Review of Recent Advances in Thiazolidin-4-One Derivatives as Promising Antitubercular Agents (2021–Present)
Abstract
1. Introduction
2. Methodology
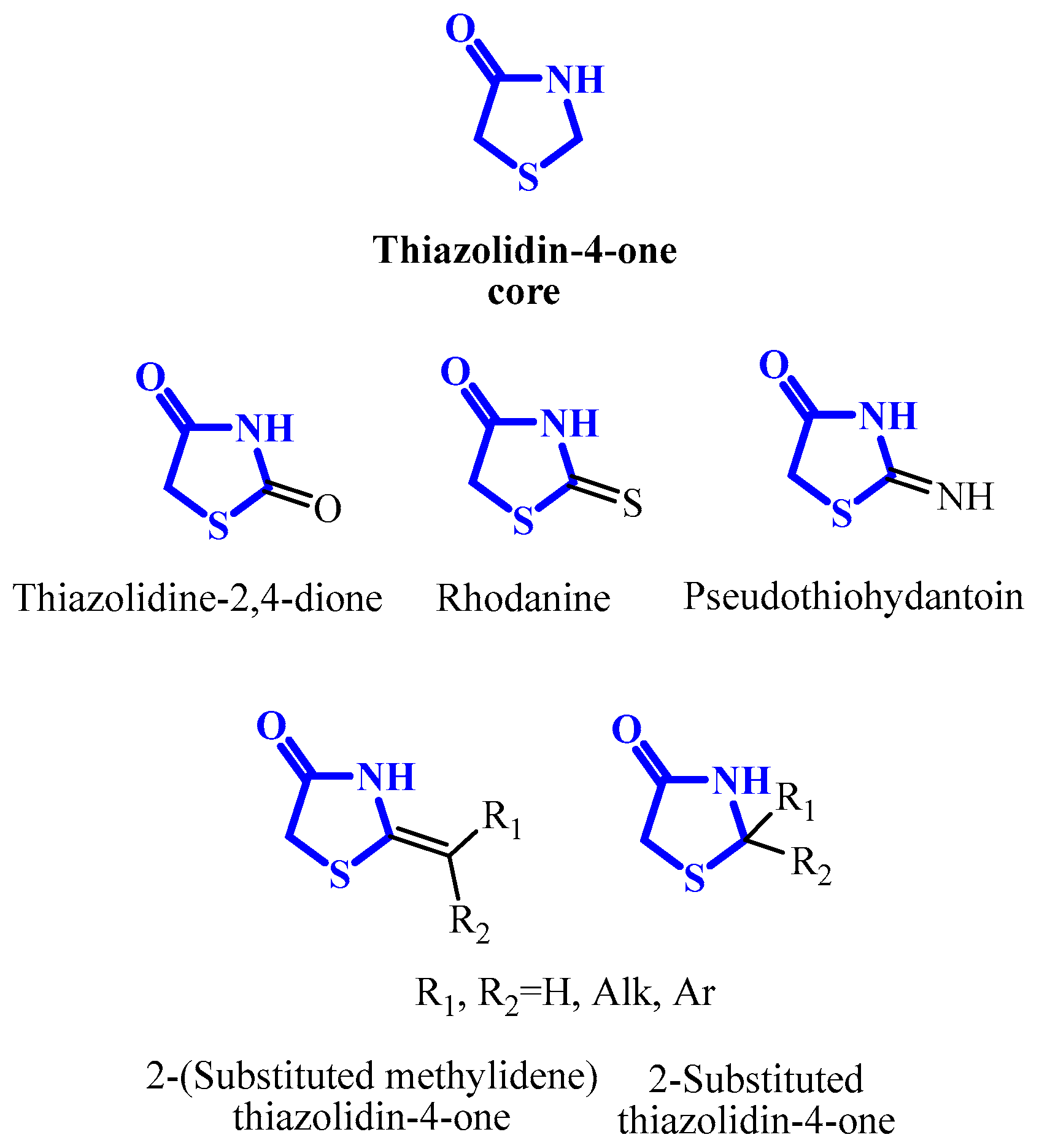
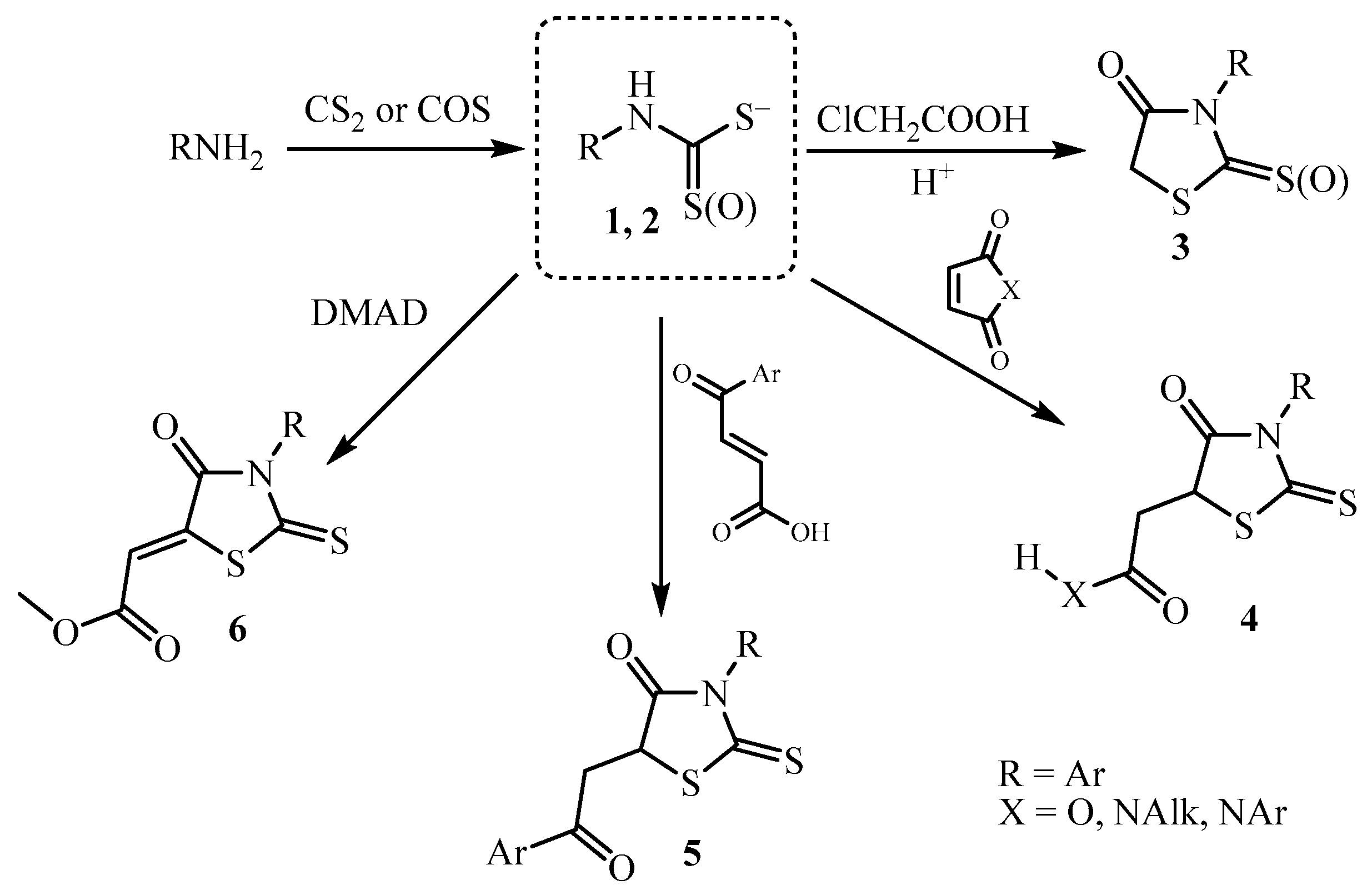
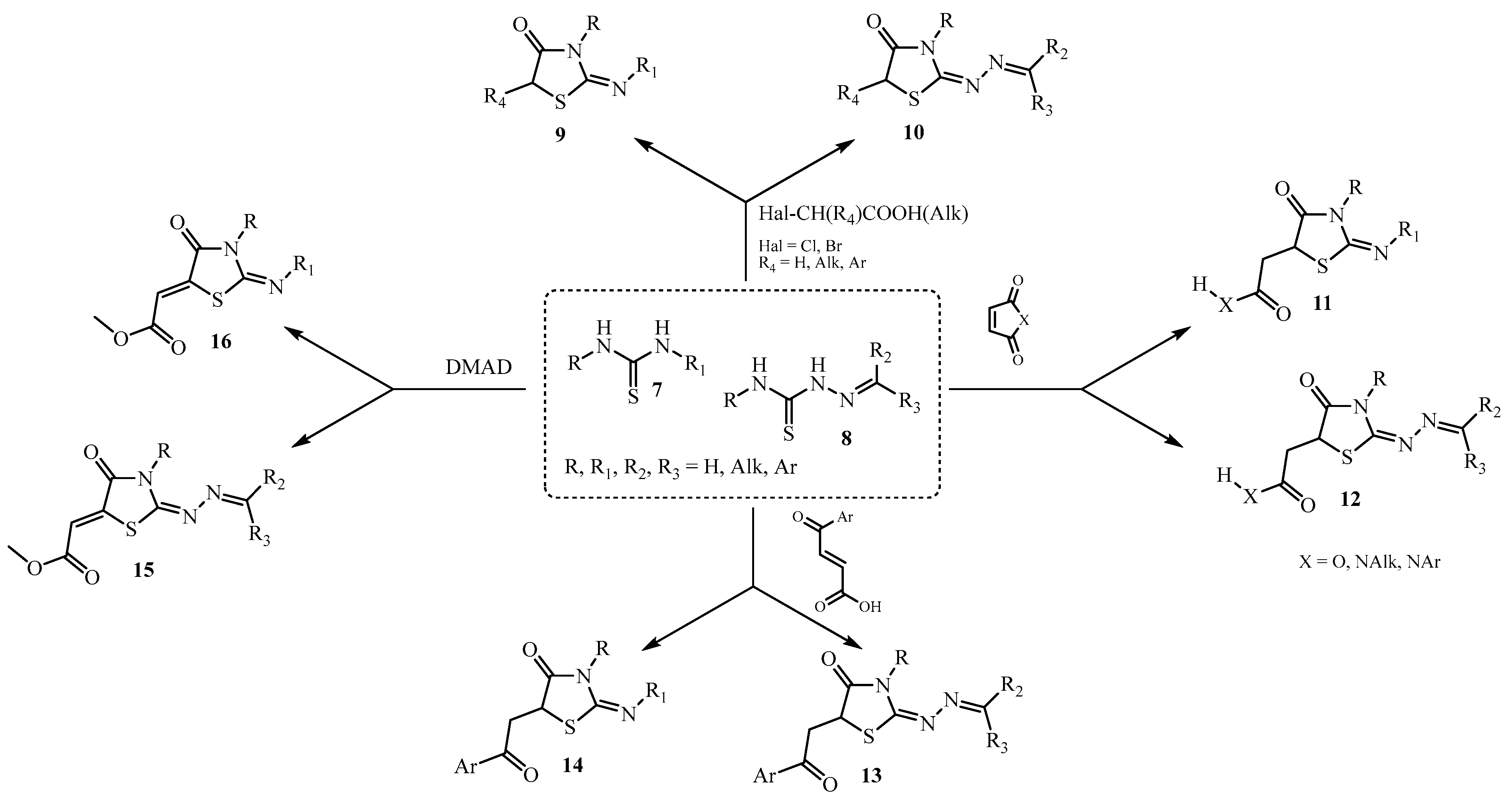
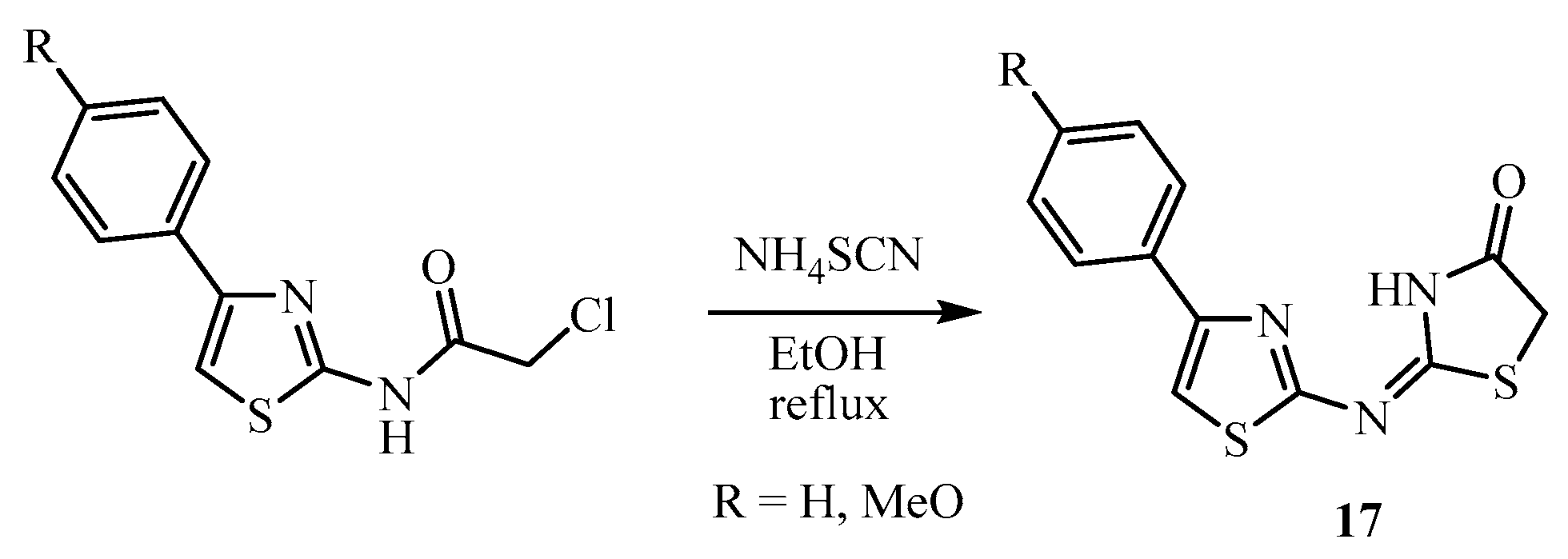
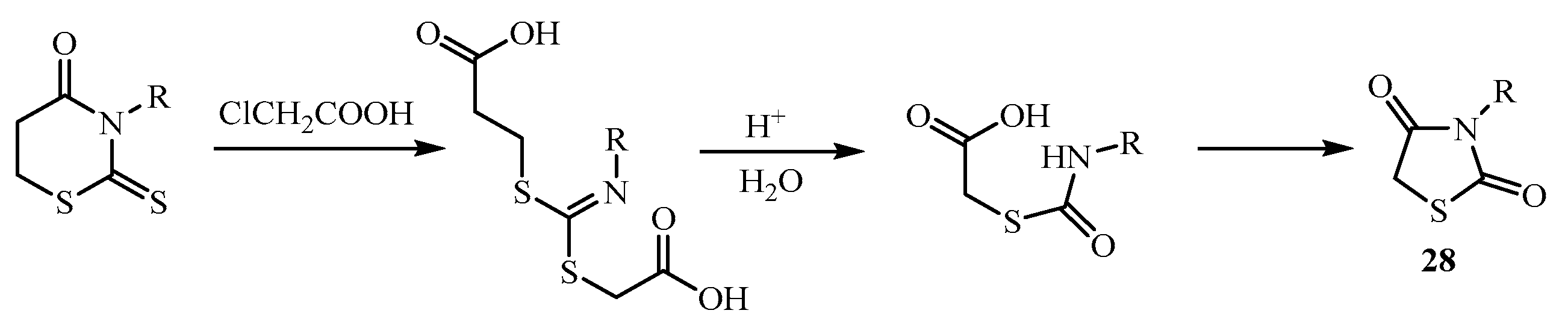
3. Main Pathways for Synthesis of Thiazolidin-4-one Ring
4. Structure–Activity Relationship (SAR)
4.1. 2-Alkyl/Arylthiazolidin-4-ones
4.2. 2-Iminothiazolidin-4-ones (Pseudothiohydantoins)
4.3. Thiazolidine-2,4-diones
4.4. 2-Thioxothiazolidin-4-ones (Rhodanines)
5. Mechanism of Action
5.1. Enoyl-Acyl Carrier Protein Reductase (InhA)
5.2. Mycobacterial Membrane Protein Large 3 (MmpL3)
5.3. DNA Gyrase
5.4. Other Promising Targets
6. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMP | adenosine monophosphate |
| ATP | adenosine triphosphate |
| CA | carbonic anhydrase |
| Dev/DosR | DevR/DosR dormancy regulator |
| DMAD | dimethyl acetylenedicarboxylate |
| DNA | deoxyribonucleic acid |
| DprE1 | decaprenylphosphoryl-β-D-ribose 2′-oxidase |
| FadD32 | fatty acid degradation protein D32 |
| FICI | fractional inhibitory concentration index |
| HadAB | (3R)-hydroxyacyl-ACP dehydratase heterodimer |
| HEK-293 | human embryonic kidney cell line |
| HIV | human immunodeficiency virus |
| IC50 | half maximal inhibitory concentration |
| InhA | enoyl-acyl carrier protein reductase |
| KasA | β-ketoacyl-acyl carrier protein synthase |
| Ki | inhibition constant |
| MDR | multidrug-resistant |
| MIC | minimum inhibitory concentration |
| MmpL3 | mycobacterial membrane protein large 3 |
| MptpA | Mycobacterium tuberculosis protein tyrosine phosphatase A |
| MptpB | Mycobacterium tuberculosis protein tyrosine phosphatase B |
| Mtb | Mycobacterium tuberculosis |
| MurB | UDP-N-acetylenolpyruvoylglucosamine reductase |
| Pks13 | polyketide synthase |
| PS | pantothenate synthetase |
| PTP1B | protein tyrosine phosphatase 1B |
| QSAR | quantitative structure–activity relationship |
| RAW 264.7 | adherent cell line isolated from a mouse tumor that was induced by Abelson murine leukemia virus |
| SAR | structure–activity relationship |
| TB | tuberculosis |
| THF | tetrahydrofuran |
| TZD | thiazolidine-2,4-dione |
| XDR | extensively drug-resistant |
| Zmp1 | zinc metalloprotease 1 |
References
- Global Tuberculosis Report 2024; World Health Organization: Geneva, Switzerland, 2024.
- Zhao, R.; Du, B.; Luo, Y.; Xue, F.; Wang, H.; Qu, D.; Han, S.; Heilbronner, S.; Zhao, Y. Antimicrobial and anti-biofilm activity of a thiazolidinone derivative against Staphylococcus aureus in vitro and in vivo. Microbiol. Spectr. 2024, 12, e02327-23. [Google Scholar] [CrossRef]
- Trotsko, N.; Kosikowska, U.; Paneth, A.; Plech, T.; Malm, A.; Wujec, M. Synthesis and antibacterial activity of new thiazolidine-2,4-dione-based chlorophenylthiosemicarbazone hybrids. Molecules 2018, 23, 1023. [Google Scholar] [CrossRef] [PubMed]
- Trotsko, N.; Kosikowska, U.; Paneth, A.; Wujec, M.; Malm, A. Synthesis and antibacterial activity of new (2,4-dioxothiazolidin-5-yl/ylidene)acetic acid derivatives with thiazolidine-2,4-dione, rhodanine and 2-thiohydantoin moieties. Saudi Pharm. J. 2018, 26, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Mukhrish, Y.E.; Amri, N.J.; Al-humaidi, J.Y.; Oubella, A.; Auhmani, A.; Itto, M.Y.A. Discovery of novel thiazolidinone-1,2,3-triazole hybrids with (D)-limonene skeleton as anticancer agents: Design, synthesis and biological evaluation. J. Mol. Struct. 2024, 1308, 138127. [Google Scholar] [CrossRef]
- Szczepański, J.; Khylyuk, D.; Korga-Plewko, A.; Michalczuk, M.; Mańdziuk, S.; Iwan, M.; Trotsko, N. Rhodanine-piperazine hybrids as potential VEGFR, EGFR, and HER2 targeting anti-breast cancer agents. Int. J. Mol. Sci. 2024, 25, 12401. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, P.; Anjali; Imtiyaz, K.; Rizvi, M.A.; Nath, V.; Kumar, V.; Husain, A.; Amir, M. Design, synthesis, biological assessment and molecular modeling studies of novel imidazothiazole-thiazolidinone hybrids as potential anticancer and anti-inflammatory agents. Sci. Rep. 2024, 14, 8457. [Google Scholar] [CrossRef]
