1. Introduction
Understanding the chemical bonding and structure of gold chloride compounds and clusters plays an important role in many fields, such as nanoscience [
1,
2,
3,
4,
5], environmental science, geological studies [
6,
7,
8,
9] and homogeneous and heterogeneous catalysis [
10,
11,
12,
13,
14,
15,
16]. Barngrover and colleagues investigated the transformation of gold(III) chloride complexes into gold(I) thiolate species—a key step in the synthesis of thiolate-stabilized gold nanoparticles. Their work highlighted that the ligand exchange between chloride and thiolate ligands occurs with a low-activation energy barrier (~0.35 eV), facilitating the formation of gold chloride–thiolate or gold–thiolate clusters or nanoparticles from gold chloride precursors [
2]. In a separate study, Davies and co-workers examined the catalytic behavior of gold supported on carbon (Au/C) in the hydrochlorination of acetylene—an important industrial process for the production of vinyl chloride monomers. Their results demonstrated that Au–Cl complexes play an essential role in catalytic activity [
11]. Accordingly, additional insight into the stability of various gold chloride clusters can offer valuable information for understanding nanoparticle formation mechanisms and for designing catalysts with tailored properties.
In general, the chemistry of gold chloride differs from that of other related metals. Metal chloride complexes have mainly ionic properties, whereas gold-containing compounds have a pronounced covalent character. These differences are due to the strong relativistic effect that occurs in gold, leading to the stabilization of the outer 6s orbital and destabilization of the 5d orbitals, resulting in a decrease in the 6s–5d energy gap and an increase in s-d hybridization, which is responsible for the presence of covalent bonds between Au and Cl [
17,
18,
19]. Gold chloride clusters also have an interesting structure and properties [
20,
21,
22,
23,
24]. Theoretical studies have shown that the anions of the Au
nCl
n+1 cluster (n = 2–7), as the most stable isomers, have a planar zigzag structure characterized by an interesting interaction between the gold atoms. At the base of the cluster structure Au
nCl
n+1 for n > 2, there is a terminal Au···Au interaction (interaction between the terminal gold atom and the neighboring gold atom) and the interaction between two inner neighboring gold atoms (so-called non-terminal Au···Au interaction) [
22]. These distances between the terminal and non-terminal gold atom are shorter than the sum of the van der Waals radii of two gold atoms, which is unexpected since the gold(I) centers have a closed-shell electronic configuration [5d
10]. Therefore, this Au···Au interaction, whose energy lies between the van der Waals and covalent bonding, has been termed the “aurophilic interaction” [
25]. An increase in n in Au
nCl
n+1 clusters leads to a stronger “aurophilic interaction” between neighboring gold atoms. It has also been shown that the “aurophilic interactions” in gold–iodine clusters (Au
nI
n+1) are stronger than in these gold–chlorine clusters [
26]. The distances of Au···Au in the clusters of Au
nCl
n+3 (n = 3–7) are somewhat shorter than the corresponding distances in Au
nCl
n+1. The main structural difference between these clusters is the presence of the AuCl
4 unit, i.e., there is an Au (III) atom at the edge connected to four Cl atoms, which is not the case in the Au
nCl
n+1 cluster [
21,
22].
The relativistic effects can be decisive for the stability of the oxidation states of the 5d elements [
27]. While the halogen chemistry of silver and copper is restricted to the oxidation states +I and +II, the oxidation states +I and +III are known for gold, while the divalent Au(II) occurs less frequently [
28]. Gold can have different oxidation states in anionic, neutral and cationic complexes such as AuCl
2 (the valence of Au is +I in AuCl
2−, the valence of Au is +II for AuCl
2 and the valence of Au is +III for AuCl
2+). Since the oxidation states of Au can be increased by adding electronegative ligands such as chlorine (e.g., AuCl, AuCl
2 and AuCl
3), AuCl
n-type gold clusters (n = 1–6) were selected as a good prototype for exploring the maximum possible oxidation states of gold [
24,
29,
30]. Theoretical studies have shown that the participation of d electrons in the bonding increases with the increase in Cl atoms, so +V is the highest possible oxidation state of Au in the AuCl
n clusters. It is also shown that with an increase in the number of Cl atoms above 3 in the AuCl
n clusters (n= 2–6), a delocalization of the additional electron over several Cl atoms occurs. The consequence of this electron delocalization is the fact that AuCl
n clusters (n ≥ 2) have an adiabatic electron affinity (EA) that is greater than the EA of Cl, so these clusters belong to the group of “superhalogens” [
31,
32,
33]. It is interesting to note that the electron affinity of Au
2n clusters increases with the addition of Cl atoms. It should be emphasized that in the group of Au
2nCl clusters (n = 1−4), the electron affinity of Au
2Cl is higher than that of Cl, which is very unusual for a multimetal cluster belonging to a group of “superhalogens” [
24].
