Error in Figure
In the original publication [1], there was a mistake in Figure 4 as published. The first picture in the second row and the second picture in the third row are mistyped. The corrected Figure 4 appears below.
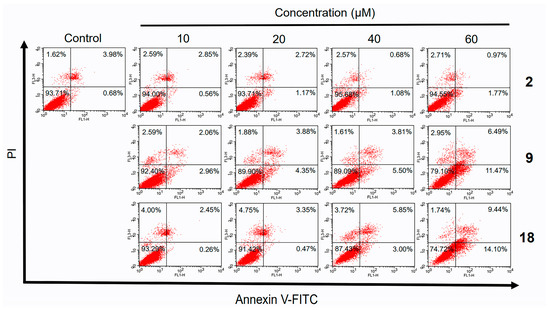
Figure 4.
Flow cytometry analysis of HepG2 cells treated with different concentrations of norcantharidin (2), compounds 9 and 18 for 48 h. Treated cells were examined for apoptotic cells using Annexin V-FITC apoptosis detection kit. Annexin V-positive/PI-negative cells were in early stages of apoptosis and double-positive cells were in late stages of apoptosis, whereas annexin V-negative/PI-positive cells were necrotic.
The authors state that the scientific conclusions are unaffected. This correction was approved by the Academic Editor. The original publication has also been updated.
Reference
- Wu, J.-Y.; Kuo, C.-D.; Chu, C.-Y.; Chen, M.-S.; Lin, J.-H.; Chen, Y.-J.; Liao, H.-F. Synthesis of Novel Lipophilic N-Substituted Norcantharimide Derivatives and Evaluation of Their Anticancer Activities. Molecules 2014, 19, 6911–6928. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).