4. Materials and Methods
All starting compounds and reagents used are commercially available. The progress of reactions was monitored by TLC on silica gel 60 F254 plates (Merk, Rahway, NJ, USA) using n-hexane/ethyl acetate eluent. The purification and isolation of the products by flash chromatography was carried out using an Isolera Four Flash chromatograph on SNAP KP-Sil 100 g cartridges (Biotage, Uppsala, Sweden) with n-hexane/ethyl acetate eluent. HPLC analysis was performed on an LC-20 Prominence (Shimadzu, Kyoto, Japan) using a Nucleodur PolarTec column (Macherey-Nagel, Düren, Germany), with a length of 150 mm, internal diameter of 3.0 mm, and particle size of 3 µm, in acetonitrile–0.1% trifluoroacetic acid (50/50), and with a flow rate of 0.4 mL/min and oven temperature of 40 °C. Mass spectra were recorded on an LCMS-2020 device (Shimadzu) with a single quadrupole detector under positive mode, electrospray ionization (ESI).
General procedure for demethylation. BBr3 (1 M in dichloromethane, 28 mL, 28 mmol) was added at −50 °C dropwise to a suspension of ether (4.6 mmol) in dichloromethane (20 mL). The reaction mixture was stirred at room temperature overnight. The mixture was concentrated to dryness, cooled on an ice bath and diluted with saturated aqueous NaHCO3. The resulting precipitate was filtered and air-dried, the crude product was used without further purification.
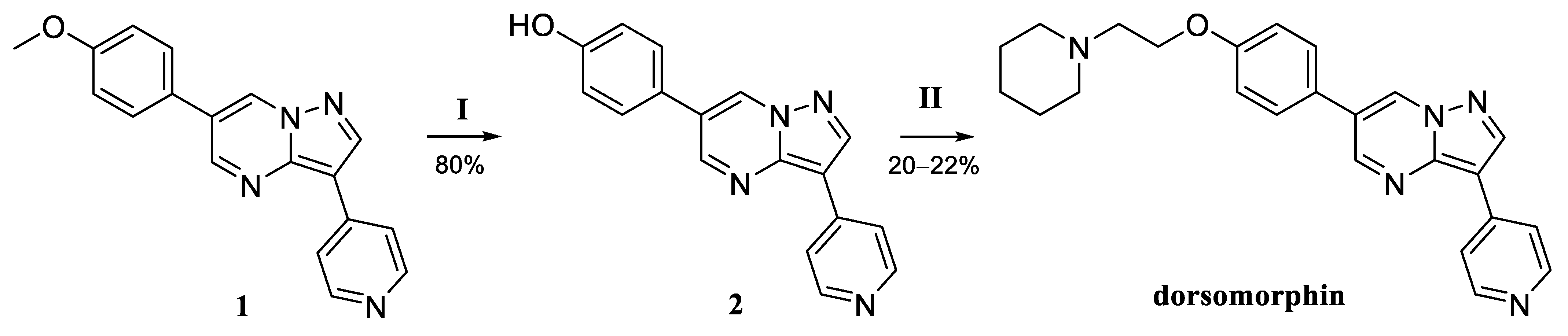
4-(3-(pyridin-4-yl)pyrazolo[1,5-a]pyrimidin-6-yl)phenol (2). Yellowish solid. 1H NMR (400 MHz, DMSO-d6) δ 9.86 (s, 1H), 9.60 (d, J = 2.3 Hz, 1H), 9.23 (d, J = 2.3 Hz, 1H), 9.22 (s, 1H), 8.82 (d, J = 6.8 Hz, 2H), 8.66 (d, J = 6.8 Hz, 2H), 7.74 (d, J = 8.7 Hz, 2H), and 6.94 (d, J = 8.7 Hz, 2H). 13C NMR (101 MHz, DMSO) δ 158.36, 152.53, 145.68, 144.82, 142.38, 142.33, 133.18, 128.47, 123.95, 123.13, 120.49, 116.14, and 104.36. MS (ESI) m/z: 289.1 (100) [M + H]+.
General procedure for nucleophilic substitution. Halide (0.05 mol), O- or N-nucleophile (0.055 mol), and finely ground K2CO3 were dissolved in 200 mL of anhydrous DMF and stirred at 60 °C for 16 h. The solution was filtered from an inorganic precipitate, and DMF was distilled off under reduced pressure. The residue was poured into 200 mL of water, extracted with 3 × 100 mL of ethyl acetate, washed twice with water and saturated NaCl solution, and dried over Na2SO4. The product was isolated by flash chromatography using hexane/ethyl acetate (6:1–9:1) as eluent.
6-(4-(2-(Piperidin-1-yl)ethoxy)phenyl)-3-(pyridin-4-yl)pyrazolo[1,5-a]pyrimidine (dorsomorphin). Yield 18% (two stages), yellowish solid. 1H NMR (400 MHz, DMSO-d6) δ 9.52 (d, J = 2.3 Hz, 1H), 9.12 (d, J = 2.3 Hz, 1H), 8.97 (s, 1H), 8.59 (d, J = 6.2 Hz, 2H), 8.16 (d, J = 6.2 Hz, 2H), 7.82 (d, J = 8.8 Hz, 2H), 7.11 (d, J = 8.8 Hz, 2H), 4.14 (t, J = 5.9 Hz, 2H), 2.68 (t, J = 5.9 Hz, 2H), 2.47–2.40 (m, 4H), 1.54–1.47 (m, 4H), and 1.41–1.36 (m, 2H). 13C NMR (101 MHz, DMSO) δ 158.97, 150.80, 149.99, 143.85, 143.81, 139.29, 132.61, 128.27, 125.30, 122.18, 119.63, 115.29, 106.08, 65.81, 57.35, 54.44, 25.61, and 23.97. MS (ESI) m/z: 400.2 (100) [M + H]+.
2-(Piperidin-1-yl)ethyl benzoate (9a). Yield 73%, colorless liquid. 1H NMR (400 MHz, DMSO-d6) δ 7.97–7.93 (m, 2H), 7.65 (t, J = 7.4 Hz, 1H), 7.52 (t, J = 7.7 Hz, 2H), 4.35 (t, J = 5.9 Hz, 2H), 2.63 (t, J = 5.9 Hz, 2H), 2.45–2.37 (m, 4H), 1.50–1.43 (m, 4H), and 1.38–1.31 (m, 2H). 13C NMR (101 MHz, DMSO) δ 165.65, 133.26, 129.83, 129.10, 128.74, 62.40, 56.79, 54.17, 25.63, and 23.89.
2-(4-Bromophenoxy)ethyl benzoate (12a). Yield 77%, colorless liquid. 1H NMR (400 MHz, DMSO-d6) δ 7.97–7.93 (m, 2H), 7.66 (tt, J = 7.0, 1.3 Hz, 1H), 7.52 (t, J = 7.7 Hz, 2H), 7.45 (d, J = 9.0 Hz, 2H), 6.97 (d, J = 9.1 Hz, 2H), 4.61–4.58 (m, 2H), and 4.35–4.32 (m, 2H). 13C NMR (101 MHz, DMSO) δ 165.66, 157.60, 133.46, 132.18, 129.45, 129.21, 128.77, 116.96, 112.30, 66.14, and 63.28.
