Specialized Pro-Resolving Lipid Mediators in Pulmonary Diseases: Molecular and Therapeutic Implications
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
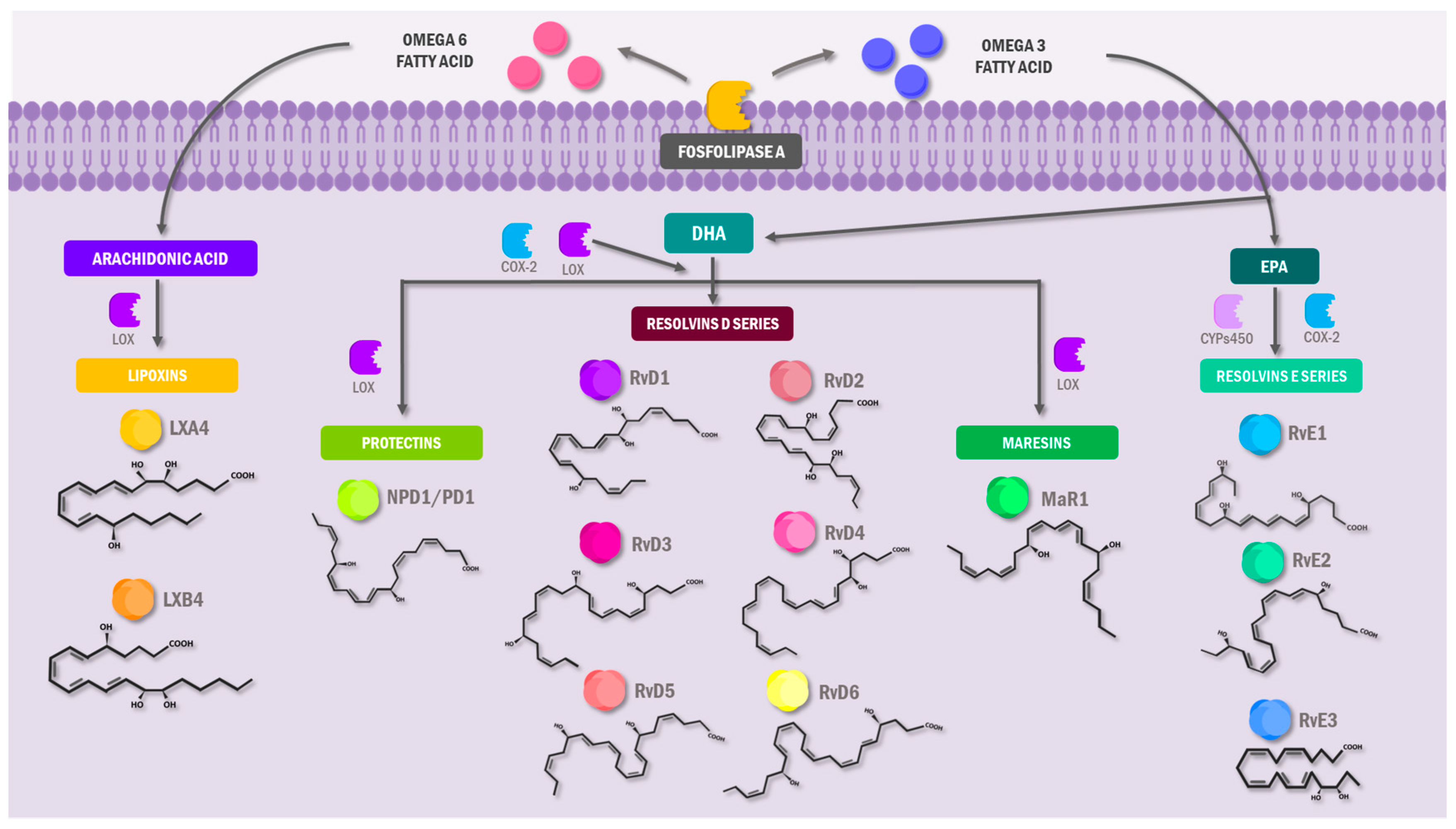
3.1. Specialized Pro-Resolving Lipid Mediators: An Overview
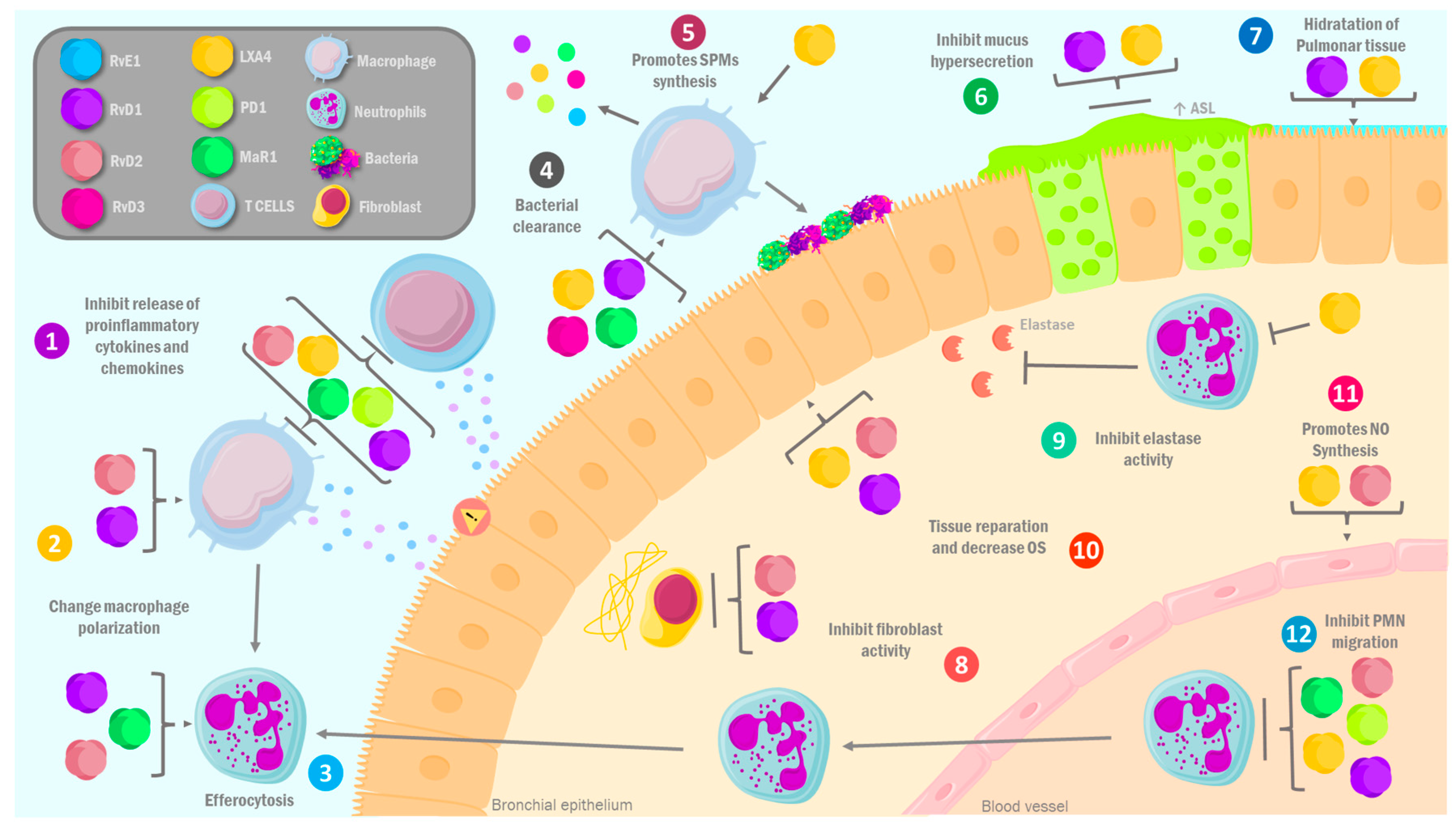
3.2. Pro-Resolving Lipid Mediators in Pulmonary Diseases: Molecular Mechanisms
3.2.1. Asthma
3.2.2. Chronic Obstructive Pulmonary Disease (COPD)
3.2.3. Cystic Fibrosis
3.2.4. COVID-19
3.3. Preclinical and Clinical Evidence of Pro-Resolving Lipid Mediators in the Management of Pulmonary Diseases
3.3.1. Asthma
3.3.2. Chronic Obstructive Pulmonary Disease
3.3.3. Cystic Fibrosis
3.3.4. COVID-19
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| ASL | Mucociliary clearing |
| CF | Cystic fibrosis |
| COX-2 | Cyclooxygenase 2 |
| COPD | Chronic obstructive pulmonary disease |
| CYPs | Cytochromes P450 |
| DHA | Docosahexaenoic acid |
| DPA | Docosapentaenoic acid |
| EPA | Eicosapentaenoic acid |
| FEV1 | Forced expiratory volume in one second |
| ILD | Inflammatory lung diseases |
| LOX | Lipoxygenases |
| LXA4 | Lipoxins A4 |
| mar-01 | Maresin 1 |
| NSAID | Non-steroidal anti-inflammatory drug |
| NO | Nitric oxide |
| NPD1 | Neuroprotectin 1 |
| OS | Oxidative stress |
| PD1 | Protectin 1 |
| PMN | Polymorphonuclear neutrophil |
| PUFAs | Polyunsaturated fatty acids |
| RvD1 | Resolvin D1 |
| RvD2 | Resolvin D2 |
| RvD3 | Resolvin D3 |
| RvD4 | Resolvin D4 |
| RvD5 | Resolvin D5 |
| RvD6 | Resolvin D6 |
| RvE1 | Resolvin E1 |
| RvE2 | Resolvin E2 |
| RvE3 | Resolvin E3 |
| ω-3 | Omega-3 |
| ω-6 | Omega-6 |
References
- Asthma. Available online: https://www.who.int/news-room/fact-sheets/detail/asthma (accessed on 18 March 2022).
- Chronic Obstructive Pulmonary Disease (COPD). Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 18 March 2022).
- Sanders, D.B.; Fink, A.K. Background and Epidemiology. Pediatr. Clin. N. Am. 2016, 63, 567–584. [Google Scholar] [CrossRef]
- COVID-19 Cases | WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 1 July 2024).
