The Multifaceted Health Benefits of Broccoli—A Review of Glucosinolates, Phenolics and Antimicrobial Peptides
Abstract
1. Introduction
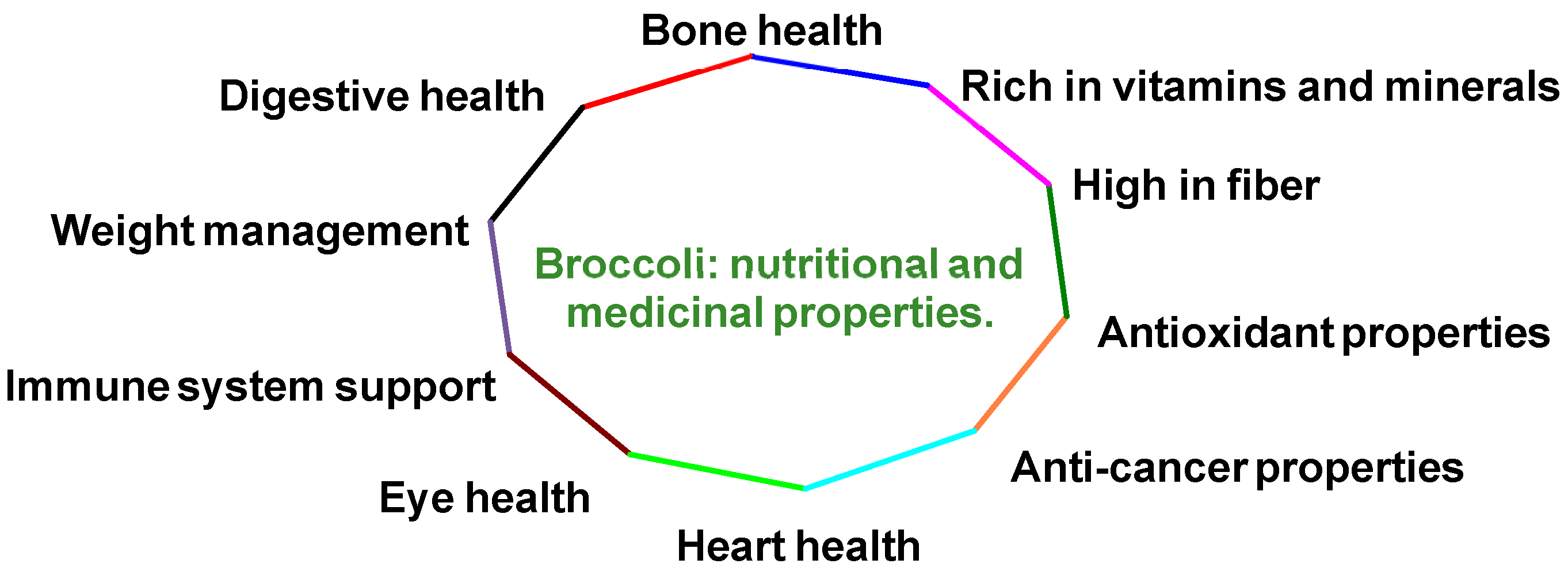
- It is rich in dietary fiber, which supports digestion, increases feelings of fullness and contributes to a healthy digestive system [6].
- It provides various antioxidants, including vitamins C and E, β-carotene and several flavonoids, which help shield cells from free radical damage [7].
- It promotes eye health through its high vitamin A content and the presence of antioxidants [12].
- It boosts the immune system while supporting collagen production, wound healing and iron absorption [13].
- It contributes to bone health as it is a significant source of calcium and contains vitamin K [14].
- It aids in weight management due to its low-calorie content and high fiber levels [15].
- It supports digestive health, with its high fiber content promoting regular bowel movements [16].
2. Bioactive Compounds in Broccoli
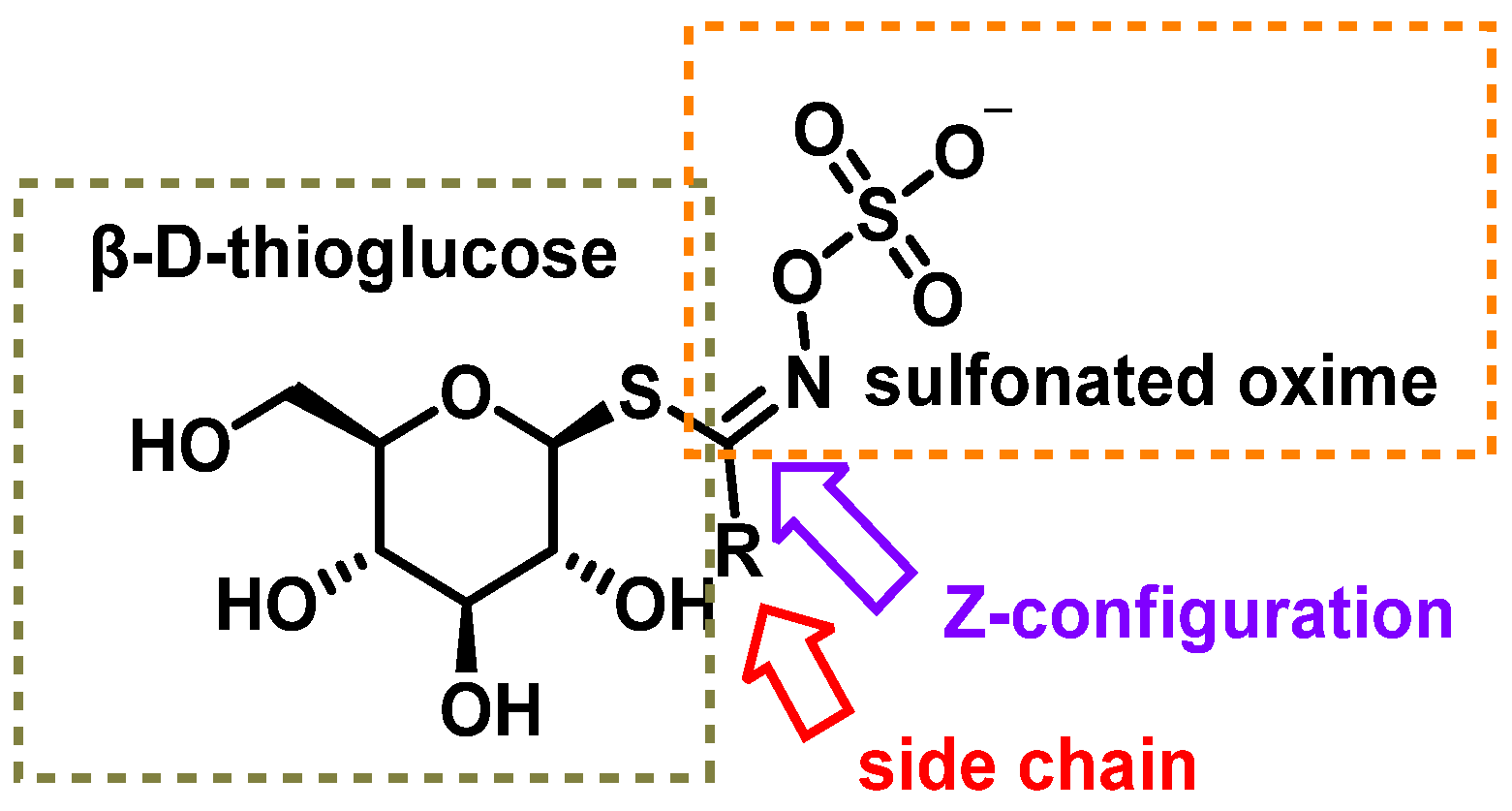
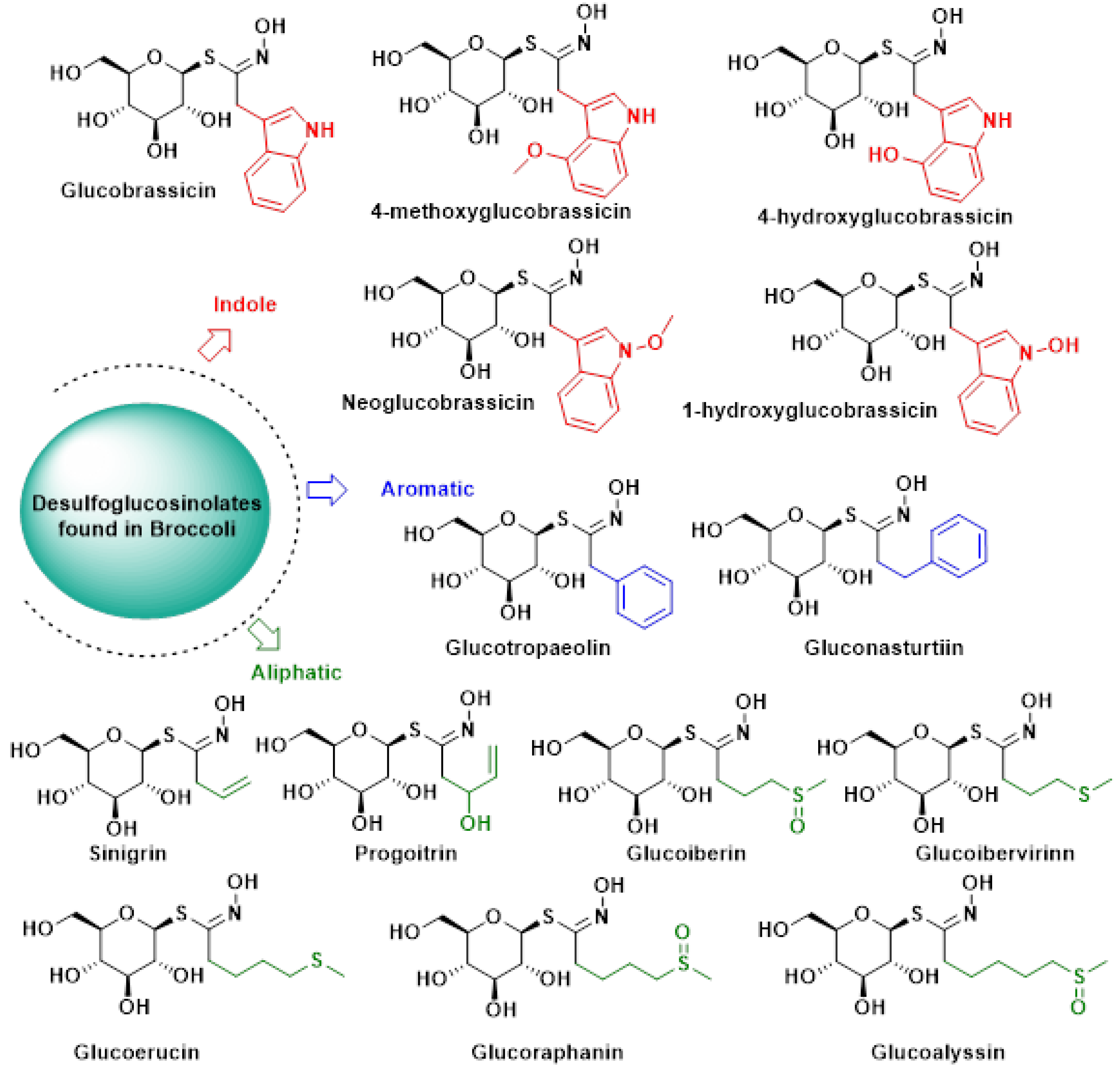
2.1. Glucosinolates
- (i)
- (ii)
- (iii)
2.1.1. Bioavailability of Glucosinolates
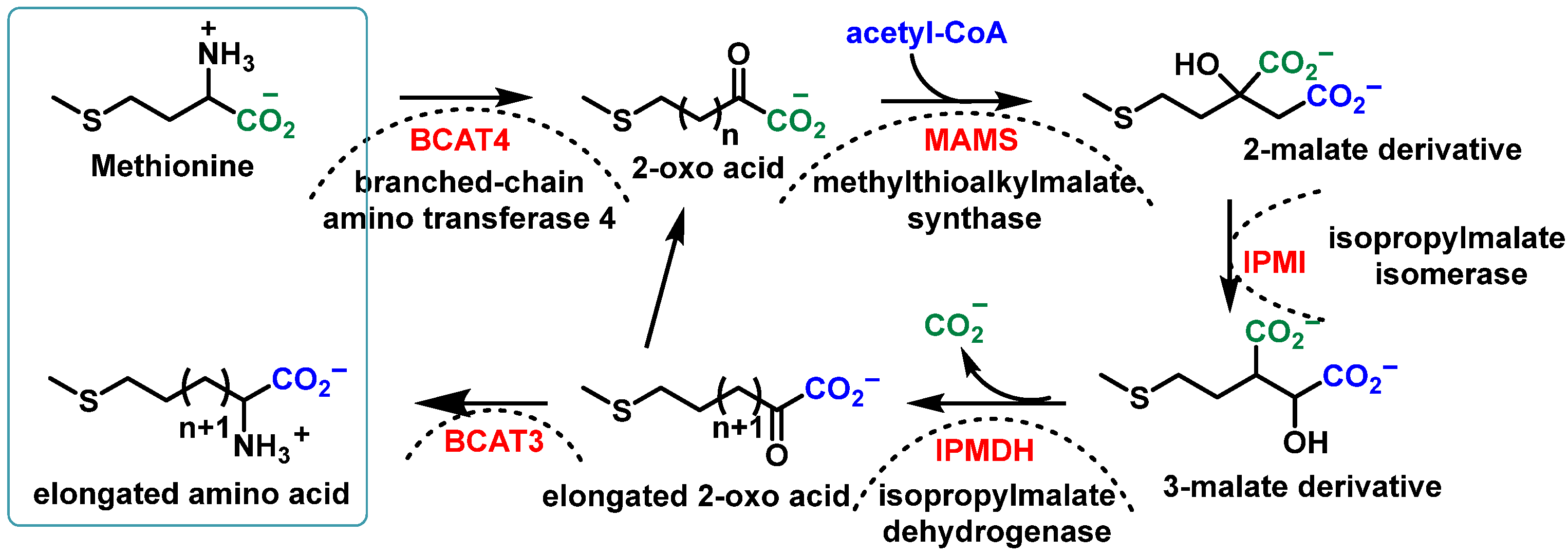
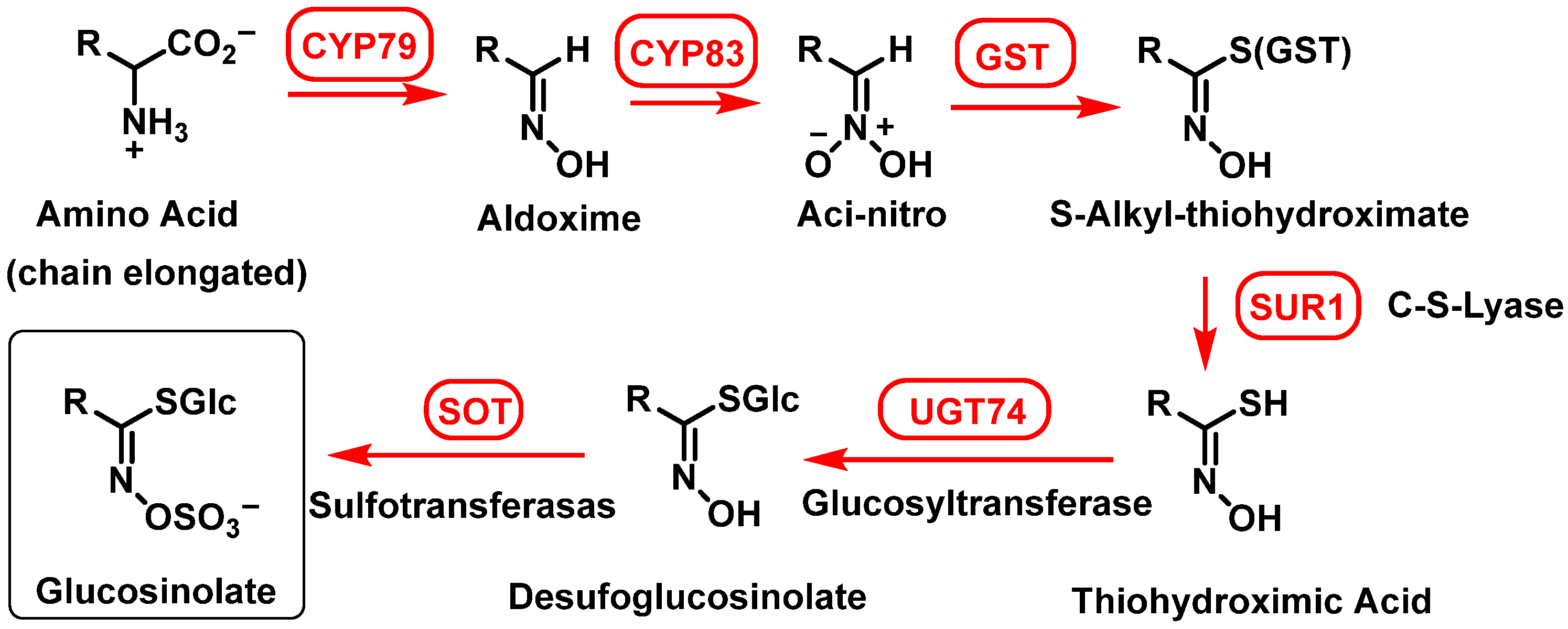
2.1.2. Biosynthesis of Glucosinolates in Plants
- (1)
- Elongation of the side chain of amino acids.
- Deamination of aliphatic or aromatic amino acids, yielding 2-oxo acids;
- Condensation of 2-oxo acids with Acetyl-CoA, forming 2-malate derivatives;
- Isomerization of 2-malate to 3-malate;
- Oxidation and decarboxylation, resulting in the loss of the initial amino acid carboxyl group and the formation of an elongated 2-oxo acid molecule.
