Influence of Ovophospholipids on Lymphocyte Subsets and Humoral Immune Response in Mice
Abstract
1. Introduction
2. Results
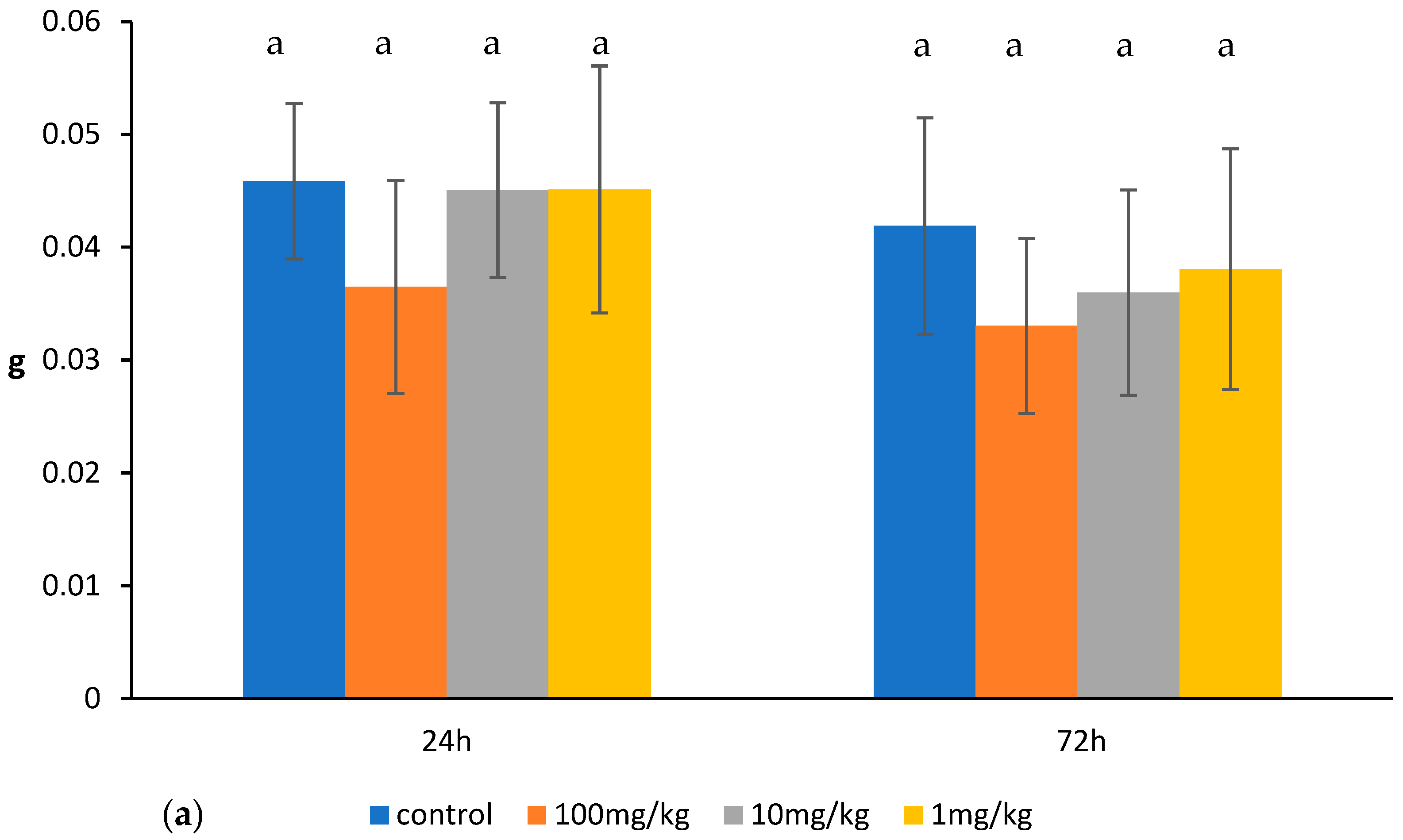
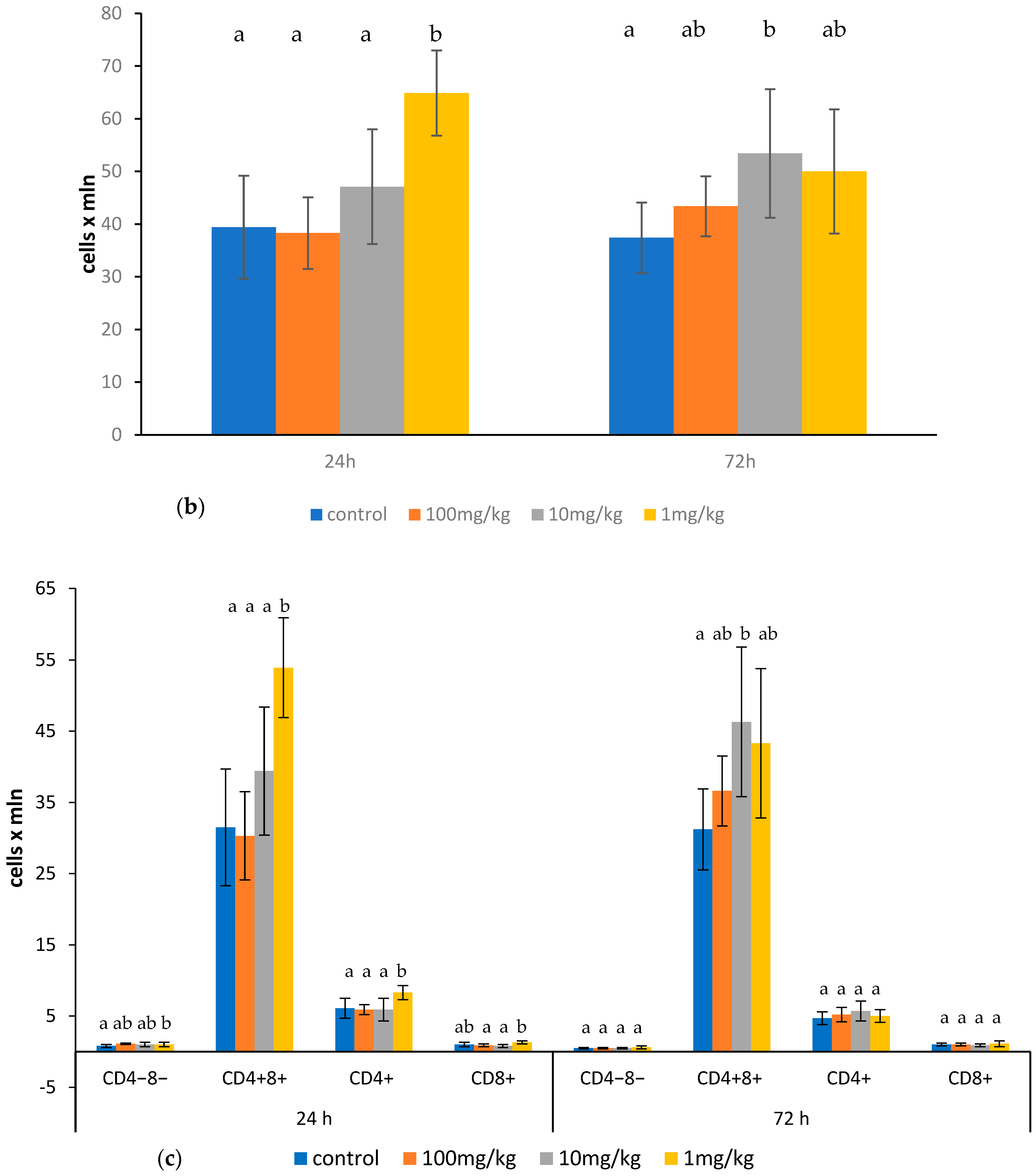
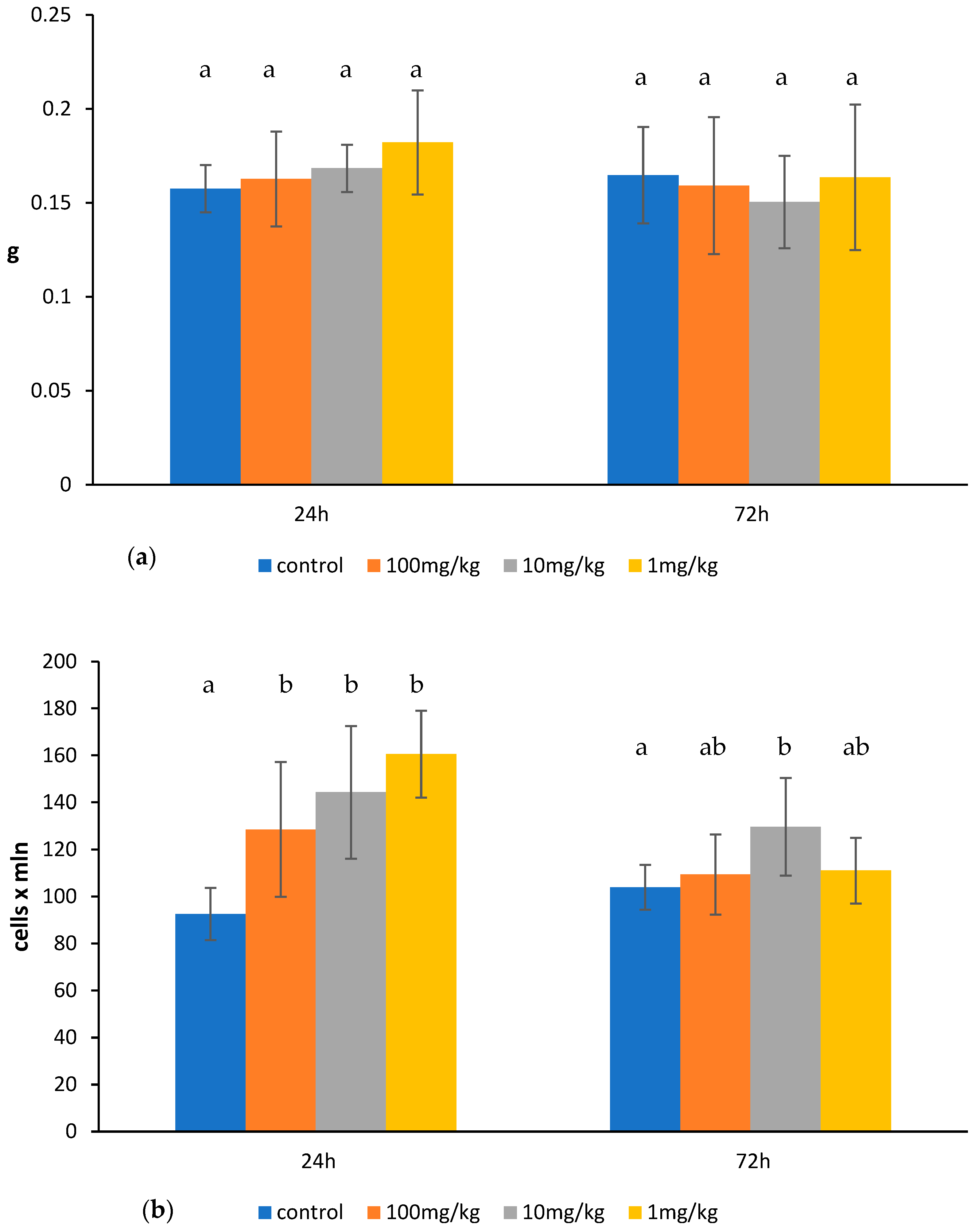
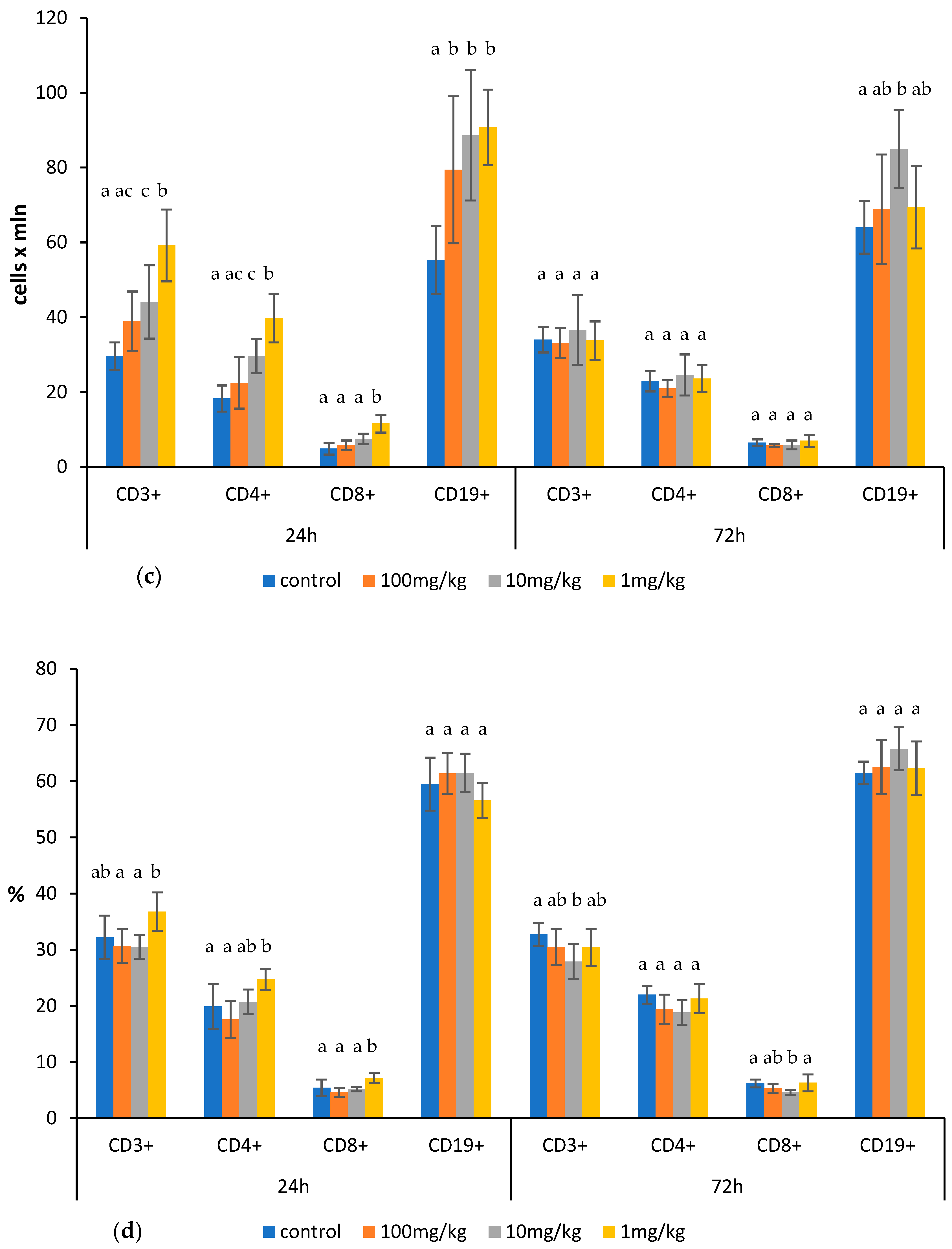
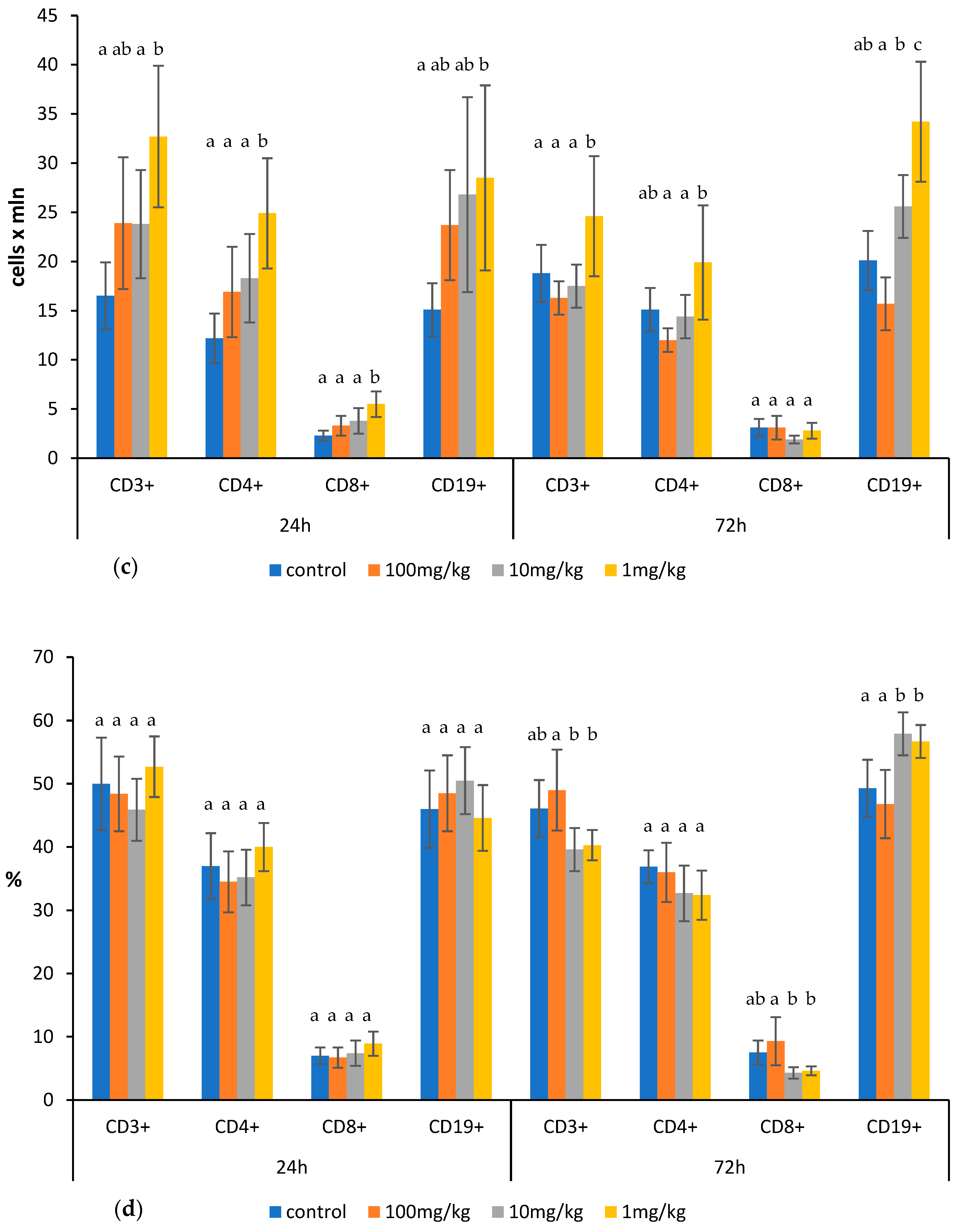
2.1. Effects of Ovophospholipids on Lymphocyte Subsets
2.2. Effect of Ovophospholipids on TNF-α and IL-1β Levels in the Culture Supernatants of Peritoneal Macrophages Stimulated In Vitro with Lipopolysaccharide (LPS) from E. coli (055:B5)
2.3. Effect of Ovophospholipids on Humoral Immune Response
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs and Treatments
4.3. Measurements
4.4. Determination of Total Count and Subsets of Lymphocytes in the Thymus, Spleen, and Mesenteric Lymph Nodes
4.5. Determination of Synthesis of TNF-α and IL-1β in the Culture Supernatants of Peritoneal Macrophages Stimulated In Vitro with LPS from E. coli (055:B5)
4.6. Determination of Plaque Forming Cells
4.7. Determination of Serum Anti-SRBC Antibodies
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bubel, F.; Dobrzański, Z.; Bykowski, P.; Patkowska-Sokoła, B.; Trziszka, T. Enrichment of hen eggs with omega-3 polyunsaturated fatty acids-physiological and nutritional aspects. Acta Sci. Pol. Med. Weterinaria 2011, 10, 5–18. [Google Scholar]
- Sun, N.; Chen, J.; Wang, D.; Lin, S. Advance in food-derived phospholipids: Sources, molecular species and structure as well as their biological activities. Trends Food Sci. Technol. 2018, 80, 199–211. [Google Scholar] [CrossRef]
