Optimization of In Vitro Shoot Culture Parameters for Enhanced Biomass and Rosmarinic Acid Production in Salvia atropatana
Abstract
1. Introduction
2. Results
2.1. Effect of Exogenous Cytokinins
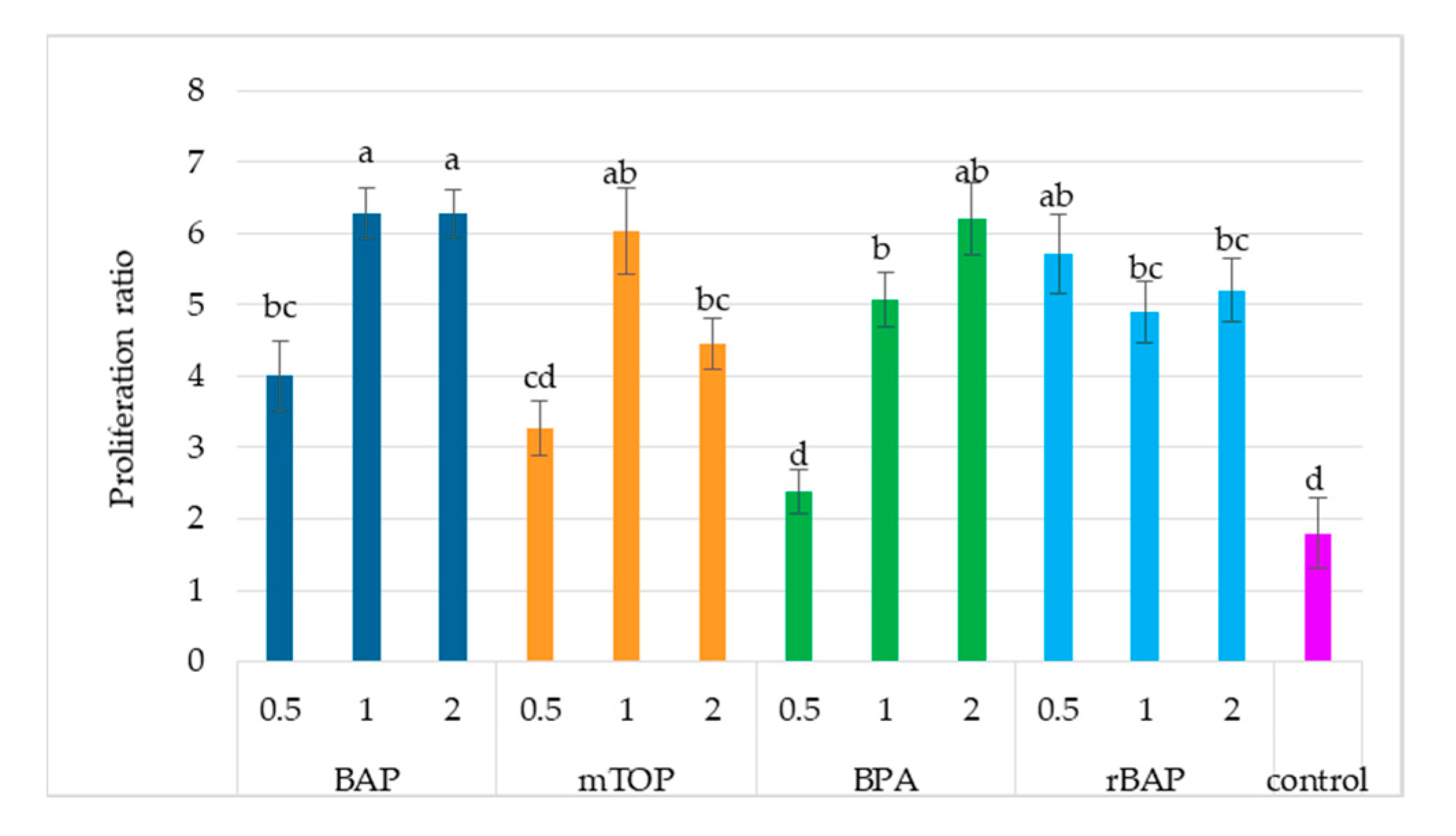
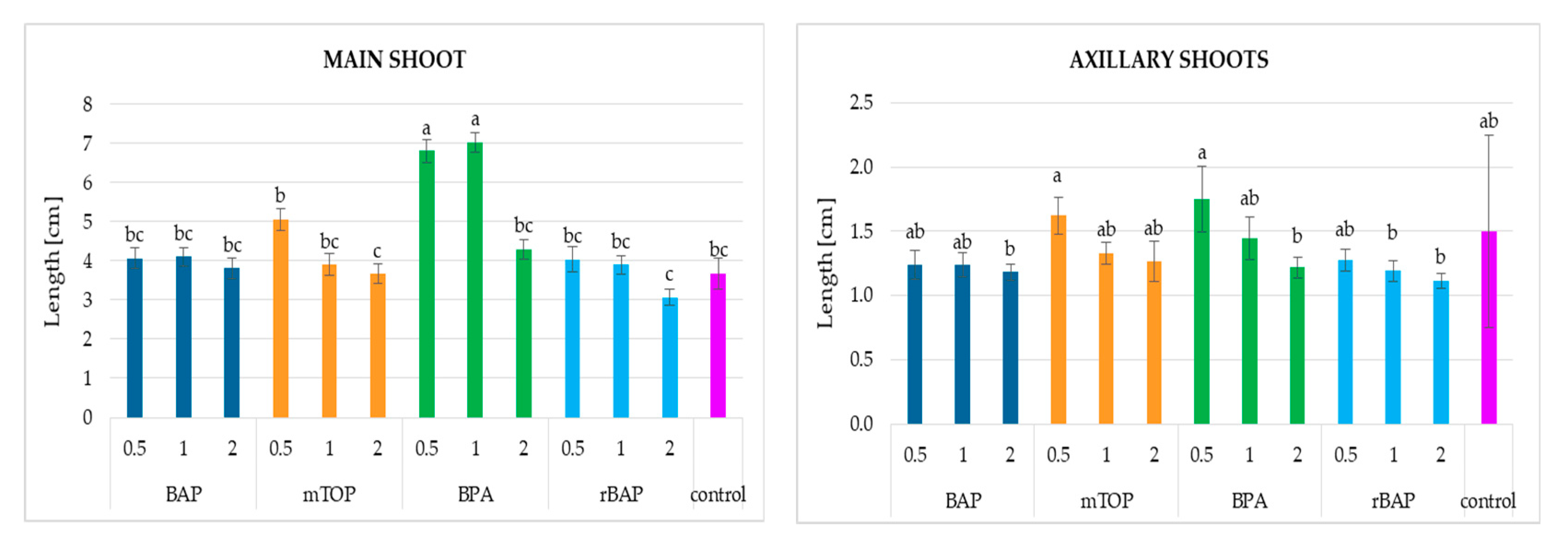
2.1.1. Culture Growth
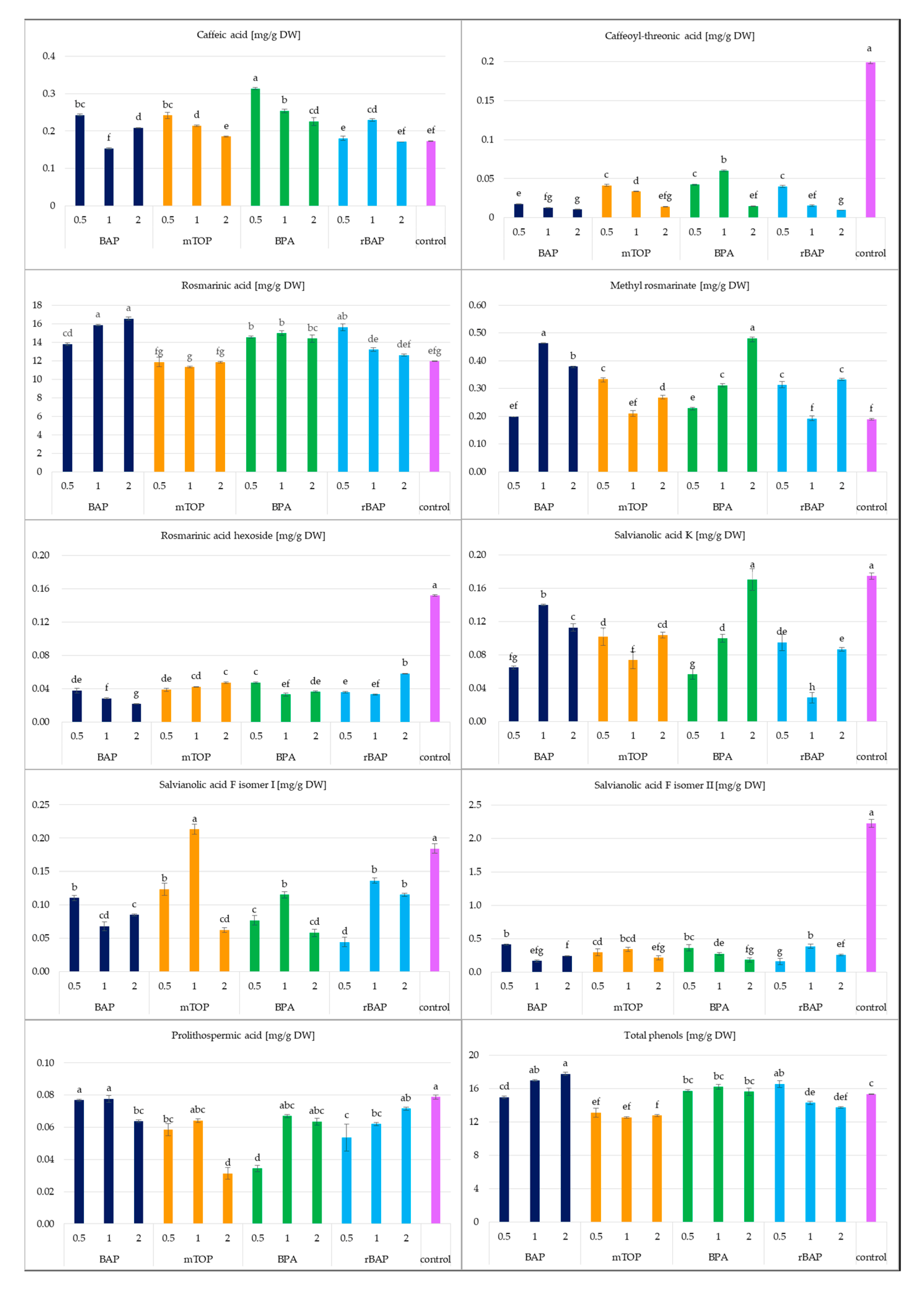
2.1.2. Secondary Metabolite Accumulation
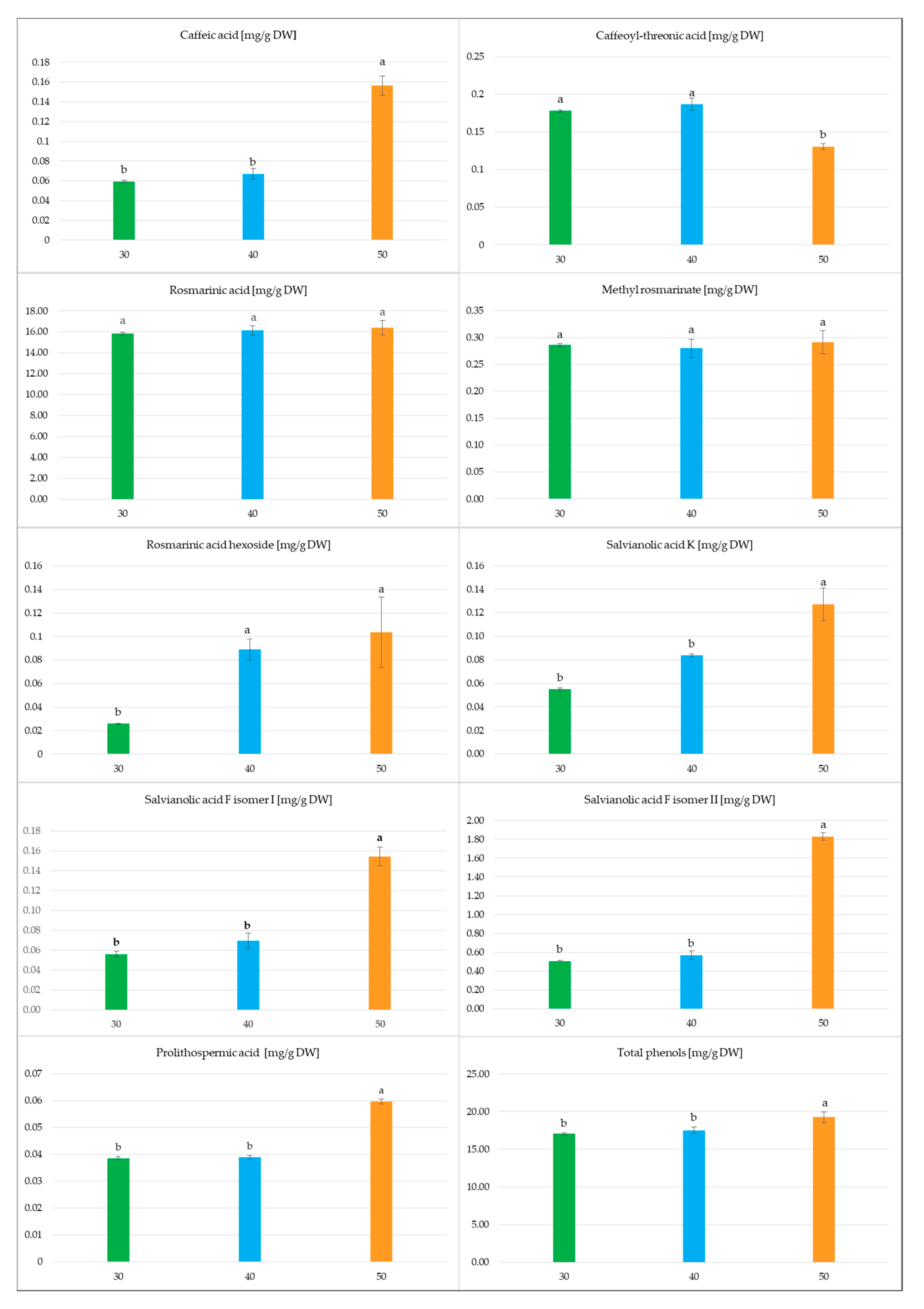
2.2. Effect of the Culture Duration
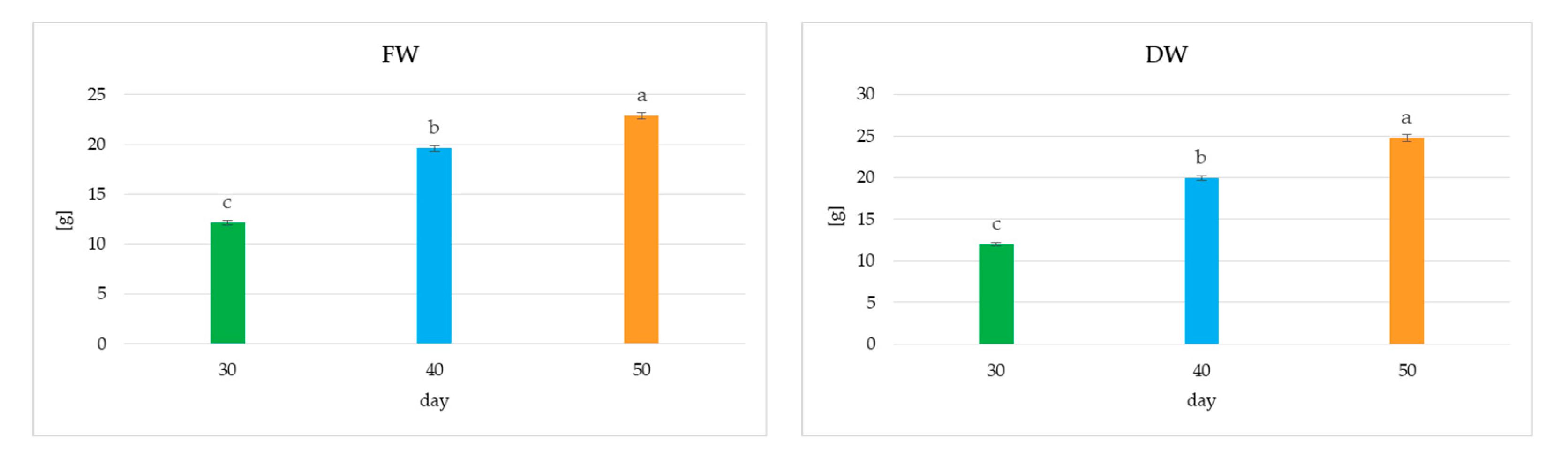
2.2.1. Culture Growth
2.2.2. Secondary Metabolite Accumulation
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Culture Proliferation, Growth, and Biomass Accumulation
4.3. Qualitative and Quantitative Polyphenolic Compound Analysis
4.4. Culture Duration
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Kantar, S.; Yassin, A.; Nehmeh, B.; Labaki, L.; Mitri, S.; Naser Aldine, F.; Hirko, A.; Caballero, S.; Monck, E.; Garcia-Maruniak, A.; et al. Deciphering the therapeutical potentials of rosmarinic acid. Sci. Rep. 2022, 12, 15489. [Google Scholar] [CrossRef] [PubMed]
- Adomako-Bonsu, A.G.; Chan, S.L.F.; Pratten, M.; Fry, J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. In Vitro 2017, 40, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Cuvelier, M.E. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J. Am. Oil Chem. Soc. 2002, 162, 981–987. [Google Scholar] [CrossRef]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci. OA 2018, 4, FSO283. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; El-Ansary, D.O.; Al-Mana, F.A.; Mahmoud, E.A. Saudi Rosmarinus officinalis and Ocimum basilicum L. polyphenols and biological activities. Processes 2020, 8, 446. [Google Scholar] [CrossRef]
- Bejenaru, L.E.; Biţă, A.; Mogoşanu, G.D.; Segneanu, A.E.; Radu, A.; Ciocîlteu, M.V.; Bejenaru, C. Polyphenols investigation and antioxidant and anticholinesterase activities of Rosmarinus officinalis L. species from southwest Romania flora. Molecules 2024, 29, 4438. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biologically active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef]