General procedure for obtaining glycol monobenzoates. To a solution of diole (0.4 mol) in 300 mL CH2Cl2 containing 55.6 mL triethylamine (40.5 g, 0.4 mol) and cooled to 0 °C, a solution of 28 g benzoyl chloride (0.2 mol) in 100 mL of CH2Cl2 was added dropwise over 2 h, maintaining the temperature no higher than 5 °C. After complete addition, the solution was stirred at room temperature for 12 h. The resulting solution was washed with water 2 × 200 mL, then with 200 mL of 5% hydrochloric acid, and again with water 2 × 200 mL, 5% NaHCO3 solution, and saturated NaCl solution, and dried over Na2SO4. The solvent was removed under reduced pressure. The residue was distilled at a pressure of 5 mbar.
2-Hydroxyethyl benzoate (7a). 74%, colorless liquid. 1H NMR (400 MHz, DMSO-d6) δ 8.02–7.99 (m, 2H), 7.64 (tt, J = 6.9, 1.3 Hz, 1H), 7.52 (t, J = 7.7 Hz, 1H), 4.90 (br s, 1H), 4.31–4.27 (m, 2H), and 3.74–3.70 (m, 2H). 13C NMR (101 MHz, DMSO) δ 165.91, 133.27, 129.92, 129.28, 128.69, 66.60, and 59.14.
General procedure for obtaining chlorides. To a solution of carbinol (0.1 mol) in 300 mL dichloromethane cooled to 4 °C, 14.3 mL thionyl chloride (23.4 g, 0.2 mol) was added dropwise with stirring at such a rate that the temperature of the mixture did not exceed 15 °C. After the addition was complete, the mixture was left at room temperature for 16 h. The solvent was distilled to dryness under reduced pressure, 100 mL of 10% NaHCO3 solution and 150 mL of ethyl acetate were added to the residue, and the mixture was stirred for 20 min. The layers were separated, and the aqueous layer was extracted with ethyl acetate 2 × 150 mL. The organic layer was dried over Na2SO4 and distilled under reduced pressure. The residue was distilled at a pressure of 5–10 mbar.
2-Chloroethyl benzoate (8a-Cl). Yield 85%, colorless liquid. 1H NMR (400 MHz, DMSO-d6) δ 8.01–7.97 (m, 2H), 7.70–7.65 (m, 1H), 7.57–7.52 (m, 2H), 4.56–4.52 (m, 2H), and 3.99–3.95 (m, 2H). 13C NMR (101 MHz, DMSO) δ 165.44, 133.57, 129.31, 129.23, 128.83, 64.63, and 42.69.
1-(2-Chloroethyl)piperidine (11a-Cl). Yield 76%, colorless liquid. 1H NMR (400 MHz, DMSO-d6) δ 3.64 (t, J = 6.9 Hz, 2H), 2.58 (t, J = 6.9 Hz, 2H), 2.44–2.33 (m, 4H), 1.51–1.43 (m, 4H), and 1.40–1.33 (m, 2H). 13C NMR (101 MHz, DMSO) δ 60.01, 53.87, 41.60, 25.51, and 23.90.
General procedure for obtaining bromides. To a solution of 13.1 g triphenylphosphine (0.05 mol) in 200 mL anhydrous acetonitrile, 2.6 mL bromine (8 g, 0.05 mol) and 0.05 mol of carbinol were added with stirring at 0 °C. The mixture was stirred at room temperature for 30 min, then refluxed for 8 h. After the reaction was complete, the solvent was distilled to dryness under reduced pressure, and 300 mL of a hexane/ethyl acetate mixture (6:1) was added to the residue and stirred for an hour. The solution was filtered from the precipitated triphenylphosphine oxide, washed with 10% NaHCO3 solution and twice with water and saturated NaCl solution. The organic layer was dried over Na2SO4 and passed through a 10 cm layer of silica gel, which was then washed with 500 mL of the same mixture. After the distillation of the solvent under reduced pressure, a crude bromine derivative suitable for further reactions was obtained.
2-Bromoethyl benzoate (8a-Br). Yield 84%, colorless liquid. 1H NMR (400 MHz, DMSO-d6) δ 8.02–7.99 (m, 2H), 7.61–7.56 (m, 1H), 7.49–7.44 (m, 2H), 4.51–4.48 (m, 2H), and 3.68–3.65 (m, 2H). 13C NMR (100 MHz, DMSO) δ 166.45, 133.06, 130.46, 129.75, 128.85, 65.27, and 29.18.
1-(2-Bromoethyl)piperidine (11a-Br). Yield 73%, colorless liquid. 1H NMR (400 MHz, DMSO-d6) δ 3.53 (t, J = 7.1 Hz, 2H), 3.00 (t, J = 7.1 Hz, 2H), 2.48–2.37 (m, 4H), 1.54–1.46 (m, 4H), and 1.42–1.35 (m, 2H). 13C NMR (101 MHz, DMSO) δ 59.41, 53.79, 29.24, 25.55, and 23.90.
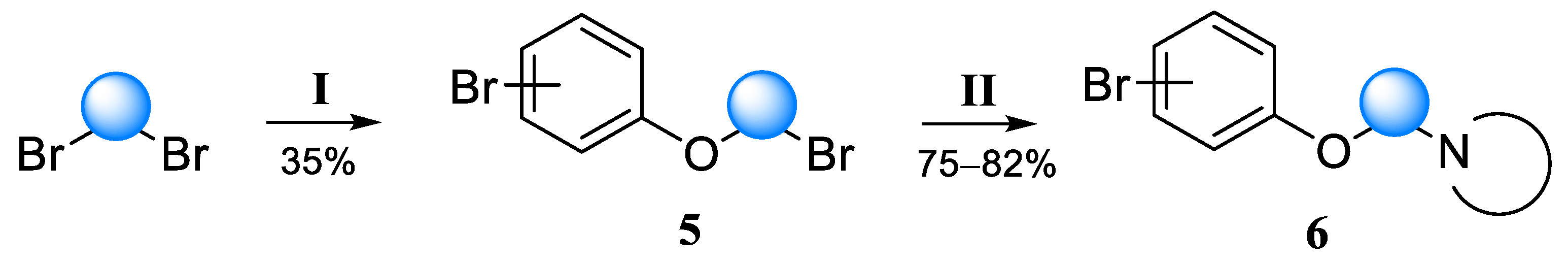
1-Bromo-4-(2-bromoethoxy)benzene (14a-Br). Yield 82%, colorless liquid. 1H NMR (400 MHz, DMSO-d6) δ 7.45 (d, J = 9.1 Hz, 2H), 6.94 (d, J = 9.1 Hz, 2H), 4.32–4.29 (m, 2H), and 3.81–3.77 (m, 2H). 13C NMR (101 MHz, DMSO) δ 157.20, 132.21, 116.94, 112.45, 67.98, and 31.25.
