Abstract
Eucalyptus camaldulensis of the Myrtaceae family shows high resistance to aluminum (Al) ions and contains various compounds such as steroids, terpenoids, saponins, flavonoids, glycosides, alkaloids, and tannins. Although the ellagitannin oenothein B (12) isolated from E. camaldulensis exhibits remarkable properties for Al detoxification, likely contributing to its Al resistance, other ellagitannin oligomers present in E. camaldulensis have not been investigated in detail. In this study, novel dimeric and trimeric ellagitannin oligomers eucarpanin D2 (1) and eucamalin A (2), together with known gallotannins (7, 8, and 10), monomeric ellagitannins (4–6, and 11), and dimeric ellagitannins (3, 9, and 12–14), were isolated from E. camaldulensis leaves. The structures of these novel compounds were elucidated based on their chemical and physicochemical properties, including the orientations of tergalloyl groups in compounds 1 and 2. Similar to compound 12, previously isolated from the roots of E. camaldulensis, the ellagitannins demonstrated good Al detoxification properties. Hence, these tannins may play a critical role in the high Al resistance of E. camaldulensis in acidic soils. This paper reports for the first time the isolation of ellagitannin oligomers from the leaves of E. camaldulensis.
1. Introduction
Eucalyptus camaldulensis Dehnh. is an evergreen tree that belongs to the Myrtaceae family. Native to Australia, it is widespread in tropical and subtropical regions and commonly grows on riverbanks. This plant is tolerant to extreme drought and high temperatures, grows rapidly, and exhibits a good coppicing ability [1,2]. Therefore, it shows significant variations among different regions. E. camaldulensis exhibits high resistance to Al ions and can grow in acidic soils, which account for 30% of the Earth land area [3,4]. We conducted an ingredient search to elucidate the mechanism of this ability of E. camaldulensis.
E. camaldulensis contains various compounds such as steroids, terpenoids, saponins, flavonoids, glycosides, alkaloids, and tannins [5,6]. Among them, we previously identified the Al-binding ligand oenothein B (12), a dimeric ellagitannin, in the roots of E. camaldulensis. It contains large amounts of compound 12, and the bioassays using Arabidopsis (Arabidopsis thaliana) showed that the inhibition of root elongation by Al was alleviated by compound 12 [7]. That is, it is suggested that compound 12 contributes to the Al detoxification in E. camaldulensis. Initially, compound 12 was isolated from the leaves of Oenothera erythrosepala (Onagraceae), which is widely distributed among species belonging to the Myrtaceae, Onagraceae, and Lythraceae families [8]. These species have oligomeric analogs, such as eucarpanin D1 (15) (dimer), oenothein T1 (trimer), eucarpanin T1 (16), and T2 (trimer) extracted from the leaves of Eucalyptus cypellocarpa and woodfordin I (dimer), E (trimer), and F (tetramer) isolated from the flowers of Woodfordia fruticosa [8,9,10]. However, the ellagitannin oligomers present in E. camaldulensis have not been investigated in detail to date. Therefore, we focused on high molecular weight tannins to identify those having higher ability to reduce the toxic effects of Al than compound 12, as well as the leaves of E. camaldulensis, which are rich in such tannins. In this study, we isolated and characterized monomeric and oligomeric ellagitannin, along with gallotannins, from the leaves of E. camaldulensis and examined their Al detoxification properties.
2. Results and Discussion
2.1. Isolation and Structural Elucidation of Novel and Known Compounds
A concentrated solution of a 70% aqueous acetone homogenate from the frozen leaves of E. camaldulensis was sequentially extracted with Et2O, EtOAc, and water-saturated n-BuOH. The EtOAc extract yielded three known ellagitannins: pedunculagin (4) [11], tellimagrandin I (5) [12], and tellimagrandin II (6) [12], two known gallotannins: 1,2,6-tri-O-galloyl-β-d-glucose (7) [13] and 1,2,3,6-tetra-O-galloyl-β-d-glucose (8) [14], and dimeric ellagitannin: eucalbanin C (9) [15]. In addition, two novel dimeric and trimeric ellagitannins: eucarpanin D2 (1) and eucamalin A (2), were isolated from this extract (Figure 1). The n-BuOH extract gave gallotannin: 1,6-di-O-galloyl-β-d-glucose (10) [16], three monomeric ellagitannins: compounds 4 and 5, and strictinin (11) [11,17], two dimeric ellagitannins: compound 9 and oenothein B (12) [8,18], and two highly oxidized ellagitannins: eurobustin C (3) [19] and eugeniflorin D2 (14) [8,18,19] (Figure 1). The water-soluble extract obtained dimeric ellagitannin: eugeniflorin D1 (13) [8,19] (Figure 1). The known compounds were identified by comparing their spectroscopic properties to data obtained from previous isolations (Figure S1).
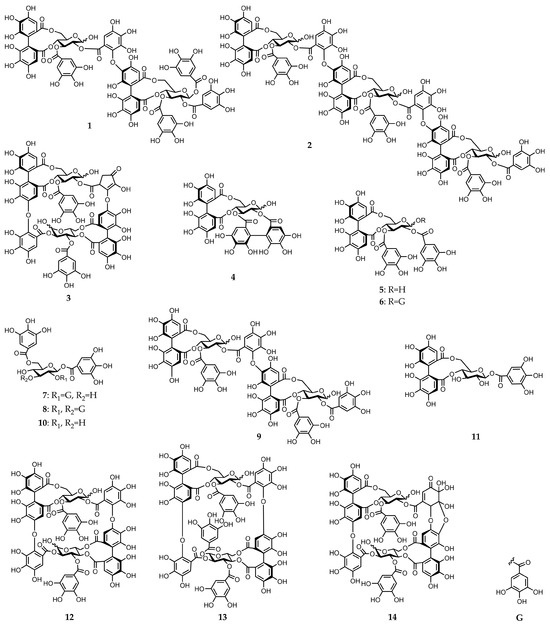
Figure 1.
Chemical structures of gallotannins and ellagitannins isolated from E. camaldulensis leaves (compounds 1–14).
We further characterized the novel dimeric and trimeric ellagitannins identified in this work and named them eucarpanin D2 (1) and eucamalin A (2), respectively. These ellagitannins contained tergalloyl groups serving as acyl units. Eurobustin C (3) was first isolated from Eucalyptus robusta, and its structure was identified by Yoshida et al. in their book, however, a detailed chemical structure determination was not reported [19]. Therefore, we performed a detailed structural characterization of compound 3. The chemical structures of these ellagitannins are described below.
Eucarpanin D2 (1) was obtained as a pale-brown amorphous powder. The molecular formula of compound 1 (C75H54O48) was determined by high-resolution electrospray ionization mass spectrometry (HR-ESI-MS) from its singly and doubly charged molecular ions at m/z 1721.1649 [M − H]− and 860.0840 [M − 2H]2−, indicating that compound 1 was an ellagitannin dimer. Its 1H-nuclear magnetic resonance (NMR) spectrum is similar to that of eucalbanin C (9) and indicated the presence of four sets of 2H singlets (δ 7.11, 7.11, 7.02, 7.02, 7.01, 7.01, 7.00, and 6.99), two sets of 1H singlets (δ 6.65, 6.64, 6.49, and 6.48), and three sets of 1H singlets (δ 6.87, 6.84, 6.60, 6.59, 6.53, and 6.51), which were ascribed to four galloyl, one hexahydroxy diphenoyl (HHDP), and one tergalloyl groups, respectively. This compound also exists as an equilibrium mixture of two tautomers and is characterized by the presence of two 4C1-conformation glucopyranose cores. The absolute configuration of glucopyranose in compound 1 was determined from the D-series using the method described by Tanaka et al. [20]. The H-1 signal of the β-anomer of compound 1 shifted to a lower field (δ 6.18, d, J = 8.4 Hz) than that of compound 9 (δ 4.98). The corresponding 13C-NMR spectrum contains a signal characteristic of the tergalloyl group in the hydroxyl carbon region; the tergalloyl-4’ carbon was present in a lower field (δ 149.0) than that of the valoneoyl group, similar to that of compound 9 (Figure S2). The enzymatic hydrolysis of compound 1 with tannase yielded compound 9. Tellimagrandin I (5), tellimagrandin II (6), and gemin D (17) [21] were produced via partial hydrolysis. This isomerization via a Smiletype rearrangement was successfully applied to establish a chemical correlation between compound 1 and eucarpanin D1 (15). The tergalloyl group in the crowded substitution mode is isomerized to the valoneoyl group, which has a less sterically hindered structure because of a Smiles-type rearrangement. An aqueous solution containing a small amount of 0.02 M phosphate buffer (pH 7.4) was left standing at room temperature for 6 h to produce an isomerized product of compound 15. The methylation of compound 1 with dimethyl sulfate and potassium carbonate in acetone followed by methanolysis yielded methyl tri-O-methylgallate (18), dimethyl hexamethoxydiphenate (19), and trimethyl octa-O-methyltergallate (20) (Figure 2). The positive cotton effect in the short wavelength region of the circular dichroism (CD) spectrum indicated that the absolute configuration of HHDP and tergalloyl group was S-series, respectively [22]. Based on these data, eucarpanin D2 is represented by the structure of compound 1.
