Physical, Chemical, and Enzymatic Pretreatment of Spent Hops and Its Impact on Xanthohumol Extraction Yield
Abstract
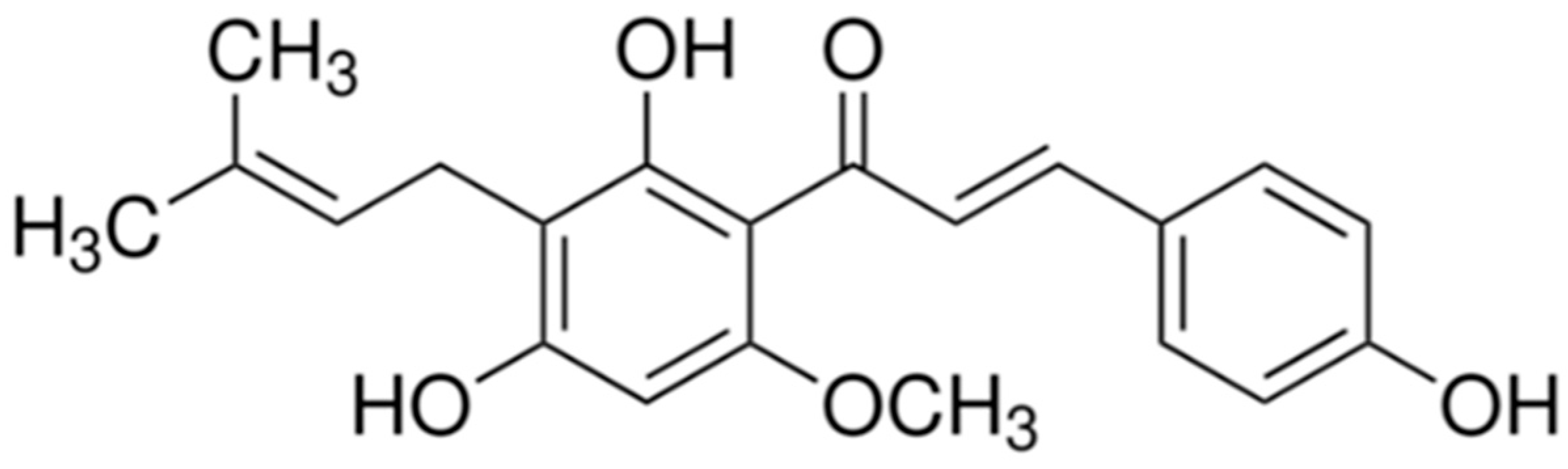
:1. Introduction
2. Results
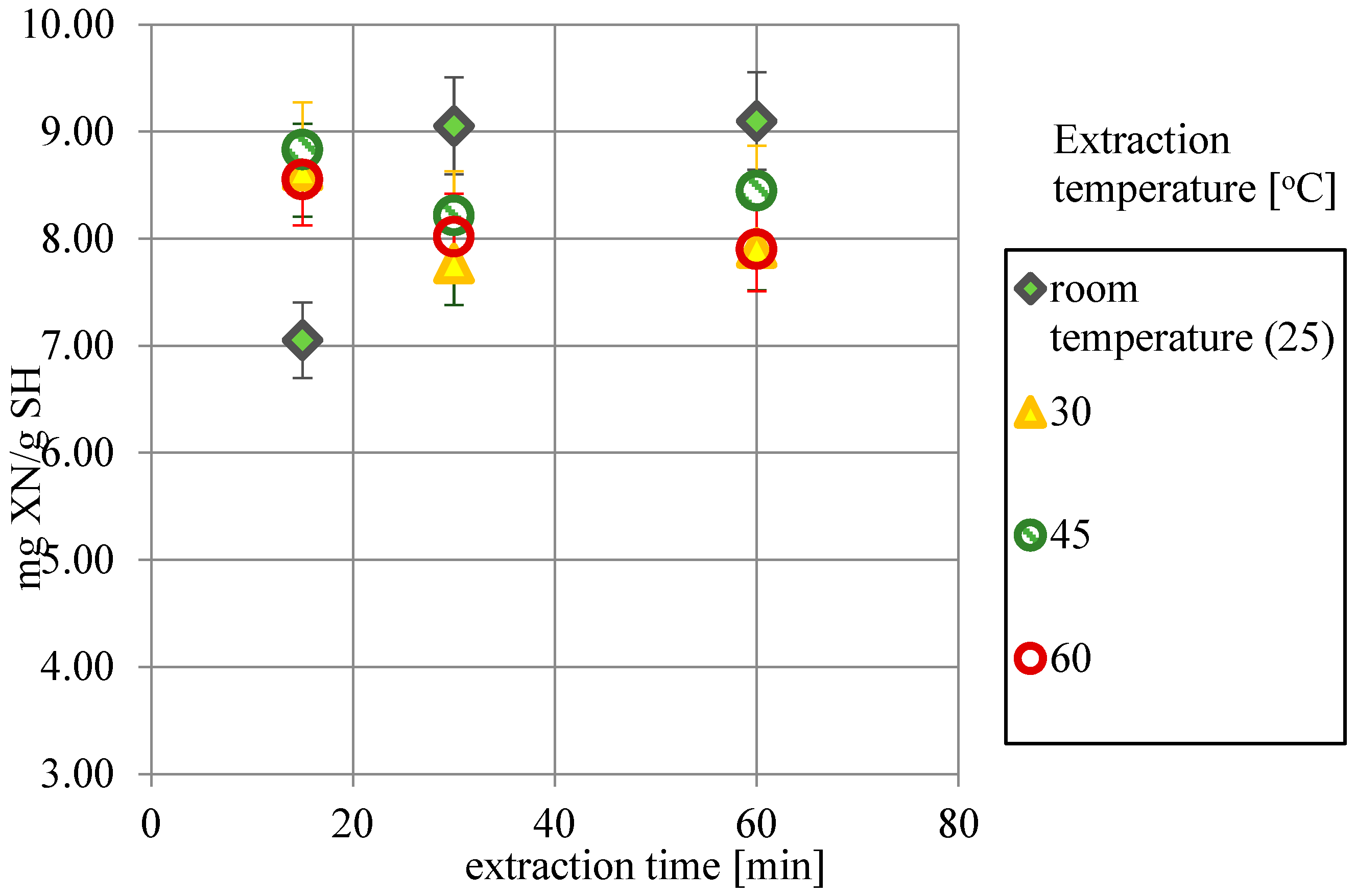