General procedure for alkaline hydrolysis. Benzoate (0.05 mol) was dissolved in 150 mL THF with stirring, and a solution of 8 g sodium hydroxide (0.2 mol) in 100 mL water was added. The resulting mixture was kept for 12 h at 40 °C with vigorous stirring. Upon the completion of the reaction, the aqueous layer was separated, and the organic layer was evaporated to dryness under reduced pressure. The residue was dissolved in 200 mL ethyl acetate, filtered, and passed through a 10 cm layer of silica gel, which was additionally washed with 400 mL ethyl acetate. The solvent was distilled off under reduced pressure. If necessary, the resulting product was distilled at 5–10 mbar.
2-(Piperidin-1-yl)ethan-1-ol (10a). Yield 92%, colorless liquid. 1H NMR (400 MHz, DMSO-d6) δ 4.32 (t, J = 5.2 Hz, 1H), 3.46 (td, J = 6.4, 5.1 Hz, 2H), 2.36–2.30 (m, 4H), 2.31 (t, J = 6.5 Hz, 2H), 1.50–1.43 (m, 4H), and 1.38–1.32 (m, 1H). 13C NMR (101 MHz, DMSO) δ 61.12, 58.58, 54.55, 25.62, and 24.09.
2-(4-Bromophenoxy)ethan-1-ol (13a). Yield 87%, colorless liquid. 1H NMR (400 MHz, DMSO-d6) δ 7.43 (d, J = 9.0 Hz, 2H), 6.91 (d, J = 9.0 Hz, 2H), 4.89 (t, J = 5.5 Hz, 1H), 3.96 (t, J = 4.9 Hz, 2H), and 3.70 (td, J = 5.4, 5.0 Hz, 2H). 13C NMR (101 MHz, DMSO) δ 158.01, 132.11, 116.76, 111.81, 69.82, and 59.48.
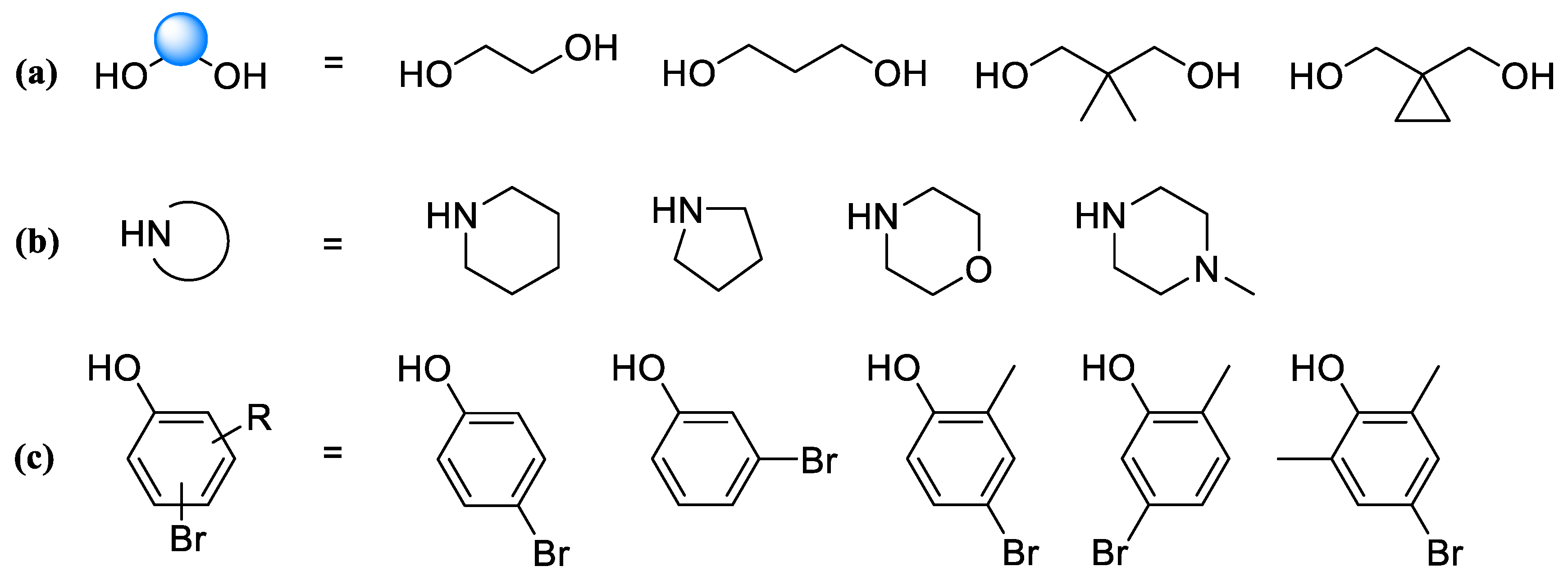
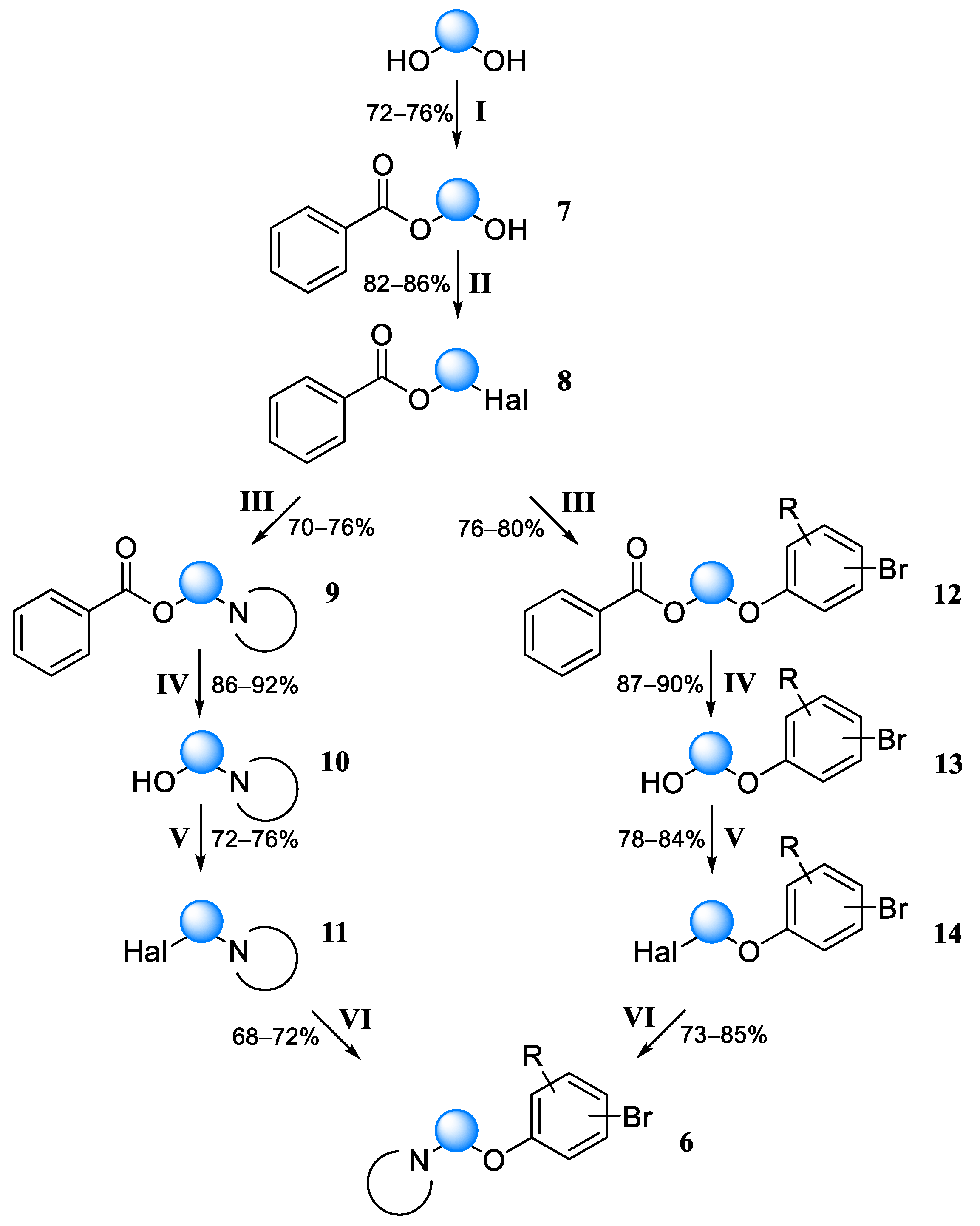
Using the above protocols, the following fragments were obtained according to the scheme presented in
Figure 6:
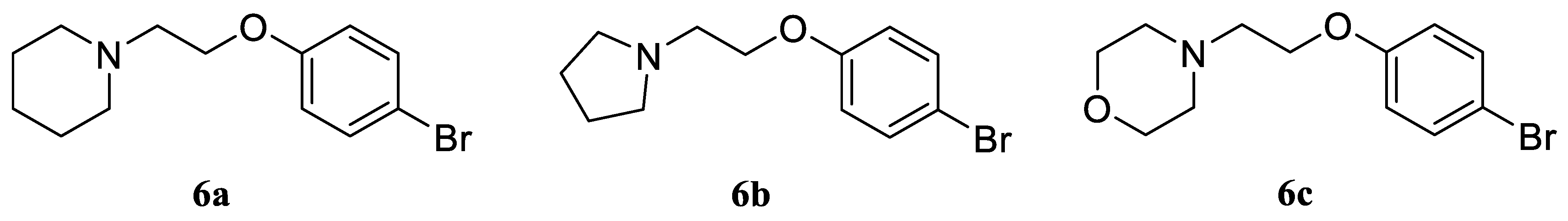
1-(2-(4-Bromophenoxy)ethyl)piperidine (6a). Yield 33% (six stages), colorless liquid. 1H NMR (400 MHz, DMSO-d6) δ 7.42 (d, J = 9.1 Hz, 2H), 6.90 (d, J = 9.1 Hz, 2H), 4.03 (t, J = 5.9 Hz, 2H), 2.62 (t, J = 5.9 Hz, 2H), 2.43–2.36 (m, 4H), 1.51–1.44 (m, 4H), and 1.39–1.32 (m, 2H). 13C NMR (101 MHz, DMSO) δ 157.79, 132.05, 116.77, 111.81, 65.89, 57.24, 54.38, 25.57, and 23.92. MS (ESI) m/z: 284.1 (100) [M + H]+.
