Metabolomic Profiling of Osteoblasts in Rat Subchondral Bone Following Anterior Cruciate Ligament Injury
Abstract
1. Introduction
2. Results
2.1. Histological Changes in Bone Tissue Following Anterior Cruciate Ligament Transection (ACLT)
2.2. Morphological Observation and Alkaline Phosphatase (ALP) Staining Results of Primary Osteoblasts
2.3. Metabolite Identification in Osteoblasts
2.4. Metabolic Profiles of Osteoblasts at Different Time Points After ACLT
2.5. Differencial Metabolites in Osteoblasts at Different Time Points After ACLT
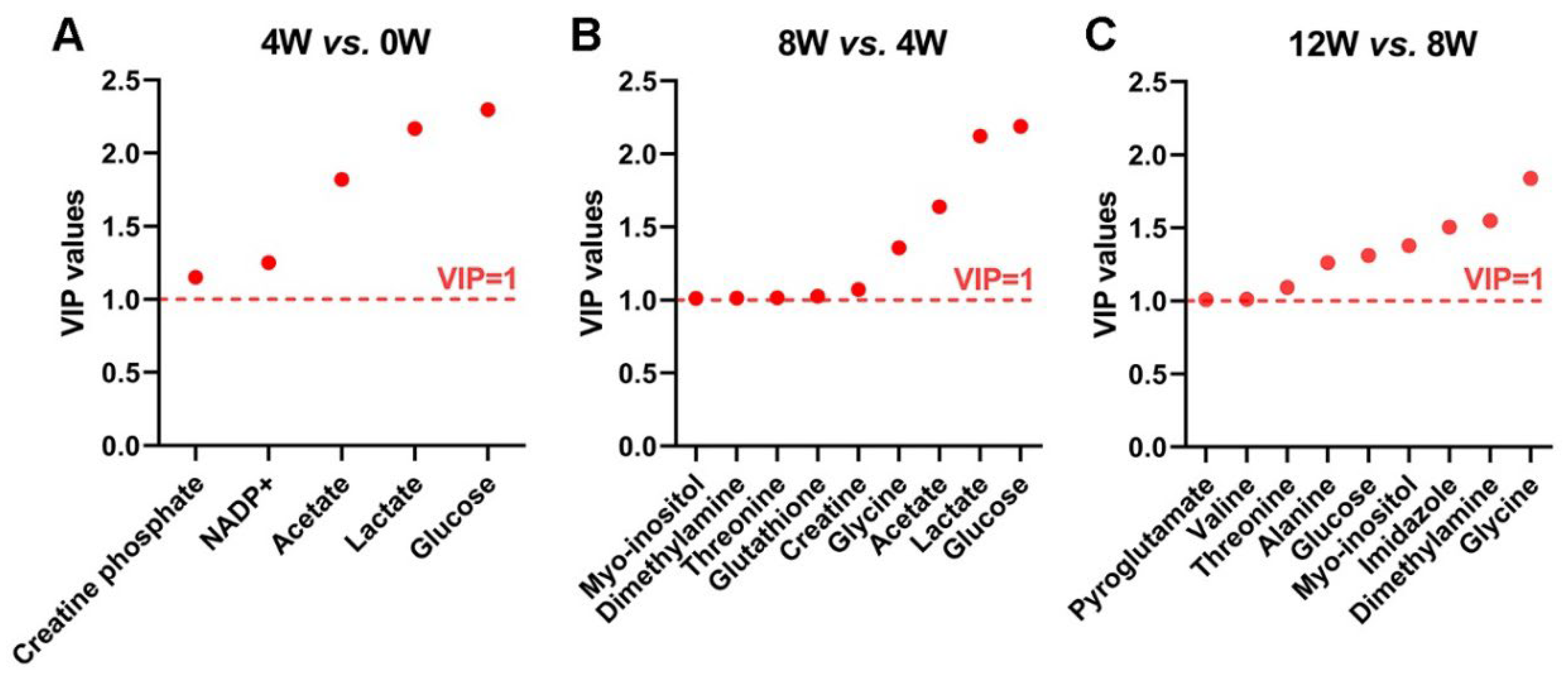
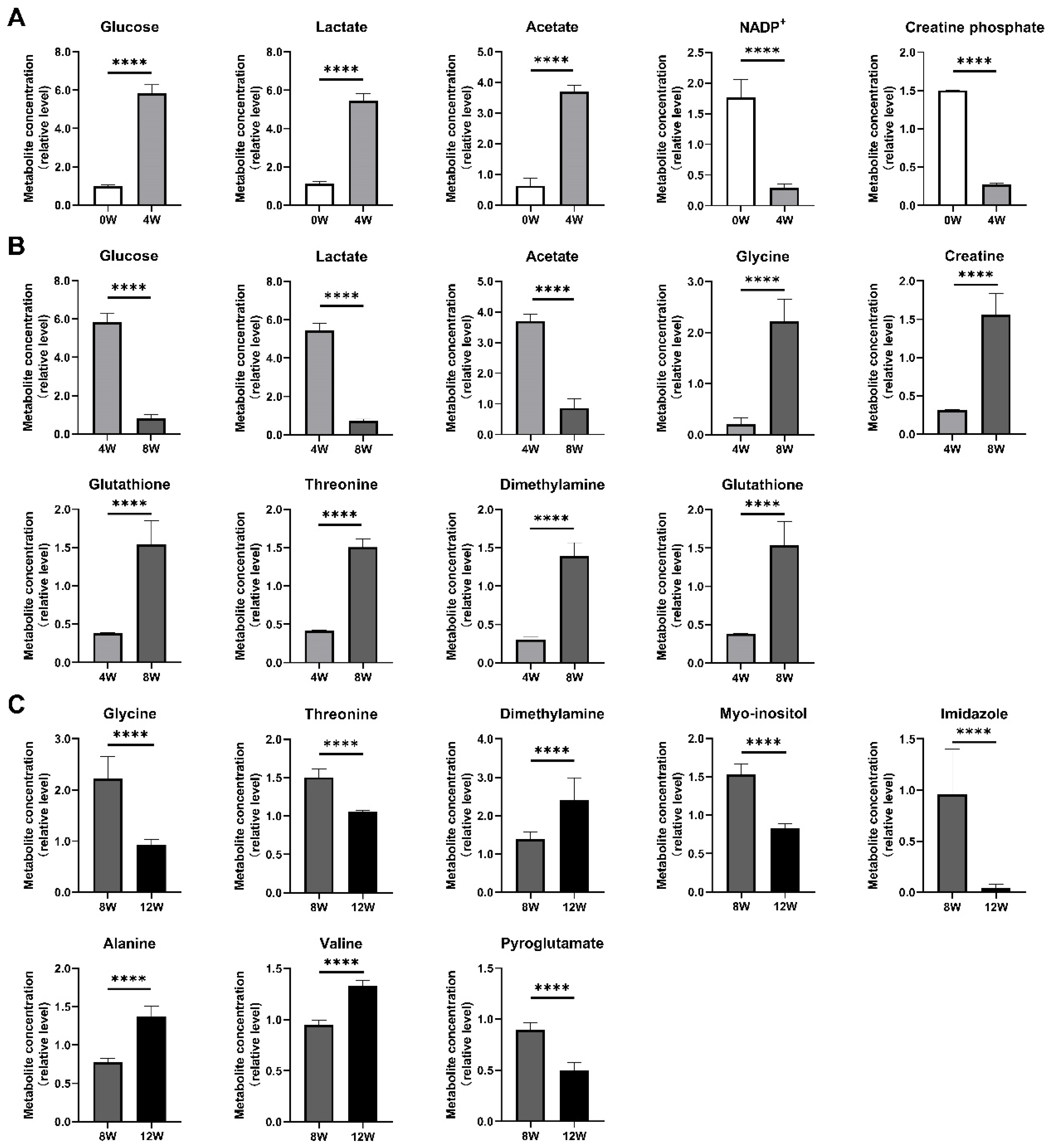
2.6. Significant Metabolites in Osteoblasts at Different Time Points After ACLT
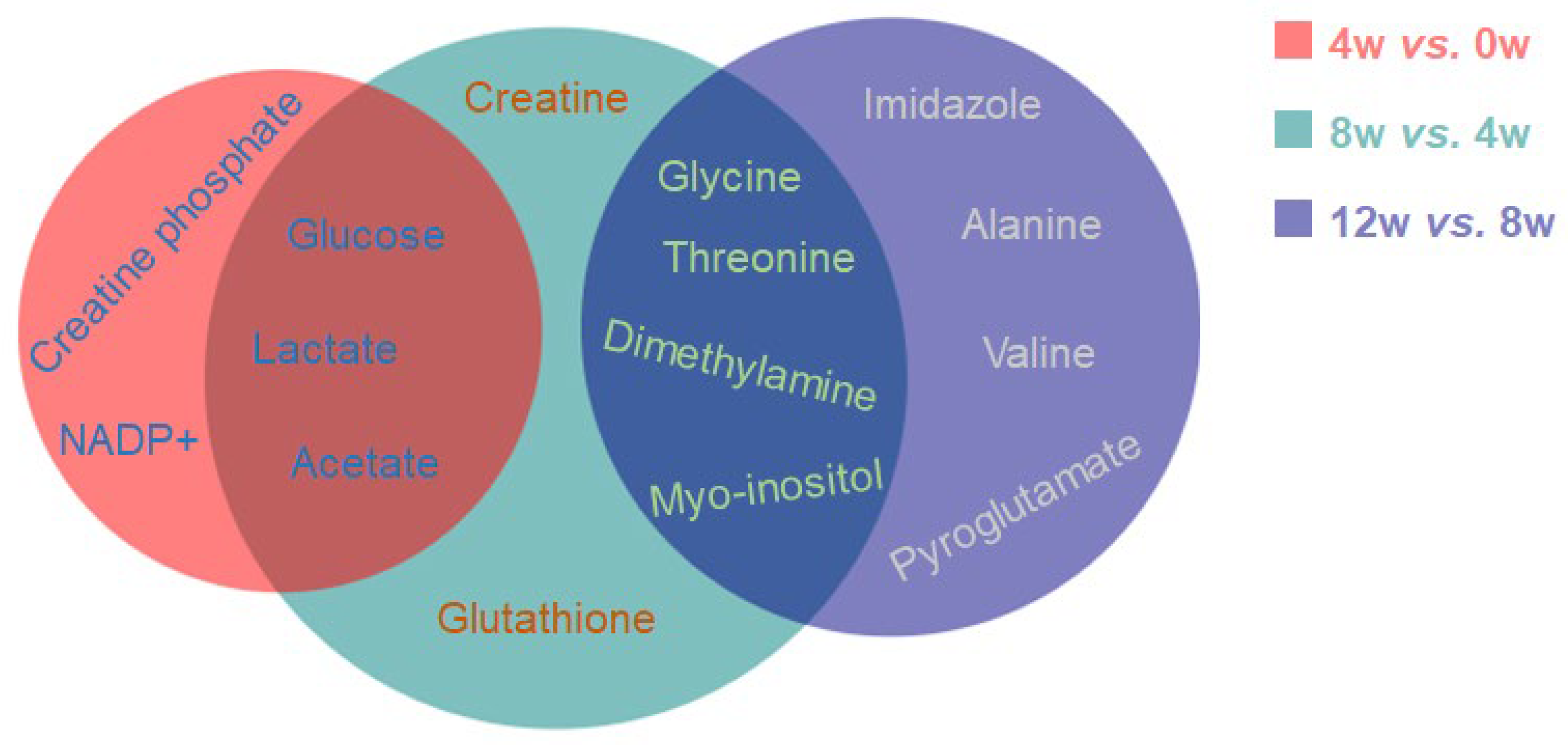
2.7. Characteristic Metabolites of Osteoblasts at Different Time Points After ACLT
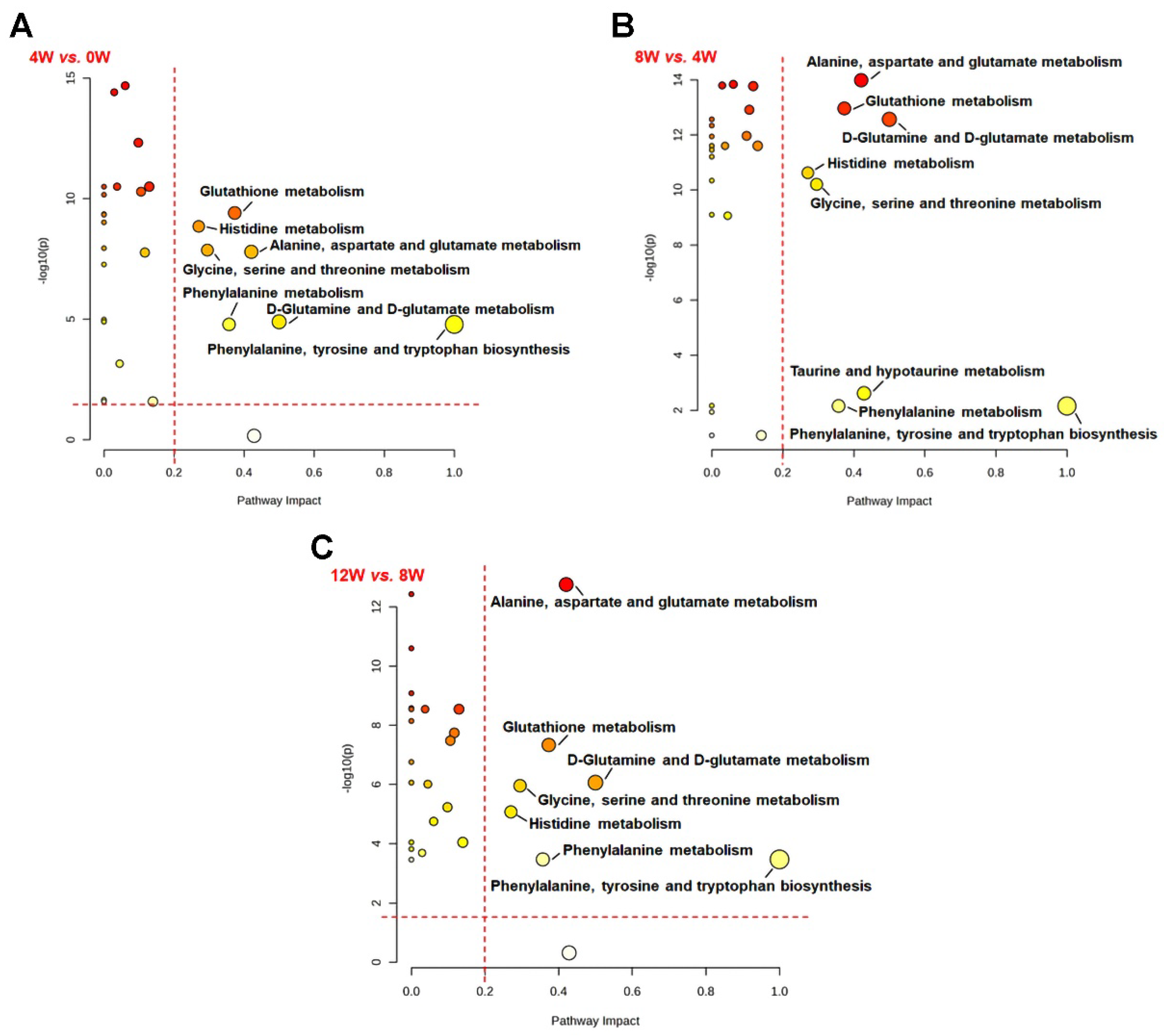
2.8. Key Metabolic Pathways in Osteoblast at Different Time Points Following ACLT
3. Discussion
3.1. Metabolic Changes in Energy Substrates
3.2. Role of BCAA Metabolism
3.3. Alanine Metabolism and Osteoblast Function
3.4. Limitations
4. Materials and Methods
4.1. Osteoarthritis (OA) Animal Models
4.2. Hematoxylin and Eosin (H&E) Staining Procedure
4.3. Acquisition of Primary Osteoblasts
4.4. Cell Culture
4.5. Identification of Primary Osteoblasts via Morphological Observation and Alkaline Phosphatase (ALP) Staining
4.6. Extraction of Intracellular Metabolites
4.7. Acquisition of NMR Spectra
4.8. Processing of NMR Spectra
4.9. Resonance Assigments of Metabolites
4.10. Multivariate and Univariate Statistical Analyses of NMR Dataset
4.11. Metabolic Pathway Analysis
4.12. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Australian Institute of Health and Welfare. Chronic Musculoskeletal Conditions; AIHW: Canberra, Australia, 2023.
- Blagojevic, M.; Jinks, C.; Jeffery, A.; Jordan, K.P. Risk factors for onset of osteoarthritis of the knee in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2010, 18, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.C.; Hubbard-Turner, T.; Wikstrom, E.A.; Palmieri-Smith, R.M. Epidemiology of Posttraumatic Osteoarthritis. J. Athl. Train. 2017, 52, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Rodeo, S. Review of current understanding of post-traumatic osteoarthritis resulting from sports injuries. J. Orthop. Res. 2017, 35, 397–405. [Google Scholar] [CrossRef]