Experimentally, gold chloride clusters are generally obtained in the gas phase using various types of mass spectrometers. Karataev et al., for example, used a high-resolution time-of-flight mass spectrometer with electron impact ionization (EI-TOF-MS) for their experiment [
34]. A sample of HAuCl
4 (weighing 1–2 mg) was placed in a quartz crucible and heated at 100–120 °C in a tantalum furnace. In this way, the following gold clusters were recorded in the positive-mode mass spectrum: Au
2Cl
+, Au
2Cl
2+, Au
2Cl
3+, Au
2Cl
4+, Au
2Cl
6+. Lemke et al. have presented that the mononuclear clusters of the types [AuCl
2]
+(H
2O)
n (n = 0–4), [AuOHCl]
+ (H
2O)
n (n = 0–1) and [AuCl
2]
+(HCl)
2(H
2O)
n (n = 0–4) and the dinuclear [Au
2Cl
5-xOH
x]
+(H
2O)
n (x = 0–1) can be obtained by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry (ESI-FTICR-MS). The sample was an aqueous AuCl
3 solution (concentration 5–50 mM). The results showed that with increasing AuCl
3 concentration, the abundance of the dinuclear gold–chloride cluster fraction increases, especially [Au
2Cl
5]
+(H
2O)
n [
35]. Ma et al. used a matrix-assisted laser desorption/ionization Fourier transform ion cyclotron resonance mass spectrometer (MALDI-FTICR-MS) in their study [
23]. The sample was HAuCl
4 at a concentration of 2 mg/mL prepared in water, while the graphene was a matrix at a concentration of 1 mg/mL dispersed in acetone. First, 1 μL of the graphene dispersion was applied to the stainless target spot, and after drying, 1 μL of the HAuCl
4 solution was added to the same spot. The mass spectrum was generated using a Nd:YAG laser with typical laser energy of 3 mJ/pulse and a wavelength of 355 nm. In contrast to the two previous cases, the mass spectrum here was recorded in negative ion mode. In this mass spectrum, three main peaks corresponding to Au
3Cl
2− > Au
4Cl
− > Au
2Cl
3− clusters and low-intensity peaks of Au
5−, Au
3−, Au
7−~Au
6Cl
−, Au
8Cl
−, Au
5Cl
2− and Au
4Cl
3− were detected. The researchers in the same group have shown that when the HAuCl
4 concentration was increased to 20 mg/mL and the laser energy was increased by 10%, the cluster anions Au
3Cl
4− > Au
2Cl
3− > Au
4Cl
5− could be identified. In this case, the cluster anions Au
nCl
n−1− and Au
nCl
nH
− were also found with low intensity [
22].
It is worth noting that previous studies reported only selected gold chloride clusters with low intensity and insufficient stability for detailed analysis, which made systematic classification difficult. Although previous work showed the detection of Au
nCl
n+1−, Au
nCl
n+3− and Au
nCl
n+5−, (n = 2–4) by LDI-TOF-MS without graphene, the focus was mainly on theoretical considerations [
21]. In contrast, this study provides the first experimental insight into how cluster size—by the sequential addition of AuCl or Cl units—affects stability. By varying the laser intensity, we systematically investigated the relative stabilities of the simultaneously formed clusters. These results provide valuable insights into the stability and transformation of gold chloride clusters and help researchers evaluate their role in relevant systems. The results were also compared with previous mass spectra to assess the influence of graphene and with theoretical data to better understand the stability of clusters.
