Abstract
Proteolytic enzymes, which play a crucial role in peptide bond cleavage, are widely applied in various industries. In this study, protease-producing bacteria were isolated and characterized from marine sediments collected from the Yellow Sea, China. Comprehensive screening and 16S rDNA sequencing identified a promising G4 strain as Bacillus atrophaeus. Following meticulous optimization of fermentation conditions and medium composition via response surface methodology, protease production using strain G4 was significantly enhanced by 64%, achieving a yield of 3258 U/mL. The G4 protease exhibited optimal activity at 50 °C and pH 7.5, demonstrating moderate thermal stability with 52% residual activity after 30-min incubation at 50 °C—characteristics typical of an alkaline protease. Notably, the enzyme retained over 79% activity across a broad pH range (6–11) and exhibited excellent salt tolerance, maintaining over 50% activity in a saturated NaCl solution. Inhibition by phenylmethylsulfonyl fluoride, a serine protease inhibitor, confirmed its classification as a serine protease. The enzyme’s potential in generating bioactive peptides was further demonstrated through hydrolysis of sheep (Ovis aries) placenta, resulting in a hydrolysate with notable antioxidant properties. The hydrolysate exhibited a 64% superoxide anion scavenging activity, surpassing that of reduced glutathione. These findings expand the current understanding of Bacillus atrophaeus G4 proteases and provide a foundation for innovative sheep placenta utilization with potential industrial applications.
1. Introduction
Proteases, a class of biocatalysts that catalyze the hydrolysis of peptide bonds, play a crucial role in various cellular metabolic pathways and are widely applied across multiple industrial sectors [1]. These enzymes are particularly important in industries such as leather processing, dairy production, diagnostic reagent development, waste management, and precious metal recovery. Notably, they are widely used in detergent formulation, food processing, and pharmaceutical manufacturing [2]. Despite the extensive characterization of protease diversity, only a limited subset exhibits the necessary stability to withstand the harsh conditions common in industrial processes, such as high temperatures, extreme pH, elevated salinity, and exposure to organic solvents [3]. Consequently, there is an urgent need in both applied and fundamental research to identify and characterize novel proteases with exceptional stability and activity under these challenging conditions.
Covering over 71% of the Earth’s surface, the ocean is a vast reservoir of biodiversity, rich in microbial life forms exhibiting a wide range of unique properties [4]. Marine environments are particularly noted for their dense microbial communities, which include extremophilic microorganisms capable of thriving under harsh conditions such as high temperature, salinity, nutrient limitation, and pressure. Such extremophiles have evolved distinct metabolic and physiological traits compared to their terrestrial counterparts [5]. Marine-derived proteases, in particular, exhibit a broader range of catalytic functions and possess distinctive enzymatic properties, including enhanced activity and stability [5]. These properties hold significant potential for driving innovation across diverse sectors, including medical applications [6], the household chemical industry, and food processing [2].
The placenta, a transient yet indispensable organ essential for fetal growth and development, serves as a rich source of essential amino acids, bioactive peptides, vitamins, trace elements, growth factors, and numerous other bioactive compounds [7,8]. Recent studies have shown that enzymatic hydrolysates derived from sheep placenta exhibit a variety of therapeutic properties, including potent antioxidant activity [9] and fatigue-alleviating effects [10]. These bioactivities highlight their potential for a wide range of applications. Despite the abundant availability of sheep placenta resources, especially in China, a large proportion is currently discarded, leading to substantial resource wastage. The Ningxia Tan sheep, a unique Chinese fur breed listed as a national Grade II protected species, has not yet been reported as a source of placenta hydrolysate. The systematic development and strategic utilization of placental materials offer significant practical advantages and considerable market potential, underscoring the need for further research and innovation in this field.
The genus Bacillus represents a major source of commercially valuable proteases, noted for their high enzymatic activity, stability across a wide pH and temperature range, and broad substrate specificity. These enzymes also offer advantages such as ease of purification and cost-effectiveness [11,12]. However, empirical studies on proteases derived from Bacillus atrophaeus remain limited [13,14]. To optimize the use of marine biological resources and promote the sustainable utilization of sheep placenta, we isolated a Bacillus atrophaeus G4 strain from Yellow Sea sediments in China, which produces a protease of notable interest. This protease was subsequently used for the enzymatic hydrolysis of sheep placenta, and the resulting hydrolysates were assessed for their antioxidant potential. The overarching objective of this study is to enhance the value and broaden the application potential of sheep placenta resources.
2. Results and Discussion
2.1. Strain Screening and Identification
2.1.1. Strain Screening
A total of 493 bacterial strains isolated from Yellow Sea sediments were initially screened using skim milk agar plates to assess proteolytic activity. Among them, 263 strains exhibited proteolytic activity, as evidenced by clear hydrolysis zones surrounding the colonies. Strains forming large hydrolysis zones were selected for further evaluation. Selected strains were cultured in fermentation medium at 30 °C and 180 rpm for 24 h. Following centrifugation at 8000× g for 10 min at 4 °C, the supernatant was collected as the crude enzyme extract. Eight strains exhibited protease activity exceeding 100 U/mL. Through successive rounds of fermentation and subculturing, strain G4 was identified as the most promising candidate, exhibiting exceptional protease activity (1759 U/mL) and stable inheritance across passages.
2.1.2. Molecular Identification of Strain G4
The 16S rRNA gene sequence of strain G4 was analyzed using the EzBioCloud database, revealing a 99.9% sequence similarity with Bacillus atrophaeus JCM 9070. Sequences of closely related species were selected and aligned using MEGA11.0 for multiple sequence comparison. A phylogenetic tree was then constructed using the neighbor-joining method, as shown in Figure 1.
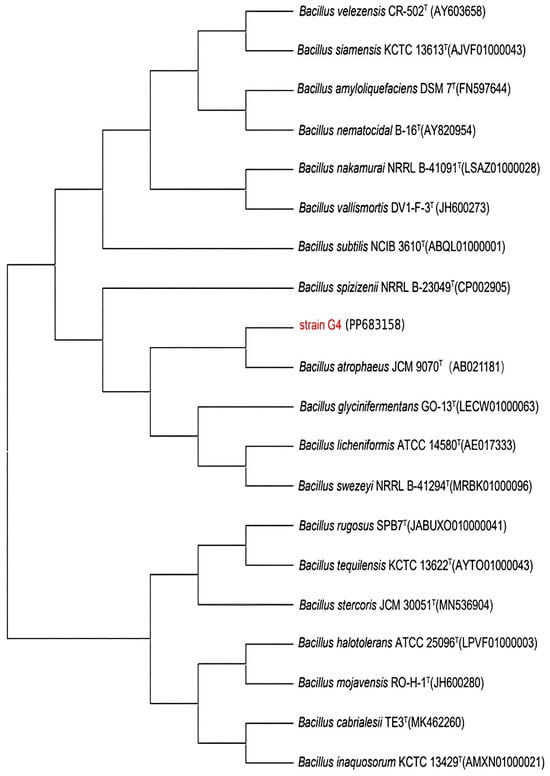
Figure 1.