- Anton, M. Composition and Structure of Hen Egg Yolk. In Bioactive Egg Compounds; Huopalahti, R., López-Fandiño, R., Anton, M., Schade, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–6. [Google Scholar]
- Xu, D.; Ren, J.; Ali, B.; Jin, Y.; Jin, Z.; Xu, X. Water-in-oil soybean concentrated phospholipids hydrolysis based on the model of enzymatic deactivation and its application in bread. Food Biosci. 2021, 44 Pt A, 101412. [Google Scholar] [CrossRef]
- Cherian, G. Egg quality and yolk polyunsaturated fatty acid status in relation to broiler breeder hen age and dietary n-3 oils. Poult. Sci. 2008, 87, 1131–1137. [Google Scholar] [CrossRef]
- Gorjão, R.; Verlengia, R.; Lima, T.M.; Soriano, F.G.; Boaventura, M.F.; Kanunfre, C.C.; Peres, C.M.; Sampaio, S.C.; Otton, R.; Folador, A.; et al. Effect of docosahexaenoic acid-rich fish oil supplementation on human leukocyte function. Clin. Nutr. 2006, 25, 923–938. [Google Scholar] [CrossRef]
- Kew, S.; Mesa, M.D.; Tricon, S.; Buckley, R.; Minihane, A.M.; Yaqoob, P. Effects of oils rich in eicosapentaenoic and docosahexaenoic acids on immune cell composition and function in healthy humans. Am. J. Clin. Nutr. 2004, 79, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Sullenbarger, B.; Prakash, R.; McDaniel, J.C. Supplementation with eicosapentaenoic acid and docosahexaenoic acid reduces high levels of circulating proinflammatory cytokines in aging adults: A randomized, controlled study. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 23–29. [Google Scholar] [CrossRef]
