOF)-derived catalysts for Fischer-Tropsch synthesis: Recent progress and future perspectives. J. Energy Chem. 2020, 51, 230–245. [Google Scholar] [CrossRef]
- Razavi, S.A.A.; Morsali, A. Linker functionalized metal-organic frameworks. Coord. Chem. Rev. 2019, 399, 213023. [Google Scholar] [CrossRef]
- Chen, L.Y.; Xu, Q. Metal-Organic Framework Composites for Catalysis. Matter 2019, 1, 57–89. [Google Scholar] [CrossRef]
- Wezendonk, T.A.; Santos, V.P.; Nasalevich, M.A.; Warringa, Q.S.E.; Dugulan, A.I.; Chojecki, A.; Koeken, A.C.J.; Ruitenbeek, M.; Meima, G.; Islam, H.U.; et al. Elucidating the Nature of Fe Species during Pyrolysis of the Fe-BTC MOF into Highly Active and Stable Fischer-Tropsch Catalysts. ACS Catal. 2016, 6, 3236–3247. [Google Scholar] [CrossRef]
- Nisa, M.U.; Chen, Y.; Li, X.; Jiang, X.; Li, Z. Modulating C5+selectivity for Fischer-Tropsch synthesis by tuning pyrolysis temperature of MOFs derived Fe-based catalyst. J. Taiwan Inst. Chem. Eng. 2022, 131, 104170. [Google Scholar] [CrossRef]
- Abbaslou, R.M.M.; Soltan, J.; Dalai, A.K. Effects of nanotubes pore size on the catalytic performances of iron catalysts supported on carbon nanotubes for Fischer-Tropsch synthesis. Appl. Catal. A-Gen. 2010, 379, 129–134. [Google Scholar] [CrossRef]
- An, B.; Cheng, K.; Wang, C.; Wang, Y.; Lin, W.B. Pyrolysis of Metal-Organic Frameworks to Fe3O4@Fe5C2 Core-Shell Nanoparticles for Fischer-Tropsch Synthesis. ACS Catal. 2016, 6, 3610–3618. [Google Scholar] [CrossRef]
- Wezendonk, T.A.; Warringa, Q.S.E.; Santos, V.P.; Chojecki, A.; Ruitenbeek, M.; Meima, G.; Makkee, M.; Kapteijn, F.; Gascon, J. Structural and elemental influence from various MOFs on the performance of Fe@C catalysts for Fischer–Tropsch synthesis. Faraday Discuss. 2017, 197, 225–242. [Google Scholar] [CrossRef]