- Muráriková, A.; Kaffková, K.; Raab, S.; Neugebauerová, J. Evaluation of content of phenolics in Salvia species cultivated in South Moravian Region. Acta Fac. Pharm. Univ. Comen. LXII 2015, 9, 18–22. [Google Scholar]
- Akdeniz, M.; Yener, I.; Dincel, D.; Firat, M.; Karatas Degirmenci, D.; Ertas, A. Determination of fingerprints contents of different extracts and parts of six endemic Salvia taxa by GC–MS: Source species for valuable compounds with drug or drug potential. Biomed. Chromatogr. 2022, 36, e5263. [Google Scholar] [CrossRef]
- Areias, F.; Valentão, P.; Andrade, P.B.; Ferreres, F.; Seabra, R.M. Flavonoids and phenolic acids of sage: Influence of some agricultural factors. J. Agric. Food Chem. 2000, 48, 6081–6084. [Google Scholar] [CrossRef]
- Askari, S.F.; Avan, R.; Tayarani-Najaran, Z.; Sahebkar, A.; Eghbali, S. Iranian Salvia species: A phytochemical and pharmacological update. Phytochemistry 2021, 183, 112619. [Google Scholar] [CrossRef] [PubMed]
- Ertas, A.; Yigitkan, S.; Orhan, I.E. A focused review on cognitive improvement by the genus Salvia L. (Sage)—From ethnopharmacology to clinical evidence. Pharmaceuticals 2023, 16, 171. [Google Scholar] [CrossRef]
- Radmanesh, H.; Tehranipour, M.; Sazgarnia, A. Effect of aqueous extract of Salvia atropatana leaf on subcutaneous tumor model of CT26 colon carcinoma in mice. J. Gorgan Univ. Med. Sci. 2021, 23, 38–46. [Google Scholar]
- Abdollahi-Ghehi, H.; Sonboli, A.; Ebrahimi, S.; Esmaeili, M.; Mirjalili, M. Triterpenic acid content and cytotoxicity of some Salvia species from Iran. Nat. Prod. Commun. 2019, 14, 1934578X19842722. [Google Scholar] [CrossRef]
- Shakeri, A.; Farahmand, S.S.; Tayarani-Najaran, Z.; Emami, S.A.; Kúsz, N.; Hohmann, J.; Boozari, M.; Tavallaie, F.Z.; Asili, J. 4,5-Seco-5,10-friedo-abietane-type diterpenoids with anticancer activity from Salvia atropatana Bunge. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 241–248. [Google Scholar] [CrossRef]
- Mirza, M.; Ahmadi, L. Composition of the essential oil of Salvia atropatana Bunge. J. Essent. Oil Res. 2000, 12, 575–576. [Google Scholar] [CrossRef]