- López-Campos, J.L.; Tan, W.; Soriano, J.B. Global burden of COPD. Respirol. Carlton Vic. 2016, 21, 14–23. [Google Scholar] [CrossRef]
- Loftus, P.A.; Wise, S.K. Epidemiology and economic burden of asthma. Int. Forum Allergy Rhinol. 2015, 5 (Suppl. S1), S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.; Stargardt, T.; Schneider, U.; Schreyögg, J. The Economic Burden of Cystic Fibrosis in Germany from a Payer Perspective. PharmacoEconomics 2019, 37, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Robles, A.J.; Kornblith, L.Z.; Hendrickson, C.M.; Howard, B.M.; Conroy, A.S.; Moazed, F.; Calfee, C.S.; Cohen, M.J.; Callcut, R.A. Health care utilization and the cost of posttraumatic acute respiratory distress syndrome care. J. Trauma Acute Care Surg. 2018, 85, 148–154. [Google Scholar] [CrossRef]
- Duvall, M.G.; Levy, B.D. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur. J. Pharmacol. 2016, 785, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Philippe, R.; Urbach, V. Specialized Pro-Resolving Lipid Mediators in Cystic Fibrosis. Int. J. Mol. Sci. 2018, 19, 2865. [Google Scholar] [CrossRef]
- Robb, C.T.; Regan, K.H.; Dorward, D.A.; Rossi, A.G. Key mechanisms governing resolution of lung inflammation. Semin. Immunopathol. 2016, 38, 425–448. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, T.S.; Kim, Y.S.; Lee, J.S.; Oh, Y.M.; Lee, S.D.; Lee, S.W. Resolvin D1 prevents smoking-induced emphysema and promotes lung tissue regeneration. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 1119–1128. [Google Scholar] [CrossRef]
- Bisgaard, H.; Stokholm, J.; Chawes, B.L.; Vissing, N.H.; Bjarnadóttir, E.; Schoos, A.-M.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Thorsteinsdóttir, S.; et al. Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N. Engl. J. Med. 2016, 375, 2530–2539. [Google Scholar] [CrossRef]
- Atlantis, E.; Cochrane, B. The association of dietary intake and supplementation of specific polyunsaturated fatty acids with inflammation and functional capacity in chronic obstructive pulmonary disease: A systematic review. Int. J. Evid. Based Healthc. 2016, 14, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Keen, C.; Olin, A.C.; Eriksson, S.; Ekman, A.; Lindblad, A.; Basu, S.; Beermann, C.; Strandvik, B. Supplementation with fatty acids influences the airway nitric oxide and inflammatory markers in patients with cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Serhan, C.N. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol. Asp. Med. 2017, 58, 114–129. [Google Scholar] [CrossRef]
- Seki, H.; Fukunaga, K.; Arita, M.; Arai, H.; Nakanishi, H.; Taguchi, R.; Miyasho, T.; Takamiya, R.; Asano, K.; Ishizaka, A.; et al. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J. Immunol. Baltim. Md 1950 2010, 184, 836–843. [Google Scholar] [CrossRef]
- Aoki, H.; Hisada, T.; Ishizuka, T.; Utsugi, M.; Kawata, T.; Shimizu, Y.; Okajima, F.; Dobashi, K.; Mori, M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem. Biophys. Res. Commun. 2008, 367, 509–515. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Jia, M.R.; Sun, T. The roles of special proresolving mediators in pain relief. Rev. Neurosci. 2018, 29, 645–660. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie 2009, 91, 791–795. [Google Scholar] [CrossRef]
- Schaller, M.S.; Zahner, G.J.; Gasper, W.J.; Harris, W.S.; Conte, M.S.; Hills, N.K.; Grenon, S.M. Relationship between the omega-3 index and specialized pro-resolving lipid mediators in patients with peripheral arterial disease taking fish oil supplements. J. Clin. Lipidol. 2017, 11, 1289–1295. [Google Scholar] [CrossRef]
- Chandrasekharan, J.A.; Sharma-Walia, N. Lipoxins: Nature’s way to resolve inflammation. J. Inflamm. Res. 2015, 8, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Cianci, E.; Simiele, F.; Recchiuti, A. Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur. J. Pharmacol. 2015, 760, 49–63. [Google Scholar] [CrossRef]
- Chiang, N.; Bermudez, E.A.; Ridker, P.M.; Hurwitz, S.; Serhan, C.N. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc. Natl. Acad. Sci. USA 2004, 101, 15178–15183. [Google Scholar] [CrossRef] [PubMed]
- Poorani, R.; Bhatt, A.N.; Dwarakanath, B.S.; Das, U.N. COX-2, aspirin and metabolism of arachidonic, eicosapentaenoic and docosahexaenoic acids and their physiological and clinical significance. Eur. J. Pharmacol. 2016, 785, 116–132. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.D. Resolvins and protectins: Natural pharmacophores for resolution biology. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 327–332. [Google Scholar] [CrossRef]
- Sun, Y.P.; Oh, S.F.; Uddin, J.; Yang, R.; Gotlinger, K.; Campbell, E.; Colgan, S.P.; Petasis, N.A.; Serhan, C.N. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J. Biol. Chem. 2007, 282, 9323–9334. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Kohli, P.; Levy, B.D. Resolvins and protectins: Mediating solutions to inflammation. Br. J. Pharmacol. 2009, 158, 960–971. [Google Scholar] [CrossRef]
- Jadapalli, J.K.; Halade, G.V. Unified nexus of macrophages and maresins in cardiac reparative mechanisms. FASEB J. 2018, 32, 5227–5237. [Google Scholar] [CrossRef]
- Tang, S.; Wan, M.; Huang, W.; Stanton, R.C.; Xu, Y. Maresins: Specialized Proresolving Lipid Mediators and Their Potential Role in Inflammatory-Related Diseases. Mediat. Inflamm. 2018, 2018, 2380319. [Google Scholar] [CrossRef] [PubMed]
- Mas, E.; Croft, K.D.; Zahra, P.; Barden, A.; Mori, T.A. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin. Chem. 2012, 58, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Mims, J.W. Asthma: Definitions and pathophysiology. Int. Forum Allergy Rhinol. 2015, 5 (Suppl. S1), S2–S6. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Brüggemann, T.R.; Peh, H.Y.; Tavares, L.P.; Nijmeh, J.; Shay, A.E.; Rezende, R.M.; Lanser, T.B.; Serhan, C.N.; Levy, B.D. Eosinophil Phenotypes Are Functionally Regulated by Resolvin D2 during Allergic Lung Inflammation. Am. J. Respir. Cell Mol. Biol. 2023, 69, 666–677. [Google Scholar] [CrossRef]
- Townsend, E.A.; Guadarrama, A.; Shi, L.; Roti Roti, E.; Denlinger, L.C. P2X7 signaling influences the production of pro-resolving and pro-inflammatory lipid mediators in alveolar macrophages derived from individuals with asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 325, L399–L410. [Google Scholar] [CrossRef]
- Serhan, C.N.; Krishnamoorthy, S.; Recchiuti, A.; Chiang, N. Novel anti-inflammatory--pro-resolving mediators and their receptors. Curr. Top. Med. Chem. 2011, 11, 629–647. [Google Scholar] [CrossRef]
- Dauletbaev, N.; Lands, L.C. Could relative abundance of airway lipoxins be the clue to restore corticosteroid sensitivity in severe asthma? J. Allergy Clin. Immunol. 2016, 137, 1807–1808. [Google Scholar] [CrossRef]
- Levy, B.D.; Lukacs, N.W.; Berlin, A.A.; Schmidt, B.; Guilford, W.J.; Serhan, C.N.; Parkinson, J.F. Lipoxin A4 stable analogs reduce allergic airway responses via mechanisms distinct from CysLT1 receptor antagonism. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 3877–3884. [Google Scholar] [CrossRef]
- Hamid, Q.; Tulic, M. Immunobiology of asthma. Annu. Rev. Physiol. 2009, 71, 489–507. [Google Scholar] [CrossRef]
- Barnig, C.; Cernadas, M.; Dutile, S.; Liu, X.; Perrella, M.A.; Kazani, S.; Wechsler, M.E.; Israel, E.; Levy, B.D. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci. Transl. Med. 2013, 5, 174ra26. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Resolution phase of inflammation: Novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 2007, 25, 101–137. [Google Scholar] [CrossRef] [PubMed]
- Kytikova, O.; Novgorodtseva, T.; Denisenko, Y.; Antonyuk, M.; Gvozdenko, T. Pro-Resolving Lipid Mediators in the Pathophysiology of Asthma. Med. Kaunas Lith. 2019, 55, 284. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chanez, P.; Chavis, C. Lipoxins in asthma: Potential therapeutic mediators on bronchial inflammation? Allergy 2004, 59, 1027–1041. [Google Scholar] [CrossRef]
- O’Meara, S.J.; Rodgers, K.; Godson, C. Lipoxins: Update and impact of endogenous pro-resolution lipid mediators. Rev. Physiol. Biochem. Pharmacol. 2008, 160, 47–70. [Google Scholar] [CrossRef]
- Barnig, C.; Levy, B.D. Innate immunity is a key factor for the resolution of inflammation in asthma. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2015, 24, 141–153. [Google Scholar] [CrossRef]
- Yonetomi, Y.; Sekioka, T.; Kadode, M.; Kitamine, T.; Kamiya, A.; Matsumura, N.; Fujita, M.; Kawabata, K. Leukotriene C4 induces bronchoconstriction and airway vascular hyperpermeability via the cysteinyl leukotriene receptor 2 in S-hexyl glutathione-treated guinea pigs. Eur. J. Pharmacol. 2015, 754, 98–104. [Google Scholar] [CrossRef]
- Miyata, J.; Arita, M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2015, 64, 27–34. [Google Scholar] [CrossRef]