2. Results and Discussion
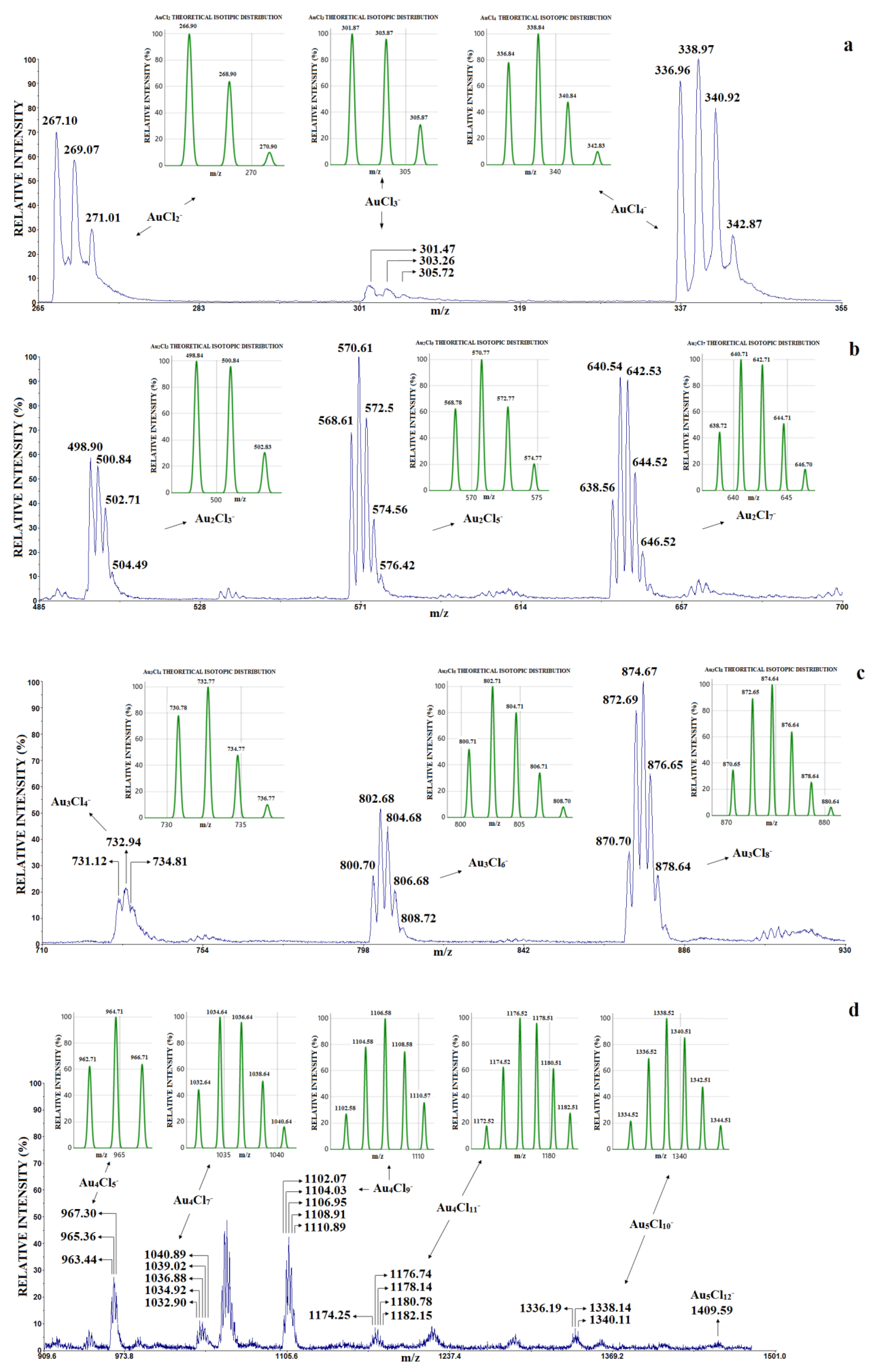
A typical LDI mass spectrum of HAuCl
4 in the positive mode is shown in
Figure 1.
For clarity, the representative LDI mass spectrum of HAuCl
4 in the negative mode is divided into four parts—
m/
z 260–360,
m/
z 480–700,
m/
z 700–930 and
m/
z 930–1500—and shown in
Figure 2. The theoretical isotopology of the assumed stoichiometry of the gold chloride clusters is shown in
Figure 2 next to the corresponding peak groups.
For an easier comparison of the results obtained in this work with the corresponding results available in the literature,
Table 1 lists the ions obtained by other authors from the EI-TOF-MS and MALDI-FTICR mass spectra of HAuCl
4. It should be noted that
Table 1 does not include the ESI-FTICR-MS [
35] and LDI-MS results (at a concentration of 2.0–0.025 mg/mL) for HAuCl
4 [
36] because both studies detected hydrated gold chloride clusters, which are not the subject of this study.