1-(2-(4-Bromophenoxy)ethyl)pyrrolidine (6b). Yield 31% (six stages), colorless liquid. 1H NMR (400 MHz, DMSO-d6) δ 7.38 (d, J = 9.0 Hz, 2H), 6.86 (d, J = 9.0 Hz, 2H), 4.03 (t, J = 5.9 Hz, 2H), 2.73 (t, J = 5.9 Hz, 2H), 2.45–2.38 (m, 4H), and 1.69–1.60 (m, 4H). 13C NMR (101 MHz, DMSO) δ 157.80, 132.01, 116.75, 111.81, 64.76, 56.93, 53.82, and 23.07. MS (ESI) m/z: 270.0 (100) [M + H]+.
4-(2-(4-Bromophenoxy)ethyl)morpholine (6c). Yield 33% (six stages), colorless liquid. 1H NMR (400 MHz, DMSO-d6) δ 7.35 (d, J = 9.0 Hz, 2H), 6.83 (d, J = 9.0 Hz, 2H), 4.06 (t, J = 5.9 Hz, 2H), 3.57–2.53 (m, 4H), 2.68 (t, J = 5.9 Hz, 2H), and 2.39–2.34 (m, 4H). 13C NMR (101 MHz, DMSO) δ 157.82, 132.03, 116.76, 111.80, 66.21, 65.55, 57.02, and 53.66. MS (ESI) m/z: 286.0 (100) [M + H]+.
General procedure for obtaining compounds by the Mitsunobu reaction. Substituted phenol or secondary amine (0.05 mol), alcohol component (0.055 mol), triphenylphosphine (14.4 g, 0.055 mol), and triethylamine (5 g, 0.05 mol) were dissolved in 100 mL THF with stirring. The mixture was cooled and a solution of DIAD (11.1 g, 0.055 mol) in 30 mL of THF was added dropwise at 4 °C over 30 min. After complete addition, the cooling was removed and the mixture was stirred for another 30 min, then refluxed for 24 h with stirring. The solvent was distilled off under reduced pressure. The residue was diluted with 400 mL hexane/ethyl acetate (6:1) mixture and stirred for an hour. The solution was filtered from the precipitated triphenylphosphine oxide, washed with 10% NaHCO3 solution, twice with water, and with saturated NaCl, and dried over Na2SO4. The solvent was distilled off under reduced pressure. The residue was dissolved in a hexane/ethyl acetate mixture (9:1–19:1) and passed through a 10 cm layer of silica gel, which was then washed with 500 mL of the same mixture. After distilling off the solvent, a viscous oil representing a product with a purity of about 95% was obtained.