- Trotsko, N.; Przekora, A.; Zalewska, J.; Ginalska, G.; Paneth, A.; Wujec, M. Synthesis and in vitro antiproliferative and antibacterial activity of new thiazolidine-2,4-dione derivatives. J. Enzym. Inhib. Med. Chem. 2018, 33, 17–24. [Google Scholar] [CrossRef]
- Mekhlef, Y.O.; AboulMagd, A.M.; Gouda, A.M. Design, synhtesis, molecular docking, and biological evaluation of novel 2,3-diaryl-1,3-thiazolidine-4-one derivatives as potential anti-inflammatory and cytotoxic agents. Bioorg. Chem. 2023, 133, 106411. [Google Scholar] [CrossRef]
- Haroun, M.; Petrou, A.; Tratrat, C.; Kolokotroni, A.; Fesatidou, M.; Zagaliotis, P.; Gavalas, A.; Venugopala, K.N.; Sreeharsha, N.; Nair, A.B.; et al. Discovery of 5-methylthiazole-thiazolidinone conjugates as potential anti-inflammatory agents: Molecular target indetification and in silico studies. Molecules 2022, 27, 8137. [Google Scholar] [CrossRef]
- Kryshchyshyn, A.; Kaminskyy, D.; Karpenko, O.; Gzella, A.; Grellier, P.; Lesyk, R. Thiazolidinone/thiazole based hybrids—New class of antitrypanosomal agents. Eur. J. Med. Chem. 2019, 174, 292–308. [Google Scholar] [CrossRef]
- Trotsko, N.; Bekier, A.; Paneth, A.; Wujec, M.; Dzitko, K. Synthesis and in vitro anti-Toxoplasma gondii activity of novel thiazolidin-4-one derivatives. Molecules 2019, 24, 3029. [Google Scholar] [CrossRef]
- Trotsko, N. Antitubercular properties of thiazolidin-4-ones—A review. Eur. J. Med. Chem. 2021, 215, 113266. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.S.; Bhole, R.P.; Khedekar, P.B.; Chikhale, R.V. Mycobacterium enoyl acyl carrier protein reductase (InhA): A key target for antitubercular drug discovery. Bioorg. Chem. 2021, 115, 105242. [Google Scholar] [CrossRef] [PubMed]
- Mdluli, K.; Ma, Z. Mycobacterium tuberculosis DNA gyrase as a target for drug discovery. Infect. Disord. Drug Targets 2007, 7, 159–168. [Google Scholar] [CrossRef]
- Shao, M.; McNeil, M.; Cook, G.M.; Lu, X. MmpL3 inhibitors as antituberculosis drugs. Eur. J. Med. Chem. 2020, 200, 112390. [Google Scholar] [CrossRef] [PubMed]
- Bolla, J.R. Targeting MmpL3 for anti-tuberculosis drug development. Biochem. Soc. Trans. 2020, 48, 1463–1472. [Google Scholar] [CrossRef]
- Seboletswe, P.; Cele, N.; Singh, P. Thiazolidinone-heterocycle frameworks: A concise review of their pharmacological significance. ChemMedChem 2023, 18, e202200618. [Google Scholar] [CrossRef]
- Manjal, S.K.; Kaur, R.; Bhatia, R.; Kumar, K.; Singh, V.; Shankar, R.; Kaur, R.; Rawal, R.K. Synthetic and medicinal perspective of thiazolidinones: A review. Bioorg. Chem. 2017, 75, 406–423. [Google Scholar] [CrossRef]
- Trotsko, N. Thiazolidin-4-ones as a promising scaffold in the development of antibiofilm agents—A review. Int. J. Mol. Sci. 2024, 25, 325. [Google Scholar] [CrossRef]
- Mech, D.; Kurowska, A.; Trotsko, N. The bioactivity of thiazolidin-4-ones: A short review of the most recent studies. Int. J. Mol. Sci. 2021, 22, 11533. [Google Scholar] [CrossRef]
- Gupta, S.; Jha, S.; Rani, S.; Arora, P.; Kumar, S. Medicinal perspective of 2,4-thiazolidinediones derivatives: An insight into recent advancements. ChemistryOpen 2024, 13, e202400147. [Google Scholar] [CrossRef] [PubMed]
- Tarasevicius, E.L. Synthesis of 3-furfurylrhodanine and its 5-arylidene derivatives. Farmatsevtychnyi Zhurnal 1966, 6, 11–14. [Google Scholar]
- Yakubich, V.I.; Gritsyuk, L.V. Synthesis of methionine-based rhodanines. Farmatsevtychnyi Zhurnal 1984, 1, 40–43. [Google Scholar]
- Yakubich, V.I.; Fedirko, Y.M. Synthesis of asparagine acid-based rhodanines. Farmatsevtychnyi Zhurnal 1983, 5, 58–60. [Google Scholar]
- Hanefeld, W.; Jalili, M.A. Synthese und reaktionen von 3-aminorhodaninen. Arch. Pharm. 1987, 320, 323–337. [Google Scholar] [CrossRef]
- Kinugawa, J.; Nagase, H. 2-Thioxo-4-thiazolidinone-5-acetic Acid Derivatives. JP 41016285 B 14 September 1966. Available online: https://scifinder-n.cas.org/searchDetail/reference/6827c08638bc51022663f787/referenceDetails (accessed on 16 May 2025).
