Laser-Powered Homogeneous Pyrolysis (LPHP) of Lignin Dispersed into Gas Phase
Abstract
1. Introduction
2. Results and Discussion
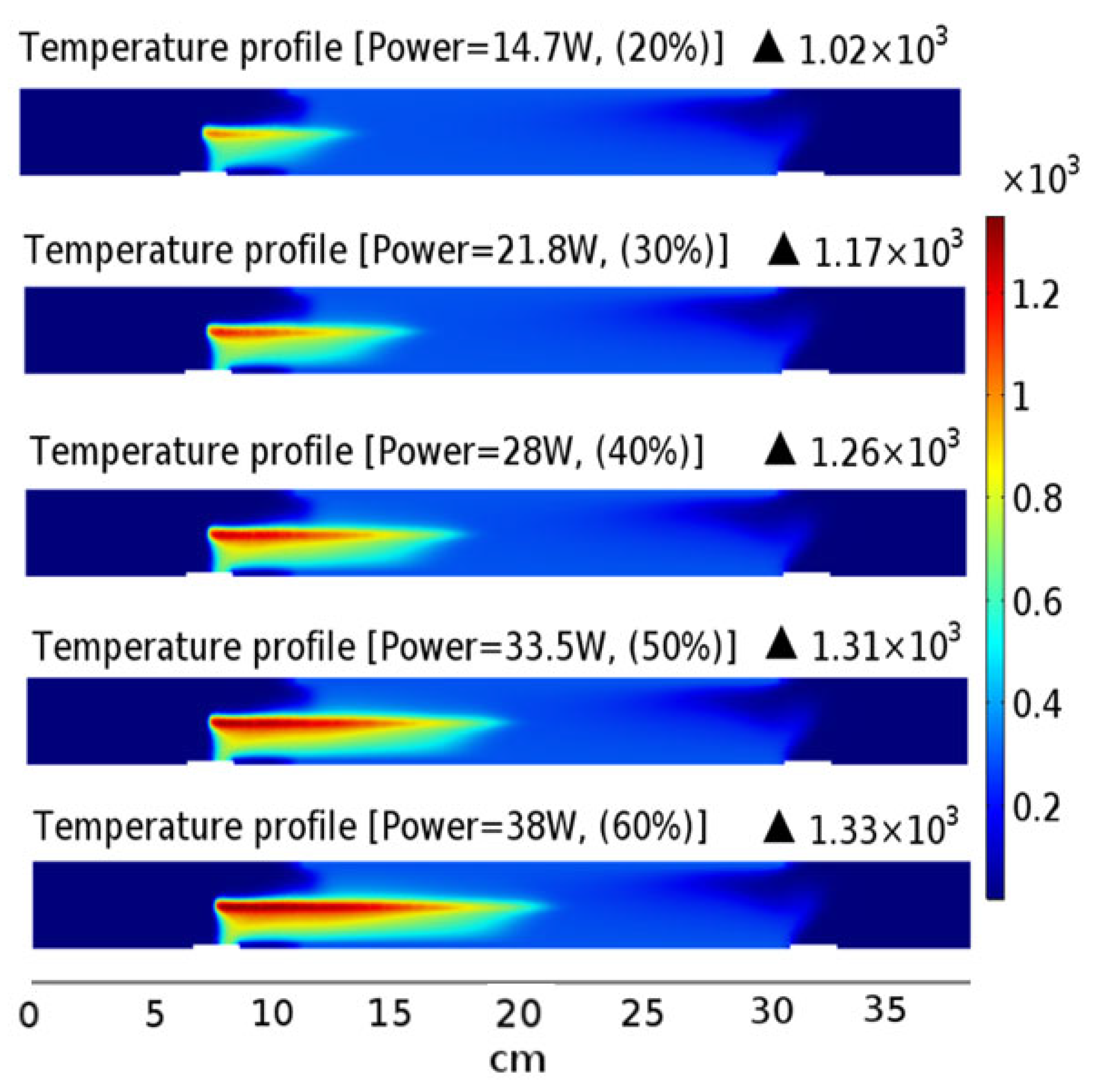
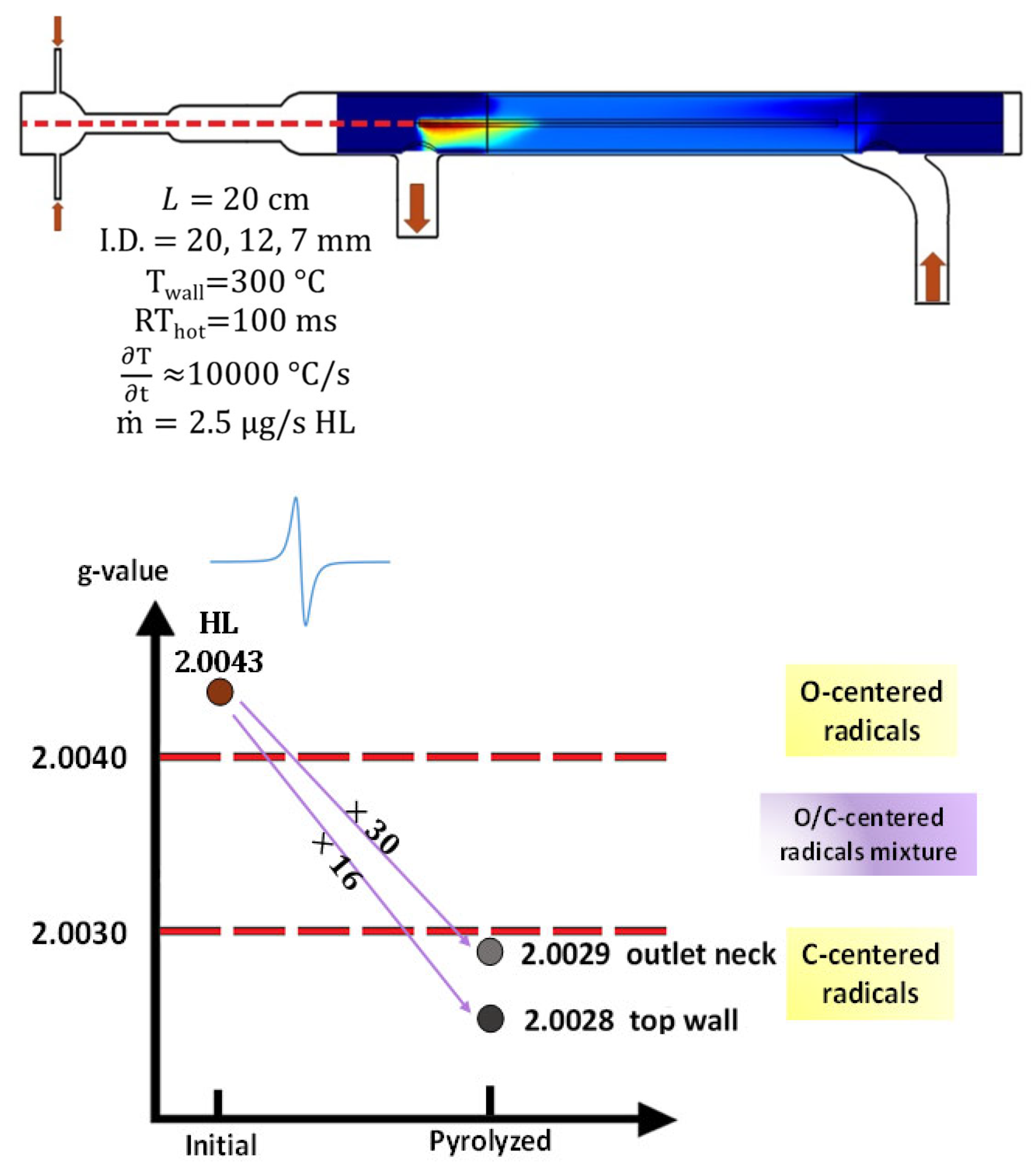
2.1. Temperature Distribution in LPHP Countercurrent Reactor
2.2. Condensable Products from Countercurrent LPHP Reactor
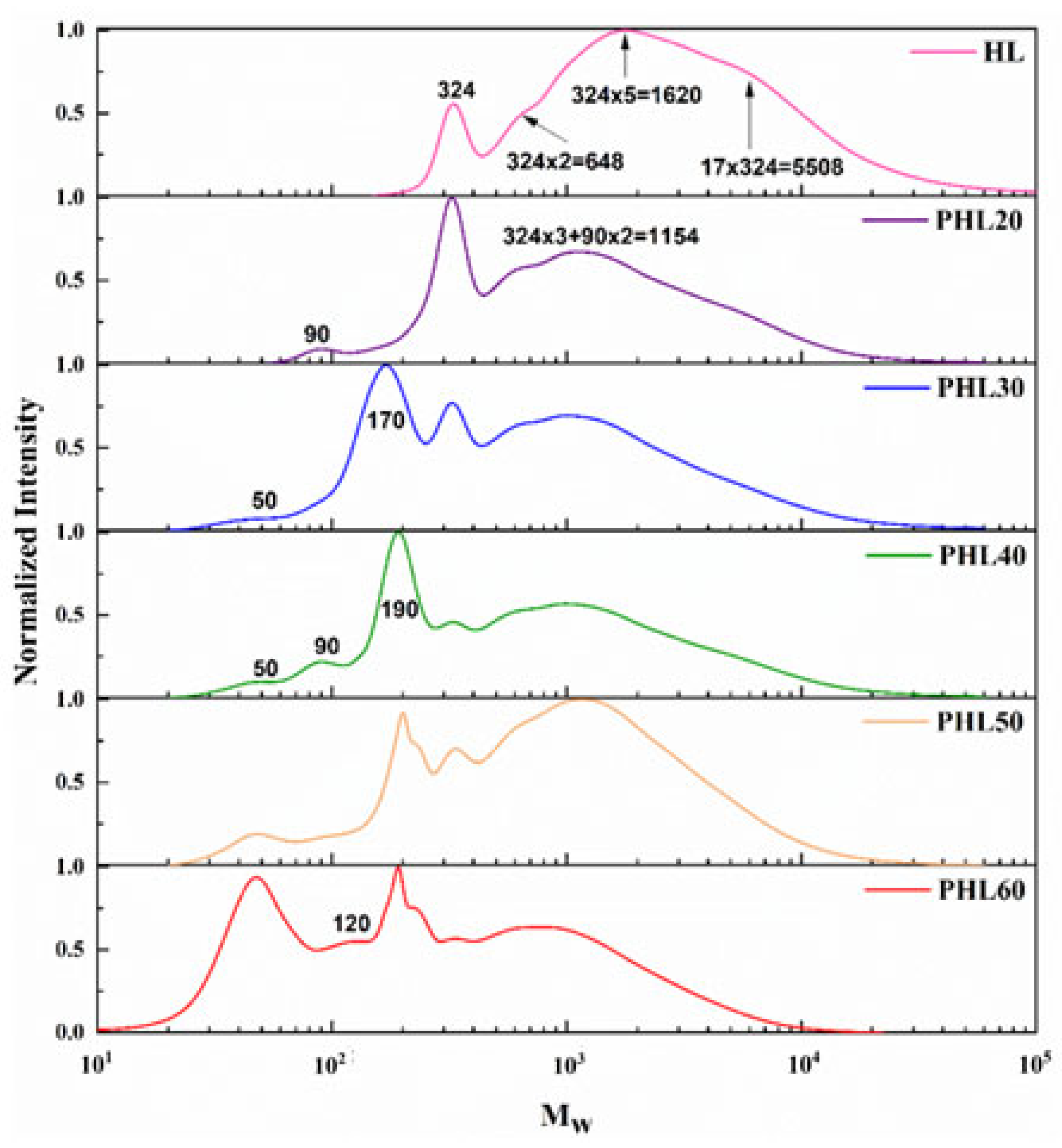
2.3. Molecular Weight Distribution of the Lignin Homogeneous Pyrolysis Products
2.3.1. GPC Analysis
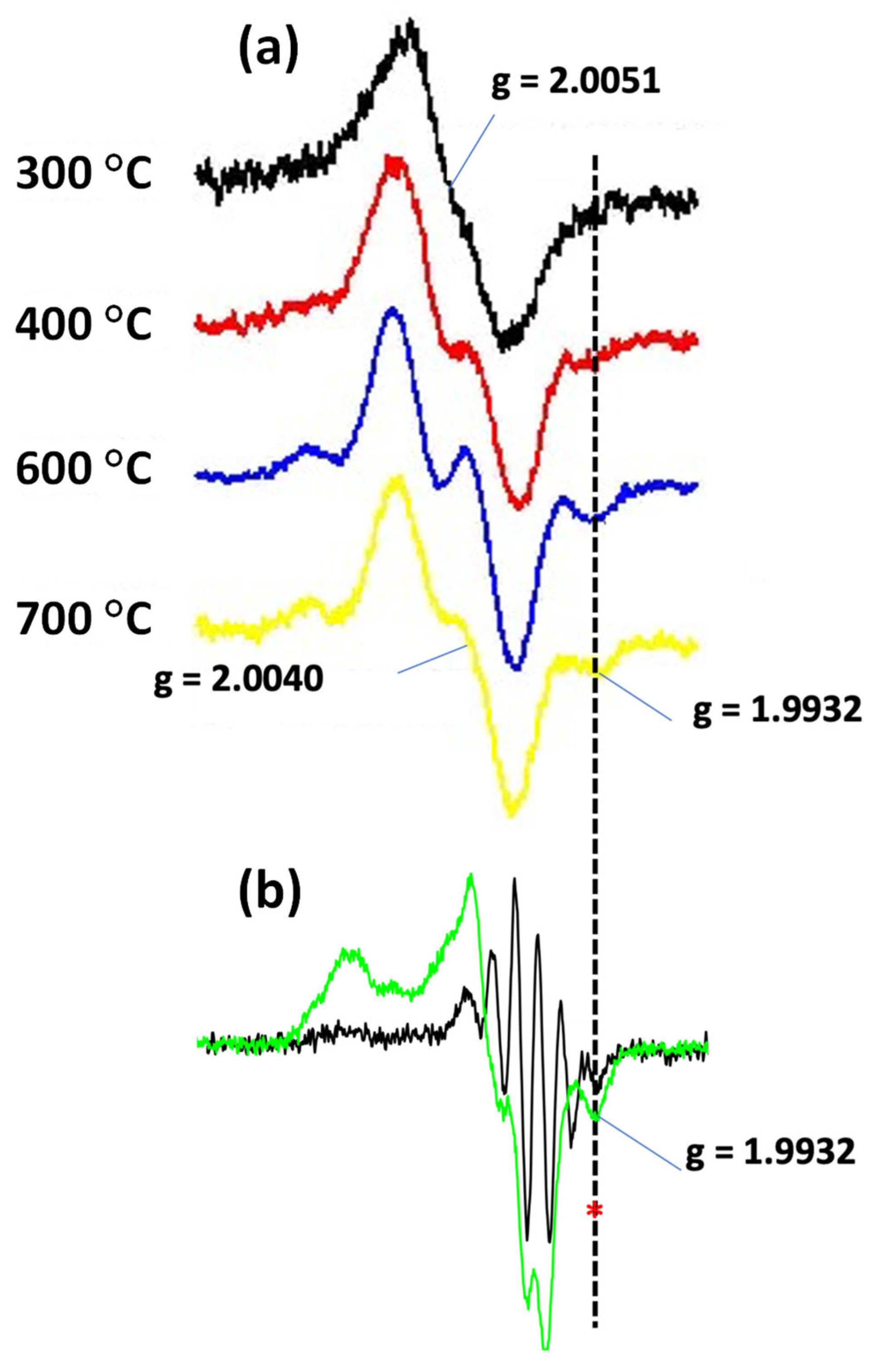
2.3.2. The Radical Character of the Decomposition of Lignin in LPHP Reactors
2.4. Formation Mechanism of PAHs from HL Gas-Phase Pyrolysis in LPHP Reactor
2.4.1. Combustion-Related Homogeneous Channels for Formation of PAHs:
2.4.2. A “Heterogenous” Mechanism for Formation of PAHs
3. Materials and Methods
3.1. Materials
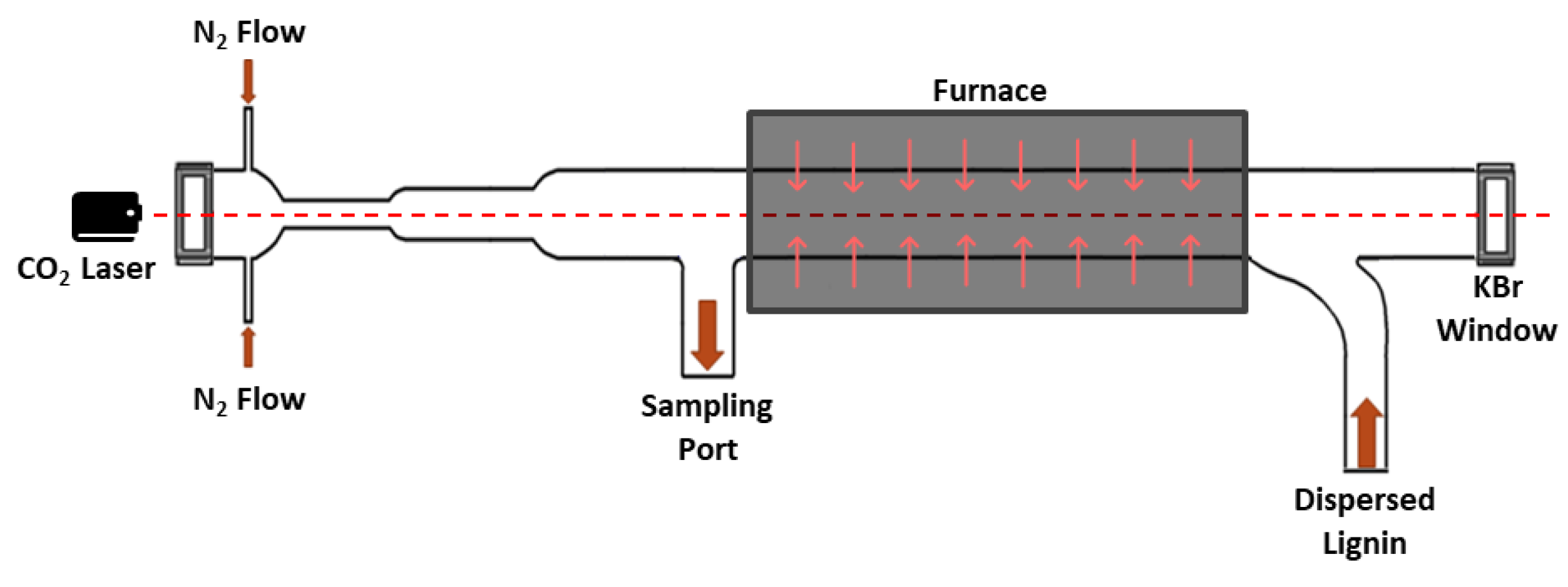
3.2. IR Laser-Powered Homogeneous Pyrolysis (LPHP) Reactor
LPHP Countercurrent Reactor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barekati-Goudarzi, M.; Boldor, D.; Marculescu, C.; Khachatryan, L. Peculiarities of Pyrolysis of Hydrolytic Lignin in Dispersed Gas Phase and in Solid State. Energy Fuels 2017, 31, 12156–12167. [Google Scholar] [CrossRef]
- Barekati-Goudarzi, M.; Boldor, D.; Khachatryan, L.; Lynn, B.; Kalinoski, R.; Shi, J. Heterogeneous and Homogeneous Components in Gas-Phase Pyrolysis of Hydrolytic Lignin. ACS Sustain. Chem. Eng. 2020, 8, 12891–12901. [Google Scholar] [CrossRef]
- Khachatryan, L.; Barekati-Goudarzi, M.; Kekejian, D.; Aguilar, G.; Asatryan, R.; Stanley, G.G.; Boldor, D. Pyrolysis of Lignin in Gas-Phase Isothermal and cw-CO2 Laser Powered Non-Isothermal Reactors. Energy Fuels 2018, 32, 12597–12606. [Google Scholar] [CrossRef]
- Kekejian, D.; Khachatryan, L.; Barekati-Goudarzi, M.; Boldor, D. Implication of COMSOL to Laser Powered Non-Isothermal Reactors for Pyrolysis in the Gas Phase. In Proceedings of the COMSOL Conference, Boston, MA, USA, 3–5 October 2018. [Google Scholar]
- Zhou, H.; Wu, C.; Onwudili, J.A.; Meng, A.; Zhang, Y.; Williams, P.T. Polycyclic Aromatic Hydrocarbon Formation from the Pyrolysis/Gasification of Lignin at Different Reaction Conditions. Energy Fuels 2014, 28, 6371–6379. [Google Scholar] [CrossRef]
- McGrath, T.; Sharma, R.; Hajaligol, M. An experimental investigation into the formation of polycyclic-aromatic hydrocarbons (PAH) from pyrolysis of biomass materials. Fuel 2001, 80, 1787–1797. [Google Scholar] [CrossRef]