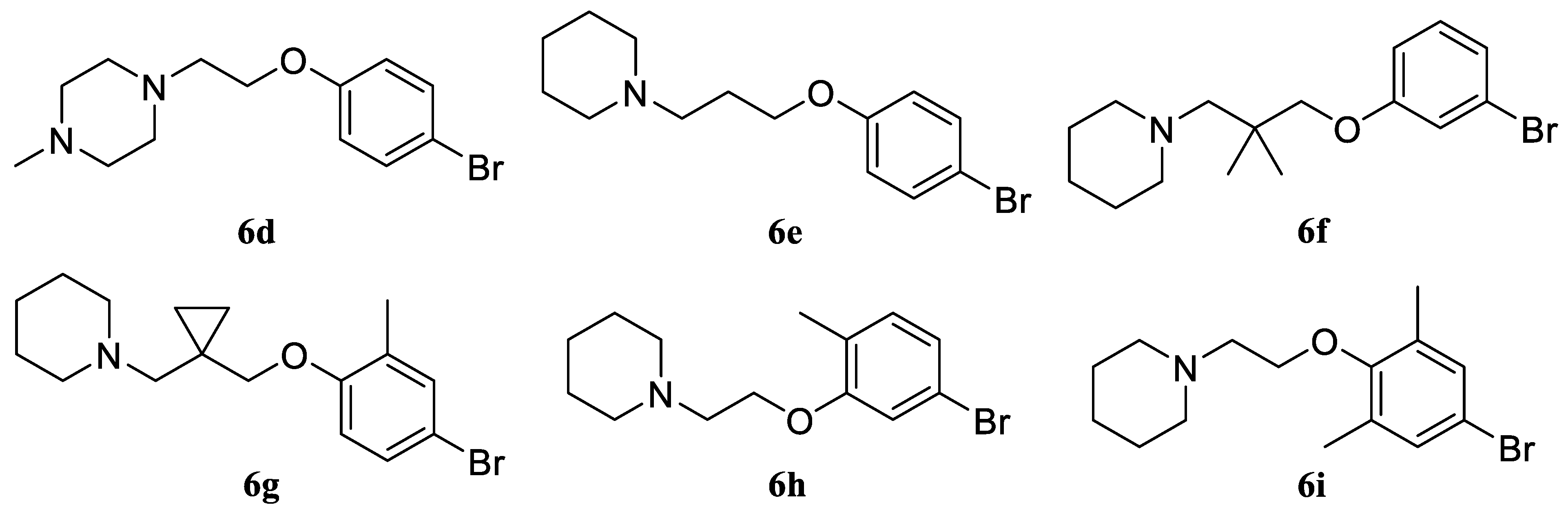
1-(2-(4-Bromophenoxy)ethyl)-4-methylpiperazine (6d). Yield 46% (four stages), colorless liquid. 1H NMR (400 MHz, DMSO-d6) δ 7.40 (d, J = 9.1 Hz, 2H), 6.85 (d, J = 9.1 Hz, 2H), 4.03 (t, J = 5.8 Hz, 2H), 2.65 (t, J = 5.8 Hz, 2H), 2.43–2.33 (m, 4H), 2.32–2.22 (m, 4H), and 2.12 (s, 3H). 13C NMR (101 MHz, DMSO) δ 157.80, 132.11, 116.74, 111.85, 65.00, 57.22, 54.74, 53.21, and 45.83. MS (ESI) m/z: 299.1 (100) [M + H]+.
1-(3-(4-Bromophenoxy)propyl)piperidine (6e). Yield 49% (four stages), colorless liquid. 1H NMR (400 MHz, DMSO-d6) δ 7.42 (d, J = 9.0 Hz, 2H), 6.91 (d, J = 9.0 Hz, 2H), 4.09 (t, J = 6.2, 2H), 2.61 (t, J = 6.2 Hz, 2H), 2.46–2.36 (m, 4H), 1.86 (p, J = 6.2 Hz, 2H), 1.55–1.48 (m, 4H), and 1.39–1.33 (m, 2H). 13C NMR (101 MHz, DMSO) δ 157.78, 132.12, 116.75, 111.86, 66.72, 62.20, 54.37, 27.88, 25.58, and 23.91. MS (ESI) m/z: 298.1 (100) [M + H]+.
1-(3-(3-Bromophenoxy)-2,2-dimethylpropyl)piperidine (6f). Yield 42% (four stages), colorless liquid. 1H NMR (400 MHz, DMSO-d6) δ 7.22 (t, J = 8.1 Hz, 1H), 7.13–7.08 (m, 2H), 6.93 (ddd, J = 8.3, 2.4, 0.9 Hz, 1H), 3.88 (s, 2H), 3.78 (s, 2H), 2.43–2.36 (m, 4H), 1.50–1.44 (m, 4H), 1.39–1.31 (m, 2H), and 0.91 (s, 6H). 13C NMR (101 MHz, DMSO) δ 160.15, 131.12, 123.16, 122.02, 117.29, 113.97, 69.33, 64.59, 54.38, 36.35, 25.57, 23.93, and 21.49. MS (ESI) m/z: 326.1 (100) [M + H]+.
1-((1-((4-Bromo-2-methylphenoxy)methyl)cyclopropyl)methyl)piperidine (6g). Yield 40% (four stages), colorless liquid. 1H NMR (400 MHz, DMSO-d6) δ 7.31–7.22 (m, 2H), 6.87 (d, J = 8.6 Hz, 1H), 3.85 (s, 2H), 3.63 (s, 2H), 2.47–2.32 (m, 4H), 2.11 (s, 3H), 1.53–1.44 (m, 4H), 1.44–1.31 (m, 2H), and 0.79–0.71 (m, 4H). 13C NMR (101 MHz, DMSO) δ 156.30, 132.52, 131.54, 129.32, 113.47, 111.31, 68.66, 65.03, 54.38, 25.99, 24.46, 22.46, 15.62, and 7.91. MS (ESI) m/z: 338.1 (100) [M + H]+.
1-(2-(5-Bromo-2-methylphenoxy)ethyl)piperidine (6h). Yield 41% (four stages), colorless liquid. 1H NMR (400 MHz, DMSO-d6) δ 7.10–7.06 (m, 2H), 7.03–6.98 (m, 1H), 4.05 (t, J = 5.8 Hz, 2H), 2.66 (t, J = 5.8 Hz, 2H), 2.45–2.33 (m, 4H), 2.10 (s, 3H), 1.55–1.43 (m, 4H), and 1.40–1.34 (m, 2H). 13C NMR (101 MHz, DMSO) δ 157.46, 131.75, 125.50, 122.81, 118.99, 114.49, 66.12, 57.38, 54.39, 25.58, 23.92, and 15.60. MS (ESI) m/z: 298.1 (100) [M + H]+.
1-(2-(4-Bromo-2,6-dimethylphenoxy)ethyl)piperidine (6i). Yield 39% (four stages), colorless liquid. 1H NMR (400 MHz, DMSO-d6) δ 7.85 (d, J = 2.3 Hz, 2H), 3.97 (t, J = 5.8 Hz, 2H), 2.64 (t, J = 5.8 Hz, 2H), 2.44–2.37 (m, 4H), 2.22 (s, 6H), 1.51–1.44 (m, 4H), and 1.39–1.33 (m, 2H). 13C NMR (101 MHz, DMSO) δ 156.13, 132.99, 120.02, 113.42, 66.01, 57.38, 54.40, 25.55, 23.90, and 15.38. MS (ESI) m/z: 312.1 (100) [M + H]+.
General procedure for obtaining aryl-, heteroarylboronic acids. Aryl bromide (10 mmol) and 40 mL THF were placed in a dried flask purged with argon. The resulting solution was cooled to −78 °C, and 10 mL of n-BuLi 1 M solution in hexane was added dropwise and stirred for 2 h at −78 °C. Then, trimethyl borate (0.98 mL, 11 mmol) in 10 mL THF was added and stirred for 1 h at −78 °C. After warming to room temperature, the reaction mixture was diluted with 20 mL water, and 10 mL of saturated NH4Cl solution was added and stirred for 1 h. Then, the solvent was distilled to dryness under reduced pressure, and the residue was extracted with ethyl acetate. The resulting solution was dried with Na2SO4 and completely evaporated under reduced pressure. The residue was dissolved in 10 mL ethyl acetate and the product was precipitated by adding hexane. The resulting oil was suitable for cross-coupling according to Suzuki–Miyaura. If necessary, the product was purified by recrystallization from a hexane-ethyl acetate mixture without identification.