Phylogenetic tree of strain G4 constructed based on 16S rRNA gene sequences, illustrating its phylogenetic placement within the Bacillus genus. GenBank accession numbers are shown in parentheses.
2.2. Optimization of Protease Production by Fermentation of Strain G4
2.2.1. Effect of Cultivation Time
As shown in Figure 2, a significant increase in the cell density of strain G4 was observed after 4 h of incubation, coinciding with exponential growth in protease production. Maximum protease activity was observed at 24 h. The growth and protease production profile of strain G4 were comparable to that of Bacillus siamensis F2, a halotolerant protease-producing strain [15], which reached the stationary phase at 24 h, with no further increase in enzyme yield. However, in contrast to B. siamensis F2, which exhibited rapid cell death and a continuous decline in enzyme activity after 24 h of fermentation, B. atrophaeus G4 showed more stable growth and sustained higher protease activity beyond 24 h.
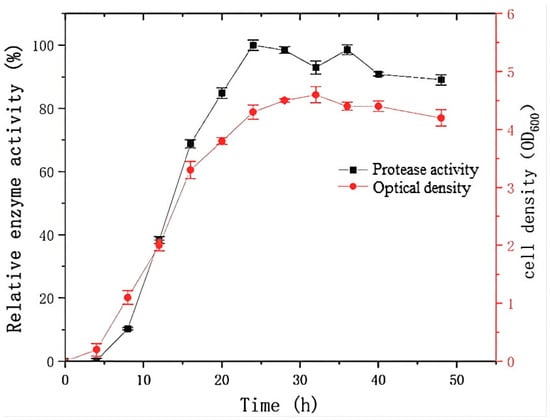
Figure 2.
Protease production and growth kinetics of strain G4. The relative activity (100%) corresponds to protease activity of 1700 U/mL.
2.2.2. Effect of Temperature and pH on Protease Production
Strain G4 exhibited protease production across a temperature range of 25–40 °C, with maximum activity observed at 30 °C (Figure 3a). The effect of pH on protease production during fermentation is shown in Figure 3b. The results indicate that neutral to mildly acidic conditions (pH 6) are most favorable for protease production.
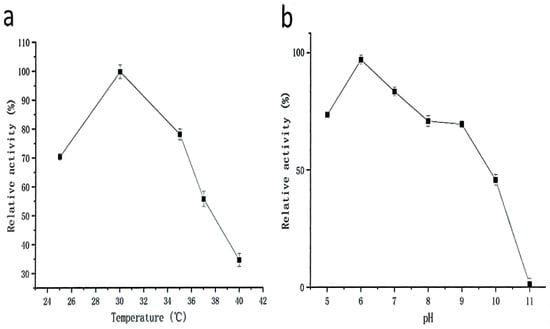
Figure 3.
Effect of temperature (a) and pH (b) on protease production (black squares represent protease activity values) during fermentation by strain G4. The relative activity (100%) corresponds to protease activity of 1700 U/mL. All cultures were grown for 24 h at 200 rpm, with (a) pH 6.0 and (b) temperature at 30 °C.
2.2.3. Optimization of the Fermentation Medium for Protease Production
As shown in Figure 4, preliminary analysis using a one-variable-at-a-time approach revealed that a fermentation medium containing 2% (w/v) casein, 0.25% (w/v) yeast extract, and 1% (w/v) NaCl was optimal for protease production by strain G4. Notably, yeast extract, serving as a carbon source, significantly enhanced protease production compared to other tested sources such as glucose, starch, sucrose, cornmeal, and bran. Even slight variations in the concentration of yeast extract led to substantial changes in protease yield. This enhancement is attributed to the rich nutritional composition of yeast extract, which contains amino acids, essential vitamins, and carbohydrates that support cell growth and enhance the metabolic activity of the bacterium [16].
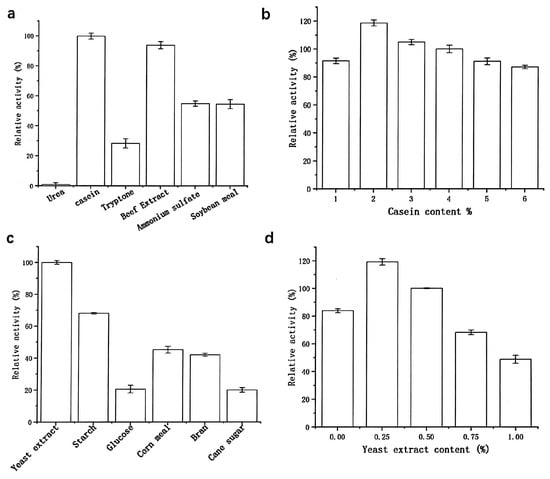
Figure 4.
Effects of nitrogen sources (a,b) and carbon sources (c,d) on protease production. Nitrogen source screening: The 100% relative activity corresponds to 1700 U/mL (basal medium: 0.5% (w/v) yeast extract, 1% (w/v) NaCl); carbon source screening: The 100% relative activity corresponds to 2500 U/mL (basal medium: 2% (w/v) casein, 1% (w/v) NaCl).
2.2.4. Optimization of Enzyme Production Using Response Surface Methodology
In a one-factor-at-a-time experiment, a single variable is varied while all others are kept constant. While this method offers preliminary insights and allows quantification of individual factor effects, it is limited in evaluating interactions among variables and identifying optimal parameter combinations. To better understand the interactions among variables and determine the optimal response region, response surface methodology was employed.
Based on the results of single-factor experiments, a central composite design was used to optimize the fermentation conditions. Using Design-Expert 10.0 software, the concentrations (w/v) of casein, yeast extract, and NaCl were selected as key influencing factors and defined as independent variables (Table 1).

Table 1.
Box-Behnken design matrix and experimental results.
The experimental data for protease activity (R) were fitted using a quadratic multiple regression model in Design-Expert software. The resulting equation, expressing protease activity (R) as a function of casein content (A), yeast extract content (B), and NaCl concentration (C), is presented below:
R = 3140.62 + 122.24A + 602.78B + 42.78C − 134.53AB + 117.39AC − 44.15BC − 498.95A2 − 717.51B2 − 207.29C2
Analysis of variance and significance testing of the quadratic model were also performed using Design-Expert. The results are summarized in Table 2. The model’s p-value (<0.0001) indicates a highly significant fit. Among the linear terms, both A (casein content) and B (yeast extract content) had significant effects on protease production, demonstrating their substantial influence within the tested ranges (casein: 1–3% (w/v); yeast extract: 0–0.5% (w/v)). In contrast, C (NaCl concentration) exhibited a p-value > 0.05, indicating no statistically significant effect on enzyme production within the range of 0.5–1.5% (w/v).