- Friel, N.A.; Chu, C.R. The role of ACL injury in the development of posttraumatic knee osteoarthritis. Clin. Sports Med. 2013, 32, 1–12. [Google Scholar] [CrossRef]
- Bendele, A.M. Animal models of osteoarthritis. J. Musculoskelet. Neuronal Interact. 2001, 1, 363–376. [Google Scholar]
- Fang, H.; Beier, F. Mouse models of osteoarthritis: Modelling risk factors and assessing outcomes. Nat. Rev. Rheumatol. 2014, 10, 413–421. [Google Scholar] [CrossRef]
- Jones, G.; Cooley, H.M.; Bellamy, N. A cross-sectional study of the association between Heberden’s nodes, radiographic osteoarthritis of the hands, grip strength, disability and pain. Osteoarthr. Cartil. 2001, 9, 606–611. [Google Scholar] [CrossRef]
- Batiste, D.L.; Kirkley, A.; Laverty, S.; Thain, L.M.F.; Spouge, A.R.; Holdsworth, D.W. Ex vivo characterization of articular cartilage and bone lesions in a rabbit ACL transection model of osteoarthritis using MRI and micro-CT. Osteoarthr. Cartil. 2004, 12, 986–996. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Mickiewicz, B.; Kelly, J.J.; Ludwig, T.E.; Weljie, A.M.; Wiley, J.P.; Schmidt, T.A.; Vogel, H.J. Metabolic analysis of knee synovial fluid as a potential diagnostic approach for osteoarthritis. J. Orthop. Res. 2015, 33, 1631–1638. [Google Scholar] [CrossRef]
- Damyanovich, A.Z.; Avery, L.; Staples, J.R.; Marshall, K.W. 1H NMR metabolic profiling of synovial fluid from patients with anterior cruciate ligament tears and hemarthrosis. Osteoarthr. Cartil. 2023, 31, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.S.; Zhuang, H.M.; Ren, X.S.; Zhang, Y.L.; Zhou, P.H. The metabolic characteristics and changes of chondrocytes and in osteoarthritis. Front. Endocrinol. 2024, 15, 1393550. [Google Scholar] [CrossRef]
- Zhen, G.; Wen, C.; Jia, X.; Li, Y.; Crane, J.L.; Mears, S.C.; Askin, F.B.; Frassica, F.J.; Chang, W.; Yao, J.; et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 2013, 19, 704–712. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Ramme, A.J.; Lendhey, M.; Raya, J.G.; Kirsch, T.; Kennedy, O.D. A novel rat model for subchondral microdamage in acute knee injury: A potential mechanism in post-traumatic osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1776–1785. [Google Scholar] [CrossRef]
