The Effect of Bromine and Iodine on the Plant Growth, Phytochemical Composition and Antioxidant Capacity of Dandelion (Taraxacum officinale F.H. Wiggers Coll.) Plants
Abstract
1. Introduction
1.1. Iodine and Bromine Biogeochemistry
1.2. Dandelion Plants—Medicinal Properties, Endogenous Content of Br and I, Iodine Biofortification of Plants
2. Results
2.1. Biomass and Dry Weight in Roots and Leaves
2.2. Content of Iodine and Bromine and Its Quantitative Ratio in Roots and Leaves
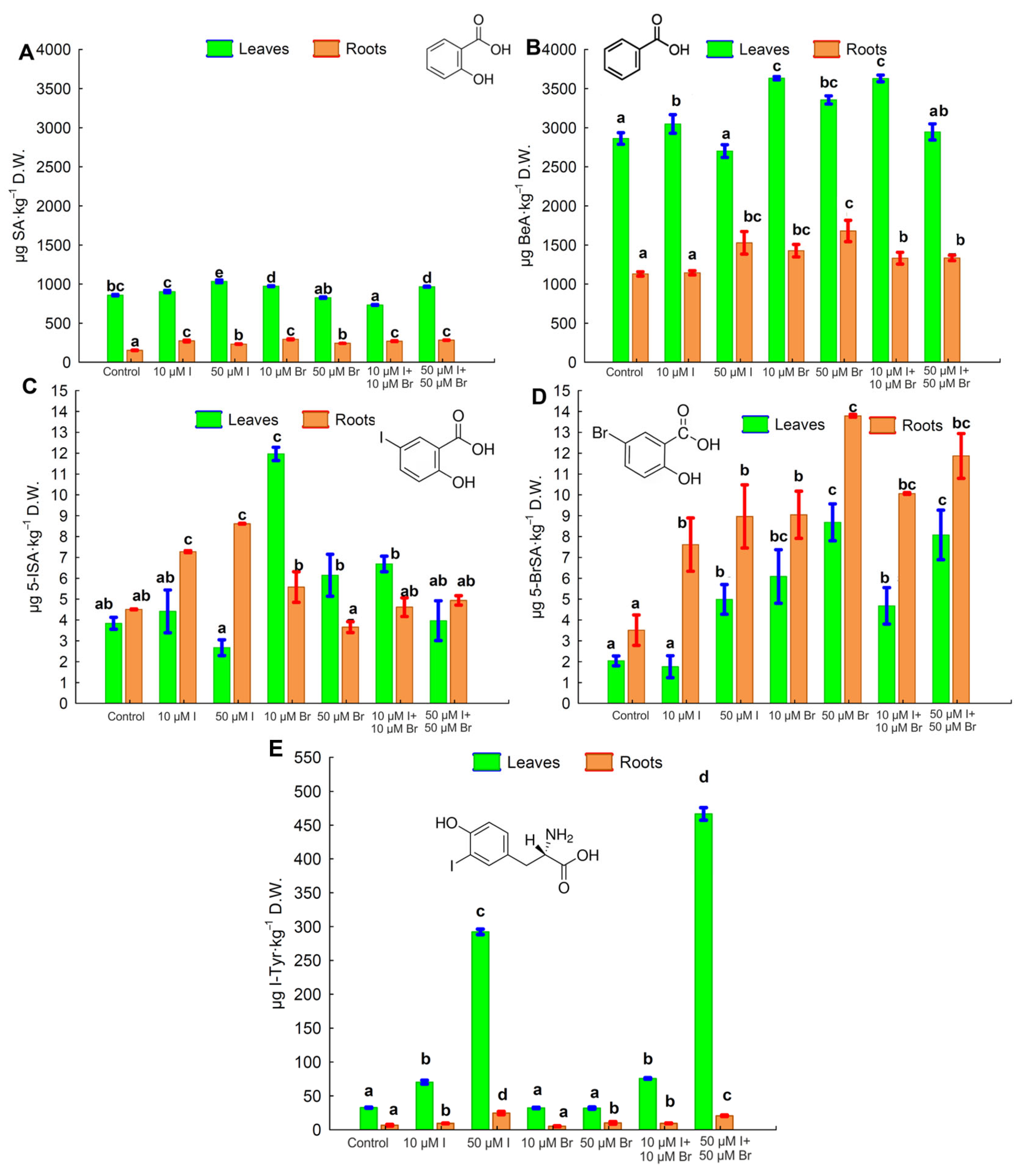
2.3. Content of Salicylic, Benzoic, 5-Iodosalicylic, and 5-Bromosalicylic Acids and Iodotyrosine
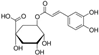
2.4. Content of Esculin, Chlorogenic Acid, and Total Phenolic Compounds
2.5. Antioxidant Capacity of Dandelion Plants
2.6. Concentrations of Proline as Well as Phytohormones: IAA, GA3, GA4, JA, and ABA
3. Discussion
3.1. Accumulation of I and Br in Dandelion Leaves and Roots
3.2. Effect of Br and I on Phytohormone Content in Dandelion Plants
3.3. Antioxidant Capacity of Dandelion Plants
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Determination of Total Iodine and Bromine Content
4.3. Determination of Iodotyrosine, Benzoic and Salicylic Acids, and Their Iodine and Bromine Derivatives
4.4. Determination of Esculin, Chlorogenic Acid, Proline, and Phytohormones Using the LC-MS/MS Technique
4.5. Determination of the Content of Phenolic Compounds and Antioxidant Capacity
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perminova, T. Bromine in the Natural Environments of the Tomsk Region and Its Toxicity Assessment. Doctoral Dissertation, Université de Technologie de Troyes, Troyes, France, Université polytechnique de Tomsk, Tomsk Oblast, Russie, 2017. [Google Scholar]
- Bojarski, L. Iodine and bromine as hydrochemical indicators of hydrocarbons in the mesozoic and palaeozoic deposits of north Poland. Geol. Q. 1966, 10, 177–193. (In Polish) [Google Scholar]
- Wishkerman, A. Bromine and Iodine in Plant-Soil Systems. Doctoral Dissertation, University of Heidelberg, Heidelberg, Germany, 2006. Available online: https://archiv.ub.uni-heidelberg.de/volltextserver/6581 (accessed on 18 September 2024).