Table 2.
Analysis of variance for the protease activity regression model.
The relative influence of the three factors on enzyme production followed the order: B > A > C. The lack-of-fit p-value (0.2296 > 0.05) was not significant, supporting the adequacy of the model. The coefficient of determination (R2 = 0.9830) and the adjusted coefficient of determination (R2adj = 0.9611) were both close to 1, indicating a strong correlation between predicted and actual values. Additionally, the predicted R-Squared (R2pred = 0.8201) was in good agreement with R2adj, with a difference of less than 0.2, confirming the model’s robustness and predictive accuracy.
In the regression equation, the negative coefficients of the quadratic terms (A2, B2, C2) indicate a downward-opening response surface, suggesting the presence of a maximum point. Among the primary terms, B was identified as significant, while A was highly significant. In the quadratic terms, A2, B2, and C2 all exhibited high significance, further supporting the model’s reliability in describing the system.
To validate the accuracy of the response surface methodology, a verification experiment was conducted under the predicted optimal conditions: 2.08% (w/v) casein, 0.36% (w/v) yeast extract, and 1.04% (w/v) NaCl, with a predicted protease activity of 3271 U/mL. To accommodate practical experimental constraints, the conditions were adjusted to 2.1% (w/v) casein, 0.36% (w/v) yeast extract, and 1% (w/v) NaCl. Three replicate experiments were conducted under these conditions, yielding a measured protease activity of 3258 U/mL. This close agreement between the predicted and experimental values confirms the validity and reliability of the response surface model. The optimized conditions enhanced protease production by 64% compared to the baseline culture system.
2.3. Characterization of the Purified Protease
Purification of the protease derived from strain G4 resulted in a 25-fold increase in purity, with a specific activity of 10,976 U/mg and a final yield of 4% (Table 3). The molecular weight of the purified protease was estimated to be approximately 29 kDa, as determined by SDS–PAGE (Figure 5). The protease derived from B. atrophaeus strain 08 exhibited minimal caseinolytic activity, demonstrating a striking contrast to the high activity observed in the G4 protease [13].

Table 3.
Summary of the purification of crude protease from strain G4.
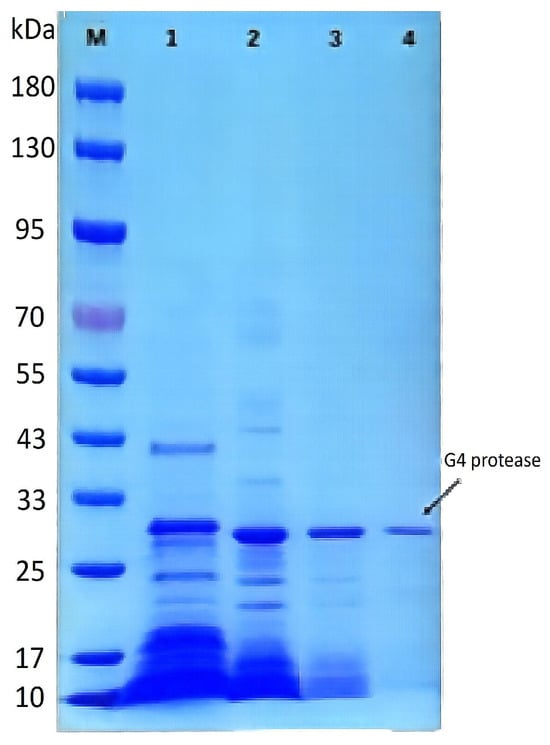
Figure 5.
SDS-PAGE analysis of the purification process of protease from strain G4. M: Pre-stained protein molecular weight marker; Lane 1: Crude extract of protease from strain G4; Lane 2: Protease fraction after 20–60% (NH4)2SO4 precipitation; Lane 3: Protease fraction after DEAE FF ion-exchange chromatography; Lane 4: Final purified protease.
2.3.1. Effect of Temperature on the Activity and Stability of Protease from Strain G4
Temperature is a key parameter affecting enzymatic activity. As shown in Figure 6a, the purified protease from strain G4 exhibited activity across a broad temperature range, retaining over 40% of its activity between 25 °C and 65 °C, with an optimal temperature of 50 °C. These findings are consistent with previous reports on proteases from B. siamensis F2 [15] and B. subtilis [17], which also exhibited maximum activity at 50–60 °C. The high relative activity at elevated temperatures suggests that the G4 protease has strong potential for industrial applications operating under medium to high temperature conditions.
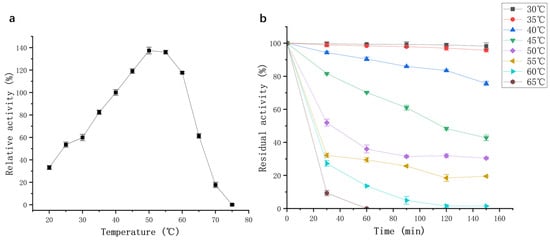
Figure 6.
(a) Effect of temperature on protease activity (black squares); (b) Effect of temperature on thermal stability of protease activity.
Thermal stability assays showed that the enzyme maintained its activity with minimal loss when incubated at 30–35 °C for 150 min (Figure 6b). At 40 °C, 80% of the initial enzyme activity was retained after 150 min. However, when incubated at 50–55 °C, enzyme activity declined sharply within the first 30 min but subsequently stabilized, with 20% residual activity remaining at 55 °C after 150 min. However, the G4 protease was not a thermophilic enzyme, exhibiting lower optimal temperature and thermostability compared to protease SAPNIJ derived from the thermophilic strain B. atrophaeus NIJ [14]. Considering the G4 protease’s optimal temperature, thermal stability, and industrial applicability, a working temperature range of 20–60 °C is recommended for practical use.
2.3.2. Effects of pH on Activity and Stability of Protease from Strain G4
The enzyme exhibited high proteolytic activity across a broad pH range of 6–11 (Figure 7a). Relative activity remained above 78%, with maximum activity observed at pH 7.5. As shown in Figure 7b, the protease from strain G4 exhibited minimal activity loss after incubation at various pH levels for 6 h and 24 h. At pH 7.5, enzyme activity decreased by only 3.8% after 6 h and 6.6% after 24 h of incubation. Furthermore, under the strongly alkaline condition (pH 11), the enzyme retained 73.4% of its initial activity after 24 h (Figure 7b). These results indicate that the enzyme exhibits remarkable stability under both neutral and alkaline pH conditions.