- Kandeel, K.A.; Youssef, A.S.A. Reaction of 5-aroylmethylene-3-benzyl-4-oxo-2-thioxo-1,3-thiazolidines with nitrile oxides. Molecules 2001, 6, 510–518. [Google Scholar] [CrossRef]
- Nagase, H. Fungicides XXII. Reaction of dimethyl acetylenedicarboxylate with dithiocarbamates, thiolcarbamates, thiosemicarbazides, and thiosemicarbazones. Chem. Pharm. Bull. 1973, 21, 279–286. [Google Scholar] [CrossRef]
- Carradori, S.; Secci, D.; Bizzarri, B.; Chimenti, P.; De Monte, C.; Guglielmi, P.; Campestre, C.; Rivanera, D.; Bordon, C.; Jones-Brando, L. Synthesis and biological evaluation of anti-Toxoplasma gondii activity of a novel scaffold of thiazolidinone derivatives. J. Enzym. Inhib. Med. Chem. 2017, 32, 746–758. [Google Scholar] [CrossRef]
- D’Ascenzio, M.; Bizzarri, B.; De Monte, C.; Carradori, S.; Bolasco, A.; Secci, D.; Rivanera, D.; Faulhaber, N.; Bordon, C.; Jones-Brando, L. Design, synthesis and biological characterization of thiazolidin-4-one derivatives as promising inhibitors of Toxoplasma gondii. Eur. J. Med. Chem. 2014, 86, 17–30. [Google Scholar] [CrossRef]
- Pan, B.; Huang, R.-Z.; Han, S.-Q.; Qu, D.; Zhu, M.-L.; Wei, P.; Ying, H.-J. Design, synthesis, and antibiofilm activity of 2-arylimino-3-aryl-thiazolidine-4-ones. Bioorg. Med. Chem. Lett. 2010, 20, 2461–2464. [Google Scholar] [CrossRef]
- Mushtaque, M.; Avecilla, F.; Azam, A. Synthesis, characterization and structure optimization of a series of thiazolidinone derivatives as Entamoeba histolytica inhibitors. Eur. J. Med. Chem. 2012, 55, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Arakelian, A.N.; Dunn, H.; Grieshammer, J.L.L.; Coleman, L.E. 2-Imino-4-oxo-5-thiazolidineacetic acid and its derivatives. J. Org. Chem. 1960, 25, 465–467. [Google Scholar] [CrossRef]
- Jangale, A.D.; Dalal, D.K. Highly efficient, combinatorial and catalyst-free approach for the synthesis of 2-benzylidenehydrazono-3-phenyl-4-thiazolidinone-5-acetates in ethanol. ChemistrySelect 2019, 4, 1323–1329. [Google Scholar] [CrossRef]
- Hosseinzadeh, N.; Hasani, M.; Foroumadi, A.; Nadsri, H.; Emami, S.; Samadi, N.; Faramarzi, M.A.; Saniee, P.; Siavoshi, F.; Abadian, N.; et al. 5-Nitro-heteroarylidene analogs of 2-thiazolyliminol-4-thiazolidinones as a novel series of antibacterial agents. Med. Chem. Res. 2013, 22, 2293–2302. [Google Scholar] [CrossRef]
- Nektegayev, I.; Lesyk, R. 3-Oxyarylthiazolidones-4 and their choleretic activity. Sci. Pharm. 1999, 67, 227–230. [Google Scholar]
- Holmberg, B.; Psilanderhielm, B. Uber einige Amidderivate von Thiocarbonglycolsauren. Chem. Zentr. 1911, Bd.I, 296–297. [Google Scholar]
- Nencki, M. Uber die Einwirkung der Monochloressigsaure auf Sulfocyansaure und Salze. J. Prakt. Chem. 1877, 16, 1–17. [Google Scholar] [CrossRef]
- Minka, A.F. Synthesis and study of 5-alkyl derivatives of rhodanine. Farmatsevtychnyi Zhurnal 1963, 5, 32–35. [Google Scholar]
- Kretov, A.E.; Bespalyi, A.S. Thiazolidine derivatives. Zhurnal Obshchei Khimii 1963, 33, 1878–1882. [Google Scholar]
- Andreasch, R. Substituted rhodanines and some of their aldehyde condensation products. XIII. J. Chem. Soc. 1917, 112, 663. [Google Scholar]