- Pauly, H.M.; Larson, B.E.; Coatney, G.A.; Button, K.D.; DeCamp, C.E.; Fajardo, R.S.; Haut, R.C.; Haut Donahue, T.L. Assessment of cortical and trabecular bone changes in two models of post-traumatic osteoarthritis. J. Orthop. Res. 2015, 33, 1835–1845. [Google Scholar] [CrossRef]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef]
- Henrotin, Y.; Pesesse, L.; Sanchez, C. Subchondral bone and osteoarthritis: Biological and cellular aspects. Osteoporos. Int. 2012, 23, 847–851. [Google Scholar] [CrossRef]
- Zhai, G. Alteration of Metabolic Pathways in Osteoarthritis. Metabolites 2019, 9, 11. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C. Systems biology: Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef]
- Showiheen, S.A.A.; Sun, A.R.; Wu, X.; Crawford, R.; Xiao, Y.; Wellard, R.M.; Prasadam, I. Application of Metabolomics to Osteoarthritis: From Basic Science to the Clinical Approach. Curr. Rheumatol. Rep. 2019, 21, 26. [Google Scholar] [CrossRef]
- Halama, A. Metabolomics in cell culture—A strategy to study crucial metabolic pathways in cancer development and the response to treatment. Arch. Biochem. Biophys. 2014, 564, 100–109. [Google Scholar] [CrossRef] [PubMed]
- McGarrah, R.W.; Crown, S.B.; Zhang, G.-F.; Shah, S.H.; Newgard, C.B. Cardiovascular metabolomics. Circ. Res. 2018, 122, 1238–1258. [Google Scholar] [CrossRef]
- Anderson, J.R.; Chokesuwattanaskul, S.; Phelan, M.M.; Welting, T.J.M.; Lian, L.Y.; Peffers, M.J.; Wright, H.L. 1H NMR Metabolomics Identifies Underlying Inflammatory Pathology in Osteoarthritis and Rheumatoid Arthritis Synovial Joints. J. Proteome Res. 2018, 17, 3780–3790. [Google Scholar] [CrossRef]
- Anderson, J.R.; Phelan, M.M.; Foddy, L.; Clegg, P.D.; Peffers, M.J. Ex Vivo Equine Cartilage Explant Osteoarthritis Model: A Metabolomics and Proteomics Study. J. Proteome Res. 2020, 19, 3652–3667. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, M.; Shi, T.; Gao, M.; Lv, Y.; Zhao, Y.; Li, J.; Zhang, M.; Zhang, H.; Guan, F.; et al. Analysis of Serum Metabolomics in Rats with Osteoarthritis by Mass Spectrometry. Molecules 2021, 26, 7181. [Google Scholar] [CrossRef]
- Hislop, B.D.; Devine, C.; June, R.K.; Heveran, C.M. Subchondral bone structure and synovial fluid metabolism are altered in injured and contralateral limbs 7 days after non-invasive joint injury in skeletally-mature C57BL/6 mice. Osteoarthr. Cartil. 2022, 30, 1593–1605. [Google Scholar] [CrossRef]