- Fabbri, D.; Adamiano, A.; Torri, C. GC-MS determination of polycyclic aromatic hydrocarbons evolved from pyrolysis of biomass. Anal. Bioanal. Chem. 2010, 397, 309–317. [Google Scholar] [CrossRef]
- Kozliak, E.I.; Kubatova, A.; Artemyeva, A.A.; Nagel, E.; Zhang, C.; Rajappagowda, R.B.; Srnirnova, A.L. Thermal Liquefaction of Lignin to Aromatics: Efficiency, Selectivity, and Product Analysis. ACS Sustain. Chem. Eng. 2016, 4, 5106–5122. [Google Scholar] [CrossRef]
- Valero-Romero, M.J.; García-Mateos, F.J.; Kapteijn, F.; Rodríguez-Mirasol, J.; Cordero, T. Fischer-Tropsch synthesis over lignin-derived cobalt-containing porous carbon fiber catalysts. Appl. Catal. B-Environ. 2023, 321, 122078. [Google Scholar] [CrossRef]
- Shen, Q.; Zhong, L. Lignin-based carbon films and controllable pore size and properties. Mater. Sci. Eng. A-Struct. 2007, 445, 731–735. [Google Scholar] [CrossRef]
- Li, X.H.; You, X.Y.; Wang, X.L.; Kang, J.; Zhang, H.J. Advanced Lignin-Based Hydrogels with Superior Stiffness, Toughness, and Sensing Capabilities. Adv. Funct. Mater. 2025, 35, 2415744. [Google Scholar] [CrossRef]
- Westerholm, R.N.; Alsberg, T.E.; Frommelin, A.B.; Strandell, M.E.; Rannug, U.; Winquist, L.; Grigoriadis, V.; Egeback, K.E. Effect of Fuel Polycyclic Aromatic Hydrocarbon Content on the Emissions of Polycyclic Aromatic-Hydrocarbons and Other Mutagenic Substances from a Gasoline-Fueled Automobile. Environ. Sci. Technol. 1988, 22, 925–930. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.V.; Corrêa, S.M. Polycyclic aromatic hydrocarbons in diesel emission, diesel fuel and lubricant oil. Fuel 2016, 185, 925–931. [Google Scholar] [CrossRef]
- Qian, Y.; Yu, L.; Li, Z.L.; Zhang, Y.H.; Xu, L.L.; Zhou, Q.Y.; Han, D.; Lu, X.C. A new methodology for diesel surrogate fuel formulation: Bridging fuel fundamental properties and real engine combustion characteristics. Energy 2018, 148, 424–447. [Google Scholar] [CrossRef]
- Guibet, J.C.; Faure-Birchem, E. Fuels and Engines: Technology, Energy, Environment; Éditions Technip: Paris, France, 1999. [Google Scholar]
- Todorciuc, T.; Capraru, A.-M.; Kratochvilova, I.; Popa, V.I. Characterization of non-wood lignin and its hydroxymethylated derivatives by spectroscopy and self-assembling investigations. Surfaces 2009, 43, 399–408. [Google Scholar]