- Cho, J.M.; Kim, B.G.; Han, G.Y.; Sun, J.; Jeong, H.K.; Bae, J.W. Effects of metal-organic framework-derived iron carbide phases for CO hydrogenation activity to hydrocarbons. Fuel 2020, 281, 6. [Google Scholar] [CrossRef]
- Santos, V.P.; Wezendonk, T.A.; Jaén, J.J.D.; Dugulan, A.I.; Nasalevich, M.A.; Islam, H.U.; Chojecki, A.; Sartipi, S.; Sun, X.; Hakeem, A.A.; et al. Metal organic framework-mediated synthesis of highly active and stable Fischer-Tropsch catalysts. Nat. Commun. 2015, 6, 6451. [Google Scholar] [CrossRef]
- Lippi, R.; Howard, S.C.; Barron, H.; Easton, C.D.; Madsen, I.C.; Waddington, L.J.; Vogt, C.; Hill, M.R.; Sumby, C.J.; Doonan, C.J.; et al. Highly active catalyst for CO2 methanation derived from a metal organic framework template. J. Mater. Chem. A 2017, 5, 12990–12997. [Google Scholar] [CrossRef]
- Ye, R.P.; Ding, J.; Gong, W.B.; Argyle, M.D.; Zhong, Q.; Wang, Y.J.; Russell, C.K.; Xu, Z.H.; Russell, A.G.; Li, Q.H.; et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 5698. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.Z.; Xiao, T.C.; Makgae, O.A.; Jie, X.Y.; Gonzalez-Cortes, S.; Guan, S.L.; Kirkland, A.I.; Dilworth, J.R.; Al-Megren, H.A.; Alshihri, S.M.; et al. Transforming carbon dioxide into jet fuel using an organic combustion-synthesized Fe-Mn-K catalyst. Nat. Commun. 2020, 11, 6395. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.T.; Zhang, Y.C.; Peng, C. Recent Advances Hydrogenation of Carbon Dioxide to Light Olefins over Iron-Based Catalysts via the Fischer-Tropsch Synthesis. ACS Omega 2024, 9, 25610–25624. [Google Scholar] [CrossRef]
- Wang, D.; Xie, Z.H.; Porosoff, M.D.; Chen, J.G.G. Recent advances in carbon dioxide hydrogenation to produce olefins and aromatics. Chem 2021, 7, 2277–2311. [Google Scholar] [CrossRef]