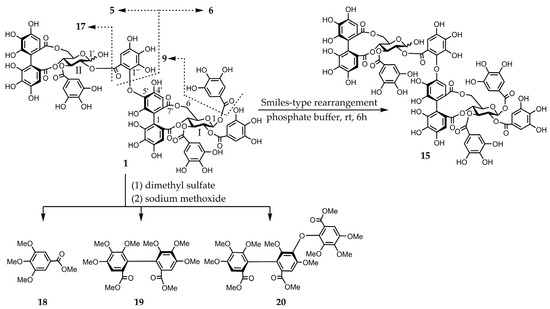
Figure 2.
Smiles-type rearrangement, partial hydrolysis, and methylation of compound 1 followed by methanolysis.
Eucamalin A (2) was obtained as a pale-brown amorphous powder. The molecular formula of this material, which was determined by HR-ESI-MS from the singly, doubly, and triply charged molecular ions at m/z 2353.2400 [M − H]−, 1176.1117 [M − 2H]2−, and 783.7383 [M − 3H]3−, respectively, was C102H74O66, indicating that compound 2 was a trimeric ellagitannin. The 1H-NMR spectrum pattern of compound 2 was strongly resembled those of compound 9 and eucarpanin T1 (16) [23], suggesting that compound 2 was linked through its tergalloyl groups. Although the 1H-NMR spectrum of compound 2 exhibited intricate signals caused by the presence of anomeric mixtures at the glucose cores, the presence of galloyl, HHDP, tergalloyl groups, and glucose moieties was confirmed by the signals at δ 7.06–6.96 (galloyl-H), δ 6.87–6.83 (tergalloyl-H), δ 6.64–6.62 (HHDP-H), δ 6.60–6.56 (tergalloyl-H), δ 6.52–6.46 (tergalloyl and HHDP-H), and δ 3.74–5.90 (glucose-H) (Figure S3). Furthermore, the signal pattern of glucose carbon atoms in the 13C-NMR spectrum of compound 2 was similar to those of compounds 9 and 16. The absolute configuration of glucopyranose in compound 2 was identified from the D-series [20]. This isomerization via a Smiles-type rearrangement was successfully applied to establish a chemical correlation between compounds 2 and 16. An aqueous solution of compound 2 containing a small amount of the 0.02 M phosphate buffer (pH 7.4) was left to stand at room temperature for 6 h and was nearly identical to those of compound 16. The phenolic acyl units of compound 2 were confirmed by the methylation of compound 2 and subsequent methanolysis to produce compounds 18–20. The configurations of the chiral biphenyl moieties of the HHDP and tergalloyl units in compound 2 represent the S-series because of the strong positive cotton effects in the CD spectrum at 228 and 220 nm (shoulder), respectively [22]. The partial hydrolysis of compound 2 generated compounds 5 and 17 (Figure 3). Based on these data, we determined the structure of compound 2.
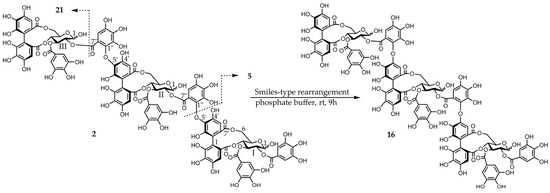
Figure 3.
Smiles-type rearrangement and partial hydrolysis of compound 2.
Eurobustin C (3) was obtained as a pale-brown amorphous powder. The molecular formula of this material (C67H48O43) was determined by HR-ESI-MS from its singly and doubly charged molecular ions with m/z 1539.1491 [M − H]− and 769.0657 [M − 2H]2−, indicating that compound 3 was an ellagitannin dimer. Some of the aromatic and sugar proton signals in the obtained 1H-NMR spectrum was broadened probably because of the poor flexibility of the macro-ring arising from restricted rotation. The 1H-NMR spectrum of compound 3 exhibited two 2H singlets at δ 7.15 and 7.08 attributable to the protons of one galloyl group. Three 1H singlets at δ 7.18, 6.85, and 6.56, and two 1H singlets at δ 6.39 and 6.26 indicated the presence of an HHDP group and a new acyl unit named a eurobustinoyl group. This unit is metabolized by the dehydrovaloneoyl group of eugeniflorin D2 (14). Therefore, the 1H-NMR spectrum of compound 3 is similar to that of compound 14. 1H-1H correlation spectroscopy (COSY) experiment revealed the presence of two 4C1 conformation glucopyranose cores. The doublet at δ 6.94 (J = 3.6 Hz) and δ 4.84 (J = 8.8 Hz) of anomeric protons resonated at a higher field than those expected for the C-1 signals of acylated α- and β-anomers, demonstrating that both anomeric centers were unacylated. A methine proton signal at δ 4.45 and methylene proton signals at δ 2.14 and 1.89 were coupled to each other. The 13C-NMR spectrum of compound 3 showed the presence of an α, β-unsaturated ketone system (δ 136.9, 160.6, and 197.2). Heteronuclear multiple bond connectivity (HMBC) experiment revealed the existence of correlations between methyl protons and carbonyl carbon (δ 197.2), which were separated by two bonds. The 13C-NMR spectrum was similar to those of brevifolincarboxylic acid [19]. Therefore, this compound contains a five-membered ring according to the HMBC experiment of compound 3. The connectivity between a methine protons (δ 4.45) and one of the methylene protons (δ 2.14), and H-2 of glucose II (δ 4.82, t, J = 8.8 Hz) was confirmed by a long-range correlation through the same ester carbonyl carbon signal (δ 170.5). Similarly, H-3’ of the valoneoyl group (δ 6.85) correlated with H-4 of glucose II (δ 4.82, t, J = 8.8 Hz) through the signal at δ 166.9. The allocations of galloyl groups at O-3 of glucose I and O-3 of glucose II were also established by the cross peaks of H-3 of glucose I (δ 5.42, t, J = 8.8 Hz) and H-3 of glucose II (δ 5.92, t, J = 6.8 Hz), respectively, formed with the ester carbonyl carbon at δ 168.2 and 166.2, which correlated with the galloyl proton signals (Figure S4). These data suggested the formation of structure 3, which was supported by the following chemical reaction. The partial hydrolysis of compound 3 in hot water containing 1% trifluoroacetic acid (TFA) produced gallic acid (21), valoneic acid dilactone (22), oenothein C (23) [24], isocoriariin F (24) [25,26], and cornusiin B (25) [26,27]. The valoneoyl group of compound 3 was of the isorugosin type because this reaction yielded compounds 23–25. The methylation of compound 3 with dimethyl sulfate and potassium carbonate in acetone followed by methanolysis resulted in compounds 18 and 19, trimethyl octa-O-methylvalonate (26), and trimethyl hexa-O-methyleurobustinate (27). Compound 19 was produced by the cleavage of the ether bond of the valoneoyl group (Figure 4). The S-configuration was identified for both the valoneoyl and eurobustinoyl group in compound 3 by the positive cotton effects in the CD spectrum [22]. Finally, the structure of compound 3 was established based on these data.
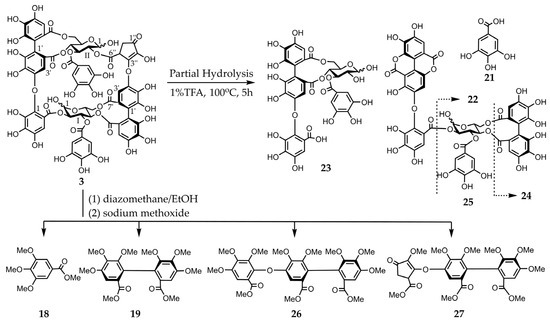
Figure 4.