- Fotovvat, M.; Radjabian, T.; Saboora, A. HPLC fingerprint of important phenolic compounds in some Salvia L. species from Iran. Rec. Nat. Prod. 2019, 13, 37–49. [Google Scholar] [CrossRef]
- Kharazian, N. Identification of flavonoids in leaves of seven wild growing Salvia L. (Lamiaceae) species from Iran. Prog. Biol. Sci. 2013, 3, 81–98. [Google Scholar]
- Grzegorczyk-Karolak, I.; Ejsmont, W.; Kiss, A.K.; Tabaka, P.; Starbała, W.; Krzemińska, M. Improvement of bioactive polyphenol accumulation in callus of Salvia atropatana Bunge. Molecules 2024, 29, 2626. [Google Scholar] [CrossRef]
- Krzemińska, M.; Hnatuszko-Konka, K.; Weremczuk-Jeżyna, I.; Owczarek-Januszkiewicz, A.; Ejsmont, W.; Olszewska, M.A.; Grzegorczyk-Karolak, I. Effect of light conditions on polyphenol production in transformed shoot culture of Salvia bulleyana Diels. Molecules 2023, 28, 4603. [Google Scholar] [CrossRef]
- Attaran Dowom, S.; Abrishamchi, P.; Radjabian, T.; Salami, S.A. Enhanced phenolic acids production in regenerated shoot cultures of Salvia virgata Jacq. after elicitation with Ag⁺ ions, methyl jasmonate and yeast extract. Ind. Crops Prod. 2017, 103, 81–88. [Google Scholar]
- Grzegorczyk-Karolak, I.; Hnatuszko-Konka, K.; Zarzycka, M.; Kuźma, Ł. The stimulatory effect of purine-type cytokinins on proliferation and polyphenolic compound accumulation in shoot culture of Salvia viridis. Biomolecules 2020, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Hano, C. (Ed.) Plant Hormones: Recent Advances, New Perspectives and Applications; IntechOpen: London, UK, 2022. [Google Scholar]
- Hallmark, H.T.; Rashotte, A.M. Cytokinin response factors: Responding to more than cytokinin. Plant Sci. 2019, 289, 110251. [Google Scholar] [CrossRef]
- Rademacher, W. Plant growth regulators: Backgrounds and uses in plant production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Pasternak, T.P.; Steinmacher, D. Plant growth regulation in cell and tissue culture in vitro. Plants 2024, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Echeverrigaray, S.; Carrer, R.P.; Andrade, L.B. Micropropagation of Salvia guaranitica Benth. through axillary shoot proliferation. Braz. Arch. Biol. Technol. 2010, 53, 883–888. [Google Scholar] [CrossRef]