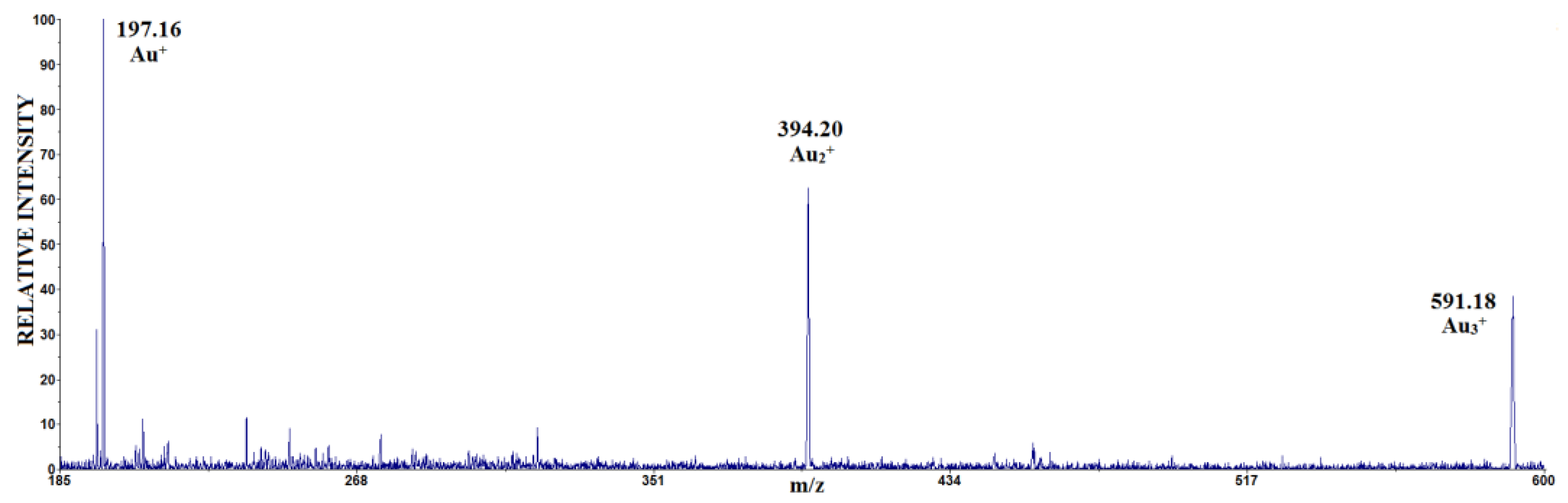
In this study, the positive mode of the LDI mass spectrum of HAuCl
4 contains three peaks at
m/
z 197, 394 and 591, which are identified as Au
+, Au
2+ and Au
3+, respectively (
Figure 1). In the laser energy range of 1100–2000 a.u., the individual intensities of the Au
n+ clusters (n = 1, 2, 3) decreased with increasing laser energy, while the ratio of their relative intensities was Au
+ > Au
2+ > Au
3+.
The comparison of the results in
Figure 1 with the results from the literature (
Table 1) shows that the choice of ionization method influences the type of clusters detected. For example, the LDI-MS method in positive mode favors the formation of Au
n+ clusters (n = 2, 3), while EI-TOF-MS favors the formation of Au
nCl
+ and Au
2Cl
n+1+ cations from the same sample [
34].
In the negative mode of the LDI mass spectrum of HAuCl
4 (
Figure 2), the following clusters were identified: a “superhalogen” mononuclear cluster of type AuCl
n+1− (n = 1, 2, 3) in the first part of
Figure 2a (
m/
z 260–360); a dinuclear cluster of type Au
2Cl
2n+1− in the second part of
Figure 2b (
m/
z 480–700); a trinuclear cluster of type Au
3Cl
2n+2− in the third part of
Figure 2c (
m/
z 700–930); and a tetranuclear cluster of type Au
4Cl
2n+1− and pentanuclear Au
5Cl
2n+2− clusters in the fourth part of
Figure 2d (
m/
z 930–1500). These clusters were detected in the laser intensity range from 1200 to 2600 a.u.
Comparing the available results for the positive and negative modes from
Table 1, it can be seen that mononuclear and dinuclear clusters can be obtained in the positive-mode EI-TOF-MS [
34], while mononuclear, dinuclear, trinuclear and tetranuclear gold chloride clusters were detected in the negative-mode LDI-TOF-MS. It should also be noted that clusters with two gold atoms and an even number of Cl atoms (Au
2Cl
4+, Au
2Cl
6+) were detected in the positive EI-TOF-MS mode, while dinuclear gold clusters with an odd number of Cl atoms (Au
2Cl
5−, Au
2Cl
7−) are stable in the negative LDI-TOF-MS mode. Interestingly, the dinuclear Au
2Cl
3 cluster is stable in both positive and negative modes.