Partial hydrolysis and methylation of compound 3 followed by methanolysis.
2.2. Detoxification of Al
The extracts in the liquid–liquid distributions and ellagitannins isolated from leaves of E. camaldulensis were added to the seedlings of Arabidopsis thaliana, and their Al detoxification properties were evaluated after 24 h. The EtOAc, n-BuOH, and water-soluble extracts in the liquid–liquid distributions demonstrated Al detoxification abilities (Figure 5). Therefore, we focused on isolating tannins from these extracts. Among the ellagitannins isolated from these extracts, oenothein B (12), eucarpanin D2 (1), eucalbanin C (9), and eugeniflorin D2 (14) exhibited the same degree of root elongation, whereas eucamalin A (2) and eugeniflorin D1 (13) at 8 μM pyrogallol-equivalent differed significantly from compound 12 in the Tukey-Kramer test (Figure 6). We also evaluated the effect of the addition of 16 μM pyrogallol-equivalent ellagitannin alone, to the seedlings of A. thaliana. Among these ellagitannins: compounds 9, 12, and 13 did not inhibit root elongation; however, compounds 1, 2, and 14 slightly inhibited the root elongation process (Figure 7). These results revealed that ellagitannin did not promote root elongation and that compounds 1, 9, and 14 possessed the same Al detoxification ability as that of compound 12, whereas the detoxification abilities of compounds 2 and 13 were stronger than that of compound 12. Ellagitannins have structure of pyrogallol, and metal ions form chelate there [28,29]. Compound 12 has eight pyrogallols, which are presumed to contribute to Al detoxification by binding to Al ions [7]. Similarly, ellagitannins isolated from the leaves of E. camaldulensis (compounds 1, 2, 9, 13 and 14) have some or greater number of pyrogallols in their molecules as compound 12. Further isolation of the compounds is necessary to elucidate the detailed mechanisms of Al detoxification potential, including differences in the number of pyrogallol and the steric structure of the compounds.
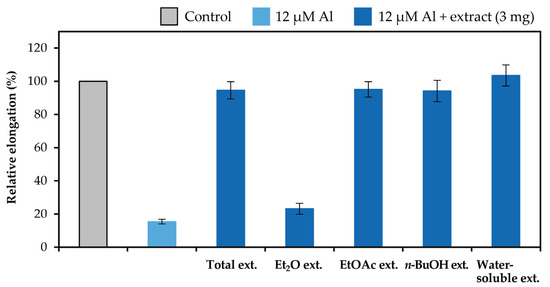
Figure 5.
Al detoxification properties of the extracts in the liquid–liquid distributions. Roots of A. thaliana were exposed to 500 mL of a 2% strength modified Molecular Genetics Research Laboratory (MGRL) medium (pH 5.0) containing 0 or 12 μM AlCl3 and 3 mg of the extracts for 24 h. The relative root elongation is expressed as the percentage of the root elongation in the control sample (0 μM Al without extract). The values and error bars are expressed as means ± SE (n = 20).
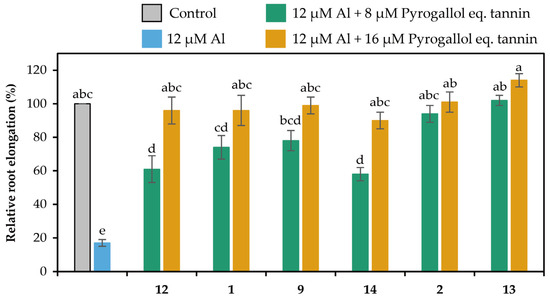
Figure 6.
Al detoxification properties of ellagitannins (compounds 1, 2, 9, 12, 13 and 14) isolated from E. camaldulensis. Roots of A. thaliana were exposed to a 2% strength modified MGRL medium (pH 5.0) containing 0 or 12 μM AlCl3 and 0, 8, or 16 μM pyrogallol-equivalent ellagitannin for 24 h. The relative root elongation is expressed as the percentage of the root elongation in the control sample (0 μM Al, 0 μM ellagitannin). The values and error bars are expressed as means ± SE (n = 20). The bars marked with the same letter do not differ significantly at p < 0.05 (Tukey-Kramer test).
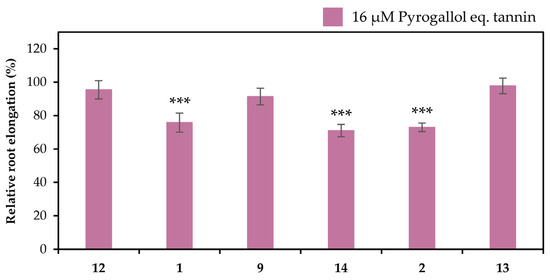
Figure 7.
Effects of ellagitannins (compounds 1, 2, 9, 12, 13 and 14) on the root elongation. Roots of A. thaliana were exposed to 0 or 16 μM pyrogallol-equivalent ellagitannin in a 2% strength modified MGRL medium (pH 5.0) for 24 h. The relative root elongation is expressed as the percentage of the root elongation in the control sample (0 μM ellagitannin). The values and error bars are expressed as means ± SE (n = 20). The asterisks indicate a significant difference compared with the control at p < 0.001 (Student’s t-test).
3. Materials and Methods
3.1. Instrumentation
Optical rotations were recorded using a Jasco DIP-1000 polarimeter (Jasco, Tokyo, Japan). The ultraviolet (UV) and CD spectra were obtained using a Jasco V-530 spectrophotometer (Jasco, Tokyo, Japan) and a Jasco J-710 spectropolarimeter (Jasco, Tokyo, Japan), respectively. The 1H-NMR (600 MHz) and 13C-NMR (151 MHz) spectra including 1H-1H COSY, heteronuclear single quantum correlation (HSQC), and HMBC were recorded on a Varian NMR instrument (Varian, Palo Alto, CA, USA), and the chemical shifts are listed in part-per-million (ppm) values relative to acetone-d6 (2.04 ppm for 1H and 29.8 ppm for 13C). The 1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectra including 1H-1H COSY, HSQC, and HMBC were recorded on a JNM-ECS400 instrument (JEOL, Akishima, Tokyo, Japan), and the chemical shifts are provided in part-per-million (ppm) values relative to acetone-d6 (2.04 ppm for 1H and 29.8 ppm for 13C). The high-resolution mass spectra were obtained using a Bruker MicrOTOF II instrument (Bruker, Billerica, MA, USA) and a Xevo G3 QTof (Waters, Milford, MA, USA) with an ESI source in negative-ion mode. Reversed-phase HPLC was conducted with a Wakosil II 5C18HG (250 × 4.6 mm i.d., Wako, Osaka, Japan) column using a mobile phase composed of 0.1% aqueous TFA (solvent A) and CH3CN (solvent B) (82:18) and isocratic program at a temperature of 35 °C, UV detection wavelength of 250 nm, and flow rate of 0.8 mL/min (System A). Reversed-phase HPLC was performed on an Inertsustain C18 column (150 × 4.6 mm i.d., 5 µm, GL Sciences, Tokyo, Japan) using a mobile phase composed of H2O:CH3CN:HCOOH (90:5:5) (solvent A) and H2O:CH3CN:HCOOH (50:45:5) (solvent B) at 40 °C, and the gradient was programmed as follows: 0–30 min (solvent B: 0–100%) (system B). Reversed-phase HPLC was performed on a YMC-pack ODS-A column (250 × 4.6 mm i.d., 5 µm, YMC Co., Ltd., Kyoto, Japan) using a mobile phase consisting of 0.01 M H3PO4:0.01 M KH2PO4:EtOH:EtOAc = 47.5:47.5:3:2 at a column temperature of 40 °C, flow rate of 1.0 mL/min, and linear gradient. Detection was performed at 280 nm (system C). Normal-phase HPLC was conducted on a CHIRALCEL OD-H column (250 × 4.6 mm i.d., 5 µm, DAICEL Co., Ltd., Osaka, Japan) using a mobile phase composed of n-hexane: EtOH = 20:1 at 24 ± 1 °C. The flow rate was 2.0 mL/min, and the UV detection wavelength was 280 nm (system D). Purification by column chromatography were conducted using Diaion-HP-20 (Mitsubishi Kasei Co., Tokyo, Japan), Toyopearl HW-40 (coarse grade) (Tosoh Co., Tokyo, Japan), Sephadex LH-20 (GE Healthcare, Chicago, IL, USA), and MCI GEL CHP-20P (75–150 µm) (Mitsubishi Chemical Co., Tokyo, Japan) resins.