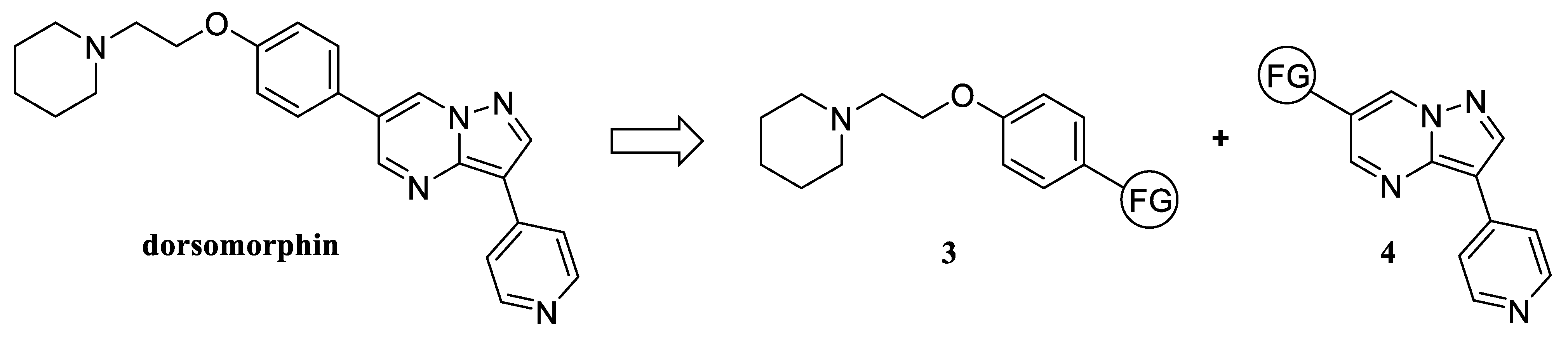
General procedure for obtaining 6-aryl-substituted 3-pyridylpyrazolo[1,5-a]pyrimidines. Substituted aryl bromide (10 mmol), 0.05 g Pd[P(Ph)3]4 (0.043 mmol, 0.3 mol%) and 3.1 g (3-pyridylpyrazolo[1,5-a]pyrimidin-6-yl)boronic acid (13 mmol) were dissolved in 100 mL THF with stirring. The resulting mixture was stirred for 30 min under a weak stream of argon, and then a solution of 5 g K2CO3 (36 mmol) in 50 mL water was added. The reaction mixture was refluxed for 4 h with vigorous stirring in a weak stream of argon. Upon the completion of the reaction, the solvent was distilled to dryness under reduced pressure. The residue was stirred in 200 mL of boiling butyl acetate, then filtered hot. The filtrate was clarified with 2 g silica gel while boiling and stirring, filtered again, and the volume of the solution was brought to 30 mL. The precipitate formed after 16 h was filtered.
General procedure for obtaining 6-aryl-5,7-dimethyl-substituted 3-pyridylpyrazolo[1,5-a]pyrimidines. A total of 0.04 g Pd2(dba)3 (0.044 mmol, 0.66 mol%) and 0.08 g 2-dicyclohexylphosphino-2′,6′-dimethoxybiphenyl (Sphos, 0.195 mmol) were dissolved in 120 mL toluene with stirring. The solution was stirred for 30 min under a slight stream of argon. 6-Bromo-5,7-dimethyl-substituted 3-pyridylpyrazolo[1,5-a]pyrimidine (2 g, 6.6 mmol), boronic acid (11 mmol), and 6 g Cs2CO3 (18.4 mmol) were added to the reaction mixture. The reaction was carried out at 100 °C with vigorous stirring in a weak stream of argon for 16 h. After the reaction was complete, the volume of toluene was brought to 200 mL, the mixture was heated until boiling and filtered hot. The filtrate was clarified with 1 g silica gel while boiling and stirring, filtered again, and the volume of the solution was brought to 15 mL. The precipitated product was filtered after 24 h.
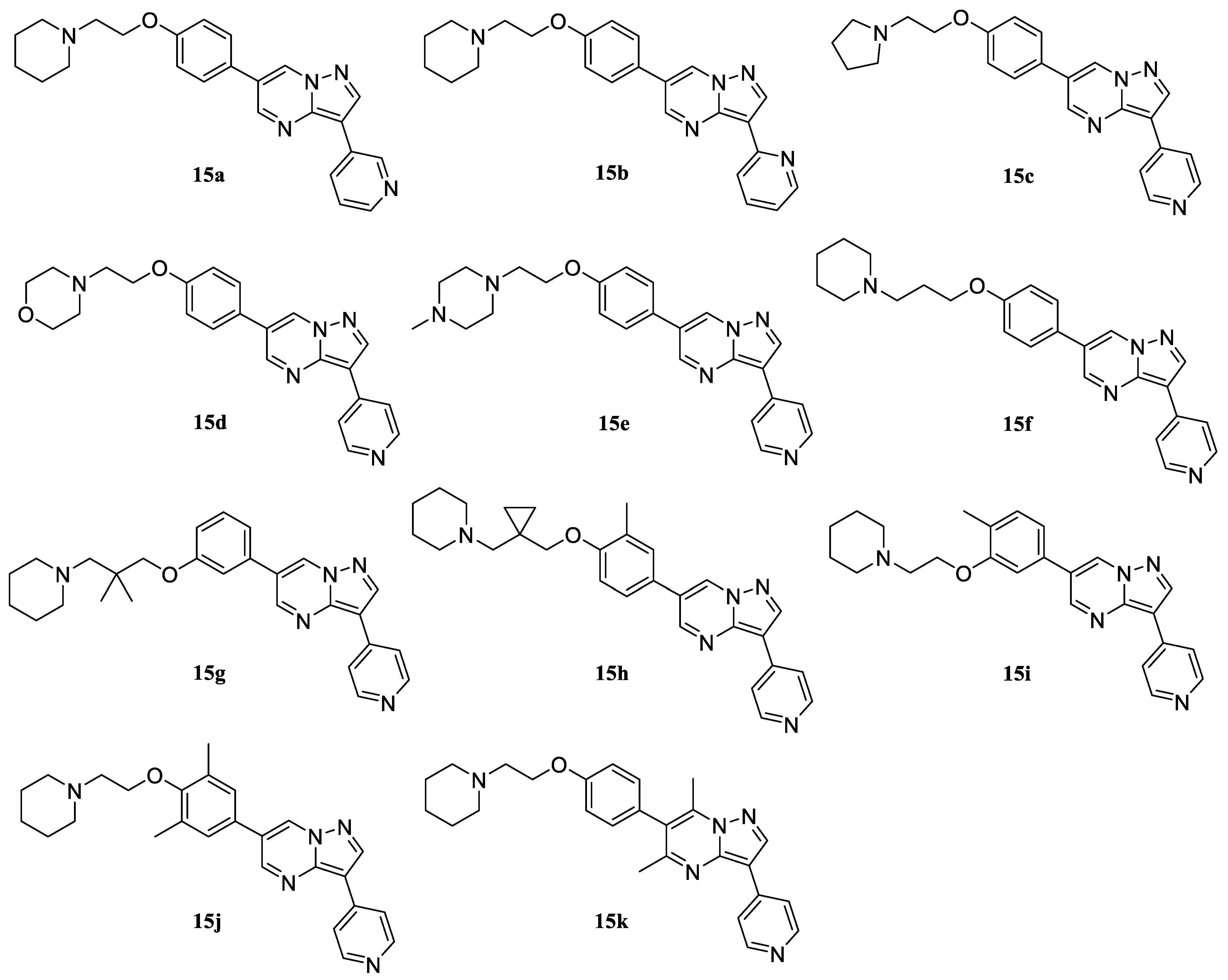
6-(4-(2-(Piperidin-1-yl)ethoxy)phenyl)-3-(pyridin-3-yl)pyrazolo[1,5-a]pyrimidine (15a). Yield 49% (from two stages), yellowish solid. 1H NMR (400 MHz, DMSO-d6) δ 9.50 (d, J = 2.3 Hz, 1H), 9.39 (dd, J = 2.3, 0.8 Hz, 1H), 9.08 (d, J = 2.3 Hz, 1H), 8.89 (s, 1H), 8.54 (ddd, J = 8.0, 2.3, 1.6 Hz, 1H), 8.46 (dd, J = 4.8, 1.6 Hz, 1H), 7.82 (d, J = 8.8 Hz, 2H), 7.49 (ddd, J = 8.0, 4.8, 0.9 Hz, 1H), 7.11 (d, J = 8.8 Hz, 2H), 4.15 (t, J = 5.8 Hz, 2H), 2.69 (t, J = 5.8 Hz, 2H), 2.50–2.45 (m, 4H), 1.55–1.47 (m, 4H), and 1.43–1.35 (m, 2H). 13C NMR (101 MHz, DMSO) δ 159.75, 150.42, 146.88, 146.66, 143.28, 142.92, 132.39, 132.30, 128.20, 128.01, 125.47, 123.77, 121.80, 115.26, 106.43, 65.80, 57.34, 54.44, 25.60, and 23.96. MS (ESI) m/z: 400.2 (100) [M + H]+.