- Andreasch, R. New synthesis of 2,4-dioxythiazole and of its 3-phenyl derivative. Monatshefte Chem. 1917, 38, 203–209. [Google Scholar] [CrossRef]
- Abhale, Y.K.; Shinde, A.; Shelke, M.; Nawale, L.; Sarkar, D.; Mhaske, P.C. Synthesis of new 2-(thiazol-4-yl)thiazolidin-4-one derivatives as potential anti-mycobacterial agents. Bioorg. Chem. 2021, 115, 105192. [Google Scholar] [CrossRef] [PubMed]
- Shawky, A.M.; Almalki, F.A.; Abdalla, A.N.; Youssif, B.G.M.; Abdel-Fattah, M.M.; Hersi, F.; El-Sherief, H.A.M.; Ibrahim, N.A.; Gouda, A.M. Discovery and optimization of 2,3-diaryl-1,3-thiazolidin-4-one-based derivatives as potent and selective cytotoxic agents with anti-inflammatory activity. Eur. J. Med. Chem. 2023, 259, 115712. [Google Scholar] [CrossRef] [PubMed]
- Isidor, J.L.; McKee, R.L. Synthesis of 2-methylene-4-thiazolidinones. J. Org. Chem. 1973, 38, 3615–3617. [Google Scholar] [CrossRef]
- Volhard, J. Ueber einige Derivate des Sulfoharnstoffs. J. Prakt. Chem. 1874, 9, 6–30. [Google Scholar] [CrossRef]
- Lozinskii, M.O.; Kukota, S.N.; Pel’kis, P.S. Condensation and cyclization of (arylazo)chloroacetic acids. VIII. New 5-(arylhydrazono)-3-aryl-2-(arylamino)-4-thiazolidones and their oxidation and hydrolysis. Khimiya Geterotsiklicheskikh Soedin. 1971, 3, 171–175. [Google Scholar]
- Turkevich, N.M.; Yakubich, V.I. Synthesis and hydrazinolysis of 3-substituted rhodanines. Khimiya Geterotsiklicheskikh Soedin. 1971, 3, 121–125. [Google Scholar]
- Nomura, M.; Kinoshita, S.; Satoh, H.; Maeda, T.; Murakami, K.; Tsunoda, M.; Miyashi, H.; Awano, K. (3-Substituted benzyl)thiazolidine-2,4-diones as structurally new antihyperglycemic agents. Bioorg. Med. Chem. Lett. 1999, 9, 533–538. [Google Scholar] [CrossRef]
- Turkevich, N.M.; Vvedenskii, V.M.; Petlichnaya, L.M. Substitutions in the azolidine ring. XIII. Method of obtaining pseudothiohydantoin and 2,4-thiazolidinedione. Ukr. Khimicheskii Zhurnal 1961, 27, 680–681. [Google Scholar]
- Lesyk, R.B.; Zimenkovsky, B.S.; Golota, S.M.; Leb’yak, M.M. Synthesis of 2,4-dioxothiazolidine-5-acetic acid and its amides—Perspective synthons for obtaining combinatorial libraries of biologically active substances. Farmatsevtychnyi Zhurnal 2001, 5, 57–63. [Google Scholar]
- Orlinskii, M.M. Recyclization of 2-thioxo-1,3-thiazan-4-one and its derivatives. Zhurnal Org. Khimii 1993, 29, 2323–2324. [Google Scholar]
- Dincel, E.D.; Şatana, D.; Özbey, S.; Ulusoy-Güzeldemirci, N. Synthesis, characterization and antimicrobial evaluation of new 2-(2-thienylcarbonyl)hydrazono-3-alkyl/aryl-4-thiazolidinone and 2-aryl-3-(2-thienylcarbonyl)amino-4-thiazolidinone derivatives. J. Res. Pharm. 2022, 26, 641–654. [Google Scholar] [CrossRef]
- Othman, D.I.A.; Hamdi, A.; Abdel-Aziz, M.M.; Elfeky, S.M. Novel 2-arylthiazolidin-4-one-thiazole hybrids with potent activity against Mycobacterium tuberculosis. Bioorg. Chem. 2022, 124, 105809. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Mital, A.; Akhir, A.; Saxena, D.; Ahmad, M.N.; Dasgupta, A.; Chopra, S.; Jain, R. Pyrrole-thiazolidinone hybrids as a new structural class of broad-spectrum anti-infectives. Eur. J. Med. Chem. 2023, 260, 115757. [Google Scholar] [CrossRef]
