Effect of Chitooligosaccharides on TLR2/NF-κB Signaling in LPS-Stimulated RAW 264.7 Macrophages
Abstract
1. Introduction
2. Results
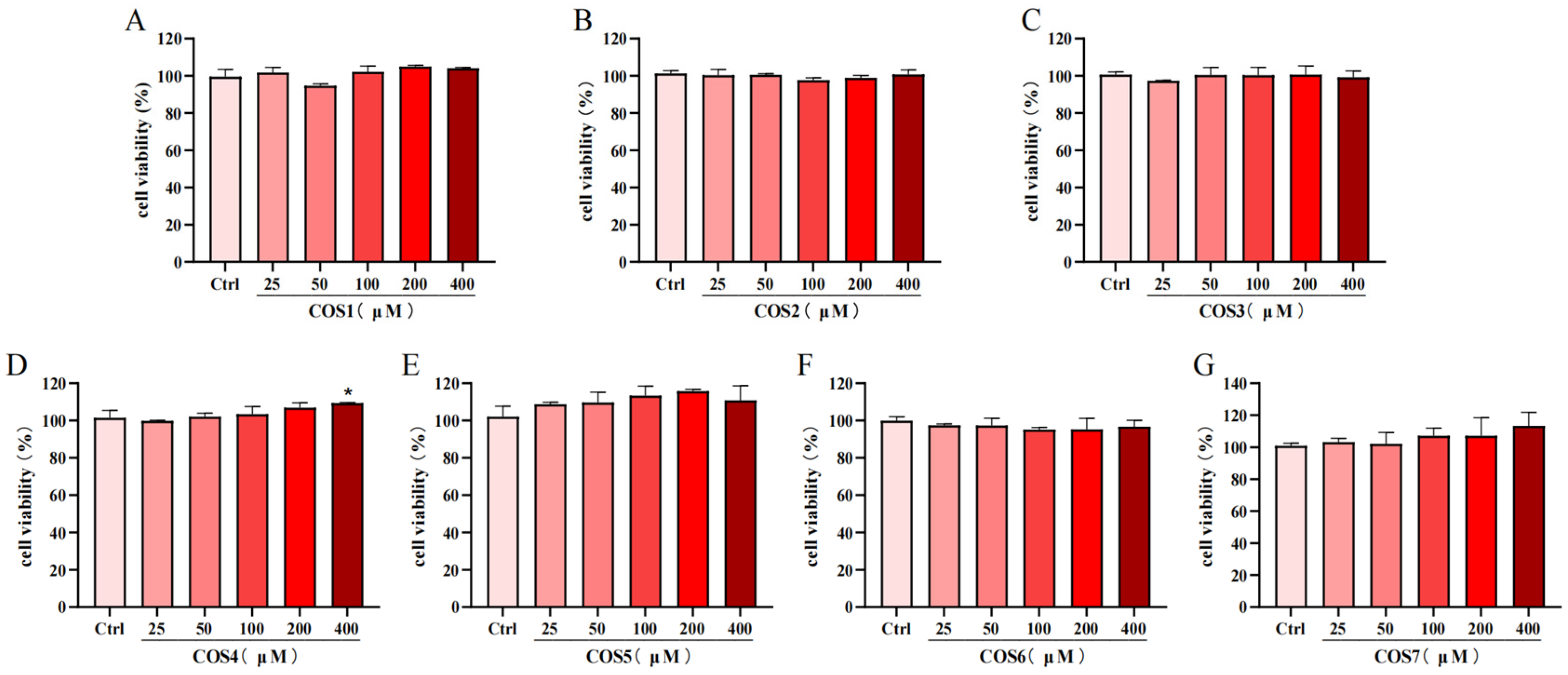
2.1. Effect of COS on the Viability of RAW 264.7 Cells
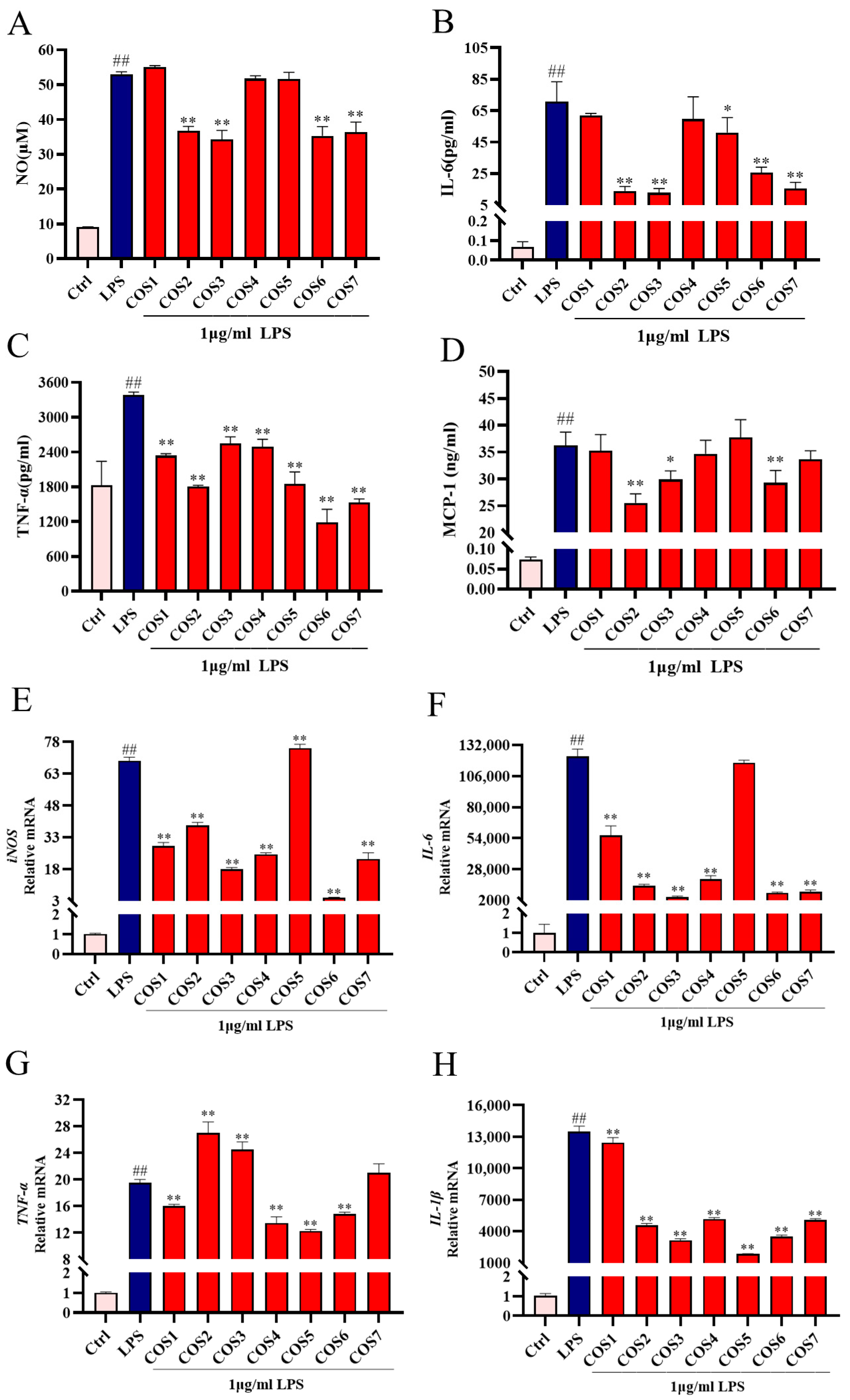
2.2. Effect of COS Polymerization Degree on LPS-Induced Inflammatory Signaling
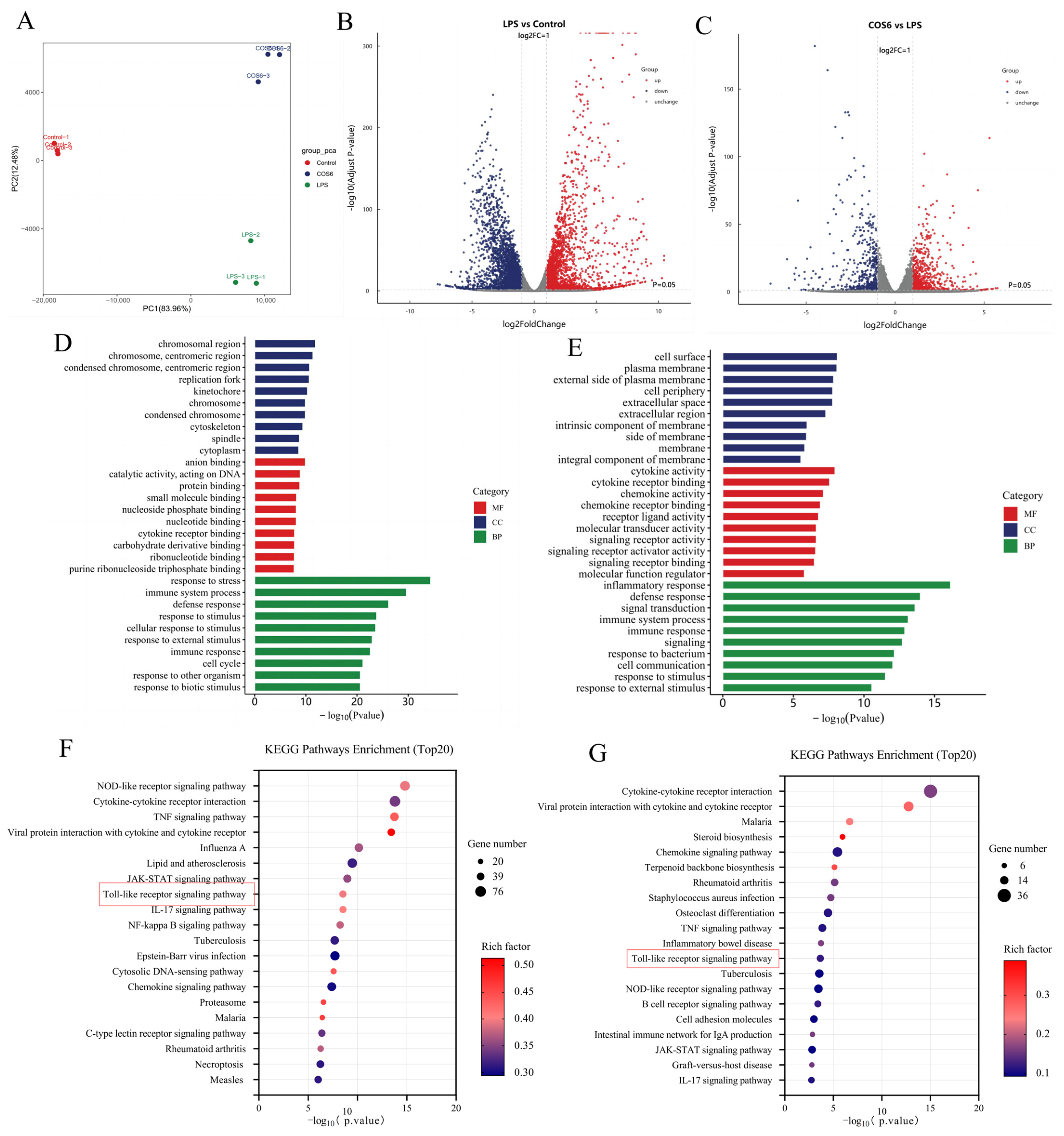
2.3. Effect of COS6 on Transcriptome of LPS-Stimulated RAW 264.7 Macrophages
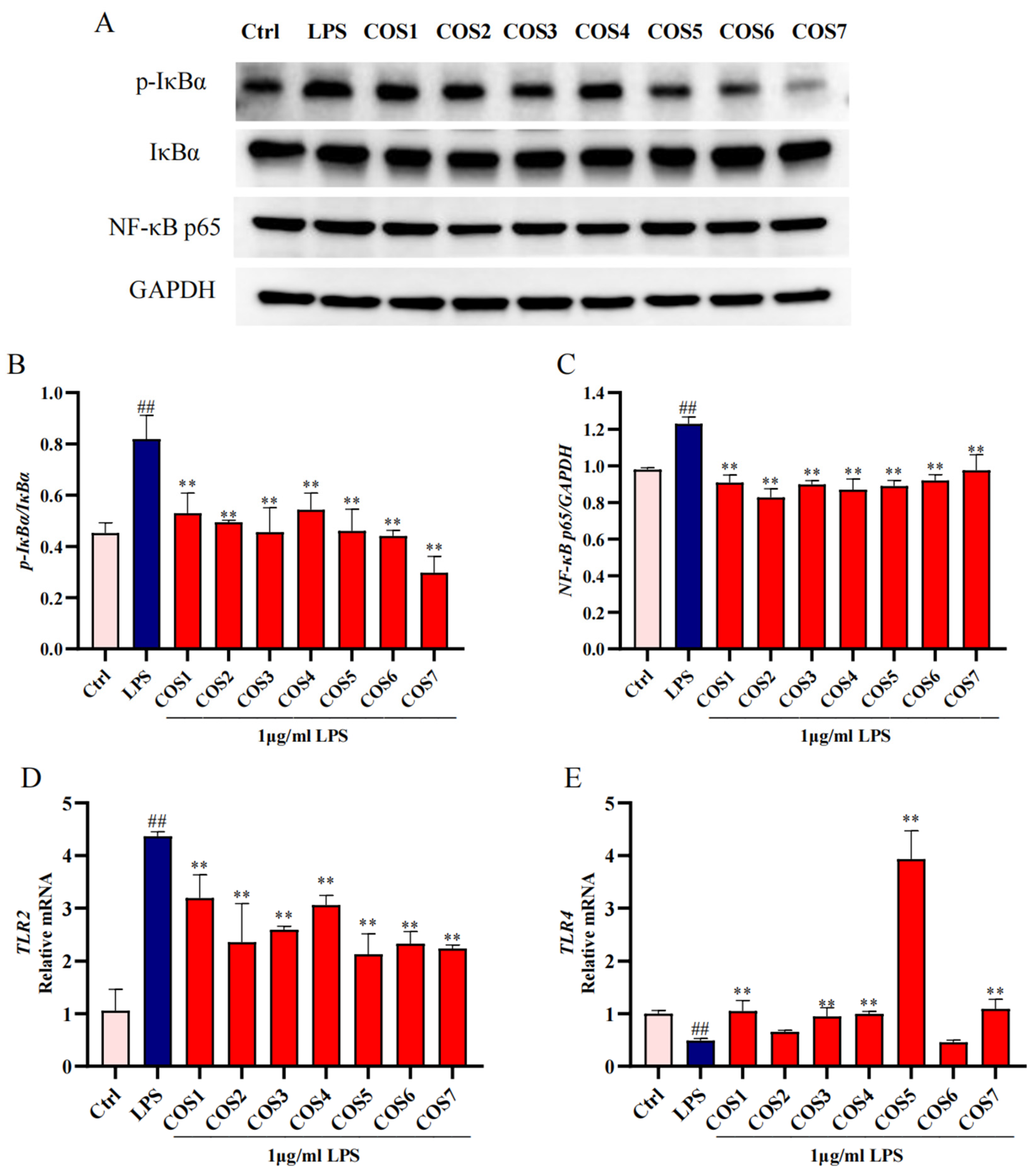
2.4. The Anti-Inflammatory Effect of COS on LPS-Stimulated RAW 264.7 Macrophages Through the TLR2/NF-κB Pathway
2.5. TLR2 and TLR4 Involved in COS Regulation of LPS-Stimulated Inflammatory Signaling
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Cell Culture and COS Treatment
4.3. Cell Viability
4.4. Griess Reaction and ELISA Kit Assay
4.5. Real-Time Quantitative PCR (RT-qPCR)
4.6. RNA-Seq
4.7. Western Blot
4.8. Molecular Docking
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, C.; Chen, L.; Yang, L.; Ji, X. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 2017, 131, 1541–1558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dehaini, D.; Zhang, Y.; Zhou, J.; Chen, X.; Zhang, L.; Fang, R.H.; Gao, W.; Zhang, L. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat. Nanotechnol. 2018, 13, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Bjorkegren, J.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Li, D.T.; Feng, Y.; Tian, M.L.; Ji, J.F.; Hu, X.S.; Chen, F. Gut microbiota-derived inosine from dietary barley leaf supplementation attenuates colitis through PPAR gamma signaling activation. Microbiome 2021, 9, 83. [Google Scholar] [CrossRef]
- Biffi, G.; Tuveson, D.A. Diversity and biology of cancer-associated fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef]
- Huynh, N.C.; Nguyen, T.T.T.; Nguyen, D.T.C.; Tran, T.V. Occurrence, toxicity, impact and removal of selected non-steroidal anti-inflammatory drugs (NSAIDs): A review. Sci. Total Environ. 2023, 898, 165317. [Google Scholar] [CrossRef]