- Levy, B.D.; Serhan, C.N. Resolution of acute inflammation in the lung. Annu. Rev. Physiol. 2014, 76, 467–492. [Google Scholar] [CrossRef]
- Duffney, P.F.; Falsetta, M.L.; Rackow, A.R.; Thatcher, T.H.; Phipps, R.P.; Sime, P.J. Key roles for lipid mediators in the adaptive immune response. J. Clin. Investig. 2018, 128, 2724–2731. [Google Scholar] [CrossRef]
- Tungen, J.E.; Gerstmann, L.; Vik, A.; De Matteis, R.; Colas, R.A.; Dalli, J.; Chiang, N.; Serhan, C.N.; Kalesse, M.; Hansen, T.V. Resolving Inflammation: Synthesis, Configurational Assignment, and Biological Evaluations of RvD1n-3 DPA. Chem. Weinh. Bergstr. Ger. 2019, 25, 1476–1480. [Google Scholar] [CrossRef]
- Zambalde, É.P.; Teixeira, M.M.; Favarin, D.C.; de Oliveira, J.R.; Magalhães, M.L.; Cunha, M.M.; Silva, W.C.; Okuma, C.H.; Rodrigues, V.; Levy, B.D.; et al. The anti-inflammatory and pro-resolution effects of aspirin-triggered RvD1 (AT-RvD1) on peripheral blood mononuclear cells from patients with severe asthma. Int. Immunopharmacol. 2016, 35, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Ramon, S.; Thatcher, T.H.; Woeller, C.F.; Sime, P.J.; Phipps, R.P. Specialized proresolving mediators (SPMs) inhibit human B-cell IgE production. Eur. J. Immunol. 2016, 46, 81–91. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta 2015, 1851, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, N.; Burkett, P.R.; Dalli, J.; Abdulnour, R.E.E.; Colas, R.; Ramon, S.; Phipps, R.P.; Petasis, N.A.; Kuchroo, V.K.; Serhan, C.N.; et al. Cutting edge: Maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J. Immunol. Baltim. Md 1950 2015, 194, 863–867. [Google Scholar] [CrossRef]
- Lotfi, R.; Rezaiemanesh, A.; Mortazavi, S.H.; Karaji, A.G.; Salari, F. Immunoresolvents in asthma and allergic diseases: Review and update. J. Cell. Physiol. 2019, 234, 8579–8596. [Google Scholar] [CrossRef]
- Levy, B.D. Resolvin D1 and Resolvin E1 Promote the Resolution of Allergic Airway Inflammation via Shared and Distinct Molecular Counter-Regulatory Pathways. Front. Immunol. 2012, 3, 390. [Google Scholar] [CrossRef]
- Qureshi, H.; Sharafkhaneh, A.; Hanania, N.A. Chronic obstructive pulmonary disease exacerbations: Latest evidence and clinical implications. Ther. Adv. Chronic Dis. 2014, 5, 212–227. [Google Scholar] [CrossRef]
- May, S.M.; Li, J.T.C. Burden of chronic obstructive pulmonary disease: Healthcare costs and beyond. Allergy Asthma Proc. 2015, 36, 4–10. [Google Scholar] [CrossRef]
- Pauwels, R.A.; Buist, A.S.; Calverley, P.M.; Jenkins, C.R.; Hurd, S.S.; GOLD Scientific Committee Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am. J. Respir. Crit. Care Med. 2001, 163, 1256–1276. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin. Chest Med. 2014, 35, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Devine, J.F. Chronic obstructive pulmonary disease: An overview. Am. Health Drug Benefits 2008, 1, 34–42. [Google Scholar] [PubMed]
- Giudetti, A.M.; Cagnazzo, R. Beneficial effects of n-3 PUFA on chronic airway inflammatory diseases. Prostaglandins Other Lipid Mediat. 2012, 99, 57–67. [Google Scholar] [CrossRef]
- Vestbo, J.; Hurd, S.S.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef]
- Brusselle, G.G.; Joos, G.F.; Bracke, K.R. New insights into the immunology of chronic obstructive pulmonary disease. Lancet Lond. Engl. 2011, 378, 1015–1026. [Google Scholar] [CrossRef]
- Joo, M.J.; Au, D.H.; Fitzgibbon, M.L.; Lee, T.A. Inhaled corticosteroids and risk of pneumonia in newly diagnosed COPD. Respir. Med. 2010, 104, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, G.; Ruprecht, A.; De Marco, C.; Mazza, R.; Nicolini, G.; Boffi, R. Inhaled steroid/tobacco smoke particle interactions: A new light on steroid resistance. Respir. Res. 2009, 10, 48. [Google Scholar] [CrossRef]
- Balode, L.; Strazda, G.; Jurka, N.; Kopeika, U.; Kislina, A.; Bukovskis, M.; Beinare, M.; Gardjušina, V.; Taivāns, I. Lipoxygenase-derived arachidonic acid metabolites in chronic obstructive pulmonary disease. Med. Kaunas Lith. 2012, 48, 292–298. [Google Scholar] [CrossRef]
- Hsiao, H.M.; Sapinoro, R.E.; Thatcher, T.H.; Croasdell, A.; Levy, E.P.; Fulton, R.A.; Olsen, K.C.; Pollock, S.J.; Serhan, C.N.; Phipps, R.P.; et al. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PLoS ONE 2013, 8, e58258. [Google Scholar] [CrossRef]
- Bozinovski, S.; Uddin, M.; Vlahos, R.; Thompson, M.; McQualter, J.L.; Merritt, A.-S.; Wark, P.A.B.; Hutchinson, A.; Irving, L.B.; Levy, B.D.; et al. Serum amyloid A opposes lipoxin A4 to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. USA 2012, 109, 935–940. [Google Scholar] [CrossRef]
- Groutas, W.C.; Dou, D.; Alliston, K.R. Neutrophil elastase inhibitors. Expert Opin. Ther. Pat. 2011, 21, 339–354. [Google Scholar] [CrossRef]
- Stevens, T.; Ekholm, K.; Gränse, M.; Lindahl, M.; Kozma, V.; Jungar, C.; Ottosson, T.; Falk-Håkansson, H.; Churg, A.; Wright, J.L.; et al. AZD9668: Pharmacological characterization of a novel oral inhibitor of neutrophil elastase. J. Pharmacol. Exp. Ther. 2011, 339, 313–320. [Google Scholar] [CrossRef]
- Guilherme, R.F.; Xisto, D.G.; Kunkel, S.L.; Freire-de-Lima, C.G.; Rocco, P.R.M.; Neves, J.S.; Fierro, I.M.; Canetti, C.; Benjamim, C.F. Pulmonary antifibrotic mechanisms aspirin-triggered lipoxin A(4) synthetic analog. Am. J. Respir. Cell Mol. Biol. 2013, 49, 1029–1037. [Google Scholar] [CrossRef]
- Planagumà, A.; Pfeffer, M.A.; Rubin, G.; Croze, R.; Uddin, M.; Serhan, C.N.; Levy, B.D. Lovastatin decreases acute mucosal inflammation via 15-epi-lipoxin A4. Mucosal Immunol. 2010, 3, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Croasdell, A.; Thatcher, T.H.; Kottmann, R.M.; Colas, R.A.; Dalli, J.; Serhan, C.N.; Sime, P.J.; Phipps, R.P. Resolvins attenuate inflammation and promote resolution in cigarette smoke-exposed human macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L888–L901. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.M.; Thatcher, T.H.; Colas, R.A.; Serhan, C.N.; Phipps, R.P.; Sime, P.J. Resolvin D1 Reduces Emphysema and Chronic Inflammation. Am. J. Pathol. 2015, 185, 3189–3201. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.C.; Schenker, M.B. Protection against breathing dust: Behavior over time in Californian farmers. J. Agric. Saf. Health 2008, 14, 189–203. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Dalli, J.; Jacobsson, A.; Battistini, L.; Maccarrone, M.; Serhan, C.N. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci. Transl. Med. 2016, 8, 353ra111. [Google Scholar] [CrossRef]
- Harizi, H.; Corcuff, J.B.; Gualde, N. Arachidonic-acid-derived eicosanoids: Roles in biology and immunopathology. Trends Mol. Med. 2008, 14, 461–469. [Google Scholar] [CrossRef]
- Noguera, A.; Gomez, C.; Faner, R.; Cosio, B.; González-Périz, A.; Clària, J.; Carvajal, A.; Agustí, A. An investigation of the resolution of inflammation (catabasis) in COPD. Respir. Res. 2012, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C. The noncanonical NF-κB pathway. Immunol. Rev. 2012, 246, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Yang, R.; Martinod, K.; Kasuga, K.; Pillai, P.S.; Porter, T.F.; Oh, S.F.; Spite, M. Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2009, 206, 15–23. [Google Scholar] [CrossRef]
- Corriveau, S.; Sykes, J.; Stephenson, A.L. Cystic fibrosis survival: The changing epidemiology. Curr. Opin. Pulm. Med. 2018, 24, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Naehrig, S.; Chao, C.-M.; Naehrlich, L. Cystic Fibrosis. Dtsch. Arztebl. Int. 2017, 114, 564–574. [Google Scholar] [CrossRef]
- Elborn, J.S. Cystic fibrosis. Lancet Lond. Engl. 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Calella, P.; Valerio, G.; Brodlie, M.; Donini, L.M.; Siervo, M. Cystic fibrosis, body composition, and health outcomes: A systematic review. Nutr. Burbank Los Angeles Cty. Calif. 2018, 55–56, 131–139. [Google Scholar] [CrossRef]
- Duvall, M.G.; Bruggemann, T.R.; Levy, B.D. Bronchoprotective mechanisms for specialized pro-resolving mediators in the resolution of lung inflammation. Mol. Asp. Med. 2017, 58, 44–56. [Google Scholar] [CrossRef]
- Mattoscio, D.; Evangelista, V.; De Cristofaro, R.; Recchiuti, A.; Pandolfi, A.; Di Silvestre, S.; Manarini, S.; Martelli, N.; Rocca, B.; Petrucci, G.; et al. Cystic fibrosis transmembrane conductance regulator (CFTR) expression in human platelets: Impact on mediators and mechanisms of the inflammatory response. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 3970–3980. [Google Scholar] [CrossRef]
- Ringholz, F.C.; Buchanan, P.J.; Clarke, D.T.; Millar, R.G.; McDermott, M.; Linnane, B.; Harvey, B.J.; McNally, P.; Urbach, V. Reduced 15-lipoxygenase 2 and lipoxin A4/leukotriene B4 ratio in children with cystic fibrosis. Eur. Respir. J. 2014, 44, 394–404. [Google Scholar] [CrossRef]