- Makunga, N.P.; Van Staden, J. An efficient system for the production of clonal plantlets of the medicinally important aromatic plant: Salvia africana-lutea L. Plant Cell Tissue Organ Cult. 2008, 92, 63–72. [Google Scholar] [CrossRef]
- Misic, D.; Grubisic, D.; Konjevic, R. Micropropagation of Salvia brachyodon through nodal explants. Biol. Plant 2006, 50, 473–476. [Google Scholar] [CrossRef]
- Koszeghi, S.; Bereczki, C.; Balog, A.; Benedek, K. Comparing the effects of benzyladenine and meta-topolin on sweet basil (Ocimum basilicum) micropropagation. Not. Sci. Biol. 2014, 6, 422–427. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Skała, E.; Kuźma, Ł.; Kiss, A.K.; Grzegorczyk-Karolak, I. The effect of purine-type cytokinin on the proliferation and production of phenolic compounds in transformed shoots of Dracocephalum forrestii. J. Biotechnol. 2019, 306, 125–133. [Google Scholar] [CrossRef]
- Piątczak, E.; Owczarek, A.; Lisiecki, P.; Gonciarz, W.; Kozłowska, W.; Szemraj, M.; Chmiela, M.; Kiss, A.K.; Olszewska, M.A.; Grzegorczyk-Karolak, I. Identification and quantification of phenolic compounds in Salvia cadmica Boiss. and their biological potential. Ind. Crops Prod. 2021, 160, 113113. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Krzemińska, M.; Kiss, A.K.; Olszewska, M.A.; Owczarek, A. Phytochemical profile and antioxidant activity of aerial and underground parts of Salvia bulleyana Diels. Metabolites 2020, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Llorent-Martínez, E.J.; Fernández-de Córdova, M.L.; Bahadori, M.B.; Mocan, A.; Locatelli, M.; Aktumsek, A. Chemical composition and biological activities of extracts from three Salvia species: S. blepharochlaena, S. euphratica var. leiocalycina, and S. verticillata subsp. amasiaca. Ind. Crops Prod. 2018, 111, 11–21. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Krzemińska, M.; Kiss, A.K.; Owczarek-Januszkiewicz, A.; Olszewska, M.A. Role of phytohormones in biomass and polyphenol accumulation in Salvia bulleyana in vitro culture. Biomolecules 2023, 13, 227. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Wysokińska, H. The influence of cytokinins on proliferation and polyphenol accumulation in shoot cultures of Scutellaria altissima L. Phytochem. Lett. 2017, 20, 449–455. [Google Scholar]