- Fatima, M.; Mir, S.; Ali, M.; Hassan, S.; Ul Haq Khan, Z.; Waqar, K. Synthesis and applications of chitosan derivatives in food preservation-A review. Eur. Polym. J. 2024, 216, 113242. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, M.; Vivekanand, V.; Pareek, N. Chitin biorefinery: A narrative and prophecy of crustacean shell waste sustainable transformation into bioactives and renewable energy. Renew. Sustain. Energy Rev. 2023, 184, 113595. [Google Scholar] [CrossRef]
- Liaqat, F.; Eltem, R. Chitooligosaccharides and their biological activities: A comprehensive review. Carbohydr. Polym. 2018, 184, 243–259. [Google Scholar] [CrossRef]
- Pan, Z.; Cheng, D.; Wei, X.; Li, S.; Guo, H.; Yang, Q. Chitooligosaccharides inhibit tumor progression and induce autophagy through the activation of the p53/mTOR pathway in osteosarcoma. Carbohydr. Polym. 2021, 258, 117596. [Google Scholar] [CrossRef] [PubMed]
- Laokuldilok, T.; Potivas, T.; Kanha, N.; Surawang, S.; Seesuriyachan, P.; Wangtueai, S.; Phimolsiripol, Y.; Regenstein, J.M. Physicochemical, antioxidant, and antimicrobial properties of chitooligosaccharides produced using three different enzyme treatments. Food Biosci. 2017, 18, 28–33. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Chanvorachote, P.; Reudhabibadh, R.; Singh, A.; Benjakul, S.; Roytrakul, S.; Hutamekalin, P. Chitooligosaccharide from Pacific White Shrimp Shell Chitosan Ameliorates Inflammation and Oxidative Stress via NF-κB, Erk1/2, Akt and Nrf2/HO-1 Pathways in LPS-Induced RAW264.7 Macrophage Cells. Foods 2023, 12, 2740. [Google Scholar] [CrossRef]
- Ouyang, A.; Zhang, M.; Yuan, G.; Liu, X.; Su, J. Chitooligosaccharide boosts the immunity of immunosuppressed blunt snout bream against bacterial infections. Int. J. Biol. Macromol. 2023, 242, 124696. [Google Scholar] [CrossRef]
- Fang, Z.; Cong, W.; Zhou, H.; Zhang, J.; Wang, M. Biological activities, mechanisms and applications of chitooligosaccharides in the food industry. J. Funct. Foods 2024, 116, 106219. [Google Scholar] [CrossRef]