- (2)
- Synthesis of GSL from the modified amino acid
- (3)
- GSL sidechain modifications
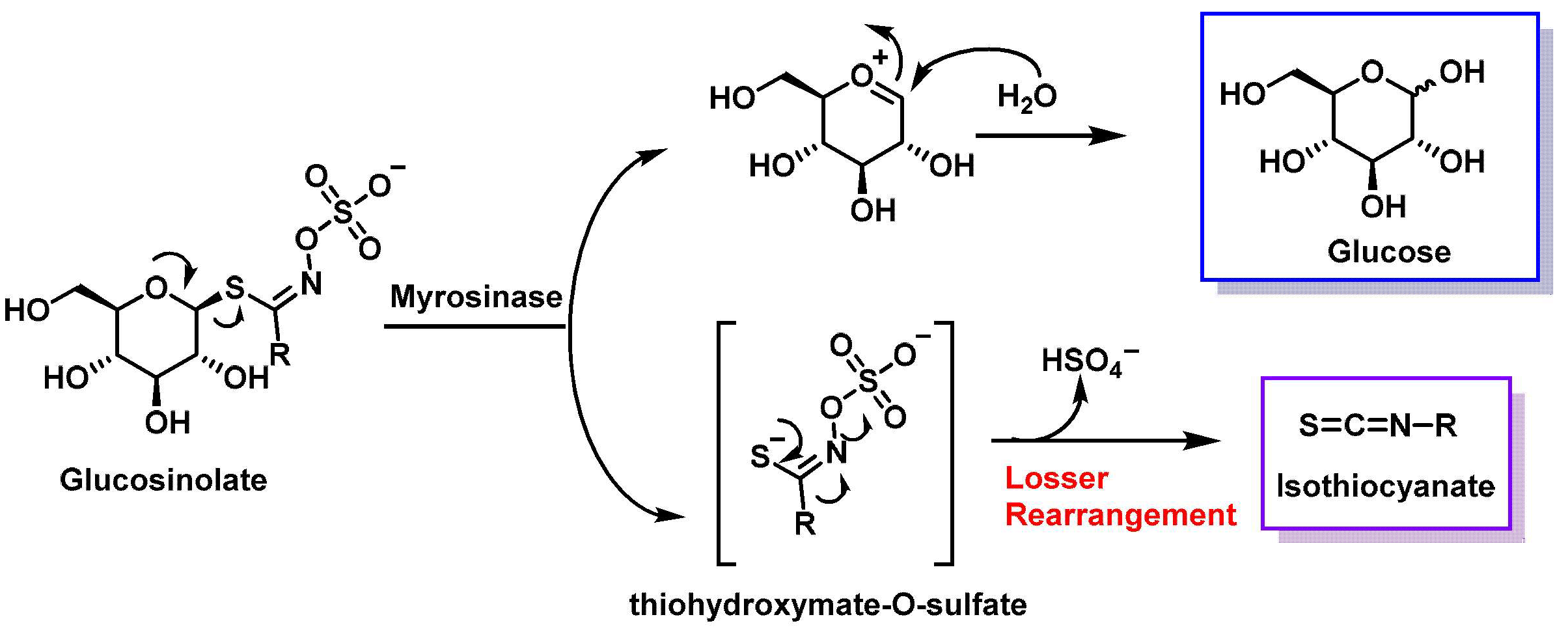
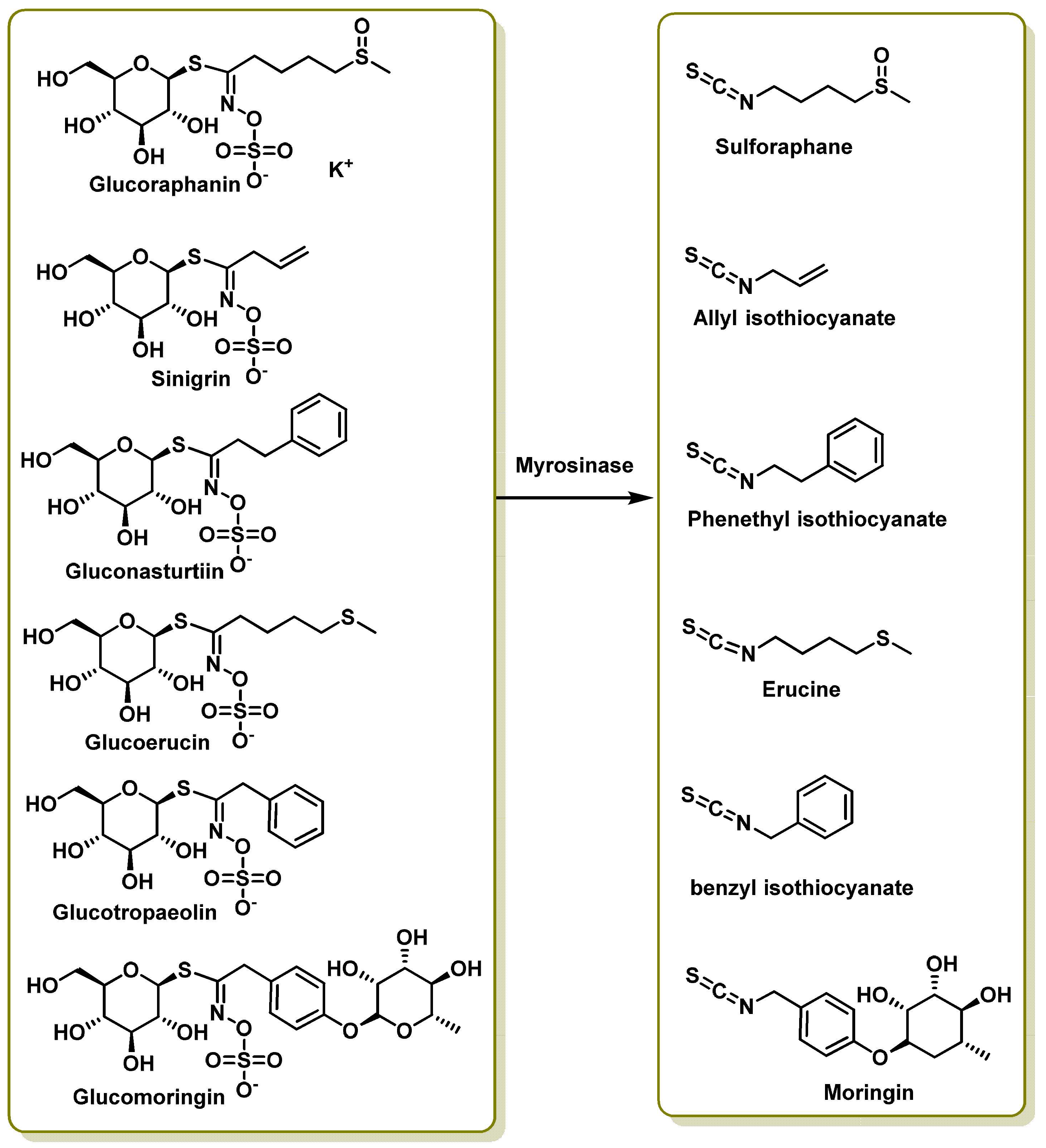
3. Most Studied Broccoli Glucosinolate Hydrolysis Compounds
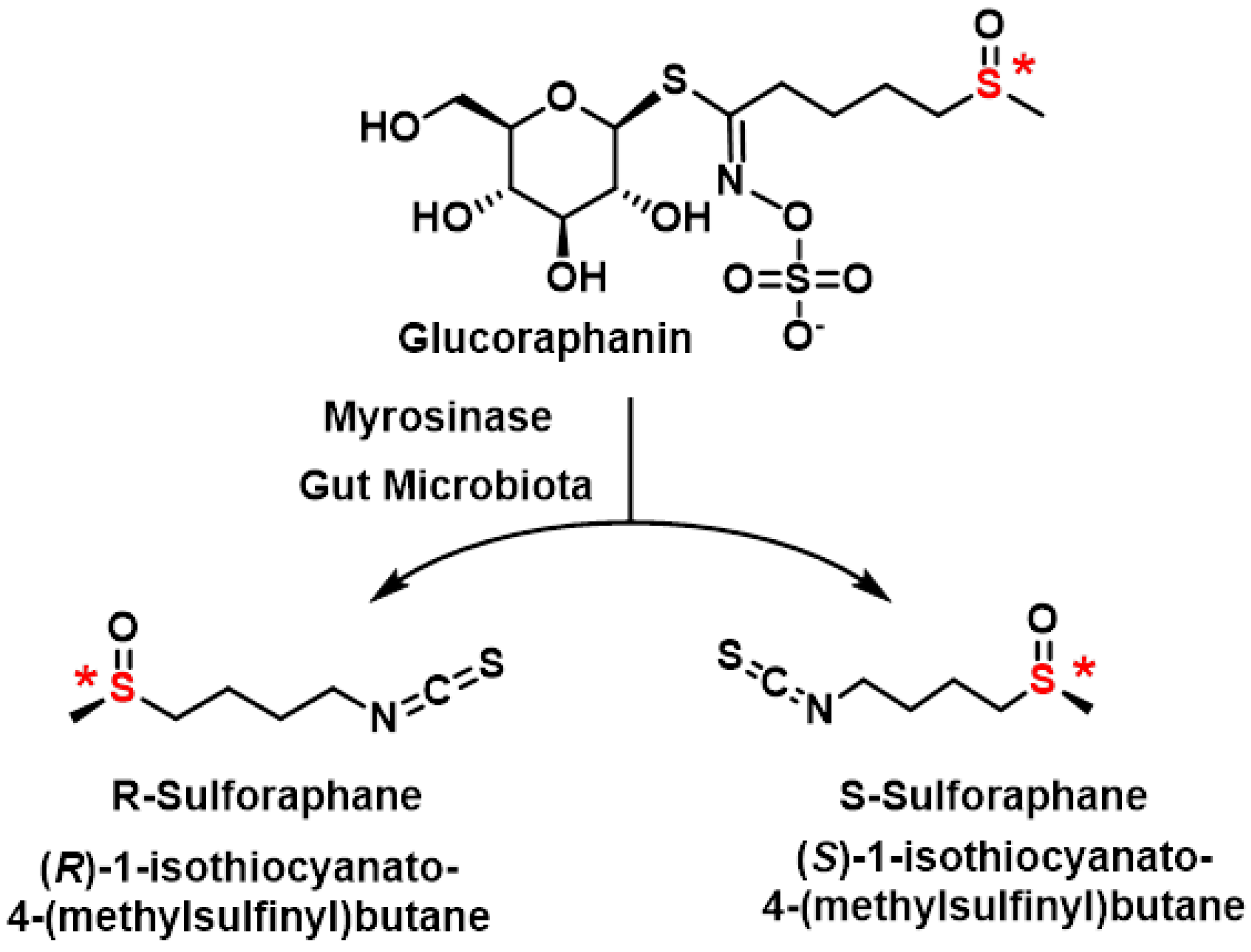
3.1. Sulforaphane
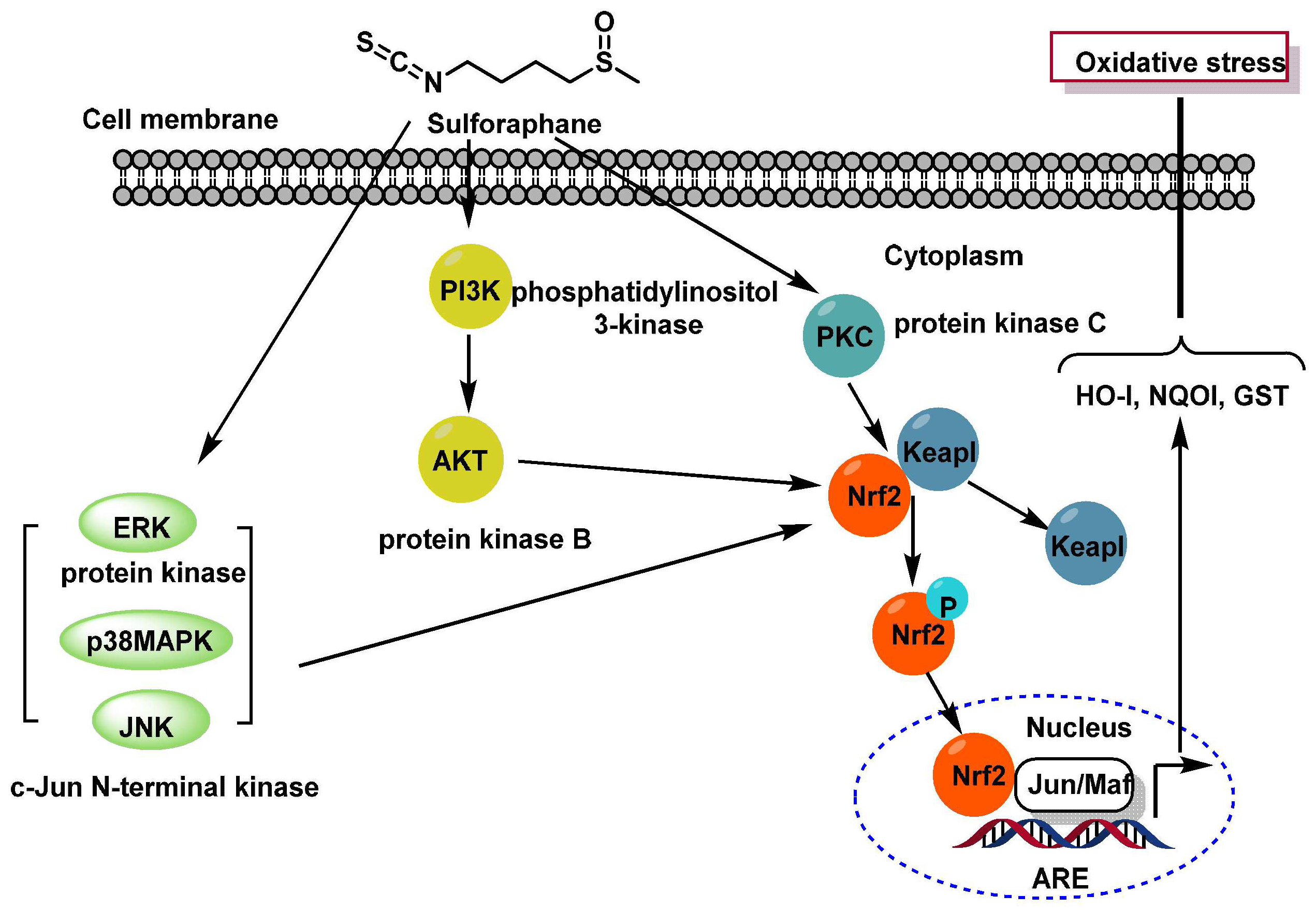
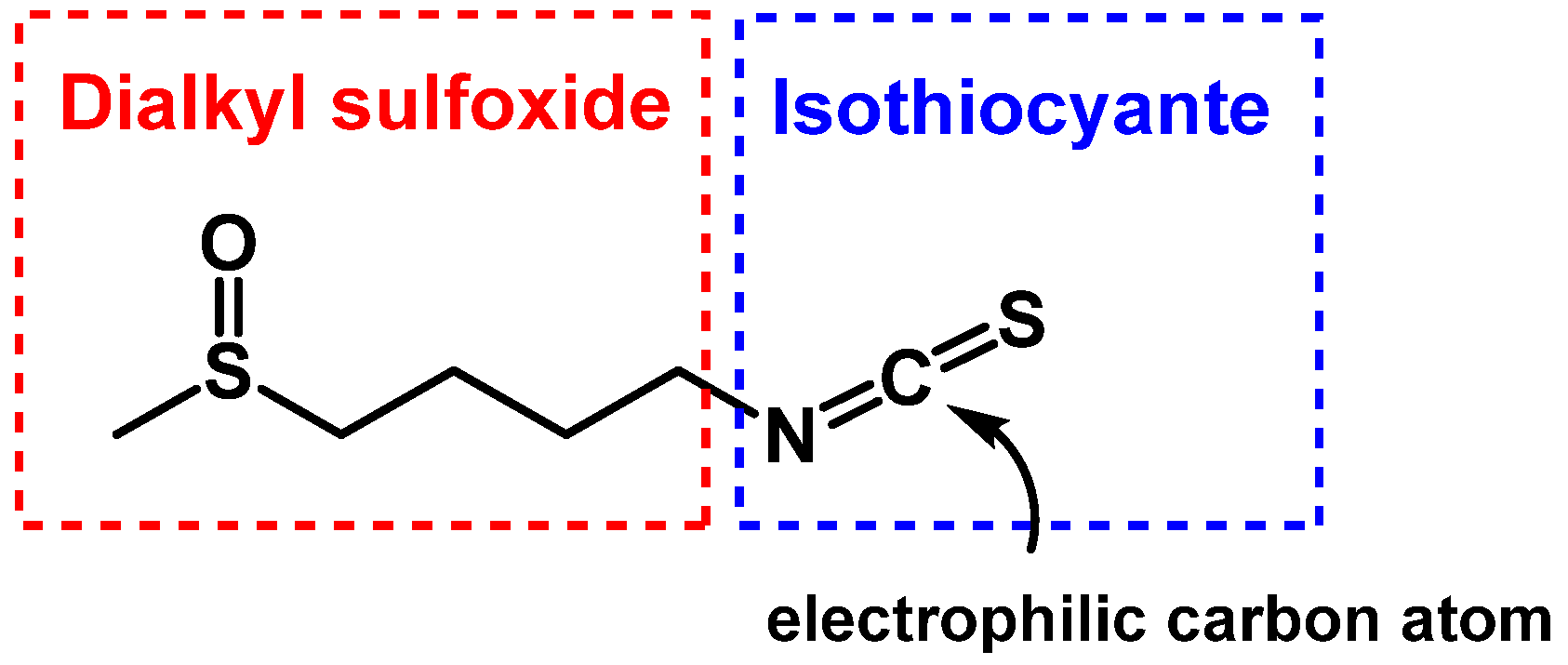
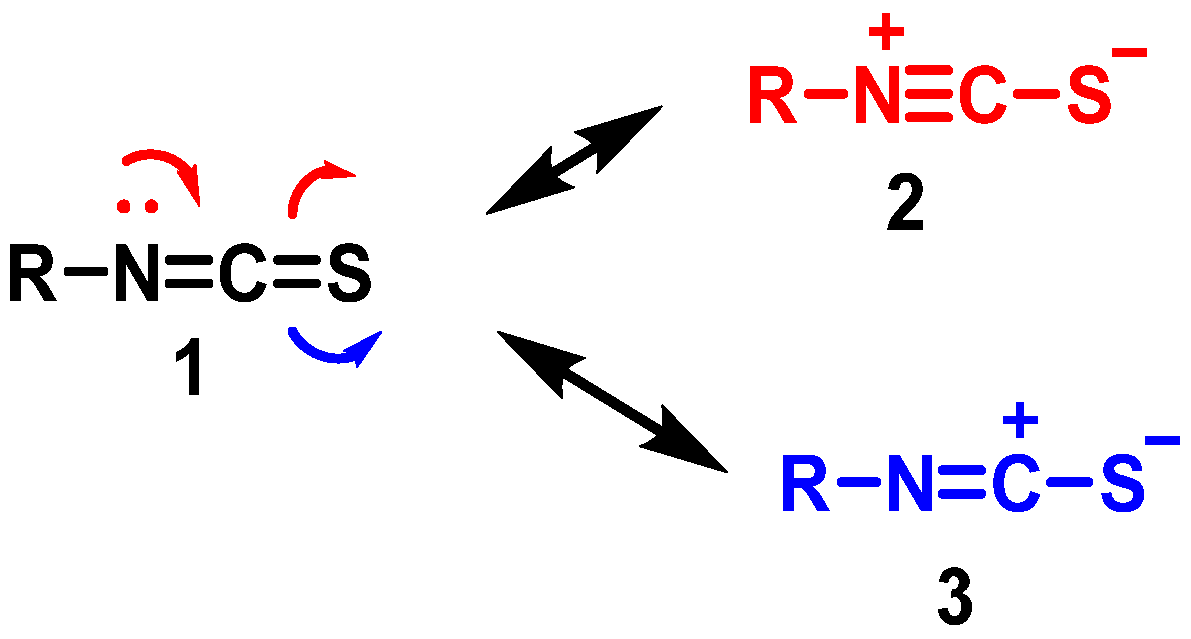
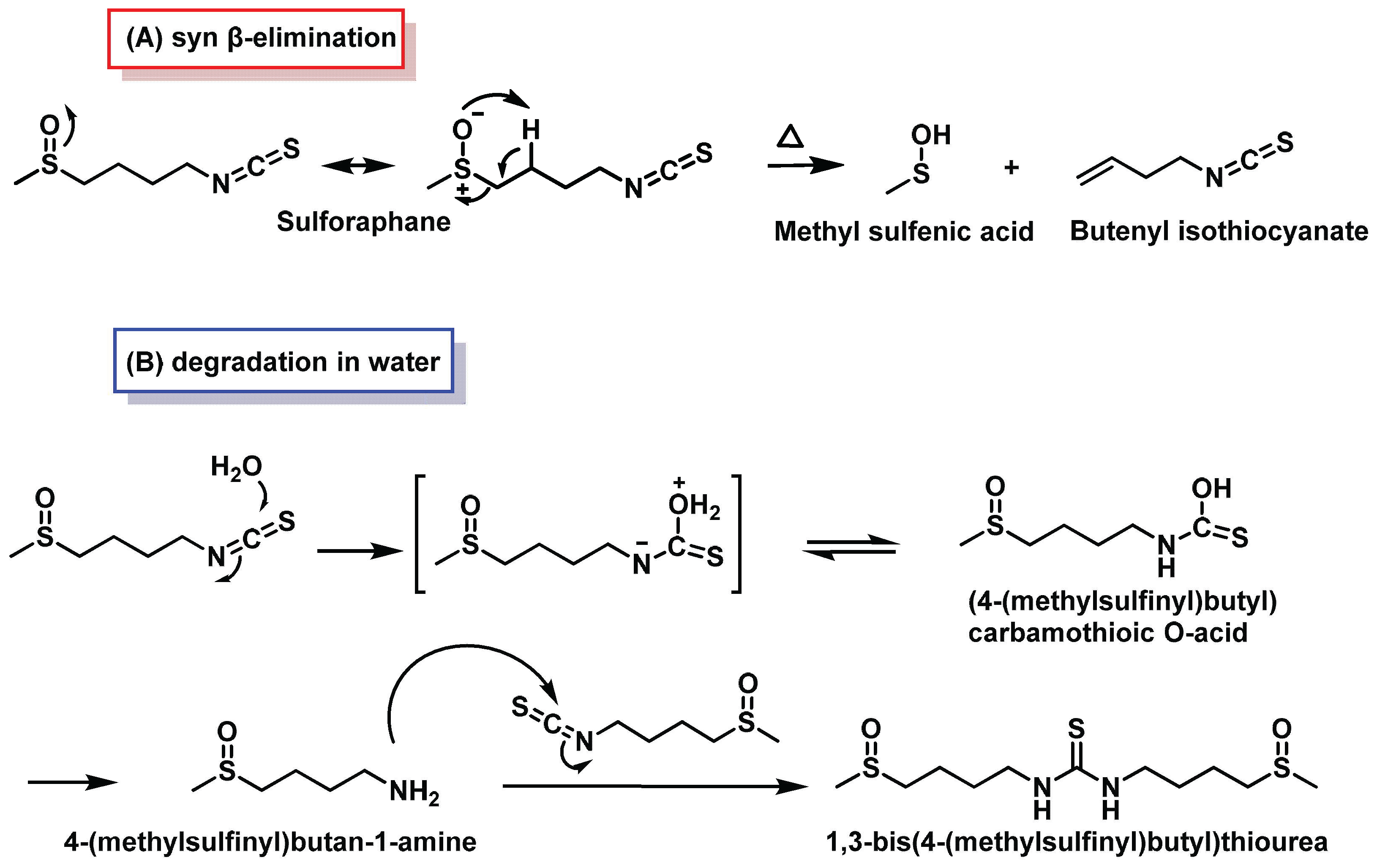
3.1.1. Sulforaphane Reactivity
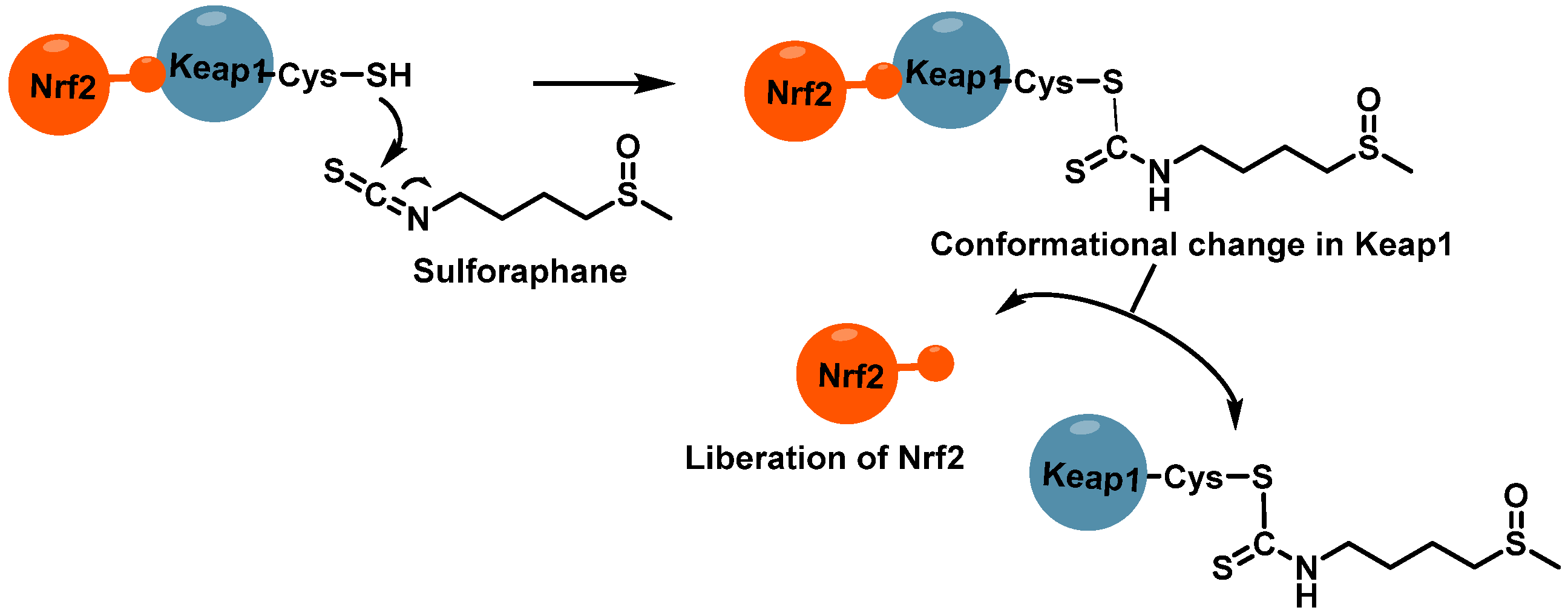
3.1.2. Interaction of Sulforaphane with Keap1
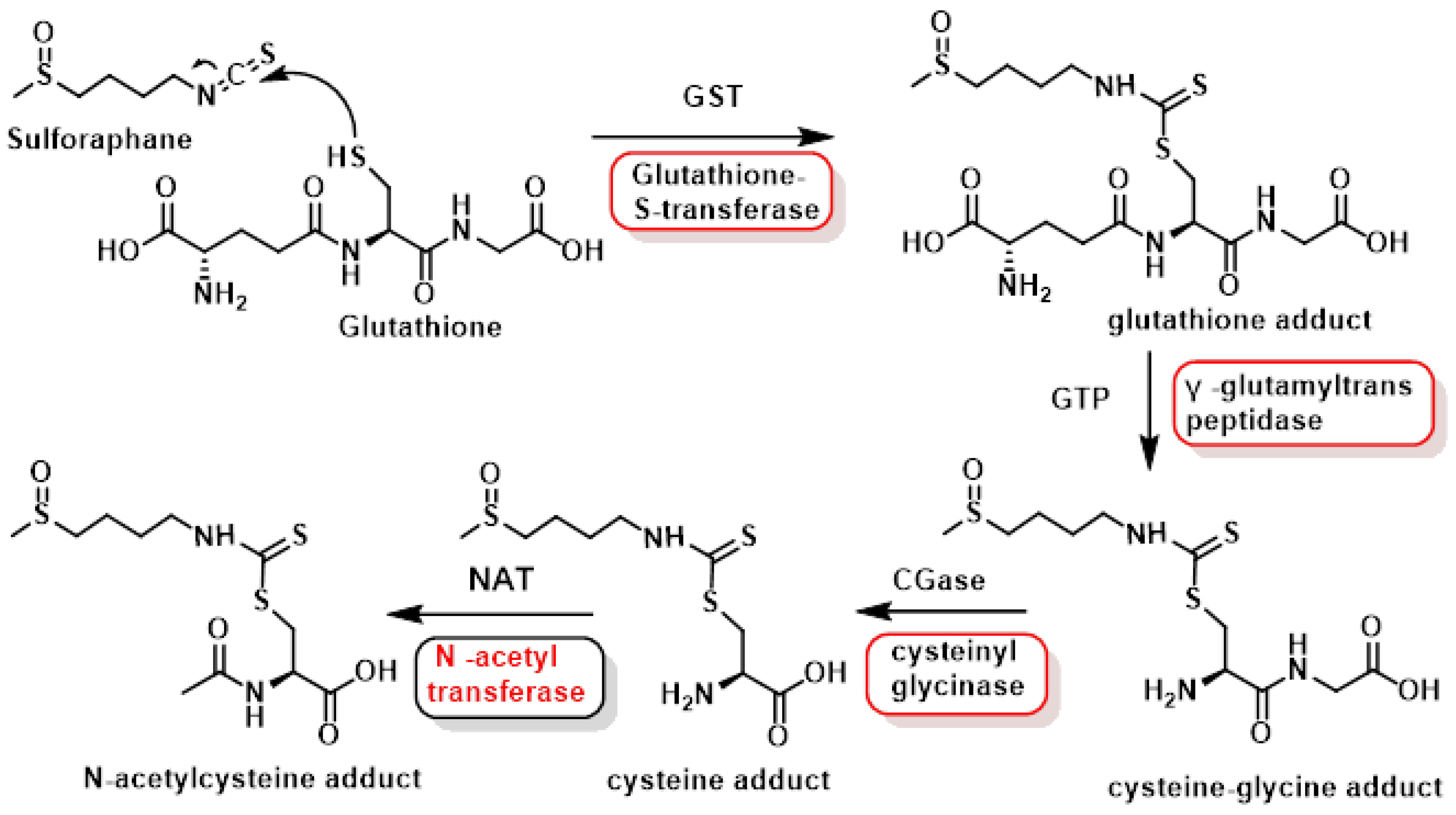
3.1.3. Interaction of Sulforaphane with GSH
3.2. The Risks of Excessive Intake of Sulforaphane
- -
- Gastrointestinal discomfort: Some people report digestive symptoms such as diarrhea, gas or stomach upset after consuming sulforaphane supplements or large amounts of broccoli and other cruciferous vegetables. These symptoms may be more pronounced if the digestive system is not accustomed to the fiber and compounds present in these vegetables. Therefore, sufferers of gastrointestinal disorders, such as irritable bowel syndrome, may experience worsening symptoms due to the fermentation of fiber in the intestinal tract [118].
- -
- Allergic reactions. Although rare, some people may experience allergic reactions to sulforaphane or its food sources. In rare cases, symptoms can range from mild skin rashes to more severe reactions [119].
- -
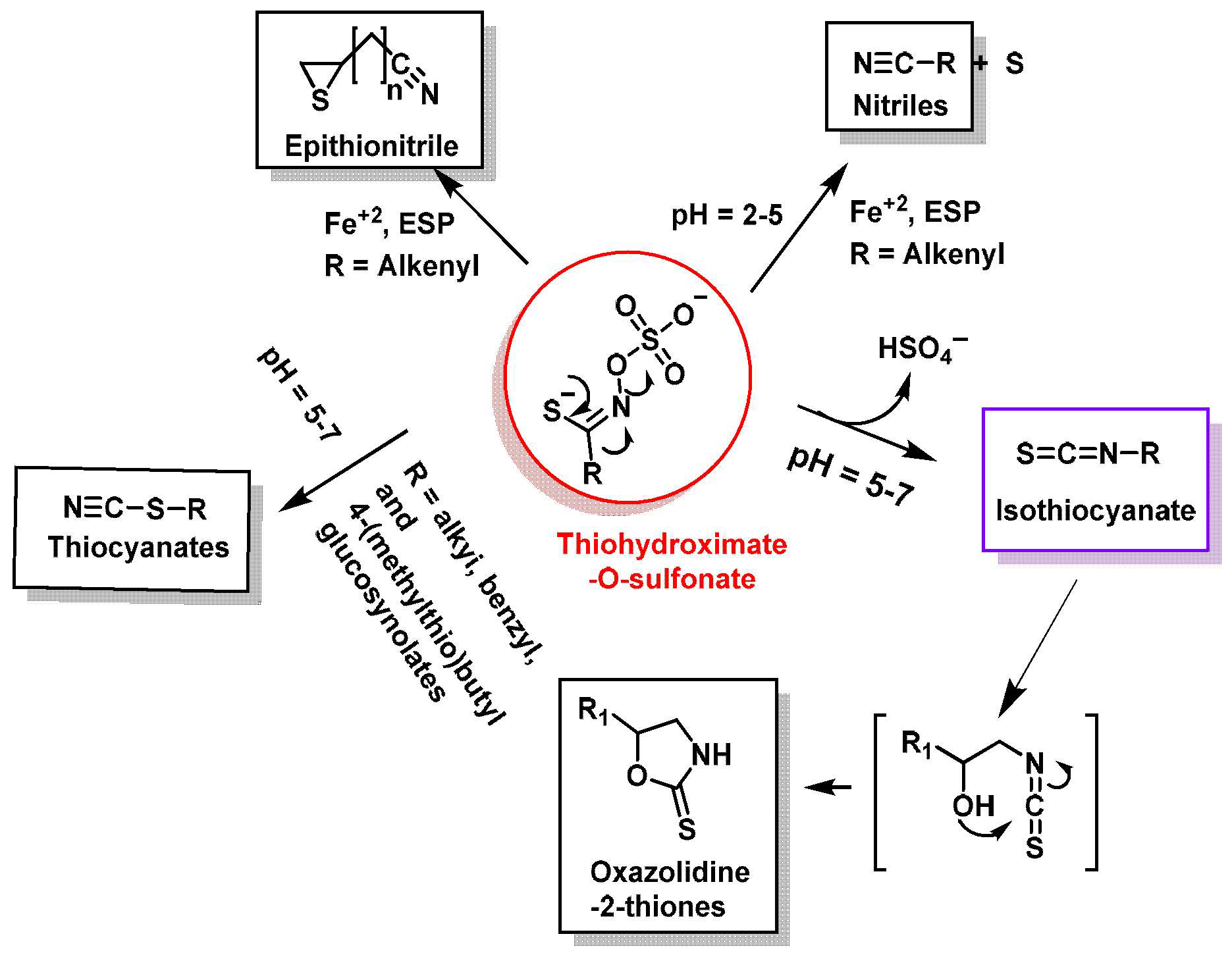
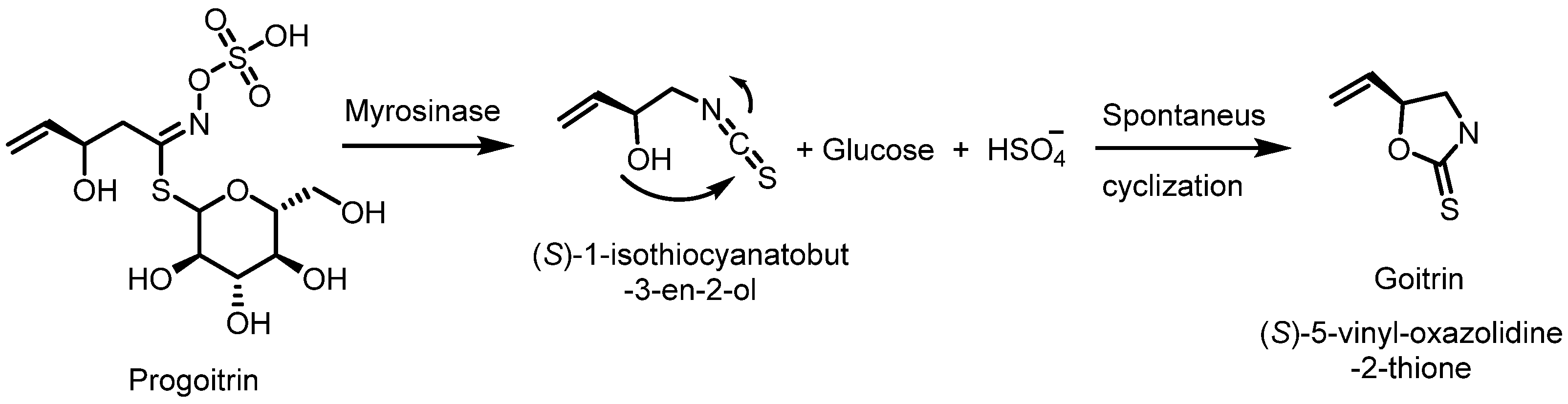
- Thyroid interaction: There are indications that sulforaphane may interfere with thyroid function, especially in people with hypothyroidism. This is due to the goitrogenic compounds present in cruciferous vegetables, which may affect iodine absorption and thus thyroid hormone production. These vegetables also contain 2-hydroxy-3-butenyl glucosinolate (progoitrin) and indole glucosinolate, which can be converted to goitrin and thiocyanate, which act as biocogens in animal models. The conversion can occur either by spontaneous cyclization, as in Figure 16, or activated by the enzyme myrosinase [120,121].
- -