- Ringholz, F.C.; Higgins, G.; Hatton, A.; Sassi, A.; Moukachar, A.; Fustero-Torre, C.; Hollenhorst, M.; Sermet-Gaudelus, I.; Harvey, B.J.; McNally, P.; et al. Resolvin D1 regulates epithelial ion transport and inflammation in cystic fibrosis airways. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2018, 17, 607–615. [Google Scholar] [CrossRef]
- Higgins, G.; Fustero Torre, C.; Tyrrell, J.; McNally, P.; Harvey, B.J.; Urbach, V. Lipoxin A4 prevents tight junction disruption and delays the colonization of cystic fibrosis bronchial epithelial cells by Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L1053–L1061. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Mainprice, B.; Chanez, P.; Bousquet, J.; Urbach, V. Lipoxin A4 stimulates a cytosolic Ca2+ increase in human bronchial epithelium. J. Biol. Chem. 2003, 278, 10879–10884. [Google Scholar] [CrossRef] [PubMed]
- Verrière, V.; Higgins, G.; Al-Alawi, M.; Costello, R.W.; McNally, P.; Chiron, R.; Harvey, B.J.; Urbach, V. Lipoxin A4 stimulates calcium-activated chloride currents and increases airway surface liquid height in normal and cystic fibrosis airway epithelia. PLoS ONE 2012, 7, e37746. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Hamblett, N.; Retsch-Bogart, G.; Kloster, M.; Accurso, F.; Rosenfeld, M.; Albers, G.; Black, P.; Brown, P.; Cairns, A.; Davis, S.D.; et al. Azithromycin for Early Pseudomonas Infection in Cystic Fibrosis. The OPTIMIZE Randomized Trial. Am. J. Respir. Crit. Care Med. 2018, 198, 1177–1187. [Google Scholar] [CrossRef]
- Codagnone, M.; Cianci, E.; Lamolinara, A.; Mari, V.C.; Nespoli, A.; Isopi, E.; Mattoscio, D.; Arita, M.; Bragonzi, A.; Iezzi, M.; et al. Resolvin D1 enhances the resolution of lung inflammation caused by long-term Pseudomonas aeruginosa infection. Mucosal Immunol. 2018, 11, 35–49. [Google Scholar] [CrossRef]
- Karp, C.L.; Flick, L.M.; Park, K.W.; Softic, S.; Greer, T.M.; Keledjian, R.; Yang, R.; Uddin, J.; Guggino, W.B.; Atabani, S.F.; et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat. Immunol. 2004, 5, 388–392. [Google Scholar] [CrossRef]
- Recchiuti, A.; Mattoscio, D.; Isopi, E. Roles, Actions, and Therapeutic Potential of Specialized Pro-resolving Lipid Mediators for the Treatment of Inflammation in Cystic Fibrosis. Front. Pharmacol. 2019, 10, 252. [Google Scholar] [CrossRef]
- Colas, R.A.; Dalli, J.; Chiang, N.; Vlasakov, I.; Sanger, J.M.; Riley, I.R.; Serhan, C.N. Identification and Actions of the Maresin 1 Metabolome in Infectious Inflammation. J. Immunol. Baltim. Md 1950 2016, 197, 4444–4452. [Google Scholar] [CrossRef]
- Zaidi, A.K.; Singh, R.B. SARS-CoV-2 variant biology and immune evasion. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2024; Volume 202, pp. 45–66. ISBN 978-0-443-13284-1. [Google Scholar]
- Chilamakuri, R.; Agarwal, S. COVID-19: Characteristics and Therapeutics. Cells 2021, 10, 206. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Karki, R.; Kanneganti, T.-D. Innate immunity, cytokine storm, and inflammatory cell death in COVID-19. J. Transl. Med. 2022, 20, 542. [Google Scholar] [CrossRef]
- Driscoll, D.F.; Bistrian, B.R. Cytokine storm associated with severe COVID-19 infections: The potential mitigating role of omega-3 fatty acid triglycerides in the ICU. FASEB J. 2023, 37, e23066. [Google Scholar] [CrossRef]
- Regidor, P.A.; De La Rosa, X.; Santos, F.G.; Rizo, J.M.; Gracia Banzo, R.; Silva, R.S. Acute severe SARS COVID-19 patients produce pro-resolving lipids mediators and eicosanoids. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6782–6796. [Google Scholar] [CrossRef] [PubMed]
- Ferri, G.; Mucci, M.; Mattoscio, D.; Recchiuti, A. Specialized pro-resolving lipid mediators and resolution of viral diseases. Prostaglandins Other Lipid Mediat. 2023, 168, 106762. [Google Scholar] [CrossRef]
- Chiang, N.; Serhan, C.N. Specialized pro-resolving mediator network: An update on production and actions. Essays Biochem. 2020, 64, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Pawelzik, S.C.; Arnardottir, H.; Sarajlic, P.; Mahdi, A.; Vigor, C.; Zurita, J.; Zhou, B.; Kolmert, J.; Galano, J.M.; Religa, D.; et al. Decreased oxidative stress and altered urinary oxylipidome by intravenous omega-3 fatty acid emulsion in a randomized controlled trial of older subjects hospitalized for COVID-19. Free Radic. Biol. Med. 2023, 194, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Libreros, S.; Nshimiyimana, R. E-series resolvin metabolome, biosynthesis and critical role of stereochemistry of specialized pro-resolving mediators (SPMs) in inflammation-resolution: Preparing SPMs for long COVID-19, human clinical trials, and targeted precision nutrition. Semin. Immunol. 2022, 59, 101597. [Google Scholar] [CrossRef]
- Recchiuti, A.; Patruno, S.; Mattoscio, D.; Isopi, E.; Pomilio, A.; Lamolinara, A.; Iezzi, M.; Pecce, R.; Romano, M. Resolvin D1 and D2 reduce SARS-Cov-2-induced inflammation in cystic fibrosis macrophages. bioRxiv 2020, bioRxiv 2020.08.28.255463. [Google Scholar] [CrossRef]
- Dalli, J.; Serhan, C.N. Pro-Resolving Mediators in Regulating and Conferring Macrophage Function. Front. Immunol. 2017, 8, 1400. [Google Scholar] [CrossRef]
- Navarini, L.; Vomero, M.; Currado, D.; Berardicurti, O.; Biaggi, A.; Marino, A.; Bearzi, P.; Corberi, E.; Rigon, A.; Arcarese, L.; et al. The specialized pro-resolving lipid mediator Protectin D1 affects macrophages differentiation and activity in Adult-onset Still’s disease and COVID-19, two hyperinflammatory diseases sharing similar transcriptomic profiles. Front. Immunol. 2023, 14, 1148268. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, N.; Selvaraj, H.; Lakhawat, S.S.; Datta, M.; Sharma, P.K.; Jain, A.; Khanna, R.; Srinivasan, J.; Kumar, V. Possibility of averting cytokine storm in SARS-COV 2 patients using specialized pro-resolving lipid mediators. Biochem. Pharmacol. 2023, 209, 115437. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Kuba, K.; Ichikawa, A.; Nakayama, M.; Katahira, J.; Iwamoto, R.; Watanebe, T.; Sakabe, S.; Daidoji, T.; Nakamura, S.; et al. The Lipid Mediator Protectin D1 Inhibits Influenza Virus Replication and Improves Severe Influenza. Cell 2013, 153, 112–125. [Google Scholar] [CrossRef]
- Goc, A.; Niedzwiecki, A.; Rath, M. Polyunsaturated ω-3 fatty acids inhibit ACE2-controlled SARS-CoV-2 binding and cellular entry. Sci. Rep. 2021, 11, 5207. [Google Scholar] [CrossRef]
- Balta, M.G.; Papathanasiou, E.; Christopoulos, P.F. Specialized Pro-Resolving Mediators as Potential Regulators of Inflammatory Macrophage Responses in COVID-19. Front. Immunol. 2021, 12, 632238. [Google Scholar] [CrossRef]
- Kumar, V.; Yasmeen, N.; Chaudhary, A.A.; Alawam, A.S.; Al-Zharani, M.; Suliman Basher, N.; Harikrishnan, S.; Goud, M.D.; Pandey, A.; Lakhawat, S.S.; et al. Specialized pro-resolving lipid mediators regulate inflammatory macrophages: A paradigm shift from antibiotics to immunotherapy for mitigating COVID-19 pandemic. Front. Mol. Biosci. 2023, 10, 1104577. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, C.M.; Payan, D.G.; Wong, M.Y.; Dohlman, J.G.; Blake, V.A.; Petri, M.A.; Offenberger, J.; Goetzl, E.J.; Gold, W.M. Effect of eicosapentaenoic acid in asthma. Clin. Allergy 1988, 18, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Scaglia, N.; Chatkin, J.; Chapman, K.R.; Ferreira, I.; Wagner, M.; Selby, P.; Allard, J.; Zamel, N. The relationship between omega-3 and smoking habit: A cross-sectional study. Lipids Health Dis. 2016, 15, 61. [Google Scholar] [CrossRef]
- Novgorodtseva, T.P.; Denisenko, Y.K.; Zhukova, N.V.; Antonyuk, M.V.; Knyshova, V.V.; Gvozdenko, T.A. Modification of the fatty acid composition of the erythrocyte membrane in patients with chronic respiratory diseases. Lipids Health Dis. 2013, 12, 117. [Google Scholar] [CrossRef]
- Lawrence, R.; Sorrell, T. Eicosapentaenoic acid in cystic fibrosis: Evidence of a pathogenetic role for leukotriene B4. Lancet Lond. Engl. 1993, 342, 465–469. [Google Scholar] [CrossRef]
- Doaei, S.; Gholami, S.; Rastgoo, S.; Gholamalizadeh, M.; Bourbour, F.; Bagheri, S.E.; Samipoor, F.; Akbari, M.E.; Shadnoush, M.; Ghorat, F.; et al. The effect of omega-3 fatty acid supplementation on clinical and biochemical parameters of critically ill patients with COVID-19: A randomized clinical trial. J. Transl. Med. 2021, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Mazidimoradi, A.; Alemzadeh, E.; Alemzadeh, E.; Salehiniya, H. The effect of polyunsaturated fatty acids on the severity and mortality of COVID patients: A systematic review. Life Sci. 2022, 299, 120489. [Google Scholar] [CrossRef]
- Miyata, J.; Fukunaga, K.; Iwamoto, R.; Isobe, Y.; Niimi, K.; Takamiya, R.; Takihara, T.; Tomomatsu, K.; Suzuki, Y.; Oguma, T.; et al. Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma. J. Allergy Clin. Immunol. 2013, 131, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.D.; Bonnans, C.; Silverman, E.S.; Palmer, L.J.; Marigowda, G.; Israel, E. Severe Asthma Research Program, National Heart, Lung, and Blood Institute Diminished lipoxin biosynthesis in severe asthma. Am. J. Respir. Crit. Care Med. 2005, 172, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Vachier, I.; Bonnans, C.; Chavis, C.; Farce, M.; Godard, P.; Bousquet, J.; Chanez, P. Severe asthma is associated with a loss of LX4, an endogenous anti-inflammatory compound. J. Allergy Clin. Immunol. 2005, 115, 55–60. [Google Scholar] [CrossRef]