- Pangestuti, R.; Bak, S.; Kim, S. Attenuation of pro-inflammatory mediators in LPS-stimulated BV2 microglia by chitooligosaccharides via the MAPK signaling pathway. Int. J. Biol. Macromol. 2011, 49, 599–606. [Google Scholar] [CrossRef]
- Hao, W.T.; Li, K.C.; Ge, X.Y.; Yang, H.Y.; Xu, C.J.; Liu, S.; Yu, H.H.; Li, P.C.; Xing, R.G. The Effect of N-Acetylation on the Anti-Inflammatory Activity of Chitooligosaccharides and Its Potential for Relieving Endotoxemia. Int. J. Mol. Sci. 2022, 23, 8205. [Google Scholar] [CrossRef]
- Chang, S.; Lin, Y.; Wu, G.; Huang, C.; Tsai, G.J. Effect of chitosan molecular weight on anti-inflammatory activity in the RAW 264.7 macrophage model. Int. J. Biol. Macromol. 2019, 131, 167–175. [Google Scholar] [CrossRef]
- Liang, T.W.; Chen, W.T.; Lin, Z.H.; Kuo, Y.H.; Nguyen, A.D.; Pan, P.S.; Wang, S.L. An Amphiprotic Novel Chitosanase from Bacillus mycoides and Its Application in the Production of Chitooligomers with Their Antioxidant and Anti-Inflammatory Evaluation. Int. J. Mol. Sci. 2016, 17, 1302. [Google Scholar] [CrossRef]
- Ma, P.; Liu, H.; Wei, P.; Xu, Q.; Bai, X.; Du, Y.; Yu, C. Chitosan oligosaccharides inhibit LPS-induced over-expression of IL-6 and TNF-α in RAW264.7 macrophage cells through blockade of mitogen-activated protein kinase (MAPK) and PI3K/Akt signaling pathways. Carbohydr. Polym. 2011, 84, 1391–1398. [Google Scholar]
- Qiao, Y.; Ruan, Y.; Xiong, C.; Xu, Q.; Wei, P.; Ma, P.; Bai, X.; Du, Y. Chitosan oligosaccharides suppressant LPS binding to TLR4/MD-2 receptor complex. Carbohydr. Polym. 2010, 82, 405–411. [Google Scholar] [CrossRef]
- Erridge, C.; Bennett-Guerrero, E.; Poxton, I.R. Structure and function of lipopolysaccharides. Microbes Infect. 2002, 4, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Fuke, N.; Nagata, N.; Suganuma, H.; Ota, T. Regulation of Gut Microbiota and Metabolic Endotoxemia with Dietary Factors. Nutrients 2019, 11, 2277. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Khosrojerdi, A.; Jamialahmadi, T.; Al-Rasadi, K.; Johnston, T.P.; Sahebkar, A. Implications for the role of lipopolysaccharide in the development of atherosclerosis. Trends Cardiovasc. Med. 2022, 32, 525–533. [Google Scholar] [CrossRef]