- Yuita, K.; Nobusawa, Y.; Shibuya, M.; Aso, S. Iodine, bromine and chlorine contents in soils and plants of Japan: I. Iodine, bromine and chlorine contents in soils and plants of the basin of the Miomote River. Soil Sci. Plant Nutr. 1982, 28, 315–336. [Google Scholar] [CrossRef]
- Maw, G.A.; Kempton, R.J. Bromine in soils and peats. Plant Soil 1982, 65, 103–109. [Google Scholar] [CrossRef]
- Duborská, E.; Matulová, M.; Vaculovič, T.; Matúš, P.; Urík, M. Iodine fractions in soil and their determination. Forests 2021, 12, 1512. [Google Scholar] [CrossRef]
- Medrano-Macías, J.; Leija-Martínez, P.; González-Morales, S.; Juárez-Maldonado, A.; Benavides-Mendoza, A. Use of iodine to biofortify and promote growth and stress tolerance in crops. Front. Plant Sci. 2016, 7, 1146. [Google Scholar] [CrossRef]
- Kiferle, C.; Martinelli, M.; Salzano, A.M. Evidences for a nutritional role of iodine in plants. Front. Plant Sci. 2021, 12, 616868. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Singh, K.; Iqbal, N.; Nisha, N.; Rani, A.; Kumar, M.; Khatri, N.; Siddiqui, M.H.; Yasheshwar; Kim, S.T.; et al. Iodine: An emerging biostimulant of growth and stress responses in plants. Plant Soil 2023, 486, 119–133. [Google Scholar] [CrossRef]
- Anke, M. Essentiality of arsenic, bromine, fluorine and titanium for animal and man. In Proceedings Book of 3rd International Symposium on Trace Elements in Human; New Perspectives: Athens, Greece, 2001. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar] [CrossRef]
- Wisniak, J. The history of bromine—From discovery to commodity. Indian J. Chem. Technol. 2002, 9, 263–271. [Google Scholar]
- Pavelka, S.; Babický, A.; Lener, J.; Vobecký, M. Impact of high bromide intake in the rat dam on iodine transfer to the sucklings. Food Chem. Toxicol. 2002, 40, 1041–1045. [Google Scholar] [CrossRef]
- Pavelka, S. Metabolism of bromide and its interference with the metabolism of iodine. Physiol. Res. 2004, 53, S81–S90. [Google Scholar] [CrossRef]
- Cazzola, M.; Page, C.P.; Calzetta, L.; Matera, M.G. Pharmacology and therapeutics of bronchodilators. Pharmacol. Rev. 2012, 64, 450–504. [Google Scholar] [CrossRef] [PubMed]
- Bor, S.; Lehert, P.; Chalbaud, A.; Tack, J. Efficacy of pinaverium bromide in the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Therapeut. Adv. Gastroenterol. 2021, 14, 17562848211033740. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO Bromide Ion. Pesticide Residues in Food—1988 Evaluations. Part II—Toxicology; Food and Agriculture Organization of the United Nations: Rome, Italy, 1989; FAO Plant Production and Protection Paper 93/2; Available online: http://www.inchem.org/documents/jmpr/jmpmono/v88pr03.htm (accessed on 28 April 2025).