- Kazani, S.; Planaguma, A.; Ono, E.; Bonini, M.; Zahid, M.; Marigowda, G.; Wechsler, M.E.; Levy, B.D.; Israel, E. Exhaled breath condensate eicosanoid levels associate with asthma and its severity. J. Allergy Clin. Immunol. 2013, 132, 547–553. [Google Scholar] [CrossRef]
- Fritscher, L.G.; Post, M.; Rodrigues, M.T.; Silverman, F.; Balter, M.; Chapman, K.R.; Zamel, N. Profile of eicosanoids in breath condensate in asthma and COPD. J. Breath. Res. 2012, 6, 026001. [Google Scholar] [CrossRef]
- Hasan, R.A.; O’Brien, E.; Mancuso, P. Lipoxin A(4) and 8-isoprostane in the exhaled breath condensate of children hospitalized for status asthmaticus. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 2012, 13, 141–145. [Google Scholar] [CrossRef]
- Tahan, F.; Saraymen, R.; Gumus, H. The role of lipoxin A4 in exercise-induced bronchoconstriction in asthma. J. Asthma Off. J. Assoc. Care Asthma 2008, 45, 161–164. [Google Scholar] [CrossRef]
- Sanak, M.; Levy, B.D.; Clish, C.B.; Chiang, N.; Gronert, K.; Mastalerz, L.; Serhan, C.N.; Szczeklik, A. Aspirin-tolerant asthmatics generate more lipoxins than aspirin-intolerant asthmatics. Eur. Respir. J. 2000, 16, 44–49. [Google Scholar] [CrossRef]
- Celik, G.E.; Erkekol, F.O.; Misirligil, Z.; Melli, M. Lipoxin A4 levels in asthma: Relation with disease severity and aspirin sensitivity. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2007, 37, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Higashi, N.; Mita, H.; Ono, E.; Komase, Y.; Nakagawa, T.; Miyazawa, T.; Akiyama, K.; Taniguchi, M. Urinary concentrations of 15-epimer of lipoxin A(4) are lower in patients with aspirin-intolerant compared with aspirin-tolerant asthma. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2011, 41, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.D.; De Sanctis, G.T.; Devchand, P.R.; Kim, E.; Ackerman, K.; Schmidt, B.A.; Szczeklik, W.; Drazen, J.M.; Serhan, C.N. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4). Nat. Med. 2002, 8, 1018–1023. [Google Scholar] [CrossRef]
- Karra, L.; Haworth, O.; Priluck, R.; Levy, B.D.; Levi-Schaffer, F. Lipoxin B4 promotes the resolution of allergic inflammation in the upper and lower airways of mice. Mucosal Immunol. 2015, 8, 852–862. [Google Scholar] [CrossRef]
- Aoki, H.; Hisada, T.; Ishizuka, T.; Utsugi, M.; Ono, A.; Koga, Y.; Sunaga, N.; Nakakura, T.; Okajima, F.; Dobashi, K.; et al. Protective effect of resolvin E1 on the development of asthmatic airway inflammation. Biochem. Biophys. Res. Commun. 2010, 400, 128–133. [Google Scholar] [CrossRef]
- Flesher, R.P.; Herbert, C.; Kumar, R.K. Resolvin E1 promotes resolution of inflammation in a mouse model of an acute exacerbation of allergic asthma. Clin. Sci. Lond. Engl. 1979 2014, 126, 805–814. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Haworth, O.; Croze, R.; Oh, S.F.; Uddin, M.; Carlo, T.; Pfeffer, M.A.; Priluck, R.; Serhan, C.N.; Levy, B.D. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J. Immunol. Baltim. Md 1950 2012, 189, 1983–1991. [Google Scholar] [CrossRef]
- Kim, N.; Thatcher, T.H.; Sime, P.J.; Phipps, R.P. Corticosteroids inhibit anti-IgE activities of specialized proresolving mediators on B cells from asthma patients. JCI Insight 2017, 2, e88588. [Google Scholar] [CrossRef] [PubMed]
- Haworth, O.; Cernadas, M.; Yang, R.; Serhan, C.N.; Levy, B.D. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat. Immunol. 2008, 9, 873–879. [Google Scholar] [CrossRef]
- Levy, B.D.; Kohli, P.; Gotlinger, K.; Haworth, O.; Hong, S.; Kazani, S.; Israel, E.; Haley, K.J.; Serhan, C.N. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J. Immunol. 2007, 178, 496–502. [Google Scholar] [CrossRef]
- Abdulnour, R.E.; Sham, H.P.; Douda, D.N.; Colas, R.A.; Dalli, J.; Bai, Y.; Ai, X.; Serhan, C.N.; Levy, B.D. Aspirin-triggered resolvin D1 is produced during self-resolving gram-negative bacterial pneumonia and regulates host immune responses for the resolution of lung inflammation. Mucosal Immunol. 2016, 9, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Nordgren, T.M.; Bauer, C.D.; Heires, A.J.; Poole, J.A.; Wyatt, T.A.; West, W.W.; Romberger, D.J. Maresin-1 reduces airway inflammation associated with acute and repetitive exposures to organic dust. Transl. Res. J. Lab. Clin. Med. 2015, 166, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.; Moreira, A.; Fonseca, J.; Delgado, L.; Castel-Branco, M.G.; Haahtela, T.; Lopes, C.; Moreira, P. Dietary intake of α-linolenic acid and low ratio of n-6:n-3 PUFA are associated with decreased exhaled NO and improved asthma control. Br. J. Nutr. 2011, 106, 441–450. [Google Scholar] [CrossRef]
- Broadfield, E.C.; McKeever, T.M.; Whitehurst, A.; Lewis, S.A.; Lawson, N.; Britton, J.; Fogarty, A. A case-control study of dietary and erythrocyte membrane fatty acids in asthma. Clin. Htmlent Glyphamp Asciiamp Exp. Allergy 2004, 34, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.S.; Dockery, D.W.; Neas, L.M.; Schwartz, J.; Coull, B.A.; Raizenne, M.; Speizer, F.E. Low dietary nutrient intakes and respiratory health in adolescents. Chest 2007, 132, 238–245. [Google Scholar] [CrossRef]
- Kompauer, I.; Demmelmair, H.; Koletzko, B.; Bolte, G.; Linseisen, J.; Heinrich, J. Association of fatty acids in serum phospholipids with lung function and bronchial hyperresponsiveness in adults. Eur. J. Epidemiol. 2008, 23, 175–190. [Google Scholar] [CrossRef]
- Schwartz, J.; Weiss, S.T. The relationship of dietary fish intake to level of pulmonary function in the first National Health and Nutrition Survey (NHANES I). Eur. Respir. J. 1994, 7, 1821–1824. [Google Scholar] [CrossRef]
- Mickleborough, T.D.; Lindley, M.R.; Ionescu, A.A.; Fly, A.D. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest 2006, 129, 39–49. [Google Scholar] [CrossRef]
- Mickleborough, T.D.; Murray, R.L.; Ionescu, A.A.; Lindley, M.R. Fish oil supplementation reduces severity of exercise-induced bronchoconstriction in elite athletes. Am. J. Respir. Crit. Care Med. 2003, 168, 1181–1189. [Google Scholar] [CrossRef]
- Adams, S.; Lopata, A.L.; Smuts, C.M.; Baatjies, R.; Jeebhay, M.F. Relationship between Serum Omega-3 Fatty Acid and Asthma Endpoints. Int. J. Environ. Res. Public. Health 2018, 16, 43. [Google Scholar] [CrossRef]
- Li, J.; Xun, P.; Zamora, D.; Sood, A.; Liu, K.; Daviglus, M.; Iribarren, C.; Jacobs, D.; Shikany, J.M.; He, K. Intakes of long-chain omega-3 (n-3) PUFAs and fish in relation to incidence of asthma among American young adults: The CARDIA study. Am. J. Clin. Nutr. 2013, 97, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Nagakura, T.; Matsuda, S.; Shichijyo, K.; Sugimoto, H.; Hata, K. Dietary supplementation with fish oil rich in omega-3 polyunsaturated fatty acids in children with bronchial asthma. Eur. Respir. J. 2000, 16, 861–865. [Google Scholar] [CrossRef]
- Hodge, L.; Salome, C.M.; Hughes, J.M.; Liu-Brennan, D.; Rimmer, J.; Allman, M.; Pang, D.; Armour, C.; Woolcock, A.J. Effect of dietary intake of omega-3 and omega-6 fatty acids on severity of asthma in children. Eur. Respir. J. 1998, 11, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Arm, J.P.; Horton, C.E.; Mencia-Huerta, J.M.; House, F.; Eiser, N.M.; Clark, T.J.; Spur, B.W.; Lee, T.H. Effect of dietary supplementation with fish oil lipids on mild asthma. Thorax 1988, 43, 84–92. [Google Scholar] [CrossRef]
- Arm, J.P.; Horton, C.E.; Spur, B.W.; Mencia-Huerta, J.M.; Lee, T.H. The effects of dietary supplementation with fish oil lipids on the airways response to inhaled allergen in bronchial asthma. Am. Rev. Respir. Dis. 1989, 139, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Mitsunobu, F.; Ashida, K.; Mifune, T.; Hosaki, Y.; Tsugeno, H.; Harada, S.; Tanizaki, Y. Effects of dietary supplementation with n-3 fatty acids compared with n-6 fatty acids on bronchial asthma. Intern. Med. Tokyo Jpn. 2000, 39, 107–111. [Google Scholar] [CrossRef]
- Thien, F.C.; Mencia-Huerta, J.M.; Lee, T.H. Dietary fish oil effects on seasonal hay fever and asthma in pollen-sensitive subjects. Am. Rev. Respir. Dis. 1993, 147, 1138–1143. [Google Scholar] [CrossRef]
- Stenius-Aarniala, B.; Aro, A.; Hakulinen, A.; Ahola, I.; Seppälä, E.; Vapaatalo, H. Evening primose oil and fish oil are ineffective as supplementary treatment of bronchial asthma. Ann. Allergy 1989, 62, 534–537. [Google Scholar]
- Emelyanov, A.; Fedoseev, G.; Krasnoschekova, O.; Abulimity, A.; Trendeleva, T.; Barnes, P.J. Treatment of asthma with lipid extract of New Zealand green-lipped mussel: A randomised clinical trial. Eur. Respir. J. 2002, 20, 596–600. [Google Scholar] [CrossRef]