- Faix, O. Classification of Lignins from Different Botanical Origins by FT-IR Spectroscopy. Holzforschung 1991, 45, 21. [Google Scholar] [CrossRef]
- Sharma, R.K.; Wooten, J.B.; Baliga, V.L.; Lin, X.; Chan, W.G.; Hajaligol, M.R. Characterization of chars from pyrolysis of lignin. Fuel 2004, 83, 1469–1482. [Google Scholar] [CrossRef]
- Boon, J.; Bobeldijk Pastorova, I.; Botto, R.E.; Arisz, P. Structural studies on cellulose pyrolysis and cellulose chars by PYMS, PYGCMS, FTIR, NMR and by wet chemical techniques. Biomass Bioenergy 1994, 7, 25–32. [Google Scholar] [CrossRef]
- Ben, H.; Ragauskas, A.J. Comparison for the compositions of fast and slow pyrolysis oils by NMR characterization. Bioresour. Technol. 2013, 147, 577–584. [Google Scholar] [CrossRef]
- Marathe, P.S.; Westerhof, R.J.M.; Kersten, S.R.A. Fast pyrolysis of lignins with different molecular weight: Experiments and modelling. Applied Energy 2019, 236, 1125–1137. [Google Scholar] [CrossRef]
- Zhou, S.; Garcia-Perez, M.; Pecha, B.; Kersten, S.R.A.; McDonald, A.G.; Westerhof, R.J.M. Effect of the Fast Pyrolysis Temperature on the Primary and Secondary Products of Lignin. Energy Fuels 2013, 27, 5867–5877. [Google Scholar] [CrossRef]
- Wang, H. Formation of nascent soot and other condensed-phase materials in flames. Proc. Combust. Inst. 2011, 33, 41–67. [Google Scholar] [CrossRef]
- Frenklach, M.; Gardiner, W.C.; Stein, S.E.; Clary, D.W.; Yuan, T. Mechanism of Soot Formation in Acetylene-Oxygen Mixtures. Combust. Sci. 1986, 50, 79–115. [Google Scholar] [CrossRef]
- Frenklach, M.; Singh, R.I.; Mebel, A.M. On the low-temperature limit of HACA. Proc. Combust. Inst. 2019, 37, 969–976. [Google Scholar] [CrossRef]
- Saggese, C.; Frassoldati, A.; Cuoci, A.; Faravelli, T.; Ranzi, E. A wide range kinetic modeling study of pyrolysis and oxidation of benzene. Combust. Flame 2013, 160, 1168–1190. [Google Scholar] [CrossRef]
- Ledesma, E.B.; Marsh, N.D.; Sandrowitz, A.K.; Wornat, M.J. Global kinetic rate parameters for the formation of polycyclic aromatic hydrocarbons from the pyrolyis of catechol, a model compound representative of solid fuel moieties. Energy Fuels 2002, 16, 1331–1336. [Google Scholar] [CrossRef]
- Mastral, A.M.; Callen, M.S. A review on polycyclic aromatic hydrocarbon (PAH) emissions from energy generation. Environ. Sci. Technol. 2000, 34, 3051–3057. [Google Scholar] [CrossRef]
- Akazawa, M.; Kojima, Y.; Kato, Y. Formation mechanism of polycyclic compounds from phenols by fast pyrolysis. EC Agric. 2015, 1, 67–85. [Google Scholar]