- Olson, M.V.; Liu, Y.C.; Dangi, B.; Paul Zimmer, J.; Salem, N., Jr.; Nauroth, J.M. Docosahexaenoic acid reduces inflammation and joint destruction in mice with collagen-induced arthritis. Inflamm. Res. 2013, 62, 1003–1013. [Google Scholar] [CrossRef]
- Che, H.; Li, H.; Song, L.; Dong, X.; Yang, X.; Zhang, T.; Wang, Y.; Xie, W. Orally Administered DHA-Enriched Phospholipids and DHA-Enriched Triglyceride Relieve Oxidative Stress, Improve Intestinal Barrier, Modulate Inflammatory Cytokine and Gut Microbiota, and Meliorate Inflammatory Responses in the Brain in Dextran Sodium Sulfate Induced Colitis in Mice. Mol. Nutr. Food Res. 2021, 65, e2000986. [Google Scholar] [CrossRef]
- Merzouk, S.A.; Saker, M.; Reguig, K.B.; Soulimane, N.; Merzouk, H.; Guermouche, B.; Berrouiguet, A.Y.; Hichami, A.; Narce, M.; Khan, N.A. N-3 polyunsaturated fatty acids modulate in-vitro T cell function in type I diabetic patients. Lipids 2008, 43, 485–497. [Google Scholar] [CrossRef]
- Chapkin, R.S.; Arrington, J.L.; Apanasovich, T.V.; Carroll, R.J.; McMurray, D.N. Dietary n-3 PUFA affect TcR-mediated activation of purified murine T cells and accessory cell function in co-cultures. Clin Exp Immunol. 2002, 130, 12–18, Erratum in Clin. Exp. Immunol. 2002, 130, 557–558. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Yen, J.H.; Ganea, D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2011, 25, 872–882. [Google Scholar] [CrossRef]
- Lian, M.; Luo, W.; Sui, Y.; Li, Z.; Hua, J. Dietary n-3 PUFA Protects Mice from Con A Induced Liver Injury by Modulating Regulatory T Cells and PPAR-γ Expression. PLoS ONE 2015, 10, e0132741. [Google Scholar] [CrossRef] [PubMed]