- Bekircan, T.; Yaşar, A.; Yıldırım, S.; Sökmen, M.; Sökmen, A. Effect of cytokinins on in vitro multiplication, volatiles composition and rosmarinic acid content of Thymus leucotrichus Hal. shoots. 3 Biotech 2018, 8, 180. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Kuźma, Ł.; Kiss, A.K.; Grzegorczyk-Karolak, I. Effect of cytokinins on shoot proliferation and rosmarinic and salvianolic acid B production in shoot culture of Dracocephalum forrestii. Acta Physiol. Plant. 2018, 40, 189. [Google Scholar] [CrossRef]
- Skała, E.; Wysokińska, H. In vitro regeneration of Salvia nemorosa L. from shoot tips and leaf explants. In Vitro Cell. Dev. Biol. Plant 2004, 40, 596–602. [Google Scholar] [CrossRef]
- Tour, J.; Khalida, K. In vitro regeneration of Salvia santolinifolia. Pak. J. Bot. 2014, 46, 325–328. [Google Scholar]
- Yadav, A.; Kothari, S.L.; Kachhwaha, S.; Joshi, A. In vitro propagation of chia (Salvia hispanica L.) and assessment of genetic fidelity using random amplified polymorphic DNA and intersimple sequence repeat molecular markers. J. Appl. Biol. Biotechnol. 2019, 7, 42–47. [Google Scholar]
- Santos-Gomes, P.C.; Seabra, R.M.; Andrade, P.B.; Fernandes-Ferreira, M. Phenolic antioxidant compounds produced by in vitro shoots of Salvia officinalis L. Plant Sci. 2002, 162, 981–987. [Google Scholar] [CrossRef]
- Bektaş, E. Changes in essential oil composition, phenylalanine ammonia lyase gene expression and rosmarinic acid content during shoot organogenesis in cytokinin-treated Satureja spicigera (C. Koch) boiss. shoots. J. Plant Biochem. Biotechnol. 2020, 29, 450–460. [Google Scholar] [CrossRef]
- Tyagi, K.; Maoz, I.; Kochanek, B.; Sela, N.; Lerno, L.; Ebeler, S.E.; Lichter, A. Cytokinin but not gibberellin application had major impact on the phenylpropanoid pathway in grape. Hortic. Res. 2021, 8, 51. [Google Scholar] [CrossRef]