- Sarofim, A.F.; Longwell, J.P.; Wornat, M.J.; Mukherjee, J. (Eds.) Soot Formation in Combustion; Springer: Berlin, Germany, 1994. [Google Scholar]
- Shukla, B.; Koshi, M. Comparative study on the growth mechanisms of PAHs. Combust Flame 2011, 158, 369–375. [Google Scholar] [CrossRef]
- Lu, M.; Mulholland, J.A. Aromatic hydrocarbon growth from indene. Chemosphere 2001, 42, 623. [Google Scholar] [CrossRef]
- Yang, B.; Hu, B.; Koylu, U.O. Mean soot volume fractions in turbulent hydrocarbon flames: A comparison of sampling and laser measurements. Combust. Sci. 2005, 177, 1603–1626. [Google Scholar] [CrossRef]
- Chu, T.C.; Smith, M.C.; Yang, J.; Liu, M.J.; Green, W.H. Theoretical study on the HACA chemistry of naphthalenyl radicals and acetylene: The formation of C12H8, C14H8, and C14H10 species. Int. J. Chem. Kinet. 2020, 52, 752–768. [Google Scholar] [CrossRef]
- Khachatryan, L.; Adounkpè, J.; Maskos, Z.; Dellinger, B. Formation of Cyclopentadienyl Radical from the Gas-Phase Pyrolysis of Hydroquinone, Catechol, and Phenol. Environ. Sci. Technol. 2006, 40, 5071–5076. [Google Scholar] [CrossRef] [PubMed]
- Adunkpe, J.; Khachatryan, L.; Dellinger, B.; Ghosh, M. Radicals from the Atmospheric Pressure Pyrolysis and Oxidative Pyrolysis of Hydroquinone, Catechol and Phenol. Energy Fuel 2009, 23, 1551–1554. [Google Scholar] [CrossRef]
- Khachatryan, L.; Xu, M.X.; Wu, A.J.; Pechagin, M.; Asatryan, R. Radicals and Molecular Products from the Gas-Phase Pyrolysis of Lignin Model Compounds. Cinnamyl Alcohol. J. Anal. Appl. Pyrolysis 2016, 121, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.X.; Khachatryan, L.; Baev, A.; Asatryan, R. Radicals from the Gas-Phase Pyrolysis of Lignin Model Compounds. p-Coumaryl Alcohol. RSC Adv. 2016, 6, 62399–62405. [Google Scholar] [CrossRef]
- Adounkpe, J.; Aina, M.; Mama, D.; Sinsin, B. Gas Chromatography Mass Spectrometry Identification of Labile Radicals Formed during Pyrolysis of Catechool, Hydroquinone, and Phenol through Neutral Pyrolysis Product Mass Analysis. Int. Sch. Res. Not. 2013, 2013, 930573. [Google Scholar] [CrossRef]
- Custodis, V.B.F.; Hemberger, P.; Ma, Z.Q.; van Bokhoven, J.A. Mechanism of Fast Pyrolysis of Lignin: Studying Model Compounds. J. Phys. Chem. B 2014, 118, 8524–8531. [Google Scholar] [CrossRef]