- Zhao, B.; Wang, J.; Sun, N.; Liu, C. Low concentration of bromide ions improves sulfadiazine phytoremoval and attenuates its phytotoxicity. Sci. Total Environ. 2023, 893, 164857. [Google Scholar] [CrossRef]
- Dong, M.; Sun, N.; Liu, C. Bromide ion enhancing the phytodegradation of emerging phenolic pollutants and its mechanisms mediating wheat resistance to phenolic pollutants stress. J. Clean. Prod. 2023, 411, 137295. [Google Scholar] [CrossRef]
- Flury, M.; Papritz, A. Bromide in the natural environment: Occurrence and toxicity. J. Environ. Qual. 1993, 22, 747–758. [Google Scholar] [CrossRef]
- Castellón, C.I.; Taboada, M.E. Leaching of copper concentrate with iodized salts in a saline acid medium: Part 1—Effect of concentrations. Materials 2023, 16, 2312. [Google Scholar] [CrossRef]
- Consentino, B.B.; Ciriello, M.; Sabatino, L.; Vultaggio, L.; Baldassano, S.; Vasto, S.; Rouphael, Y.; La Bella, S.; De Pascale, S. Current acquaintance on agronomic biofortification to modulate the yield and functional value of vegetable crops: A review. Horticulturae 2023, 9, 219. [Google Scholar] [CrossRef]
- Duborská, E.; Urík, M.; Šeda, M. Iodine biofortification of vegetables could improve iodine supplementation status. Agronomy 2020, 10, 1574. [Google Scholar] [CrossRef]
- Kiferle, C.; Ascrizzi, R.; Martinelli, M.; Gonzali, S.; Mariotti, L.; Pistelli, L.; Flamini, G.; Perata, P. Effect of iodine treatments on Ocimum basilicum L.: Biofortification, phenolics production and essential oil composition. PLoS ONE 2019, 14, e0226559, Erratum in PLoS ONE 2019, 15, e0229016. [Google Scholar] [CrossRef]
- Halka, M.; Smoleń, S.; Czernicka, M.; Klimek-Chodacka, M.; Pitala, J.; Tutaj, K. Iodine biofortification through expression of HMT, SAMT and S3H genes in Solanum lycopersicum L. Plant Physiol. Biochem. 2019, 144, 35–48. [Google Scholar] [CrossRef]
- Grzanka, M.; Smoleń, S.; Kováčik, P. Effect of vanadium on the uptake and distribution of organic and inorganic forms of iodine in sweetcorn plants during early-stage development. Agronomy 2020, 10, 1666. [Google Scholar] [CrossRef]
- Smoleń, S.; Czernicka, M.; Kowalska, I.; Kȩska, K.; Halka, M.; Grzebelus, D.; Grzanka, M.; Skoczylas, Ł.; Pitala, J.; Koronowicz, A.; et al. New aspects of uptake and metabolism of non-organic and organic iodine compounds—The role of vanadium and plant-derived thyroid hormone analogs in lettuce. Front. Plant Sci. 2021, 12, 653168. [Google Scholar] [CrossRef] [PubMed]
- Tagami, K.; Uchida, S.; Hirai, I.; Tsukada, H.; Takeda, H. Determination of chlorine, bromine and iodine in plant samples by inductively coupled plasma-mass spectrometry after leaching with tetramethyl ammonium hydroxide under a mild temperature condition. Anal. Chim. Acta 2006, 570, 88–92. [Google Scholar] [CrossRef]
- Nascimento, M.S.; Mendes, A.L.G.; Henn, A.S.; Picoloto, R.S.; Mello, P.A.; Flores, E.M. Accurate determination of bromine and iodine in medicinal plants by inductively coupled plasma-mass spectrometry after microwave-induced combustion. Spectrochim. Acta Part B At. Spectrosc. 2017, 138, 58–63. [Google Scholar] [CrossRef]
- Tong, Y.; Liu, Z.Z.; Lu, J.F.; Zhang, H.Y.; Shi, K.Q.; Chen, G.R.; Liu, Y.-Q.; Feng, H.-R.; Pan, Y.J. Detection and quantification of water-soluble inorganic chlorine, bromine and iodine by MALDI-MS. J. Anal. Test. 2022, 6, 419–423. [Google Scholar] [CrossRef]
- Di Napoli, A.; Zucchetti, P. A comprehensive review of the benefits of Taraxacum officinale on human health. Bull. Natl. Res. Cent. 2021, 45, 110. [Google Scholar] [CrossRef]