6-(4-(2-(Piperidin-1-yl)ethoxy)phenyl)-3-(pyridin-2-yl)pyrazolo[1,5-a]pyrimidine (15b). Yield 48% (from two stages), yellowish solid. 1H NMR (400 MHz, DMSO-d6) δ 9.49 (d, J = 2.2 Hz, 1H), 9.09 (d, J = 2.2 Hz, 1H), 8.81 (s, 1H), 8.60 (ddd, J = 4.9, 1.8, 1.0, 1H), 8.47 (dt, J = 7.7, 1.1 Hz, 1H), 7.88 (td, J = 7.7, 1.8 Hz, 1H), 7.82 (d, J = 8.7 Hz, 2H), 7.24 (ddd, J = 7.6, 4.8, 1.2 Hz, 1 H), 7.10 (d, J = 8.7 Hz, 2H), 4.13 (t, J = 5.6 Hz, 2H), 2.67 (t, J = 5.6 Hz, 2H), 2.49–2.39 (m, 4H), 1.55–1.46 (m, 4H), and 1.42–1.34 (m, 2H). 13C NMR (101 MHz, DMSO) δ 158.90, 150.93, 150.44, 149.39, 144.06, 143.55, 136.75, 132.54, 128.22, 125.42, 121.80, 121.11, 120.31, 115.26, 109.65, 65.79, 57.34, 54.43, 25.60, and 23.95. MS (ESI) m/z: 400.2 (100) [M + H]+.
3-(Pyridin-4-yl)-6-(4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)pyrazolo[1,5-a]pyrimidine (15c). Yield 50% (from two stages), yellowish solid. 1H NMR (400 MHz, DMSO-d6) δ 9.54 (d, J = 2.3 Hz, 1H), 9.14 (d, J = 2.3 Hz, 1H), 8.99 (s, 1H), 8.60 (d, J = 5.3 Hz, 2H), 8.15 (d, J = 5.3 Hz, 2H), 7.85 (d, J = 8.9 Hz, 2H), 7.12 (d, J = 8.9 Hz, 2H), 4.14 (t, J = 5.7 Hz, 2H), 2.79 (t, J = 5.7 Hz, 2H), 2.46–2.39 (m, 4H), and 1.68–1.60 (m, 4H). 13C NMR (101 MHz, DMSO) δ 158.98, 150.75, 149.98, 143.86, 143.82, 139.31, 132.60, 128.28, 125.35, 122.20, 119.65, 115.30, 106.07, 64.67, 57.03, 53.88, and 23.11. MS (ESI) m/z: 386.2 (100) [M + H]+.
4-(2-(4-(3-(Pyridin-4-yl)pyrazolo[1,5-a]pyrimidin-6-yl)phenoxy)ethyl)morpholine (15d). Yield 46% (from two stages), yellowish solid. 1H NMR (400 MHz, DMSO-d6) δ 9.53 (d, J = 2.3 Hz, 1H), 9.13 (d, J = 2.3 Hz, 1H), 8.97 (s, 1H), 8.59 (d, J = 5.2 Hz, 2H), 8.16 (d, J = 5.2 Hz, 2H), 7.83 (d, J = 8.8 Hz, 2H), 7.11 (d, J = 8.8 Hz, 2H), 4.17 (t, J = 5.8 Hz, 2H), 3.56–3.52 (m, 4H), 2.74 (t, J = 5.8 Hz, 2H), and 2.40–2.35 (m, 4H). 13C NMR (101 MHz, DMSO) δ 158.82, 150.60, 149.99, 143.84, 143.80, 139.27, 132.58, 128.27, 125.31, 122.17, 119.63, 115.28, 106.71, 66.27, 65.50, 57.12, and 53.81. MS (ESI) m/z: 402.2 (100) [M + H]+.
6-(4-(2-(4-Methylpiperazin-1-yl)ethoxy)phenyl)-3-(pyridin-4-yl)pyrazolo[1,5-a]pyrimidine (15e). Yield 50% (from two stages), yellowish solid. 1H NMR (400 MHz, DMSO-d6) δ 9.54 (d, J = 2.2 Hz, 1H), 9.14 (d, J = 2.2 Hz, 1H), 8.98 (s, 1H), 8.60 (d, J = 5.2 Hz, 2H), 8.18 (d, J = 5.2 Hz, 2H), 7.85 (d, J = 8.8 Hz, 2H), 7.12 (d, J = 8.8 Hz, 2H), 4.17 (t, J = 5.8 Hz, 2H), 2.69 (t, J = 5.8 Hz, 2H), 2.49–2.39 (m, 4H), 2.38–2.28 (m, 4H), and 2.12 (s, 3H). 13C NMR (101 MHz, DMSO) δ 158.90, 150.70, 149.98, 143.84, 143.79, 139.25, 132.55, 128.26, 125.29, 122.16, 119.65, 115.29, 106.53, 64.91, 57.32, 54.80, 53.25, and 45.83. MS (ESI) m/z: 415.2 (100) [M + H]+.