- Herring, P.; Khachatryan, L.; Lomnicki, S.; Dellinger, B. Paramagnetic centers in particulate formed from the oxidative pyrolysis of 1-methylnaphthalene in the presence of Fe(III)(2)O-3 nanoparticles. Combust. Flame 2013, 160, 2996–3003. [Google Scholar] [CrossRef]
- Asatryan, R.; Bennadji, H.; Bozzelli, J.W.; Ruckenstein, E.; Khachatryan, L. Molecular Products and Fundamentally Based Reaction Pathways in the Gas-Phase Pyrolysis of the Lignin Model Compound p-Coumaryl Alcohol. J. Phys. Chem. A 2017, 121, 3352–3371. [Google Scholar] [CrossRef]
- Shukla, B.; Koshi, M. A novel route for PAH growth in HACA based mechanisms. Combust Flame 2012, 159, 3589–3596. [Google Scholar] [CrossRef]
- Bockhorn, H.; Fetting, F.; Wenz, H. Investigation of the Formation of High Molecular Hydrocarbons and Soot in Premixed Hydrocarbon-Oxygen Flames. Ber. Bunsenges. Phys. Chem. 1983, 87, 1067–1073. [Google Scholar] [CrossRef]
- Frenklach, M.; Warnatz, J. Detailed modeling of PAH profiles in a sooting low-pressure acetylene flame. Combust. Sci. 1987, 51, 265–283. [Google Scholar] [CrossRef]
- Khachatryan, L.; Mascos, Z.; Dellinger, B. The Tar and Tar Radicals from Lignin Pyrolysis. Free. Radic. Biol. Med. 2014, 76, S140. [Google Scholar] [CrossRef]
- Khachatryan, L.; Adounkpe, J.; Dellinger, B. Formation of Phenoxy and cyclopentadienyl radicals from the gas-phase pyrolysis of phenol. J. Phys. Chem. A 2008, 112, 481–487. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Jiang, B.; Chen, H.; Wu, W.J.; Wu, S.F.; Jin, Y.C.; Xiao, H.N. Recent advances in understanding the effects of lignin structural characteristics on enzymatic hydrolysis. Biotechnol. Biofuels 2021, 14, 205. [Google Scholar] [CrossRef]
- Smith, E.A.; Lee, Y.J. Petroleomic Analysis of Bio-oils from the Fast Pyrolysis of Biomass: Laser Desorption Ionization–Linear Ion Trap–Orbitrap Mass Spectrometry Approach. Energy Fuels 2010, 24, 5190–5198. [Google Scholar] [CrossRef]
- Shaub, W.M.; Bauer, S.H. Laser-Powered Homogeneous Pyrolysis. Int. J. Chem. Kinet. 1975, 7, 509–529. [Google Scholar] [CrossRef]
- Kubat, P.; Pola, J. Spatial Temperature Distribution in Cw Co2-Laser Photosensitized Reactions. Collect. Czech. Chem. C. 1984, 49, 1354–1359. [Google Scholar] [CrossRef]
- Molin, Y.N.; Panfilov, V.N.; Petrov, A.K. Infrared Photochemistry; Novosibirsk Izd. Nauka: Novosibirsk, Russia, 1985. [Google Scholar]