3.2. Plant Material
A clone of Eucalyptus camaldulensis Dehnh. (seed lot: 19708; Australian Tree Seed Center, CSIRO) was propagated by cutting as described previously. The obtained plantlets were transferred to vermiculite in plastic pots (18.4 × 11.3 cm i.d.) and cultured in a growth chamber (16 h light/8 h dark; 28/25 °C; photosynthetic photon flux density: 200 µmol m−2 s−1). The plantlets were watered twice a day with a nutrient solution [7]. After two months of cultivation, leaves were harvested from the plantlets with heights of approximately 1 m, frozen in liquid nitrogen, and stored at −80 °C until use.
3.3. Extraction and Isolation
The frozen leaves of E. camaldulensis (2.4 kg) were homogenized with 70% aqueous acetone (4.0 L × 3), filtered, and evaporated in vacuo. Extracts were obtained sequentially with Et2O (3.0 L × 3), EtOAc (3.0 L × 3), and water-saturated n-BuOH (3.0 L × 3) to yield Et2O (1.7 g), EtOAc (15.1 g), n-BuOH (40.7 g), and a water-soluble fraction (117 g). A portion of the EtOAc extract (7.0 g) was subjected to column chromatography over Toyopearl HW-40 (coarse grade) (40 × 2.2 cm i.d.) using 30%, 40%, 50%, 60%, and 70% aqueous MeOH; MeOH; MeOH:H2O:acetone (7:2:1), MeOH:H2O:acetone (7:1:2), and 70% aqueous acetone as eluents. The 50%MeOH fraction resulted in tellimagrandin I (5) (924 mg), which was purified over MCI GEL CHP-20P (30 × 1.1 cm i.d.) with aqueous MeOH and Mega Bond Elut C18 cartridge column with aqueous MeOH to yield pedunculagin (4) (148 mg) and 1,2,6-tri-O-galloyl-β-d-glucose (7) (143 mg). 1,2,3,6-Tetra-O-galloyl-β-d-glucose (8) (104 mg) and tellimagrandin II (6) (30 mg) was obtained from the 60% and 100% MeOH fractions, respectively. The MeOH:H2O:acetone (7:1:2) fraction yielded eucalbanin C (9) (682 mg) and a novel ellagitannin: eucarpanin D2 (1) (175 mg). The 70% aqueous acetone fraction resulted in eucamalin A (2) (37 mg). A portion of the n-BuOH extract (6.3 g) was chromatographed over Diaion HP-20 (70 × 1.5 cm i.d.) using H2O; 10%, 30%, and 50% aqueous MeOH; MeOH; and 70% aqueous acetone as eluents. The 30% MeOH fraction (1.5 g) was subjected to column chromatography over Toyopearl HW-40 (coarse grade) (40 × 2.2 cm i.d.) using 30%, 40%, 50%, 60%, and 70% aqueous MeOH; MeOH; MeOH:H2O:acetone (7:2:1), MeOH:H2O:acetone (7:1:2), and 70% aqueous acetone as eluents to give 1,6-di-O-galloyl-β-d-glucose (10) (20 mg), strictinin (11) (28 mg), compounds 4 (44 mg) and 5 (53 mg), and oenothein B (12) (360 mg). The water-soluble extract (120 g) was chromatographed over Diaion HP-20 (60 × 5 cm i.d.) using H2O; 10%, 30%, and 50% aqueous MeOH; MeOH; and 70% aqueous acetone as eluents. The 50% MeOH fraction (19 g) was further chromatographed over Toyopearl HW-40 (coarse grade) (40 × 2.2 cm i.d.) using 30%, 40%, 50%, 60%, and 70% aqueous MeOH; MeOH; MeOH:H2O:acetone (7:2:1); MeOH:H2O:acetone (7:1:2); and 70% aqueous acetone as eluents. The 60%MeOH fraction purified over MCI GEL CHP-20P (30 × 1.1 cm i.d.) with aqueous MeOH yielded Eugeniflorin D1 (13) (39 mg). For further ellagitannin studies, the leaves of E. camaldulensis were extracted again. The frozen leaves of E. camaldulensis (1.6 kg) were homogenized in 70% aqueous acetone (4.0 L × 3), filtered, and evaporated in vacuo. Extracts were obtained sequentially with Et2O (2.0 L × 3), EtOAc (2.0 L × 3), and water-saturated n-BuOH (2.0 L × 3) to produce Et2O (2.3 g), EtOAc (12.3 g), n-BuOH (31.8 g), and a water-soluble fraction (92.4 g). The EtOAc extract (10.0 g) was subjected to column chromatography over Toyopearl HW-40 (coarse grade) (40 × 2.2 cm i.d.) using 30%, 40%, 50%, 60%, and 70% aqueous MeOH; MeOH; MeOH:H2O:acetone (7:2:1); MeOH:H2O:acetone (7:1:2); and 70% aqueous acetone as eluents. The 50% MeOH fraction produced compound 5 (758 mg); the MeOH:H2O:acetone (7:1:2) fraction resulted in compounds 1 (340 mg) and 9 (677 mg); and the 70% aqueous acetone fraction produced compound 2 (20 mg). A portion of the n-BuOH extract (31.8 g) was separated by column chromatography over Diaion HP-20 (20 × 8 cm i.d.) using H2O with increasing amounts of MeOH (H2O, 10%, 20%, 40% and 100% MeOH) in a stepwise mode followed by 70% aqueous acetone. The 50% MeOH fraction (1.0 g) was chromatographed over Toyopearl HW-40 (coarse grade) (30 × 2.2 cm i.d.) with 30%, 40%, 50%, 60%, and 70% aqueous MeOH; MeOH:H2O:acetone (7:2:1); MeOH:H2O:acetone (7:1:2); and 70% aqueous acetone. The 50% aqueous MeOH fraction produced compound 12 (1.0 g) and was purified by MCI GEL CHP-20P (30 × 1.1 cm i.d.) and preparative reversed-phase HPLC to yield eurobustin C (3) (14 mg) and eugeniflorin D2 (14) (35 mg).
The physicochemical data obtained for compounds 1–3, 9, 13, and 14 are provided below.
Eucarpanin D2 (1): pale brown amorphous powder; [a + 24.5° (c 0.002, MeOH); UV (MeOH) λmax (log ε) 218 (5.17), 275 (4.83) nm; CD (MeOH) [θ] (nm) + 1.6 × 105 (225), +1.7 × 105 (237), −8.7 × 105 (262), +7.9 × 105 (285), −1.4 × 105 (322); 1H-NMR [600 MHz, acetone-d6-D2O (9:1)] δ 7.11, 7.02, 7.02, 7.01, 6.10, 6.99 (2H, s, galloyl-H), 6.65, 6.64, 6.49, 6.48 (1H, s, HHDP-H), 6.87, 6.84, 6.60, 6.59, 6.53, 6.51 (1H, s, tergalloyl-H), 6.18 (1H, d, J = 8.4 Hz, Glc (I) H-1), 5.87 (1H, t, J = 9.6 Hz, Glc (II) H-3α), 5.85 (1H, t, J = 10.6 Hz, Glc (I) H-3), 5.67 (1H, t, J = 9.6 Hz, Glc (II) H-3β), 5.63 (1H, dd, J = 1.2, 8.4 Hz, Glc (I) H-2), 5.60 (1H, d, J = 5.4 Hz, Glc (II) H-1α), 5.33–5.23 (1H, m, Glc (I) H-4, H-6, Glc (II) H-2β, 6αβ), 5.16–5.11 (1H, m, Glc (II) H-2α, 4αβ), 5.00 (1H, d, J = 7.8 Hz, Glc (II) H-1β), 4.66 (1H, m, Glc (II) H-5α), 4.57 (1H, m, Glc (I) H-5), 4.26 (1H, m, Glc (II) H-5β), 3.93 (1H, brd, J = 13.2 Hz, Glc (I) H-6), 3.89–3.87 (2H, d, J = 12.6 Hz, Glc (II) H-6αβ); 13C-NMR [151 MHz, acetone-d6-D2O] δ 96.3 (Glc (II) C-1β), 93.5 (Glc (I) C-1), 90.8 (Glc (II) C-1α), 74.8 (Glc (II) C-2β), 73.5 (Glc (II) C-2α, 3β), 73.1 (Glc (II) C-3α), 72.7 (Glc (II) C-5α), 71.9 (Glc (II) C-5β), 71.8 (Glc (I) C-2), 71.3 (Glc (I) C-3), 71.0 (Glc (I) C-4, Glc (II) 3C-4β), 71.0 (Glc (II) C-4α), 67.0 (Glc (I) C-5), 63.4 (Glc (I) H-6, Glc (II) C-6αβ), 168.5, 168.0, 168.0, 167.8, 167.6, 167.2, 166.6, 166.0, 165.1 (ester carbonyl-C); HR-ESI-MS: m/z 1721.1649 [M − H]−, 860.0840 [M − 2H]2− (calcd for C75H54O48 − H, 1721.1712).