- Birgül, S.I.D.; Kumari, J.; Tamhaev, R.; Mourey, L.; Lherbet, C.; Sriram, D.; Akdemir, A.; Küçükgüzel, I. In silico design, synthesis and antitubercular activity of novel 2-acylhydrazono-5-arylmethylene-4-thiazolidinones as enoyl-acyl carrier protein reductase inhibitors. J. Biomol. Struct. Dyn. 2024, 42, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zou, Y.; Chen, X.; Chen, J.; Wang, B.; Tian, J.; Ye, F.; Lu, Y.; Huang, H.; Lu, Y.; et al. Design, synthesis and biological evaljuation of 3-substituted-2-thioxothiazolidin-4-one (rhodanine) derivatives as antitubercular agents against Mycobacterium tuberculosis protein tyrosine phosphatase B. Eur. J. Med. Chem. 2023, 258, 115571. [Google Scholar] [CrossRef]
- Verma, V.A.; Saundane, A.R. Synthesis of some novel 5-(8-substituted-11H-indolo [3,2-c]isoquinolin-5-ylthio)-1′,3′,4′-oxadiazol-2-amines bearing thiazolidinones and azetidinones as potential antimicrobial, antioxidant, antituberculosis, and anticancer agents. Polycycl. Aromat. Compd. 2021, 41, 871–896. [Google Scholar] [CrossRef]
- Verma, V.A.; Saundane, A.R.; Meti, R.S.; Shamrao, R.; Katkar, V. Synthesis, biological evaluation and docking studies of some new indolyl-pyridine containing thiazolidinone and azetidinone analogs. Polycycl. Aromat. Compd. 2022, 42, 1545–1559. [Google Scholar] [CrossRef]
- Alghamdi, S.; Almehmadi, M.M.; Asif, M.; Alshehri, M.M.; Kamal, M. Amtimycobacterial evaluation and microwave-assisted synthesis of N-(5-methyl-4-oxo-2-arylthiazolidin-3-yl) isonicotinamide derivatives. Pharm. Chem. J. 2022, 56, 215–219. [Google Scholar] [CrossRef]
- Dunga, A.K.; Rao Allaka, T.; Shaik, A.; Nemuri, R.; Thandlam, A.K.; Nechipadappu, S.K.; Pothana, P.; Kishore, P.V.N.N. Synthesis of novel antimicrobial indazole-linked 1,2,4-triazolylthiadiazole and 4-thiazolidinone derivatives and study of their molecular modelling. Russ. J. Gen. Chem. 2023, 93, 949–961. [Google Scholar] [CrossRef]
- Chintakunta, R.; Subbareddy, G.V. Synthesis docking studies, characterization and anti-tubercular activity of ofloxacin containing thiazolidinone derivatives. J. Young Pharm. 2022, 14, 77–81. [Google Scholar] [CrossRef]
- Salve, P.S.; Parchure, P.; Araujo, L.; Kavalapure, R.S.; Jalapure, S.S.; Sriram, D.; Krishna, V.S.; Estharla, M.R.; Alegaon, S.G. Design and synthesis of new 3-((7-chloroquinolin-4-yl)amino)thiazolidin-4-one analogs as Mycobacterium tuberculosis DNA gyrase inhibitors. Future J. Pharm. Sci. 2021, 7, 10. [Google Scholar] [CrossRef]
- Alghamdi, S.; Kamal, M.; Asif, M. Antimycobacterial assessment and microwave-assisted synthesis of 2-aryl-3-(4-methylphenylamino)thiazolidin-4-one derivatives. Lett. Org. Chem. 2022, 19, 250–255. [Google Scholar] [CrossRef]
- Bhoge, N.; Magare, B.; Mohite, P. Synthesis and biological evaluation of 3-(benzo[d]thiazol-2-yl)-2-(substituted aryl)thiazolidin-4-one derivatives. Lett. Appl. NanoBioScience 2023, 13, 19. [Google Scholar] [CrossRef]