- Han, S.C.; Koo, D.H.; Kang, N.J.; Yoon, W.J.; Kang, G.J.; Kang, H.K.; Yoo, E.S. Docosahexaenoic Acid Alleviates Atopic Dermatitis by Generating Tregs and IL-10/TGF-β-Modified Macrophages via a TGF-β-Dependent Mechanism. J. Investig. Dermatol. 2015, 135, 1556–1564. [Google Scholar] [CrossRef]
- Carlsson, J.A.; Wold, A.E.; Sandberg, A.S.; Östman, S.M. The Polyunsaturated Fatty Acids Arachidonic Acid and Docosahexaenoic Acid Induce Mouse Dendritic Cells Maturation but Reduce T-Cell Responses In Vitro. PLoS ONE 2015, 10, e0143741. [Google Scholar] [CrossRef]
- Monk, J.M.; Hou, T.Y.; Turk, H.F.; McMurray, D.N.; Chapkin, R.S. n3 PUFAs reduce mouse CD4+ T-cell ex vivo polarization into Th17 cells. J. Nutr. 2013, 143, 1501–1508. [Google Scholar] [CrossRef]
- Teague, H.; Rockett, B.D.; Harris, M.; Brown, D.A.; Shaikh, S.R. Dendritic cell activation, phagocytosis and CD69 expression on cognate T cells are suppressed by n-3 long-chain polyunsaturated fatty acids. Immunology 2013, 139, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Vedin, I.; Cederholm, T.; Freund Levi, Y.; Basun, H.; Garlind, A.; Faxén Irving, G.; Jönhagen, M.E.; Vessby, B.; Wahlund, L.O.; Palmblad, J. Effects of docosahexaenoic acid-rich n-3 fatty acid supplementation on cytokine release from blood mononuclear leukocytes: The OmegAD study. Am. J. Clin. Nutr. 2008, 87, 1616–1622. [Google Scholar] [CrossRef]
- Rusnak, T.; Azarcoya-Barrera, J.; Makarowski, A.; Jacobs, R.L.; Richard, C. Plant- and Animal-Derived Dietary Sources of Phosphatidylcholine Have Differential Effects on Immune Function in The Context of A High-Fat Diet in Male Wistar Rats. J. Nutr. 2024, 154, 1936–1944. [Google Scholar] [CrossRef]