- Andersen, B.R.; Jin, G.; Chen, R.; Ertl, J.R.; Chen, C.M. Transcriptional regulation of hydroxypyruvate reductase gene expression by cytokinin in etiolated pumpkin cotyledons. Planta 1996, 198, 1–5. [Google Scholar] [CrossRef]
- Esmaeili, H.; Karami, A.; Maggi, F. Essential oil composition, total phenolic and flavonoids contents, and antioxidant activity of Oliveria decumbens Vent. (Apiaceae) at different phenological stages. J. Clean. Prod. 2018, 198, 91–95. [Google Scholar] [CrossRef]
- Hazrati, S.; Mollaei, S.; Rabbi Angourani, H.; Hosseini, S.J.; Sedaghat, M.; Nicola, S. How do essential oil composition and phenolic acid profile of Heracleum persicum fluctuate at different phenological stages? Food Sci. Nutr. 2020, 8, 6192–6206. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.R.; Bhattacharya, A.; Kaul, P.; Ramesh, S. Yield and chemical composition of rose-scented geranium (Pelargonium species) oil at different times of harvesting. J. Essent. Oil Res. 2001, 13, 456–459. [Google Scholar] [CrossRef]
- Hazrati, S.; Beidaghi, P.; Beyraghdar Kashkooli, A.; Hosseini, S.J.; Nicola, S. Effect of harvesting time variations on essential oil yield and composition of Salvia officinalis. Horticulturae 2022, 8, 149. [Google Scholar] [CrossRef]
- Farhat, M.B.; Chaouch-Hamada, R.; Sotomayor, J.A.; Landoulsi, A.; Jordán, M.J. Antioxidant potential of Salvia officinalis L. residues as affected by the harvesting time. Ind. Crops Prod. 2014, 54, 78–85. [Google Scholar] [CrossRef]
- Sellami, I.H.; Maamouri, E.; Chahed, T.; Wannes, W.A.; Kchouk, M.E.; Marzouk, B. Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoram (Origanum majorana L.). Ind. Crops Prod. 2009, 30, 395–402. [Google Scholar] [CrossRef]
- Ayan, A.K.; Yanar, P.; Cirak, C.; Bilgener, M. Morphogenetic and diurnal variation of total phenols in some Hypericum species from Turkey during their phenological cycles. Bangladesh J. Bot. 2007, 36, 39–46. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Hosseini, S.J.; Tahmasebi-Sarvestani, Z.; Pirdashti, H.; Modarres-Sanavy, S.A.M.; Mokhtassi-Bidgoli, A.; Hazrati, S.; Nicola, S. Investigation of yield, phytochemical composition, and photosynthetic pigments in different mint ecotypes under salinity stress. Food Sci. Nutr. 2021, 9, 2620–2643. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, M.; Pavlov, A. Rosmarinic acid production by Lavandula vera MM cell-suspension culture. Appl. Microbiol. Biotechnol. 1997, 47, 683–688. [Google Scholar] [CrossRef]