- Potential drug interactions. Sulforaphane potentiates the anticonvulsant efficacy of carbamazepine in a seizure test, indicating possible pharmacokinetic interactions. Sulforaphane may interact with drugs that are metabolized in the liver, particularly those that are substrates of cytochrome P450 enzymes, such as CYP3A4 and CYP1A2. The interaction between SFN and fu-rosemide, verapamil and ketoprofen modifies the activity of the enzyme system involved in drug metabolism and transport. This can lead to altered drug effectiveness and also to the development of multidrug resistance [123].
- -
- Variable effects on tumorigenesis. Several studies with higher doses of sulforaphane in mice describe toxicities that require careful attention to risk-benefit analyses and determination of therapeutic or prophylactic indices. Shorey et al., 2013, observed increased morbidity and no reduction in lung tumorigenesis in offspring born to mothers receiving transplacental and lactational exposure to the carcinogen dibenzo[def,p]chrysene and supplemented with dietary sulforaphane (400 ppm) in contrast to many reports of chemoprotection in adult animal models [124].
- -
- Tao et al., 2018, used a vinyl carbamate chemical carcinogenesis model (A/J mice) and a genetic model (LSL-K-rasG12D/+ mice) to induce lung cancers [125]. In the genetic model, pretreatment with SF had no effect on the number of tumors, but post-treatment increased the number and size of tumors. Kombairaju et al. reported that prolonged treatment with sulforaphane (0.5 mg, 5 days/week for 3 months did not improve tumorigenesis in the same LSL-K-rasG12D/+ murine model [125].
- -
- A toxicity study of SFN supplementation carried out in mouse models showed that ingestion of high doses of sulforaphane produced: marked sedation (at 150–300 mg/kg), hypothermia (at 150–300 mg/kg), impaired motor coordination (at 200–300 mg/kg), decreased skeletal muscle strength (at 250–300 mg/kg) and deaths (at 200–300 mg/kg). In addition, blood analysis showed leukopenia in mice injected with sulforaphane at 200 mg/kg [118].
- -
- Yagishita et al., 2019, provide an excellent review assessing the current state of knowledge on the relationships between formulation (e.g., plants, sprouts, drinks, supplements), bioavailability and efficacy, and the doses of glucoraphanin and/or sulforaphane that have been used in preclinical and clinical studies, paying particular attention to better integration of animal models and clinical studies, particularly with regard to dose selection and route of administration [126].