- Dry, J.; Vincent, D. Effect of a Fish Oil Diet on Asthma: Results of a 1-Year Double-Blind Study. Int. Arch. Allergy Immunol. 1991, 95, 156–157. [Google Scholar] [CrossRef]
- Thien, F.C.; De Luca, S.; Woods, R.K.; Abramson, M.J. Cochrane Review: Dietary marine fatty acids (fish oil) for asthma in adults and children. Evid.-Based Child Health Cochrane Rev. J. 2011, 6, 984–1012. [Google Scholar] [CrossRef]
- Venter, C.; Meyer, R.W.; Nwaru, B.I.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; Akdis, C.A.; Bischoff, S.C.; et al. EAACI position paper: Influence of dietary fatty acids on asthma, food allergy, and atopic dermatitis. Allergy 2019, 74, 1429–1444. [Google Scholar] [CrossRef] [PubMed]
- Mickleborough, T.D.; Vaughn, C.L.; Shei, R.-J.; Davis, E.M.; Wilhite, D.P. Marine lipid fraction PCSO-524 (lyprinol/omega XL) of the New Zealand green lipped mussel attenuates hyperpnea-induced bronchoconstriction in asthma. Respir. Med. 2013, 107, 1152–1163. [Google Scholar] [CrossRef]
- Wood, L.G.; Hazlewood, L.C.; Foster, P.S.; Hansbro, P.M. Lyprinol reduces inflammation and improves lung function in a mouse model of allergic airways disease. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2010, 40, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Masoli, M.; Fabian, D.; Holt, S.; Beasley, R. Global Initiative for Asthma (GINA) Program The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy 2004, 59, 469–478. [Google Scholar] [CrossRef]
- Bisgaard, H.; Szefler, S. Prevalence of asthma-like symptoms in young children. Pediatr. Pulmonol. 2007, 42, 723–728. [Google Scholar] [CrossRef]
- Prescott, S.L.; Barden, A.E.; Mori, T.A.; Dunstan, J.A. Maternal fish oil supplementation in pregnancy modifies neonatal leukotriene production by cord-blood-derived neutrophils. Clin. Sci. Lond. Engl. 1979 2007, 113, 409–416. [Google Scholar] [CrossRef]
- Dunstan, J.A.; Mori, T.A.; Barden, A.; Beilin, L.J.; Taylor, A.L.; Holt, P.G.; Prescott, S.L. Maternal fish oil supplementation in pregnancy reduces interleukin-13 levels in cord blood of infants at high risk of atopy. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2003, 33, 442–448. [Google Scholar] [CrossRef]
- Krauss-Etschmann, S.; Hartl, D.; Rzehak, P.; Heinrich, J.; Shadid, R.; Del Carmen Ramírez-Tortosa, M.; Campoy, C.; Pardillo, S.; Schendel, D.J.; Decsi, T.; et al. Decreased cord blood IL-4, IL-13, and CCR4 and increased TGF-beta levels after fish oil supplementation of pregnant women. J. Allergy Clin. Immunol. 2008, 121, 464–470.e6. [Google Scholar] [CrossRef]
- Blümer, N.; Renz, H. Consumption of omega3-fatty acids during perinatal life: Role in immuno-modulation and allergy prevention. J. Perinat. Med. 2007, 35 (Suppl. S1), S12–S18. [Google Scholar] [CrossRef]
- Willers, S.M.; Devereux, G.; Craig, L.C.A.; McNeill, G.; Wijga, A.H.; Abou El-Magd, W.; Turner, S.W.; Helms, P.J.; Seaton, A. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax 2007, 62, 773–779. [Google Scholar] [CrossRef]
- Dunstan, J.A.; Mori, T.A.; Barden, A.; Beilin, L.J.; Taylor, A.L.; Holt, P.G.; Prescott, S.L. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: A randomized, controlled trial. J. Allergy Clin. Immunol. 2003, 112, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.F.; Østerdal, M.L.; Salvig, J.D.; Mortensen, L.M.; Rytter, D.; Secher, N.J.; Henriksen, T.B. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am. J. Clin. Nutr. 2008, 88, 167–175. [Google Scholar] [CrossRef]
- Palmer, D.J.; Sullivan, T.; Gold, M.S.; Prescott, S.L.; Heddle, R.; Gibson, R.A.; Makrides, M. Effect of n-3 long chain polyunsaturated fatty acid supplementation in pregnancy on infants’ allergies in first year of life: Randomised controlled trial. BMJ 2012, 344, e184. [Google Scholar] [CrossRef]
- Palmer, D.J.; Sullivan, T.; Gold, M.S.; Prescott, S.L.; Heddle, R.; Gibson, R.A.; Makrides, M. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy 2013, 68, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Lumia, M.; Luukkainen, P.; Tapanainen, H.; Kaila, M.; Erkkola, M.; Uusitalo, L.; Niinistö, S.; Kenward, M.G.; Ilonen, J.; Simell, O.; et al. Dietary fatty acid composition during pregnancy and the risk of asthma in the offspring. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2011, 22, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Best, K.P.; Gold, M.; Kennedy, D.; Martin, J.; Makrides, M. Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: A systematic review and meta-analysis of observational studies and randomized controlled trials. Am. J. Clin. Nutr. 2016, 103, 128–143. [Google Scholar] [CrossRef]
- Hansen, S.; Strøm, M.; Maslova, E.; Dahl, R.; Hoffmann, H.J.; Rytter, D.; Bech, B.H.; Henriksen, T.B.; Granström, C.; Halldorsson, T.I.; et al. Fish oil supplementation during pregnancy and allergic respiratory disease in the adult offspring. J. Allergy Clin. Immunol. 2017, 139, 104–111.e4. [Google Scholar] [CrossRef]
- Furuhjelm, C.; Warstedt, K.; Fagerås, M.; Fälth-Magnusson, K.; Larsson, J.; Fredriksson, M.; Duchén, K. Allergic disease in infants up to 2 years of age in relation to plasma omega-3 fatty acids and maternal fish oil supplementation in pregnancy and lactation. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2011, 22, 505–514. [Google Scholar] [CrossRef]
- Best, K.P.; Sullivan, T.; Palmer, D.; Gold, M.; Kennedy, D.J.; Martin, J.; Makrides, M. Prenatal Fish Oil Supplementation and Allergy: 6-Year Follow-up of a Randomized Controlled Trial. Pediatrics 2016, 137, e20154443. [Google Scholar] [CrossRef]
- Gunaratne, A.W.; Makrides, M.; Collins, C.T. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst. Rev. 2015, 7, CD010085. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.L.; Sharp, D.S.; Abbott, R.D.; Burchfiel, C.M.; Masaki, K.; Chyou, P.-H.; Huang, B.; Yano, K.; Curb, J.D. Fish Intake May Limit the Increase in Risk of Coronary Heart Disease Morbidity and Mortality Among Heavy Smokers: The Honolulu Heart Program. Circulation 1996, 94, 952–956. [Google Scholar] [CrossRef]
- Shahar, E.; Folsom, A.R.; Melnick, S.L.; Tockman, M.S.; Comstock, G.W.; Gennaro, V.; Higgins, M.W.; Sorlie, P.D.; Ko, W.J.; Szklo, M. Dietary n-3 polyunsaturated fatty acids and smoking-related chronic obstructive pulmonary disease. Atherosclerosis Risk in Communities Study Investigators. N. Engl. J. Med. 1994, 331, 228–233. [Google Scholar] [CrossRef]
- Leng, S.; Picchi, M.A.; Tesfaigzi, Y.; Wu, G.; Gauderman, W.J.; Xu, F.; Gilliland, F.D.; Belinsky, S.A. Dietary nutrients associated with preservation of lung function in Hispanic and non-Hispanic white smokers from New Mexico. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 3171–3181. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Attia, J.; McElduff, P.; McEvoy, M.; Gibson, P.G. Assessment of dietary fat intake and innate immune activation as risk factors for impaired lung function. Eur. J. Clin. Nutr. 2010, 64, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Haghighat, N.; Hakimrabet, M.; Tolide-ie, H. Nutritional evaluation in chronic obstructive pulmonary disease patients. Pak. J. Biol. Sci. PJBS 2012, 15, 501–505. [Google Scholar] [CrossRef]
- Broekhuizen, R.; Wouters, E.F.M.; Creutzberg, E.C.; Weling-Scheepers, C.a.P.M.; Schols, A.M.W.J. Polyunsaturated fatty acids improve exercise capacity in chronic obstructive pulmonary disease. Thorax 2005, 60, 376–382. [Google Scholar] [CrossRef]
- de Batlle, J.; Sauleda, J.; Balcells, E.; Gómez, F.P.; Méndez, M.; Rodriguez, E.; Barreiro, E.; Ferrer, J.J.; Romieu, I.; Gea, J.; et al. Association between Ω3 and Ω6 fatty acid intakes and serum inflammatory markers in COPD. J. Nutr. Biochem. 2012, 23, 817–821. [Google Scholar] [CrossRef]
- Sugawara, K.; Takahashi, H.; Kasai, C.; Kiyokawa, N.; Watanabe, T.; Fujii, S.; Kashiwagura, T.; Honma, M.; Satake, M.; Shioya, T. Effects of nutritional supplementation combined with low-intensity exercise in malnourished patients with COPD. Respir. Med. 2010, 104, 1883–1889. [Google Scholar] [CrossRef]
- Sugawara, K.; Takahashi, H.; Kashiwagura, T.; Yamada, K.; Yanagida, S.; Homma, M.; Dairiki, K.; Sasaki, H.; Kawagoshi, A.; Satake, M.; et al. Effect of anti-inflammatory supplementation with whey peptide and exercise therapy in patients with COPD. Respir. Med. 2012, 106, 1526–1534. [Google Scholar] [CrossRef]
- Lemoine, S.C.M.; Brigham, E.P.; Woo, H.; Hanson, C.K.; McCormack, M.C.; Koch, A.; Putcha, N.; Hansel, N.N. Omega-3 fatty acid intake and prevalent respiratory symptoms among U.S. adults with COPD. BMC Pulm. Med. 2019, 19, 97. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. National Institute on Minority Health and Health Disparities (NIMHD). The National Institute on Minority Health and Health Disparities Research Framework. NIMHD Research Framework. 2021. Available online: https://www.nih.gov/ (accessed on 22 August 2024).