- Karam, N.S.; Jawad, F.M.; Arikat, N.A.; Shibl, R.A. Growth and rosmarinic acid accumulation in callus, cell suspension, and root cultures of wild Salvia fruticosa. Plant Cell Tissue Organ Cult. 2003, 73, 117–121. [Google Scholar] [CrossRef]
- Hakkim, F.L.; Kalyani, S.; Essa, M.; Girija, S.; Song, H. Production of rosmarinic acid in Ocimum sanctum (L.) cell suspension cultures by the influence of growth regulators. Int. J. Biol. Med. Res. 2011, 2, 1158–1161. [Google Scholar]
- De-Eknamkul, W.; Ellis, B.E. Rosmarinic acid production and growth characteristics of Anchusa officinalis cell suspension cultures. Planta Med. 1984, 50, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Xu, H.; Kim, Y.K.; Park, S.U. Rosmarinic acid production in hairy root cultures of Agastache rugosa Kuntze. World J. Microbiol. Biotechnol. 2008, 24, 969–972. [Google Scholar] [CrossRef]
- Roy, D.; Mukhopadhyay, S. Enhanced rosmarinic acid production in cultured plants of two species of Mentha. Indian J. Exp. Biol. 2012, 50, 817–825. [Google Scholar]
- Moumou, Y.; Trotin, F.; Dubois, J.; Vasseur, J.; El-Boustani, E. Influence of culture conditions on polyphenol production by Fagopyrum esculentum tissue cultures. J. Nat. Prod. 1992, 55, 33–38. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]





| Cytokinin (mg/L) | % of Response | % of Shoots | % of Buds |
|---|---|---|---|
| BAP 0.5 | 100 ± 0.0 | 57.76 ± 7.06 | 42.24 ± 7.06 |
| BAP 1 | 96.67 ± 5.77 | 48.35 ± 4.23 | 51.65 ± 4.23 |
| BAP 2 | 100 ± 0.0 | 56.59 ± 9.76 | 43.41 ± 9.76 |
| mTOP 0.5 | 93.33 ± 3.33 | 58.82 ± 8.37 | 41.18 ± 8.37 |
| mTOP 1 | 100 ± 0.0 | 54.14 ± 8.45 | 45.86 ± 8.45 |
| mTOP 2 | 96.67 ± 5.77 | 50.39 ± 9.19 | 49.61 ± 9.19 |
| BPA 0.5 | 100 ± 0.0 | 54.84 ± 9.00 | 45.16 ± 9.00 |
| BPA 1 | 96.67 ± 5.77 | 44.22 ± 6.90 | 55.78 ± 6.90 |
| BPA 2 | 100 ± 0.0 | 46.77 ± 2.26 | 53.23 ± 2.26 |
| rBAP 0.5 | 93.33 ± 6.67 | 63.13 ± 6.23 | 36.88 ± 6.23 |