- Yessoufou, A.; Plé, A.; Moutairou, K.; Hichami, A.; Khan, N.A. Docosahexaenoic acid reduces suppressive and migratory functions of CD4+CD25+ regulatory T-cells. J. Lipid Res. 2009, 50, 2377–2388. [Google Scholar] [CrossRef]
- Wu, S.; Peng, H.; Li, S.; Huang, L.; Wang, X.; Li, Y.; Liu, Y.; Xiong, P.; Yang, Q.; Tian, K.; et al. The ω-3 Polyunsaturated Fatty Acid Docosahexaenoic Acid Enhances NK-Cell Antitumor Effector Functions. Cancer Immunol. Res. 2024, 12, 744–758. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Calder, P.C. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br. J. Nutr. 2012, 107 (Suppl. S2), S171–S184. [Google Scholar] [CrossRef]
- Tomasdottir, V.; Thorleifsdottir, S.; Vikingsson, A.; Hardardottir, I.; Freysdottir, J. Dietary omega-3 fatty acids enhance the B1 but not the B2 cell immune response in mice with antigen-induced peritonitis. J. Nutr. Biochem. 2014, 25, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Juman, S.; Hashimoto, M.; Katakura, M.; Inoue, T.; Tanabe, Y.; Arita, M.; Miki, T.; Shido, O. Effects of long-term oral administration of arachidonic acid and docosahexaenoic acid on the immune functions of young rats. Nutrients 2013, 5, 1949–1961. [Google Scholar] [CrossRef]
- Sasaki, T.; Kanke, Y.; Kudoh, K.; Misawa, Y.; Shimizu, J.; Takita, T. Effects of dietary docosahexaenoic acid on surface molecules involved in T cell proliferation. Biochim. Biophys. Acta 1999, 1436, 519–530. [Google Scholar] [CrossRef]
- Woodworth, H.L.; McCaskey, S.J.; Duriancik, D.M.; Clinthorne, J.F.; Langohr, I.M.; Gardner, E.M.; Fenton, J.I. Dietary fish oil alters T lymphocyte cell populations and exacerbates disease in a mouse model of inflammatory colitis. Cancer Res. 2010, 70, 7960–7969. [Google Scholar] [CrossRef]