- Svoboda, S.J. ACL injury and posttraumatic osteoarthritis. Clin. Sports Med. 2014, 33, 633–640. [Google Scholar] [CrossRef]
- Chang, J.C.; Sebastian, A.; Murugesh, D.K.; Hatsell, S.; Economides, A.N.; Christiansen, B.A.; Loots, G.G. Global molecular changes in a tibial compression induced ACL rupture model of post-traumatic osteoarthritis. J. Orthop. Res. 2017, 35, 474–485. [Google Scholar] [CrossRef]
- Riordan, E.A.; Little, C.; Hunter, D. Pathogenesis of post-traumatic OA with a view to intervention. Best Pract. Res. Clin. Rheumatol. 2014, 28, 17–30. [Google Scholar] [CrossRef]
- Beresford, J.N.; Bennett, J.H.; Devlin, C.; Leboy, P.S.; Owen, M.E. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J. Cell Sci. 1992, 102, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Aubin, J.E.; Turksen, K.; Heersche, J.N.M. Osteoblastic Cell Lineage. In Cellular and Molecular Biology of Bone; Academic Press: Cambridge, MA, USA, 1993; pp. 1–45. [Google Scholar]
- Zhang, H.; Wang, L.; Cui, J.; Wang, S.; Han, Y.; Shao, H.; Wang, C.; Hu, Y.; Li, X.; Zhou, Q.; et al. Maintaining hypoxia environment of subchondral bone alleviates osteoarthritis progression. Sci. Adv. 2023, 9, eabo7868. [Google Scholar] [CrossRef]
- Burr, D.B.; Gallant, M.A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef]
- Lories, R.J.; Luyten, F.P. The bone-cartilage unit in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 43–49. [Google Scholar] [CrossRef]
- Jung, J.W.; Macalino, S.J.Y.; Cui, M.; Kim, J.E.; Kim, H.-J.; Song, D.-G.; Nam, S.H.; Kim, S.; Choi, S.; Lee, J.W. Transmembrane 4 L Six Family Member 5 Senses Arginine for mTORC1 Signaling. Cell Metab. 2019, 29, 1306–1319.e7. [Google Scholar] [CrossRef]
- Bassit, R.A.; Sawada, L.A.; Bacurau, R.F.P.; Navarro, F.; Rosa, L.F.B.P.C. The effect of BCAA supplementation upon the immune response of triathletes. Med. Sci. Sports Exerc. 2000, 32, 1214–1219. [Google Scholar] [CrossRef]
- Surowiec, I.; Arlestig, L.; Rantapaa-Dahlqvist, S.; Trygg, J. Metabolite and Lipid Profiling of Biobank Plasma Samples Collected Prior to Onset of Rheumatoid Arthritis. PLoS ONE 2016, 11, e0164196. [Google Scholar] [CrossRef]
- Hernvann, A.; Jaffray, P.; Hilliquin, P.; Cazalet, C.; Menkes, C.-J.; Ekindjian, O.G. Interleukin-1β-mediated glucose uptake by chondrocytes. Inhibition by cortisol. Osteoarthr. Cartil. 1996, 4, 139–142. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N.; Stockinger, B.; Tak, P.P. The resolution of inflammation. Nat. Rev. Immunol. 2012, 13, 59–66. [Google Scholar] [CrossRef]