Absolute configuration of glucopyranose. A solution of compound 1 (5.0 mg) in 5% H2SO4 was heated in boiling water for 6 h. The reaction mixture was purified using Sep-Pack Plus tC18 cartridges with H2O and MeOH. The aqueous layer was neutralized with Amberlite IRA-400 (OH− form) and the resin was removed by filtration. After the removal of the solvent in vacuo, the residue was heated with l-cysteine methyl ester hydrochloride (0.5 mg) in pyridine (0.5 mL) to 60 °C for 1 h. Subsequently, O-tolyl isothiocyanate (0.5 mg) in pyridine (0.1 mL) added to the reaction mixture and further heated to 60 °C for 1 h. After the reaction, the solution was analyzed via reversed-phase HPLC (system A) (tR: 9.8 min).
Isomerization of compound 1 to eucarpanin D1 (15): A solution of compound 1 (1.0 mg) in the 0.02 M phosphate buffer (pH 7.4) (1.0 mL) was left standing at room temperature for 6 h. The isomerized product was identical to compound 15 according to the results of reversed-phase HPLC (system B) (tR: 10.13 min, 10.39 min).
Enzymatic hydrolysis of compound 1: A solution of compound 1 (1.0 mg) in H2O (1.0 mL) was incubated with tannase (Aspergillus ficuum) at 37 °C for 2 h. The product was identified as compound 9 using reversed-phase HPLC (system B) (tR: 7.02 min, 8.11 min).
Partial hydrolysis of compound 1: A solution of compound 1 (20 mg) in water was heated to 98 °C for 5 h. The reaction mixture was purified using Sephadex LH-20 (30 × 1.0 cm i.d.) and MCI GEL CHP-20P (30 × 1.0 cm i.d.) and subjected to reversed-phase HPLC (system B) to reveal the presence of compounds 5 (1.6 mg), 6 (3.5 mg), and 17 (1.5 mg) in the hydrolysate.
Methylation of compound 1 followed by methanolysis: A mixture of compound 1 (20 mg), potassium carbonate (200 mg), unhydrate, and dimethyl sulfate (100 µL) in super dehydrate acetone (2.0 mL) was stirred and refluxed for 6 h. After removing the inorganic material via centrifugation, the supernatant was evaporated. The reaction mixture was directly methanolyzed in 1% sodium methoxide (0.5 mL) and superdehydrated MeOH (0.1 mL) and left standing overnight at room temperature. After adding acetic acid (two drops), the solution was evaporated under nitrogen gas. The solution was treated with excess diazomethane (0.8 mL) and Et2O (0.4 mL) for 3 h and then evaporated in vacuo. The residue was subjected to preparative TLC (TLC Silica gel 60 PF254, Merck KGaA) with toluene:acetone (4:1) to compounds 18 (6.0 mg), 19 (1.5 mg), and 20 (1.7 mg). All three materials were characterized by HR-ESI-MS, normal-phase HPLC (system D), and CD spectroscopy, which revealed that these compounds were identical to the authentic samples. Compound 19: CD (MeOH) [θ] (nm) + 5.3 × 104 (229), −4.0 × 104 (252), +6.9 × 103 (313); HR-ESI-MS: m/z 473.1419 [M + Na]+ (calcd for C22H26O10 + Na, 473.1418); normal-phase HPLC (system D): tR 6.1 min. Compound 20: CD (MeOH) [θ] (nm) + 4.6 × 104 (221), −3.6 × 104 (254), +7.5 × 103 (317); HR-ESI-MS: m/z 683.1937 [M + Na]+ (calcd for C32H36O15 + Na, 683.1946); normal-phase HPLC (system D): tR 6.5 min.
Eucamalin A (2): pale brown amorphous powder; [a + 49.2° (c 0.002, MeOH); UV (MeOH) λmax (log ε) 218 (5.33) nm, 272 (4.50) nm; CD (MeOH) [θ] (nm) + 2.9 × 105 (222), +2.7 × 105 (237), −1.7 × 105 (262), +1.4 × 105 (286), −2.5 × 104 (323); 1H-NMR [400 MHz, acetone-d6-D2O (9:1)] δ 7.06–6.96 (2H, s, galloyl-H), 6.87–6.83 (1H, s, tergalloyl-H), 6.65–6.62 (1H, s, HHDP-H), 6.60–6.56 (1H, s, Tergalloyl-H), 6.52–6.47 (1H, s, tergalloyl and HHDP-H), 5.90–5.82 (m, Glc H-3αβ), 5.67 (t, J = 9.6 Hz, Glc H-3β), 5.63–5.54 (m, Glc H-1α), 5.56 (d, J = 3.6 Hz, Glc H-1α), 5.28–5.22 (m, Glc H-2α, 6αβ), 5.16–5.09 (m, Glc H-1β, 2α, 4), 5.00 (d, J = 7.2 Hz, Glc H-1β), 4.70–4.64 (m, Glc H-5α), 4.30–4.24 (m, Glc H-1β, H-5β), 3.91–3.79 (Glc H-6αβ); 13C-NMR [100 MHz, acetone-d6-D2O (9:1)]: δ 96.6, 96.4 (Glc C-1β), 91.1, 90.8 (Glc C-1α), 75.1 (Glc C-2β), 74.1 (Glc C-2β), 73.7 (Glc C-3, 4), 73.5 (Glc C-2α), 73.0 (Glc C-2α), 71.9, 71.7 (Glc C-5β), 71.2 (Glc C-3αβ), 71.0 (Glc C-4), 67.0, 66.91 (Glc C-5α), 63.9, 63.4 (Glc C-6αβ), 168.3, 168.1, 167.8, 167.8, 169.0, 166.9, 166.6, 166.3, 165.9 (ester carbonyl-C); HR-ESI-MS: m/z 2353.2400 [M − H]−, 1176.1117 [M − 2H]2−, 783.7383 [M − 3H]3−.
Absolute configuration of glucopyranose. A solution of compound 2 (2.0 mg) in 5% H2SO4 was heated in boiling water for 1 h. The reaction mixture was purified using Sep-Pack Plus tC18 cartridges with H2O and MeOH. The aqueous layer was neutralized with Amberlite IRA-400 (OH− form) and the resin was removed by filtration. After removal of the solvent in vacuo, the residue was heated with l-cysteine methyl ester hydrochloride (0.5 mg) in pyridine (0.5 mL) to 60 °C for 1 h. Subsequently, O-tolyl isothiocyanate (0.5 mg) in pyridine (0.1 mL) added to the reaction mixture and further heated to 60 °C for 1 h. After the reaction, the solution was analyzed via reversed-phase HPLC (system A) (tR: 9.8 min).
Isomerization of compound 2 to eucarpanin T1 (16): A solution of compound 2 (4.0 mg) in the 0.02 M phosphate buffer (pH 7.4) (2.0 mL) was stored at room temperature for 9 h. The isomerized product was identical to compound 16 according to 1H-NMR.
Partial hydrolysis of compound 2: A solution of compound 2 (10 mg) in water was heated to 98 °C for 2 h. The reaction mixture was purified by MCI GEL CHP-20P (20 × 1.0 cm i.d.) and subjected to reversed-phase HPLC (system B) to reveal the presence of compounds 5 (0.5 mg) and 17 (0.7 mg) in the hydrolysate.