- Trawally, M.; Demir-Yazici, K.; Dingiş-Birgül, S.I.; Kaya, K.; Akdemir, A.; Güzel-Akdemir, Ö. Mandelic acid-based spirothiazolidinones targeting M. tuberculosis: Synthesis, in vitro and in silico investigations. Bioorg. Chem. 2022, 121, 105688. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.K.; Yadava, U.; Sharma, M.I.; Upadhyay, A.; Gupt, M.P.; Dwivedi, A.R.; Khatoon, A. Synthesis, molecular structure investigation, biological evaluation and docking studies of novel spirothiazolidinones. Results Chem. 2023, 5, 100726. [Google Scholar] [CrossRef]
- Moulishankar, A.; Thirugnanasambandam, S. Quantitative structure activity relationship (QSAR) modeling study of some novel thiazolidine-4-one derivatives as potent anti-tubercular agents. J. Recept. Signal Transduct. 2023, 43, 83–92. [Google Scholar] [CrossRef]
- Ahmed, S.; Mital, A.; Akhir, A.; Saxena, D.; Jahan, K.; Dubey, G.; Bharatam, P.V.; Dasgupta, A.; Chopra, S.; Jain, R. Pyrrole-thiazolidin-4-one analogues exhibit promising anti-tuberculosis activity. Asian J. Org. Chem. 2024, 13, e202400054. [Google Scholar] [CrossRef]
- Kumar, V.; Shetty, P.; HS, A.; Chandra, K.S.; Ramu, R.; Patil, S.M.; Baliga, A.; Rai, V.M.; Shenoy, M.S.; Udupi, V.; et al. Potential fluorinated anti-MRSA thiazolidinone derivatives with antibacterial, antitubercular activity and molecular docking studies. Chem. Biodivers. 2022, 19, e202100532. [Google Scholar] [CrossRef]
- Hart, D.; Legoabe, L.J.; Jesumoroti, O.J.; Jordaan, A.; Warner, D.F.; Steventon, R.; Beteck, R.M. Nitrothiazole-thiazolidinone hybrids: Synthesis and in vitro antimicrobial evaluation. Chem. Biodivers. 2022, 19, e202200729. [Google Scholar] [CrossRef]
- Kamat, V.; Poojary, B.; Puthran, D.; Das, V.B.; Kumar, B.K.; Sankaranarayan, M.; Shetye, G.; Ma, R.; Franzblau, S.G.; Nayak, S.P. Synthesis, antimycobacterial, cytotoxicity, anti-inflammatory, in silico studies and molecular dynamics of pyrazole-embedded thiazolidin-4-one hybrids. Arch. Pharm. 2023, 356, e2200444. [Google Scholar] [CrossRef]
- Nayak, S.G.; Poojary, B.; Kamat, V.; Puthran, D. Novel thiazolidin-4-one clubbed thiophene derivatives via Gewald synthesis as anti-tubercular and anti-inflammatory agents. J. Chin. Chem. Soc. 2021, 68, 1116–1127. [Google Scholar] [CrossRef]
- Nefisath, P.; Kumar, V.; Shashiprabha; Patil, S.M.; Ramu, R.; Shradha, S.; Dasappa, J.P. 2,3,5-Trisubstituted thiazolidinone derivatives as potential antimicrobial and antitubercular agents and in silico studies. ChemistrySelect 2024, 9, e202303188. [Google Scholar] [CrossRef]
- Pasha, M.A.; Mondal, S.; Panigrahi, N.; Shetye, G.; Ma, R.; Franzblau, S.G.; Zheng, Y.-T.; Murugesan, S. One-pot synthesis of novel hydrazono-1,3-thiazolidin-4-one derivatives as anti-HIV and anti-tubercular agents: Synthesis, biological evaluation, molecular modelling and admet studies. Curr. HIV Res. 2022, 20, 255–271. [Google Scholar] [CrossRef]
- Younis, M.H.; Mohammed, E.R.; Mohamed, A.R.; Abdel-Aziz, M.M.; Georgey, H.H.; Gawad, N.M.A. Design, synthesis and anti-Mycobacterium tuberculosis evaluation of new thiazolidin-4-one and thiazolo[3,2-a][1,3,5]triazine derivatives. Bioorg. Chem. 2022, 124, 105807. [Google Scholar] [CrossRef] [PubMed]