- Hersoug, L.G.; Moller, P.; Loft, S. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: Implications for inflammation and obesity. Obes. Rev. 2016, 17, 297–312. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, X.; Wang, Y.; Yan, Y.; Wang, Y.; Su, M.; Lv, H.; Li, K.; Hao, X.; Xing, X.; et al. Application of lipopolysaccharide in establishing inflammatory models. Int. J. Biol. Macromol. 2024, 279, 135371. [Google Scholar] [CrossRef]
- Triantafilou, M.; Triantafilou, K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002, 23, 301–304. [Google Scholar] [CrossRef]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.S.; Lee, H.; Lee, J.O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009, 458, 1130–1191. [Google Scholar] [CrossRef]
- Yang, R.B.; Mark, M.R.; Gray, A.; Huang, A.; Xie, M.H.; Zhang, M.; Goddard, A.; Wood, W.I.; Gurney, A.L.; Godowski, P.J. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 1998, 395, 284–288. [Google Scholar] [CrossRef]
- Jin, M.S.; Kim, S.E.; Heo, J.Y.; Lee, M.E.; Kim, H.M.; Paik, S.; Lee, H.; Lee, J. Crystal Structure of the TLR1-TLR2 Heterodimer Induced by Binding of a Tri-Acylated Lipopeptide. Cell 2007, 130, 1071–1082. [Google Scholar] [CrossRef]
- Jin, Y.; Nguyen, T.L.L.; Myung, C.; Heo, K. Ginsenoside Rh1 protects human endothelial cells against lipopolysaccharide-induced inflammatory injury through inhibiting TLR2/4-mediated STAT3, NF-κB, and ER stress signaling pathways. Life Sci. 2022, 309, 120973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liang, J.; Fan, H.; Lei, K.; Li, H.; Liu, D.; Zheng, F.; He, M.; Chen, Y. Study on anti-inflammatory effect of Shangkehuangshui in vitro and in vivo based on TLR4/TLR2-NF-κB signaling pathway. J. Ethnopharmacol. 2024, 323, 117709. [Google Scholar] [CrossRef]