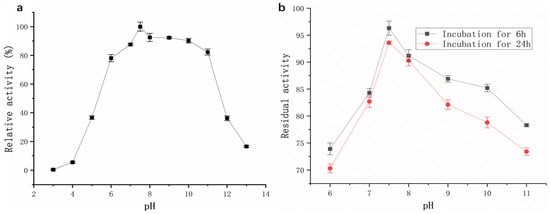
Figure 7.
(a) Effect of pH on protease activity (black squares); (b) Effect of pH on the pH stability of protease activity.
The pH stability of the G4 protease was markedly superior to that of the protease from B. velezensis Z-1 reported by Lu et al. [18] as well as a protease from Bacillus sp. [19]. This robust pH tolerance makes the G4 protease a promising candidate for use in detergent formulations, as laundry detergents typically function within a pH range of 7–11 [20].
2.3.3. Effect of NaCl on the Activity and Salt Stability of the Protease
The protease retained >97% of its initial activity across a broad NaCl concentration range (0–10% (w/v)) (Figure 8). As the NaCl concentration increased, enzymatic activity gradually declined; nevertheless, the enzyme still maintained 57% relative activity in a 40% (w/v) NaCl-saturated solution (5.4 M). Short-term stability assays (24 h, 4 °C) further confirmed negligible activity loss at ≤5% (w/v) NaCl. These results indicate that the protease exhibits remarkable stability under high-salt conditions.
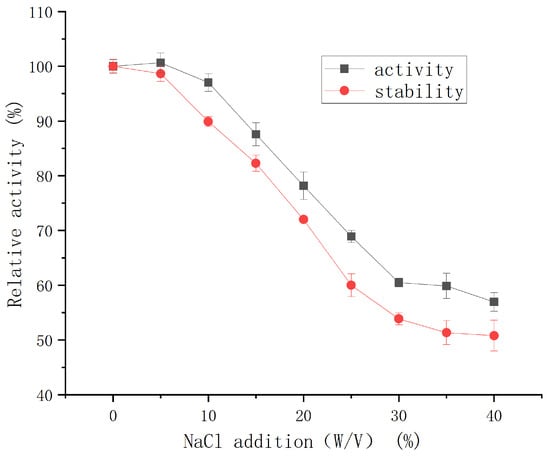
Figure 8.
Effect of NaCl concentration on the activity and salt stability of protease from strain G4.
This level of salt tolerance is superior to that of a halotolerant metalloprotease from Vibrio sp. LA-05 [21], which retained less than 20% of its initial activity under comparable conditions. G4 protease also exhibited better salt tolerance (50% activity retention in 5.4 M NaCl, 4 °C for 24 h) than halotolerant protease SC-lasB (30% retention in 3 M NaCl) [22]. Salt-tolerant proteases (STPs) are considered highly promising for industrial applications due to their superior salt resistance, broader adaptability, and efficient catalytic performance under extreme conditions compared to conventional proteases. In the food processing industry, STPs are especially valuable in the production of high-salt foods such as soy sauce, cheese, shrimp paste, and sausages [23,24]. Given its exceptional salt tolerance, the G4 protease exhibits strong potential for application in high-salt food processing.
2.3.4. Effect of Metal Ions on the Activity of Protease from Strain G4
As shown in Table 4, Mn2+ exhibited a slight activating effect on the activity of the protease. In contrast, Fe3+ and Al3+ completely inhibited protease activity at 10 mM. Similarly, Cu2+ and Zn2+ significantly suppressed protease activity. G4 protease is inhibited by Zn2+, Ni2+, Fe3+, and Al3+, consistent with reports of other proteases (e.g., Zn2+ inhibition [14,18,21]; Ni2+ [14,18,21]; Fe3+ [1]; Al3+ [21]). In contrast, Mn2+ exhibits divergent effects across proteases: it activates G4 (this study), SAPNIJ [14], strain Z-1 protease [18], and Nocardiopsis xinjiangensis protease [3], while demonstrating inhibitory effects on some Bacillus proteases [1,11,17], and additional reported cases [12,21]. This dual regulatory role of Mn2+—acting as either an activator or inhibitor depending on the protease—warrants further investigation to elucidate the underlying mechanisms.

Table 4.
Effect of metal ions on the activity of protease from strain G4.
2.3.5. Effect of Inhibitors, Denaturants, and Surfactants on the Activity of the G4 Protease
The effects of various inhibitors, denaturants, and surfactants on the activity of the G4 protease are summarized in Table 5. Phenylmethylsulfonyl fluoride (PMSF), a widely used inhibitor of serine proteases, significantly suppressed the activity of the G4 protease. At 1 mM, PMSF inhibited 80% of protease activity, whereas at 10 mM, the enzyme was almost completely inactivated. This concentration-dependent inhibition aligns with numerous reports of serine proteases being fully inactivated by 1–5 mM PMSF in the literature [11,12,14,23,25,26]. These results suggest the involvement of serine residues at the active site, confirming that the G4 protease is a serine-type enzyme.

Table 5.
Effect of protease inhibitors, denaturants, and surfactants on the activity of the G4 protease.
Similar to the proteases from Bacillus mojavensis (BM1/BM2) [11] and marine Vibrio sp. LA-05 [21], the activity of G4 protease was inhibited by EDTA in a concentration-dependent manner (Table 5). Treatment with EDTA at 1 mM and 10 mM resulted in residual activities of 62.5% and 48.9%, respectively. In contrast, 1,10-phenanthroline, another metal-chelating agent, had negligible effect (<5% inhibition) on the activity of the G4 protease, matching typical serine protease behavior [23,25]. These results suggest that specific metal ions may be involved in maintaining the active conformation of the enzyme. Dithiothreitol treatment (1–10 mM) inhibited G4 protease activity by less than 10%, mirroring the dithiothreitol-insensitive nature characteristic of serine-type proteases [14].
Common nonionic surfactants, such as Tween 80 and OP-10, showed no significant effect on the activity of the G4 protease. This observation aligns with the findings of Suwannaphan et al. [25], who reported that protease activity remained largely unaffected in the presence of 0.5% and 1.0% Tween 20, Tween 80, and Triton X-100, with over 80% activity retained.
2.4. Antioxidant Activity of Sheep Placenta Hydrolysates
2.4.1. Effect of Hydrolysis Time on the Antioxidant Activity of Sheep Placenta Hydrolysates
The productivity curve is a valuable tool for monitoring product yield over time under specific conditions, thereby enabling optimization and cost reduction [27]. As shown in Figure 9, the free amino acid content in the enzymatically hydrolyzed sheep placenta reached its maximum at 4 h. The enzymatic efficiency observed in this study exceeded that of the protease from B. velezensis Z-1, as reported by Lu et al. [18].