- Marcucci, G.; Domazetovic, V.; Nediani, C.; Ruzzolini, J.; Favre, C.; Brandi, M.L. Oxidative Stress and Natural Antioxidants in Osteoporosis: Novel Preventive and Therapeutic Approaches. Antioxidants 2023, 12, 373. [Google Scholar] [CrossRef]
- Ameye, L.; Young, M.F. Mice deficient in small leucine-rich proteoglycans: Novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology 2002, 12, 107R–116R. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Chakraborty, D.; Kumar, V.; Malhotra, R.; Biswas, S. Upregulation of leucine-rich alpha-2 glycoprotein: A key regulator of inflammation and joint fibrosis in patients with severe knee osteoarthritis. Front. Immunol. 2022, 13, 1028994. [Google Scholar] [CrossRef]
- Maher, A.D.; Coles, C.; White, J.; Bateman, J.F.; Fuller, E.S.; Burkhardt, D.; Little, C.B.; Cake, M.; Read, R.; McDonagh, M.B.; et al. 1H NMR spectroscopy of serum reveals unique metabolic fingerprints associated with subtypes of surgically induced osteoarthritis in sheep. J. Proteome Res. 2012, 11, 4261–4268. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Liu-Bryan, R.; Terkeltaub, R. The growing array of innate inflammatory ignition switches in osteoarthritis. Arthritis Rheum. 2012, 64, 2055–2058. [Google Scholar] [CrossRef]
- van den Bosch, M.H.J.; van Lent, P.; van der Kraan, P.M. Identifying effector molecules, cells, and cytokines of innate immunity in OA. Osteoarthr. Cartil. 2020, 28, 532–543. [Google Scholar] [CrossRef]
- Harris, R.C.; Tallon, M.; Dunnett, M.; Boobis, L.; Coakley, J.; Kim, H.J.; Fallowfield, J.L.; Hill, C.; Sale, C.; Wise, J.A. The absorption of orally supplied β-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 2006, 30, 279–289. [Google Scholar] [CrossRef]
- Quesnele, J.J.; Laframboise, M.A.; Wong, J.J.; Kim, P.; Wells, G.D. The effects of beta-alanine supplementation on performance: A systematic review of the literature. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 14–27. [Google Scholar] [CrossRef]
- McIlwraith, C.W.; Frisbie, D.D.; Kawcak, C.E.; Fuller, C.J.; Hurtig, M.; Cruz, A. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the horse. Osteoarthr. Cartil. 2010, 18, S93–S105. [Google Scholar] [CrossRef]