- Asanka Sanjeewa, K.K.; Jayawardena, T.U.; Kim, H.; Kim, S.; Shanura Fernando, I.P.; Wang, L.; Abetunga, D.T.U.; Kim, W.; Lee, D.; Jeon, Y. Fucoidan isolated from Padina commersonii inhibit LPS-induced inflammation in macrophages blocking TLR/NF-κB signal pathway. Carbohydr. Polym. 2019, 224, 115195. [Google Scholar] [CrossRef] [PubMed]
- Mao, N.; Yu, Y.; Lu, X.; Yang, Y.; Liu, Z.; Wang, D. Preventive effects of matrine on LPS-induced inflammation in RAW 264.7 cells and intestinal damage in mice through the TLR4/NF-κB/MAPK pathway. Int. Immunopharmacol. 2024, 143, 113432. [Google Scholar] [PubMed]
- Chen, J.; Szodoray, P.; Zeher, M. Toll-Like Receptor Pathways in Autoimmune Diseases. Clin. Rev. Allergy Immunol. 2016, 50, 1–17. [Google Scholar] [CrossRef]
- Hao, W.T.; Li, K.C.; Liu, S.; Yu, H.H.; Li, P.C.; Xing, R.E. Pleiotropic Modulation of Chitooligosaccharides on Inflammatory Signaling in LPS-Induced Macrophages. Polymers 2023, 15, 1613. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Xu, Z.; Li, F.; Yang, M.; Shi, F.; Lin, L.; Qin, Z. Characterization of effects of chitooligosaccharide monomer addition on immunomodulatory activity in macrophages. Food Res. Int. 2023, 163, 112268. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 2014, 26, 253–266. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.Z.; Chen, X.Y.; Ye, X.M.; Shen, X.; Lin, M.X.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Ma, M.; Chen, L.; Tang, Z.; Song, Z.; Kong, X. Hepatoprotective effect of total flavonoids from Carthamus tinctorius L. leaves against carbon tetrachloride-induced chronic liver injury in mice. Fitoterapia 2023, 171, 105605. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Duda, K.A.; Lanzetta, R.; Silipo, A.; De Castro, C.; Molinaro, A. A Journey from Structure to Function of Bacterial Lipopolysaccharides. Chem. Rev. 2022, 122, 15767–15821. [Google Scholar] [CrossRef] [PubMed]