3.3. Optical Isomers of Sulforaphane
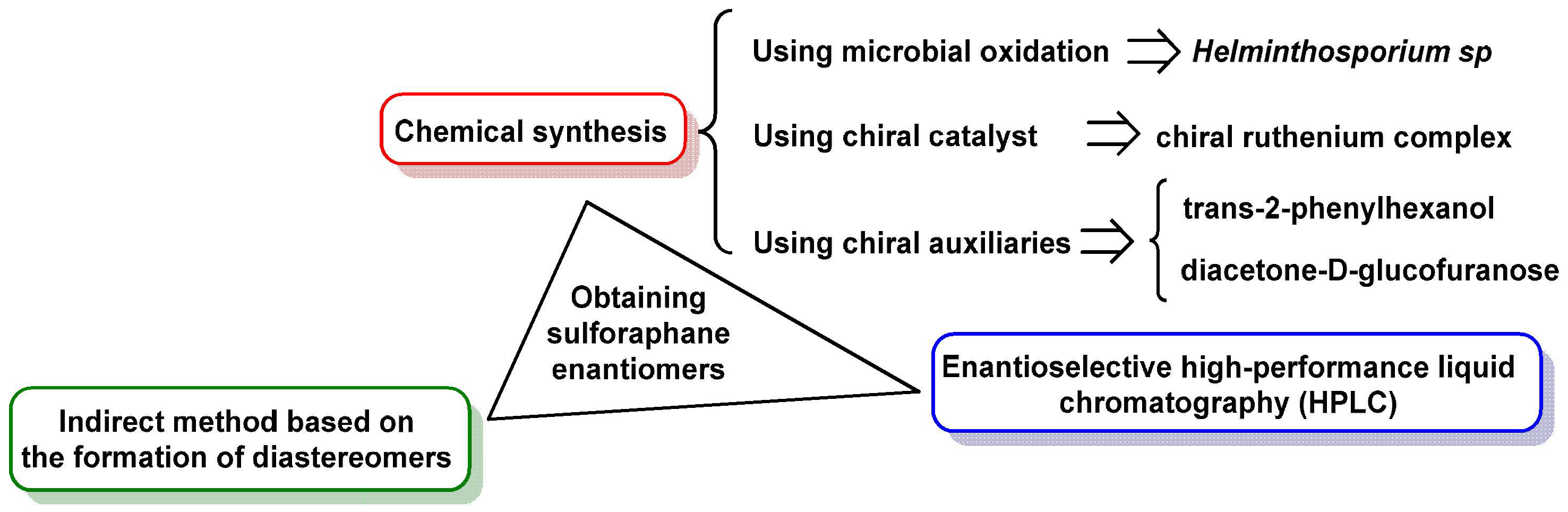
3.3.1. Obtaining Sulforaphane Enantiomers
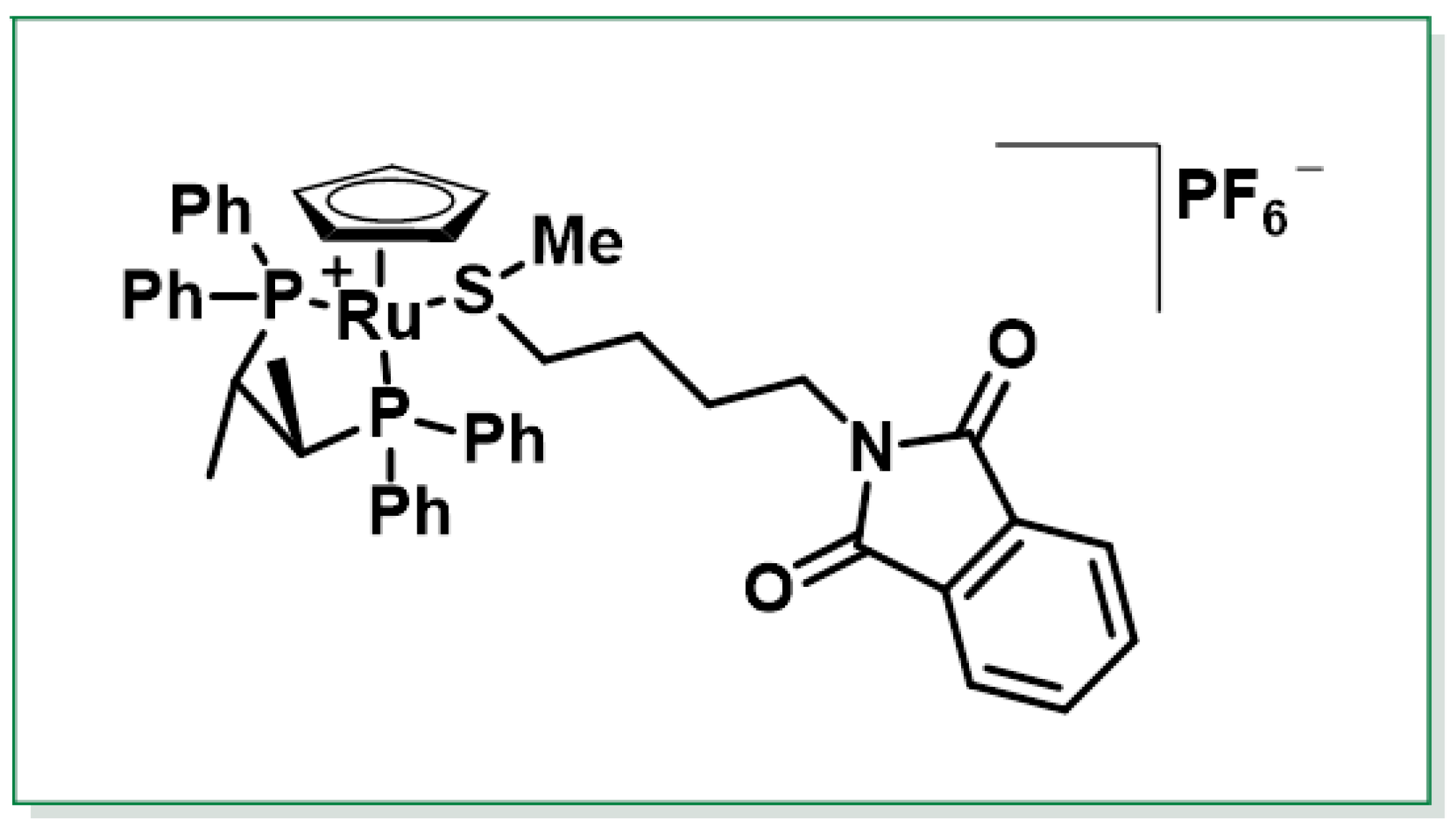
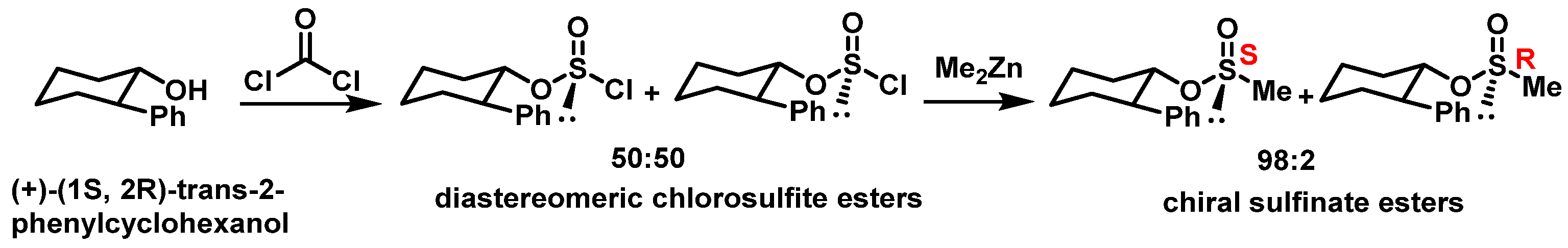
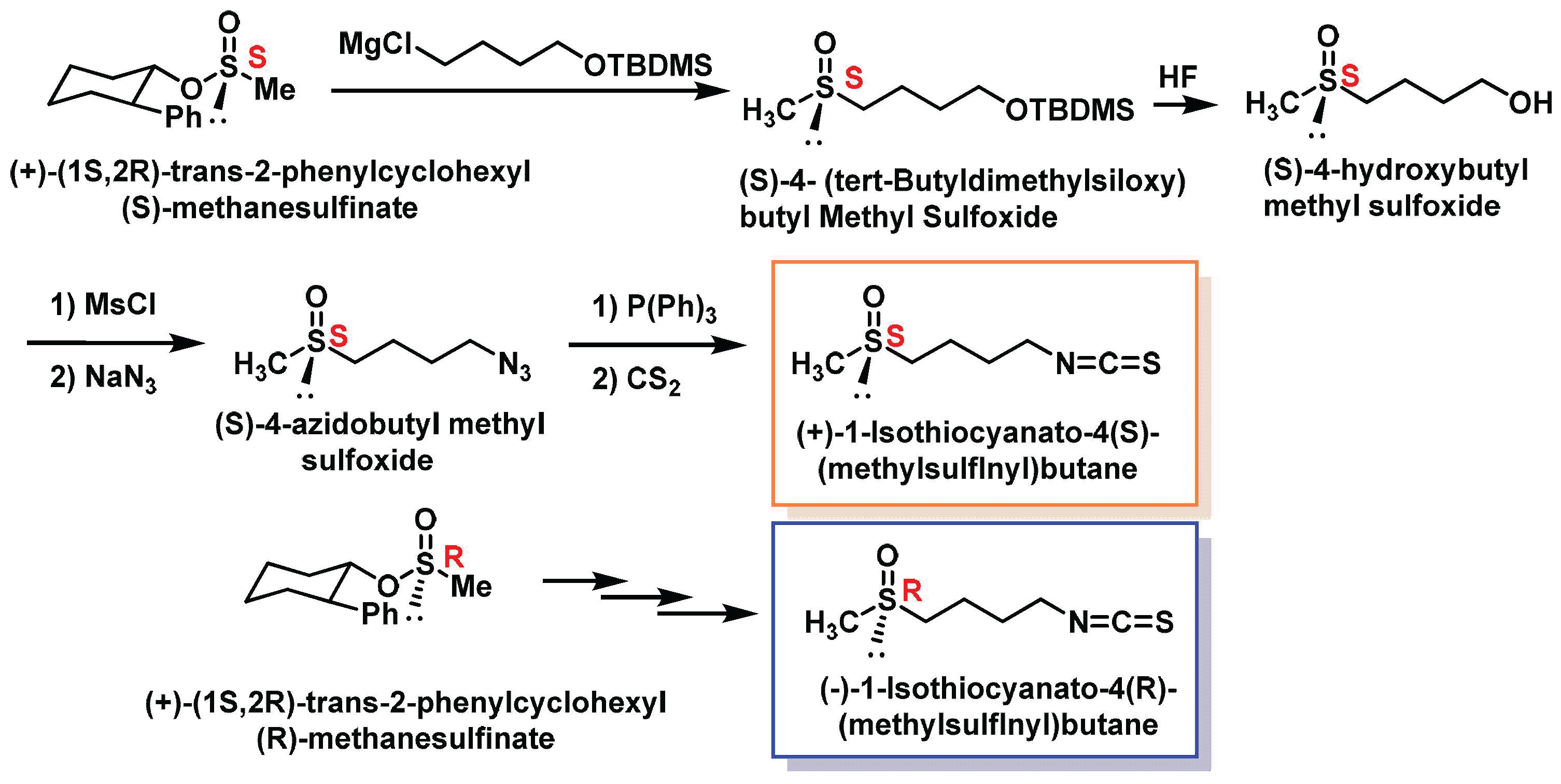
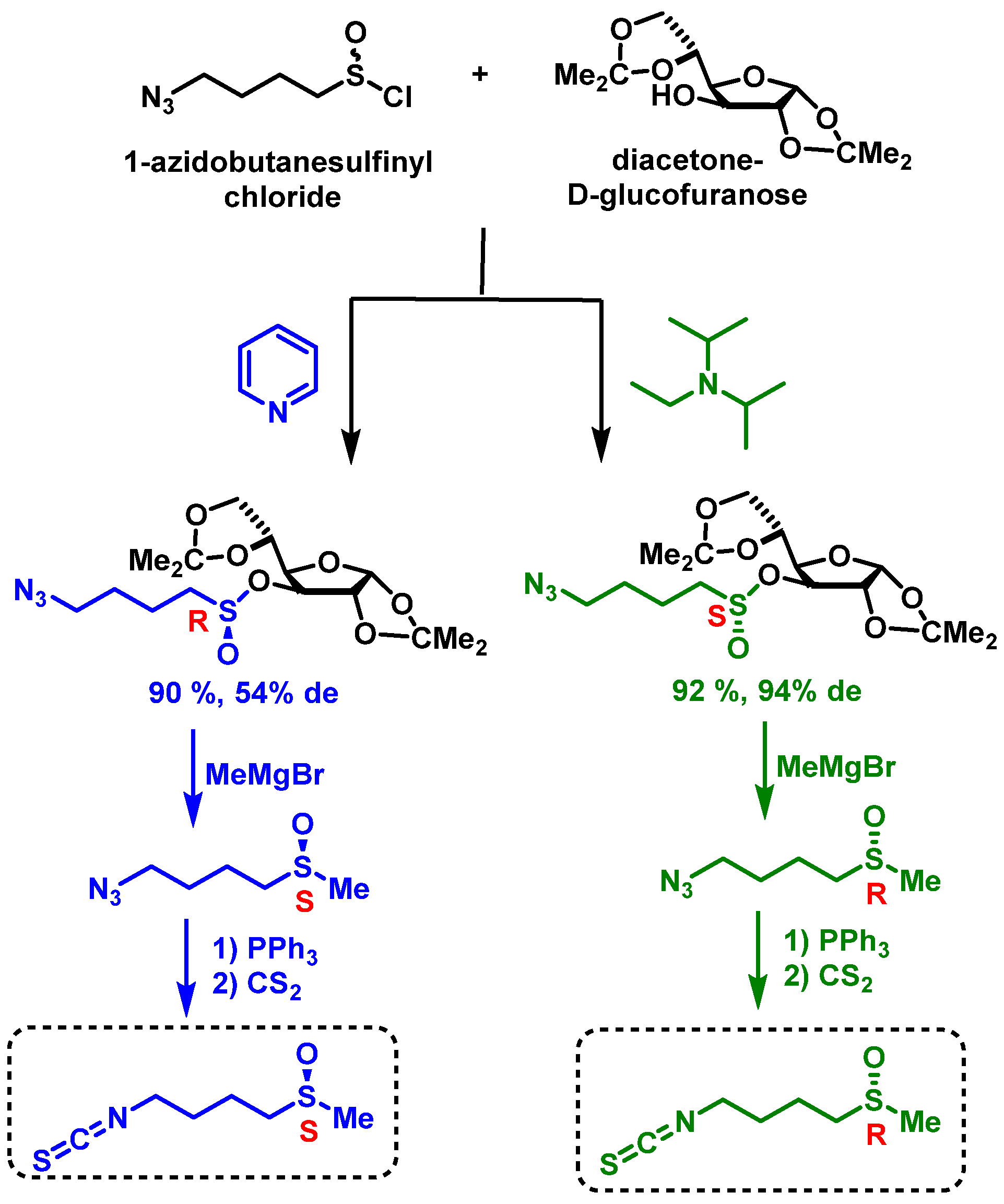
3.3.2. Chemical Synthesis of Sulforaphane Enantiomers
- Reaction of (+)-(1S,2R)-trans-2-phenylcyclohexyl(S)-methanesulfinate with a Grignard reagent derived from 4-chlorobutyl tert-butyldimethylsilyl ether.
- Removal of the tert-butyldimethylsiloxy protective group to yield (S)-4-hydroxybutyl methyl sulfoxide.
- Conversion of the alcohol to a mesylate.
- Reaction with sodium azide to form (S)-4-azidobutyl methylsulfoxide.
- Reaction of the azide with triphenylphosphine to form an iminophosphorane.
- Reaction of the iminophosphorane with carbon disulfide to produce enantiomerically pure (S)-SFN.
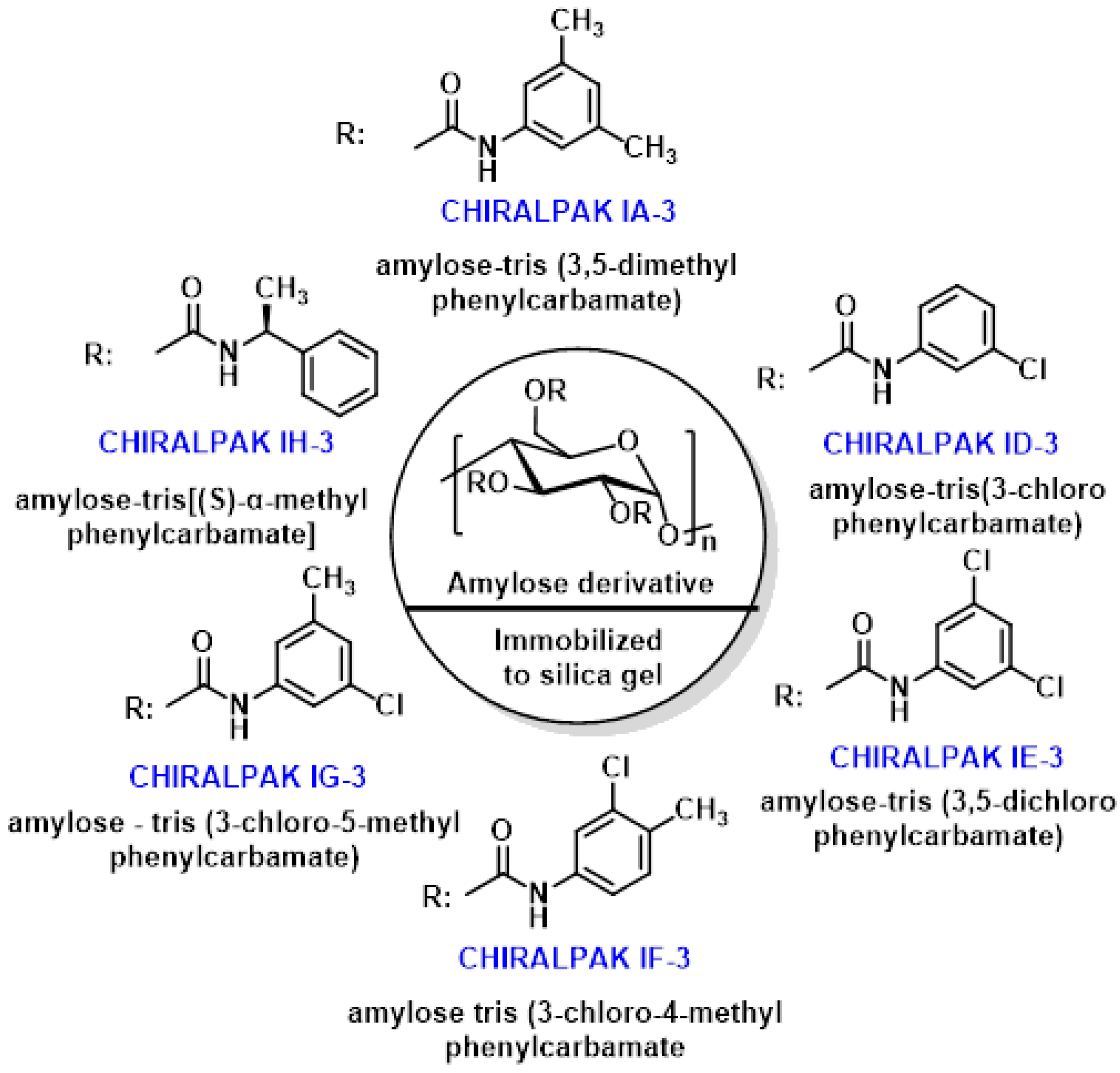
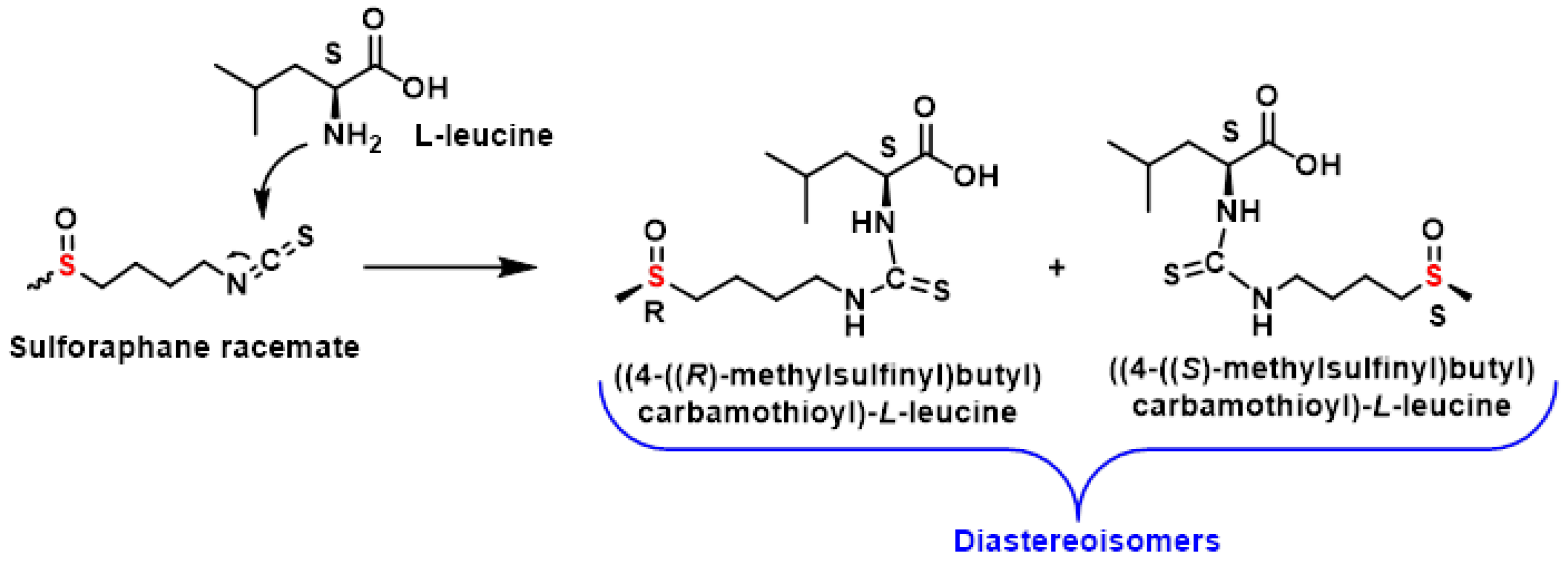
3.3.3. HPLC Separation of Sulforaphane Enantiomers in Broccoli
3.3.4. Importance of Sulfur Chirality in the Biological Activity of SFN
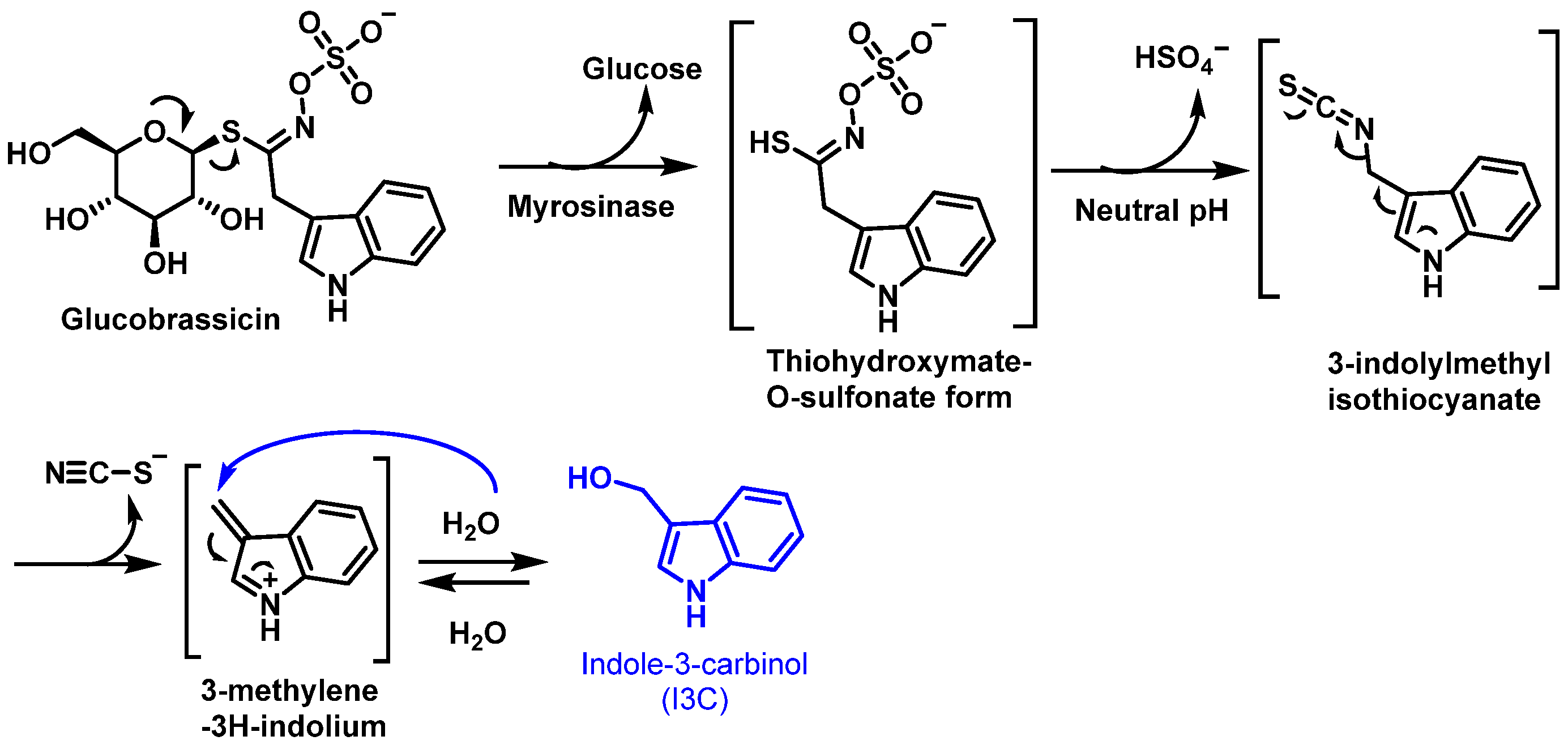
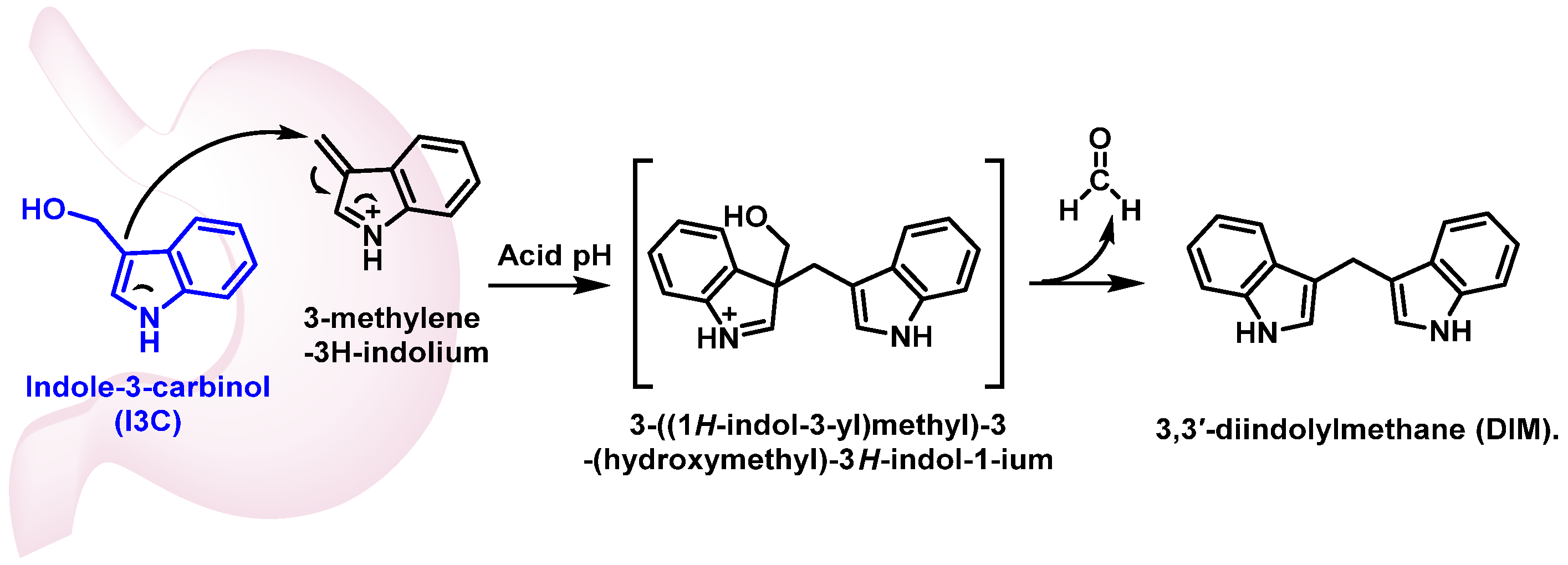
4. Indole-3-Carbinol (I3C)
- Nrf2 activation: I3C positively stimulates the transcription factor Nrf2, which plays a crucial role in cellular defense against oxidative stress. Activation of the Nrf2-ARE signaling pathway by I3C and DIM leads to increased expression of cytoprotective genes, reducing inflammation and oxidative damage [151].