2.1. The Influence of Time and Temperature on XN Extraction Yield
2.2. Physical Pretreatment
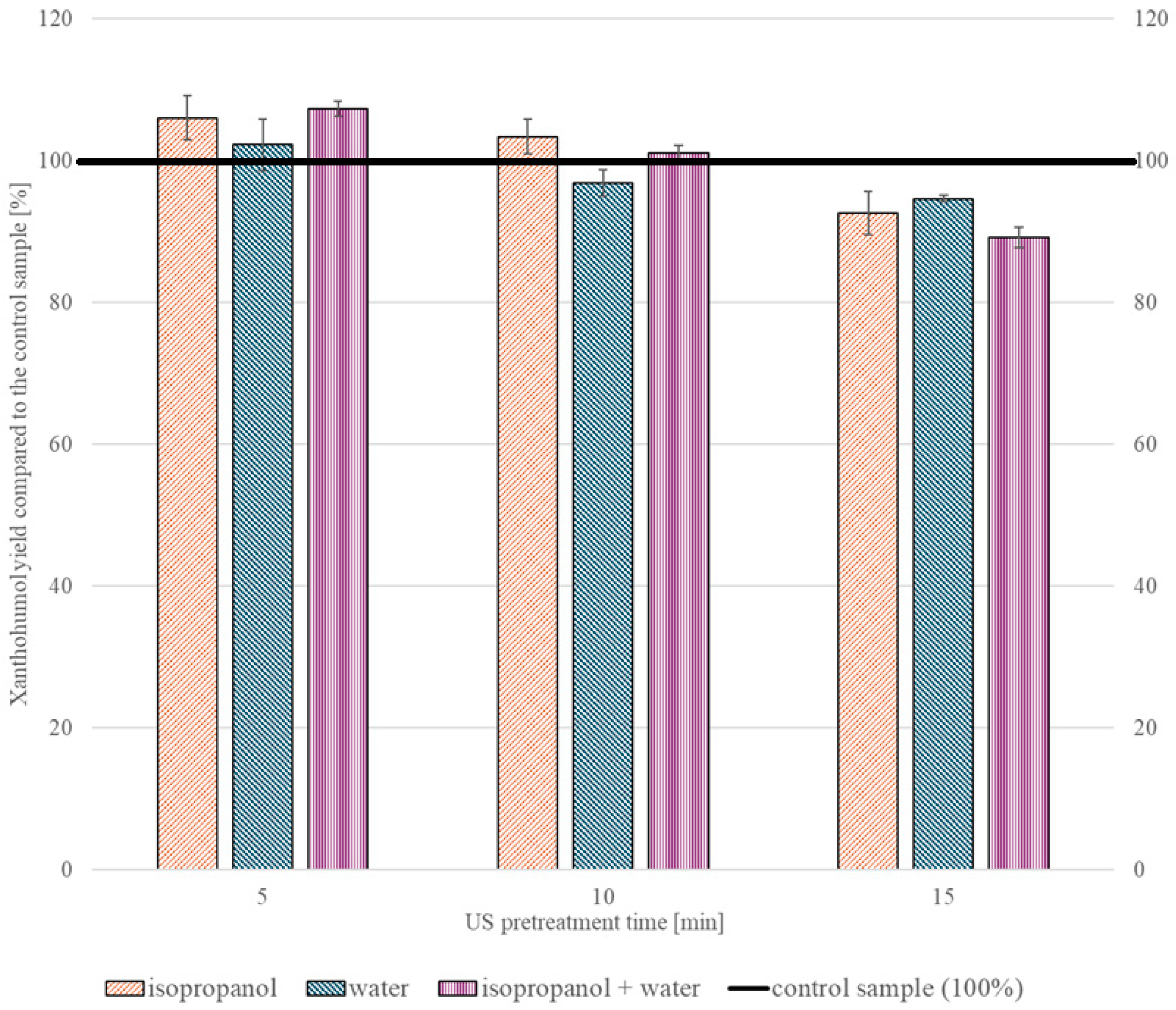
2.2.1. Ultrasonic Treatment
2.2.2. Microwave Treatment
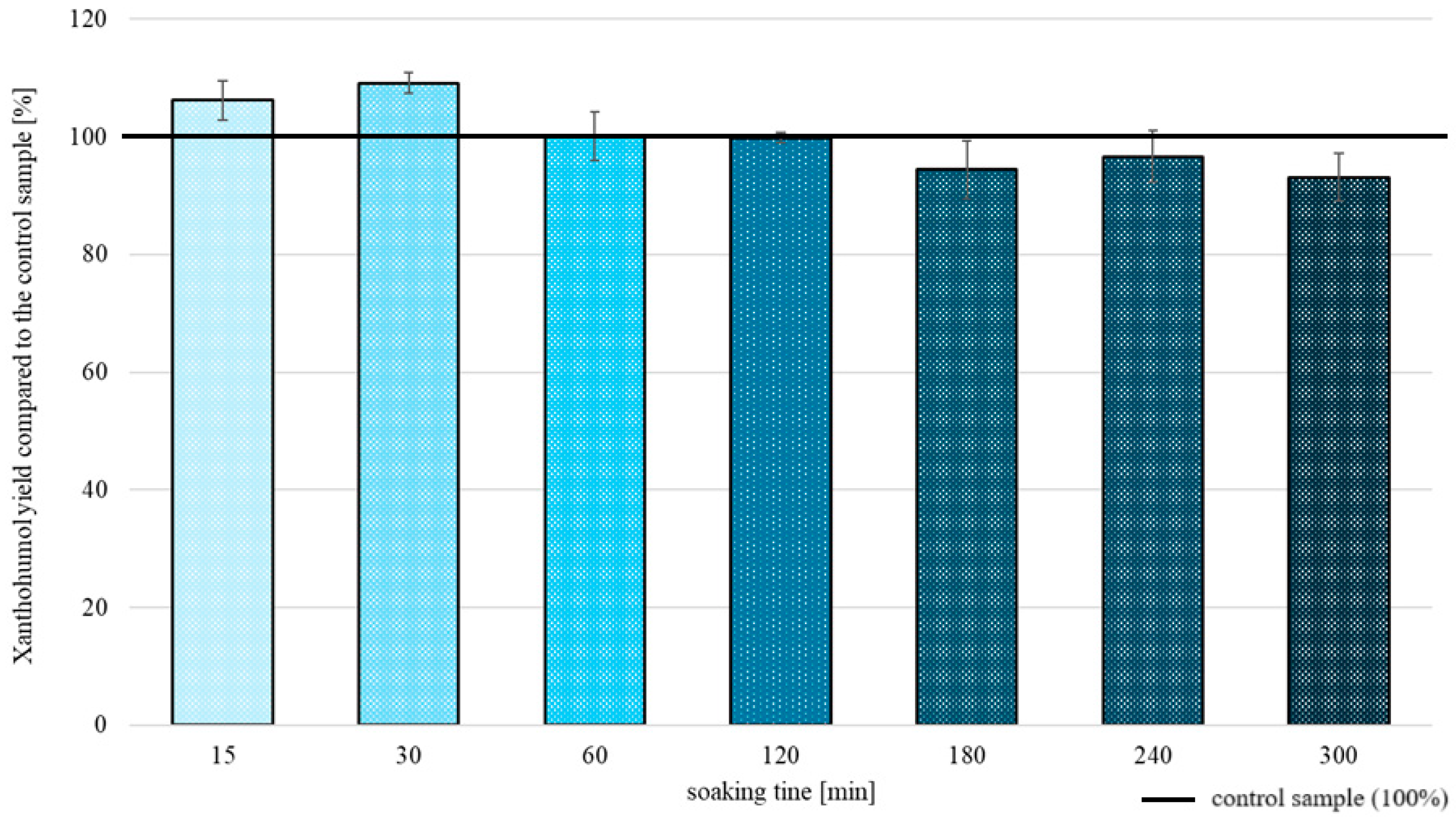
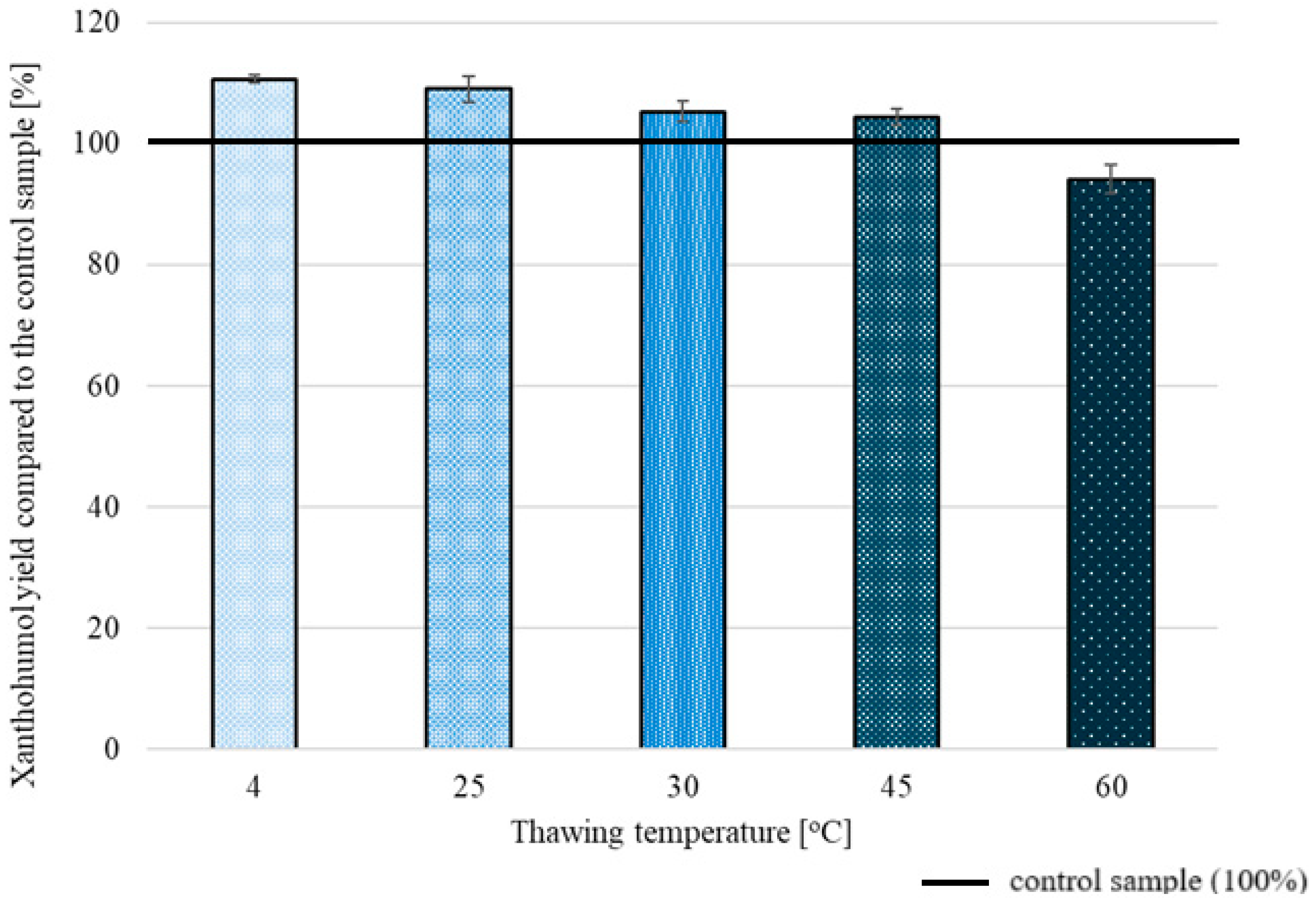