- Liu, Y.H.; Cheng, Q.P.; Xiong, S.H.; Zhang, Y.T.; Tan, L.; Song, S.; Ding, T.; Tian, Y.; Li, X.G. Enhancing CO2 hydrogenation performance via the synergistic effects of iron carbides and iron oxides. Int. J. Hydrogen Energy 2025, 104, 66–75. [Google Scholar] [CrossRef]
- Meng, W.X.; de Jong, B.C.A.; van de Bovenkamp, H.; Boer, G.J.; Bezemer, G.L.; Dugulan, A.I.; Xie, J.X. Selectivity control between reverse water-gas shift and fischer-tropsch synthesis in carbon-supported iron-based catalysts for CO2 hydrogenation. Chem. Eng. J. 2024, 489, 151166. [Google Scholar] [CrossRef]
- Minett, D.R.; O’Byrne, J.P.; Pascu, S.I.; Plucinski, P.K.; Owen, R.E.; Jones, M.D.; Mattia, D. Fe@CNT-monoliths for the conversion of carbon dioxide to hydrocarbons: Structural characterisation and Fischer-Tropsch reactivity investigations. Catal. Sci. Technol. 2014, 4, 3351–3358. [Google Scholar] [CrossRef]
- Suslova, E.; Savilov, S.; Egorov, A.; Shumyantsev, A.; Lunin, V. Carbon nanotube frameworks by spark plasma sintering. Microporous Mesoporous Mater. 2020, 293, 109807. [Google Scholar] [CrossRef]
- Chernyak, S.A.; Ivanov, A.S.; Maksimov, S.V.; Maslakov, K.I.; Isaikina, O.Y.; Chernavskii, P.A.; Kazantsev, R.V.; Eliseev, O.L.; Savilov, S.S. Fischer-Tropsch synthesis over carbon-encapsulated cobalt and iron nanoparticles embedded in 3D-framework of carbon nanotubes. J. Catal. 2020, 389, 270–284. [Google Scholar] [CrossRef]
- Chernyak, S.A.; Ivanov, A.S.; Stolbov, D.N.; Maksimov, S.V.; Maslakov, K.I.; Chernavskii, P.A.; Pokusaeva, Y.A.; Koklin, A.E.; Bogdan, V.I.; Savilov, S.V. Sintered Fe/CNT framework catalysts for CO2 hydrogenation into hydrocarbons. Carbon 2020, 168, 475–484. [Google Scholar] [CrossRef]
- Williamson, D.L.; Herdes, C.; Torrente-Murciano, L.; Jones, M.D.; Mattia, D. N-Doped Fe@CNT for Combined RWGS/FT CO2 Hydrogenation. ACS Sustain. Chem. Eng. 2019, 7, 7395–7402. [Google Scholar] [CrossRef]