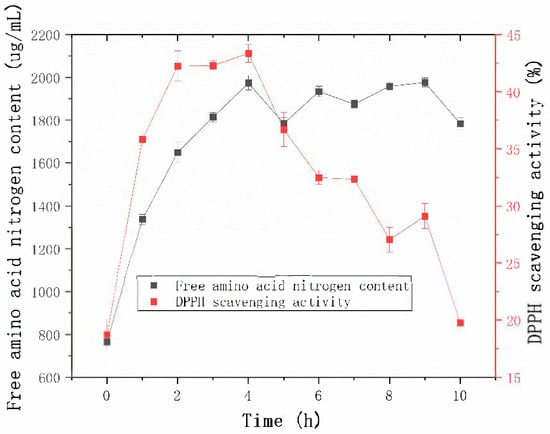
Figure 9.
Effect of hydrolysis time on free amino acid content and DPPH radical scavenging activity.
The 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity was used as a marker of antioxidant capacity to assess the effect of hydrolysis time on the antioxidant properties of the hydrolysates [28]. The DPPH radical scavenging activity increased steadily during the first 2 h of hydrolysis and stabilized between 2 h and 4 h. At 4 h, the DPPH radical scavenging activity reached its maximum value. These results suggest that prolonged hydrolysis may lead to the degradation or inactivation of antioxidant peptides, thereby reducing the overall antioxidant activity of the hydrolysates [29].
2.4.2. Evaluation of the Antioxidant Activity of the Hydrolysate
DPPH is a stable free radical widely used to evaluate the antioxidant capacity of various food-related substances due to its chemical stability and cost-effectiveness [30]. In this assay, antioxidant compounds mediate radical scavenging through hydrogen atom transfer or single electron transfer mechanisms, with the reaction progress monitored spectrophotometrically at 517 nm following 30 min of incubation under standardized conditions. Glutathione (GSH), a well-known reducing peptide, has been extensively studied for its potent antioxidant properties [31,32]. As shown in Figure 10a, the DPPH radical scavenging activity of the sheep placenta hydrolysate increased with increasing concentration. At 10 mg/mL, the DPPH radical scavenging activity reached 78.3%, showing a 15.7% improvement over the non-hydrolyzed sample and only 12% lower than that of GSH. These results suggest that enzymatic hydrolysis significantly enhances the DPPH scavenging activity of sheep placenta, yielding antioxidant effects comparable to GSH.
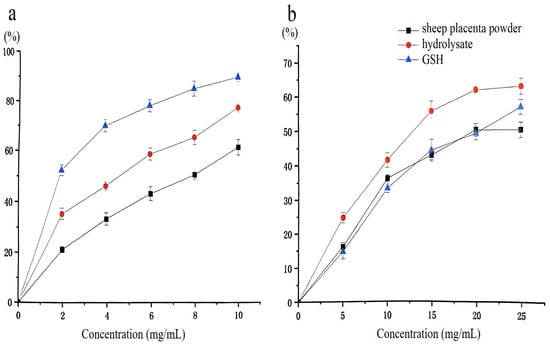
Figure 10.
(a) DPPH radical scavenging activity of the hydrolysate; (b) Superoxide anion radical scavenging activity of the hydrolysate.
Hydrolysis of sheep placenta using papain (4000 U/g) and extraction by-products (10 mg/mL) yielded peptides with a DPPH radical scavenging activity of up to 87% [33]. In this study, untreated sheep placenta was used as the raw material. Although the resulting hydrolysate exhibited a slightly lower DPPH scavenging activity (78.3%), the enzyme dosage (1600 U/g) was more economical.
Superoxide anions, generated during biological metabolic processes, can cause crosslinking or degradation of macromolecules such as proteins, lipids, and nucleic acids. This oxidative damage leads to loss of structural integrity and biological function, accelerates aging, and contributes to the development of various diseases [34]. As shown in Figure 10b, the sheep placenta hydrolysate produced using protease from strain G4 exhibited superior superoxide radical scavenging activity compared to GSH. At 25 mg/mL, the superoxide radical scavenging activity of the hydrolysate reached 64.1%, which was 5.9% higher than that of GSH.
Although bioactive peptides can be directly extracted from sheep placenta [8,35], antioxidant peptides from this source are typically obtained through enzymatic hydrolysis. Previous studies have reported the preparation of sheep placenta antioxidant peptides using dual proteases [9,10] or ultrasound-assisted enzymatic digestion [10]. However, this study demonstrates that comparable antioxidant results can be achieved using a single G4 protease, offering a simpler and more efficient approach.
3. Materials and Methods
3.1. Screening of Protease-Producing Strains
Primary Screening: The screening medium consisted of 2% (w/v) skim milk, 0.25% (w/v) yeast extract, 2% (w/v) agar, and 1% (w/v) NaCl. Wells were created in the solidified medium using a sterile hole punch. A total of 493 bacterial isolates, previously activated from Yellow Sea sediment samples, were inoculated by adding 50 μL of each bacterial suspension into individual wells. The inoculated plates were incubated at 30 °C for 24 h. Isolates exhibiting clear zones of proteolysis, indicated by transparent halos in the medium, were selected for further purification and cryopreservation.
Re-screening Process: Isolates exhibiting significant proteolytic activity in the primary screening were subcultured in seed medium composed of 1% (w/v) tryptone, 0.5% (w/v) yeast extract, and 1% (w/v) NaCl, followed by incubation at 30 °C for 24 h. Subsequently, 2% (v/v) of each culture was inoculated into fermentation medium (4% (w/v) casein, 0.5% (w/v) yeast extract, and 1% (w/v) NaCl) for protease production. Fermentation was carried out in a shaking incubator (Zhichu, Shanghai, China) at 30 °C and 180 rpm for 24 h. Following centrifugation (10,000× g, 10 min, 4 °C), the supernatant was collected for protease activity assay. The isolate exhibiting the highest enzymatic activity, designated as strain G4, was selected for further characterization.
3.2. Protease Activity Assay
Protease activity was quantified using a modified version of the method described by Lowry et al. [36]. Briefly, 200 μL of enzyme solution (600 U) was mixed with an equal volume of 10 g/L casein solution and incubated at 40 °C for 10 min. The reaction was terminated by the addition of 400 μL of 400 mM trichloroacetic acid. Then, 100 μL of the reaction supernatant was mixed with 500 μL of 400 mM Na2CO3 and 100 μL of Folin–Ciocalteu reagent. After 20 min of incubation at room temperature, absorbance was measured at 680 nm. One unit of enzyme activity (U) was defined as the amount of enzyme that catalyzes the release of 1 µg of tyrosine-equivalent amino acids per minute under the specified experimental conditions.