2.2.3. Freeze/Thaw
2.2.4. Ultrasound and Microwave Thawing
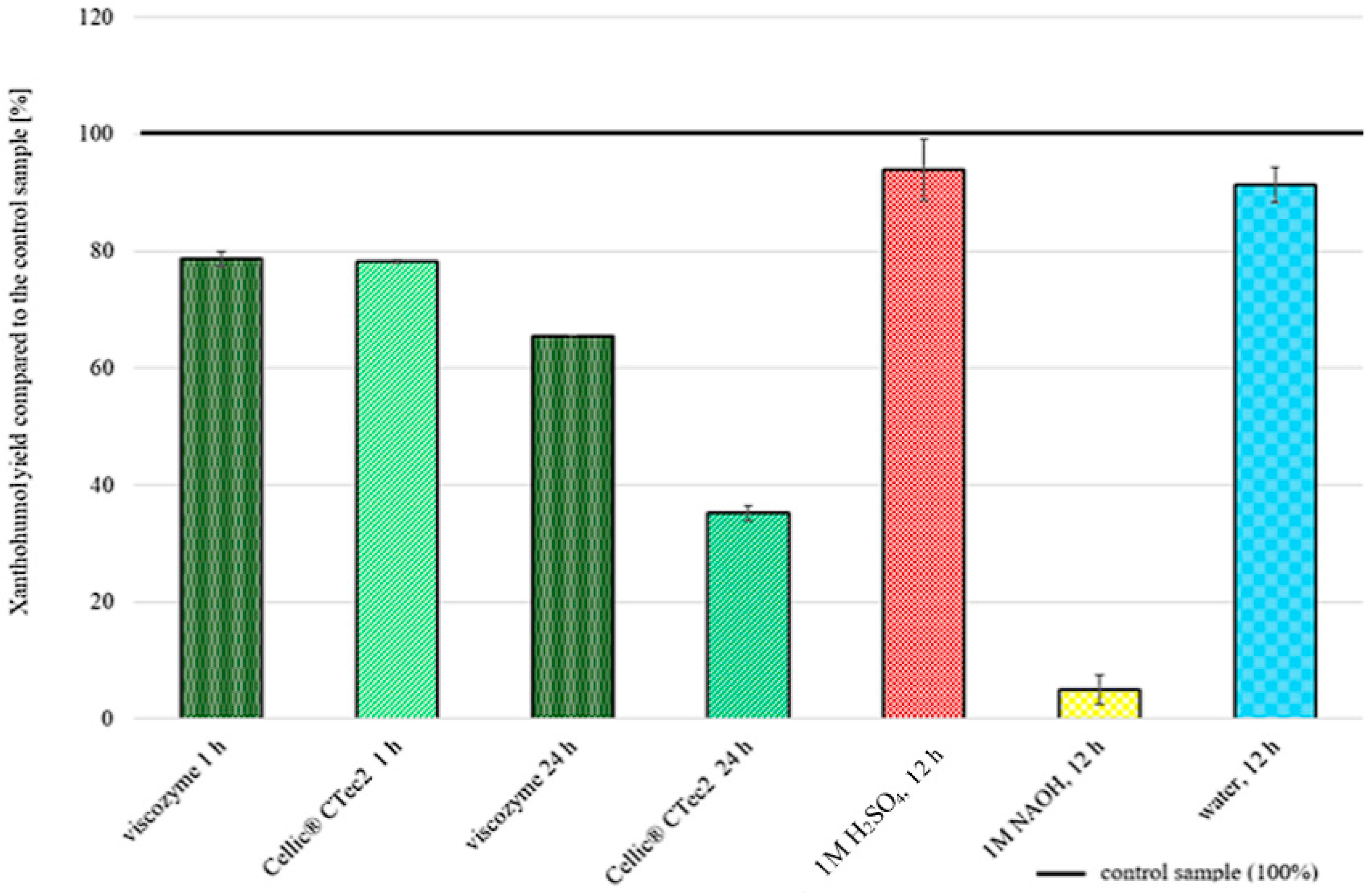
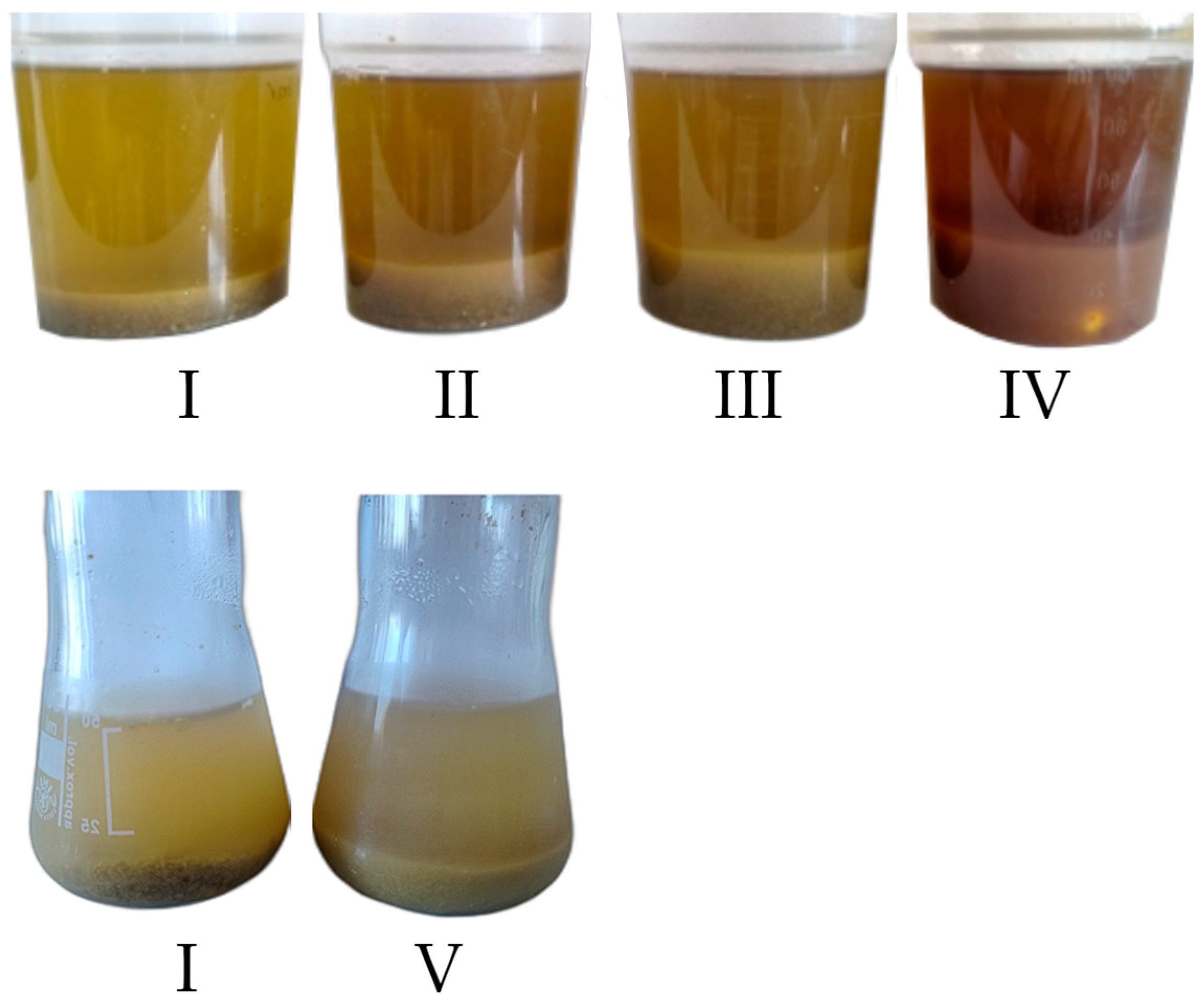
2.3. Chemical and Enzymatic Pretreatment
3. Discussion
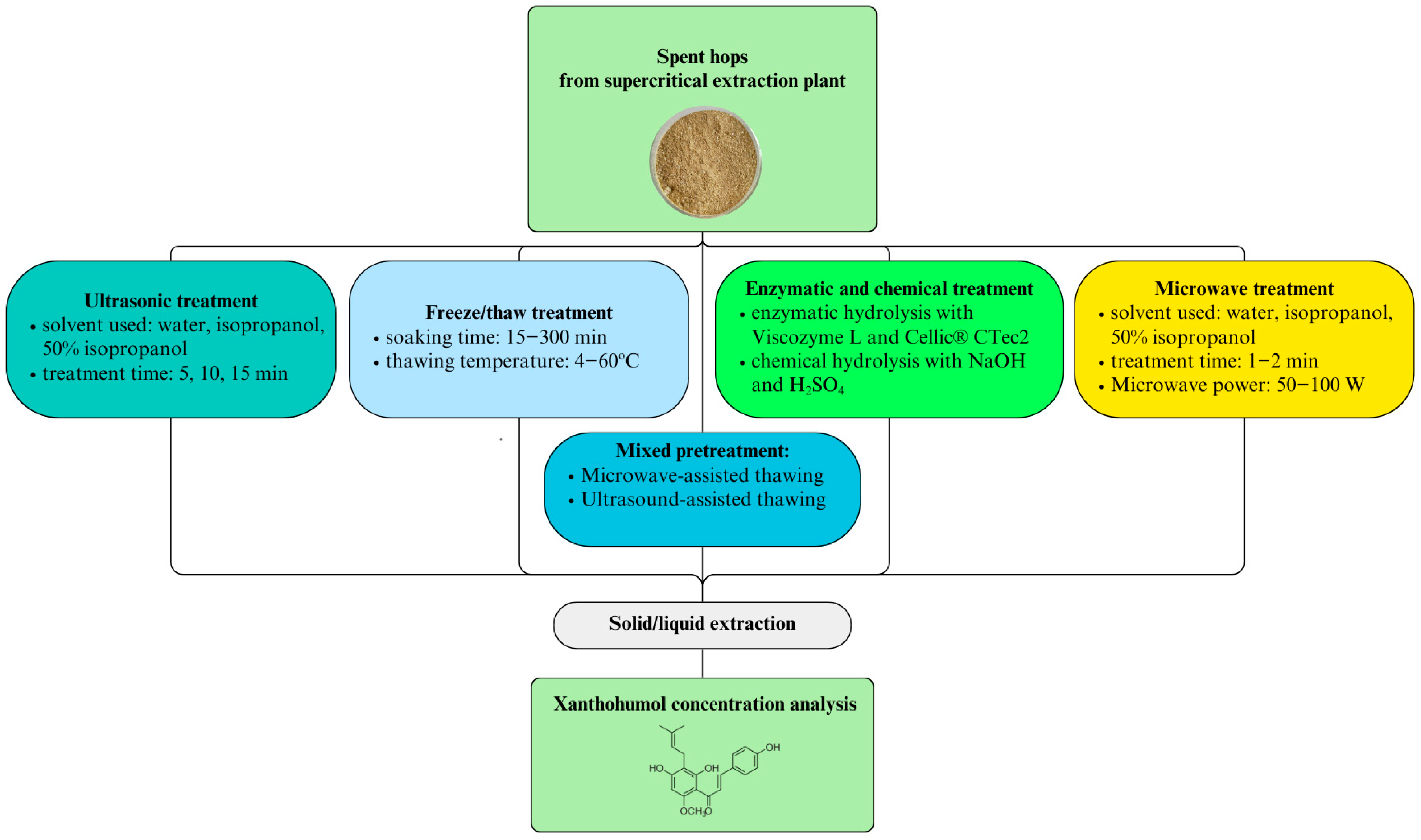
4. Materials and Methods