- Angelova, V.T.; Pencheva, T.; Buyukliev, R.; Yovkova, E.K.; Valkova, I.; Momekov, G.; Vulcheva, V. Antimycobacterial activity, in silico ADME evaluation, and docking study of novel thiazolidinedione and imidazolidinone conjugates. Russ. J. Bioorg. Chem. 2021, 47, 122–133. [Google Scholar] [CrossRef]
- Angelova, V.T.; Valcheva, V.; Vassilev, N.; Buyukliev, R.; Mihaylova, R.; Momekov, G. Synthesis, in vitro antiproliferative and antimycobacterial activity of thiazolidine-2,4-dione and hydantoin derivatives. Bulg. Chem. Commun. 2017, 49, 643–651. [Google Scholar]
- Trotsko, N.; Głogowska, A.; Kaproń, B.; Kozieł, K.; Augustynowicz-Kopeć, E.; Paneth, A. The new thiazolidine-2,4-dione-based hybrids with promising antimycobacterial activity: Design, synthesis, biological evaluation, and drug interaction analysis. J. Enzym. Inhib. Med. Chem. 2025, 40, 2442703. [Google Scholar] [CrossRef]
- Dak, M.; Šlachtova, V.; Šebela, M.; Bazgier, V.; Berka, K.; Smiejkowska, N.; Oorts, L.; Cappoen, D.; Brulikova, L. Novel heterocyclic hydroxamates as inhibitors of the mycobacterial zinc metalloprotease Zmp1 to probe its mechanism of function. Eur. J. Med. Chem. 2022, 244, 114831. [Google Scholar] [CrossRef]
- Raghu, M.S.; Kumar, C.B.P.; Yogesh Kumar, K.; Prashanth, M.K.; Alharethy, F.; Jeon, B.-H. Synthesis, biologicalevaluation and molecular docking study of pyrimidine linked thiazolidinedione derivatives as potential antimicrobial and antitubercular agents. Bioorg. Med. Chem. Lett. 2024, 103, 129707. [Google Scholar] [CrossRef]
- Maddipatla, S.; Bakchi, B.; Gadhave, R.R.; Ammara, A.; Sau, S.; Rani, B.; Nanduri, S.; Kalia, N.P.; Supuran, C.T.; Yaddanapudi, V.M. Exploring rhodanine linked enamine-carbohydrazide derivatives as mycobacterial carbonic anhydrase inhibitors: Design, synthesis, biological evaluation, and molecular docking studies. Arch. Pharm. 2024, 357, e2400064. [Google Scholar] [CrossRef] [PubMed]
- Chollet, A.; Maveyraud, L.; Lherbet, C.; Bernardes-Genisson, V. An overview on crystal structures of InhA protein: Apo-form, in complex with its natural ligands and inhibitors. Eur. J. Med. Chem. 2018, 146, 318. [Google Scholar] [CrossRef]
- Amado, P.S.M.; Woodley, C.; Cristiano, M.L.S.; O’neill, P.M. Recent advances of DprE1 inhibitors against Mycobacterium tuberculosis: Computational analysis of physicochemical and ADMET properties. ACS Omega 2022, 7, 40659–40681. [Google Scholar] [CrossRef]
- Yadav, S.; Soni, A.; Tanwar, O.; Bhadane, R.; Besra, G.S.; Kawathekar, N. DprE1 Inhibitors: Enduring aspirations for future antituberculosis drug discovery. ChemMedChem 2023, 18, e202300099. [Google Scholar] [CrossRef]
- Liang, W.G.; Mancl, J.M.; Zhao, M.; Tang, W.-J. Structural analysis of Mycobacterium tuberculosis M13 metalloprotease Zmp1 open states. Structure 2021, 29, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.; Srinivasarao, S.; Khetmalis, Y.M.; Nizalapur, S.; Sankaranarayanan, M.; Sekhar, K.V.G.C. Inhibitors of pantothenate synthetase of Mycobacterium tuberculosis—A medicinal chemist perspective. RSC Adv. 2020, 10, 37098. [Google Scholar] [CrossRef]