3.3. Molecular Identification of Bacillus atrophaeus G4
Genomic DNA from strain G4 was extracted using a commercial bacterial DNA extraction kit (Tiangen, Beijing, China). The 16S rRNA gene was amplified via PCR using universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-ACGGGCGGTGTGTACAAG-3′). The amplified 16S rRNA gene sequence (GenBank accession number: PP683158) was analyzed using the EzBioCloud database for sequence homology comparison [37]. Nineteen closely related strains with the highest sequence similarity were selected for phylogenetic analysis. Phylogenetic relationships were inferred using the neighbor-joining method with 1000 bootstrap replicates in MEGA 11.0 software [38], thereby determining the taxonomic position of strain G4 within the genus Bacillus.
3.4. Optimization of Protease Production by Bacillus atrophaeus G4 Fermentation
Initial fermentation conditions were established using a basal medium containing 4% (w/v) casein, 0.5% (w/v) yeast extract, and 1% (w/v) NaCl, with incubation at 30 °C and pH 7.0 for 24 h.
A one-factor-at-a-time approach was employed to optimize fermentation parameters, including temperature, pH, incubation time, NaCl concentration, and various carbon and nitrogen sources. Following fermentation, cultures were centrifuged (8000× g, 10 min, 4 °C), and protease activity in the resulting supernatant was measured using the standard assay.
Based on the results of single-factor experiments, a Box–Behnken design was implemented using Design-Expert 10.0 software, following the response surface methodology described by Hassabo [39]. Three independent variables—casein content, yeast extract concentration, and NaCl concentration—were optimized, with protease activity as the response variable. Experimental data were analyzed through response surface analysis and model validation to determine the optimal fermentation conditions.
3.5. Purification of Protease from Strain G4
Protease purification was carried out using a three-step procedure comprising ammonium sulfate fractionation, ion-exchange chromatography, and ultrafiltration [40]. The fermentation broth was centrifuged (8000× g, 10 min, 4 °C) to remove cells. The resulting supernatant was sequentially precipitated with ammonium sulfate at 20% and 60% saturation. The 60% saturation precipitate was dissolved in 20 mM phosphate buffer (pH 7.5) and dialyzed overnight at 4 °C.
The dialyzed sample was loaded onto a HiTrap™ DEAE FF anion-exchange column (Cytiva, Uppsala, Sweden) pre-equilibrated with 20 mM phosphate buffer (pH 7.5). Bound proteins were eluted with a linear NaCl gradient (0–1 M) at a flow rate of 3 mL/min. Fractions exhibiting protease activity were pooled and concentrated using an Amicon® Ultra-15 (Merck, Billerica, MA, USA) centrifugal filter (30 kDa cutoff, 5000× g, 15 min, 4 °C). Protein concentration and protease activity were monitored at each step of the purification process [26].
3.6. Protein Analysis
Protein concentration was measured using a BCA Protein Assay Kit (Solarbio, Beijing, China), following the manufacturer’s instructions. The purity and molecular weight of the purified protease were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 4–20% precast gradient gel (Tsingke, Beijing, China) [41]. Before electrophoresis, samples were mixed with 5× loading buffer containing 5% (v/v) 2-mercaptoethanol and heated at 95 °C for 5 min to ensure complete protein denaturation. Electrophoresis was carried out at a constant voltage of 120 V until the dye front reached the bottom of the gel. Protein bands were visualized using Coomassie Brilliant Blue R-250 staining.
3.7. Enzymatic Characterization of G4 Protease
Temperature and pH Optima: Optimal temperature and pH values for protease activity were determined by incubating the purified enzyme at temperatures ranging from 20 °C to 75 °C (in 5 °C intervals) and pH values from 3.0 to 13.0.
Thermal and pH Stability: Thermal stability was evaluated by incubating the enzyme at temperatures ranging from 30 °C to 65 °C (in 5 °C intervals) for 30–150 min. pH stability was assessed by incubating the enzyme at pH 6.0–11.0 for 1–24 h. Residual activity was measured after each treatment to establish the enzyme’s stability profile.
Metal Ion Effects: The influence of various metal ions (Cu2+, Fe3+, Mg2+, K+, Zn2+, Ni2+, Co2+, Mn2+, Al3+, and Ca2+) on protease activity was assessed at final concentrations of 1 mM and 10 mM. Enzyme activity was measured relative to a control without metal ions.
NaCl Tolerance: The effect of NaCl on protease activity was evaluated at 40 °C and pH 7.5, using NaCl concentrations ranging from 0% to 40% (w/v). Salt tolerance was assessed by measuring residual activity after 24 h of incubation with NaCl.
Inhibitor and Surfactant Effects: The impact of various compounds—including protease inhibitors, reducing agents, and surfactants—on protease activity was investigated. Each compound was tested at appropriate concentrations to evaluate its individual effect on enzymatic activity.
3.8. Preparation of Sheep Placenta Hydrolysates Using G4 Protease
Fifty grams of fresh Ningxia Tan sheep placenta powder (Yibaisheng, Yinchuan, China) was homogenized in 50 mM phosphate buffer (pH 7.5) on ice for 20 min. The homogenate was enzymatically digested with G4 protease (1600 U/g substrate) at 40 °C for 4 h with continuous shaking at 150 rpm. The reaction was terminated by boiling for 10 min, followed by centrifugation at 8000× g for 15 min at 4 °C. The resulting supernatant was collected and freeze-dried to obtain powdered hydrolysates.
Polypeptide content was quantified using Biuret test reagents (Yuanye, Shanghai, China). The concentration of free amino acid nitrogen was determined using the ninhydrin method [42]. Briefly, 100 μL of ninhydrin reagent (containing 0.5% ninhydrin, 0.3% fructose, 10% Na2HPO4·10H2O, and 6% KH2PO4) was mixed with 200 μL of hydrolysate solution. After heating at 100 °C for 15 min and cooling to room temperature, 500 μL of 40% ethanol was added. Absorbance was measured at 570 nm using appropriate standard curves for quantification.
3.9. Evaluation of Antioxidant Activity of G4 Protease Hydrolysates
Preparation of Peptide Solutions: Lyophilized sheep placenta hydrolysates were dissolved in deionized water to obtain peptide solutions at concentrations of 2.0, 4.0, 6.0, 8.0, and 10.0 mg/mL, based on their measured polypeptide content.
DPPH Radical Scavenging Activity: Antioxidant activity was assessed using a DPPH assay kit (Solarbio, Beijing, China). Briefly, 50 μL of each peptide solution was mixed with 200 μL of DPPH working solution and incubated in the dark at room temperature for 30 min. Absorbance was measured at 517 nm using a spectrophotometer. Glutathione (1 mg/mL) served as the positive control. The scavenging activity was calculated using the following equation:
where Asample and Acontrol represent the absorbance of the sample and control, respectively.
Scavenging activity (%) = (1 − Asample/Acontrol) × 100
Superoxide Anion Scavenging Activity: Superoxide anion radical scavenging activity was measured using a commercial assay kit (Epsilon, Beijing, China), following the manufacturer’s instructions. The reaction mixture was incubated at 37 °C for 40 min, and absorbance was measured at 550 nm.