Methanolysis of compound 2: A mixture of compound 2 (5.0 mg), potassium carbonate (100 mg), unhydrate, and dimethyl sulfate (100 µL) in super dehydrated acetone (1.0 mL) was stirred and refluxed for 6 h. After removing the inorganic material via centrifugation, the supernatant was evaporated. The reaction mixture was directly methanolyzed in 1% sodium methoxide (0.5 mL) and superdehydrated MeOH (0.1 mL) and left standing overnight at room temperature. After adding acetic acid (two drops), the solution was evaporated under nitrogen gas. The solution was treated with an excess of diazomethane (0.4 mL) and Et2O (0.2 mL) for 3 h and then evaporated in vacuo. The residue was subjected to preparative TLC (TLC Silica gel 60 PF254, Merck KGaA) with toluene: acetone (4:1) to yield compounds 18 (1.6 mg), 19 (1.3 mg), and 20 (0.9 mg). All three compounds were characterized by HR-ESI-MS and CD spectroscopy, which revealed that they were identical to the authentic samples. Compound 18: HR-ESI-MS: m/z 227.0968 [M + H]+ (calcd for C11H15O5 + H: 227.0919). Compound 19: CD (MeOH) [θ] (nm) + 5.3 × 104 (228), −4.1 × 104 (252), +7.1 × 103 (313); HR-ESI-MS: m/z 450.1517 [M]+ (calcd for C22H26O10, 450.1526); normal-phase HPLC (system D): tR 6.1 min. Compound 20: CD (MeOH) [θ] (nm) + 5.0 × 104 (220), −4.0 × 104 (254), +8.6 × 103 (314); HR-ESI-MS: m/z 661.2113 [M + H]+ (calcd for C32H35O15 + H, 661.2132); normal-phase HPLC (system D): tR 6.4 min.
Eurobustin C (3): pale brown amorphous powder; [α + 13° (c 1, MeOH); UV (MeOH) λmax (log ε) 219 (4.94), 685 (4.69) nm; CD (MeOH) [θ] (nm) − 2.4×104 (206), +1.9 × 105 (218), +8.4 × 104 (228), +2.0 × 105 (240), −1.3 × 105 (263), +5.7 × 104 (284), −1.7 × 104 (308); 1H-NMR [400 MHz, acetone-d6-D2O (9:1)] δ 7.15, 7.08 (2H, s, galloyl-H), 7.20, 6.85, 6.56 (1H, s, valoneoyl-H), 6.39, 6.26 (1H, s, eurobustinoyl-H), 5.94 (1H, d, J = 3.6 Hz, Glc (I) H-1α), 5.93 (1H, t, J = 4.0 Hz, Glc (I) H-2), 5.92 (1H, t, J = 9.6 Hz, Glc (I) H-3α), 5.42 (1H, t, J = 8.8 Hz, Glc (II) H-3β), 5.21 (1H, dd, J = 6,13 Hz, Glc (I) H-6α), 5.08 (1H, dd, J = 6.4, 13.2 Hz, Glc (II) H-6β), 4.99 (1H, t, J = 10.0 Hz, Glc (I) H-4α), 4.90 (1H d, J = 8.8 Hz, Glc (II) H-1β), 4.82 (1H, t, J = 8.8 Hz, Glc (II) H-2β, 4β), 4.80 (1H, dd, J = 6.4, 10.0 Hz, Glc (I) H-5α), 4.45 (1H, dd, J = 2.0, 8.0 Hz, methin-H), 3.65 (2H, d, J = 13.2 Hz, Glc (II) H-6β), 3.57 (2H, d, J = 12.8 Hz, Glc (I) H-6α), 2.14 (1H, dd, J = 1.6, 18.8 Hz, methylene-H), 1.89 (1H, dd, J = 8.0, 19.2 Hz, methylene-H); 13C-NMR [100 MHz, acetone-d6-D2O] δ 96.2 (Glc (II) C-1), 91.9 (Glc (I) C-1), 74.7 (Glc (I) C-2), 73.9 (Glc (II) C-2), 73.3 (Glc (II) C-3), 72.1 (Glc (II) C-4), 70.6 (Glc (I) C-4), 70.4 (Glc (II) C-5), 69.8 (Glc (I) C-3),68.2 (Glc (I) C-5), 63.2, 62.9 (Glc (I) (II) C-6), 170.5, 168.2, 167.9, 167.6, 166.9, 166.2, 165.0 (ester carbonyl-C), 197.2 (ketone-C); HR-ESI-MS: m/z 1539.1423 [M − H]−, 769.0657 [M − 2H]2− (calcd for C67H48O43 − H, 1539.1491).
Partial hydrolysis of compound 3: A solution of compound 3 (0.7 mg) in hot water containing 1% TFA (0.7 mL) was heated in a water bath for 5 h. The reaction mixture was subjected to reversed-phase HPLC (System C) to reveal the presence of compounds 21–25 in the hydrolysate.
Methylation of compound 3 followed by methanolysis: diazomethane-EtOH (1 mL) was added to an EtOH solution (0.5 mL) of compound 3 (10 mg), and the obtained mixture was stored for 3 h at room temperature. After removing the solvent using an evaporator, a MeOH solution (1.0 mL) of the residue was added to 1% sodium methoxide (20 drops) and left standing overnight at room temperature. After the addition of AcOH, the solution was evaporated, and the residue was partitioned between EtOAc and H2O. An EtOAc layer was added, the residue was further treated with diazomethane (10 drops)–Et2O for 2 h, and the solvent was evaporated. The residue was subjected to preparative thin layer chromatography (TLC) (TLC silica gel 60 PF254, Merck KGaA) with toluene:acetone (4:1) to yield compounds 18, 19, 26, and 27. All four materials were characterized via CD spectroscopy, which revealed that these compounds were identical to the authentic samples. Compound 26: CD (MeOH) [θ] (nm) − 4.4 × 104 (202), +1.5 × 105 (220), −7.3 × 104 (251), +2.0 × 103 (278), −2.0 × 103 (294), +1.7 × 104 (315). Compound 27: CD (MeOH) [θ] (nm) − 5.7 × 104 (204), +1.1 × 105 (228), −8.2 × 104 (254), +1.0 × 104 (278), −1.1 × 104 (297), +5.1 × 103 (316).
Eucalbanin C (9): pale brown amorphous powder; 1H-NMR [600 MHz, acetone-d6-D2O (9:1)] δ 7.06, 7.05, 7.05, 7.04, 7.02, 7.02, 7.01, 6.99, 6.99, 6.96 (2H in total each, s, galloyl-H), 6.65, 6.64, 6.64, 6.63, 6.49, 6.48 (1H, s, HHDP-H), 6.87, 6.86, 6.84, 6.83, 6.59, 6.58, 6.58, 6.52, 6.50, 6.50 (1H, s, tergalloyl-H), 5.86 (2H, t, Glc (I) H-3α, Glc (I) H-3β), 5.66 (1H, t, Glc (I) H-3β), 5.60 (2H, m, Glc (II) H-1α, H-3α), 5.54 (1H, d, J = 3.6 Hz, Glc (I) H-1α), 5.28–5.20 (1H, m, Glc (I) H-2β, Glc (II) H-2β, Glc (I) (II) H-6αβ), 5.17–5.07 (1H, m, Glc (I) H-2α, Glc (II) H-2α, Glc (I) (II) H-4αβ), 4.37 (1H, m, J = 7.8 Hz, Glc (II) H-1β), 4.98 (1H, d, J = 7.8 Hz, Glc (I) H-1β), 4.66 (2H, m, Glc (I) (II) H-5α), 4.26 (2H, m, Glc (I) (II) H-5β), 3.83 (2H, m, Glc (I) (II) H-6’αβ); 13C-NMR [151 MHz, acetone-d6-D2O] δ 96.5 (Glc (II) C-1β), 96.3 (Glc (I) C-1β), 91.1 (Glc (I) C-1α), 90.7 (Glc (II) C-1α), 74.8 (Glc (I) (II) C-2β), 74.0 (Glc (I) (II) C-2α), 73.7, 73.6 (Glc C-3β), 71.9, 71.7 (Glc (I) (II) C-5β), 71.4 (Glc (I) (II) C-3α), 73.0, 71.2, 71.1 (Glc (I) (II) C-4β), 71.0 (Glc (I) (II) C-4α), 66.9, 66.8 (Glc (I) (II) C-5α), 63.9, 63.5 (Glc (I) (II) C-6αβ); HR-ESI-MS: m/z 1569.1569 [M − H]−, 785.0816 [M − 2H]2− (calcd for C68H50O44 − H, 1569.1602).