The influence of graphene on the type of the gold chloride clusters can be observed by comparing the results of the LDI and MALDI methods in negative mode (
Figure 2 and
Table 1). With the MALDI method, clusters of type Au
nCl (n = 2, 4, 6, 8), Au
n (n = 1–9) and Au
nCl
n−1− (n = 3, 4) were detected (the matrix was graphene and the concentration of HAuCl
4 was 2 mg/mL,
Table 1). The mentioned clusters were not found in the LDI mass spectrum (without graphene and the concentration of HAuCl
4 was 2.5 mg/mL,
Figure 2).
On the other hand, as mentioned above, the anions Au
nCl
n+1, Au
nCl
n+3 and Au
nCl
n+5 were detected in the LDI mass spectrum (without graphene, the concentration of HAuCl
4 was 2.5 mg/mL,
Table 1 and
Figure 2). However, Au
nCl
n+1−, Au
nCl
n−1−, Au
nCl
nH
− anions (n = 1–4) were detected in the MALDI mass spectrum and at a much higher concentration (the concentration of HAuCl
4 was 20 mg/mL,
Table 1) than in the LDI method.
These indicates that in the negative mode, the presence of graphene is an important factor affecting the stability of some clusters, such as Aun− and AunCl−, AunCln− and AuCln+1−, while graphene suppresses the formation of the anions AuCln+1−, AuCln+3− and AuCln+5−.
To avoid confusion, it must be emphasized that the clusters identified in
Figure 2 can be grouped in two ways: as Au
nCl
n+m− (n = 2–5, m = 1, 3, 5, 7), i.e., as clusters of type AuCl
n+1−, AuCl
n+3− and AuCl
n+5− and Au
nCl
n+7− (as shown in previous work, which is consistent with previous theoretical results), or as mononuclear, dinuclear, trinuclear, tetranuclear and pentanuclear gold chloride clusters. In accordance with the above, two issues are discussed below: (1) How the increase in Au
nCl
n+1, Au
nCl
n+3 and Au
nCl
n+5 clusters for the AuCl unit affects its stability in the range of laser intensity from 1200 to 2600 a.u.; (2) How the increase in the number of chlorine atoms affects the stability of AuCl
n+1−, Au
2Cl
2n+1−, Au
3Cl
2n+2−, Au
4Cl
2n+1− and Au
5Cl
2n+2− clusters due to the increase in laser intensity.
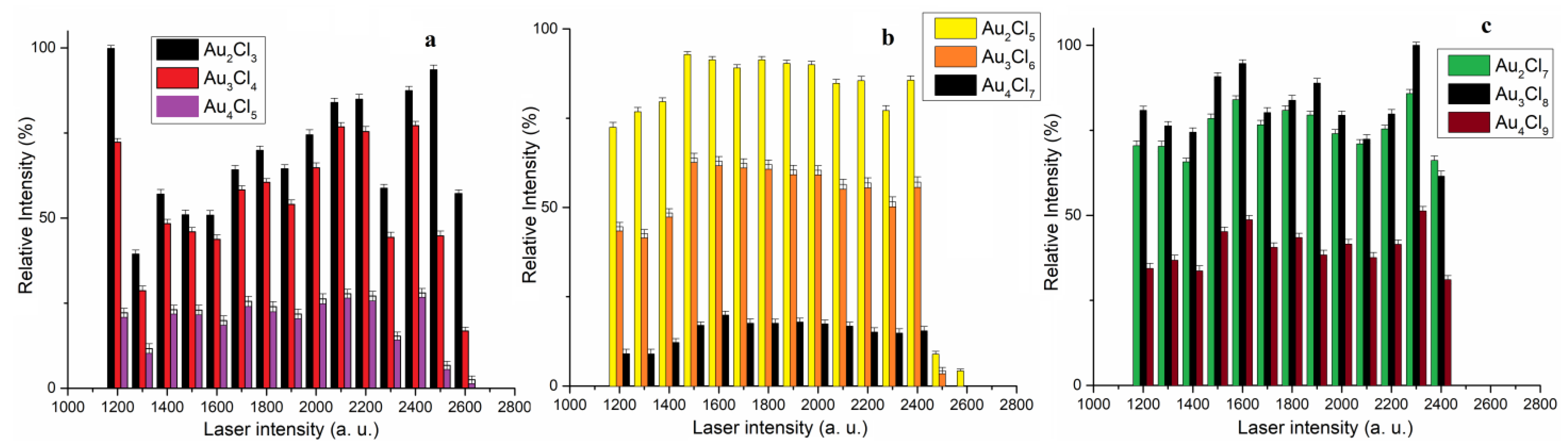
Under our experimental conditions, the Au
5Cl
10− and Au
5Cl
12− (pentanuclear Au
5Cl
2n+2 clusters) were of low intensity (
Figure 2d) and could therefore not be included in further investigations. The dependence of the relative intensity of the most abundant isotope vs. the laser intensity (in the range of 1200 do 2600 a.u.) for AuCl
n+1−, AuCl
n+3− and AuCl
n+5− clusters is presented in
Figure 3a, b and c, respectively.