- Amin Mir, M.; Sawhney, S.S.; Jassal, M.M.S. Qualitative and quantitative analysis of phytochemicals of Taraxacum officinale. Wudpecker J. Pharm. Pharmocol. 2013, 2, 1–5. [Google Scholar]
- Singh, A.; Malhotra, S.; Subban, R. Dandelion (Taraxacum officinale)—Hepatoprotective herb with therapeutic potential. Pharmacogn. Rev. 2008, 2, 163–167. [Google Scholar]
- Kabata-Pendias, A.; Dudka, S. Trace metal contents of Taraxacum officinale (dandelion) as a convenient environmental indicator. Environ. Geochem. Health 1991, 13, 108–113. [Google Scholar] [CrossRef]
- Respondek, Z.; Isinkaralar, O.; Świsłowski, P.; Isinkaralar, K.; Rajfur, M. Biomonitoring with the use of the herbal plant Taraxacum officinale as a source of information on environmental contamination. Plants 2024, 13, 1805. [Google Scholar] [CrossRef]
- Królak, E. Accumulation of Zn, Cu, Pb and Cd by dandelion (Taraxacum officinale Web.) in environments with various degrees of metallic contamination. Polish J. Environ. Studies 2003, 12, 713–721. [Google Scholar]
- Hartmans, J. Factors affecting the herbage iodine content. Netherlands J. Agric. Sci. 1974, 22, 195–206. [Google Scholar] [CrossRef]
- Baidoo, I.K.; Fletcher, J.J.; Mensah, P.K.; Quagraine, R.E.; Opata, N.S. Determination of mineral element composition of Ayoyo, Baobab and Dandelion vegetable green leaves in Ghana using instrumental neutron activation analysis. J. Food Meas. Charact. 2014, 8, 389–397. [Google Scholar] [CrossRef]
- Krzepiłko, A.; Prażak, R.; Skwaryło-Bednarz, B.; Molas, J. Agronomic biofortification as a means of enriching plant foodstuffs with iodine. Acta Agrobot. 2019, 72, 1766. [Google Scholar] [CrossRef]
- Djingova, R.; Kuleff, I.; Penev, I.; Sansoni, B. Bromine, copper, manganese, and lead content of the leaves of Taraxacum officinale (dandelion). Sci. Total Environ. 1986, 50, 197–208. [Google Scholar] [CrossRef]
- Shtangeeva, I.; Niemelä, M.; Perämäki, P.; Ryumin, A.; Timofeev, S.; Chukov, S.; Kasatkina, G. Phytoextraction of bromine from contaminated soil. J. Geochem. Explor. 2017, 174, 21–28. [Google Scholar] [CrossRef]
- Shtangeeva, I.; Niemelä, M.; Perämäki, P.; Timofeev, S. Response of wheat and pea seedlings on increase of bromine concentration in the growth medium. Environ. Sci. Poll. Res. 2015, 22, 19060–19068. [Google Scholar] [CrossRef]
- Shtangeeva, I.; Niemelä, M.; Perämäki, P. Bioavailability and toxicity of bromine and neodymium for plants grown in soil and water. Environ. Geochem. Health 2022, 44, 285–293. [Google Scholar] [CrossRef]
- Yuita, K. Overview and dynamics of iodine and bromine in the environment, 2: Iodine and bromine toxicity and environmental hazards. Jpn. Agric. Res. Q. 1994, 28, 90–111. [Google Scholar]
- Yamada, H.; Takeda, C.; Mizushima, A.; Yoshino, K.; Yonebayashi, K. Effect of oxidizing power of roots on iodine uptake by rice plants. Soil Sci. Plant Nutr. 2005, 51, 141–145. [Google Scholar] [CrossRef]
- Blasco, B.; Rios, J.J.; Cervilla, L.M.; Sánchez-Rodrigez, E.; Ruiz, J.M.; Romero, L. Iodine biofortification and antioxidant capacity of lettuce: Potential benefits for cultivation and human health. Ann. Appl. Biol. 2008, 152, 289–299. [Google Scholar] [CrossRef]
- Cortés-Flores, C.; Rodríguez-Mendoza, M.N.; Benavides-Mendoza, A.; García-Cué, J.L.; Tornero-Campante, M.; Sánchez-García, P. Iodine increases the growth and mineral concentration in sweet pepper seedlings. Agrociencia 2016, 50, 747–758. [Google Scholar]