- Sukiasyan, A.A.; Khachatryan, L.A.; Il’in, S.D. Measuring of the kinetic parameters of homogeneous decomposition of Azomethane under CO2-laser irradiation. Arm. Khim. Zhur. 1988, 41, 104. [Google Scholar]
- Russell, D.K. Infrared-Laser Powered Homogeneous Pyrolysis. Chem. Soc. Rev. 1990, 19, 407–437. [Google Scholar] [CrossRef]
- Mantashyan, A.A. Peculiarities of the slow combustion of a hydrocarbon in a “wall-less” reactor with laser heating. Combust Flame 1998, 112, 261–265. [Google Scholar] [CrossRef]
- Swihart, M.T.; Carr, R.W. Pulsed laser powered homogeneous pyrolysis for reaction kinetics studies: Probe laser measurement of reaction time and temperature. Int. J. Chem. Kinet. 1996, 28, 817–828. [Google Scholar] [CrossRef]
- Liu, E.; Das, L.; Zhao, B.; Crocker, M.; Shi, J. Impact of Dilute Sulfuric Acid, Ammonium Hydroxide, and Ionic Liquid Pretreatments on the Fractionation and Characterization of Engineered Switchgrass. Bioenergy Res. 2017, 10, 1079–1093. [Google Scholar] [CrossRef]
- Faix, O. Investigation of Lignin Polymer Models (Dhps) by Ftir Spectroscopy. Holzforschung 1986, 40, 273–280. [Google Scholar] [CrossRef]
- Yang, H.P.; Yan, R.; Chen, H.P.; Lee, D.H.; Zheng, C.G. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Clapp, R.; deFur, P.; Silbergeld, E.; Washburn, P. EPA on the right track. Environ. Sci. Technol. 1995, 29, 29–30. [Google Scholar] [CrossRef]
- Kislov, V.; Islamova, N.; Kolker, A.; Lin, S.; Mebel, A. Hydrogen Abstraction Acetylene Addition and Diels–Alder Mechanisms of PAH Formation: A Detailed Study Using First Principles Calculations. J. Chem. Theory Comput. 2005, 1, 908–924. [Google Scholar] [CrossRef]
- Hansen, N.; Schenk, M.; Moshammer, K.; Kohse-Hoinghaus, K. Investigating repetitive reaction pathways for the formation of polycyclic aromatic hydrocarbons in combustion processes. Proc. Combust. Flame 2017, 180, 250–261. [Google Scholar] [CrossRef]
- Frenklach, M. Reaction mechanism of soot formation in flames. Phys. Chem. Chem. Phys. 2002, 4, 2028–2037. [Google Scholar] [CrossRef]
- Frenklach, M.; Schuetz, C.A.; Ping, J. Migration mechanism of aromatic-edge growth. Proc. Combust. Inst. 2005, 30, 1389–1396. [Google Scholar] [CrossRef]
- Kholghy, M.R.; Eaves, N.A.; Veshkini, A.; Thomson, M.J. The role of reactive PAH dimerization in reducing soot nucleation reversibility. Proc. Combust. Inst. 2019, 37, 1003–1011. [Google Scholar] [CrossRef]