The relative intensity of the Au
nCl
n+1− (n = 2–4) cluster changes in a similar way with increasing laser intensity (
Figure 3a). This can be explained by the fact that all clusters of the group Au
nCl
n+1− have similar zigzag structures with the characteristic chemical bond Au(I)-A(I). In this case, the Au
2Cl
3− cluster shows the highest stability in the observed range of laser intensity, which is consistent with previous experimental results [
22]. Results have shown that increasing the Au
2Cl
3 cluster by one AuCl unit leads to a small decrease in stability, and with the addition of another AuCl unit, the stability decreases significantly (
Figure 3a). Therefore, the ratio of the relative intensities of Au
nCl
n+1− clusters was Au
2Cl
3− > Au
3Cl
4− > Au
4Cl
5− and does not change with the change in laser intensity. This is consistent with theoretical calculations of their binding energies based on the formula Au
n−1Cl
n− + AuCl = AuCl
n+1, where the binding energies decrease in the series Au
2Cl
3 > Au
3Cl
4 > Au
4Cl
5 [
22]. In our experimental conditions, we have shown that the Au
2Cl
3 clusters without “aurophilic” interaction are more stable than Au
3Cl
4 and Au
4Cl
5 clusters with “aurophilic” interaction.
The relative intensity of the Au
nCl
n+3− clusters changes in the same way with increasing laser intensity, as in the previous case (
Figure 3b). The most stable cluster in this group is the Au
2Cl
5 cluster, which can be described as a fusion of the structural units of AuCl
4− and AuCl
2− by the elimination of a Cl atom, without “aurophilic” Au(I)-Au(I) interaction [
21]. Also in this case, similar to Au
nCl
n+1−, the stability of the cluster decreases with an increasing number of AuCl units, so the Au
4Cl
7− cluster has the lowest intensity and could not be detected at laser intensities of 2500 and 2600 a.u. (
Figure 3b). In the laser intensity range from 1200 to 2400, the ratio of the relative intensities of the Au
nCl
n+3−-type clusters is the same, i.e., Au
2Cl
5− > Au
3Cl
6− > Au
4Cl
7− (
Figure 3b).
A comparison of the results in
Figure 3a,b shows that increasing the Au
nCl
n+3− cluster by one AuCl unit leads to greater instability than for the Au
nCl
n+1− cluster. It should be noted that for the Au
nCl
n+1− and Au
nCl
n+3− clusters, the “aurophilic” interaction is present when n > 2 [
21,
22]. Accordingly, the experimental results indicate that the “aurophilic” Au(I)-Au(I) interaction has a more favorable effect on the stability of the Au
nCl
n+1− clusters than the combination of Au(III)-Au(I) and an “aurophilic” Au(I)-Au(I) interaction in the Au
nCl
n+3− cluster.
The Au
nCl
n+5− clusters were detected in a slightly narrower range of laser intensity from 1200 to 2400 (
Figure 3c), indicating their lower stability compared to Au
nCl
n+1− and Au
nCl
n+3− (
Figure 3a,b). However, it should be noted that the relative intensity of the Au
2Cl
7− and Au
3Cl
8− clusters was more than 60% in the observed laser intensity ranges. It is interesting to note that four clusters (Au
2Cl
7−, Au
3Cl
8−, Au
4Cl
9− and Au
5Cl
10−) were identified in the Au
nCl
n+5− cluster group, in contrast to the previous two groups where three clusters were identified (
Figure 2d). The Au
5Cl
10− cluster was of low intensity; its relative intensity was between 6 and 10%, but like the other Au
nCl
n+5− clusters, it was detected in the same laser range. In addition, the Au
3Cl
8− cluster (the second largest cluster in this group) is more stable than A
2Cl
7− and Au
4Cl
9−. However, it should be noted that the Au
2Cl
− and Au
3Cl
8− clusters, which differ from AuCl, do not show a significant difference in relative intensity, as is the case for the Au
nCl
n+3− clusters (
Figure 3c). Unfortunately, the results of the theoretical studies on the structure of the Au
nCl
n+5− clusters are missing in the literature, so we cannot compare the structure of the Au
nCl
n+5− clusters with the other two groups.