- Lacitignola, L.; Fanizzi, F.P.; Francioso, E.; Crovace, A. 1H NMR investigation of normal and osteo-arthritic synovial fluid in the horse. Vet. Comp. Orthop. Traumatol. 2008, 21, 85–88. [Google Scholar]
- Yang, G.; Zhang, H.; Chen, T.; Zhu, W.; Ding, S.; Xu, K.; Xu, Z.; Guo, Y.; Zhang, J. Metabolic analysis of osteoarthritis subchondral bone based on UPLC/Q-TOF-MS. Anal. Bioanal. Chem. 2016, 408, 4275–4286. [Google Scholar] [CrossRef] [PubMed]
- Wennberg, C.; Hessle, L.; Lundberg, P.; Mauro, S.; Narisawa, S.; Lerner, U.H.; Millán, J.L. Functional Characterization of Osteoblasts and Osteoclasts from Alkaline Phosphatase Knockout Mice. J. Bone Miner. Res. 2000, 15, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Bhinderwala, F.; Roth, H.E.; Noel, H.; Feng, D.; Powers, R. Chemical shift variations in common metabolites. J. Magn. Reson. 2022, 345, 107335. [Google Scholar] [CrossRef]
- Ito, K.; Tsutsumi, Y.; Date, Y.; Kikuchi, J. Fragment Assembly Approach Based on Graph/Network Theory with Quantum Chemistry Verifications for Assigning Multidimensional NMR Signals in Metabolite Mixtures. ACS Chem. Biol. 2016, 11, 1030–1038. [Google Scholar] [CrossRef]




| Metabolite | Mean ± SD | One-Way ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 W | 4 W | 8 W | 12 W | 4 W vs. 0 W | 8 W vs. 4 W | 12 W vs. 8 W | F | P | |
| Leucine | 0.948 ± 0.061 | 0.979 ± 0.012 | 1.014 ± 0.036 | 1.259 ± 0.079 | ns | ns | ****↑ | 56.538 | <0.0001 |
| Isoleucine | 0.940 ± 0.064 | 1.031 ± 0.014 | 1.021 ± 0.048 | 1.245 ± 0.043 | ***↑ | ns | ****↑ | 64.255 | <0.0001 |
| Valine | 0.973 ± 0.060 | 1.015 ± 0.018 | 0.951 ± 0.044 | 1.330 ± 0.052 | ns | *↓ | ****↑ | 116.284 | <0.0001 |
| Alanine | 1.000 ± 0.044 | 0.829 ± 0.015 | 0.776 ± 0.050 | 1.378 ± 0.130 | ****↓ | ns | ****↑ | 110.373 | <0.0001 |
| Acetate | 0.631 ± 0.252 | 3.702 ± 0.219 | 0.866 ± 0.296 | 0.588 ± 0.043 | ****↑ | ****↓ | *↓ | 363.034 | <0.0001 |
| Glutamate | 1.086 ± 0.314 | 0.340 ± 0.071 | 1.298 ± 0.075 | 0.912 ± 0.107 | ****↓ | ****↑ | ***↓ | 44.734 | <0.0001 |
| Pyroglutamate | 1.113 ± 0.061 | 1.509 ± 0.004 | 0.897 ± 0.067 | 0.501 ± 0.072 | ****↑ | ****↓ | ****↓ | 419.480 | <0.0001 |
| Dimethylamine | 1.230 ± 0.268 | 0.303 ± 0.031 | 1.395 ± 0.172 | 2.418 ± 0.575 | ****↓ | ****↑ | ****↑ | 55.457 | <0.0001 |