- Shi, J.; Li, H.; Yuan, C.; Luo, M.; Wei, J.; Liu, X. Cigarette Smoke-Induced Acquired Dysfunction of Cystic Fibrosis Transmembrane Conductance Regulator in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Oxid. Med. Cell. Longev. 2018, 2018, 6567578. [Google Scholar] [CrossRef]
- Raju, S.V.; Jackson, P.L.; Courville, C.A.; McNicholas, C.M.; Sloane, P.A.; Sabbatini, G.; Tidwell, S.; Tang, L.P.; Liu, B.; Fortenberry, J.A.; et al. Cigarette Smoke Induces Systemic Defects in Cystic Fibrosis Transmembrane Conductance Regulator Function. Am. J. Respir. Crit. Care Med. 2013, 188, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Courville, C.A.; Raju, S.V.; Liu, B.; Accurso, F.J.; Dransfield, M.T.; Rowe, S.M. Recovery of Acquired Cystic Fibrosis Transmembrane Conductance Regulator Dysfunction after Smoking Cessation. Am. J. Respir. Crit. Care Med. 2015, 192, 1521–1524. [Google Scholar] [CrossRef]
- Bodas, M.; Min, T.; Vij, N. Critical role of CFTR-dependent lipid rafts in cigarette smoke-induced lung epithelial injury. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2011, 300, L811–L820. [Google Scholar] [CrossRef] [PubMed]
- Kaza, N.; Lin, V.Y.; Stanford, D.; Hussain, S.S.; Falk Libby, E.; Kim, H.; Borgonovi, M.; Conrath, K.; Mutyam, V.; Byzek, S.A.; et al. Evaluation of a novel CFTR potentiator in COPD ferrets with acquired CFTR dysfunction. Eur. Respir. J. 2022, 60, 2101581. [Google Scholar] [CrossRef]
- Brown, M.B.; Hunt, W.R.; Noe, J.E.; Rush, N.I.; Schweitzer, K.S.; Leece, T.C.; Moldobaeva, A.; Wagner, E.M.; Dudek, S.M.; Poirier, C.; et al. Loss of Cystic Fibrosis Transmembrane Conductance Regulator Impairs Lung Endothelial Cell Barrier Function and Increases Susceptibility to Microvascular Damage from Cigarette Smoke. Pulm. Circ. 2014, 4, 260–268. [Google Scholar] [CrossRef]
- Carlstedt-Duke, J.; Brönnegård, M.; Strandvik, B. Pathological regulation of arachidonic acid release in cystic fibrosis: The putative basic defect. Proc. Natl. Acad. Sci. USA 1986, 83, 9202–9206. [Google Scholar] [CrossRef]
- Farrell, P.M.; Mischler, E.H.; Engle, M.J.; Jeannette Brown, D.; Lau, S.-M. Fatty Acid Abnormalities in Cystic Fibrosis. Pediatr. Res. 1985, 19, 104–109. [Google Scholar] [CrossRef]
- Andersson, C.; Al-Turkmani, M.R.; Savaille, J.E.; Alturkmani, R.; Katrangi, W.; Cluette-Brown, J.E.; Zaman, M.M.; Laposata, M.; Freedman, S.D. Cell culture models demonstrate that CFTR dysfunction leads to defective fatty acid composition and metabolism. J. Lipid Res. 2008, 49, 1692–1700. [Google Scholar] [CrossRef]
- Guilbault, C.; De Sanctis, J.B.; Wojewodka, G.; Saeed, Z.; Lachance, C.; Skinner, T.A.A.; Vilela, R.M.; Kubow, S.; Lands, L.C.; Hajduch, M.; et al. Fenretinide Corrects Newly Found Ceramide Deficiency in Cystic Fibrosis. Am. J. Respir. Cell Mol. Biol. 2008, 38, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Guilbault, C.; Wojewodka, G.; Saeed, Z.; Hajduch, M.; Matouk, E.; De Sanctis, J.B.; Radzioch, D. Cystic Fibrosis Fatty Acid Imbalance Is Linked to Ceramide Deficiency and Corrected by Fenretinide. Am. J. Respir. Cell Mol. Biol. 2009, 41, 100–106. [Google Scholar] [CrossRef]
- Garić, D.; Dumut, D.C.; Shah, J.; De Sanctis, J.B.; Radzioch, D. The role of essential fatty acids in cystic fibrosis and normalizing effect of fenretinide. Cell. Mol. Life Sci. 2020, 77, 4255–4267. [Google Scholar] [CrossRef] [PubMed]
- Garić, D.; De Sanctis, J.B.; Dumut, D.C.; Shah, J.; Peña, M.J.; Youssef, M.; Petrof, B.J.; Kopriva, F.; Hanrahan, J.W.; Hajduch, M.; et al. Fenretinide favorably affects mucins (MUC5AC/MUC5B) and fatty acid imbalance in a manner mimicking CFTR-induced correction. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2020, 1865, 158538. [Google Scholar] [CrossRef]
- Centorame, A.; Dumut, D.C.; Youssef, M.; Ondra, M.; Kianicka, I.; Shah, J.; Paun, R.A.; Ozdian, T.; Hanrahan, J.W.; Gusev, E.; et al. Treatment With LAU-7b Complements CFTR Modulator Therapy by Improving Lung Physiology and Normalizing Lipid Imbalance Associated With CF Lung Disease. Front. Pharmacol. 2022, 13, 876842. [Google Scholar] [CrossRef]
- Rouzer, C.A.; Marnett, L.J. Cyclooxygenases: Structural and functional insights. J. Lipid Res. 2009, 50, S29–S34. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, B.; Dahlén, S.-E.; Lindgren, J.Å.; Rouzer, C.A.; Serhan, C.N. Leukotrienes and Lipoxins: Structures, Biosynthesis, and Biological Effects. Science 1987, 237, 1171–1176. [Google Scholar] [CrossRef]
- Freedman, S.D.; Blanco, P.G.; Zaman, M.M.; Shea, J.C.; Ollero, M.; Hopper, I.K.; Weed, D.A.; Gelrud, A.; Regan, M.M.; Laposata, M.; et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N. Engl. J. Med. 2004, 350, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Jeanson, L.; Guerrera, I.C.; Papon, J.-F.; Chhuon, C.; Zadigue, P.; Prulière-Escabasse, V.; Amselem, S.; Escudier, E.; Coste, A.; Edelman, A. Proteomic analysis of nasal epithelial cells from cystic fibrosis patients. PLoS ONE 2014, 9, e108671. [Google Scholar] [CrossRef]
- Eickmeier, O.; Fussbroich, D.; Mueller, K.; Serve, F.; Smaczny, C.; Zielen, S.; Schubert, R. Pro-resolving lipid mediator Resolvin D1 serves as a marker of lung disease in cystic fibrosis. PLoS ONE 2017, 12, e0171249. [Google Scholar] [CrossRef]
- Spite, M.; Norling, L.V.; Summers, L.; Yang, R.; Cooper, D.; Petasis, N.A.; Flower, R.J.; Perretti, M.; Serhan, C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 2009, 461, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Hanssens, L.; Thiébaut, I.; Lefèvre, N.; Malfroot, A.; Knoop, C.; Duchateau, J.; Casimir, G. The clinical benefits of long-term supplementation with omega-3 fatty acids in cystic fibrosis patients—A pilot study. Prostaglandins Leukot. Essent. Fat. Acids 2016, 108, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Henderson, W.R.; Astley, S.J.; McCready, M.M.; Kushmerick, P.; Casey, S.; Becker, J.W.; Ramsey, B.W. Oral absorption of omega-3 fatty acids in patients with cystic fibrosis who have pancreatic insufficiency and in healthy control subjects. J. Pediatr. 1994, 124, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Panchaud, A.; Sauty, A.; Kernen, Y.; Decosterd, L.A.; Buclin, T.; Boulat, O.; Hug, C.; Pilet, M.; Roulet, M. Biological effects of a dietary omega-3 polyunsaturated fatty acids supplementation in cystic fibrosis patients: A randomized, crossover placebo-controlled trial. Clin. Nutr. Edinb. Scotl. 2006, 25, 418–427. [Google Scholar] [CrossRef]
- Oliver, C.; Watson, H. Omega-3 fatty acids for cystic fibrosis. Cochrane Database Syst. Rev. 2016, CD002201. [Google Scholar] [CrossRef]
- Watson, H.; Stackhouse, C. Omega-3 fatty acid supplementation for cystic fibrosis. Cochrane Database Syst. Rev. 2020, 4, CD002201. [Google Scholar] [CrossRef]
- Elborn, J.S.; Horsley, A.; MacGregor, G.; Bilton, D.; Grosswald, R.; Ahuja, S.; Springman, E.B. Phase I Studies of Acebilustat: Biomarker Response and Safety in Patients with Cystic Fibrosis. Clin. Transl. Sci. 2017, 10, 28–34. [Google Scholar] [CrossRef]
- Elborn, J.S.; Ahuja, S.; Springman, E.; Mershon, J.; Grosswald, R.; Rowe, S.M. EMPIRE-CF: A phase II randomized placebo-controlled trial of once-daily, oral acebilustat in adult patients with cystic fibrosis—Study design and patient demographics. Contemp. Clin. Trials 2018, 72, 86–94. [Google Scholar] [CrossRef]
- Zurier, R.B.; Sun, Y.-P.; George, K.L.; Stebulis, J.A.; Rossetti, R.G.; Skulas, A.; Judge, E.; Serhan, C.N. Ajulemic acid, a synthetic cannabinoid, increases formation of the endogenous proresolving and anti-inflammatory eicosanoid, lipoxin A4. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 1503–1509. [Google Scholar] [CrossRef]