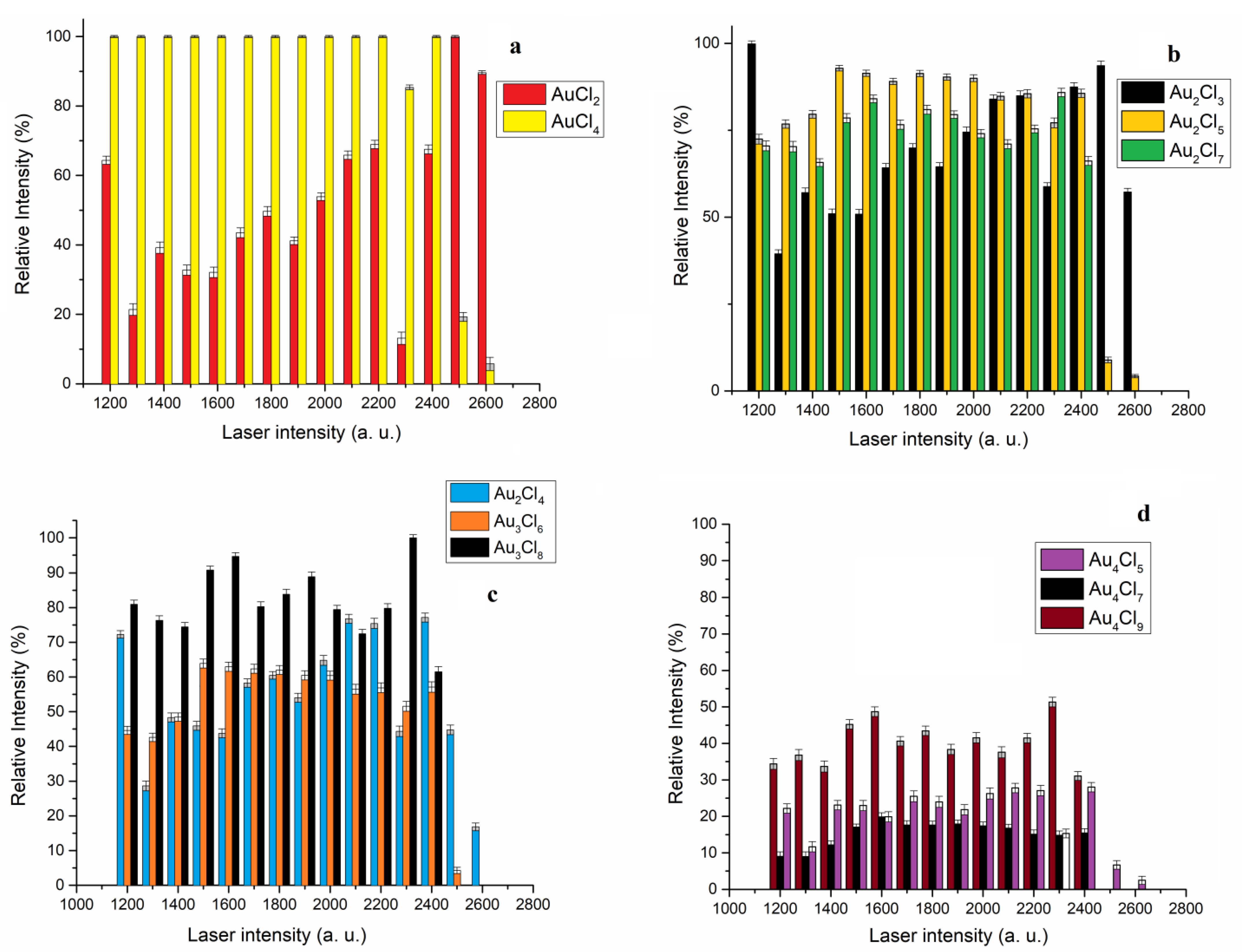
The dependence of the relative intensity of the most abundant isotope of AuCl
n+1−, Au
2Cl
2n+1−, Au
3Cl
2n+2− and Au
4Cl
2n+1− on the laser intensity is shown in
Figure 4a, b, c and d, respectively.