- He, X.M.; Liu, L.Y.; Gu, F.L.; Huang, R.S.; Liu, L.; Nian, Y.T.; Zhang, Y.Y.; Song, C. Exploration of the anti-inflammatory, analgesic, and wound healing activities of Bletilla Striata polysaccharide. Int. J. Biol. Macromol. 2024, 261, 129874. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xia, H.; Zou, X.; Li, X.; Zhang, Z.; Yao, X.; Yin, M.; Tian, D.; Liu, H. N-acetyl-chitooligosaccharide attenuates inflammatory responses by suppression of NF-κB signaling, MAPK and NLRP3 inflammasome in macrophages. J. Funct. Foods 2021, 78, 104364. [Google Scholar] [CrossRef]
- Sun, C.C.; Hao, B.F.; Pang, D.R.; Li, Q.; Li, E.R.; Yang, Q.; Zou, Y.X.; Liao, S.T.; Liu, F. Diverse Galactooligosaccharides Differentially Reduce LPS-Induced Inflammation in Macrophages. Foods 2022, 11, 3973. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, P.; Hao, C.; Wu, L.; Wan, W.; Mao, X. Neoagaro-oligosaccharide monomers inhibit inflammation in LPS-stimulated macrophages through suppression of MAPK and NF-κB pathways. Sci. Rep. 2017, 7, 44252. [Google Scholar] [CrossRef]
- Liu, H.L.; Zhou, L.; Wang, X.F.; Zheng, Q.C.; Zhan, F.F.; Zhou, L.Q.; Dong, Y.; Xiong, Y.H.; Yi, P.C.; Xu, G.H.; et al. Dexamethasone upregulates macrophage PIEZO1 via SGK1, suppressing inflammation and increasing ROS and apoptosis. Biochem. Pharmacol. 2024, 222, 116050. [Google Scholar] [CrossRef]
- Chen, L.; Xie, L.M.; Zhang, J.; Feng, Y.F.; Wu, X. GC-MS analysis of fatty acid metabolomics in RAW264.7 cell inflammatory model intervened by non-steroidal anti-inflammatory drugs and a preliminary study on the anti-inflammatory effects of NLRP3 signaling pathway. PLoS ONE 2023, 18, e290051. [Google Scholar]
- Liu, Y.T.; Fang, S.L.; Li, X.Y.; Feng, J.; Du, J.; Guo, L.J.; Su, Y.Y.; Zhou, J.; Ding, G.; Bai, Y.X.; et al. Aspirin inhibits LPS-induced macrophage activation via the NF-κB pathway. Sci. Rep. 2017, 7, 11549. [Google Scholar] [CrossRef]