- Landau, M.V.; Meiri, N.; Utsis, N.; Nehemya, R.V.; Herskowitz, M. Conversion of CO2, CO, and H2 in CO2 Hydrogenation to Fungible Liquid Fuels on Fe-Based Catalysts. Ind. Eng. Chem. Res. 2017, 56, 13335–13356. [Google Scholar] [CrossRef]
- Witoon, T.; Numpilai, T.; Nueangnoraj, K.; Cheng, C.K.; Chareonpanich, M.; Limtrakul, J. Light olefins synthesis from CO2 hydrogenation over mixed Fe-Co-K supported on micro-mesoporous carbon catalysts. Int. J. Hydrogen Energy 2022, 47, 42185–42199. [Google Scholar] [CrossRef]
- Peng, L.; Jurca, B.; Primo, A.; Gordillo, A.; Parvulescu, V.I.; García, H. Co–Fe Clusters Supported on N-Doped Graphitic Carbon as Highly Selective Catalysts for Reverse Water Gas Shift Reaction. ACS Sustain. Chem. Eng. 2021, 9, 9264–9272. [Google Scholar] [CrossRef]
- Liang, J.M.; Liu, J.T.; Guo, L.S.; Wang, W.H.; Wang, C.W.; Gao, W.Z.; Guo, X.Y.; He, Y.L.; Yang, G.H.; Yasuda, S.; et al. CO2 hydrogenation over Fe-Co bimetallic catalysts with tunable selectivity through a graphene fencing approach. Nat. Commun. 2024, 15, 13. [Google Scholar] [CrossRef]
- Wang, H.; Sun, K.; Tao, F.; Stacchiola, D.J.; Hu, Y.H. 3D Honeycomb-Like Structured Graphene and Its High Efficiency as a Counter-Electrode Catalyst for Dye-Sensitized Solar Cells. Angew. Chem.-Int. Ed. 2013, 52, 9210–9214. [Google Scholar] [CrossRef]
- Felgueiras, M.B.S.; Pereira, M.F.R.; Soares, O. Effect of the support on the CO2 hydrogenation to C2-C4 products. Catal. Today 2024, 441, 9. [Google Scholar] [CrossRef]
- Chen, J.Y.; Han, S.J.; Park, H.G.; Nasriddinov, K.; Zhang, C.D.; Jun, K.W.; Kim, S.K. Benchmarking promoters of Fe/activated carbon catalyst for stable hydrogenation of CO2 to liquid hydrocarbons. Appl. Catal. B-Environ. Energy 2023, 325, 12. [Google Scholar] [CrossRef]
- Svidersky, S.A.; Dement’eva, O.S.; Ivantsov, M.I.; Grabchak, A.A.; Kulikova, M.V.; Maximov, A.L. Hydrogenation of CO2 over Biochar-Supported Catalysts. Pet. Chem. 2023, 63, 443–452. [Google Scholar] [CrossRef]