- Marrakchi, H.; Laneelle, M.-A.; Daffe, M. Mycolic acids: Structures, biosynthesis, and beyond. Chem. Biol. 2014, 21, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.T.; Abramovitch, R.B. Molecular mechanisms of MmpL3 function and inhibition. Microb. Drug Resist. 2023, 29, 190–212. [Google Scholar] [CrossRef]
- Sajid, Z.; Akhtar, T.; Ahmad, K.; Haroon, M. Molecular docking simulation and ADMET/pharmacokinetic screening of newly designed 2-(2-aryl-4-oxo-4,5-dihydrothiazol-5-yl)acetohydrazides as potential antitubercular agents. ChemistrySelect 2024, 9, e202403715. [Google Scholar] [CrossRef]
- Sharma, D.; Sharma, S.; Sinha, N.; Jain, N.; Kumar, A.; Sarkar, A.; Tyagi, J.S.; Gupta, R.K. Novel benzoic thiazolidin-4-one derivatives targeting DevR/DosR dormancy regulator of Mycobacterium tuberculosis. J. Mol. Struct. 2022, 1254, 132278. [Google Scholar] [CrossRef]
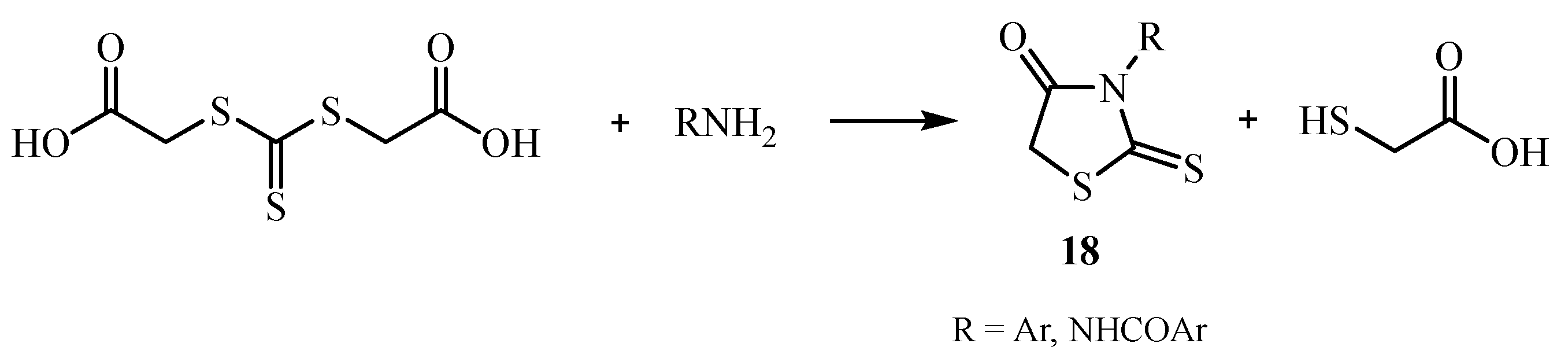
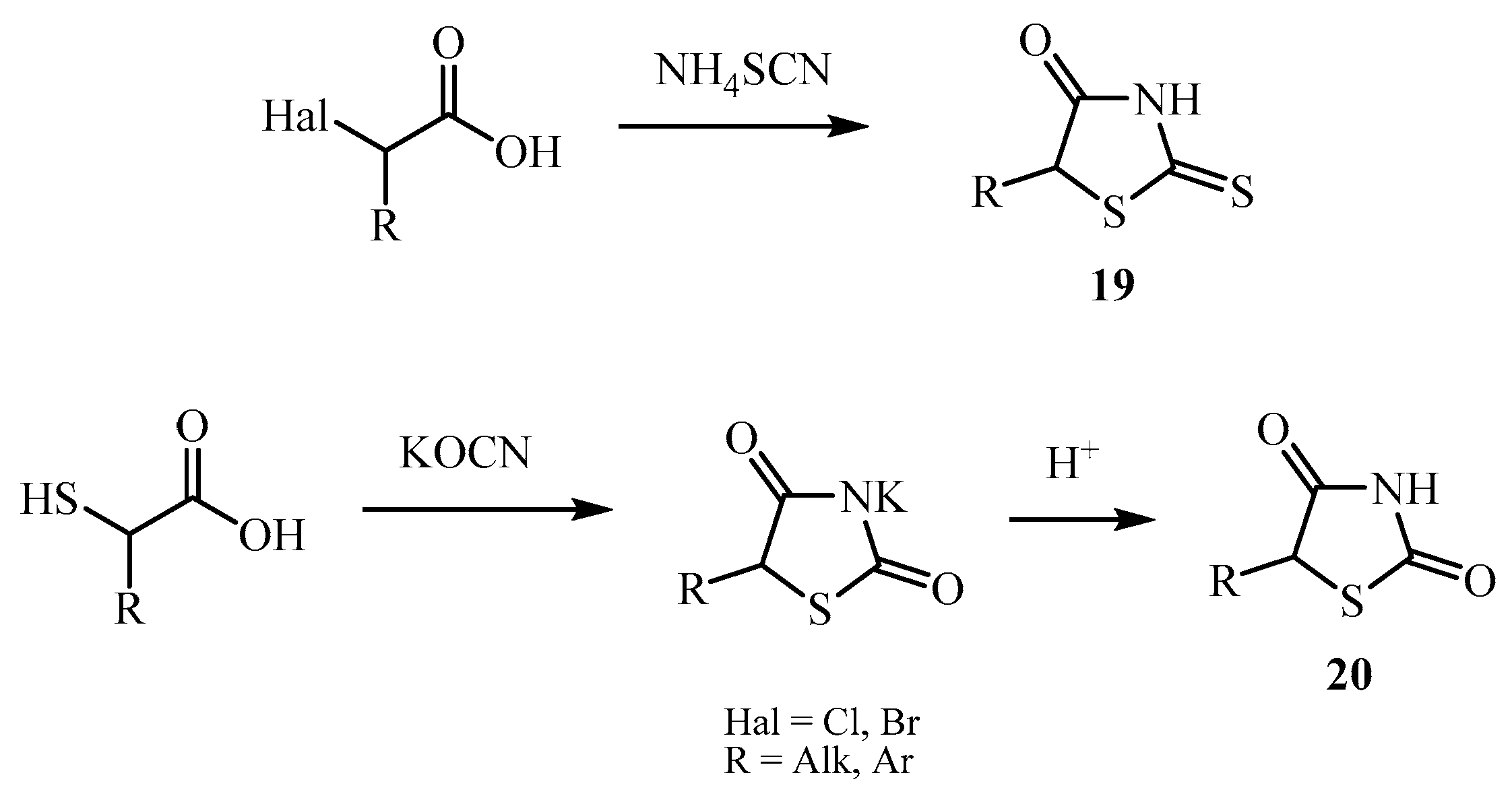


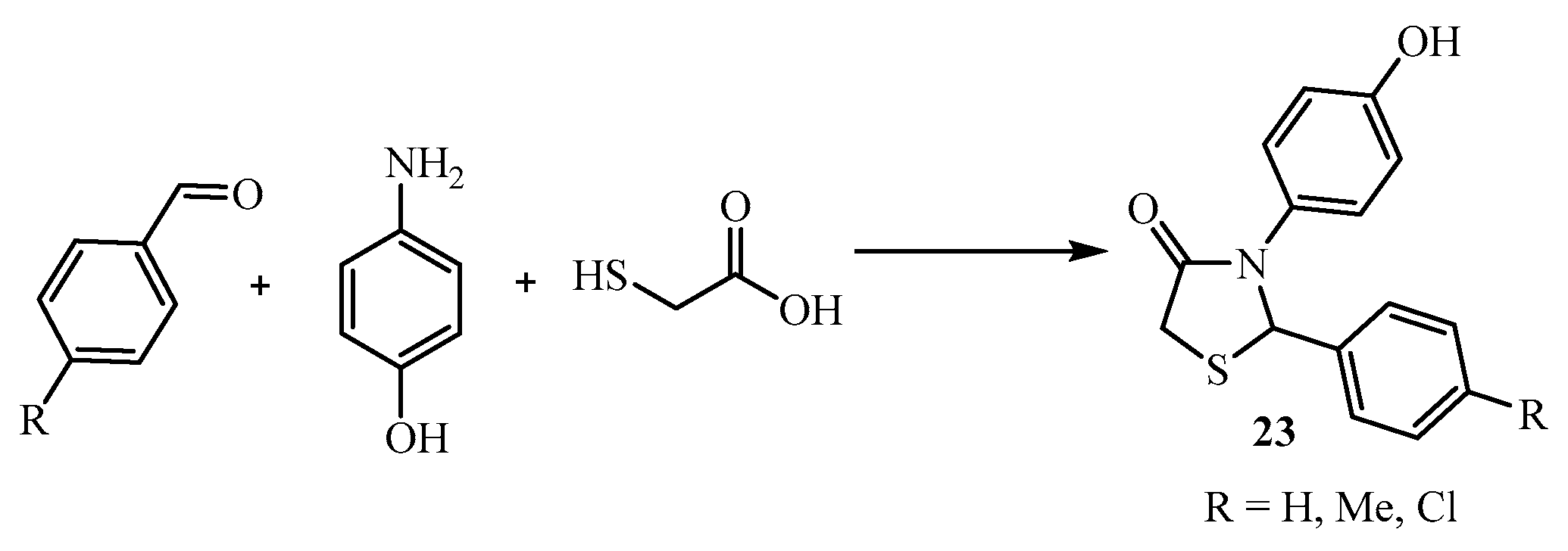

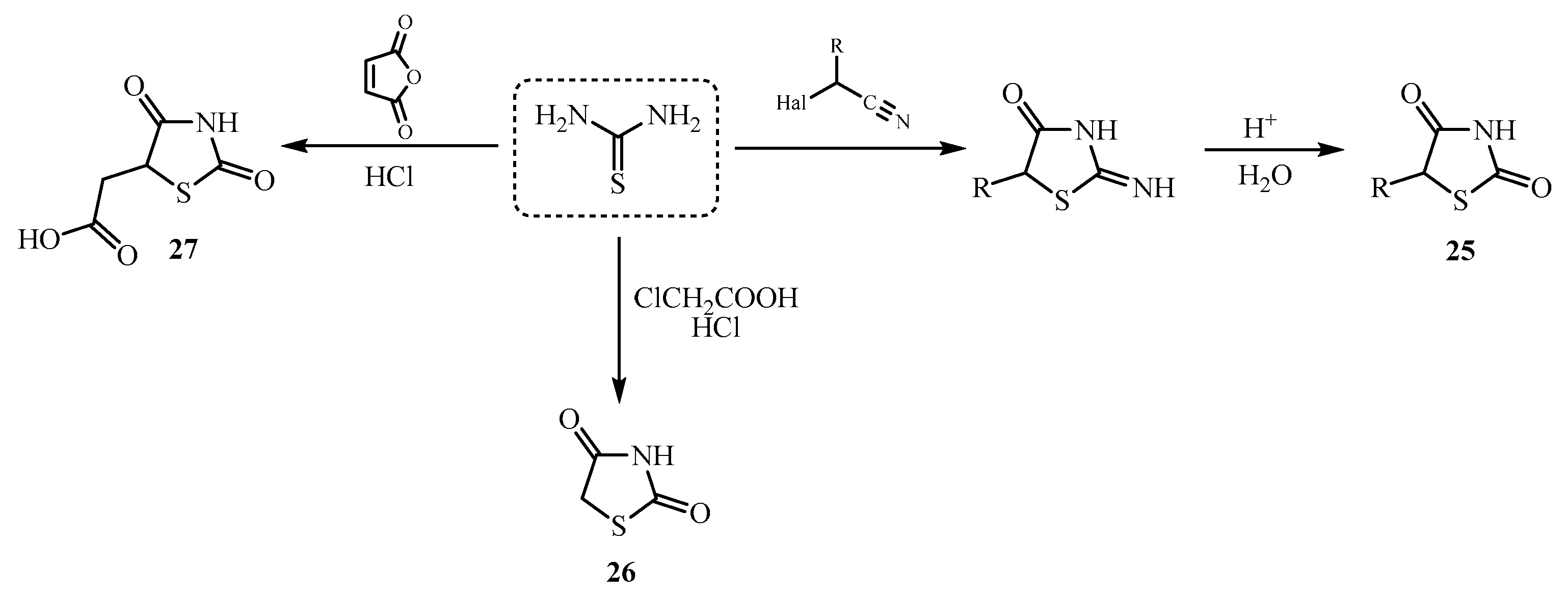
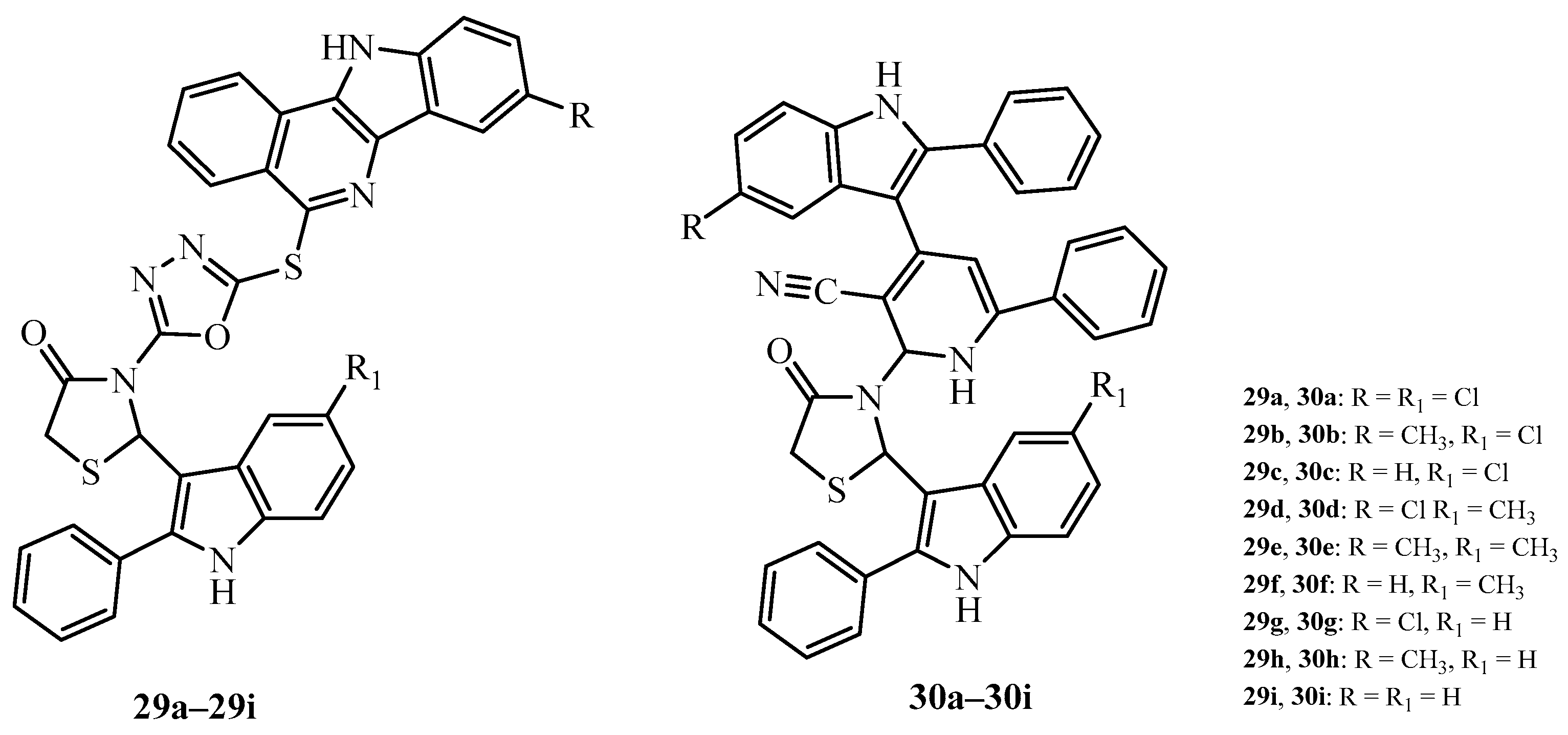
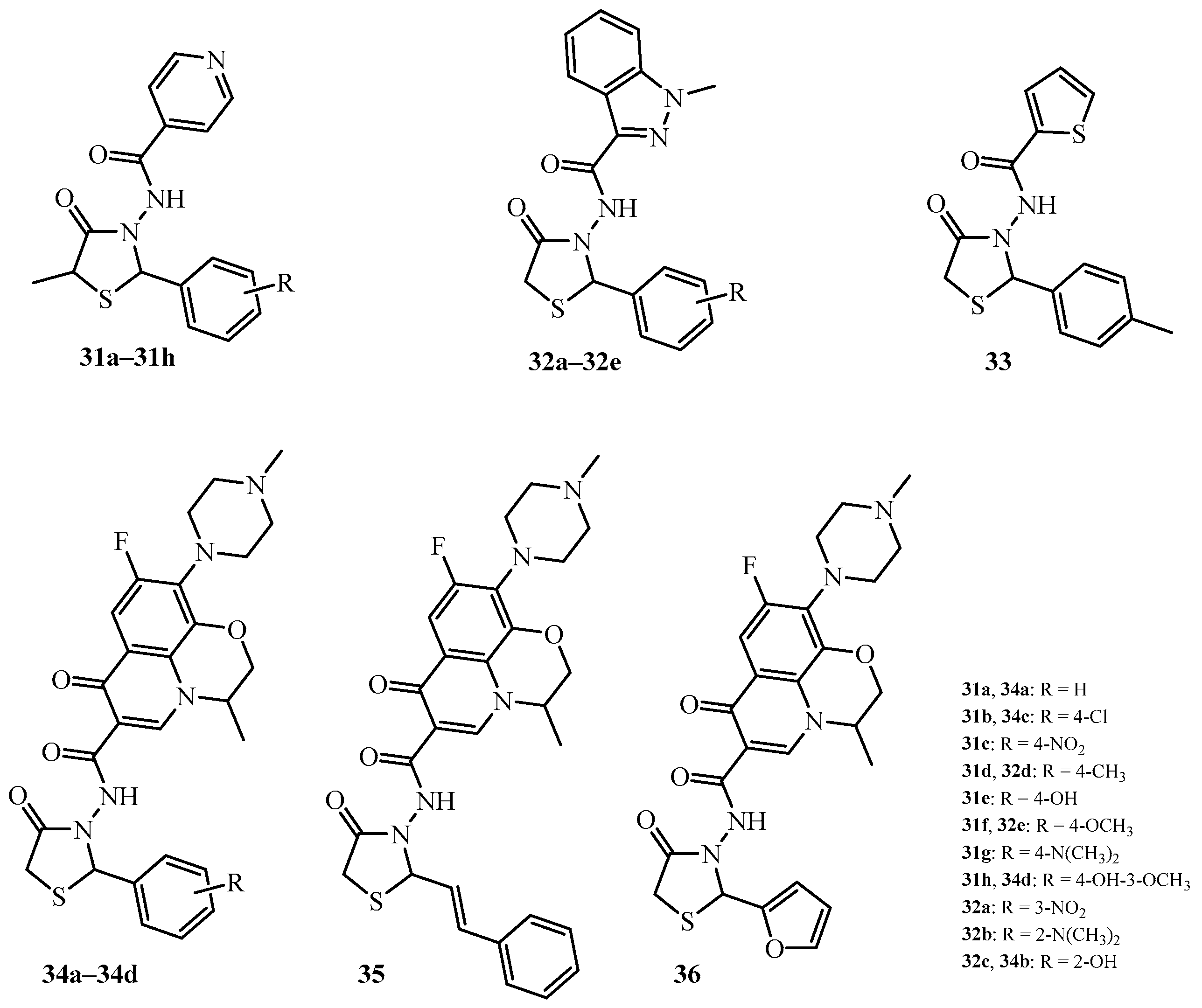
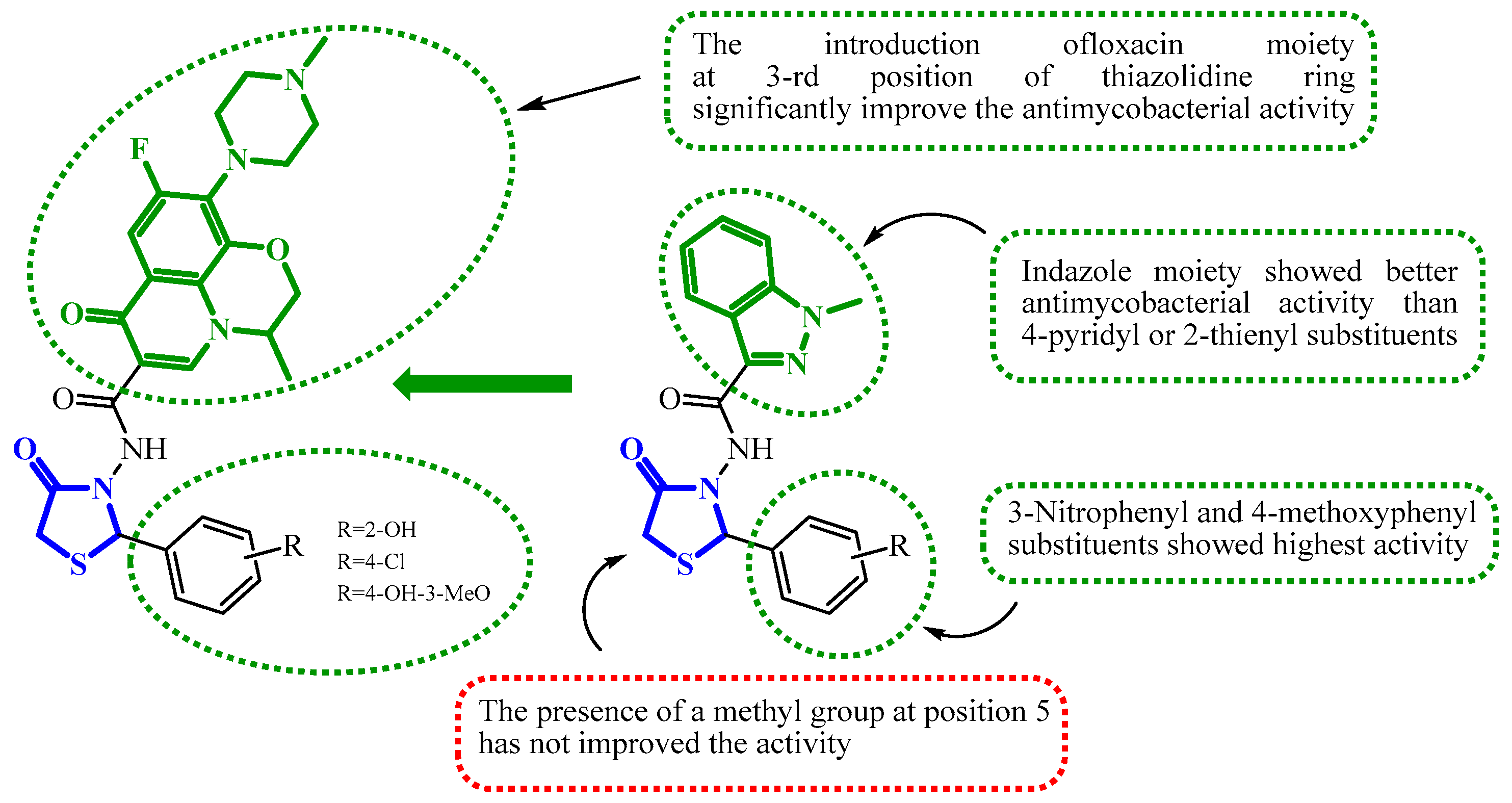
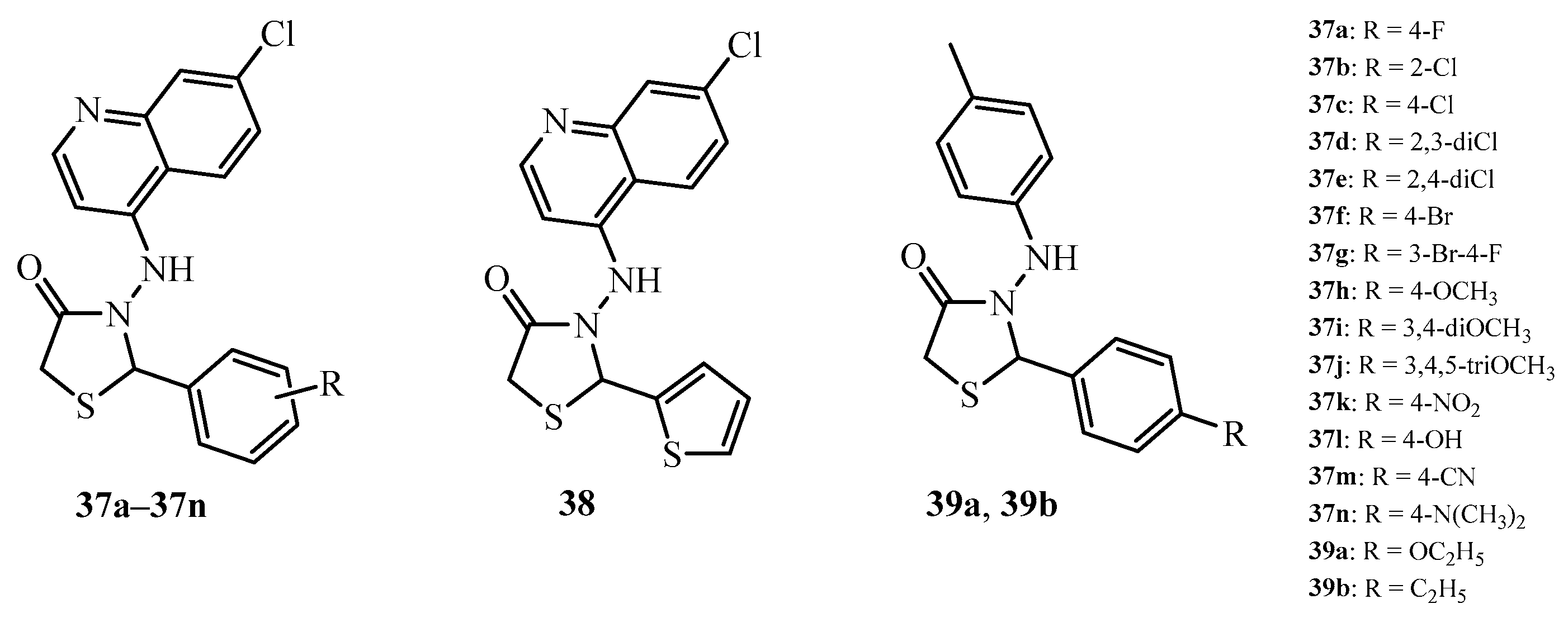
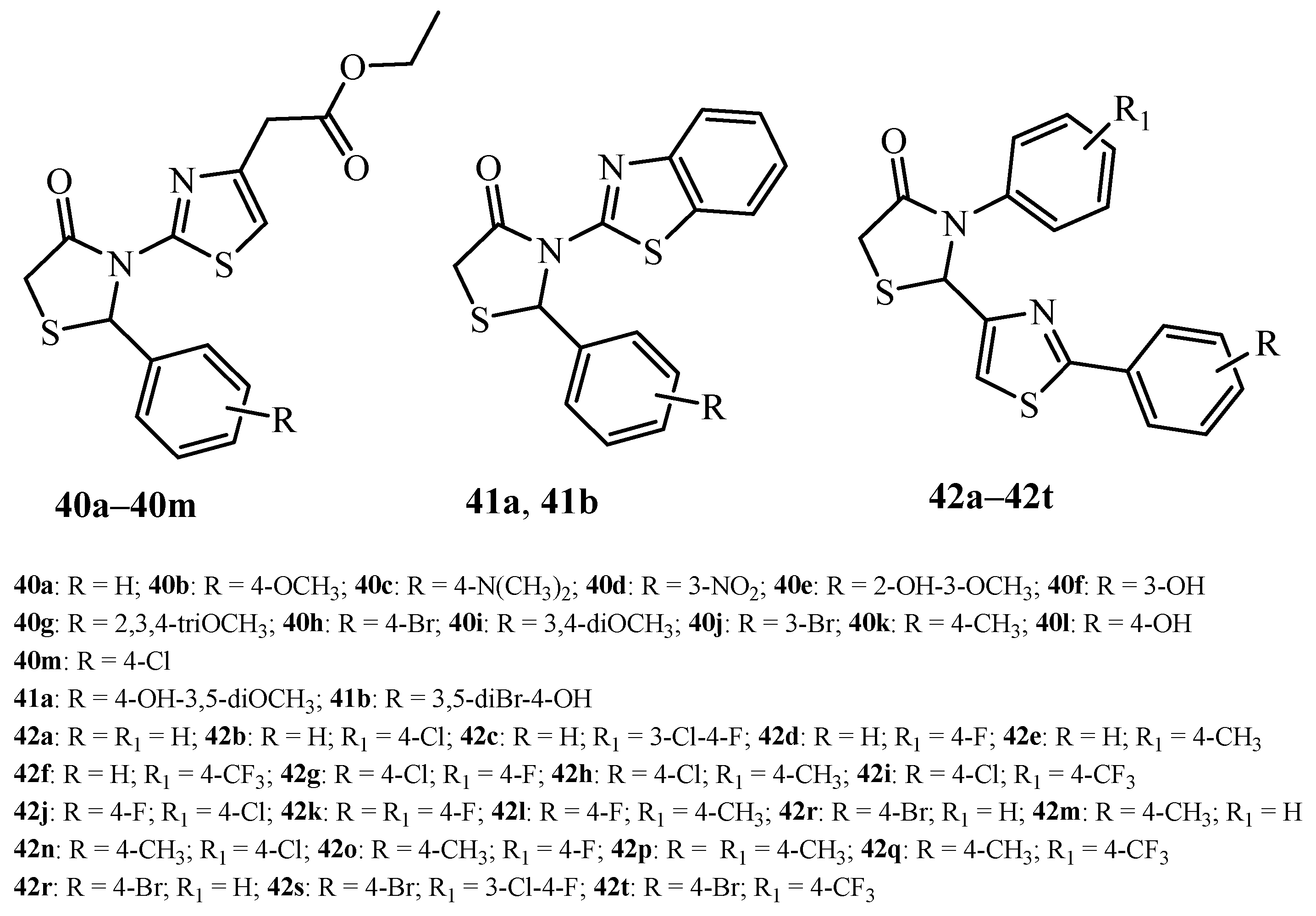
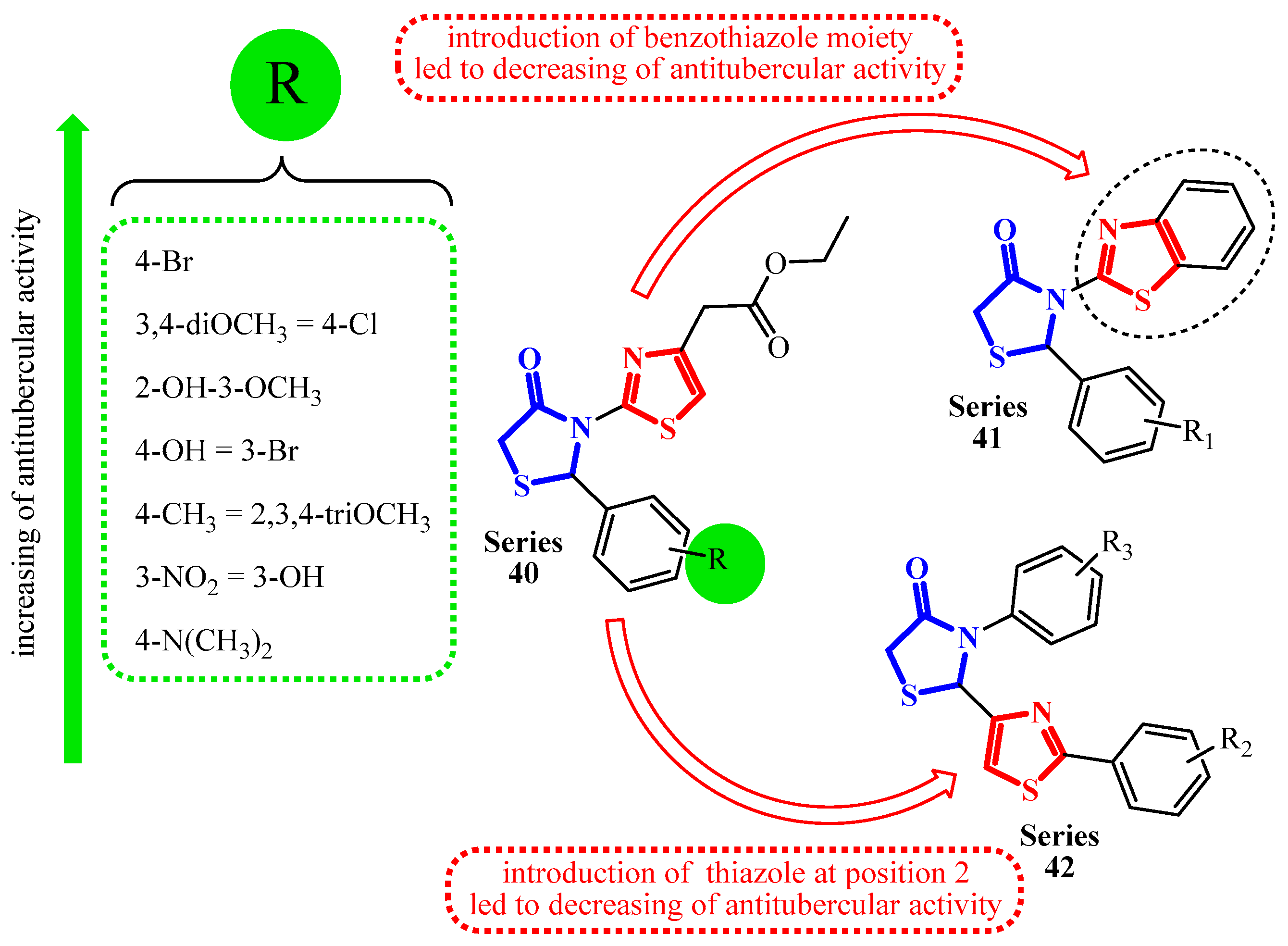
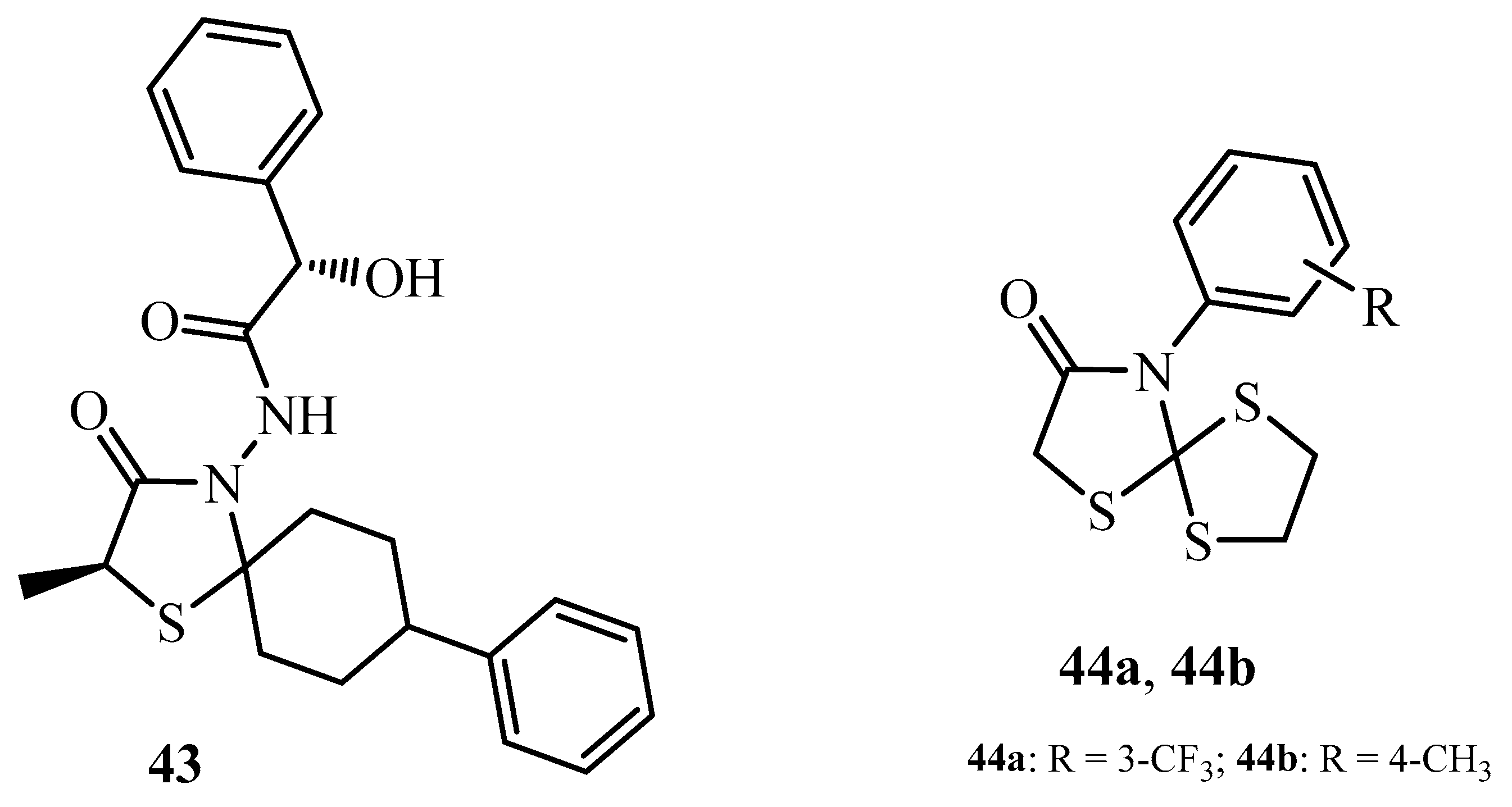
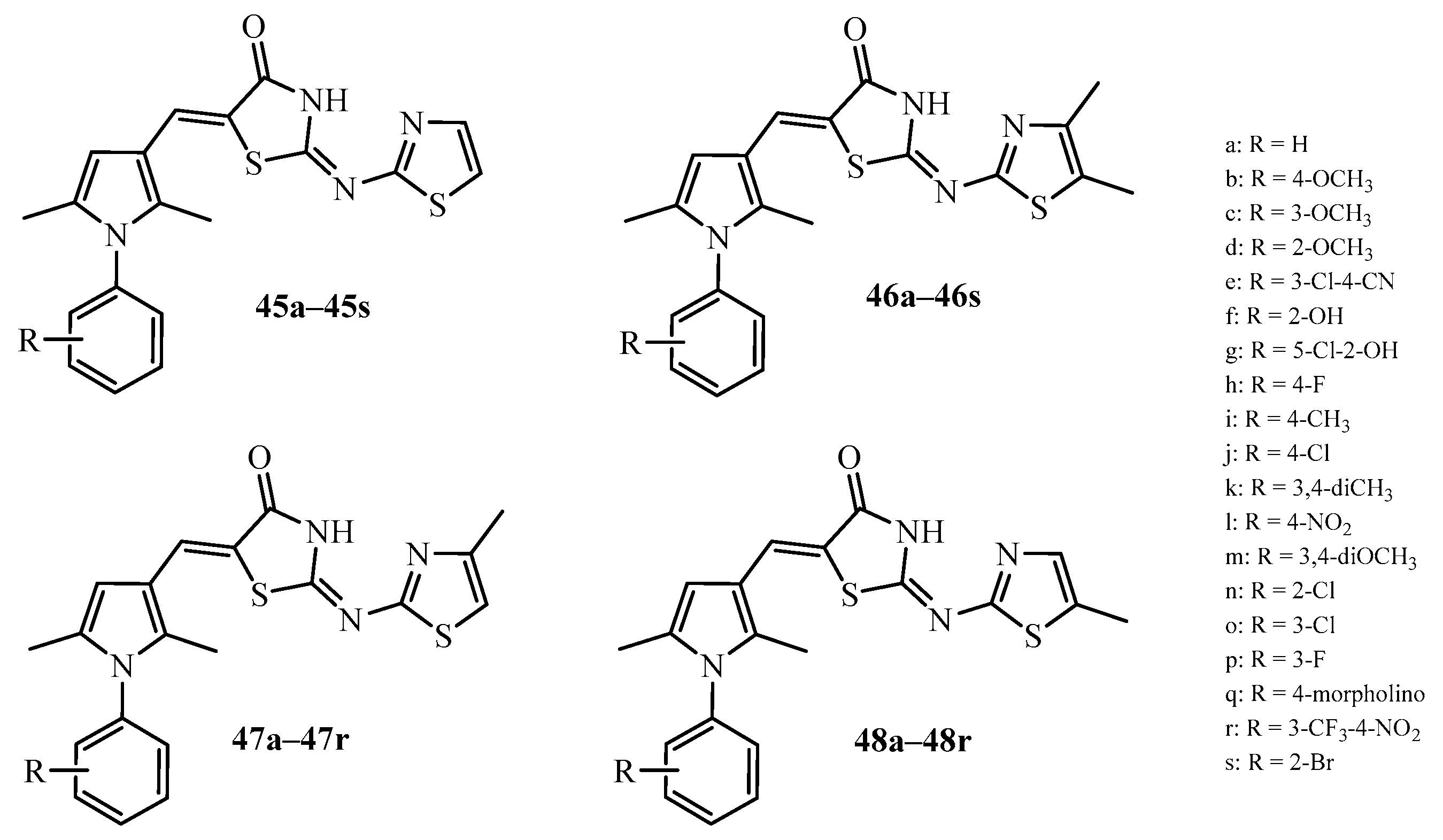
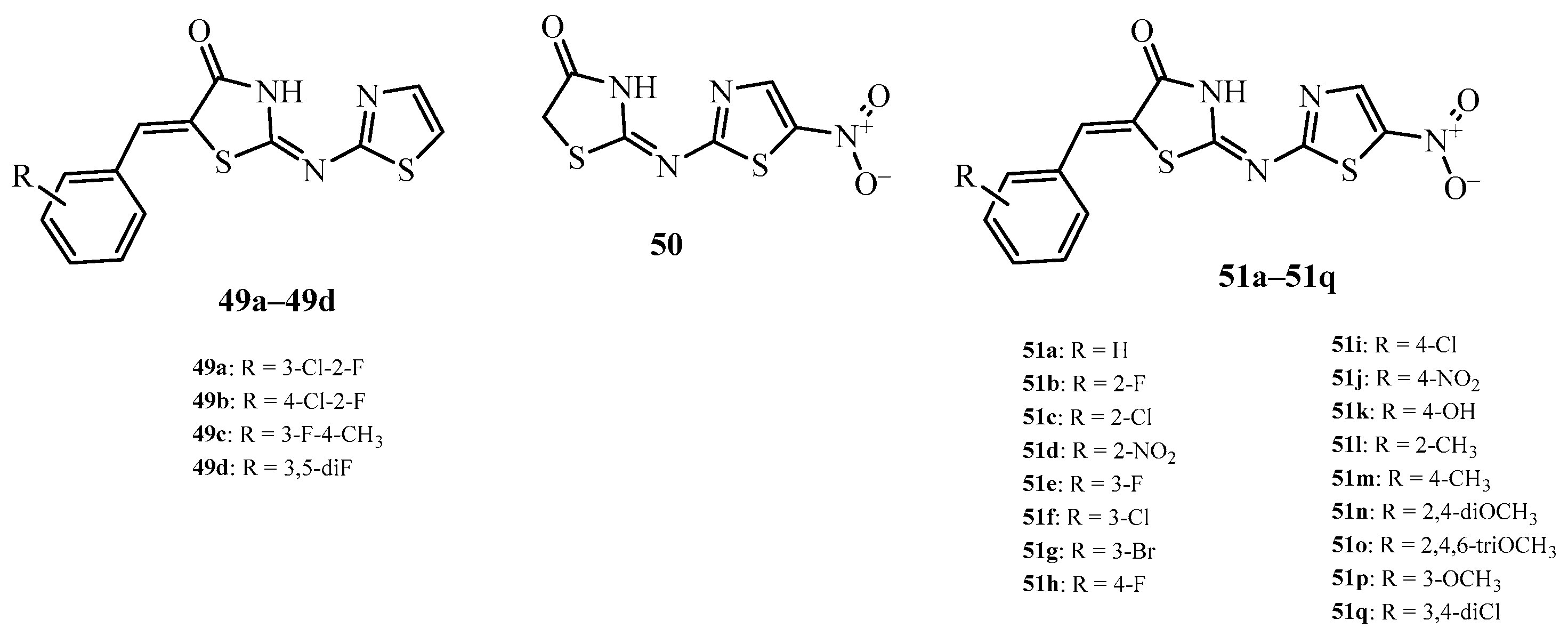
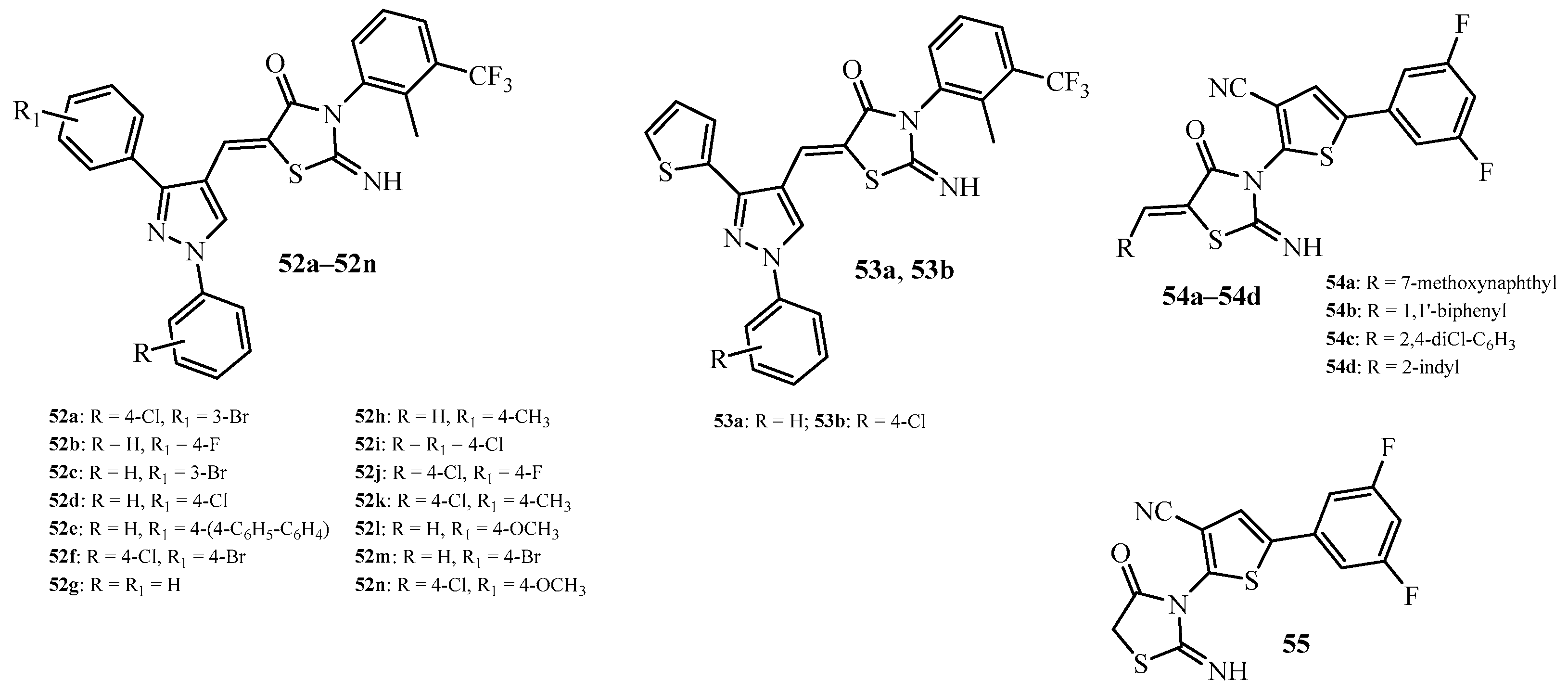
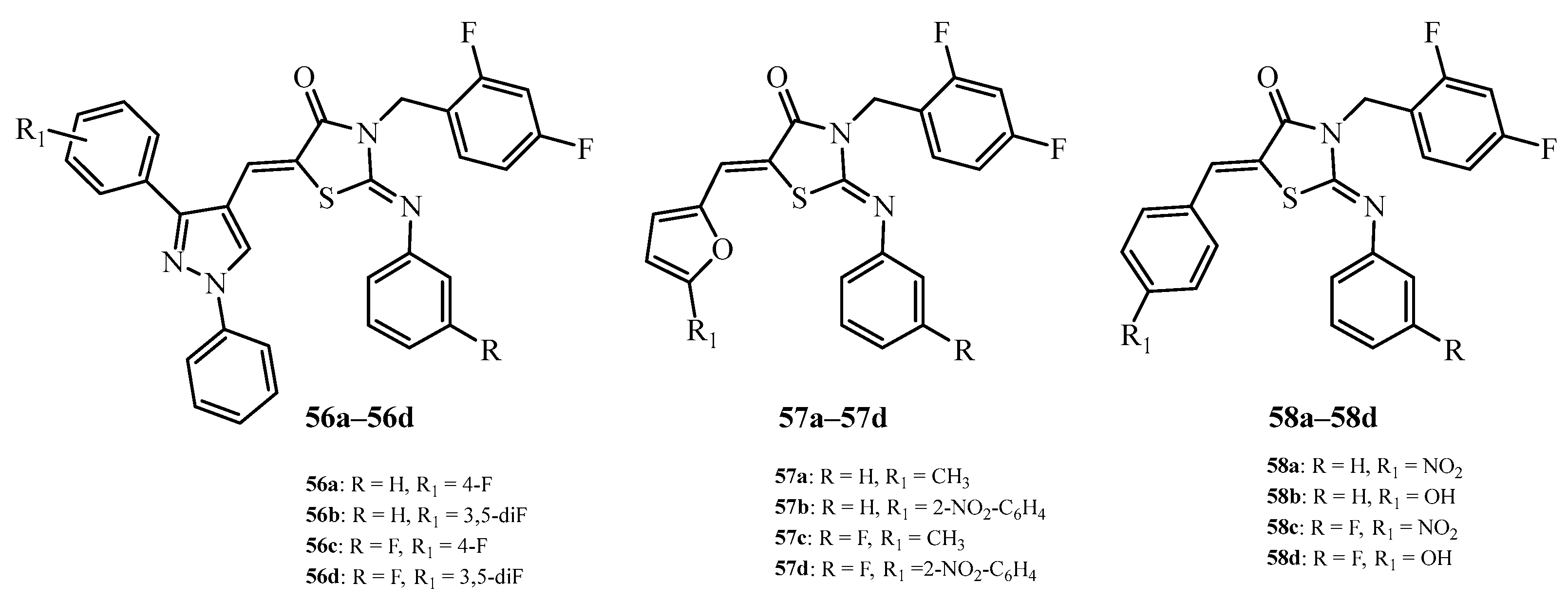
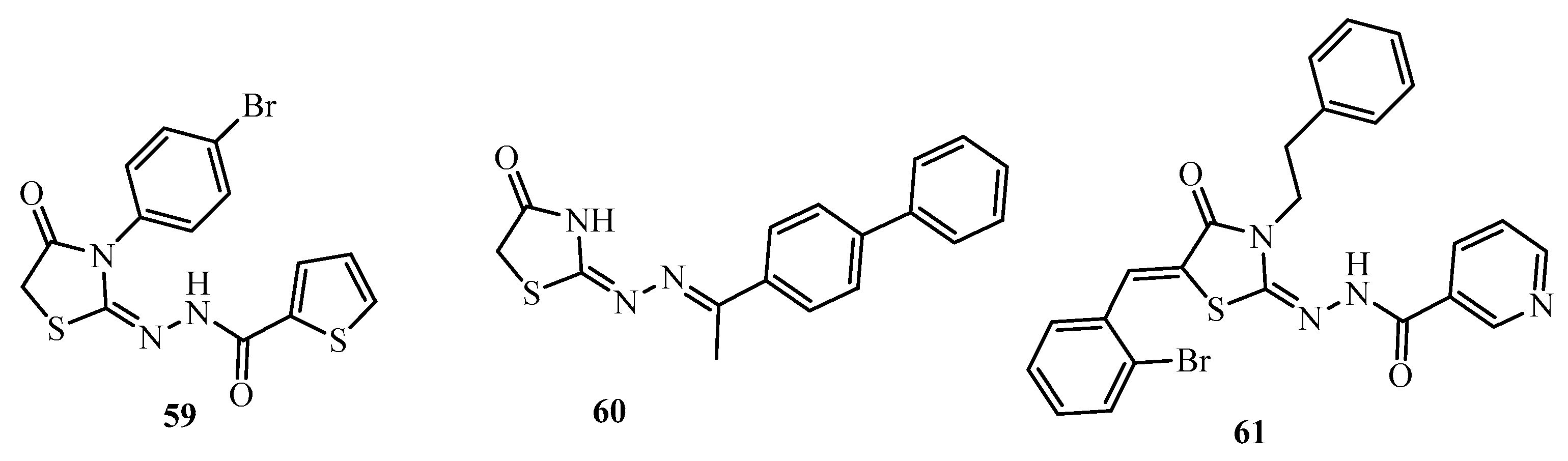
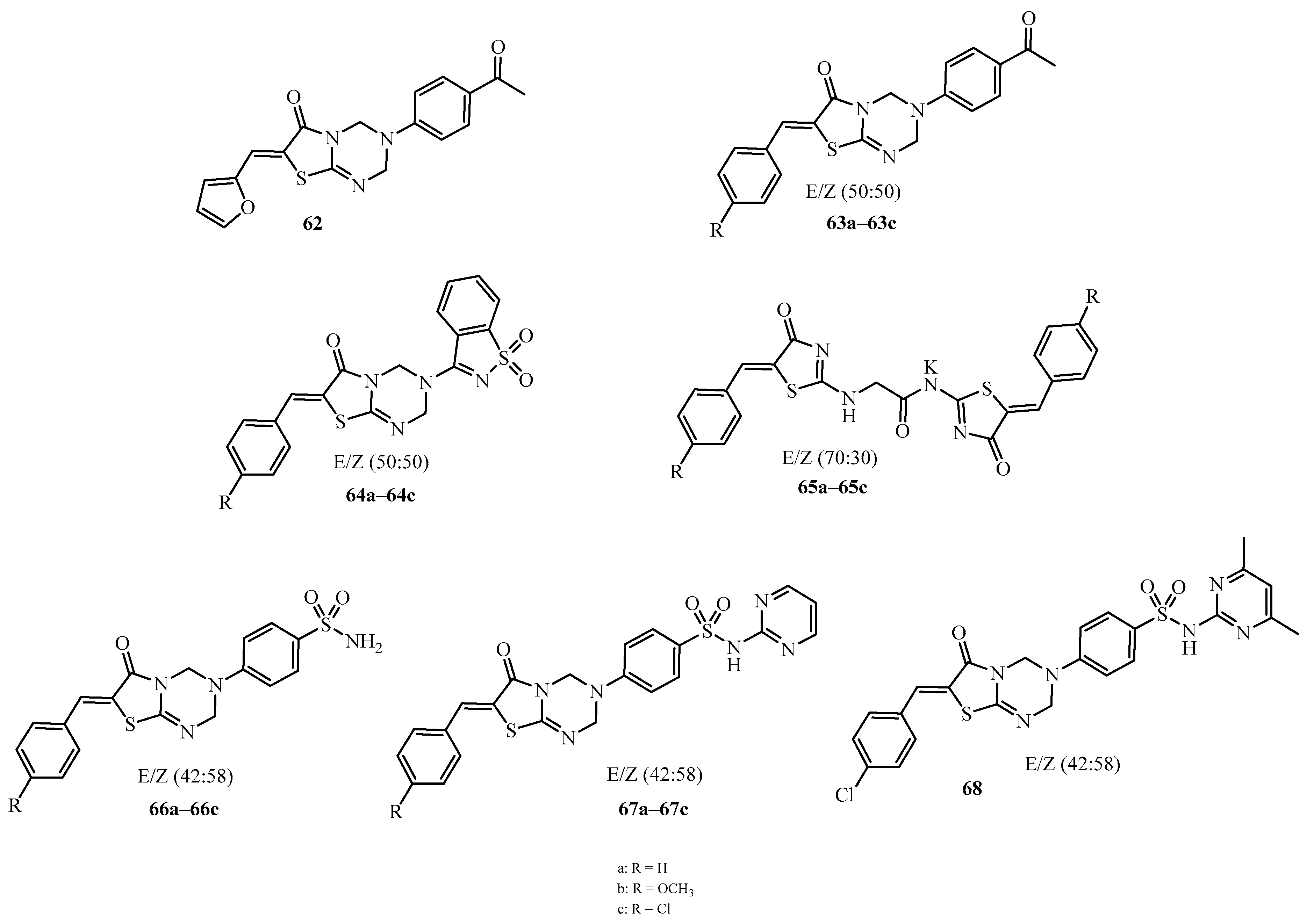

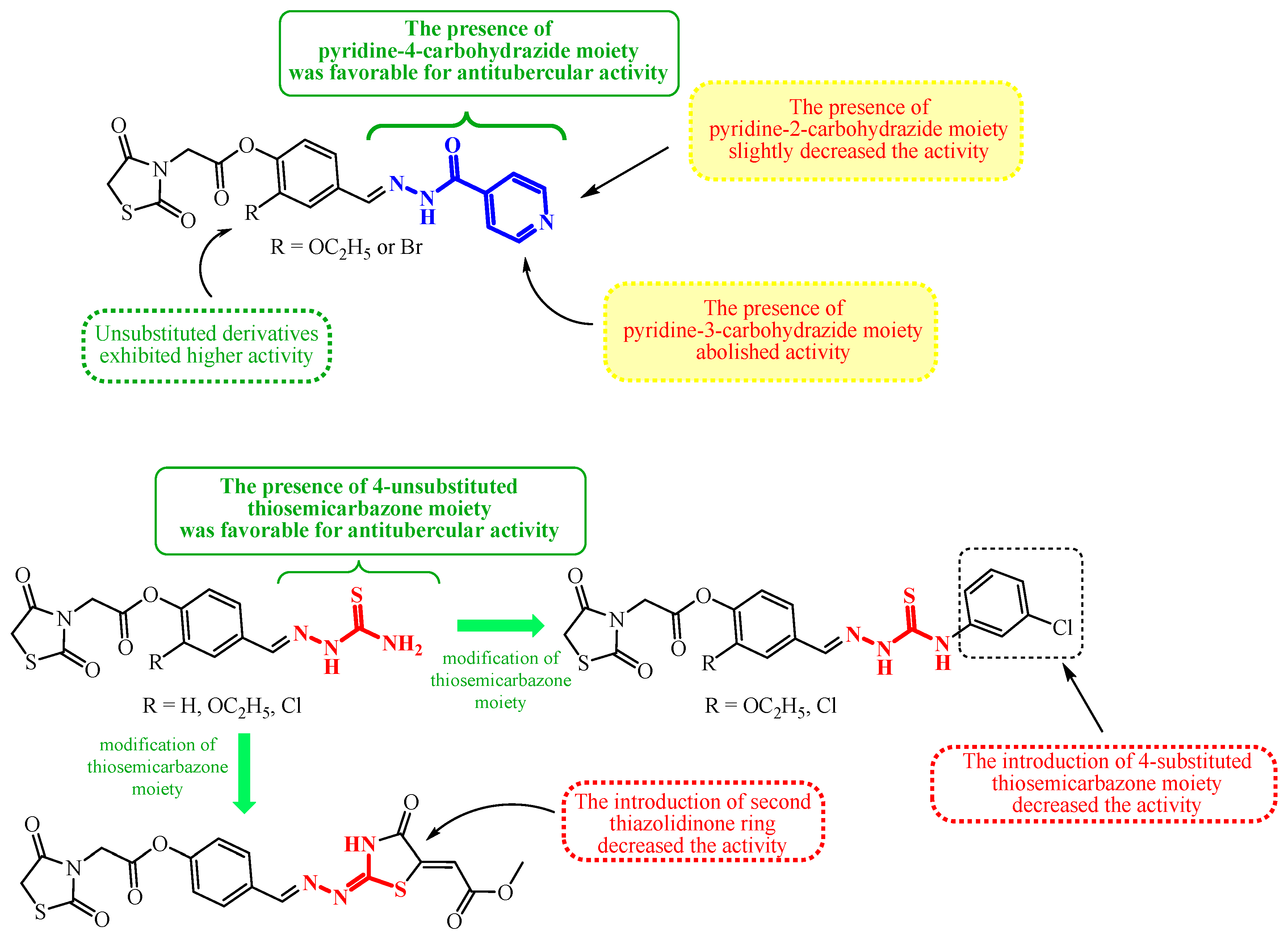
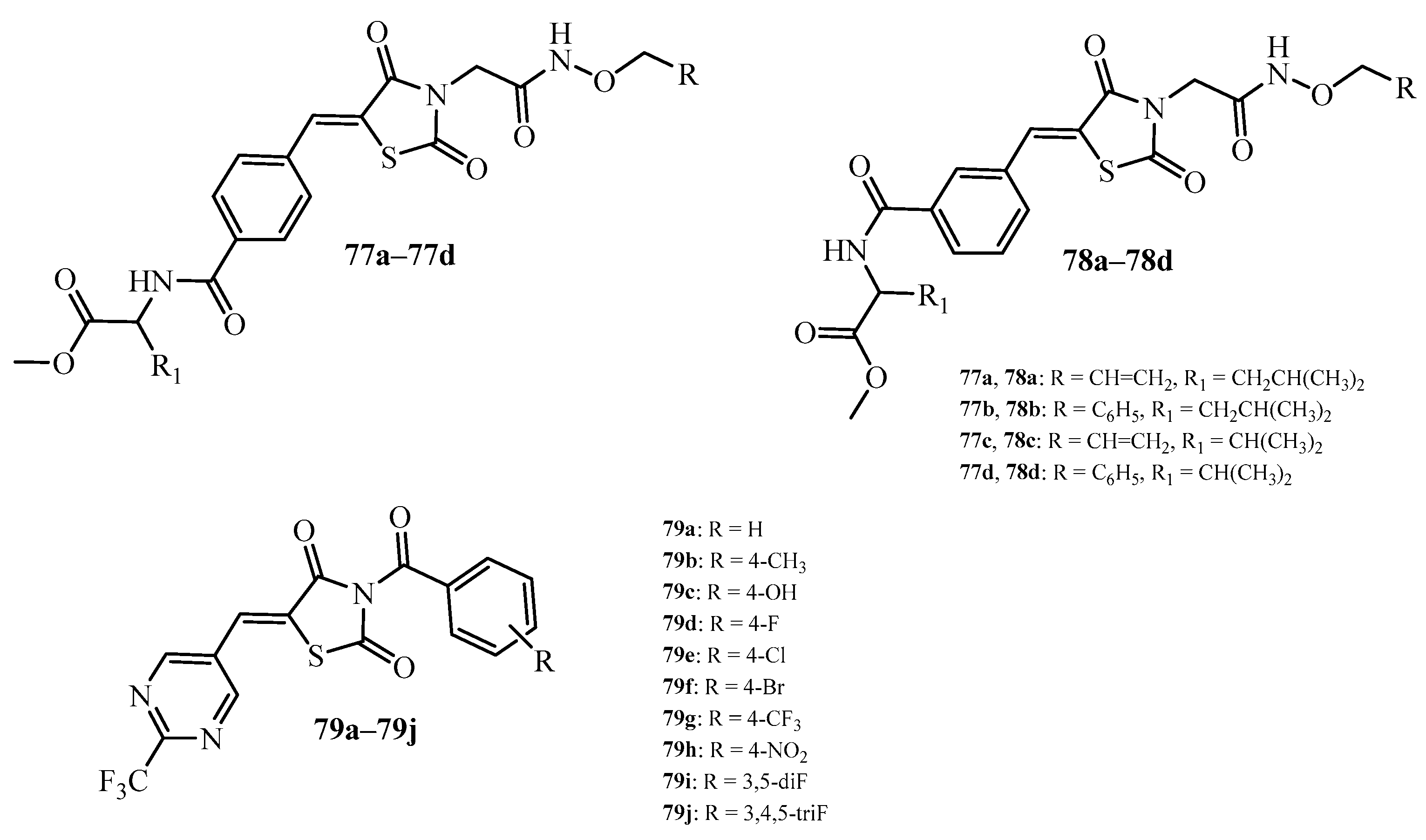
| Compound | Mycobacterium tuberculosis Strains, MIC (µg/mL) | ||
|---|---|---|---|
| Drug-Sensitive H37Ra | MDR | XDR | |
| 40a | 62.5 | >125 | na |
| 40b | 0.48 | 3.9 | 31.25 |
| 40c | 31.25 | na | na |
| 40d | 15.63 | na | na |
| 40e | 1.95 | 15.63 | >125 |
| 40f | 15.63 | 125 | na |
| 40g | 7.81 | 62.5 | na |
| 40h | 0.12 | 1.95 | 7.81 |
| 40i | 0.98 | 15.63 | 125 |
| 40j | 3.9 | 31.25 | na |
| 40k | 7.81 | 125 | na |
| 40l | 3.9 | 62.5 | na |
| 40m | 0.48 | 7.81 | 15.63 |
| isoniazid | 0.12 | na | na |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drzał, W.; Trotsko, N. Review of Recent Advances in Thiazolidin-4-One Derivatives as Promising Antitubercular Agents (2021–Present). Molecules 2025, 30, 2201. https://doi.org/10.3390/molecules30102201
Drzał W, Trotsko N. Review of Recent Advances in Thiazolidin-4-One Derivatives as Promising Antitubercular Agents (2021–Present). Molecules. 2025; 30(10):2201. https://doi.org/10.3390/molecules30102201
Chicago/Turabian StyleDrzał, Wiktoria, and Nazar Trotsko. 2025. "Review of Recent Advances in Thiazolidin-4-One Derivatives as Promising Antitubercular Agents (2021–Present)" Molecules 30, no. 10: 2201. https://doi.org/10.3390/molecules30102201
APA StyleDrzał, W., & Trotsko, N. (2025). Review of Recent Advances in Thiazolidin-4-One Derivatives as Promising Antitubercular Agents (2021–Present). Molecules, 30(10), 2201. https://doi.org/10.3390/molecules30102201