- Herdman, J.D.; Miller, J.H. Intermolecular potential calculations for polynuclear aromatic hydrocarbon clusters. J. Phys. Chem. A 2008, 112, 6249–6256. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, R.; Kollias, A.C.; Domin, D.; Lester, W.A.; Frenklach, M. Graphene layer growth: Collision of migrating five-member rings. Proc. Combust. Inst. 2007, 31, 539–546. [Google Scholar] [CrossRef]
- Cavallotti, C.; Mancarella, S.; Rota, R.; Carra, S. Conversion of C5 into C6 cyclic species through the formation of C7 intermediates. J. Phys. Chem. A 2007, 111, 3959–3969. [Google Scholar] [CrossRef]
- Agafonov, G.L.; Naydenova, I.; Vlasov, P.A.; Warnatz, J. Detailed Kinetic Modeling of Soot Formation in Shok Tube Pyrolysis and Oxidation of Toluene and N-Heptane. Proc. Combust. Inst. 2007, 31, 575–583. [Google Scholar] [CrossRef]
- Violi, A.; Izvekov, S. Soot Primary Particle Formation from Multiscale Coarse-Grained Molecular Dynamics Simulation. Proc. Combust. Inst. 2007, 31, 529–537. [Google Scholar] [CrossRef]
- Sinha, S.; Rahman, R.K.; Raj, A. On the role of resonantly stabilized radicals in polycyclic aromatic hydrocarbon (PAH) formation: Pyrene and fluoranthene formation from benzyl-indenyl addition. Phys. Chem. Chem. Phys. 2017, 19, 19262–19278. [Google Scholar] [CrossRef]
- Johansson, K.O.; Head-Gordon, M.P.; Schrader, P.E.; Wilson, K.R.; Michelsen, H.A. Resonance-stabilized hydrocarbon-radical chain reactions may explain soot inception and growth. Science 2018, 361, 997–1000. [Google Scholar] [CrossRef]
- Marinov, N.M.; Pitz, M.J.; Westbrook, C.K.; Vincitore, A.M.; Castaldy, M.J.; Senkan, S.M. Aromatic and polycyclic aromatic hydrocarbon formation in a laminar premixed n-butane flame. Combust. Flame 1998, 114, 192. [Google Scholar] [CrossRef]
- Ihm, H.; White, J.M. Stepwise Dissociation of Thermally Activated Phenol on Pt(III). J. Phys. Chem. B 2000, 104, 6202–6211. [Google Scholar] [CrossRef]
- Netzer, F.P. Low-Temperature Polymerization of Condensed Cyclopentadiene Induced by Uv Irradiation. Chem. Phys. Lett. 1988, 146, 566–569. [Google Scholar] [CrossRef]
- Welipitiya, D.; Dowben, P.A.; Zhang, J.D.; Pai, W.W.; Wendelken, J.F. The adsorption and desorption of ferrocene on Ag(100). Surf. Sci. 1996, 367, 20–32. [Google Scholar] [CrossRef]
- Pai, W.W.; Zhang, Z.Y.; Zhang, J.D.; Wendelken, J.F. Direct visualization in manipulation of stable molecular radicals at room temperature. Surf. Sci. 1997, 393, L106–L112. [Google Scholar] [CrossRef]