- Niu, L.W.; Liu, X.W.; Wen, X.D.; Yang, Y.; Xu, J.; Li, Y.W. Effect of potassium promoter on phase transformation during H2 pretreatment of a Fe2O3 Fischer Tropsch synthesis catalyst precursor. Catal. Today 2020, 343, 101–111. [Google Scholar] [CrossRef]
- Amoyal, M.; Vidruk-Nehemya, R.; Landau, M.V.; Herskowitz, M. Effect of potassium on the active phases of Fe catalysts for carbon dioxide conversion to liquid fuels through hydrogenation. J. Catal. 2017, 348, 29–39. [Google Scholar] [CrossRef]
- Hu, S.; Liu, M.; Ding, F.; Song, C.; Zhang, G.; Guo, X. Hydrothermally stable MOFs for CO2 hydrogenation over iron-based catalyst to light olefins. J. CO2 Util. 2016, 15, 89–95. [Google Scholar] [CrossRef]
- Assfour, B.; Leoni, S.; Seifert, G. Hydrogen Adsorption Sites in Zeolite Imidazolate Frameworks ZIF-8 and ZIF-11. J. Phys. Chem. C 2010, 114, 13381–13384. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, W.; Yildirim, T. Hydrogen storage in a prototypical zeolitic imidazolate framework-8. J. Am. Chem. Soc. 2007, 129, 5314–5315. [Google Scholar] [CrossRef]
- Ramirez, A.; Gevers, L.; Bavykina, A.; Ould-Chikh, S.; Gaston, J. Metal Organic Framework-Derived Iron Catalysts for the Direct Hydrogenation of CO2 to Short Chain Olefins. ACS Catal. 2018, 8, 9174–9182. [Google Scholar] [CrossRef]
- Feng, S.S.; Li, W.; Shi, Q.; Li, Y.H.; Chen, J.C.; Ling, Y.; Asiri, A.M.; Zhao, D.Y. Synthesis of nitrogen-doped hollow carbon nanospheres for CO2 capture. Chem. Commun. 2014, 50, 329–331. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, D.D.; Sun, Z.K.; Deng, Y.H.; Xia, Y.Y.; Zhao, D.Y. A Controllable Synthesis of Rich Nitrogen-Doped Ordered Mesoporous Carbon for CO2 Capture and Supercapacitors. Adv. Funct. Mater. 2013, 23, 2322–2328. [Google Scholar] [CrossRef]