- Paixão, E.M.D.S.; Oliveira, A.C.M.; Pizato, N.; Muniz-Junqueira, M.I.; Magalhães, K.G.; Nakano, E.Y.; Ito, M.K. The effects of EPA and DHA enriched fish oil on nutritional and immunological markers of treatment naïve breast cancer patients: A randomized double-blind controlled trial. Nutr. J. 2017, 16, 71. [Google Scholar] [CrossRef]
- Teague, H.; Fhaner, C.J.; Harris, M.; Duriancik, D.M.; Reid, G.E.; Shaikh, S.R. n-3 PUFAs enhance the frequency of murine B-cell subsets and restore the impairment of antibody production to a T-independent antigen in obesity. J. Lipid Res. 2013, 54, 3130–3138. [Google Scholar] [CrossRef]
- Teague, H.; Harris, M.; Fenton, J.; Lallemand, P.; Shewchuk, B.M.; Shaikh, S.R. Eicosapentaenoic and docosahexaenoic acid ethyl esters differentially enhance B-cell activity in murine obesity. J. Lipid Res. 2014, 55, 1420–1433. [Google Scholar] [CrossRef]
- Gurzell, E.A.; Teague, H.; Harris, M.; Clinthorne, J.; Shaikh, S.R.; Fenton, J.I. DHA-enriched fish oil targets B cell lipid microdomains and enhances ex vivo and in vivo B cell function. J. Leukoc. Biol. 2013, 93, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg Med. Chem. 2020, 28, 115327. [Google Scholar] [CrossRef] [PubMed]
- Weise, C.; Hilt, K.; Milovanovic, M.; Ernst, D.; Rühl, R.; Worm, M. Inhibition of IgE production by docosahexaenoic acid is mediated by direct interference with STAT6 and NFκB pathway in human B cells. J. Nutr. Biochem. 2011, 22, 269–275. [Google Scholar] [CrossRef]