- NF-κB pathway inhibition: DIM’s main target is the NF-κB signaling pathway. By inhibiting this pathway, DIM decreases the production of proinflammatory cytokines such as TNF-α and IL-6, as well as prostaglandins. This results in a reduced inflammatory response [152].
- Modulation of inflammatory mediators: I3C treatment has been shown to decrease the expression of pro-inflammatory factors like IL-1β and IL-6 while increasing anti-inflammatory factors such as IL-4 and IL-10. This modulation of inflammatory mediators contributes to the overall anti-inflammatory effect [153].
- Regulation of microglia: I3C has been found to reduce the number of activated microglia and increase the number of M2-type microglia, which have anti-inflammatory properties. This regulation of microglia populations further contributes to the anti-inflammatory effects of I3C [154].
- Cancer chemoprevention: both I3C and DIM have been extensively studied for their chemopreventive properties against various types of cancer [155].
5. Other Compounds in Broccoli
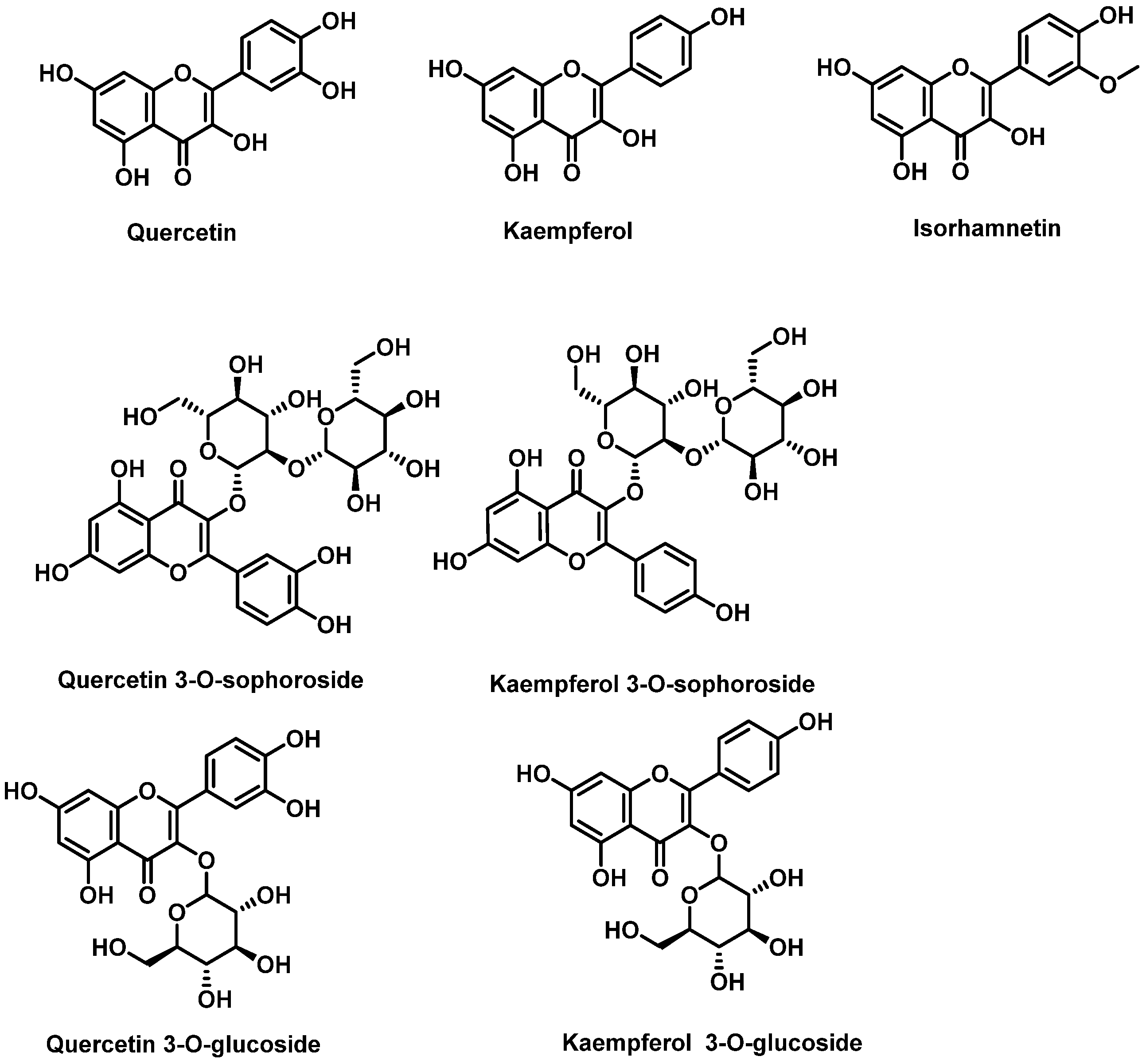
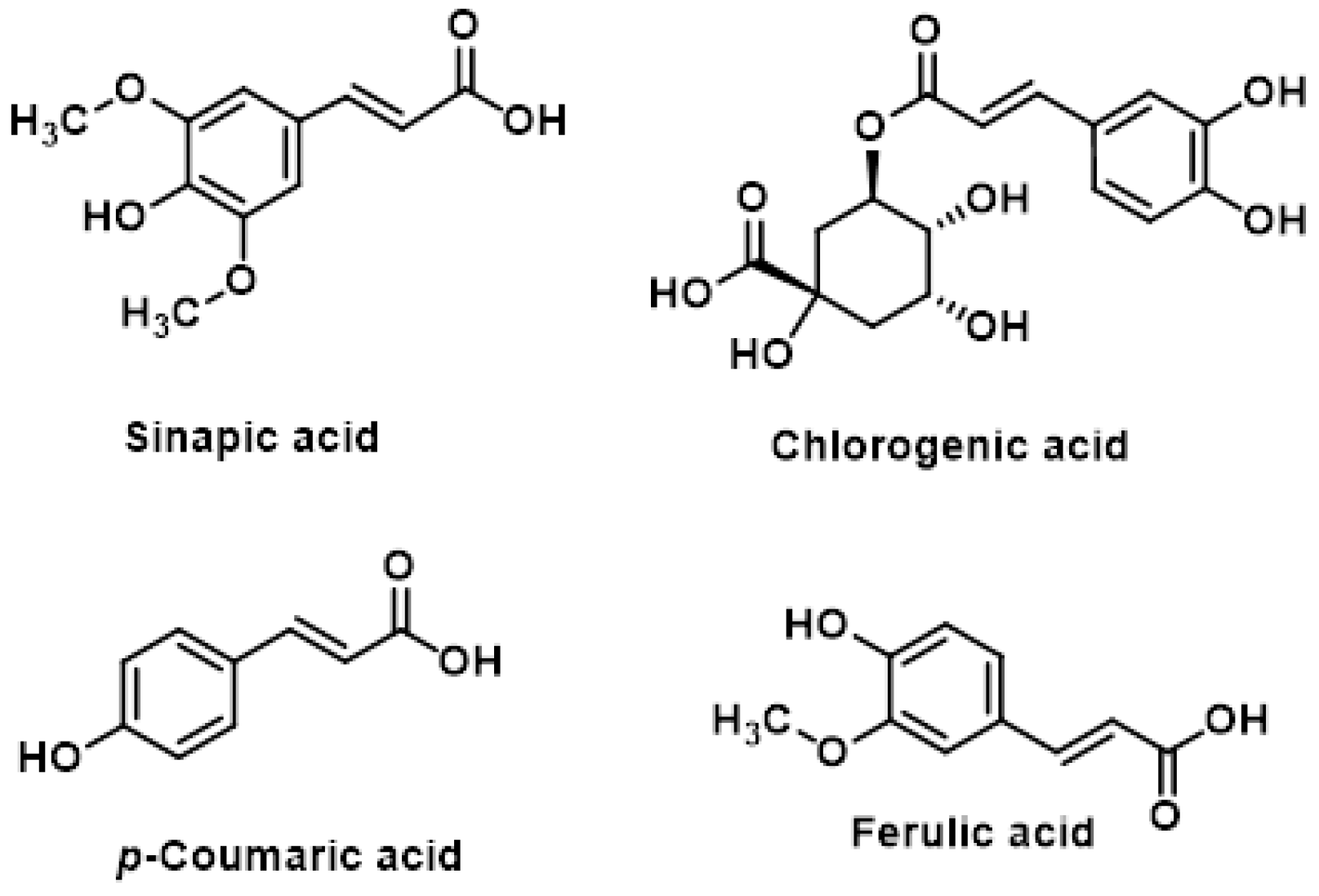
5.1. Polyphenols
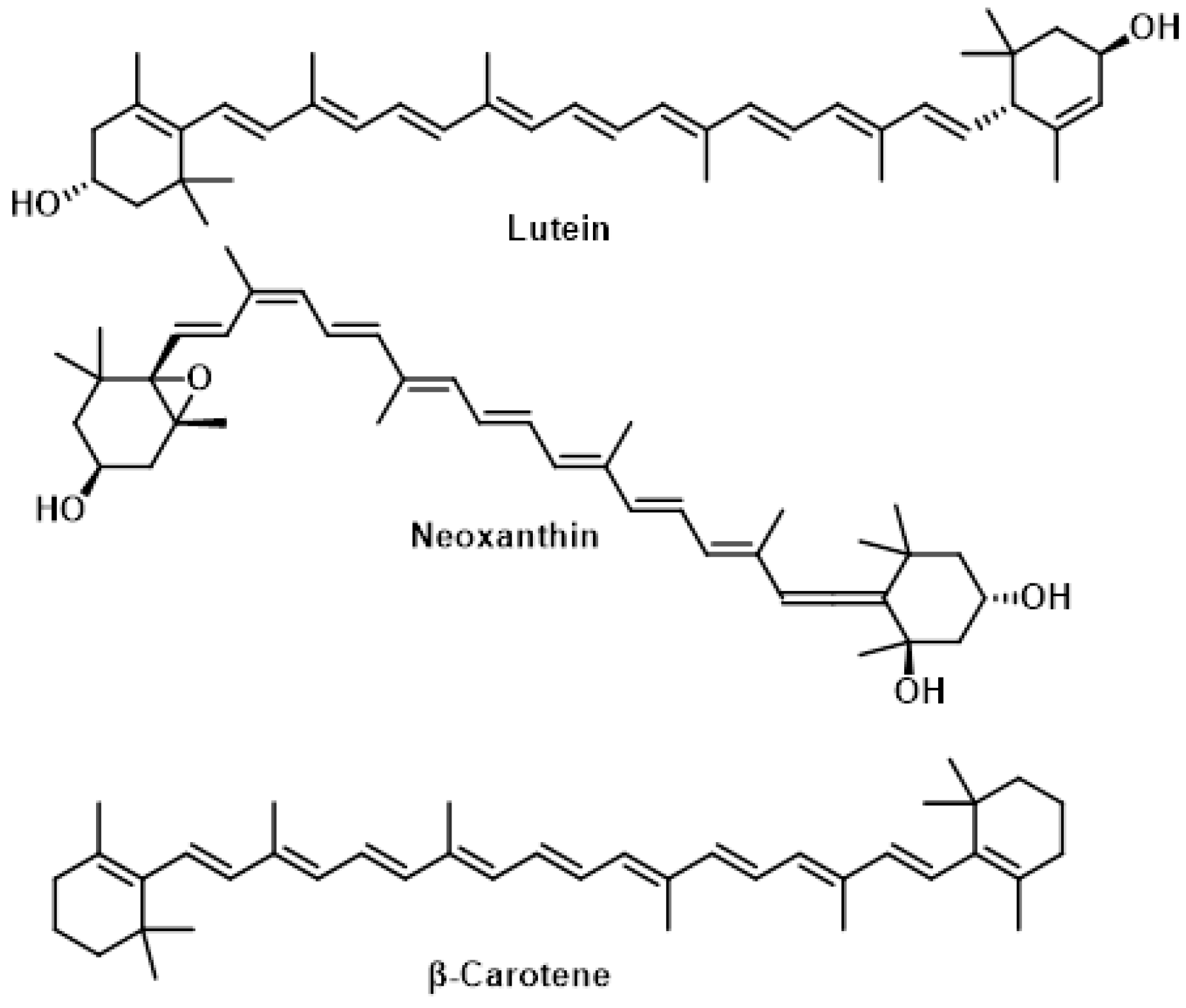
5.2. Carotenoids
- In eye health, lutein and zeaxanthin form the macular pigment in the retina, protecting photoreceptor cells from oxidative stress caused by sunlight exposure. This may help reduce the risk of age-related macular degeneration [169].
- In skin protection, dietary carotenoids accumulate in the skin and offer measurable photo-protective benefits against UV-induced damage. Carotenoids can quench singlet oxygen and scavenge toxic free radicals, preventing or reducing oxidative stress [170].
- Some carotenoids, like β-carotene, serve as precursors to vitamin A, which is essential for various biological processes, including vision [171].
- Florets and leaves, that contain high levels of lutein, neoxanthin, and β-carotene.
- Stems, that contain lutein and neoxanthin, but lack β-carotene.