- Li, Y.H.; Hung, T.H.; Chen, C.W. A first-principles study of nitrogen- and boron-assisted platinum adsorption on carbon nanotubes. Carbon 2009, 47, 850–855. [Google Scholar] [CrossRef]
- He, L.; Weniger, F.; Neumann, H.; Beller, M. Synthesis, Characterization, and Application of Metal Nanoparticles Supported on Nitrogen-Doped Carbon: Catalysis beyond Electrochemistry. Angew. Chem.-Int. Ed. 2016, 55, 12582–12594. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Jiang, X.; Zhang, A.; Song, C.; Guo, X. Pyrolyzing ZIF-8 to N-doped porous carbon facilitated by iron and potassium for CO2 hydrogenation to value-added hydrocarbons. J. CO2 Util. 2018, 25, 120–127. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, A.; Jiang, X.; Zhang, G.; Sun, Y.; Liu, M.; Ding, F.; Song, C.; Guo, X. Overcoating the Surface of Fe-Based Catalyst with ZnO and Nitrogen-Doped Carbon toward High Selectivity of Light Olefins in CO2 Hydrogenation. Ind. Eng. Chem. Res. 2019, 58, 4017–4023. [Google Scholar] [CrossRef]
- Xu, F.; Meng, X.; Zhao, R.; Jin, D.M.; Dai, W.H.; Xu, B.W.; Yang, D.D.; Xin, Z. Metal-organic framework-derived Fe3O4-FeCx catalyst for direct CO2 hydrogenation to light olefins. Appl. Catal. A-Gen. 2024, 670, 119537. [Google Scholar] [CrossRef]
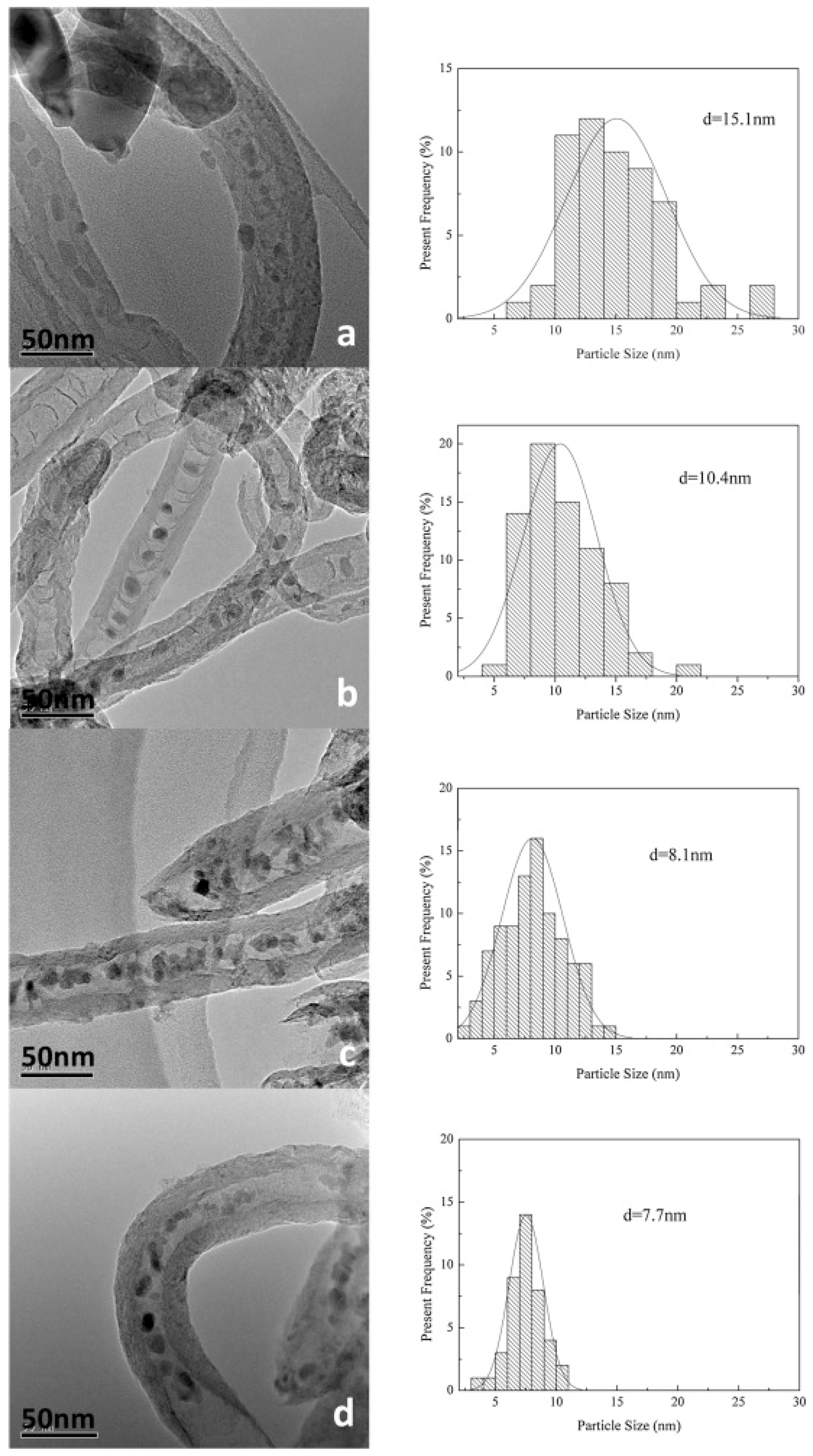
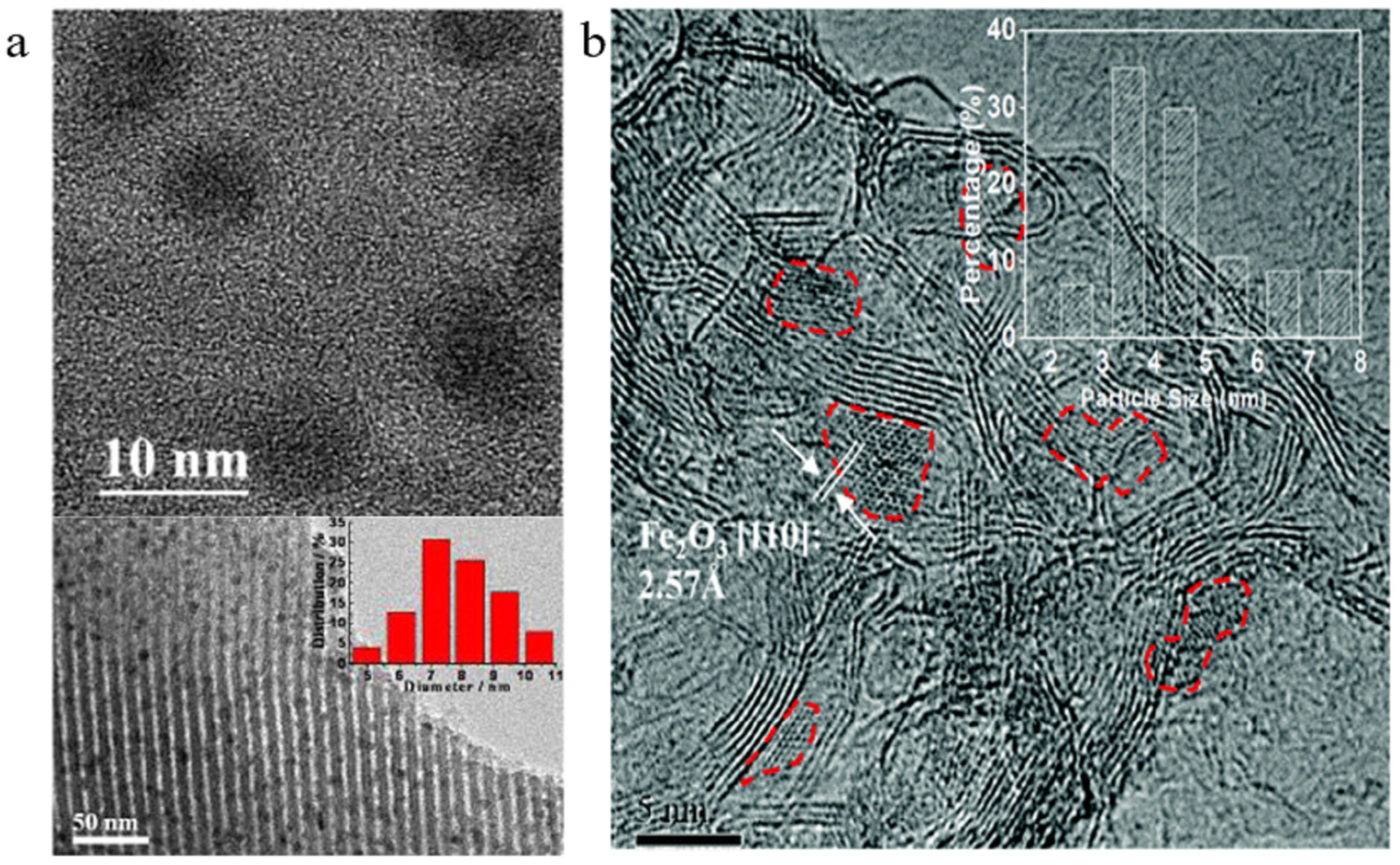
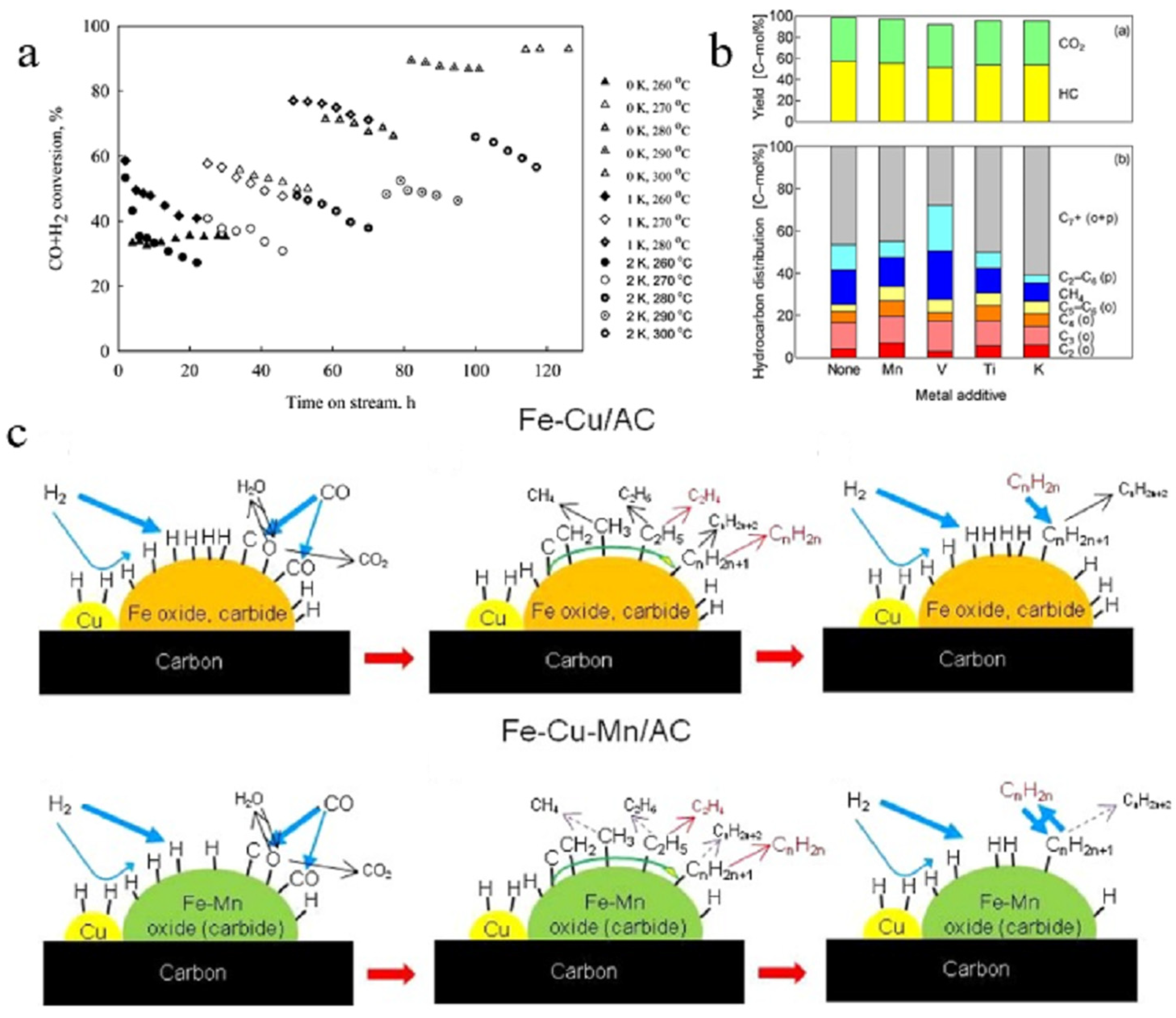
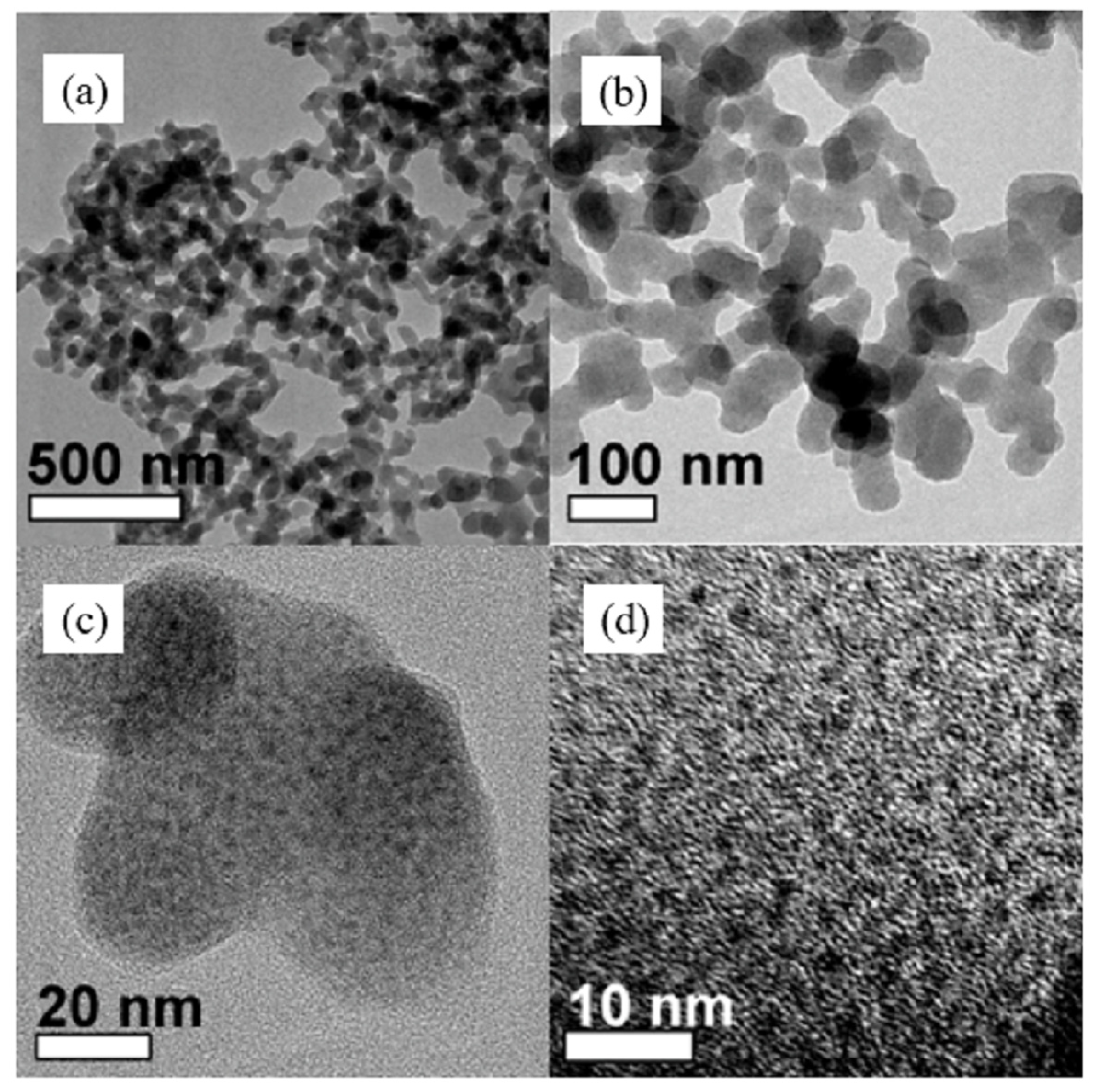
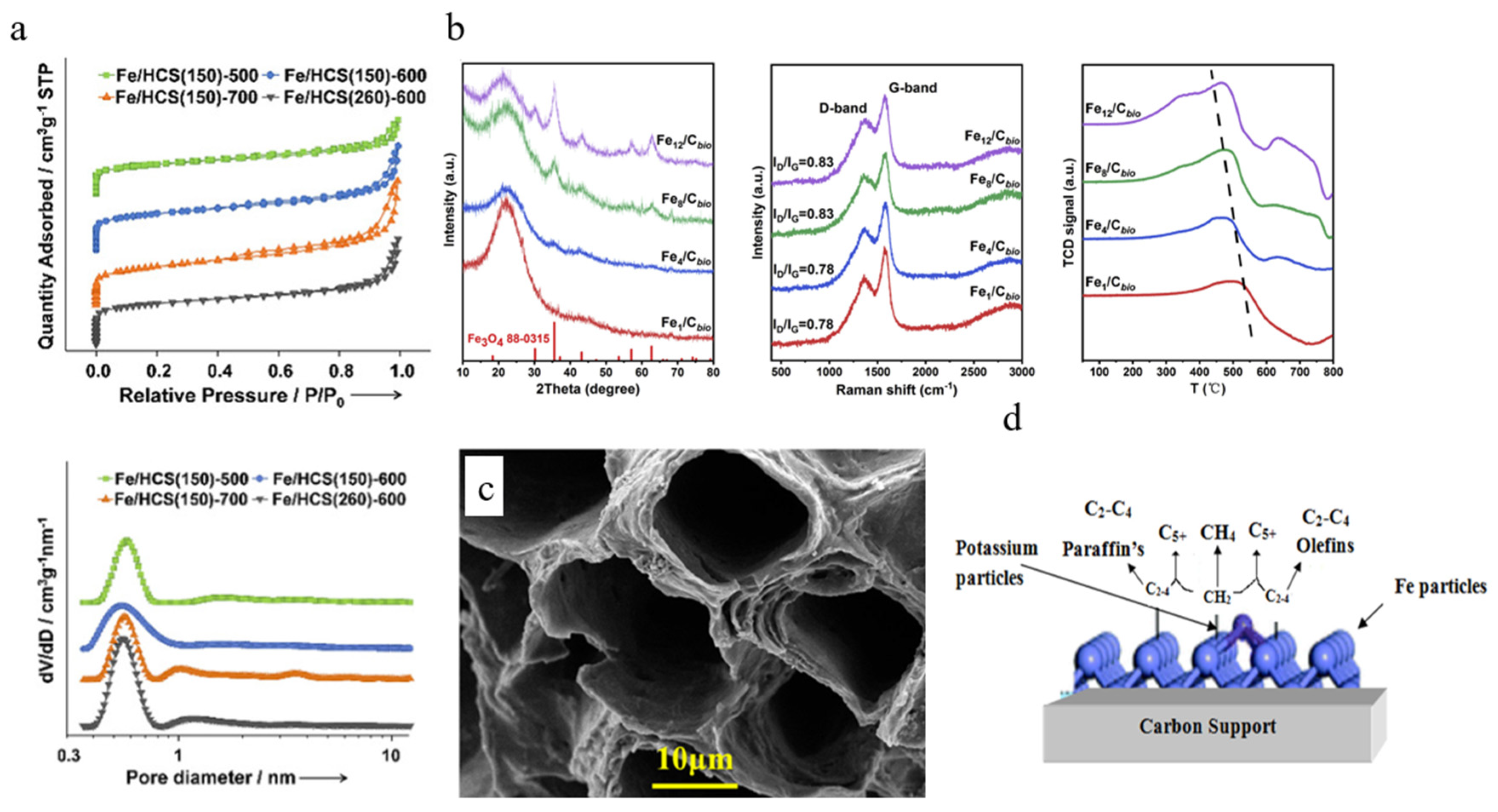
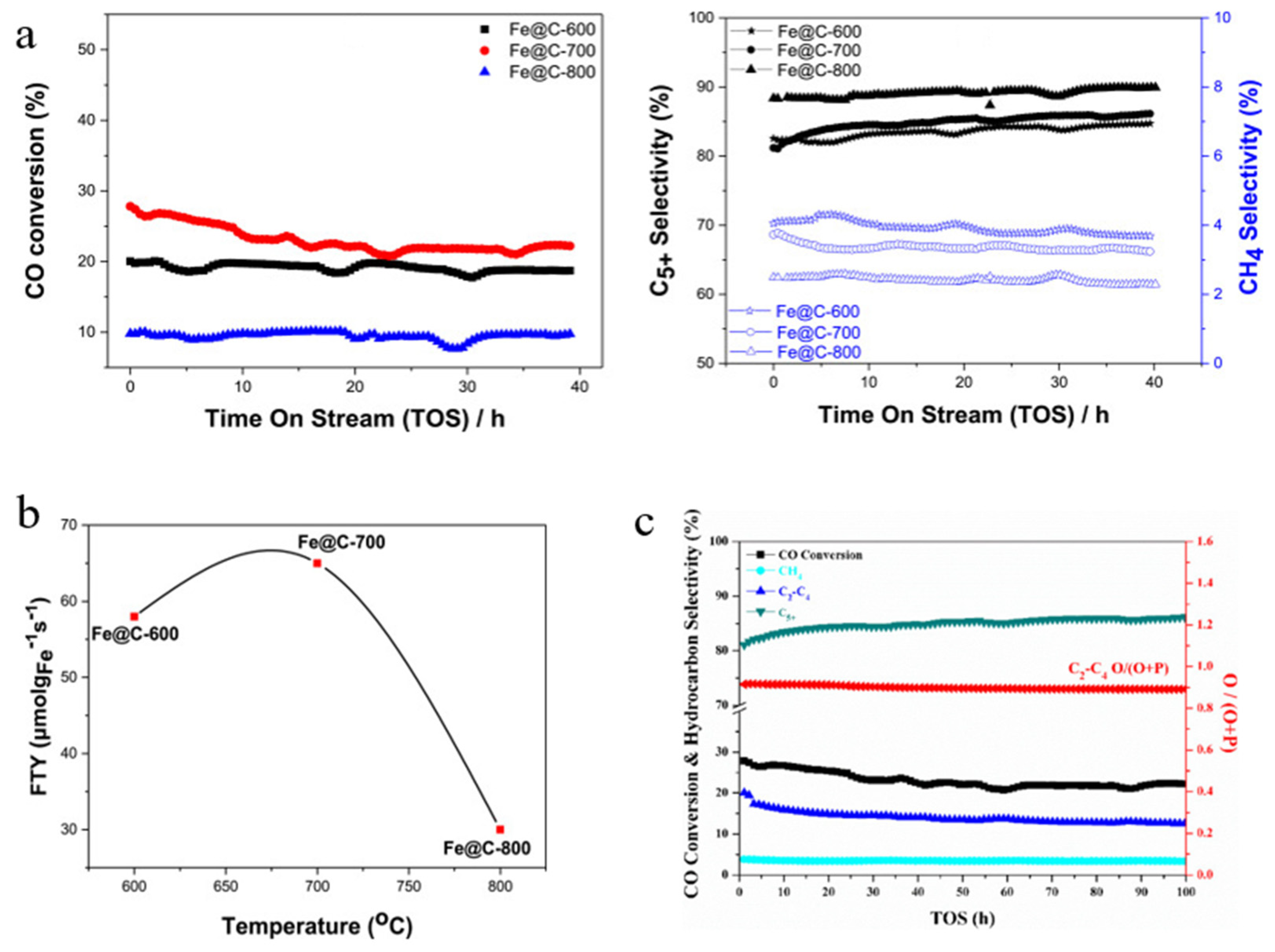

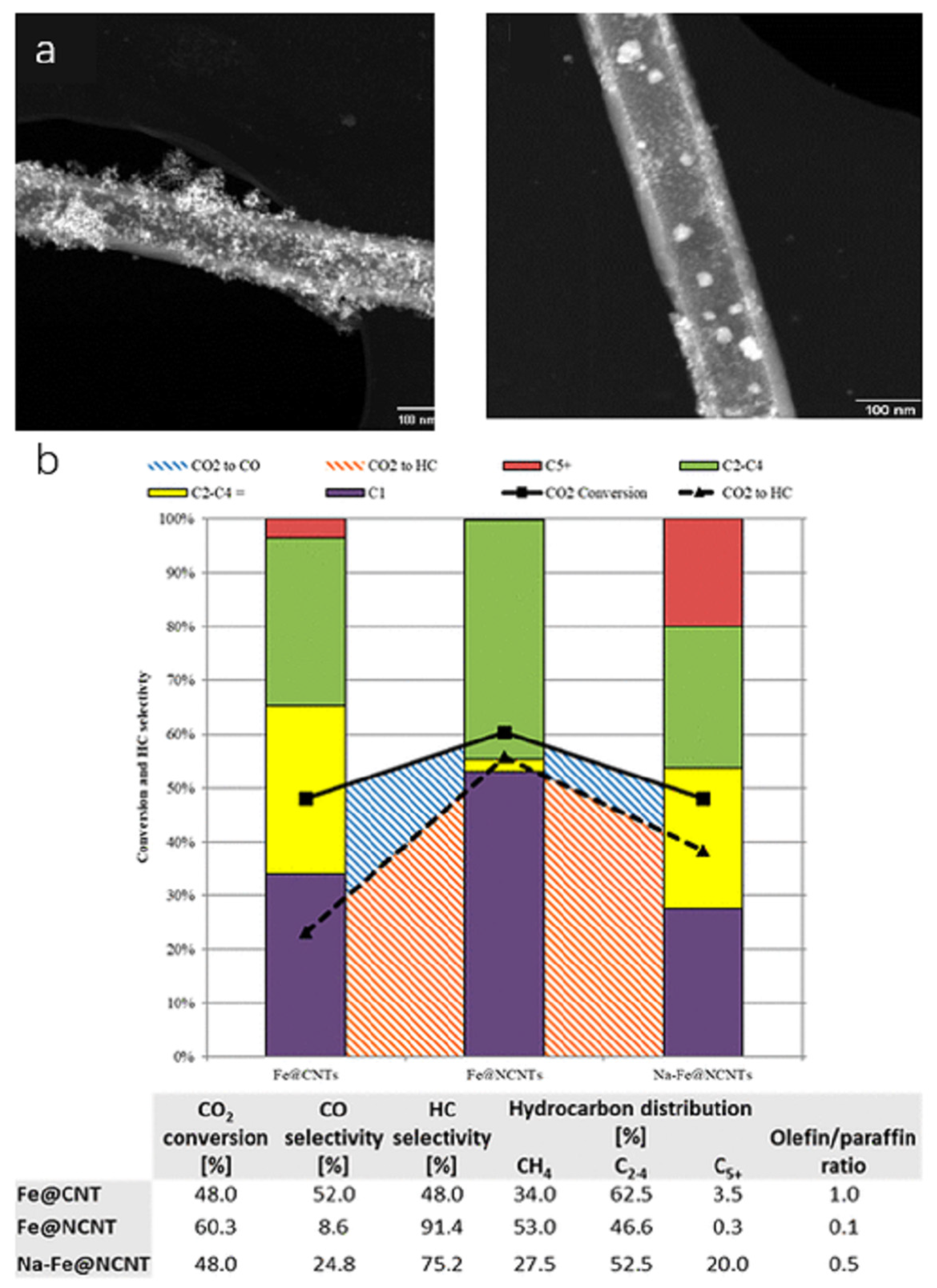
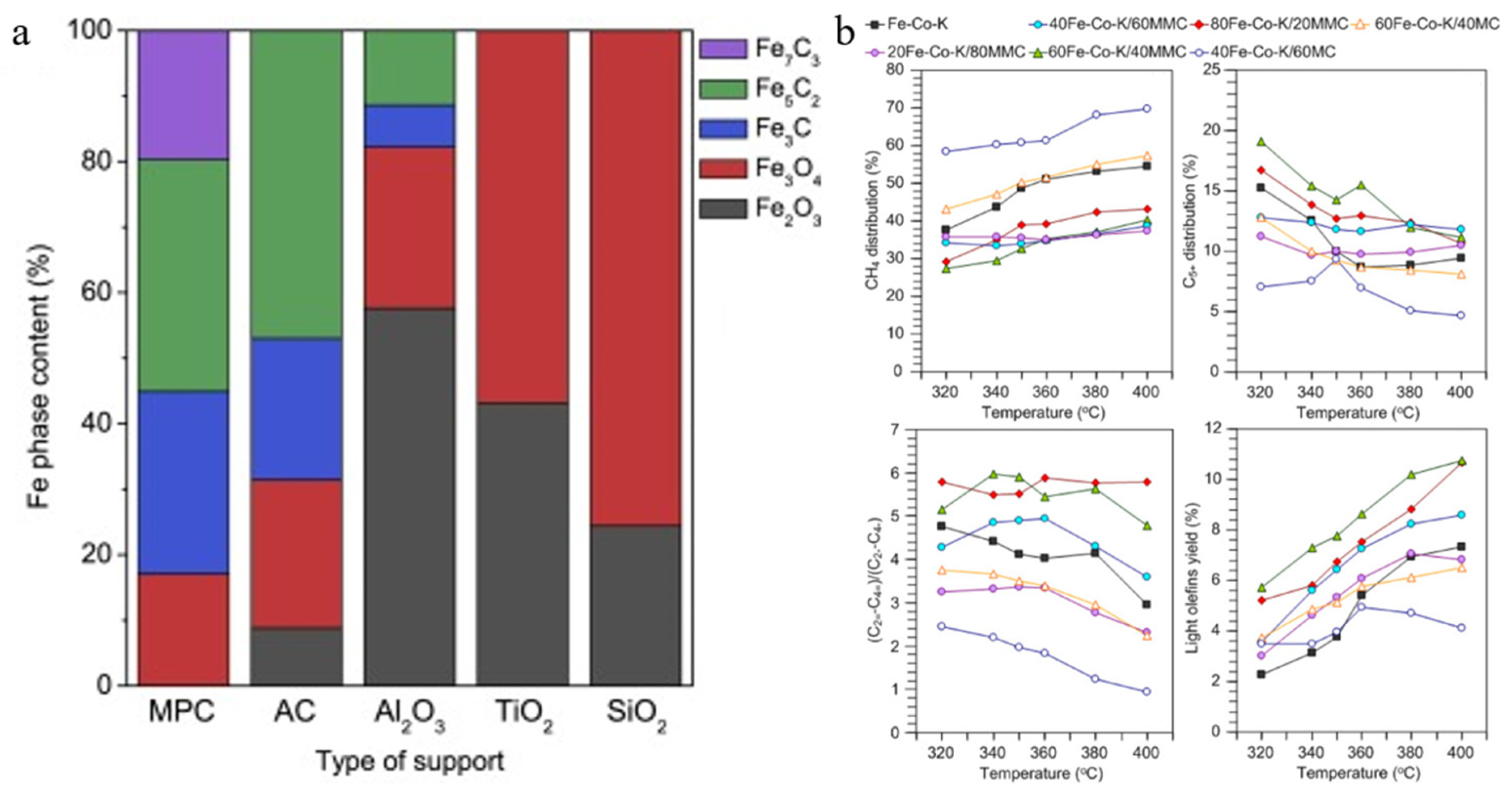




| Catalyst | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | Ref. |
|---|---|---|---|---|
| 20Fe/CNTs-Syn | 267 | 1.29 | 17.4 | [48] |
| Fe/NCNTs-10 | 110 | 0.29 | 10.3 | [50] |
| N-CNT-800 | 59 | 0.22 | / | [53] |
| CMK-3-N | 1530 | 1.62 | 3.5 | [61] |