| Aspartate | 0.810 ± 0.106 | 0.179 ± 0.040 | 0.750 ± 0.036 | 0.899 ± 0.008 | ****↓ | ****↑ | ****↑ | 240.328 | <0.0001 |
| Glutathione | 1.276 ± 0.032 | 0.382 ± 0.007 | 1.539 ± 0.307 | 1.344 ± 0.244 | ****↓ | ****↑ | ns | 54.639 | <0.0001 |
| DMF | 0.885 ± 0.140 | 0.409 ± 0.071 | 1.213 ± 0.183 | 1.165 ± 0.363 | ***↓ | ****↑ | ns | 22.968 | <0.0001 |
| β-alanine | 0.910 ± 0.065 | 0.366 ± 0.051 | 1.348 ± 0.254 | 1.068 ± 0.221 | ****↓ | ****↑ | **↓ | 45.568 | <0.0001 |
| Taurine | 0.963 ± 0.121 | 0.982 ± 0.051 | 0.827 ± 0.107 | 0.865 ± 0.101 | ns | **↓ | ns | 4.608 | <0.01 |
| Glycine | 0.994 ± 0.493 | 0.212 ± 0.114 | 2.220 ± 0.436 | 0.921 ± 0.115 | ****↓ | ****↑ | ****↓ | 48.372 | <0.0001 |
| Threonine | 1.047 ± 0.043 | 0.416 ± 0.009 | 1.506 ± 0.108 | 1.061 ± 0.011 | ****↓ | ****↑ | ****↓ | 470.833 | <0.0001 |
| Creatine | 1.056 ± 0.075 | 0.315 ± 0.004 | 1.558 ± 0.279 | 1.362 ± 0.250 | ****↓ | ****↑ | *↓ | 65.303 | <0.0001 |
| Creatine phosphate | 1.502 ± 0.003 | 0.272 ± 0.017 | 1.134 ± 0.081 | 1.519 ± 0.419 | ****↓ | ****↑ | **↑ | 59.916 | <0.0001 |
| Myo-inositol | 1.167 ± 0.109 | 0.445 ± 0.022 | 1.529 ± 0.136 | 0.826 ± 0.066 | ****↓ | ****↑ | ****↓ | 196.117 | <0.0001 |
| Lactate | 1.116 ± 0.119 | 5.450 ± 0.375 | 0.713 ± 0.107 | 1.135 ± 0.227 | ****↑ | ****↓ | **↑ | 736.619 | <0.0001 |
| Glucose | 0.976 ± 0.081 | 5.847 ± 0.430 | 0.814 ± 0.200 | 1.454 ± 0.611 | ****↑ | ****↓ | **↑ | 304.263 | <0.0001 |
| Tyrosine | 1.025 ± 0.033 | 1.067 ± 0.036 | 1.160 ± 0.136 | 1.488 ± 0.104 | ns | *↑ | ****↑ | 44.589 | <0.0001 |
| Histidine | 1.158 ± 0.402 | 0.967 ± 0.367 | 1.131 ± 0.127 | 1.453 ± 0.162 | ns | ns | *↑ | 3.867 | 0.020 |
| Imidazole | 0.898 ± 0.352 | 0.029 ± 0.014 | 0.960 ± 0.446 | 0.047 ± 0.034 | ****↓ | ****↑ | ****↓ | 26.218 | <0.0001 |
| Phenylalanine | 1.009 ± 0.050 | 0.921 ± 0.030 | 1.110 ± 0.123 | 1.296 ± 0.073 | *↓ | ****↑ | ****↑ | 34.683 | <0.0001 |
| NADP+ | 1.767 ± 0.293 | 0.295 ± 0.056 | 0.954 ± 0.107 | 1.045 ± 0.103 | ****↓ | ****↑ | ns | 104.224 | <0.0001 |
| Formate | 1.104 ± 0.042 | 0.613 ± 0.022 | 1.077 ± 0.051 | 1.189 ± 0.062 | ****↓ | ****↑ | ****↑ | 244.659 | <0.0001 |
| Metabolite | 4 W vs. 0 W | 8 W vs. 4 W | 12 W vs. 8 W | |||
|---|---|---|---|---|---|---|
| VIP | Significance | VIP | Significance | VIP | Significance | |
| Glucose | 2.297 | ****↑ | 2.190 | ****↓ | 1.311 | **↑ |
| Lactate | 2.167 | ****↑ | 2.122 | ****↓ | 0.949 | **↑ |