- Rockett, B.D.; Salameh, M.; Carraway, K.; Morrison, K.; Shaikh, S.R. n-3 PUFA improves fatty acid composition, prevents palmitate-induced apoptosis, and differentially modifies B cell cytokine secretion in vitro and ex vivo. J. Lipid Res. 2010, 51, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Fuentes, N.R.; Hou, T.Y.; Barhoumi, R.; Li, X.C.; Deutz, N.E.P.; Engelen, M.P.K.J.; McMurray, D.N.; Chapkin, R.S. Remodelling of primary human CD4+ T cell plasma membrane order by n-3 PUFA. Br. J. Nutr. 2018, 119, 163–175. [Google Scholar] [CrossRef]
- Jine, Y.; Lis, M.; Szczypka, M.; Obmińska-Mrukowicz, B. Influence of betulinic acid on lymphocyte subsets and humoral immune response in mice. Pol. J. Vet. Sci. 2012, 15, 305–313. [Google Scholar] [CrossRef][Green Version]
- Lis, M.; Szczypka, M.; Suszko, A.; Obmińska-Mrukowicz, B. Influence of bestatin, an inhibitor of aminopeptidases, on T and B lymphocyte subsets in mice. Pol. J. Vet. Sci. 2011, 14, 393–403. [Google Scholar] [CrossRef]
- Mishell, R.I.; Dutton, R.W. Immunization of dissociated spleen cell cultures from normal mice. J. Exp. Med. 1967, 126, 423–442. [Google Scholar] [CrossRef]
- Hudson, L.; Hay, F.C. Practical Immunology; Blackwell Scientific Publications: Oxford, UK; London, UK; Edinburgh, UK; Boston, MA, USA; Melbourne, VIC, Australia, 1980. [Google Scholar]
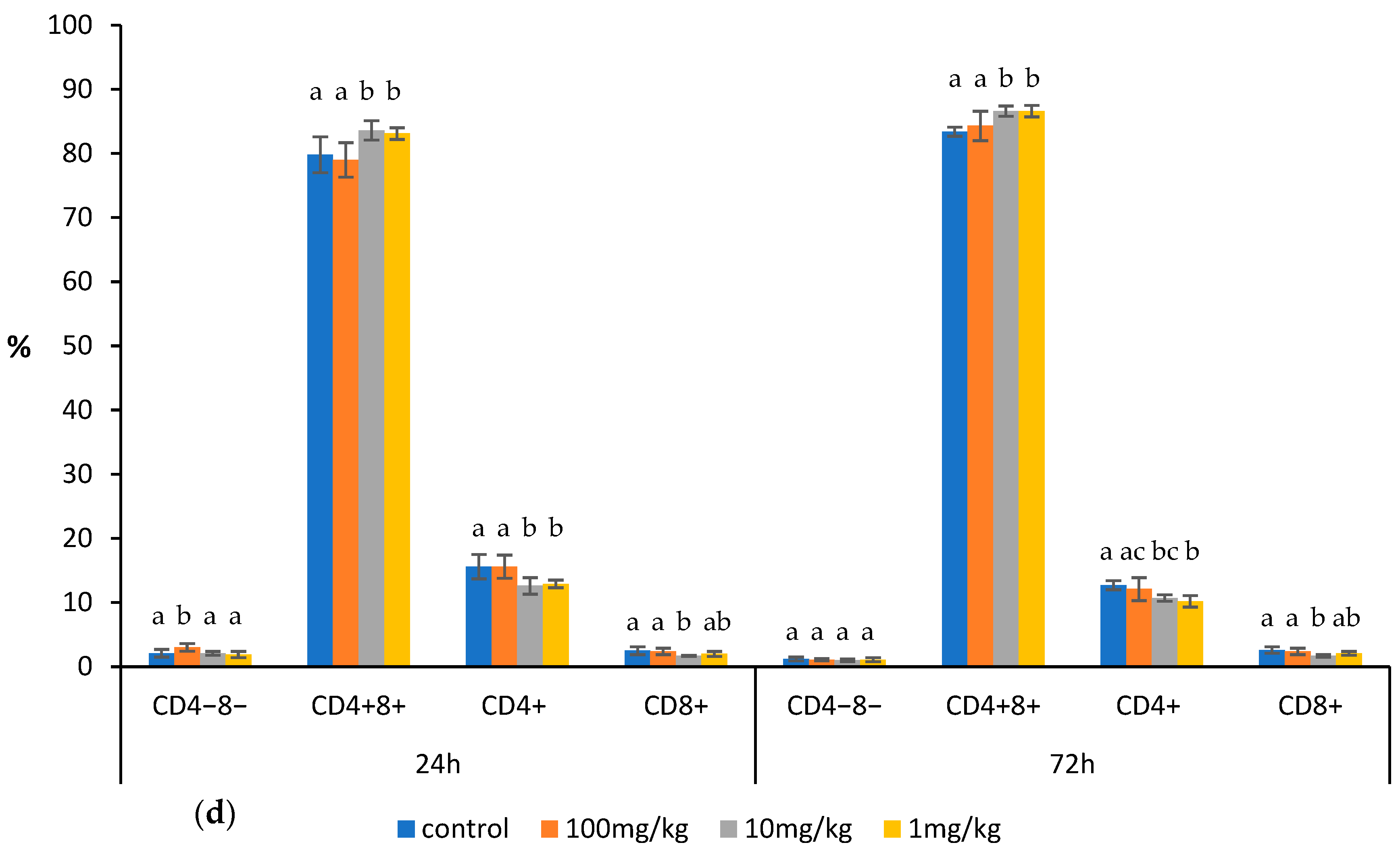
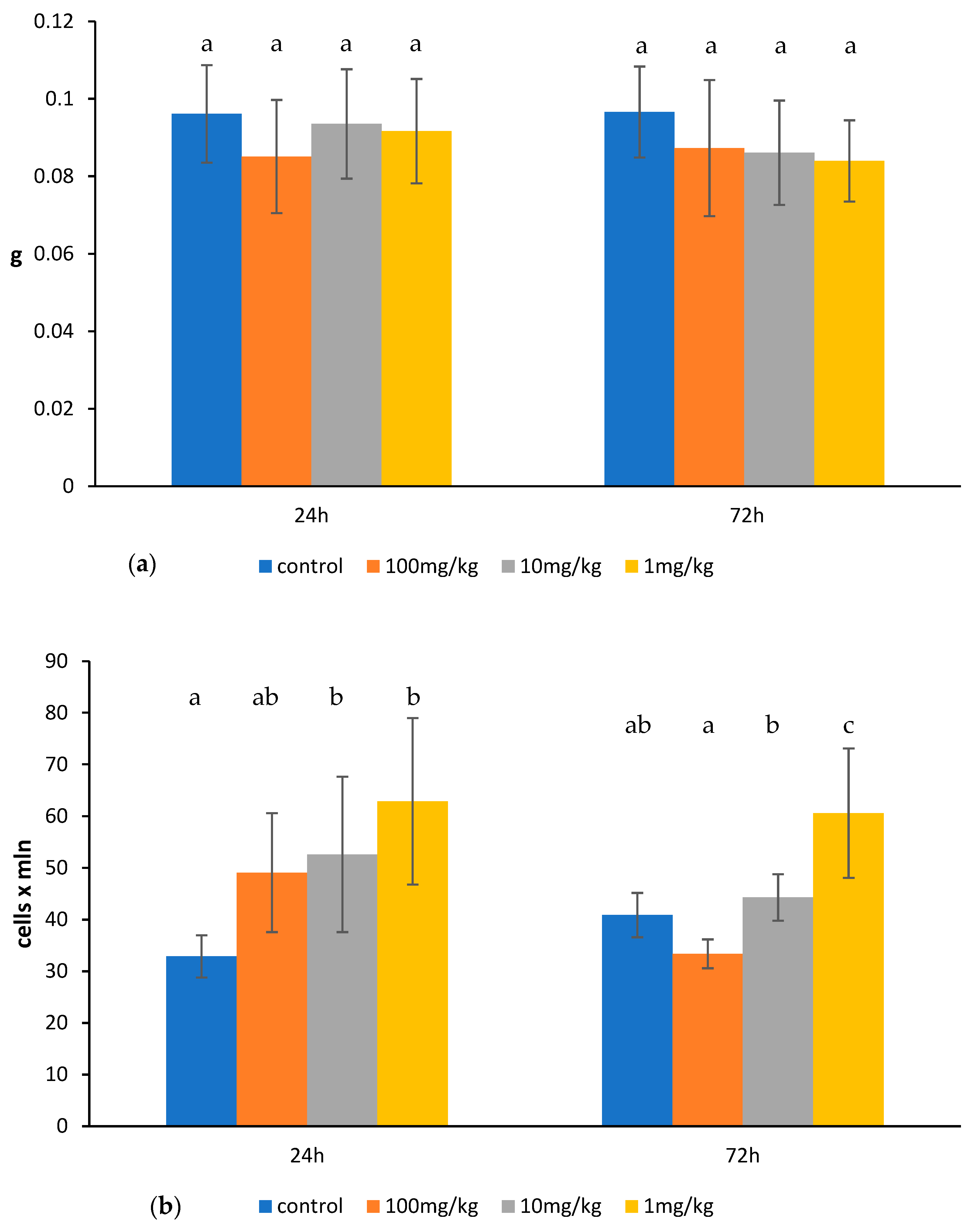

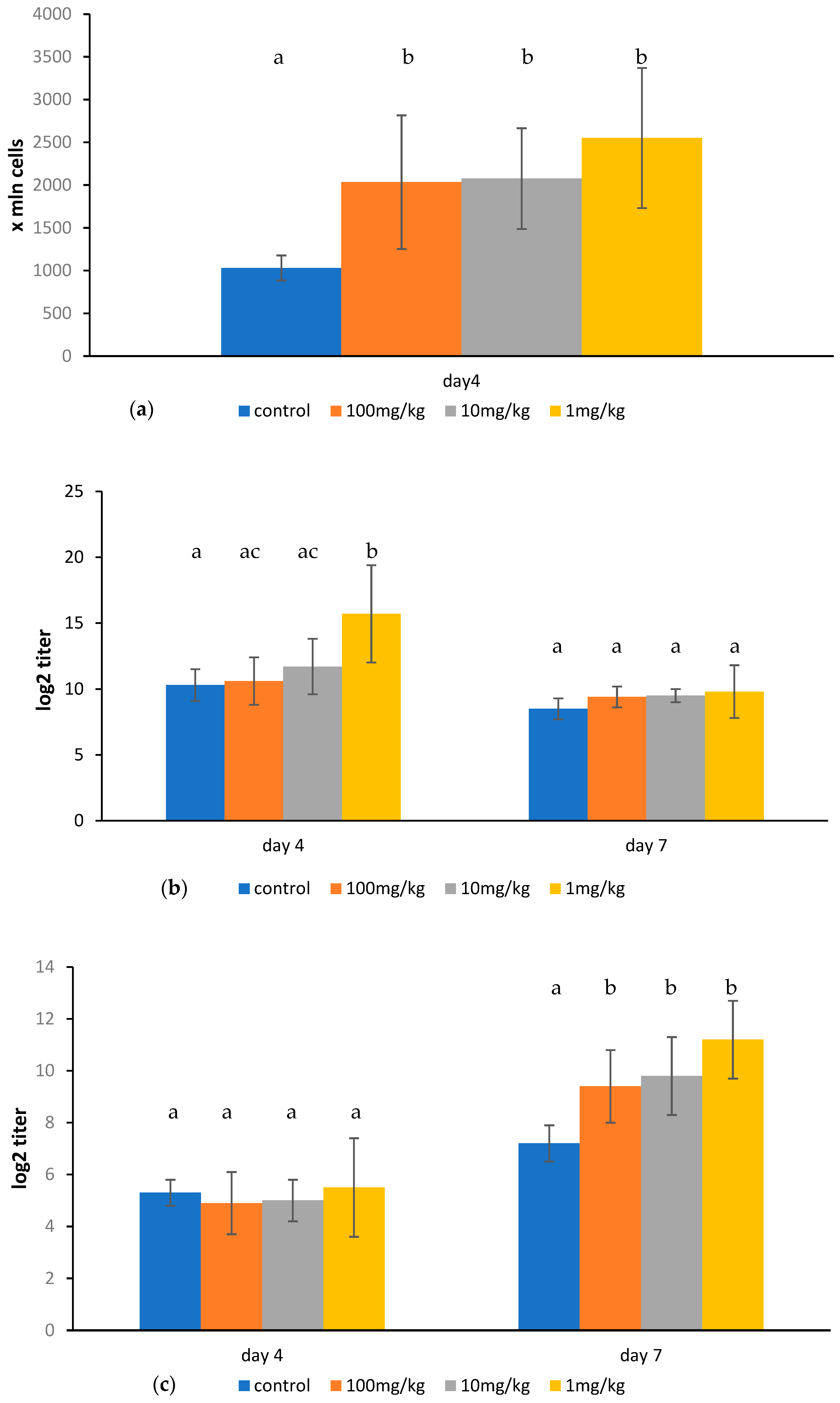
| Acetone Insoluble Matter 75.68% Phosphatidylcholine (Lecithin) 75.6% Phosphatidylethanolamine (Cephalin) 24.4% | Amount (%) |
|---|---|
| Acids | |