5.3. Tocopherols
5.4. Vitamin C
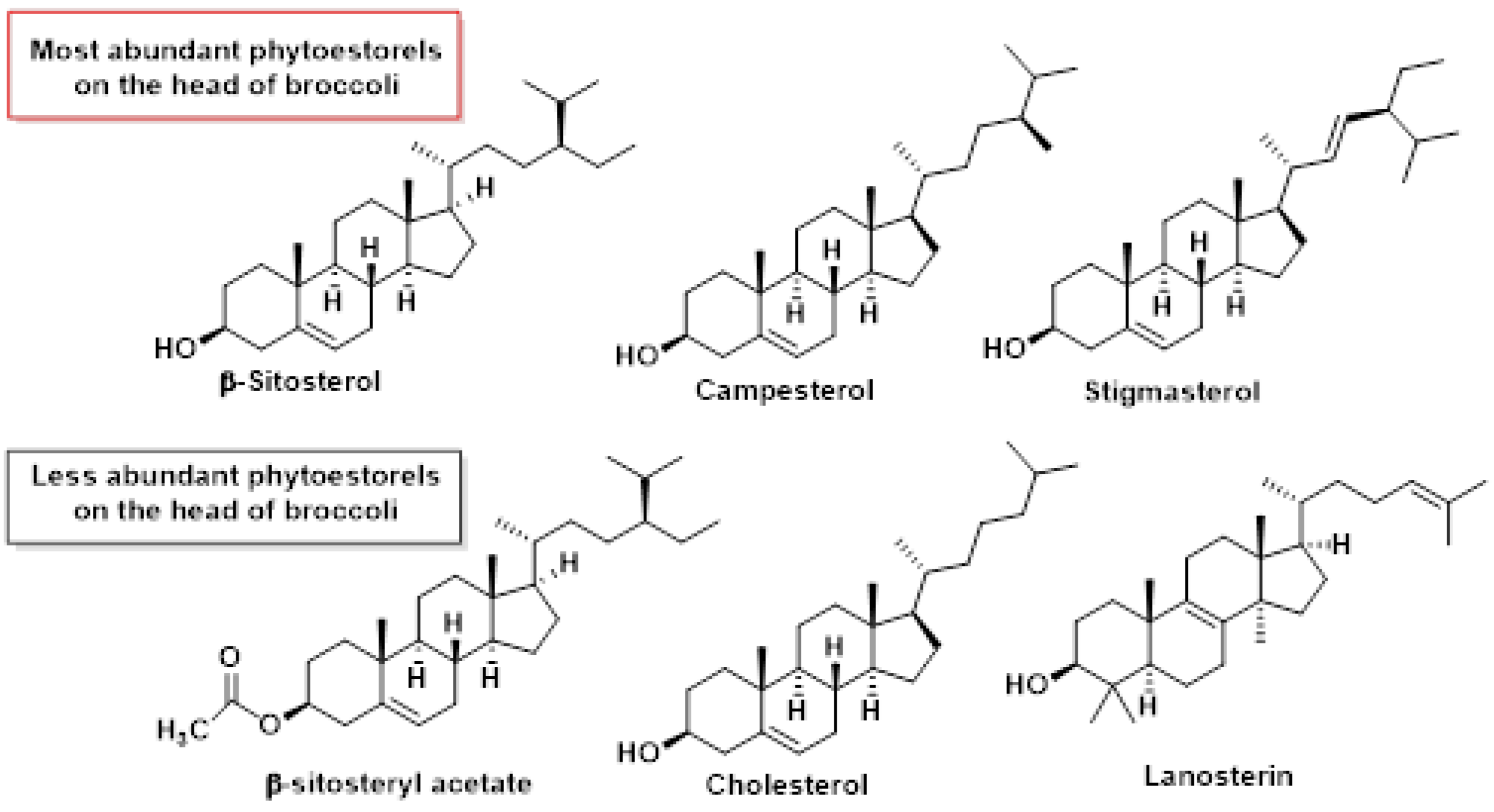
5.5. Phytosterols
6. Antimicrobial Peptides
6.1. Distribution and Sources of Broccoli AMPs
6.2. Mechanisms of Action
6.3. Potential Applications
7. Use of Broccoli By-Products as a Source of Bioactive Products
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Xia, Y.; Liu, H.-Y.; Guo, H.; He, X.-Q.; Liu, Y.; Wu, D.-T.; Mai, Y.-H.; Li, H.-B.; Zou, L.; et al. Nutritional values, beneficial effects, and food applications of broccoli (Brassica oleracea var. italica Plenck). Trends Food Sci. Technol. 2022, 119, 288–308. [Google Scholar] [CrossRef]
- Liu, B.; Tao, Y.; Manickam, S.; Li, D.; Han, Y.; Yu, Y.; Liu, D. Influence of sequential exogenous pretreatment and contact ultrasound-assisted air drying on the metabolic pathway of glucoraphanin in broccoli florets. Ultrason. Sonochem. 2022, 84, 105977. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Dolan, K.D.; Almenar, E. Effect of steamable bag microwaving versus traditional cooking methods on nutritional preservation and physical properties of frozen vegetables: A case study on broccoli (Brassica oleracea). Innov. Food Sci. Emerg. Technol. 2015, 31, 116–122. [Google Scholar] [CrossRef]
- Quizhpe, J.; Ayuso, P.; Rosell, M.d.l.Á.; Peñalver, R.; Nieto, G. Brassica oleracea var italica and Their By-Products as Source of Bioactive Compounds and Food Applications in Bakery Products. Foods 2024, 13, 3513. [Google Scholar] [CrossRef]
- Jacques, P.F.; Lyass, A.; Massaro, J.M.; Vasan, R.S.; D’Agostino, R.B., Sr. Relationship of lycopene intake and consumption of tomato products to incident CVD. Br. J. Nutr. 2013, 110, 545–551. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Kim, J.S.; Cuong, D.M.; Bae, Y.B.; Cho, S.K. Antioxidant and antiproliferative activities of solvent fractions of broccoli (Brassica oleracea L.) sprout. Appl. Biol. Chem. 2022, 65, 34. [Google Scholar] [CrossRef]
- Wang, T.T.; Schoene, N.W.; Milner, J.A.; Kim, Y.S. Broccoli-derived phytochemicals indole-3-carbinol and 3,3′-diindolylmethane exerts concentration-dependent pleiotropic effects on prostate cancer cells: Comparison with other cancer preventive phytochemicals. Mol. Carcinog. 2012, 51, 244–256. [Google Scholar] [CrossRef]
- Kapusta-Duch, J.; Kopeć, A.; Piatkowska, E.; Borczak, B.; Leszczyńska, T. The beneficial effects of Brassica vegetables on human health. Rocz. Panstw. Zakl. Hig. 2012, 63, 389–395. [Google Scholar]
- Le, T.N.; Chiu, C.H.; Hsieh, P.C. Bioactive Compounds and Bioactivities of Brassica oleracea L. var. Italica Sprouts and Microgreens: An Updated Overview from a Nutraceutical Perspective. Plants 2020, 9, 946. [Google Scholar] [CrossRef]
- Tang, G.Y.; Meng, X.; Li, Y.; Zhao, C.N.; Liu, Q.; Li, H.B. Effects of Vegetables on Cardiovascular Diseases and Related Mechanisms. Nutrients 2017, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.M.; Johnson, E.J. Nutrients for the aging eye. Clin. Interv. Aging 2013, 8, 741–748. [Google Scholar] [CrossRef]
- Mahn, A.; Castillo, A. Potential of Sulforaphane as a Natural Immune System Enhancer: A Review. Molecules 2021, 26, 752. [Google Scholar] [CrossRef]
- Akbari, S.; Rasouli-Ghahroudi, A.A. Vitamin K and Bone Metabolism: A Review of the Latest Evidence in Preclinical Studies. Biomed. Res. Int. 2018, 2018, 4629383. [Google Scholar] [CrossRef] [PubMed]
- Syed, R.U.; Moni, S.S.; Break, M.K.B.; Khojali, W.M.A.; Jafar, M.; Alshammari, M.D.; Abdelsalam, K.; Taymour, S.; Alreshidi, K.S.M.; Elhassan Taha, M.M.; et al. Broccoli: A Multi-Faceted Vegetable for Health: An In-Depth Review of Its Nutritional Attributes, Antimicrobial Abilities, and Anti-inflammatory Properties. Antibiotics 2023, 12, 1157. [Google Scholar] [CrossRef]
- Kaczmarek, J.L.; Liu, X.; Charron, C.S.; Novotny, J.A.; Jeffery, E.H.; Seifried, H.E.; Ross, S.A.; Miller, M.J.; Swanson, K.S.; Holscher, H.D. Broccoli consumption affects the human gastrointestinal microbiota. J. Nutr. Biochem. 2019, 63, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Guijarro-Real, C.; Fita, A.; Prohens, J.; Moreno, D.A. Conventional and Innovative Processing in the Stability of Glucosinolates. Nutraceutical Funct. Food Compon. 2022, 411–460. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Ser, S.L.; Cumming, J.R.; Ku, K.M. Comparative Phytonutrient Analysis of Broccoli By-Products: The Potentials for Broccoli By-Product Utilization. Molecules 2018, 23, 900. [Google Scholar] [CrossRef]
- Lv, X.; Meng, G.; Li, W.; Fan, D.; Wang, X.; Espinoza-Pinochet, C.A.; Cespedes-Acuña, C.L. Sulforaphane and its antioxidative effects in broccoli seeds and sprouts of different cultivars. Food Chem. 2020, 316, 126216. [Google Scholar] [CrossRef]
- Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional Ingredients From Brassicaceae Species: Overview and Perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef]
- Gudiño, I.; Casquete, R.; Martín, A.; Wu, Y.; Benito, M.J. Comprehensive Analysis of Bioactive Compounds, Functional Properties, and Applications of Broccoli By-Products. Foods 2024, 13, 3918. [Google Scholar] [CrossRef] [PubMed]
- Ares, A.M.; Nozal, M.J.; Bernal, J. Extraction, chemical characterization and biological activity determination of broccoli health promoting compounds. J. Chromatogr. A 2013, 1313, 78–95. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. A practical guide for designing effective nutraceutical combinations in the form of foods, beverages, and dietary supplements against chronic degenerative diseases. Trends Food Sci. Technol. 2019, 88, 179–193. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef]
- Jaki, B.; Sticher, O.; Veit, M.; Fröhlich, R.; Pauli, G.F. Evaluation of glucoiberin reference material from Iberis amara by spectroscopic fingerprinting. J. Nat. Prod. 2002, 65, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Waser, J.; Watson, W.H. Crystal structure of sinigrin. Nature 1963, 198, 1297–1298. [Google Scholar] [CrossRef]
- Sánchez-Pujante, P.J.; Borja-Martínez, M.; Pedreño, M.; Almagro, L. Biosynthesis and bioactivity of glucosinolates and their production in plant in vitro cultures. Planta 2017, 246, 19–32. [Google Scholar] [CrossRef]
- Moreno, D.A.; Carvajal, M.; López-Berenguer, C.; García-Viguera, C. Chemical and biological characterisation of nutraceutical compounds of broccoli. J. Pharm. Biomed. Anal. 2006, 41, 1508–1522. [Google Scholar] [CrossRef]
- Baldelli, S.; Lombardo, M.; D’Amato, A.; Karav, S.; Tripodi, G.; Aiello, G. Glucosinolates in Human Health: Metabolic Pathways, Bioavailability, and Potential in Chronic Disease Prevention. Foods 2025, 14, 912. [Google Scholar] [CrossRef]
- Uda, Y.; Kurata, T.; Arakawa, N. Effects of pH and ferrous ion on the degradation of glucosinolates by myrosinase. Agric. Biol. Chem. 1986, 50, 2735–2740. [Google Scholar]
- Rungapamestry, V.; Duncan, A.J.; Fuller, Z.; Ratcliffe, B. Changes in glucosinolate concentrations, myrosinase activity, and production of metabolites of glucosinolates in cabbage (Brassica oleracea Var. capitata) cooked for different durations. J. Agric. Food Chem. 2006, 54, 7628–7634. [Google Scholar] [CrossRef] [PubMed]
- Burow, M.; Losansky, A.; Müller, R.; Plock, A.; Kliebenstein, D.J.; Wittstock, U. The genetic basis of constitutive and herbivore-induced ESP-independent nitrile formation in Arabidopsis. Plant Physiol. 2009, 149, 561–574. [Google Scholar] [CrossRef]
- Wittstock, U.; Burow, M. Glucosinolate breakdown in Arabidopsis: Mechanism, regulation and biological significance. In The Arabidopsis Book; BioOne: Washington, DC, USA, 2010; Volume 2010, p. e0134. [Google Scholar] [CrossRef]
- Nguyen, V.P.T.; Stewart, J.; Lopez, M.; Ioannou, I.; Allais, F. Glucosinolates: Natural Occurrence, Biosynthesis, Accessibility, Isolation, Structures, and Biological Activities. Molecules 2020, 25, 4537. [Google Scholar] [CrossRef] [PubMed]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Avato, P.; Argentieri, M. Brassicaceae: A rich source of health improving phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Verhoeven, D.T.; Verhagen, H.; Goldbohm, R.A.; van den Brandt, P.A.; van Poppel, G. A review of mechanisms underlying anticarcinogenicity by brassica vegetables. Chem. Biol. Interact. 1997, 103, 79–129. [Google Scholar] [CrossRef]
- Jeffery, E.H.; Araya, M. Physiological effects of broccoli consumption. Phytochem. Rev. 2009, 8, 283–298. [Google Scholar] [CrossRef]
- Ambrosone, C.B.; McCann, S.E.; Freudenheim, J.L.; Marshall, J.R.; Zhang, Y.; Shields, P.G. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J. Nutr. 2004, 134, 1134–1138. [Google Scholar] [CrossRef]
- Brennan, P.; Hsu, C.C.; Moullan, N.; Szeszenia-Dabrowska, N.; Lissowska, J.; Zaridze, D.; Rudnai, P.; Fabianova, E.; Mates, D.; Bencko, V.; et al. Effect of cruciferous vegetables on lung cancer in patients stratified by genetic status: A mendelian randomisation approach. Lancet 2005, 366, 1558–1560. [Google Scholar] [CrossRef]
- Wang, L.I.; Giovannucci, E.L.; Hunter, D.; Neuberg, D.; Su, L.; Christiani, D.C. Dietary intake of Cruciferous vegetables, Glutathione S-transferase (GST) polymorphisms and lung cancer risk in a Caucasian population. Cancer Causes Control 2004, 15, 977–985. [Google Scholar] [CrossRef]
- Rungapamestry, V.; Duncan, A.J.; Fuller, Z.; Ratcliffe, B. Effect of cooking brassica vegetables on the subsequent hydrolysis and metabolic fate of glucosinolates. Proc. Nutr. Soc. 2007, 66, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, D.T.; Goldbohm, R.A.; van Poppel, G.; Verhagen, H.; van den Brandt, P.A. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol. Biomark. Prev. 1996, 5, 733–748. [Google Scholar]
- Joseph, M.A.; Moysich, K.B.; Freudenheim, J.L.; Shields, P.G.; Bowman, E.D.; Zhang, Y.; Marshall, J.R.; Ambrosone, C.B. Cruciferous vegetables, genetic polymorphisms in glutathione S-transferases M1 and T1, and prostate cancer risk. Nutr. Cancer 2004, 50, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.J.; Probst-Hensch, N.M.; Louie, A.D.; Kau, I.H.; Witte, J.S.; Ingles, S.A.; Frankl, H.D.; Lee, E.R.; Haile, R.W. Glutathione transferase null genotype, broccoli, and lower prevalence of colorectal adenomas. Cancer Epidemiol. Biomark. Prev. 1998, 7, 647–652. [Google Scholar]
- Fowke, J.H.; Chung, F.L.; Jin, F.; Qi, D.; Cai, Q.; Conaway, C.; Cheng, J.R.; Shu, X.O.; Gao, Y.T.; Zheng, W. Urinary isothiocyanate levels, brassica, and human breast cancer. Cancer Res. 2003, 63, 3980–3986. [Google Scholar]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed. Sci. 2014, 64, 48–59. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Bell, L.; Wagstaff, C. Glucosinolates, myrosinase hydrolysis products, and flavonols found in rocket (Eruca sativa and Diplotaxis tenuifolia). J. Agric. Food Chem. 2014, 62, 4481–4492. [Google Scholar] [CrossRef]
- Rollin, P.; Tatibouët, A. Glucosinolates: The synthetic approach. Comptes Rendus Chim. 2011, 14, 194–210. [Google Scholar] [CrossRef]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; De Schrijver, R.; Hansen, M.; Gerhäuser, C.; Mithen, R.; et al. Glucosinolates in Brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009, 53, S219. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, F.; Paredes-Gonzalez, X.; Kong, A.N. Dietary Glucosinolates Sulforaphane, Phenethyl Isothiocyanate, Indole-3-Carbinol/3,3′-Diindolylmethane: Anti-Oxidative Stress/Inflammation, Nrf2, Epigenetics/Epigenomics and In Vivo Cancer Chemopreventive Efficacy. Curr. Pharmacol. Rep. 2015, 1, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Kushad, M.M.; Brown, A.F.; Kurilich, A.C.; Juvik, J.A.; Klein, B.P.; Wallig, M.A.; Jeffery, E.H. Variation of glucosinolates in vegetable crops of Brassica oleracea. J. Agric. Food Chem. 1999, 47, 1541–1548. [Google Scholar] [CrossRef]
- Jaafaru, M.S.; Abd Karim, N.A.; Enas, M.E.; Rollin, P.; Mazzon, E.; Abdull Razis, A.F. Protective Effect of Glucosinolates Hydrolytic Products in Neurodegenerative Diseases (NDDs). Nutrients 2018, 10, 580. [Google Scholar] [CrossRef]
- Jaafaru, M.S.; Abd Karim, N.A.; Mohamed Eliaser, E.; Maitalata Waziri, P.; Ahmed, H.; Mustapha Barau, M.; Kong, L.; Abdull Razis, A.F. Nontoxic Glucomoringin-Isothiocyanate (GMG-ITC) Rich Soluble Extract Induces Apoptosis and Inhibits Proliferation of Human Prostate Adenocarcinoma Cells (PC-3). Nutrients 2018, 10, 1174. [Google Scholar] [CrossRef]
- Fahey, J.W.; Wehage, S.L.; Holtzclaw, W.D.; Kensler, T.W.; Egner, P.A.; Shapiro, T.A.; Talalay, P. Protection of humans by plant glucosinolates: Efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev. Res. 2012, 5, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Sikorska-Zimny, K.; Beneduce, L. The glucosinolates and their bioactive derivatives in Brassica: A review on classification, biosynthesis and content in plant tissues, fate during and after processing, effect on the human organism and interaction with the gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 2544–2571. [Google Scholar] [CrossRef]
- Abdel-Massih, R.M.; Debs, E.; Othman, L.; Attieh, J.; Cabrerizo, F.M. Glucosinolates, a natural chemical arsenal: More to tell than the myrosinase story. Front. Microbiol. 2023, 14, 1130208. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, Y.; Wu, X.; Zhu, Y.; Mupunga, J.; Bao, W.; Huang, J.; Mao, J.; Liu, S.; You, Y. Hydrolysis before Stir-Frying Increases the Isothiocyanate Content of Broccoli. J. Agric. Food Chem. 2018, 66, 1509–1515. [Google Scholar] [CrossRef]
- Sarvan, I.; Verkerk, R.; Dekker, M. Modelling the fate of glucosinolates during thermal processing of Brassica vegetables. LWT-Food Sci. Technol. 2012, 49, 178–183. [Google Scholar] [CrossRef]
- Johnson, I.T. Glucosinolates: Bioavailability and importance to health. Int. J. Vitam. Nutr. Res. 2002, 72, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.; Klöpping-Ketelaars, I.W.; van den Berg, R.; Vaes, W.H. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J. Agric. Food Chem. 2008, 56, 10505–10509. [Google Scholar] [CrossRef]
- Conaway, C.C.; Getahun, S.M.; Liebes, L.L.; Pusateri, D.J.; Topham, D.K.; Botero-Omary, M.; Chung, F.L. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr. Cancer 2000, 38, 168–178. [Google Scholar] [CrossRef]
- Fahey, J.W.; Holtzclaw, W.D.; Wehage, S.L.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Sulforaphane Bioavailability from Glucoraphanin-Rich Broccoli: Control by Active Endogenous Myrosinase. PLoS ONE 2015, 10, e0140963. [Google Scholar] [CrossRef] [PubMed]
- Ghawi, S.K.; Methven, L.; Niranjan, K. The potential to intensify sulforaphane formation in cooked broccoli (Brassica oleracea var. italica) using mustard seeds (Sinapis alba). Food Chem. 2013, 138, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Kitainda, V.; Jez, J.M. Structural Studies of Aliphatic Glucosinolate Chain-Elongation Enzymes. Antioxidants 2021, 10, 1500. [Google Scholar] [CrossRef]
- Grubb, C.D.; Abel, S. Glucosinolate metabolism and its control. Trends Plant Sci. 2006, 11, 89–100. [Google Scholar] [CrossRef]
- Bednarek, P.; Pislewska-Bednarek, M.; Svatos, A.; Schneider, B.; Doubsky, J.; Mansurova, M.; Humphry, M.; Consonni, C.; Panstruga, R.; Sanchez-Vallet, A.; et al. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 2009, 323, 101–106. [Google Scholar] [CrossRef]
- Schlaeppi, K.; Bodenhausen, N.; Buchala, A.; Mauch, F.; Reymond, P. The glutathione-deficient mutant pad2-1 accumulates lower amounts of glucosinolates and is more susceptible to the insect herbivore Spodoptera littoralis. Plant J. 2008, 55, 774–786. [Google Scholar] [CrossRef]
- Piotrowski, M.; Schemenewitz, A.; Lopukhina, A.; Müller, A.; Janowitz, T.; Weiler, E.W.; Oecking, C. Desulfoglucosinolate sulfotransferases from Arabidopsis thaliana catalyze the final step in the biosynthesis of the glucosinolate core structure. J. Biol. Chem. 2004, 279, 50717–50725. [Google Scholar] [CrossRef]
- Grubb, C.D.; Zipp, B.J.; Ludwig-Müller, J.; Masuno, M.N.; Molinski, T.F.; Abel, S. Arabidopsis glucosyltransferase UGT74B1 functions in glucosinolate biosynthesis and auxin homeostasis. Plant J. 2004, 40, 893–908. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, R.J.; van Dam, N.M.; van Loon, J.J. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Saito, S.; Teramoto, T.; Maruyama-Nakashita, A.; Kakuta, Y. Crystal structure of Arabidopsis thaliana sulfotransferase SOT16 involved in glucosinolate biosynthesis. Biochem. Biophys. Res. Commun. 2023, 677, 149–154. [Google Scholar] [CrossRef]
- Prieto, M.A.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular structure, breakdown, genetic, bioavailability, properties and healthy and adverse effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar] [CrossRef]
- Wittstock, U.; Kurzbach, E.; Herfurth, A.-M.; Stauber, E. Glucosinolate breakdown. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2016; Volume 80, pp. 125–169. [Google Scholar]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane: Translational research from laboratory bench to clinic. Nutr. Rev. 2013, 71, 709–726. [Google Scholar] [CrossRef]