Eugeniflorin D1 (13): pale brown amorphous powder; 1H-NMR [600 MHz, acetone-d6-D2O (9:1)] δ 6.94, 6.92, 6.91 (2H in total each, s, galloyl-H), 6.97, 6.82, 6.65, 6.61, 6.52, 6.33 (1H, s, valoneoyl-H), 6.40 (1H, d, J = 8.4 Hz, Glc (I) H-1), 5.77 (1H, t, J = 9.6 Hz, Glc (I) H-3), 5.40 (1H, dd, J = 7.2 Hz, Glc (II) H-6), 5.36 (1H, t, J = 9.0 Hz, Glc (II) H-3), 5.22–5.15 (3H, m, Glc (I) H-2, Glc (II) H-4, 6), 5.03 (1H, t, J = 9.0 Hz, Glc (II) H-2), 4.61–4.58 (1H, m, Glc (I) H-5), 4.42 (1H, d, J = 7.8 Hz, Glc (II) H-1), 4.38 (1H, dd, J = 6.0, 10.2 Hz, Glc (II) H-5), 4.00 (1H, dd, J = 2.4, 13.2 Hz, Glc (I) H-6), 3.91 (1H, d, J = 13.2 Hz, Glc (II) H-6’), 3.88–3.84 (1H, m, Glc (I) H-6’); 13C-NMR [151 MHz, acetone-d6-D2O] 95.8 (Glc (II) C-1), 93.0 (Glc (I) C-1), 75.6 (Glc (I) C-2), 74.5 (Glc (II) C-2), 73.9 (Glc (II) C-4), 72.1 (Glc (II) C-3), 72.0 (Glc (I) C-5), 71.8 (Glc (II) C-5), 71.7 (Glc (I) C-3), 71.3 (Glc (I) C-4), 66.0 (Glc (II) C-6), 63.1 (Glc (I) C-6), 170.4, 168.9, 168.8, 168.1, 166.9, 166.9, 165.9, 164.8 (ester carbonyl-C); HR-ESI-MS: m/z 1719.1532 [M − H]−, 859.0759 [M − 2H]2− (calcd for C75H52O28 − H, 1719.1555).
Eugeniflorin D2 (14): pale brown amorphous powder; 1H-NMR [400 MHz, acetone-d6-D2O (9:1)] δ 7.30, 7.16 (2H in total each, s, galloyl-H), 7.17, 6.77 (1H, s, dehydrovaloneoyl-H), 7.10 (1H, d, J = 2.0 Hz, dehydrovaloneoyl-H), 6.57, 6.36, 5.87 (1H, s, valoneoyl-H), 5.98 (1H, d, J = 3.2 Hz, Glc (I) H-1α), 5.90 (1H, t, J = 10.0 Hz, Glc (I) H-3α), 5.69 (1H, dd, J = 3.2, 10.0 Hz, Glc (I) H-2α), 5.55 (1H, t, J = 10.0 Hz, Glc (II) H-3β), 5.24 (1H, t, J = 8.0 Hz, Glc (II) H-2β), 5.19 (1H, d, J = 8.0 Hz, Glc (II) H-1β), 5.09 (1H, dd, J = 6.4, 13.6 Hz, Glc (II) H-6β), 4.91 (1H, t, J = 10.0 Hz, Glc (II) H-4αβ), 4.70 (1H, dd, J = 5.6, 12.8 Hz, Glc (I) H-6α), 4.49 (1H, dd, J = 5.6, 10.0 Hz, Glc (I) H-5α), 4.05 (1H, dd, J = 6.4, 10.0, Glc (II) H-5β), 3.74–3.70 (2H, m, Glc (I) H-6α, Glc (II) H-6β); 13C-NMR [100 MHz, acetone-d6-D2O] δ 96.2 (Glc (II) C-1β), 91.7 (Glc (I) C-1α), 75.4 (Glc (I) C-2α), 74.9 (Glc (II) C-2β), 72.5 (Glc (I) C-4α), 72.4 (Glc (II) C-3β), 71.3 (Glc (II) C-5β), 71.0 (Glc (I) C-4α), 70.3 (Glc (I) C-3α), 68.5 (Glc (I) C-5α), 64.4 (Glc (I) C-6α), 63.5 (Glc (II) C-6β), 168.4, 168.3, 167.4, 166.2, 164.5, 163.9 (ester carbonyl-C), 194.1 (ketone-C); HR-ESI-MS: m/z 1583.1389 [M − H]−, 791.0640 [M − 2H]2− (calcd for C68H48O45 − H, 1583.148).
3.4. Bioassay for Detoxification Studies
The Al detoxification properties of the fractionated extracts and isolated ellagitannins were evaluated by a bioassay using the Al-sensitive model plant Arabidopsis thaliana, as described previously [7]. Briefly, pretreated A. thaliana seeds were germinated on culture equipment comprising glass slides and nylon mesh inside a growth chamber. Seedlings were grown hydroponically in a 2% strength-modified Molecular Genetics Research Laboratory (MGRL) medium (without phosphate ions and with 200 μM CaCl2 instead of calcium salts). After 4 d of culture, the seedlings were treated with a modified MGRL medium (pH 5.0) containing 0 or 12 μM AlCl3 and 0, 8, or 16 μM ellagitannin for 24 h [30]. To evaluate the detoxification properties of the extracts, 3 mg of each dried extract was added to 500 mL of the medium instead of the ellagitannin. Images of the root were taken with a scanner before and after treatment and expressed relative to controls (0 µM Al, 0 µM ellagitannin). The elongation of each primary root was measured using the WinRHIZO Pro image analysis software (https://regent.qc.ca/assets/winrhizo_software.html, Régent Instruments, Quebec, QC, Canada).
4. Conclusions
In summary, we isolated two undescribed ellagitannin oligomers, i.e., eucarpanin D2 (1, dimer) and eucamalin A (2, trimer), along with 14 known compounds from the leaves of E. camaldulensis, including gallotannins (7, 8 and 10), monomeric ellagitannins (4–6 and 11), and dimeric ellagitannins (3, 9, 12 and 14). Compounds 1–3 were identified via HR-ESI-MS, NMR, chemical reactions, and physicochemical data. The detailed chemical structure of 3 was elucidated for the first time. Furthermore, the ellagitannins obtained from E. camaldulensis, especially compound 2 and eugeniflorin D1 (13), demonstrated high abilities to alleviate Al toxicity. These results suggest that ellagitannins other than oenothein B (12) possess good Al detoxification properties. Therefore, further studies should focus on ellagitannins that are present not only in the leaves, but also in the roots of E. camaldulensis. Although further studies are required to achieve a better understanding of the Al detoxification mechanism of ellagitannins, these results suggest that these tannins promote the growth of E. camaldulensis in acidic soils.
Supplementary Materials
The following materials are available online: https://www.mdpi.com/article/10.3390/molecules30102216/s1, Figure S1: Separation procedure of leaves of E. camaludulensis. First time (A), second time (B). Figure S2: 1D- and 2D-NMR spectra of eucarpanin D2 (1). 1H-NMR (A), 13C-NMR (B), COSY (C), HSQC (D), and HMBC (E) spectrum. Figure S3: 1D-NMR spectra of eucamalin A (2). 1H-NMR (A), 13C-NMR (B), COSY (C), HSQC (D), and HMBC (E) spectrum. Figure S4: 1D-NMR spectra of eurobustin C (3). 1H-NMR (A), 13C-NMR (B), COSY (C), HSQC (D), and HMBC (E) spectrum.
Author Contributions
Conceptualization, H.I.; Methodology and Validation, H.U. and K.T.; Formal analysis, H.U., S.S., A.I., Y.A. and M.Y.; Investigation, H.U., T.H. and K.T.; Data curation, H.U. and Visualization, H.U.; Plant resources, K.T.; Funding acquisition, K.T.; Writing—original draft preparation, H.U.; Writing—review and editing, Y.I., K.T., T.M. and H.I.; Supervision and Project administration, H.I. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by JSPS KAKENHI Grant Number 18H02246 and 23K23656.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors are grateful to the SC–NMR Laboratory of Okayama University for the NMR measurements.