4. Conclusions
In this study, a high-yield protease-producing strain, G4, was isolated from sediments of the Yellow Sea. Under optimized fermentation conditions, the protease activity reached 3258 U/mL. The protease exhibited high activity and stability across a temperature range of 25–55 °C and a pH range of 6.0–11.0, with notable retention of 20% activity after 2.5 h at 55 °C and 78% activity following 6 h incubation at pH 11.0. Comparative analysis revealed superior thermal resistance to papain (90% vs. 75% residual activity at 40 °C 1 h) [43]. These properties confirm its suitability for industrial applications under moderate temperatures and neutral to alkaline conditions. Additionally, the protease demonstrated remarkable salt tolerance, suggesting its applicability in high-salt food processing.
The enzymatic hydrolysis of sheep placenta using the G4 protease was successfully conducted, yielding hydrolysates with significantly enhanced scavenging activity against both DPPH and superoxide radicals. Notably, the superoxide radical scavenging activity of the hydrolysate surpassed that of GSH, highlighting the potential of G4 protease in the production of bioactive peptides.
While these findings demonstrate the promising applications of G4 protease, its potential likely extends beyond the current scope of investigation. Further research is warranted to explore its commercial applications and optimize its use in various industries.
Author Contributions
Conceptualization, J.H. and X.Z.; methodology, W.W.; software, W.W.; validation, G.P., J.S. and C.J.; formal analysis, W.W.; investigation, G.P.; resources, G.P.; data curation, G.P.; writing—original draft preparation, G.P.; writing—review and editing, W.W.; visualization, G.P.; supervision, W.W. and J.H.; project administration, J.H.; funding acquisition, J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Central Public-interest Scientific Institution Basal Research Fund, CAFS, grant number 2023TD71.
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author or the first author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Guo, C.; Sun, C.; Wu, S. Screening and characterization of proteases produced by deep-sea cold seep bacteria. J. Oceanol. Limnol. 2021, 40, 678–689. [Google Scholar] [CrossRef]
- Tavano, O.L.; Berenguer-Murcia, A.; Secundo, F.; Fernandez-Lafuente, R. Biotechnological applications of proteases in food technology. Compr. Rev. Food Sci. Food Saf. 2018, 17, 412–436. [Google Scholar] [CrossRef]
- Gohel, S.D.; Singh, S.P. Thermodynamics of a Ca2+ dependent, highly thermostable and detergent compatible purified alkaline serine protease from Nocardiopsis xinjiangensis strain OM-6. Int. J. Biol. Macromol. 2018, 113, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q. Genome-based studies of marine microorganisms to maximize the diversity of natural products discovery for medical treatments. Evid. Based Complement Alternat. Med. 2011, 2011, 384572. [Google Scholar] [CrossRef] [PubMed]
- Imada, C. Enzyme inhibitors of marine microbial origin with pharmaceutical importance. Mar. Biotechnol. 2004, 6, 193–198. [Google Scholar] [CrossRef]
- Barzkar, N. Marine microbial alkaline protease: An efficient and essential tool for various industrial applications. Int. J. Biol. Macromol. 2020, 161, 1216–1229. [Google Scholar] [CrossRef]
- Sun, C.; Groom, K.M.; Oyston, C.; Chamley, L.W.; Clark, A.R.; James, J.L. The placenta in fetal growth restriction: What is going wrong? Placenta 2020, 96, 10–18. [Google Scholar] [CrossRef]
- Chou, M.-Y.; Ou Yang, C.-P.; Li, W.-C.; Yang, Y.-M.; Huang, Y.-J.; Wang, M.-F.; Lin, W.-T. Evaluation of antiaging effect of sheep placenta extract using SAMP8 mice. Processes 2022, 10, 2242. [Google Scholar] [CrossRef]
- Ren, H.; Qin, T.; Zhou, Q.; Pan, L.; Yu, C.; Wang, Y.; Fan, W.; Li, Z.; Zheng, Y. Preparation, purification and identification of antioxidant peptides from the placenta of Tibetan sheep. Food Biosci. 2025, 63, 105560. [Google Scholar] [CrossRef]
- Wang, L.; Song, X.; Cui, H.; Man, S.; Li, W.; Muluye, R.A.; Bian, Y.; Chu, X.; Yan, D.; Cai, Y. Antifatigue effects of peptide isolated from sheep placenta. Chin. Herb. Med. 2018, 10, 279–284. [Google Scholar] [CrossRef]
- Haddar, A.; Agrebi, R.; Bougatef, A.; Hmidet, N.; Sellami-Kamoun, A.; Nasri, M. Two detergent stable alkaline serine-proteases from Bacillus mojavensis A21: Purification, characterization and potential application as a laundry detergent additive. Bioresour. Technol. 2009, 100, 3366–3373. [Google Scholar] [CrossRef]
- Jellouli, K.; Bougatef, A.; Manni, L.; Agrebi, R.; Siala, R.; Younes, I.; Nasri, M. Molecular and biochemical characterization of an extracellular serine-protease from Vibrio metschnikovii J1. J. Ind. Microbiol. Biotechnol. 2009, 36, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Gudzenko, O.V.; Varbanets, L.D.; Chernyshenko, V.O.; Stohnii, Y.M.; Ostapchuk, A.M.; Ivanytsia, V.O. Purification and physico-chemical properties of Bacillus atrophaeus protease with elastolytic and fibrinogenolytic activity. Ukr. Biochem. J. 2024, 96, 36–46. [Google Scholar] [CrossRef]
- Rahem, F.Z.; Badis, A.; Zenati, B.; Mechri, S.; Hadjidj, R.; Rekik, H.; Eddouaouda, K.; Annane, R.; Jaouadi, B. Characterization of a novel serine alkaline protease from Bacillus atrophaeus NIJ as a thermophilic hydrocarbonoclastic strain and its application in laundry detergent formulations. Algerian J. Environ. Sci. Technol. 2021, 7, 1707–1724. [Google Scholar]
- Rathod, B.G.; Poosarla, V.G.; Kuppili, S.K.; Chouhan, K.S.Y.R.; Shivshetty, N. Production and characterization of a halotolerant protease from Bacillus siamensis F2 for chicken byproducts valorization. Biomass Conv. Bioref. 2024, 14, 1343–1358. [Google Scholar] [CrossRef]
- Zhu, X.; Hua, Y.; Li, X.; Kong, X.; Zhang, C.; Chen, Y. Isolation and characterization of an activator-dependent protease from Aspergillus ochraceus screened from low denatured defatted soybean meal and the proteolysis of soy proteins. LWT 2021, 150, 112026. [Google Scholar] [CrossRef]