| Fe/CMK-3S | 1326 | 1.32 | 3.8 | [21] |
| Fe–C-8 | 545 | 0.33 | 4.3 | [64] |
| Fe/HCS(150)-500 | 388 | 0.334 | 4.4 | [94] |
| Fe/LC-0.2 (800 °C) | 404.2 | 0.09 | 6.4 | [102] |
| Fe4/Cbio | 34.3 | 0.11 | 4.3 | [95] |
| Fe@C-F-700 | 212.9 | 0.24 | 4.51 | [115] |
| Catalyst | Pressure (bar) | Temperature (°C) | H2/CO | CO Conversion (%) | Selectivity (%) | FTY * | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| CH4 | C2-C4 | C5+ | |||||||
| Fe15Mn5-G | 15 | 325 | 2 | 92 | 13.5 | / | / | 147 | [39] |
| FeK-OX | 20 | 270 | 1 | 28.8 | 19.5 | 53.6 | 26.9 | / | [46] |
| 20Fe/CNTs-Syn | 20 | 280 | 1 | 90.4 | 54.8 | 23.6 | 21.6 | 313 | [48] |
| Fe/NCNTs-10 | 20 | 270 | 1 | 45 | 13.7 | 9.8 | 76.5 | / | [50] |
| Fe/N-CNT-h | 8 | 275 | 2 | 70.1 | / | 25.6 | 60.9 | 55 | [53] |
| Fe/NCSver | 8 | 275 | / | 50 | 22.3 | 26.1 | 51.6 | / | [24] |
| Fe/CMK-3S | 20 | 300 | 2.1 | 49.7 | 12.7 | 39 | 48.3 | 340.3 | [21] |
| Fe–C-8 | 20 | 270 | 2 | 90.1 | 13.4 | / | 60.1 | / | [64] |
| 15.7 Fe/2 K/AC (2 K) | 20.7 | 270 | 0.9 | 41.1 | 5.7 | 33.2 | 61.1 | / | [84] |
| Fe-2MnK-AC | 20 | 320 | 1 | 96.8 | 14.3 | 32.36 | 37.4 | / | [83] |
| Fe/HCS(150)-500 | 20 | 340 | 1 | 80.3 | 16 | 31.5 | 49 | / | [94] |
| Fe4/Cbio | 20 | 300 | 1 | 80.9 | 11.6 | 22.3 | 17.5 | 1198.9 | [95] |
| Fe/NDPCbio-3 | 20 | 300 | 1 | 92 | 14.6 | 41.5 | 43.9 | / | [96] |
| Fe@C-500-Carb | 20 | 230 | 1 | 11.4 | 8.5 | 22 | 60.3 | 40.2 | [31] |
| Fe@C-700 | 20 | 300 | 1 | 23.15 | 3.39 | 11.73 | 84.88 | 65 | [115] |
| KFe@C-F300 | 20 | 340 | 1 | 91.7 | 7.8 | / | / | 459 | [118] |
| Catalyst | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | Ref. |
|---|---|---|---|---|
| FeK/MPC | 91.22 | 0.2046 | 8.1 | [66] |
| FeK/AC | 632.55 | 0.3274 | 7.7 | |
| 20Fe-Co-K/80MMC | 496 | 0.5 | 4.2 | [134] |
| Fe/LC-0.2 (700 °C) | 339.1 | 0.08 | 7.18 | [102] |
| Fe@NC-400 | 595 | 0.38 | / | [152] |