Conflicts of Interest
Authors Haruna Uemori and Toshiyuki Murakami were employed by the company Maruzen Pharmaceuticals, Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HPLC | High-performance liquid chromatography |
| HR–ESI–MS | High-resolution electrospray ionization mass spectrometry |
| NMR | Nuclear magnetic resonance |
| HHDP | Hexahydroxydiphenoyl |
| COSY | Correlation spectroscopy |
| HMBC | Heteronuclear multiple bond correlation |
| CD | Circular dichroism |
| UV | Ultraviolet |
| HSQC | Heteronuclear single quantum correlations |
| TLC | Thin layer chromatography |
References
- Hirsch, H.; Allsopp, M.H.; Canavan, S.; Cheek, M.; Geerts, S.; Geldenhuys, C.J.; Harding, G.; Hurley, B.P.; Jones, W.; Keet, J.-H.; et al. Eucalyptus camaldulensis in South Africa—Past, present, future. Trans. R. Soc. S. Afr. 2020, 75, 1–22. [Google Scholar] [CrossRef]
- Sabo, A.V.; Knezevic, P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind. Crops Prod. 2019, 132, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Kochian, L.V.; Piñeros, M.A.; Liu, J.P.; Magalhaes, J.V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Ikkaa, T.; Ogawa, T.; Lia, D.; Hiradateb, S.; Morita, A. Effect of aluminum on metabolism of organic acids and chemical forms of aluminum in root tips of Eucalyptus camaldulensis Dehnh. Phytochemistry 2013, 94, 142–147. [Google Scholar] [CrossRef]
- Marsh, K.J.; Kulheim, C.; Blomberg, S.P.; Thornhill, A.H.; Miller, J.T.; Wallis, I.R.; Nicolle, D.; Salminen, J.P.; Foley, W.J. Genus-wide variation in foliar polyphenolics in eucalypts. Phytochemistry 2017, 144, 197–207. [Google Scholar] [CrossRef]
- Sani, I.; Abdulhamid, A.; Bello, F. Eucalyptus camaldulensis: Phytochemical composition of ethanolic and aqueous ex-tracts of the leaves, stem-bark, root, fruits and seeds. J. Sci. Innov. Res. 2014, 3, 523–526. [Google Scholar] [CrossRef]
- Tahara, K.; Hashida, K.; Otsuka, Y.; Ohara, S.; Kojima, K.; Shinohara, K. Identification of a hydrolyzable tannin, oenothein B, as an aluminum-detoxifying ligand in a highly aluminum-resistant tree, Eucalyptus camaldulensis. Plant Physiol. 2014, 164, 683–693. [Google Scholar] [CrossRef]
- Yoshida, T.; Yoshimura, M.; Amakura, Y. Chemical and Biological Significance of Oenothein B and Related Ellagitannin Oligomers with Macrocyclic Structure. Molecules 2018, 23, 552. [Google Scholar] [CrossRef]
- Yoshida, T.; Hatano, T.; Ito, H. Chemistry and function of vegetable polyphenols with high molecular weights. BioFactors 2000, 13, 121–125. [Google Scholar] [CrossRef]
- Yoshida, T.; Chou, T.; Nitta, A.; Okuda, T. Tannins and related polyphenols of lythraceous plants. III. Hydrolyzable tannin oligomers with macrocyclic structures, and accompanying tannins from Woodfordia fruticosa KURZ. Chem. Pharm. Bull. 1992, 40, 2023–2030. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Ashida, M.; Yazaki, K. Tannis of Casuarina and Stachyurus species. Part 1. Structures of pedunculagin, casuarictin, strictinin, casuarinin, casuariin and stachyurin. J. Chem. Soc. Perkin Trans. 1983, 1, 1765–1772. [Google Scholar] [CrossRef]
- Wilkins, C.; Bohm, B. Ellagitannins from Tellima grandiflora. Phytochemistry 1976, 15, 211–214. [Google Scholar] [CrossRef]
- Yakubu, F.O.; Adebayo, H.A.; Dokunmu, M.T.; Zhang, Y.; Iweala, E.J.E. Cytotoxic Effects of Compounds Isolated from Ricinodendron heudelotii. Molecules 2019, 24, 145. [Google Scholar] [CrossRef]
- Wang, L.; Yin, D.; Fan, Y.; Min, T.; Yi, Y.; Wang, H. Molecular mechanism of the anti-gastric cancer activity of 1,2,3,6-tetra-O-galloyl-β-Dglucose isolated from Trapa bispinosa Roxb. Shell In Vitro. PLoS ONE 2022, 17, e0269013. [Google Scholar] [CrossRef]
- Yoshida, T.; Maruyama, T.; Nitta, A.; Okuda, T. Eucalbanins A, B and C, Monomeric and Dimeric hydrolyzable tannins from Eucalypyus alba REINW. Chem. Pharm. Bull. 1992, 40, 1750–1754. [Google Scholar] [CrossRef]
- Nonaka, G.; Nishioka, I. Tannins and Related Compounds. X. Rhubarb (2): Isolation and Structures of a Glycerol Gallate, Gallic Acid Glucoside Gallates, Galloylglucoses and Isolindleyin. Chem. Pharm. Bull. 1983, 31, 1652–1658. [Google Scholar] [CrossRef]
- Yagi, K.; Goto, K.; Nanjo, F. Identification of a Major Polyphenol and Polyphenolic Composition in Leaves of Camellia irrawadiensis. Chem. Pharm. Bull. 2009, 57, 1284–1288. [Google Scholar] [CrossRef]
- Franco, A.M.; Tocci, N.; Guella, G.; Dell’Agli, M.; Sangiovanni, E.; Perenzoni, D.; Manca, G. Myrtle seeds (Myrtus communis L.) as a rich source of the bioactive ellagitannins oenothein B and eugeniflorin D2. ACS Omega 2019, 4, 15966–15974. [Google Scholar] [CrossRef]
- Yoshida, T.; Hatano, T.; Ito, H.; Okuda, T. Highly oxidized ellagitannin and their biological activity. In Plant Pholyphenol 2; Gross, G., Hemingway, R., Yoshida, T., Branham, S., Eds.; Basic Life Sciences; Springer: Boston, MA, USA, 1999; Volume 66, pp. 127–144. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef]
- Yoshida, T.; Maruyama, Y.; Memon, M.U.; Shingu, T.; Okuda, T. Gemins D, E and F, ellagitannins from Geum japonicum. Phytochemistry 1985, 24, 1041–1046. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T.; Koga, T.; Toh, N.; Kuriyama, K. Circular dichroism of hydrolysable tannins-I ellagitannins and gallotannins. Tetrahedron Lett. 1982, 23, 3937–3940. [Google Scholar] [CrossRef]
- Ito, H.; Li, P.; Koreishi, M.; Nagatomo, A.; Nishida, N.; Yoshida, T. Ellagitannin oligomers and a neolignan from pomegranate arils and their inhibitory effects on the formation of advanced glycation end products. Food Chem. 2014, 152, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Chou, T.; Shingu, T.; Okuda, T. Oenotheins D, F and G, hydrolysable tannin dimers from Oenothera laciniata. Phytochemistry 1995, 40, 555–561. [Google Scholar] [CrossRef]
- Tian, L.; Yang, C.; Zhang, Y. Phenolic Compounds from the Fresh Leaves of Eucalyptus maideni. Helv. Chim. Acta 2010, 93, 2194–2202. [Google Scholar] [CrossRef]
- Przybylska, D.; Kucharska, Z.A.; Cybulska, I.; Sozański, T.; Piórecki, N.; Fecka, I. Cornus mas L. Stones: A Valuable by-Product as an Ellagitannin Source with High Antioxidant Potential. Molecules 2020, 25, 4646. [Google Scholar] [CrossRef]
- Abe, H.; Imai, H.; Ogura, D.; Horino, Y. Synthesis of lactonized valoneoyl group-containing ellagitannins, oenothein C and cornusiin B. Heterocycles 2020, 101, 524–535. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, R.; Gung, B.W.; Tindall, S.; Gonzalez, J.M.; Halvorson, J.J.; Hagerman, A.E. Polyphenol-aluminum complex formation: Implications for aluminum tolerance in plants. J. Agric. Food Chem. 2016, 64, 3025–3033. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, Q.; Jiang, J.; Khan, M.S. Tannin complexation with metal ions and its implication on human health, environment and industry: An overview. Int. J. Biol. Macromol. 2023, 253, 127485. [Google Scholar] [CrossRef]
- Koyama, H.; Toda, T.; Kojima, H.; Hara, T. Direct observation of root-elongation of Arabidopsis thaliana seedlings grown in hydroponic culture. Soil Sci. Plant Nutr. 1994, 41, 173–176. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).