- Motwani, M.P.; Bennett, F.; Norris, P.C.; Maini, A.A.; George, M.J.; Newson, J.; Henderson, A.; Hobbs, A.J.; Tepper, M.; White, B.; et al. Potent Anti-Inflammatory and Pro-Resolving Effects of Anabasum in a Human Model of Self-Resolving Acute Inflammation. Clin. Pharmacol. Ther. 2018, 104, 675–686. [Google Scholar] [CrossRef]
- Springman, E.; Grosswald, R.; Philpot, E.; MacGregor, G.; Horsley, A.; Bilton, D.; Stewart, J.; Elborn, J.S. 126 A phase 1 clinical study of CTX-4430 in cystic fibrosis patients. J. Cyst. Fibros. 2015, 14, S90. [Google Scholar] [CrossRef]
- A double-blind, placebo-controlled phase 2 study in adults with cystic fibrosis of anabasum, a selective cannabinoid receptor type 2 agonist. Pediatr. Pulmonol. 2017, 52, S214–S516. [CrossRef]
- Chmiel, J.F.; Flume, P.; Downey, D.G.; Dozor, A.J.; Colombo, C.; Mazurek, H.; Sapiejka, E.; Rachel, M.; Constantine, S.; Conley, B.; et al. Safety and efficacy of lenabasum in a phase 2 randomized, placebo-controlled trial in adults with cystic fibrosis. J. Cyst. Fibros 2021, 20, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, J.F.; Elborn, J.S.; Constantine, S.; White, B. WS01.5 A Phase 2 study of the safety, pharmacokinetics, and efficacy of anabasum (JBT-101) in cystic fibrosis (CF). J. Cyst. Fibros. 2017, 16, 1–13. [Google Scholar] [CrossRef]
- Cilloniz, C.; Pantin-Jackwood, M.J.; Ni, C.; Goodman, A.G.; Peng, X.; Proll, S.C.; Carter, V.S.; Rosenzweig, E.R.; Szretter, K.J.; Katz, J.M.; et al. Lethal Dissemination of H5N1 Influenza Virus Is Associated with Dysregulation of Inflammation and Lipoxin Signaling in a Mouse Model of Infection. J. Virol. 2010, 84, 7613–7624. [Google Scholar] [CrossRef]
- Lee, C.H. Role of specialized pro-resolving lipid mediators and their receptors in virus infection: A promising therapeutic strategy for SARS-CoV-2 cytokine storm. Arch. Pharm. Res. 2021, 44, 84–98. [Google Scholar] [CrossRef]
- Louca, P.; Murray, B.; Klaser, K.; Graham, M.S.; Mazidi, M.; Leeming, E.R.; Thompson, E.; Bowyer, R.; Drew, D.A.; Nguyen, L.H.; et al. Modest effects of dietary supplements during the COVID-19 pandemic: Insights from 445 850 users of the COVID-19 Symptom Study app. BMJ Nutr. Prev. Health 2021, 4, 149–157. [Google Scholar] [CrossRef]
- Zapata, B.R.; Müller, J.M.; Vásquez, J.E.; Ravera, F.; Lago, G.; Cañón, E.; Castañeda, D.; Pradenas, M.; Ramírez-Santana, M. Omega-3 Index and Clinical Outcomes of Severe COVID-19: Preliminary Results of a Cross-Sectional Study. Int. J. Environ. Res. Public. Health 2021, 18, 7722. [Google Scholar] [CrossRef]
- Berger, A.A.; Sherburne, R.; Urits, I.; Patel, H.; Eskander, J. Icosapent Ethyl—A Successful Treatment for Symptomatic COVID-19 Infection. Cureus 2020, 12, e10211. [Google Scholar] [CrossRef]
- Hackensack Meridian Health. Feasibility Pilot Clinical Trial of Omega-3 (EPA+DHA) Supplement vs. Placebo for Post-Acute Sequelae of Coronavirus-19 (COVID-19) Recovery Among Health Care Workers. 2024. Available online: https://clinicaltrials.gov/study/NCT05121766 (accessed on 25 August 2024).
- S.L.A. Pharma AG. A Randomised, Double-blind, Placebo Controlled Study of Eicosapentaenoic Acid (EPA-FFA) Gastro-resistant Capsules to Treat Hospitalised Subjects With Confirmed SARS-CoV-2. 2021. Available online: https://clinicaltrials.gov/study/NCT04335032 (accessed on 25 August 2024).
- Hamad Medical Corporation. Omega-3 Oil Use in COVID-19 Patients in Qatar: A Randomized Controlled Trial. 2021. Available online: https://clinicaltrials.gov/study/NCT04836052 (accessed on 25 August 2024).
| Authors | ILD | Methodology | Results |
|---|---|---|---|
| Thien et al. [121] | Asthma | A meta-analysis evaluating high marine ω-3 fatty acid diet in asthma. | No beneficial effect from ω-3 fatty acid intake was observed on FEV1, peak flow rate, asthma symptoms, asthma medication use, or bronchial hyperreactivity. |
| Bisgaard et al. [13] | Asthma | A randomized, double-blind, placebo-controlled study assessed 2.4 g of ω-3 fatty acid intake on the risk of asthma in the offspring of 736 pregnant women. | There was a 30.7% statistically significant relative reduction of risk of persistent wheezing for asthma in the offspring (HR, 0.69; 95% CI, 0.49 to 0.97; p = 0.035). |
| Broekhuizen et al. [122] | COPD | A randomized, double-masked, placebo-controlled, 8-week study evaluated the effects of PUFA treatment in 80 COPD patients. | PUFA supplementation significantly improved exercise capacity (MD 9.7 W; 95% CI 2.5 to 17.0; p = 0.009) but did not affect FEV1 or inflammation. |
| Sugawara et al. [123] | COPD | EPA and DPA supplementation on lung function in COPD patients. | Supplementation of EPA (p = 0.006) and DPA (p = 0.022) was significantly associated with a slower FEV1 decline. |
| Watson et al. [124] | CF | A meta-analysis of five randomized controlled trials was conducted to evaluate the benefit of omega-3 fatty acids in CF. | One study reported reduced pulmonary exacerbations and antibiotic use when the subjects were treated with PUFAs. In another six-week study, sputum levels were reduced, and lung function and clinical status improved when taking omega-3 supplements. |
| Recchiuti et al. [98] | CF | A randomized, double-masked, placebo-controlled, phase-1 study that evaluated the effects of acebilustat (CTX-4430) in CF patients. | The treated group had a 58% and 65% reduction in elastase and sputum neutrophil counts, respectively (p < 0.05). |
| Doaei et al. [125] | COVID-19 | A randomized, double-masked clinical trial study evaluated the effects of omega-3 supplementation on 128 critically ill patients infected with COVID-19. | Patients with COVID-19 who were critically ill showed improved respiratory and renal function, leading to 1-month-higher survival rates (p < 0.05). |
| Mazidimoradi et al. [126] | COVID-19 | A systematic review evaluating PUFA administration on COVID-19. | Omega-3 supplements reduced the risk of COVID-19 by 12–21%. On the other hand, a deficiency in omega-3 has been linked to severe COVID-19 symptoms, a higher need for mechanical ventilation, hospitalization, and increased mortality. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, Á.; Duran, P.; Garrido, B.; Manzano, A.; Navarro, C.; Silva, A.; Rojas, M.; De Sanctis, J.B.; Radzioch, D.; Rivera-Porras, D.; et al. Specialized Pro-Resolving Lipid Mediators in Pulmonary Diseases: Molecular and Therapeutic Implications. Molecules 2025, 30, 2212. https://doi.org/10.3390/molecules30102212
Ortega Á, Duran P, Garrido B, Manzano A, Navarro C, Silva A, Rojas M, De Sanctis JB, Radzioch D, Rivera-Porras D, et al. Specialized Pro-Resolving Lipid Mediators in Pulmonary Diseases: Molecular and Therapeutic Implications. Molecules. 2025; 30(10):2212. https://doi.org/10.3390/molecules30102212
Chicago/Turabian StyleOrtega, Ángel, Pablo Duran, Bermary Garrido, Alexander Manzano, Carolina Navarro, Aljadis Silva, Milagros Rojas, Juan Bautista De Sanctis, Danuta Radzioch, Diego Rivera-Porras, and et al. 2025. "Specialized Pro-Resolving Lipid Mediators in Pulmonary Diseases: Molecular and Therapeutic Implications" Molecules 30, no. 10: 2212. https://doi.org/10.3390/molecules30102212
APA StyleOrtega, Á., Duran, P., Garrido, B., Manzano, A., Navarro, C., Silva, A., Rojas, M., De Sanctis, J. B., Radzioch, D., Rivera-Porras, D., Paredes, C. S., & Bermúdez, V. (2025). Specialized Pro-Resolving Lipid Mediators in Pulmonary Diseases: Molecular and Therapeutic Implications. Molecules, 30(10), 2212. https://doi.org/10.3390/molecules30102212