- Shtangeeva, I.; Perämäki, P.; Niemelä, M.; Kurashov, E.; Krylova, Y. Potential of wheat (Triticum aestivum L.) and pea (Pisum sativum) for remediation of soils contaminated with bromides and PAHs. Int. J. Phytoremediat. 2018, 20, 560–566. [Google Scholar] [CrossRef]
- Shtangeeva, I.; Niemelä, M.; Perämäki, P. Effects of bromides of potassium and ammonium on some crops. J. Plant Nutr. 2019, 42, 2209–2220. [Google Scholar] [CrossRef]
- Alarcón, M.V.; Salguero, J.; Lloret, P.G. Auxin modulated initiation of lateral roots is linked to pericycle cell length in maize. Front. Plant Sci. 2019, 10, 11. [Google Scholar] [CrossRef]
- Edelmann, H.G. Plant root development: Is the classical theory for auxin-regulated root growth false? Protoplasma 2022, 259, 823–832. [Google Scholar] [CrossRef]
- To, H.T.M.; Nguyen, H.T.; Dang, N.T.M.; Nguyen, N.H.; Bui, T.X.; Lavarenne, J.; Phung, N.T.P.; Gantet, P.; Lebrun, M.; Bellafiore, S.; et al. Unraveling the genetic elements involved in shoot and root growth regulation by jasmonate in rice using a genome-wide association study. Rice 2019, 12, 69. [Google Scholar] [CrossRef]
- Sharp, R.E.; LeNoble, M.E. ABA, ethylene and the control of shoot and root growth under water stress. J. Exp. Bot. 2002, 53, 33–37. [Google Scholar] [CrossRef]
- Brookbank, B.P.; Patel, J.; Gazzarrini, S.; Nambara, E. Role of basal ABA in plant growth and development. Genes 2021, 12, 1936. [Google Scholar] [CrossRef]
- Halka, M.; Smoleń, S.; Ledwożyw-Smoleń, I. Antioxidant potential and iodine accumulation in tomato (Solanum lycopersicum L.) seedlings as the effect of the application of three different iodobenzoates. Folia Hortic. 2020, 32, 203–219. [Google Scholar] [CrossRef]
- Brown, P.H.; Zhao, F.J.; Dobermann, A. What is a plant nutrient? Changing definitions to advance science and innovation in plant nutrition. Plant Soil 2022, 476, 11–23. [Google Scholar] [CrossRef]
- Nascimento, V.L.; Souza, B.C.; Lopes, G.; Guilherme, L.R. On the role of iodine in plants: A commentary on benefits of this element. Front. Plant Sci. 2022, 13, 836835. [Google Scholar] [CrossRef] [PubMed]
- Claussen, W. Proline as a measure of stress in tomato plants. Plant Sci. 2005, 168, 241–248. [Google Scholar] [CrossRef]
- Fletcher, R.A.; Oegema, T.; Horton, R.F. Endogenous gibberellin levels and senescence in Taraxacum officinale. Planta 1969, 86, 98–102. [Google Scholar] [CrossRef]
- Eriksson, S.; Böhlenius, H.; Moritz, T.; Nilsson, O. GA4 is the active gibberellin in the regulation of LEAFY transcription and Arabidopsis floral initiation. Plant Cell 2006, 18, 2172–2181. [Google Scholar] [CrossRef]
- Kim, Y.H.; Hamayun, M.; Khan, A.L.; Na, C.I.; Kang, S.M.; Han, H.H.; Lee, I. Exogenous application of plant growth regulators increased the total flavonoid content in Taraxacum officinale Wigg. Afr. J. Biotech. 2009, 8, 5727–5736. [Google Scholar] [CrossRef]
- Dávila Rangel, I.E.; Trejo Téllez, L.I.; Ortega Ortiz, H.; Juárez Maldonado, A.; González Morales, S.; Companioni González, B.; De la Fuente, M.C.; Benavides Mendoza, A. Comparison of iodide, iodate, and iodine-chitosan complexes for the biofortification of lettuce. Appl. Sci. 2020, 10, 2378. [Google Scholar] [CrossRef]
- Krzepiłko, A.; Święciło, A.; Zych-Wężyk, I. The antioxidant properties and biological quality of radish seedlings biofortified with iodine. Agronomy 2021, 11, 2011. [Google Scholar] [CrossRef]
- Fuentes, J.E.G.; Castellanos, B.F.H.; Martínez, E.N.R.; Ortiz, W.A.N.; Mendoza, A.B.; Macías, J.M. Outcomes of foliar iodine application on growth, minerals and antioxidants in tomato plants under salt stress. Folia Hortic. 2022, 34, 27–37. [Google Scholar] [CrossRef]