- Houghton, C.A. Sulforaphane: Its “Coming of Age” as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease. Oxid. Med. Cell. Longev. 2019, 2019, 2716870. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, X.; Zhao, S.; Ma, C.; Cui, J.; Zheng, Y. Sulforaphane protects against cardiovascular disease via Nrf2 activation. Oxid. Med. Cell. Longev. 2015, 2015, 407580. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, P.; Wang, Q.; Sun, F.; Liu, F. Sulforaphane Attenuates H2O2-induced Oxidant Stress in Human Trabecular Meshwork Cells (HTMCs) via the Phosphatidylinositol 3-Kinase (PI3K)/Serine/Threonine Kinase (Akt)-Mediated Factor-E2-Related Factor 2 (Nrf2) Signaling Activation. Med. Sci. Monit. 2019, 25, 811–818. [Google Scholar] [CrossRef]
- Greaney, A.J.; Maier, N.K.; Leppla, S.H.; Moayeri, M. Sulforaphane inhibits multiple inflammasomes through an Nrf2-independent mechanism. J. Leukoc. Biol. 2016, 99, 189–199. [Google Scholar] [CrossRef]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician’s Expectation Be Matched by the Reality? Oxid. Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef]
- Johansson, N.L.; Pavia, C.S.; Chiao, J.W. Growth inhibition of a spectrum of bacterial and fungal pathogens by sulforaphane, an isothiocyanate product found in broccoli and other cruciferous vegetables. Planta Med. 2008, 74, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.E.; Baniasadi, M.; Giansiracusa, D.; Giansiracusa, M.; Garcia, M.; Fryda, Z.; Wong, T.L.; Bishayee, A. Sulforaphane: A Broccoli Bioactive Phytocompound with Cancer Preventive Potential. Cancers 2021, 13, 4796. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, B.; Li, Y.; Yuan, Q. Potential mechanisms of cancer prevention and treatment by sulforaphane, a natural small molecule compound of plant-derived. Mol. Med. 2024, 30, 94. [Google Scholar] [CrossRef]
- Li, Z.; Guo, H.; Li, J.; Ma, T.; Zhou, S.; Zhang, Z.; Miao, L.; Cai, L. Sulforaphane prevents type 2 diabetes-induced nephropathy via AMPK-mediated activation of lipid metabolic pathways and Nrf2 antioxidative function. Clin. Sci. 2020, 134, 2469–2487. [Google Scholar] [CrossRef] [PubMed]
- Men, X.; Han, X.; Lee, S.J.; Oh, G.; Park, K.T.; Han, J.K.; Choi, S.I.; Lee, O.H. Anti-Obesogenic Effects of Sulforaphane-Rich Broccoli (Brassica oleracea var. italica) Sprouts and Myrosinase-Rich Mustard (Sinapis alba L.) Seeds In Vitro and In Vivo. Nutrients 2022, 14, 3814. [Google Scholar] [CrossRef]
- Men, X.; Han, X.; Lee, S.J.; Park, K.T.; Han, J.K.; Choi, S.I.; Lee, O.H. Anti-adipogenic Effects of Sulforaphane-rich Ingredient with Broccoli Sprout and Mustard Seed in 3T3-L1 Preadipocytes. Planta Med. 2023, 89, 526–538. [Google Scholar] [CrossRef]
- Bricker, G.V.; Riedl, K.M.; Ralston, R.A.; Tober, K.L.; Oberyszyn, T.M.; Schwartz, S.J. Isothiocyanate metabolism, distribution, and interconversion in mice following consumption of thermally processed broccoli sprouts or purified sulforaphane. Mol. Nutr. Food Res. 2014, 58, 1991–2000. [Google Scholar] [CrossRef]
- Fimognari, C.; Hrelia, P. Sulforaphane as a promising molecule for fighting cancer. Mutat. Res. 2007, 635, 90–104. [Google Scholar] [CrossRef]
- Kelloff, G.J.; Crowell, J.A.; Steele, V.E.; Lubet, R.A.; Malone, W.A.; Boone, C.W.; Kopelovich, L.; Hawk, E.T.; Lieberman, R.; Lawrence, J.A.; et al. Progress in cancer chemoprevention: Development of diet-derived chemopreventive agents. J. Nutr. 2000, 130, 467s–471s. [Google Scholar] [CrossRef]
- Tian, S.; Li, X.; Wang, Y.; Lu, Y. The protective effect of sulforaphane on type II diabetes induced by high-fat diet and low-dosage streptozotocin. Food Sci. Nutr. 2021, 9, 747–756. [Google Scholar] [CrossRef]
- Li, Y.P.; Wang, S.L.; Liu, B.; Tang, L.; Kuang, R.R.; Wang, X.B.; Zhao, C.; Song, X.D.; Cao, X.M.; Wu, X.; et al. Sulforaphane prevents rat cardiomyocytes from hypoxia/reoxygenation injury in vitro via activating SIRT1 and subsequently inhibiting ER stress. Acta Pharmacol. Sin. 2016, 37, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.D.; Dashwood, R.H.; Ho, E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008, 269, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, J.; Olejnik, A. Sulforaphane—A possible agent in prevention and therapy of cancer. Postep. Hig. Med. Dosw. 2010, 64, 590–603. [Google Scholar]
- Jiang, X.; Liu, Y.; Ma, L.; Ji, R.; Qu, Y.; Xin, Y.; Lv, G. Chemopreventive activity of sulforaphane. Drug Des. Devel Ther. 2018, 12, 2905–2913. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Diorio, G.; Sexton, W.; Schell, M.; Alexandrow, M.; Fahey, J.W.; Kumar, N.B. Sulforaphane for the chemoprevention of bladder cancer: Molecular mechanism targeted approach. Oncotarget 2017, 8, 35412–35424. [Google Scholar] [CrossRef]
- Janczewski, Ł. Sulforaphane and its bifunctional analogs: Synthesis and biological activity. Molecules 2022, 27, 1750. [Google Scholar] [CrossRef]
- Perschke, W. On the structure of the triatomic radicals of rhodan hydrogen and nitrogen hydroacid. Ber. Dtsch. Chem. Ges. 1929, 62, 3054–3056. [Google Scholar] [CrossRef]
- Cubbage, J.W.; Guo, Y.; McCulla, R.D.; Jenks, W.S. Thermolysis of alkyl sulfoxides and derivatives: A comparison of experiment and theory. J. Org. Chem. 2001, 66, 8722–8736. [Google Scholar] [CrossRef]
- Dubois, J.; Marchal, A.; Lacroix, D.; Cabou, J. Sulforaphane Stabilization. U.S. Patent No. 9,254,331, 2 September 2016. [Google Scholar]
- Drobnica, Ľ.; Kristian, P.; Augustin, J. The chemistry of the—NCS group. Cyanates Their Thio Deriv. 1977, 2, 1003–1221. [Google Scholar]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Keum, Y.S. Regulation of the Keap1/Nrf2 system by chemopreventive sulforaphane: Implications of posttranslational modifications. Ann. N. Y. Acad. Sci. 2011, 1229, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Freeman, M.L.; Liebler, D.C. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem. Res. Toxicol. 2005, 18, 1917–1926. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Eggler, A.L.; Mesecar, A.D.; van Breemen, R.B. Modification of keap1 cysteine residues by sulforaphane. Chem. Res. Toxicol. 2011, 24, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, S.E.; Melton, T.F.; Olson, E.R.; Zhang, J.; Saboda, K.; Bowden, G.T. Inhibition of activator protein-1 by sulforaphane involves interaction with cysteine in the cFos DNA-binding domain: Implications for chemoprevention of UVB-induced skin cancer. Cancer Res. 2009, 69, 7103–7110. [Google Scholar] [CrossRef]
- Li, S.; Dina Kuo, H.-C.; Wang, L.; Wu, R.; Sargsyan, D.; Kong, A.-N. UVB drives metabolic rewiring and epigenetic reprograming and protection by sulforaphane in human skin keratinocytes. Chem. Res. Toxicol. 2022, 35, 1220–1233. [Google Scholar] [CrossRef]
- Hoch, C.C.; Shoykhet, M.; Weiser, T.; Griesbaum, L.; Petry, J.; Hachani, K.; Multhoff, G.; Bashiri Dezfouli, A.; Wollenberg, B. Isothiocyanates in medicine: A comprehensive review on phenylethyl-, allyl-, and benzyl-isothiocyanates. Pharmacol. Res. 2024, 201, 107107. [Google Scholar] [CrossRef]
- Rajendran, P.; Williams, D.E.; Ho, E.; Dashwood, R.H. Metabolism as a key to histone deacetylase inhibition. Crit. Rev. Biochem. Mol. Biol. 2011, 46, 181–199. [Google Scholar] [CrossRef]
- Kim, B.R.; Hu, R.; Keum, Y.S.; Hebbar, V.; Shen, G.; Nair, S.S.; Kong, A.N. Effects of glutathione on antioxidant response element-mediated gene expression and apoptosis elicited by sulforaphane. Cancer Res. 2003, 63, 7520–7525. [Google Scholar]
- Otoo, R.A.; Allen, A.R. Sulforaphane’s Multifaceted Potential: From Neuroprotection to Anticancer Action. Molecules 2023, 28, 6902. [Google Scholar] [CrossRef]
- Clarke, J.D.; Hsu, A.; Williams, D.E.; Dashwood, R.H.; Stevens, J.F.; Yamamoto, M.; Ho, E. Metabolism and tissue distribution of sulforaphane in Nrf2 knockout and wild-type mice. Pharm. Res. 2011, 28, 3171–3179. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Angeloni, C.; Malaguti, M.; Morroni, F.; Hrelia, S.; Hrelia, P. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013, 2013, 415078. [Google Scholar] [CrossRef] [PubMed]
- Yepes-Molina, L.; Carvajal, M. Nanoencapsulation of sulforaphane in broccoli membrane vesicles and their in vitro antiproliferative activity. Pharm. Biol. 2021, 59, 1490–1504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, W.; Zhao, Y.; Peng, R.; Zhang, Z.; Xu, Z.; Simal-Gandara, J.; Yang, H.; Deng, J. Bioactive sulforaphane from cruciferous vegetables: Advances in biosynthesis, metabolism, bioavailability, delivery, health benefits, and applications. Crit. Rev. Food Sci. Nutr. 2024, 2024, 2354937. [Google Scholar] [CrossRef]
- Socała, K.; Nieoczym, D.; Kowalczuk-Vasilev, E.; Wyska, E.; Wlaź, P. Increased seizure susceptibility and other toxicity symptoms following acute sulforaphane treatment in mice. Toxicol. Appl. Pharmacol. 2017, 326, 43–53. [Google Scholar] [CrossRef]
- Scott, O.; Galicia-Connolly, E.; Adams, D.; Surette, S.; Vohra, S.; Yager, J.Y. The safety of cruciferous plants in humans: A systematic review. J. Biomed. Biotechnol. 2012, 2012, 503241. [Google Scholar] [CrossRef]
- Petroski, W.; Minich, D.M. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients 2020, 12, 2929. [Google Scholar] [CrossRef]
- Bajaj, J.K.; Salwan, P.; Salwan, S. Various Possible Toxicants Involved in Thyroid Dysfunction: A Review. J. Clin. Diagn. Res. 2016, 10, Fe01–Fe03. [Google Scholar] [CrossRef]
- Oloyede, O.O.; Wagstaff, C.; Methven, L. The Impact of Domestic Cooking Methods on Myrosinase Stability, Glucosinolates and Their Hydrolysis Products in Different Cabbage (Brassica oleracea) Accessions. Foods 2021, 10, 2908. [Google Scholar] [CrossRef]
- Lubelska, K.; Milczarek, M.; Modzelewska, K.; Krzysztoń-Russjan, J.; Fronczyk, K.; Wiktorska, K. Interactions between drugs and sulforaphane modulate the drug metabolism enzymatic system. Pharmacol. Rep. 2012, 64, 1243–1252. [Google Scholar] [CrossRef]
- Shorey, L.E.; Madeen, E.P.; Atwell, L.L.; Ho, E.; Löhr, C.V.; Pereira, C.B.; Dashwood, R.H.; Williams, D.E. Differential modulation of dibenzo[def,p]chrysene transplacental carcinogenesis: Maternal diets rich in indole-3-carbinol versus sulforaphane. Toxicol. Appl. Pharmacol. 2013, 270, 60–69. [Google Scholar] [CrossRef]
- Tao, S.; Rojo de la Vega, M.; Chapman, E.; Ooi, A.; Zhang, D.D. The effects of NRF2 modulation on the initiation and progression of chemically and genetically induced lung cancer. Mol. Carcinog. 2018, 57, 182–192. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef] [PubMed]
- Vergara, F.; Wenzler, M.; Hansen, B.G.; Kliebenstein, D.J.; Halkier, B.A.; Gershenzon, J.; Schneider, B. Determination of the absolute configuration of the glucosinolate methyl sulfoxide group reveals a stereospecific biosynthesis of the side chain. Phytochemistry 2008, 69, 2737–2742. [Google Scholar] [CrossRef] [PubMed]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef]
- Okada, M.; Yamamoto, A.; Aizawa, S.I.; Taga, A.; Terashima, H.; Kodama, S. HPLC Separation of Sulforaphane Enantiomers in Broccoli and Its Sprouts by Transformation into Diastereoisomers Using Derivatization with (S)-Leucine. J. Agric. Food Chem. 2017, 65, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Abdull Razis, A.F.; Iori, R.; Ioannides, C. The natural chemopreventive phytochemical R-sulforaphane is a far more potent inducer of the carcinogen-detoxifying enzyme systems in rat liver and lung than the S-isomer. Int. J. Cancer 2011, 128, 2775–2782. [Google Scholar] [CrossRef]
- Mithen, R.; Faulkner, K.; Magrath, R.; Rose, P.; Williamson, G.; Marquez, J. Development of isothiocyanate-enriched broccoli, and its enhanced ability to induce phase 2 detoxification enzymes in mammalian cells. Theor. Appl. Genet. 2003, 106, 727–734. [Google Scholar] [CrossRef]
- Alcarranza, M.; Villegas, I.; Muñoz-García, R.; Recio, R.; Fernández, I.; Alarcón-de-la-Lastra, C. Immunomodulatory Effects of (R)-Sulforaphane on LPS-Activated Murine Immune Cells: Molecular Signaling Pathways and Epigenetic Changes in Histone Markers. Pharmaceuticals 2022, 15, 966. [Google Scholar] [CrossRef]
- Mthembu, S.X.; Mazibuko-Mbeje, S.E.; Silvestri, S.; Orlando, P.; Nkambule, B.B.; Muller, C.J.; Tiano, L.; Dludla, P.V. Dietary Supplements as Modulators of the Nrf2 Pathway to Enhance Intracellular Antioxidant Responses and Protect Against Dyslipidemia-Associated Cardiovascular Complications. Food Rev. Int. 2025, 1–29. [Google Scholar] [CrossRef]
- Holland, H.L.; Brown, F.M.; Larsen, B.G.; Zabic, M. Biotransformation of organic sulfides. Part 7. Formation of chiral isothiocyanato sulfoxides and related compounds by microbial biotransformation. Tetrahedron Asymmetry 1995, 6, 1569–1574. [Google Scholar] [CrossRef]
- Schenk, W.A.; Dürr, M. Synthesis of (R)-Sulforaphane Using [cpru(R, R)-CHIRAPHOS)]+ as Chiral Auxiliary. Chem.—A Eur. J. 1997, 3, 713–716. [Google Scholar] [CrossRef]
- Pal, S.; Badireenath Konkimalla, V. Hormetic potential of sulforaphane (SFN) in switching cells’ fate towards survival or death. Mini Rev. Med. Chem. 2016, 16, 980–995. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, S.F.; Berhow, M.A. Glucosinolate hydrolysis products from various plant sources: pH effects, isolation, and purification. Ind. Crops Prod. 2005, 21, 193–202. [Google Scholar] [CrossRef]
- Yoshida, S. Recent advances in the synthesis and transformations of sulfinate esters. Chem. Commun. 2025, 61, 5084–5093. [Google Scholar] [CrossRef]
- Whitesell, J.K.; Wong, M.-S. Asymmetric synthesis of chiral sulfinate esters and sulfoxides. Synthesis of sulforaphane. J. Org. Chem. 1994, 59, 597–601. [Google Scholar] [CrossRef]
- Khiar, N.; Werner, S.; Mallouk, S.; Lieder, F.; Alcudia, A.; Fernández, I. Enantiopure sulforaphane analogues with various substituents at the sulfinyl sulfur: Asymmetric synthesis and biological activities. J. Org. Chem. 2009, 74, 6002–6009. [Google Scholar] [CrossRef]
- Elhalem, E.; Recio, R.; Werner, S.; Lieder, F.; Calderón-Montaño, J.M.; López-Lázaro, M.; Fernández, I.; Khiar, N. Sulforaphane homologues: Enantiodivergent synthesis of both enantiomers, activation of the Nrf2 transcription factor and selective cytotoxic activity. Eur. J. Med. Chem. 2014, 87, 552–563. [Google Scholar] [CrossRef]
- Balcells, D.; Ujaque, G.; Fernández, I.; Khiar, N.; Maseras, F. How does the achiral base decide the stereochemical outcome in the dynamic kinetic resolution of sulfinyl chlorides? A computational study. Adv. Synth. Catal. 2007, 349, 2103–2110. [Google Scholar] [CrossRef]
- Khiar, N.; Alcudia, F.; Espartero, J.-L.; Rodríguez, L.; Fernández, I. Dynamic Kinetic Resolution of Bis-Sulfinyl Chlorides: A General Enantiodivergent Synthesis of C2-Symmetric Bis-Sulfinate Esters and Bis-Sulfoxides. J. Am. Chem. Soc. 2000, 122, 7598–7599. [Google Scholar] [CrossRef]
- Mammone, F.R.; Panusa, A.; Risoluti, R.; Cirilli, R. Green HPLC Enantioseparation of Chemopreventive Chiral Isothiocyanates Homologs on an Immobilized Chiral Stationary Phase Based on Amylose tris-[(S)-α-Methylbenzylcarbamate]. Molecules 2024, 29, 2895. [Google Scholar] [CrossRef] [PubMed]
- Panusa, A.; Rosetti, A.; Villani, C.; Cirilli, R. Direct HPLC enantioseparation of chemopreventive chiral isothiocyanates sulforaphane and iberin on immobilized amylose-based chiral stationary phases under normal-phase, polar organic and aqueous conditions. Talanta 2020, 218, 121151. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.; Elbarbry, F. A new validated HPLC method for the determination of sulforaphane: Application to study pharmacokinetics of sulforaphane in rats. Biomed. Chromatogr. 2016, 30, 1016–1021. [Google Scholar] [CrossRef]
- Abdull Razis, A.F.; Bagatta, M.; De Nicola, G.R.; Iori, R.; Ioannides, C. Induction of epoxide hydrolase and glucuronosyl transferase by isothiocyanates and intact glucosinolates in precision-cut rat liver slices: Importance of side-chain substituent and chirality. Arch. Toxicol. 2011, 85, 919–927. [Google Scholar] [CrossRef]
- Li, Q.; Xia, B.; Wu, J.; Yuan, X.; Lu, X.; Huang, C.; Gu, H.; Zheng, K.; You, Q.; Liu, K. Indole-3-Carbinol (I3C) Protects the Heart From Ischemia/Reperfusion Injury by Inhibiting Oxidative Stress, Inflammation, and Cellular Apoptosis in Mice. Front. Pharmacol. 2022, 13, 924174. [Google Scholar] [CrossRef] [PubMed]
- Prado, N.J.; Ramirez, D.; Mazzei, L.; Parra, M.; Casarotto, M.; Calvo, J.P.; Cuello Carrión, D.; Ponce Zumino, A.Z.; Diez, E.R.; Camargo, A.; et al. Anti-inflammatory, antioxidant, antihypertensive, and antiarrhythmic effect of indole-3-carbinol, a phytochemical derived from cruciferous vegetables. Heliyon 2022, 8, e08989. [Google Scholar] [CrossRef]
- Xue, L.; Pestka, J.J.; Li, M.; Firestone, G.L.; Bjeldanes, L.F. 3,3′-Diindolylmethane stimulates murine immune function in vitro and in vivo. J. Nutr. Biochem. 2008, 19, 336–344. [Google Scholar] [CrossRef]
- Singh, A.A.; Yadav, D.; Khan, F.; Song, M. Indole-3-Carbinol and Its Derivatives as Neuroprotective Modulators. Brain Sci. 2024, 14, 674. [Google Scholar] [CrossRef]