- Huo, J.Y.; Pei, W.H.; Liu, G.Y.; Sun, W.Z.; Wu, J.H.; Huang, M.Q.; Lu, W.; Sun, J.Y.; Sun, B.G. Huangshui Polysaccharide Exerts Intestinal Barrier Protective Effects through the TLR4/MyD88/NF-κB and MAPK Signaling Pathways in Caco-2 Cells. Foods 2023, 12, 450. [Google Scholar] [CrossRef]
- Fu, X.D.; Huang, X.R.; Tan, H.Z.; Huang, X.J.; Nie, S.P. Regulatory Effect of Fucoidan Hydrolysates on Lipopolysaccharide-Induced Inflammation and Intestinal Barrier Dysfunction in Caco-2 and RAW264.7 Cells Co-Cultures. Foods 2024, 13, 3532. [Google Scholar]
- Hoffmann, A.; Levchenko, A.; Scott, M.L.; Baltimore, D. The IκB-NF-κB signaling module: Temporal control and selective gene activation. Science 2002, 298, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, F.; Shen, S.; Yang, Z.; Ji, X.; Wang, X.; Liao, X.; Zhang, Y. More simple, efficient and accurate food research promoted by intermolecular interaction approaches: A review. Food Chem. 2023, 416, 135726. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Park, B.S.; Kim, J.; Kim, S.E.; Lee, J.; Oh, S.C.; Enkhbayar, P.; Matsushima, N.; Lee, H.; Yoo, O.J.; et al. Crystal Structure of the TLR4-MD-2 Complex with Bound Endotoxin Antagonist Eritoran. Cell 2007, 130, 906–917. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. Software News and Update AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
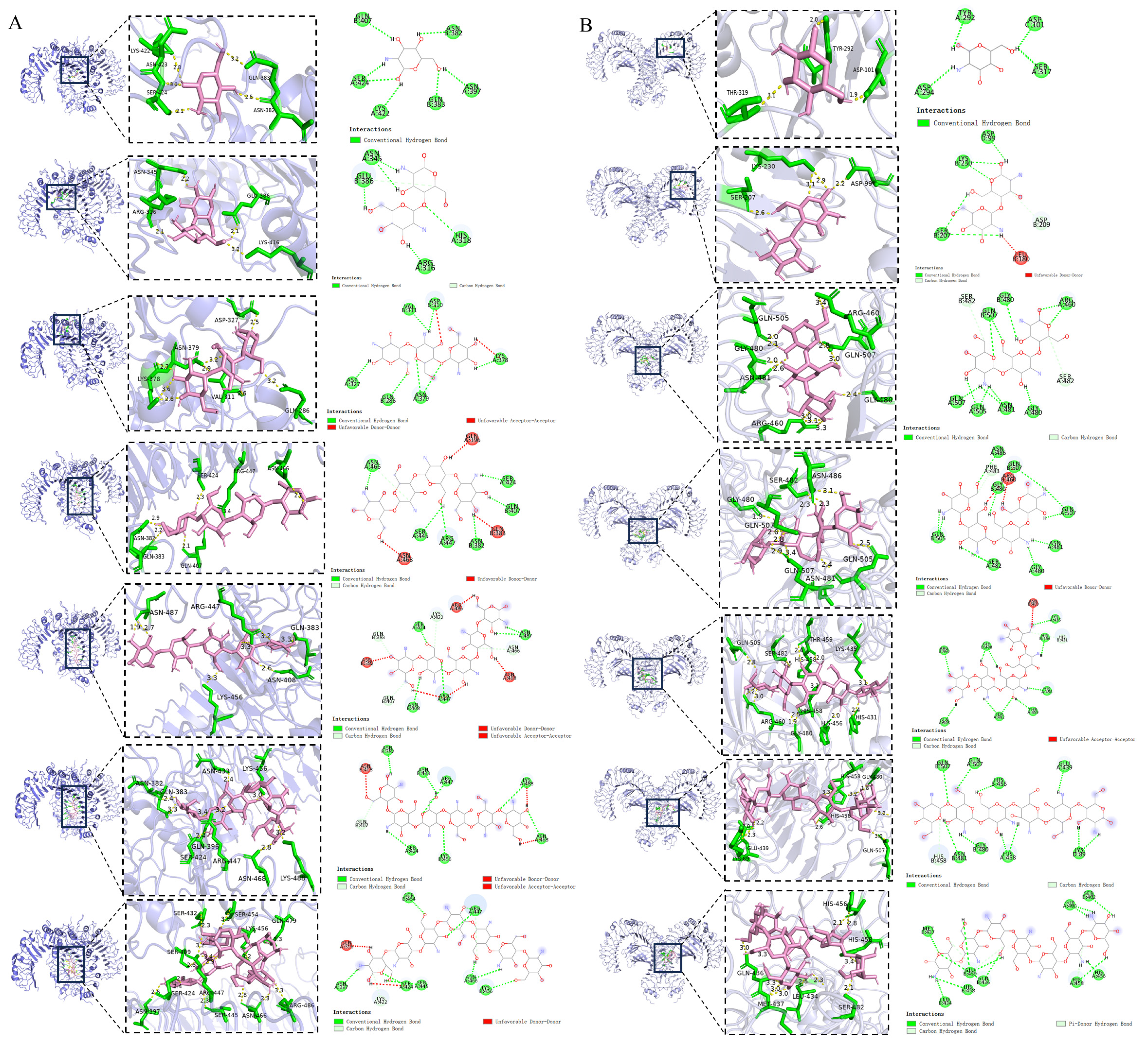
| Sample Name | TLR2 Binding Energy (kal/mol) | Potential Binding Sites |
|---|---|---|
| COS1 | −5.8 | ASN382; GLN383; ASN397; GLN407; LYS422; SER424 |
| COS2 | −6.0 | ARG316; HIS318; ASN345; GLU386 |
| COS3 | −6.0 | GLN286; ASP310; VAL311; ASP327; LYS378; ASN379 |
| COS4 | −8.0 | ASN382; GLN383; GLN396; GLN407; SER424; SER445; ARG447; ASN466; ASN468 |
| COS5 | −8.5 | ASN382; GLN383; GLN407; ASN408; LYS422; SER424; ARG447; ASN466; ASN468; ARG486; ASN487 |
| COS6 | −7.8 | ASN382; GLN407; ASN408; SER424; ASN433; ARG447; LYS456; ASN468; LYS488 |
| COS7 | −7.3 | GLN396; ASN397; LYS422; SER424; SER445; ARG447; SER454; LYS456; ASN466 |