6-(4-(3-(Piperidin-1-yl)propoxy)phenyl)-3-(pyridin-4-yl)pyrazolo[1,5-a]pyrimidine (15f). Yield 48% (from two stages), yellowish solid. 1H NMR (400 MHz, DMSO-d6) δ 9.53 (d, J = 2.2 Hz, 1H), 9.14 (d, J = 2.2 Hz, 1H), 8.97 (s, 1H), 8.59 (d, J = 5.2 Hz, 2H), 8.16 (d, J = 5.2 Hz, 2H), 7.83 (d, J = 8.9 Hz, 2H), 7.11 (d, J = 8.9 Hz, 2H), 4.13 (t, J = 5.9, 2H), 2.72 (t, J = 5.9 Hz, 2H), 2.45–2.35 (m, 4H), 1.89 (p, J = 5.9 Hz, 2H), 1.54–1.47 (m, 4H), and 1.39–1.32 (m, 2H). 13C NMR (101 MHz, DMSO) δ 158.98, 150.77, 149.99, 143.85, 143.81, 139.29, 132.61, 128.27, 125.30, 122.18, 119.62, 115.23, 106.03, 66.67, 62.30, 54.45, 27.88, 25.60, and 23.98. MS (ESI) m/z: 414.2 (100) [M + H]+.
6-(3-(2,2-Dimethyl-3-(piperidin-1-yl)propoxy)phenyl)-3-(pyridin-4-yl)pyrazolo[1,5-a]pyrimidine (15g). Yield 49% (from two stages), yellowish solid. 1H NMR (400 MHz, DMSO-d6) δ 9.62 (d, J = 2.3 Hz, 1H), 9.16 (d, J = 2.3 Hz, 1H), 8.99 (s, 1H), 8.59 (d, J = 6.2 Hz, 2H), 8.16 (d, J = 6.2 Hz, 2H), 7.48–7.44 (m, 3H), 7.07–7.00 (m, 1H), 3.90 (s, 2H), 3.76 (s, 2H), 2.46–2.36 (m, 4H), 1.53–1.41 (m, 4H), 1.41–1.32 (m, 2H), and 0.91 (s, 6H). 13C NMR (101 MHz, DMSO) δ 159.95, 150.93, 149.99, 144.14, 144.12, 139.20, 134.54, 133.66, 130.34, 122.15, 119.66, 119.17, 114.35, 112.35, 106.19, 68.81, 63.75, 54.44, 36.39, 25.62, 23.98, and 21.48. MS (ESI) m/z: 442.3 (100) [M + H]+.
6-(3-Methyl-4-((1-(piperidin-1-ylmethyl)cyclopropyl)methoxy)phenyl)-3-(pyridin-4-yl)pyrazolo[1,5-a]pyrimidine (15h). Yield 45% (from two stages), yellowish solid. 1H NMR (400 MHz, DMSO-d6) δ 9.59 (d, J = 2.3 Hz, 1H), 9.12 (d, J = 2.3 Hz, 1H), 8.98 (s, 1H), 8.61 (d, J = 5.2 Hz, 2H), 8.15 (d, J = 5.2 Hz, 2H), 7.63–7.55 (m, 2H), 7.07 (d, J = 8.6 Hz, 1H), 3.89 (s, 2H), 3.61 (s, 2H), 2.48–2.35 (m, 4H), 2.11 (s, 3H), 1.52–1.45 (m, 4H), 1.44–1.35 (m, 2H), and 0.75–0.68 (m, 4H). 13C NMR (101 MHz, DMSO) δ 158.12, 150.78, 149.99, 143.86, 143.84, 139.29, 133.15, 132.62, 131.40, 128.77, 122.18, 120.45, 119.65, 116.50, 106.84, 67.99, 64.23, 54.50, 25.63, 23.90, 22.40, 15.60, and 7.92. MS (ESI) m/z: 454.3 (100) [M + H]+.
6-(4-Methyl-3-(2-(piperidin-1-yl)ethoxy)phenyl)-3-(pyridin-4-yl)pyrazolo[1,5-a]pyrimidine (15i). Yield 47% (from two stages), yellowish solid. 1H NMR (400 MHz, DMSO-d6) δ 9.53 (d, J = 2.3 Hz, 1H), 9.13 (d, J = 2.3 Hz, 1H), 8.98 (s, 1H), 8.60 (d, J = 6.2 Hz, 2H), 8.17 (d, J = 6.2 Hz, 2H), 7.53–7.49 (m, 1H), 7.14–7.09 (m, 2H), 4.17 (t, J = 5.9 Hz, 2H), 2.70 (t, J = 5.9 Hz, 2H), 2.45–2.31 (m, 4H), 2.10 (s, 3H), 1.55–1.46 (m, 4H), and 1.41–1.32 (m, 2H). 13C NMR (101 MHz, DMSO) δ 158.56, 150.77, 149.96, 143.86, 143.80, 139.29, 132.57, 131.77, 125.60, 124.27, 122.75, 122.18, 119.63, 115.45, 106.29, 66.10, 57.35, 54.42, 25.60, 23.95, and 15.60. MS (ESI) m/z: 414.2 (100) [M + H]+.
6-(3,5-Dimethyl-4-(2-(piperidin-1-yl)ethoxy)phenyl)-3-(pyridin-4-yl)pyrazolo[1,5-a]pyrimidine (15j). Yield 50% (from two stages), yellowish solid. 1H NMR (400 MHz, DMSO-d6) δ 9.50 (d, J = 2.2 Hz, 1H), 9.11 (d, J = 2.2 Hz, 1H), 8.96 (s, 1H), 8.58 (d, J = 5.2 Hz, 2H), 8.14 (d, J = 5.2 Hz, 2H), 7.49 (d, J = 2.4 Hz, 2H), 4.08 (t, J = 5.8 Hz, 2H), 2.70 (t, J = 5.8 Hz, 2H), 2.48–2.40 (m, 4H), 2.22 (s, 6H), 1.52–1.45 (m, 4H), and 1.40–1.35 (m, 2H). 13C NMR (101 MHz, DMSO) δ 156.22, 150.75, 149.97, 143.86, 143.82, 139.30, 132.94, 129.80, 127.78, 125.30, 122.18, 119.60, 106.15, 65.98, 57.42, 54.44, 25.61, 23.97, and 15.38. MS (ESI) m/z: 428.2 (100) [M + H]+.
5,7-Dimethyl-6-(4-(2-(piperidin-1-yl)ethoxy)phenyl)-3-(pyridin-4-yl)pyrazolo[1,5-a]pyrimidine (15k). Yield 35% (from two stages), yellowish solid. 1H NMR (400 MHz, DMSO-d6) δ 8.91 (s, 1H), 8.56 (d, J = 6.2 Hz, 2H), 8.19 (d, J = 6.2 Hz, 2H), 7.32 (d, J = 8.7 Hz, 2H), 7.11 (d, J = 8.7 Hz, 2H), 4.14 (t, J = 5.6 Hz, 2H), 2.68 (t, J = 5.6 Hz, 2H), 2.48 (s, 3H), 2.48–2.38 (m, 4H), 2.35 (s, 3H), 1.53–1.44 (m, 4H), and 1.40–1.33 (m, 2H). 13C NMR (101 MHz, DMSO) δ 159.63, 159.23, 149.85, 144.14, 143.84, 142.82, 139.81, 131.25, 126.90, 122.23, 119.42, 114.82, 105.89, 65.81, 57.34, 54.44, 25.60, 24.87, 23.96, and 14.89. MS (ESI) m/z: 428.2 (100) [M + H]+.