- Keshapaga, U.R.; Jathoth, K.; Singh, S.S.; Gogada, R.; Burgula, S. Characterization of high-yield Bacillus subtilis cysteine protease for diverse industrial applications. Braz. J. Microbiol. 2023, 54, 739–752. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, Y.; Hu, R.; Cheng, Y.; Qin, J.; Yang, J.; Fang, Y.; Lyu, M.; Wang, S. Screening and characteristics of marine Bacillus velezensis Z-1 protease and its application of enzymatic hydrolysis of mussels to prepare antioxidant active substances. Molecules 2022, 27, 6570. [Google Scholar] [CrossRef]
- Thomas, N.N.; Archana, V.; Shibina, S.; Edwin, B.T. Isolation and characterization of a protease from Bacillus sps. Mater. Today Proc. 2021, 41, 685–691. [Google Scholar] [CrossRef]
- Gupta, R.; Beg, Q.K.; Lorenz, P. Bacterial alkaline proteases: Molecular approaches and industrial applications. Appl. Microbiol. Biotechnol. 2002, 59, 15–32. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Lang, D.A.; Xu, H.; Zhu, H. Purification, characterization and potential applications of a novel halotolerant metalloprotease from marine bacterium Vibrio sp. LA-05. J. Chem. Technol. Biotechnol. 2018, 93, 3627–3637. [Google Scholar] [CrossRef]
- Liu, X.; Lu, Q.; Xiao, H.; Feng, Y.; Su, G.; Zhao, M.; Huang, M. Expression of a salt-tolerant pseudolysin in yeast for efficient protein hydrolysis under high-salt conditions. Biomolecules 2023, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yin, Y.; Yan, J.; Zhang, J.; Ma, H.; Zhou, C. Separation, biochemical characterization and salt-tolerant mechanisms of alkaline protease from Aspergillus oryzae. J. Sci. Food Agric. 2019, 99, 3359–3366. [Google Scholar] [CrossRef]
- Gao, X.; Yin, Y.; Zhou, C. Purification, characterisation and salt-tolerance molecular mechanisms of aspartyl aminopeptidase from Aspergillus oryzae 3.042. Food Chem. 2018, 240, 377–385. [Google Scholar] [CrossRef]
- Suwannaphan, S.; Fufeungsombut, E.; Promboon, A.; Chim-anage, P. A serine protease from newly isolated Bacillus sp. for efficient silk degumming, sericin degrading and colour bleaching activities. Int. Biodeterior. Biodegrad. 2017, 117, 141–149. [Google Scholar] [CrossRef]
- Pereira, J.Q.; Ambrosini, A.; Passaglia, L.M.P.; Brandelli, A. A new cold-adapted serine peptidase from Antarctic Lysobacter sp. A03: Insights about enzyme activity at low temperatures. Int. J. Biol. Macromol. 2017, 103, 854–862. [Google Scholar] [CrossRef]
- Siddiqui, K.S.; Ertan, H.; Poljak, A.; Bridge, W.J. Evaluating enzymatic productivity—The missing link to enzyme utility. Int. J. Mol. Sci. 2022, 23, 6908. [Google Scholar] [CrossRef]
- Nikhita, R.; Sachindra, N.M. Optimization of chemical and enzymatic hydrolysis for production of chicken blood protein hydrolysate rich in angiotensin-I converting enzyme inhibitory and antioxidant activity. Poult. Sci. 2021, 100, 101047. [Google Scholar] [CrossRef] [PubMed]
- Babini, E.; Tagliazucchi, D.; Martini, S.; Più, L.D.; Gianotti, A. LC-ESI-QTOF-MS identification of novel antioxidant peptides obtained by enzymatic and microbial hydrolysis of vegetable proteins. Food Chem. 2017, 228, 186–196. [Google Scholar] [CrossRef]
- Chen, X.; Liang, L.; Han, C. Borate suppresses the scavenging activity of gallic acid and plant polyphenol extracts on DPPH radical: A potential interference to DPPH assay. LWT 2020, 131, 109769. [Google Scholar] [CrossRef]
- Guo, X.; Dong, Z.; Li, Q.; Wan, D.; Zhong, J.; Dongzhi, D.; Huang, M. Flavonoids from Rhododendron nivale Hook. f delay aging via modulation of gut microbiota and glutathione metabolism. Phytomedicine 2022, 104, 154270. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, U.; Elumalai, S.; Moon, J.S.; Won, K.C. c-Abl tyrosine kinase inhibition attenuate oxidative stress-induced pancreatic β-Cell dysfunction via glutathione antioxidant system. Transl. Res. 2022, 249, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, H.; Cao, G.; Yang, C.; Cao, Y. Enzymolysis of by-product derived from sheep placenta to production of highly active antioxidant peptide. Synth. Catal. 2017, 2, 3. [Google Scholar] [CrossRef]
- Lv, J.Y.; Nawaz, M.A.H.; Liu, N.; Zhou, H.P.; Hussain, E.; Wen, X.; Gou, X.Y.; Jin, X.; Yu, C. A Nile red-based near-infrared fluorescent probe for the detection of superoxide radical anion in living cells. Chin. J. Anal. Chem. 2022, 50, 100140. [Google Scholar] [CrossRef]
- Lee, E.J.; Jang, Y.S.; Hong, I.K. Effects of lamb placenta peptide powder on skin moisturizing and wrinkle formation. J. Korean Soc. Food Sci. Nutr. 2023, 52, 1013–1034. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.; Randall, R. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Chalita, M.; Kim, Y.O.; Park, S.; Oh, H.S.; Cho, J.H.; Moon, J.; Baek, N.; Moon, C.; Lee, K.; Yang, J.; et al. EzBioCloud: A genome-driven database and platform for microbiome identification and discovery. Int. J. Syst. Evol. Microbiol. 2024, 74, 006421. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Hassabo, A.A.; Selim, M.H.; Saad, M.M.; Abdelraof, M. Optimization of L-methioninase and L-arginase production by newly isolated marine yeast using response surface methodology. Biocatal. Agric. Biotechnol. 2022, 42, 102383. [Google Scholar] [CrossRef]
- Karray, A.; Alonazi, M.; Horchani, H.; Bacha, A.B. A novel thermostable and alkaline protease produced from Bacillus stearothermophilus isolated from olive oil mill sols suitable to industrial biotechnology. Molecules 2021, 26, 1139. [Google Scholar] [CrossRef]
- Weber, K.; Osborn, M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J. Biol. Chem. 1969, 244, 4406–4412. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Feng, Z. The measurement of the degree of hydrolysis of hydrolysed protein. Food Sci. 1994, 179, 65–67. (In Chinese) [Google Scholar]
- Skelton, G.S. Papaya proteinases. I. Temperature-and pH-stability curves. Enzymologia 1968, 35, 270–274. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).