| C14:0 | 0.53 |
| C14:1 | 0.16 |
| C16:0 | 25.51 |
| C16:1 | 2.69 |
| C17:0 | 0.19 |
| C18:0 | 14.48 |
| C18:1 | 27.09 |
| C18:2; ω–6 | 13.43 |
| C18:3; ω–3 | 3.32 |
| C20:2; ω–6 | 0.16 |
| C20:3; ω–6 | 0.30 |
| C20:4; ω–6 | 1.99 |
| C20:5; ω–3 | 0.92 |
| C22:6; ω–3 | 9.24 |
| Sum of ω–3 fatty acids | 13.48 |
| Sum of ω–6 fatty acids | 15.88 |
| ω–6/ω–3 | 1.18 |
| Total saturated fatty acids (SFAs) | 40.71 |
| Total unsaturated fatty acids (UFAs) | 59.29 |
| Total MUFAs | 29.94 |
| Total PUFAs | 29.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lis, M.; Szczypka, M.; Suszko-Pawłowska, A.; Pawlak, A.; Bobak, Ł.; Obmińska-Mrukowicz, B. Influence of Ovophospholipids on Lymphocyte Subsets and Humoral Immune Response in Mice. Molecules 2025, 30, 2253. https://doi.org/10.3390/molecules30112253
Lis M, Szczypka M, Suszko-Pawłowska A, Pawlak A, Bobak Ł, Obmińska-Mrukowicz B. Influence of Ovophospholipids on Lymphocyte Subsets and Humoral Immune Response in Mice. Molecules. 2025; 30(11):2253. https://doi.org/10.3390/molecules30112253
Chicago/Turabian StyleLis, Magdalena, Marianna Szczypka, Agnieszka Suszko-Pawłowska, Aleksandra Pawlak, Łukasz Bobak, and Bożena Obmińska-Mrukowicz. 2025. "Influence of Ovophospholipids on Lymphocyte Subsets and Humoral Immune Response in Mice" Molecules 30, no. 11: 2253. https://doi.org/10.3390/molecules30112253
APA StyleLis, M., Szczypka, M., Suszko-Pawłowska, A., Pawlak, A., Bobak, Ł., & Obmińska-Mrukowicz, B. (2025). Influence of Ovophospholipids on Lymphocyte Subsets and Humoral Immune Response in Mice. Molecules, 30(11), 2253. https://doi.org/10.3390/molecules30112253