| Fe/C-K@NC-350-600 | 42.1 | 0.06 | 9.6 | [153] |
| Catalyst | Pressure (bar) | Temperature (°C) | H2/CO2 | CO2 Conversion (%) | Selectivity (%) | FTY * | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| CH4 | C2-C4 | C5+ | |||||||
| Fe1200SPS | 85 | 350 | 2 | 21 | 34 | 34 | 12 | 113 | [131] |
| FeK/MPC | 25 | 300 | 3 | 50.6 | / | 31.9 | 44.5 | / | [66] |
| FeK1.5/HSG | 20 | 340 | 3 | / | 31 | 65.9 | 3.7 | 73 | [98] |
| Na-Fe@NCNT | 15 | 370 | 3 | 48 | 27.5 | 52.5 | 20 | / | [132] |
| GO/K-Fe-Co | 30 | 320 | 2.5 | 55.4 | 13 | 63.7 | 23.3 | / | [136] |
| Fe/LC-0.2 (700 °C) | 15 | 320 | 3 | 30 | / | 47 | / | / | [102] |
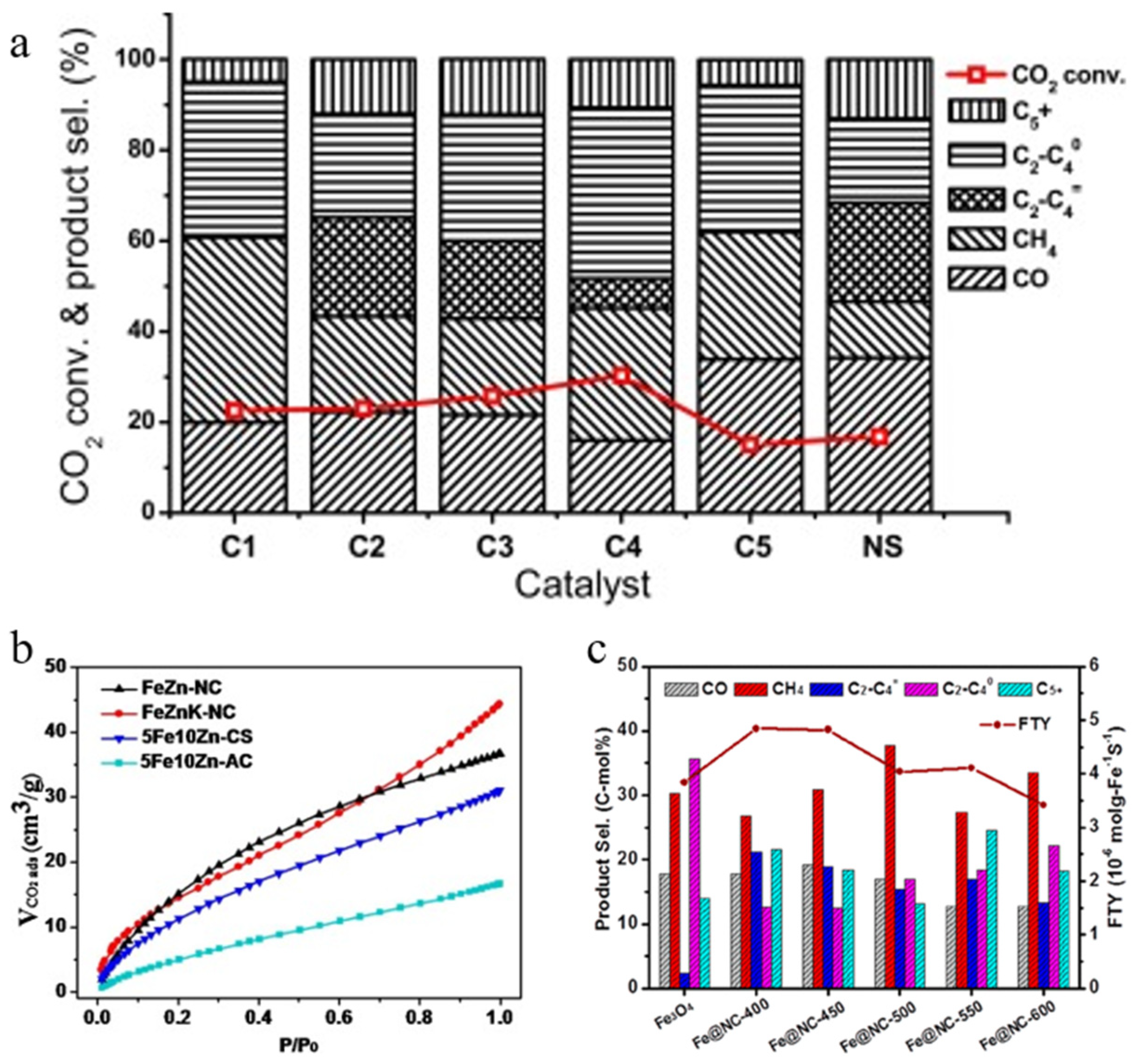
| FeZnK-NC | 30 | 320 | 3 | 34.6 | 19.1 | 37.6 | 22.1 | / | [151] |
| Fe@NC-400 | 30 | 320 | 3 | 28 | 26.8 | 33.8 | 21.6 | 4.84 | [152] |
| Fe/C-K@NC-350/600 | 30 | 340 | 3 | 25.5 | 16.2 | 28.4 | 55.4 | / | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, S.; Deng, C.; Xu, L.; Li, J.; Gao, R. Research Advances in COx Hydrogenation to Valuable Hydrocarbons over Carbon-Supported Fe-Based Catalysts. Molecules 2025, 30, 2268. https://doi.org/10.3390/molecules30112268
Peng S, Deng C, Xu L, Li J, Gao R. Research Advances in COx Hydrogenation to Valuable Hydrocarbons over Carbon-Supported Fe-Based Catalysts. Molecules. 2025; 30(11):2268. https://doi.org/10.3390/molecules30112268
Chicago/Turabian StylePeng, Shuai, Chao Deng, Lujing Xu, Junli Li, and Ruxing Gao. 2025. "Research Advances in COx Hydrogenation to Valuable Hydrocarbons over Carbon-Supported Fe-Based Catalysts" Molecules 30, no. 11: 2268. https://doi.org/10.3390/molecules30112268
APA StylePeng, S., Deng, C., Xu, L., Li, J., & Gao, R. (2025). Research Advances in COx Hydrogenation to Valuable Hydrocarbons over Carbon-Supported Fe-Based Catalysts. Molecules, 30(11), 2268. https://doi.org/10.3390/molecules30112268