- Leyva, R.; Sánchez-Rodríguez, E.; Ríos, J.J.; Rubio-Wilhelmi, M.M.; Romero, L.; Ruiz, J.M.; Blasco, B. Beneficial effects of exogenous iodine in lettuce plants subjected to salinity stress. Plant Sci. 2011, 181, 195–202. [Google Scholar] [CrossRef]
- Küpper, F.C.; Carpenter, L.J.; Leblanc, C.; Toyama, C.; Uchida, Y.; Maskrey, B.H.; Robinson, J.; Verhaeghe, E.F.; Malin, G.; Luther, G.W.; et al. In vivo speciation studies and antioxidant properties of bromine in Laminaria digitata reinforce the significance of iodine accumulation for kelps. J. Exp. Bot. 2013, 64, 2653–2664. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sahin, O.; Taskin, M.B.; Kadioglu, Y.K.; Inal, A.; Gunes, A.; Pilbeam, D.J. Influence of chloride and bromate interaction on oxidative stress in carrot plants. Sci. Hortic. 2012, 137, 81–86. [Google Scholar] [CrossRef]
- Yalçin, E.; Çavuşoğlu, K. Toxicity assessment of potassium bromate and the remedial role of grape seed extract. Sci. Rep. 2022, 12, 20529. [Google Scholar] [CrossRef] [PubMed]
- Smoleń, S.; Kowalska, I.; Sady, W. Assessment of biofortification with iodine and selenium of lettuce cultivated in the NFT hydroponic system. Sci. Hortic. 2014, 166, 9–16. [Google Scholar] [CrossRef]
- Smolen, S.; Kowalska, I.; Skoczylas, Ł.; Tabaszewska, M.; Pitala, J.; Mrozek, J.; Kovácik, P. Effectiveness of enriching lettuce with iodine using 5-iodosalicylic and 3, 5-diiodosalicylic acids and the chemical composition of plants depending on the type of soil in a pot experiment. Food Chem. 2022, 382, 132347. [Google Scholar] [CrossRef] [PubMed]
- Ledwożyw-Smoleń, I.; Pitala, J.; Smoleń, S.; Liszka-Skoczylas, M.; Kováčik, P. Iodine biofortification of dandelion plants (Taraxacum officinale FH Wiggers Coll.) with the use of inorganic and organic iodine compounds. Molecules 2023, 28, 5638. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Szteke, B. Trace Elements in Abiotic and Biotic Environments; CRC Press: Boca Raton, FL, USA, 2015; p. 468. [Google Scholar] [CrossRef]
- Smoleń, S.; Kowalska, I.; Kováčik, P.; Halka, M.; Sady, W. Biofortification of six varieties of lettuce (Lactuca sativa L.) with iodine and selenium in combination with the application of salicylic acid. Front. Plant Sci. 2019, 10, 143. [Google Scholar] [CrossRef]
- Yalçın, S.; Şükran Okudan, E.; Karakaş, Ö.; Önem, A.N.; Sözgen Başkan, K. Identification and quantification of some phytohormones in seaweeds using UPLC-MS/MS. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 475–484. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. Phenolic constituents of Prunus domestica. I. Quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–71. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Apak, R.; Güclü, K.; Özyürek, M.; Esin Karademir, S. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E. using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2010, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Alhilo, I.; Alhilo, S.; Alkhatib, B.; Al-Shorman, A. Hyperthyroidism treatment by alternative therapies based on cupping and dietary-herbal supplementation: A case report. Drug Metab. Pers. Ther. 2021, 37, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Sularz, O.; Koronowicz, A.; Boycott, C.; Smoleń, S.; Stefanska, B. Molecular effects of iodine-biofortified lettuce in human gastrointestinal cancer cells. Nutrients 2022, 14, 4287. [Google Scholar] [CrossRef]
| Treatment | Biomass | Dry Matter | |||
|---|---|---|---|---|---|
| Roots (g·plant−1) | Leaves (g·plant−1) | Whole Plant (g) | Roots (%) | Leaves (%) | |