| rBAP 1 | 100 ± 0.0 | 59.86 ± 2.30 | 40.14 ± 2.30 |
| rBAP 2 | 100 ± 0.0 | 49.01 ± 2.20 | 50.99 ± 2.20 |
| Control | 25 ± 2.89 | 44.44 ± 13.48 | 55.56 ± 13.48 |
| Peak No. | Rt | (M-H)− | Main Fragments | Tentative Compound |
|---|---|---|---|---|
| 1 | 14.9 | 297 | 135 | Caffeoyl-threonic acid |
| 2 | 19 | 179 | 135 | Caffeic acid |
| 3 | 25.2 | 357 | 313, 269, 203 | Prolithospermic acid |
| 4 | 32.2 | 521 | 223, 197, 179, 161 | Rosmarinic acid hexoside |
| 5 | 37.5 | 359 | 223, 197, 179, 161 | Rosmarinic acid |
| 6 | 38.1 | 555 | 537, 493, 359, 161 | Salvianolic acid K |
| 7 | 44.0 | 373 | 311, 197, 179, 135 | Methyl rosmarinate |
| 8 | 51.9 | 313 | 269, 161 | Salvianolic acid F isomer I |
| 9 | 53.9 | 313 | 269, 203, 161 | Salvianolic acid F isomer II |
| Treatment | Performance Score |
|---|---|
| C | 0.132 |
| 0.5 BAP | 0.635 |
| 1 BAP | 0.782 |
| 2 BAP | 0.773 |
| 0.5 mTOP | 0.347 |
| 1 mTOP | 0.683 |
| 2 mTOP | 0.569 |
| 0.5 BPA | 0.260 |
| 1 BPA | 0.600 |
| 2 BPA | 0.744 |
| 0.5 rBAP | 0.586 |
| 1 rBAP | 0.456 |
| 2 rBAP | 0.639 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ejsmont, W.; Kiss, A.K.; Grzegorczyk-Karolak, I. Optimization of In Vitro Shoot Culture Parameters for Enhanced Biomass and Rosmarinic Acid Production in Salvia atropatana. Molecules 2025, 30, 2654. https://doi.org/10.3390/molecules30122654
Ejsmont W, Kiss AK, Grzegorczyk-Karolak I. Optimization of In Vitro Shoot Culture Parameters for Enhanced Biomass and Rosmarinic Acid Production in Salvia atropatana. Molecules. 2025; 30(12):2654. https://doi.org/10.3390/molecules30122654
Chicago/Turabian StyleEjsmont, Wiktoria, Anna K. Kiss, and Izabela Grzegorczyk-Karolak. 2025. "Optimization of In Vitro Shoot Culture Parameters for Enhanced Biomass and Rosmarinic Acid Production in Salvia atropatana" Molecules 30, no. 12: 2654. https://doi.org/10.3390/molecules30122654
APA StyleEjsmont, W., Kiss, A. K., & Grzegorczyk-Karolak, I. (2025). Optimization of In Vitro Shoot Culture Parameters for Enhanced Biomass and Rosmarinic Acid Production in Salvia atropatana. Molecules, 30(12), 2654. https://doi.org/10.3390/molecules30122654