| Sample Name | TLR4 Binding Energy (kal/mol) | Potential Binding Sites |
|---|---|---|
| COS1 | −5.6 | ASP101; TYR292; ASP294; SER317 |
| COS2 | −6.8 | ASP99; LEU180; SER207; ASP209; LYS230 |
| COS3 | −7.8 | GLY480; ASN481; SER482; ARG460; GLN505; GLN507 |
| COS4 | −8.4 | ARG460; GLY480; ASN481; SER482; PHE483; ASN486; GLN505; GLN507 |
| COS5 | −8.8 | ASN409; HIS431; LYS435; HIS456; HIS458; THR459; ARG460; GLY480; SER482; GLN505 |
| COS6 | −8.2 | LYS89; GLU439; HIS456; HIS458; GLY480; ASN481; GLN507 |
| COS7 | −7.5 | LEU434; GLN436; MET437; GLU439; HIS456; GLY480; SER482; HIS458 |
| Genes | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| β-actin | CTAGGCGGACTGTTACTGAGC | ATGTTTGCTCCAACCAACTGC |
| iNOS | GTCCGAAGCAAACATCACATTCA | GGTAAACACGTTCTTTGCATGGA |
| IL-6 | GTATGAACAACGATGATGCACTTG | CTCTCTGAAGGACTCTGGCTTTG |
| TNF-α | GACCCTCACACTCAGATCATCTT | CCTTGAAGAGAACCTGGGAGTAG |
| IL-1β | CAGCACATCAACAAGAGCTTCAG | GAGGATGGGCTCTTCTTCAAAGA |
| TLR2 | GGAGATGTGTCCGCAATCATAGT | CCAAGATCCAGAAGAGCCAAAGA |
| TLR4 | TCCCTGCATAGAGGTGTGAAATT | CCACAGCCACCAGATTCTCTAAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Pang, S.; Gao, Y.; Li, T.; Jiang, H. Effect of Chitooligosaccharides on TLR2/NF-κB Signaling in LPS-Stimulated RAW 264.7 Macrophages. Molecules 2025, 30, 2226. https://doi.org/10.3390/molecules30102226
Zhao M, Pang S, Gao Y, Li T, Jiang H. Effect of Chitooligosaccharides on TLR2/NF-κB Signaling in LPS-Stimulated RAW 264.7 Macrophages. Molecules. 2025; 30(10):2226. https://doi.org/10.3390/molecules30102226
Chicago/Turabian StyleZhao, Mengting, Shurong Pang, Yiqing Gao, Ting Li, and Hongrui Jiang. 2025. "Effect of Chitooligosaccharides on TLR2/NF-κB Signaling in LPS-Stimulated RAW 264.7 Macrophages" Molecules 30, no. 10: 2226. https://doi.org/10.3390/molecules30102226
APA StyleZhao, M., Pang, S., Gao, Y., Li, T., & Jiang, H. (2025). Effect of Chitooligosaccharides on TLR2/NF-κB Signaling in LPS-Stimulated RAW 264.7 Macrophages. Molecules, 30(10), 2226. https://doi.org/10.3390/molecules30102226