| Control | 32.8 ± 2.13 a | 32.4 ± 5.37 a | 65.2 ± 3.70 a | 15.4 ± 2.35 a | 12.2 ± 0.74 a |
| 10 µM I | 37.5 ± 4.37 b | 34.3 ± 5.97 a | 71.8 ± 2.25 b | 16.0 ± 2.58 a | 11.8 ± 0.49 a |
| 50 µM I | 37.8 ± 3.27 b | 32.8 ± 5.30 a | 70.6 ± 2.14 b | 16.5 ± 2.57 a | 11.6 ± 0.57 a |
| 10 µM Br | 38.5 ± 4.91 b | 32.1 ± 5.68 a | 70.6 ± 1.39 b | 14.6 ± 1.74 a | 11.2 ± 0.26 a |
| 50 µM Br | 41.4 ± 6.10 bc | 34.3 ± 6.82 a | 75.7 ± 1.88 b | 15.8 ± 2.78 a | 12.1 ± 0.53 a |
| 10 µM I + 10 µM Br | 40.9 ± 4.43 bc | 36.3 ± 6.83 a | 77.2 ± 2.96 bc | 14.3 ± 2.54 a | 12.1 ± 0.73 a |
| 50 µM I + 50 µM Br | 45.0 ± 8.35 c | 39.0 ± 6.25 a | 84.0 ± 2.85 c | 17.2 ± 1.67 a | 11.2 ± 0.32 a |
| Part of Plant | Treatment | Total Content | Quantitative Ratio Br:I | |
|---|---|---|---|---|
| mg I·kg−1 DW | mg Br·kg−1 DW | |||
| Leaves | Control | 0.34 ± 0.009 a | 9.04 ± 1.677 a | 26.6:1 |
| 10 µM I | 4.32 ± 0.810 b | 8.36 ± 0.872 a | 1.9:1 | |
| 50 µM I | 26.63 ± 8.702 c | 11.08 ± 1.227 a | 0.4:1 | |
| 10 µM Br | 0.44 ± 0.033 a | 31.08 ± 0.531 b | 70.6:1 | |
| 50 µM Br | 0.27 ± 0.016 a | 156.46 ± 11.062 d | 579.5:1 | |
| 10 µM I + 10 µM Br | 3.80 ± 0.969 b | 52.22 ± 4.975 c | 13.7:1 | |
| 50 µM I + 50 µM Br | 30.39 ± 5.191 d | 241.01 ± 49.417 e | 7.9:1 | |
| Roots | Control | 0.29 ± 0.050 a | 4.06 ± 0.443 a | 14.0:1 |
| 10 µM I | 1.20 ± 0.201 b | 4.38 ± 0.048 a | 3.6:1 | |
| 50 µM I | 5.81 ± 1.250 d | 5.40 ± 0.217 a | 0.9:1 | |
| 10 µM Br | 0.35 ± 0.018 a | 11.59 ± 1.286 c | 33.1:1 | |
| 50 µM Br | 0.33 ± 0.019 a | 41.42 ± 5.779 d | 125.5:1 | |
| 10 µM I + 10 µM Br | 1.03 ± 0.153 b | 12.21 ± 0.880 c | 11.8:1 | |
| 50 µM I + 50 µM Br | 5.11 ± 1.184 c | 38.12 ± 5.358 d | 7.5:1 | |
| Part of Plant | Treatment | Esculin (mg·kg−1 DW) | Chlorogenic Acid (mg·kg−1 DW) | Total Phenols (g·kg−1 DW) |
|---|---|---|---|---|
 |  | |||
| Leaves | Control | 34.7 ± 1.35 a | 365.0 ± 14.16 ab | 42.8 ± 0.96 bc |
| 10 µM I | 26.5 ± 0.83 a | 387.8 ± 10.26 b | 42.6 ± 1.08 c | |
| 50 µM I | 30.6 ± 2.37 a | 399.5 ± 4.62 c | 51.3 ± 2.23 d | |
| 10 µM Br | 28.0 ± 2.67 a | 305.5 ± 9.92 a | 39.3 ± 0.90 a | |
| 50 µM Br | 29.8 ± 1.48 a | 354.6 ± 13.44 ab | 40.6 ± 1.84 ab | |
| 10 µM I + 10 µM Br | 24.3 ± 0.76 a | 324.3 ± 16.15 ab | 41.6 ± 0.24 bc | |
| 50 µM I + 50 µM Br | 28.8 ± 1.65 a | 393.3 ± 15.17 bc | 41.0 ± 0.48 ab | |
| Roots | Control | 0.082 ± 0.005 a | 186.4 ± 3.65 a | 8.1 ± 0.20 ab |
| 10 µM I | 0.104 ± 0.005 a | 203.4 ± 4.49 abc | 8.0 ± 0.32 a | |
| 50 µM I | 0.104 ± 0.007 a | 222.6 ± 6.12 c | 8.7 ± 0.38 ab | |
| 10 µM Br | 0.104 ± 0.007 a | 266.0 ± 5.01 d | 11.3 ± 0.41 d | |
| 50 µM Br | 0.111 ± 0.003 a | 213.2 ± 4.17 bc | 9.5 ± 0.22 c | |
| 10 µM I + 10 µM Br | 0.104 ± 0.001 a | 189.0 ± 4.15 ab | 8.6 ± 0.45 ab | |
| 50 µM I + 50 µM Br | 0.096 ± 0.004 a | 194.3 ± 1.26 ab | 8.4 ± 0.33 ab |
| Part of Plant | Treatments | Antioxidant Capacity (µmol TE·g−1 DW) | ||
|---|---|---|---|---|
| CUPRAC | FRAP | DPPH | ||
| Leaves | Control | 186.1 ± 5.31 ab | 151.6 ± 6.50 a | 68.8 ± 4.18 ab |
| 10 µM I | 202.4 ± 12.19 bc | 152.9 ± 4.30 a | 72.2 ± 2.63 b | |
| 50 µM I | 204.8 ± 8.19 c | 167.4 ± 6.17 cd | 73.6 ± 3.76 b | |
| 10 µM Br | 188.1 ± 6.45 ab | 162.4 ± 4.65 bc | 67.8 ± 3.56 ab | |
| 50 µM Br | 184.3 ± 12.00 a | 155.5 ± 5.29 ab | 64.7 ± 2.49 ab | |
| 10 µM I + 10 µM Br | 210.9 ± 10.32 c | 178.1 ± 6.07 e | 65.2 ± 6.62 ab | |
| 50 µM I + 50 µM Br | 199.4 ± 9.37 bc | 171.2 ± 1.11 de | 62.4 ± 8.62 a | |
| Roots | Control | 67.8 ± 6.12 ab | 24.5 ± 0.53 a | 24.5 ± 1.38 a |
| 10 µM I | 65.7 ± 3.54 a | 23.6 ± 0.89 a | 26.2 ± 1.65 b | |
| 50 µM I | 75.3 ± 2.89 cd | 25.7 ± 0.58 b | 27.5 ± 1.23 bc | |