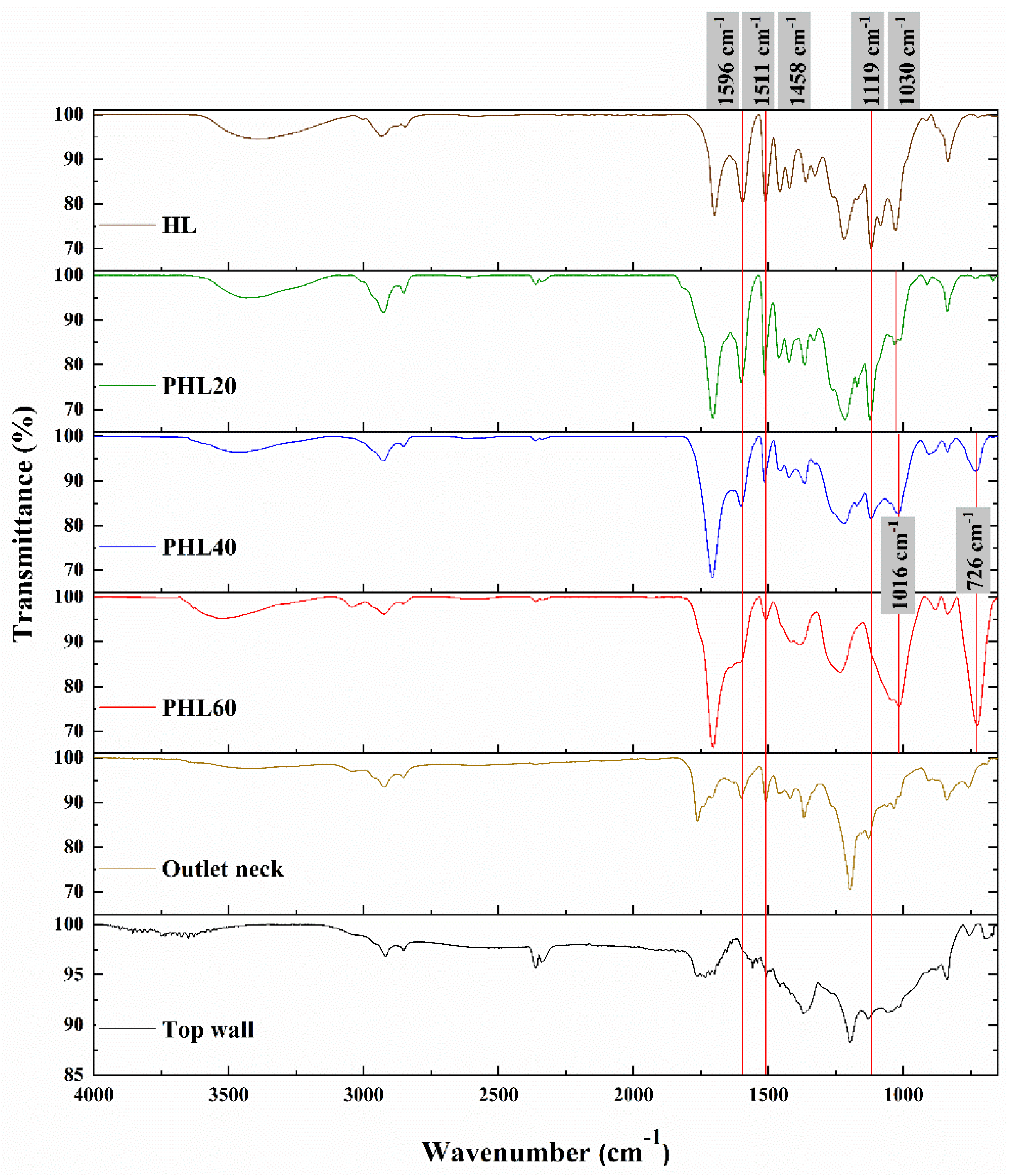

| Wavenumber (cm−1) | Peak Assignment |
|---|---|
| 3394 | O–H stretching in hydroxyl groups in phenolic and aliphatic structures [16,17] |
| 2936 | C–H stretching in methoxy, methyl, and methylene [16] |
| 2846 | C=O stretching in p-substituted aryl ketones [16] |
| 1700 | C=C stretching in aromatic ring (S) [16] |
| 1596 | C=C stretching in skeletal aromatic ring (S > G) [17,18] |
| 1511 | C=C stretching in skeletal aromatic ring (G > S) [17,18] |
| 1458 | C–H bending in methoxy and methylene [17] |
| 1422 | C–H in-plane deformation combined with skeletal aromatic ring [17] |
| 1367 | O–H in-plane bending [18];O–H in phenol, aliphatic C–H stretch in CH3 [17] |
| 1326 | C–H bending in aromatic ring (S or condensed G) [17] |
| 1220 | Aryl–O of aryl–OH and aryl–OCH3 [17] |
| 1119 | C–H bending in aromatic ring (S > condensed G) [17] |
| 1085 | C–O deformation in secondary alcohols and aliphatic ethers [17] |
| 1030 | C–O stretch in O–CH3 and C–OH [18] |
| 915 | C–H out-of-plane deformation of aromatic ring [17] |
| 834 | C–H out-of-plane in H unit and C2,6 of S unit [17] |
| 726 | C–H wags for an aromatic fused ring [18] |
| HL | PHL20 | PHL30 | PHL40 | PHL50 | PHL60 | |
|---|---|---|---|---|---|---|
| Mw (g/mol) | 5591 | 2298 | 1985 | 1947 | 1601 | 763 |
| Mn (g/mol) | 1397 | 614 | 325 | 309 | 354 | 113 |
| PDI | 4.0 | 3.7 | 6.1 | 6.3 | 5.1 | 6.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barekati-Goudarzi, M.; Khachatryan, L.; Asatryan, R.; Boldor, D.; Lynn, B.C. Laser-Powered Homogeneous Pyrolysis (LPHP) of Lignin Dispersed into Gas Phase. Molecules 2025, 30, 2215. https://doi.org/10.3390/molecules30102215
Barekati-Goudarzi M, Khachatryan L, Asatryan R, Boldor D, Lynn BC. Laser-Powered Homogeneous Pyrolysis (LPHP) of Lignin Dispersed into Gas Phase. Molecules. 2025; 30(10):2215. https://doi.org/10.3390/molecules30102215
Chicago/Turabian StyleBarekati-Goudarzi, Mohamad, Lavrent Khachatryan, Rubik Asatryan, Dorin Boldor, and Bert C. Lynn. 2025. "Laser-Powered Homogeneous Pyrolysis (LPHP) of Lignin Dispersed into Gas Phase" Molecules 30, no. 10: 2215. https://doi.org/10.3390/molecules30102215
APA StyleBarekati-Goudarzi, M., Khachatryan, L., Asatryan, R., Boldor, D., & Lynn, B. C. (2025). Laser-Powered Homogeneous Pyrolysis (LPHP) of Lignin Dispersed into Gas Phase. Molecules, 30(10), 2215. https://doi.org/10.3390/molecules30102215