The theoretical studies of Srivastava and Misra have shown that the mononuclear AuCl
n species (n = 2–6) belong to the group of “superhalogens”, i.e., clusters whose electron affinity is higher than that of Cl (3.6 eV); therefore, it was expected that this type of cluster would dominate in the mass spectrum in the negative mode [
24]. Under our experimental conditions, three “superhalogens” of AuCl
n+1− (n = 1–3) were detected, but the relative intensity of the AuCl
3− was very low in the observed laser intensity range (
Figure 2a). For that reason, the dependence of the relative intensity of the most abundant isotope on the laser intensity for two “superhalogen” (AuCl
4− and AuCl
2−) is shown in
Figure 4a. In the laser intensity range from 1200 to 2400, the dominant anion is AuCl
4−. In this laser intensity range, the relative intensity of AuCl
4− is significantly higher than the relative intensity of AuCl
2−. This is consistent with the fact that the electron affinity of AuCl
4 is higher than that of AuCl
2. However, with increasing laser intensity above 2400 a.u., the dominant ion in the mass spectrum is AuCl
2−. It should be kept in mind that the intensity of the ions in the negative mode is influenced not only by the electron affinity but also by the dissociation energy of the observed clusters. Theoretical studies also show that the dissociation energies of the AuCl
n anion for the Cl atom and the Cl
2 molecule exhibit a similar trend, decreasing in the order AuCl
2 > AuCl
4 > AuCl
3 > AuCl
5 > AuCl
6. The dissociation energy for the Cl atom and the Cl
2 molecule is low for the AuCl
5, which, despite having the highest EA in the AuCl
n series, was not detected in the mass spectrum, and nor was AuCl
6. The structure of the AuCl
5 and AuCl
6 ions is described as (AuCl
4)Cl
n [
24]. Theoretical studies by Xu and others have also shown that the structure of the Au
nCl
n+3 cluster is such that it contains an Au(III) ion surrounded by four Cl atoms. Therefore, the AuCl
4 ion could have been formed by the dissociation of the above clusters. It should be mentioned that the Au
nCl
n+1 clusters have a zigzag structure (without an AuCl
4 unit), which could be one of the reasons why the AuCl
4 ion was not detected in the previous work [
22]. Regardless of the origin of the “superhalogens” AuCl
2 and AuCl
4, their stability under these experimental conditions is more significant than the stability of the other detected clusters.
Theoretical studies have shown that there is no “aurophilic” interaction in the most stable isomers of the dinuclear Au
2Cl
2n+1− clusters: Au
2Cl
3− and Au
2Cl
5−. The Au(III)-Au(I) interaction (3.99A) in the Au
2Cl
5− cluster ensures that this cluster is more stable than Au
2Cl
3− with Au(I)-Au(I) interaction (3.69A) in the laser intensity range from 1300 to 2000 a.u. (
Figure 4b). With the increase in laser intensity above 2000 a.u., the intensities of Au
2Cl
3− and Au
2Cl
5− clusters are very similar, while above 2400 a.u., the Au
2Cl
3− cluster is much more stable than Au
2Cl
5− and Au
2Cl
7−. Increasing the number of chlorine atoms in the dinuclear clusters does not significantly affect the stability, so the intensity of the Au
2Cl
7 cluster is higher than that of the Au
2Cl
3− cluster in the range from 1300 to 1900 and at 2300 a.u., but above 2400 a.u., the Au
2Cl
7− cluster is not recorded (
Figure 4b).
In the group of Au
3Cl
n+1− clusters, the cluster with the largest number of chlorine atoms, Au
3Cl
8−, shows the highest stability in the range of laser intensity from 1200 a.u. to 2400 a.u.; above 2400 a.u., this cluster was not detected (
Figure 4c). The calculated geometrical parameters for the most stable structures available in the literature show that the Au
3Cl
6− structure is formed by Au(III)-Au(I) interaction and “aurophilic” Au(I)-Au(I) interaction [
21]. This structure is more stable than the structure of the Au
3Cl
4− cluster, which is only formed by “aurophilic” interactions in the laser intensity range from 1300 to 2100 a.u. Above 2400 a.u., the intensity of Au
3Cl
6− decreases, while the Au
3Cl
4− cluster is detected at 2600 a.u.
The tetranuclear Au
4Cl
2n+1− clusters exhibit lower stability than the dinuclear and trinuclear clusters in the observed range of laser intensity (
Figure 4d). In the group of tetranuclear gold chloride clusters, the largest cluster, Au
4Cl
9−, is the most stable in the laser intensity range from 1200 to 2400 a.u. In this laser intensity range, the Au
4Cl
7− cluster, which contains both Au(III)-Au(I) and thermal and non-thermal “aurophilic” Au(I)-Au(I) bonds, exhibits the lowest stability in the Au
4Cl
2n+1− group. The cluster with only thermal and non-thermal “aurophilic” bonds, Au
4Cl
5−, is between Au
4Cl
9− and Au
4Cl
7− in intensity. However, it should be noted that the Au
4Cl
5− cluster is the only one detected above the laser intensity of 2400 a.u., suggesting that the clusters with only “aurophilic” interactions exhibit greater stability than others with increasing laser intensity.