| 10 µM Br | 94.7 ± 2.97 e | 33.2 ± 1.50 d | 32.6 ± 0.49 d | |
| 50 µM Br | 80.7 ± 1.82 d | 29.3 ± 1.02 c | 29.5 ± 0.93 c | |
| 10 µM I + 10 µM Br | 73.3 ± 4.02 bc | 26.6 ± 0.97 b | 25.2 ± 1.32 ab | |
| 50 µM I + 50 µM Br | 72.0 ± 2.39 abc | 25.3 ± 1.21 ab | 25.6 ± 0.99 ab | |
| Part of Plant | Treatment | IAA | GA3 | GA4 | JA | ABA | Proline |
|---|---|---|---|---|---|---|---|
| (µg·kg−1 DW) | (mg·kg−1 DW) | ||||||
| Leaves | Control | 599.3 ± 24.08 b | 41.9 ± 1.41 bc | 283.4 ± 18.26 a | 412.2 ± 12.22 e | 421.8 ± 10.17 bc | 8.59 ± 0.056 a |
| 10 µM I | 548.1 ± 52.17 b | 133.5 ± 12.05 d | 250.2 ± 12.07 a | 347.3 ± 4.35 d | 551.2 ± 20.71 d | 12.31 ± 1.036 b | |
| 50 µM I | 645.6 ± 26.72 b | 23.9 ± 1.21 ab | 261.1 ± 13.37 a | 166.2 ± 2.29 a | 455.6 ± 18.07 c | 11.01 ± 0.926 b | |
| 10 µM Br | 602.8 ± 31.02 b | 9.9 ± 0.26 a | 315.7 ± 30.09 a | 284.4 ± 3.67 c | 363.2 ± 4.06 ab | 10.54 ± 0.569 ab | |
| 50 µM Br | 568.3 ± 40.57 b | 25.7 ± 2.31 ab | 270.3 ± 16.21 a | 145.1 ± 6.09 a | 392.2 ± 16.43 bc | 11.94 ± 0.700 b | |
| 10 µM I + 10 µM Br | 487.7 ± 29.26 b | 159.5 ± 1.20 d | 410.8 ± 7.77 b | 201.3 ± 3.26 b | 300.8 ± 7.63 a | 12.29 ± 1.124 b | |
| 50 µM I + 50 µM Br | 281.5 ± 6.55 a | 71.8 ± 6.90 c | 273.3 ± 9.70 a | 261.7 ± 5.49 c | 392.2 ± 21.05 bc | 11.16 ± 0.761 b | |
| Roots | Control | 72.49 ± 1.20 a | 311.8 ± 19.98 ab | 446.7 ± 7.91 a | 285.9 ± 19.96 a | 45.1 ± 5.43 bc | 10.84 ± 0.276 b |
| 10 µM I | 149.2 ± 15.72 b | 386.9 ± 6.91 ab | 569.6 ± 63.93 a | 525.8 ± 21.93 ab | 29.6 ± 2.51 ab | 8.15 ± 0.249 a | |
| 50 µM I | 282.2 ± 36.03 bc | 363.4 ± 30.67 ab | 484.4 ± 13.69 a | 946.2 ± 76.13 c | 33.7 ± 3.11 abc | 7.59 ± 0.368 a | |
| 10 µM Br | 239.4 ± 13.34 bc | 416.9 ± 24.22 b | 594.3 ± 7.08 a | 673.0 ± 41.23 b | 51.0 ± 6.43 c | 10.40 ± 0.650 b | |
| 50 µM Br | 329.2 ± 44.82 c | 316.9 ± 26.51 ab | 507.6 ± 34.26 a | 660.1 ± 48.64 b | 29.1 ± 0.21 ab | 10.37 ± 0.420 b | |
| 10 µM I + 10 µM Br | 403.1 ± 51.73 c | 371.1 ± 2.75 ab | 520.5 ± 3.40 a | 995.3 ± 69.71 c | 52.4 ± 0.13 c | 10.24 ± 0.514 b | |
| 50 µM I + 50 µM Br | 281.0 ± 23.97 bc | 288.2 ± 16.91 a | 551.3 ± 20.10 a | 595.7 ± 7.45 b | 19.4 ± 1.81 a | 10.69 ± 0.586 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledwożyw-Smoleń, I.; Smoleń, S.; Liszka-Skoczylas, M.; Pitala, J.; Skoczylas, Ł. The Effect of Bromine and Iodine on the Plant Growth, Phytochemical Composition and Antioxidant Capacity of Dandelion (Taraxacum officinale F.H. Wiggers Coll.) Plants. Molecules 2025, 30, 2239. https://doi.org/10.3390/molecules30102239
Ledwożyw-Smoleń I, Smoleń S, Liszka-Skoczylas M, Pitala J, Skoczylas Ł. The Effect of Bromine and Iodine on the Plant Growth, Phytochemical Composition and Antioxidant Capacity of Dandelion (Taraxacum officinale F.H. Wiggers Coll.) Plants. Molecules. 2025; 30(10):2239. https://doi.org/10.3390/molecules30102239
Chicago/Turabian StyleLedwożyw-Smoleń, Iwona, Sylwester Smoleń, Marta Liszka-Skoczylas, Joanna Pitala, and Łukasz Skoczylas. 2025. "The Effect of Bromine and Iodine on the Plant Growth, Phytochemical Composition and Antioxidant Capacity of Dandelion (Taraxacum officinale F.H. Wiggers Coll.) Plants" Molecules 30, no. 10: 2239. https://doi.org/10.3390/molecules30102239
APA StyleLedwożyw-Smoleń, I., Smoleń, S., Liszka-Skoczylas, M., Pitala, J., & Skoczylas, Ł. (2025). The Effect of Bromine and Iodine on the Plant Growth, Phytochemical Composition and Antioxidant Capacity of Dandelion (Taraxacum officinale F.H. Wiggers Coll.) Plants. Molecules, 30(10), 2239. https://doi.org/10.3390/molecules30102239