- Takada, Y.; Andreeff, M.; Aggarwal, B.B. Indole-3-carbinol suppresses NF-kappaB and IkappaBalpha kinase activation, causing inhibition of expression of NF-kappaB-regulated antiapoptotic and metastatic gene products and enhancement of apoptosis in myeloid and leukemia cells. Blood 2005, 106, 641–649. [Google Scholar] [CrossRef]
- Jiang, J.; Kang, T.B.; do Shim, W.; Oh, N.H.; Kim, T.J.; Lee, K.H. Indole-3-carbinol inhibits LPS-induced inflammatory response by blocking TRIF-dependent signaling pathway in macrophages. Food Chem. Toxicol. 2013, 57, 256–261. [Google Scholar] [CrossRef]
- Peng, L.; Zhu, X.; Wang, C.; Jiang, Q.; Yu, S.; Song, G.; Liu, Q.; Gong, P. Indole-3-carbinol (I3C) reduces apoptosis and improves neurological function after cerebral ischemia-reperfusion injury by modulating microglia inflammation. Sci. Rep. 2024, 14, 3145. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Hernández, O.D.; Figueroa-González, G.; Quintas-Granados, L.I.; Gutiérrez-Ruíz, S.C.; Hernández-Parra, H.; Romero-Montero, A.; Del Prado-Audelo, M.L.; Bernal-Chavez, S.A.; Cortés, H.; Peña-Corona, S.I.; et al. 3,3′-Diindolylmethane and indole-3-carbinol: Potential therapeutic molecules for cancer chemoprevention and treatment via regulating cellular signaling pathways. Cancer Cell Int. 2023, 23, 180. [Google Scholar] [CrossRef]
- Safe, S.; Wormke, M.; Samudio, I. Mechanisms of inhibitory aryl hydrocarbon receptor-estrogen receptor crosstalk in human breast cancer cells. J. Mammary Gland. Biol. Neoplasia 2000, 5, 295–306. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomás-Barberán, F.A.; Ferreres, F. Characterisation of flavonols in broccoli (Brassica oleracea L. var. italica) by liquid chromatography–UV diode-array detection–electrospray ionisation mass spectrometry. J. Chromatogr. A 2004, 1054, 181–193. [Google Scholar] [CrossRef]
- Koh, E.; Wimalasiri, K.M.S.; Chassy, A.W.; Mitchell, A.E. Content of ascorbic acid, quercetin, kaempferol and total phenolics in commercial broccoli. J. Food Compos. Anal. 2009, 22, 637–643. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, F.; Gil-Izquierdo, A.; Pérez-Vicente, A.; García-Viguera, C. In Vitro Gastrointestinal Digestion Study of Broccoli Inflorescence Phenolic Compounds, Glucosinolates, and Vitamin C. J. Agric. Food Chem. 2004, 52, 135–138. [Google Scholar] [CrossRef]
- Afnan; Saleem, A.; Akhtar, M.F.; Sharif, A.; Akhtar, B.; Siddique, R.; Ashraf, G.M.; Alghamdi, B.S.; Alharthy, S.A. Anticancer, Cardio-Protective and Anti-Inflammatory Potential of Natural-Sources-Derived Phenolic Acids. Molecules 2022, 27, 7286. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Murata, T.; El-Rayes, B.F.; Shoji, M. The flavonoid p-hydroxycinnamic acid exhibits anticancer effects in human pancreatic cancer MIA PaCa-2 cells in vitro: Comparison with gemcitabine. Oncol. Rep. 2015, 34, 3304–3310. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Murata, T.; Shoji, M.; Weitzmann, M.N. The flavonoid p-hydroxycinnamic acid mediates anticancer effects on MDA-MB-231 human breast cancer cells in vitro: Implications for suppression of bone metastases. Int. J. Oncol. 2015, 47, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Plumb, G.W.; Price, K.R.; Rhodes, M.J.; Williamson, G. Antioxidant properties of the major polyphenolic compounds in broccoli. Free Radic. Res. 1997, 27, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Burhanoğlu, T.; Halbutoğulları, Z.S.; Turhal, G.; Demiroglu-Zergeroglu, A. Evaluation of the anticancer effects of hydroxycinnamic acid isomers on breast cancer stem cells. Med. Oncol. 2025, 42, 73. [Google Scholar] [CrossRef]
- Kapusta-Duch, J.; Borczak, B.; Kopeć, A.; Filipiak-Florkiewicz, A.; Leszczyńska, T. The Influence of Packaging Type and Time of Frozen Storage on Antioxidative Properties of Brussels Sprouts. J. Food Process. Preserv. 2014, 38, 1089–1096. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Bian, Q.; Gao, S.; Zhou, J.; Qin, J.; Taylor, A.; Johnson, E.J.; Tang, G.; Sparrow, J.R.; Gierhart, D.; Shang, F. Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Radic. Biol. Med. 2012, 53, 1298–1307. [Google Scholar] [CrossRef]
- Anbualakan, K.; Tajul Urus, N.Q.; Makpol, S.; Jamil, A.; Mohd Ramli, E.S.; Md Pauzi, S.H.; Muhammad, N. A Scoping Review on the Effects of Carotenoids and Flavonoids on Skin Damage Due to Ultraviolet Radiation. Nutrients 2022, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.A.; Biesalski, H.K. Beta-carotene is an important vitamin A source for humans. J. Nutr. 2010, 140, 2268s–2285s. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Namitha, K.K.; Negi, P.S. Chemistry and biotechnology of carotenoids. Crit. Rev. Food Sci. Nutr. 2010, 50, 728–760. [Google Scholar] [CrossRef]
- Jeffery, E.; Brown, A.; Kurilich, A.; Keck, A.; Matusheski, N.; Klein, B.; Juvik, J. Variation in content of bioactive components in broccoli. J. Food Compos. Anal. 2003, 16, 323–330. [Google Scholar] [CrossRef]
- Ochoa Becerra, M.; Mojica Contreras, L.; Hsieh Lo, M.; Mateos Díaz, J.; Castillo Herrera, G. Lutein as a functional food ingredient: Stability and bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabińska, N.; Bączek, N.; Šimková, K.; Starowicz, M.; Jeliński, T. Application of Broccoli Leaf Powder in Gluten-Free Bread: An Innovative Approach to Improve Its Bioactive Potential and Technological Quality. Foods 2021, 10, 819. [Google Scholar] [CrossRef]
- Shahidi, F.; de Camargo, A.C. Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef]
- Jiang, Q.; Im, S.; Wagner, J.G.; Hernandez, M.L.; Peden, D.B. Gamma-tocopherol, a major form of vitamin E in diets: Insights into antioxidant and anti-inflammatory effects, mechanisms, and roles in disease management. Free Radic. Biol. Med. 2022, 178, 347–359. [Google Scholar] [CrossRef]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in disease prevention and cure: An overview. Indian J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Zehiroglu, C.; Ozturk Sarikaya, S.B. The importance of antioxidants and place in today’s scientific and technological studies. J. Food Sci. Technol. 2019, 56, 4757–4774. [Google Scholar] [CrossRef]
- Soundararajan, P.; Kim, J.S. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef]
- Lauer, A.-C.; Groth, N.; Haag, S.F.; Darvin, M.E.; Lademann, J.; Meinke, M.C. Dose-Dependent Vitamin C Uptake and Radical Scavenging Activity in Human Skin Measured with in vivo Electron Paramagnetic Resonance Spectroscopy. Ski. Pharmacol. Physiol. 2013, 26, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.F.; Sun, B.; Yuan, J.; Wang, Q.M. Effects of different cooking methods on health-promoting compounds of broccoli. J. Zhejiang Univ. Sci. B 2009, 10, 580–588. [Google Scholar] [CrossRef]
- AbuMweis, S.S.; Marinangeli, C.P.; Frohlich, J.; Jones, P.J. Implementing phytosterols into medical practice as a cholesterol-lowering strategy: Overview of efficacy, effectiveness, and safety. Can. J. Cardiol. 2014, 30, 1225–1232. [Google Scholar] [CrossRef]
- Shi, L.; Li, Y.; Lin, M.; Liang, Y.; Zhang, Z. Profiling the Bioactive Compounds in Broccoli Heads with Varying Organ Sizes and Growing Seasons. Plants 2024, 13, 1329. [Google Scholar] [CrossRef]
- Armah, C.N.; Derdemezis, C.; Traka, M.H.; Dainty, J.R.; Doleman, J.F.; Saha, S.; Leung, W.; Potter, J.F.; Lovegrove, J.A.; Mithen, R.F. Diet rich in high glucoraphanin broccoli reduces plasma LDL cholesterol: Evidence from randomised controlled trials. Mol. Nutr. Food Res. 2015, 59, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Ağagündüz, D.; Şahin, T.; Yılmaz, B.; Ekenci, K.D.; Duyar Özer, Ş.; Capasso, R. Cruciferous Vegetables and Their Bioactive Metabolites: From Prevention to Novel Therapies of Colorectal Cancer. Evid. Based Complement. Altern. Med. 2022, 2022, 1534083. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Cano, R.; Salcedo-Hernández, R.; López-Meza, J.; Bideshi, D.; Barboza-Corona, J. Antimicrobial activity of broccoli (Brassica oleracea var. italica) cultivar Avenger against pathogenic bacteria, phytopathogenic filamentous fungi and yeast. J. Appl. Microbiol. 2018, 124, 126–135. [Google Scholar] [CrossRef]
- Le, T.N.; Luong, H.Q.; Li, H.-P.; Chiu, C.-H.; Hsieh, P.-C. Broccoli (Brassica oleracea L. var. italica) Sprouts as the Potential Food Source for Bioactive Properties: A Comprehensive Study on In Vitro Disease Models. Foods 2019, 8, 532. [Google Scholar] [CrossRef]
- Pacheco-Cano, R.D.; Salcedo-Hernández, R.; Casados-Vázquez, L.E.; Wrobel, K.; Bideshi, D.K.; Barboza-Corona, J.E. Class I defensins (BraDef) from broccoli (Brassica oleracea var. italica) seeds and their antimicrobial activity. World J. Microbiol. Biotechnol. 2020, 36, 1–12. [Google Scholar] [CrossRef]
- Chen, H.; Xia, L.-S.; Zhang, X.-D.; Wei, Z.-J. Antioxidant and Hypolipidemic Potential of Peptides from Broccoli Stems and Leaves. Curr. Top. Nutraceutical Res. 2020, 18, 16–21. [Google Scholar]
- Nicolas-Espinosa, J.; Lucía, Y.-M.; Carvajal, M. Bioactive peptides from broccoli stems strongly enhance regenerative keratinocytes by stimulating controlled proliferation. Pharm. Biol. 2022, 60, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Ochiai, A. Characterization and production of multifunctional cationic peptides derived from rice proteins. Biosci. Biotechnol. Biochem. 2017, 81, 634–650. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Y.; Gao, X.; Pan, D.; Liu, Z.; Xiao, C.; Xiong, Y.; Du, L.; Cai, Z.; Lu, W.; Dang, Y. Identification and virtual screening of novel anti-inflammatory peptides from broccoli fermented by Lactobacillus strains. Front. Nutr. 2023, 9, 1118900. [Google Scholar] [CrossRef]
- Thery, T.; Lynch, K.M.; Zannini, E.; Arendt, E.K. Isolation, characterisation and application of a new antifungal protein from broccoli seeds—New food preservative with great potential. Food Control 2020, 117, 107356. [Google Scholar] [CrossRef]
- Favela-González, K.M.; Hernández-Almanza, A.Y.; De la Fuente-Salcido, N.M. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J. Food Biochem. 2020, 44, e13414. [Google Scholar] [CrossRef]
- Fahey, J.W.; Haristoy, X.; Dolan, P.M.; Kensler, T.W.; Scholtus, I.; Stephenson, K.K.; Talalay, P.; Lozniewski, A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7610–7615. [Google Scholar] [CrossRef] [PubMed]
- Gagandeep, K.R.; Balenahalli Narasingappa, R.; Vishnu Vyas, G. Unveiling mechanisms of antimicrobial peptide: Actions beyond the membranes disruption. Heliyon 2024, 10, e38079. [Google Scholar] [CrossRef]
- Liu, Y.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W. Antimicrobial peptides and their application in food packaging. Trends Food Sci. Technol. 2021, 112, 471–483. [Google Scholar] [CrossRef]
- Agrillo, B.; Balestrieri, M.; Gogliettino, M.; Palmieri, G.; Moretta, R.; Proroga, Y.T.; Rea, I.; Cornacchia, A.; Capuano, F.; Smaldone, G. Functionalized polymeric materials with bio-derived antimicrobial peptides for “active” packaging. Int. J. Mol. Sci. 2019, 20, 601. [Google Scholar] [CrossRef]
- Ghanbarzadeh, Z.; Mohagheghzadeh, A.; Hemmati, S. The Roadmap of Plant Antimicrobial Peptides Under Environmental Stress: From Farm to Bedside. Probiotics Antimicrob. Proteins 2024, 16, 2269–2304. [Google Scholar] [CrossRef]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Wang, L.; Mahe, J.; Li, L.; Jiao, S.; Wang, H.; Xie, Y.; Liu, X.; Zeng, X.; Hu, X. Effect of aqueous extract of seed of broccoli on inflammatory cytokines and Helicobacter pylori infection: A randomized, double-blind, controlled trial in patients without atrophic gastritis. Inflammopharmacology 2022, 30, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Mechchate, H.; El Allam, A.; El Omari, N.; El Hachlafi, N.; Shariati, M.A.; Wilairatana, P.; Mubarak, M.S.; Bouyahya, A. Vegetables and Their Bioactive Compounds as Anti-Aging Drugs. Molecules 2022, 27, 2316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, Z.; Wang, L.; Xu, L. Extraction optimization, antioxidant, and hypoglycemic activities in vitro of polysaccharides from broccoli byproducts. J. Food Biochem. 2017, 41, e12387. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Cardoso, S.M.; Wessel, D.F.; Coimbra, M.A. Microwave assisted dehydration of broccoli by-products and simultaneous extraction of bioactive compounds. Food Chem. 2018, 246, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Hlaing, M.M.; Ying, D.; Ye, J.; Sanguansri, L.; Augustin, M.A. New food ingredients from broccoli by-products: Physical, chemical and technological properties. Int. J. Food Sci. Technol. 2019, 54, 1423–1432. [Google Scholar] [CrossRef]
- Castelão-Baptista, J.P.; Barros, A.; Martins, T.; Rosa, E.; Sardão, V.A. Three in One: The Potential of Brassica By-Products against Economic Waste, Environmental Hazard, and Metabolic Disruption in Obesity. Nutrients 2021, 13, 4194. [Google Scholar] [CrossRef]
- Rafael Audino, Z.; Bruna Caroline Venceslau, P.; Evellheyn Reboucas, P.; Marina Lisboa, S.; Edilberto Cordeiro dos Santos, J.; Luan Icaro Freitas, P.; Cicera Alyne Lemos, M.; Maryana Monteiro, F.; da Cristiano Silva, C.; da Ana Caroline, S. Broccoli and Carrot Industrial Solid Waste Characterization and Application in the Bread Food Matrix. Int. J. Nutr. Food Sci. 2017, 6, 9–15. [Google Scholar] [CrossRef]
- Angiolillo, L.; Spinelli, S.; Conte, A.; Del Nobile, M.A. Extract from Broccoli Byproducts to Increase Fresh Filled Pasta Shelf Life. Foods 2019, 8, 621. [Google Scholar] [CrossRef]
- Thomas, M.; Badr, A.; Desjardins, Y.; Gosselin, A.; Angers, P. Characterization of industrial broccoli discards (Brassica oleracea var. italica) for their glucosinolate, polyphenol and flavonoid contents using UPLC MS/MS and spectrophotometric methods. Food Chem. 2018, 245, 1204–1211. [Google Scholar] [CrossRef]
- Rivas Muñoz, M.Á.; Benito Bernáldez, M.J.; Martín González, A.; Córdoba Ramos, M.G.; Ruiz-Moyano Seco de Herrera, S.; Casquete Palencia, R. Improve the functional properties of dietary fibre isolated from broccoli by-products by using different technologies. Innov. Food Sci. Emerg. Technol. 2022, 80, 103075. [Google Scholar] [CrossRef]
- Gudiño, I.; Martín, A.; Casquete, R.; Prieto, M.H.; Ayuso, M.C.; Córdoba, M.G. Evaluation of broccoli (Brassica oleracea var. italica) crop by-products as sources of bioactive compounds. Sci. Hortic. 2022, 304, 111284. [Google Scholar] [CrossRef]
- Borja-Martínez, M.; Lozano-Sánchez, J.; Borrás-Linares, I.; Pedreño, M.A.; Sabater-Jara, A.B. Revalorization of Broccoli By-Products for Cosmetic Uses Using Supercritical Fluid Extraction. Antioxidants 2020, 9, 1195. [Google Scholar] [CrossRef] [PubMed]
| Mechanism | Concentration | Concentration |
|---|---|---|
| In Vitro | In Vivo | |
| Modulation of phase I and phase II enzymes | 0.5–25 μM | 6 μmol/day to 1 mmol/kg |
| DNA protection from chemical insult | 0.06–20 μM | 10–450 μmol/kg |
| Induction of apoptosis | 0.1–300 μM | 2.4 μmol to 15 nmol/day |
| Cell-cycle modulation | <1 μM–2 mM | 10–100 μmol/day |
| Inhibition of angiogenesis | 0.1–50 μM | |
| Inhibition of metastasis formation | 6–28 μM | 2.8 μmol/kg |
| Peptide Name/Sequence | Activity | Target Pathogens/Effects | Reference |
|---|---|---|---|
| ARFEELNMDLFR | Antimicrobial activity | Porphyromonas gingivalis, Candida albicans | [194,195] |
| SIWYGPDRP | Anti-inflammatory (52% NO inhibition) | Reduces inflammation in macrophages | [196] |
| RFR | TNF-α inhibition (75%) | Chronic inflammatory diseases | [196] |
| KASFAFAGL | IL-6 inhibition (30%) | Autoimmune disorders | [196] |
| KSVLLKF | Antioxidant, hypolipidemic | Oxidative stress, lipid metabolism | [193] |
| BoNap | Antifungal inhibition | Fusarium culmorum, Penicillium expansum | [197] |
| BraDef (Class I) | Broad-spectrum antimicrobial | B. cereus, C. albicans | [192] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrés, C.M.C.; Pérez de la Lastra, J.M.; Munguira, E.B.; Juan, C.A.; Pérez-Lebeña, E. The Multifaceted Health Benefits of Broccoli—A Review of Glucosinolates, Phenolics and Antimicrobial Peptides. Molecules 2025, 30, 2262. https://doi.org/10.3390/molecules30112262
Andrés CMC, Pérez de la Lastra JM, Munguira EB, Juan CA, Pérez-Lebeña E. The Multifaceted Health Benefits of Broccoli—A Review of Glucosinolates, Phenolics and Antimicrobial Peptides. Molecules. 2025; 30(11):2262. https://doi.org/10.3390/molecules30112262
Chicago/Turabian StyleAndrés, Celia María Curieses, José Manuel Pérez de la Lastra, Elena Bustamante Munguira, Celia Andrés Juan, and Eduardo Pérez-Lebeña. 2025. "The Multifaceted Health Benefits of Broccoli—A Review of Glucosinolates, Phenolics and Antimicrobial Peptides" Molecules 30, no. 11: 2262. https://doi.org/10.3390/molecules30112262
APA StyleAndrés, C. M. C., Pérez de la Lastra, J. M., Munguira, E. B., Juan, C. A., & Pérez-Lebeña, E. (2025). The Multifaceted Health Benefits of Broccoli—A Review of Glucosinolates, Phenolics and Antimicrobial Peptides. Molecules, 30(11), 2262. https://doi.org/10.3390/molecules30112262