| Acetate | 1.820 | ****↑ | 1.638 | ****↓ | 0.690 | *↓ |
| NADP+ | 1.251 | ****↓ | 0.788 | ****↓ | 0.440 | ns |
| Creatine phosphate | 1.151 | ****↓ | 0.904 | ****↓ | 0.922 | **↑ |
| Glycine | 0.886 | ****↓ | 1.358 | ****↑ | 1.839 | ****↓ |
| Creatine | 0.891 | ****↓ | 1.073 | ****↑ | 0.716 | *↓ |
| Glutathione | 0.980 | ****↓ | 1.026 | ****↑ | 0.730 | ns |
| Threonine | 0.824 | ****↓ | 1.016 | ****↑ | 1.094 | ****↓ |
| Dimethylamine | 0.988 | ****↓ | 1.014 | ****↑ | 1.550 | ****↑ |
| Myo-inositol | 0.880 | ****↓ | 1.012 | ****↑ | 1.377 | ****↓ |
| Imidazole | 0.931 | ****↓ | 0.875 | ****↑ | 1.505 | ****↓ |
| Alanine | 0.421 | ****↓ | 0.178 | ns | 1.260 | ****↑ |
| Valine | 0.207 | ns | 0.237 | *↓ | 1.012 | ****↑ |
| Pyroglutamate | 0.648 | ****↑ | 0.757 | ****↓ | 1.009 | ****↓ |
| No. | Metabolic Pathway | 4 W vs. 0 W | 8 W vs. 4 W | 12 W vs. 8 W |
|---|---|---|---|---|
| 1 | Glutathione metabolism | √ | √ | √ |
| 2 | Histidine metabolism | √ | √ | √ |
| 3 | Alanine, aspartate, and glutamate metabolism | √ | √ | √ |
| 4 | Glycine, serine, and threonine metabolism | √ | √ | √ |
| 5 | Phenylalanine metabolism | √ | √ | √ |
| 6 | D-glutamine and D-glutamate metabolism | √ | √ | √ |
| 7 | Phenylalanine, tyrosine, and tryptophan biosynthesis | √ | √ | √ |
| 8 | Taurine and hypotaurine metabolism | √ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, X.; Deng, H.; Zhou, X.; Ni, G.; Huang, C.; Lin, D. Metabolomic Profiling of Osteoblasts in Rat Subchondral Bone Following Anterior Cruciate Ligament Injury. Molecules 2025, 30, 2255. https://doi.org/10.3390/molecules30112255
Qiu X, Deng H, Zhou X, Ni G, Huang C, Lin D. Metabolomic Profiling of Osteoblasts in Rat Subchondral Bone Following Anterior Cruciate Ligament Injury. Molecules. 2025; 30(11):2255. https://doi.org/10.3390/molecules30112255
Chicago/Turabian StyleQiu, Xu, Huili Deng, Xuchang Zhou, Guoxin Ni, Caihua Huang, and Donghai Lin. 2025. "Metabolomic Profiling of Osteoblasts in Rat Subchondral Bone Following Anterior Cruciate Ligament Injury" Molecules 30, no. 11: 2255. https://doi.org/10.3390/molecules30112255
APA StyleQiu, X., Deng, H., Zhou, X., Ni, G., Huang, C., & Lin, D. (2025). Metabolomic Profiling of Osteoblasts in Rat Subchondral Bone Following Anterior Cruciate Ligament Injury. Molecules, 30(11), 2255. https://doi.org/10.3390/molecules30112255