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanz, V.; Torres, M.D.; López Vilariño, J.M.; Domínguez, H. What Is New on the Hop Extraction? Trends Food Sci. Technol. 2019, 93, 12–22. [Google Scholar] [CrossRef]
- Kostrzewa, D.; Dobrzyńska-Inger, A.; Rój, E. Experimental Data on Xanthohumol Solubility in Supercritical Carbon Dioxide. Fluid Phase Equilibria 2013, 360, 445–450. [Google Scholar] [CrossRef]
- Hieronymus, S. For the Love of Hops the Practical Guide to Aroma, Bitterness and the Culture of Hops; Brewers Association: Boulder, CO, USA, 2012; ISBN 1-938469-01-1. [Google Scholar]
- Patil, Amol Persistence Market Research—Hop Extracts Market. Available online: https://www.persistencemarketresearch.com/market-research/hop-extracts-market.asp (accessed on 1 April 2025).
- Liu, M.; Hansen, P.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological Profile of Xanthohumol, a Prenylated Flavonoid from Hops (Humulus lupulus). Molecules 2015, 20, 754–779. [Google Scholar] [CrossRef]
- Gomes, D.; Rodrigues, L.R.; Rodrigues, J.L. Perspectives on the Design of Microbial Cell Factories to Produce Prenylflavonoids. Int. J. Food Microbiol. 2022, 367, 109588. [Google Scholar] [CrossRef]
- Jiang, C.-H.; Sun, T.-L.; Xiang, D.-X.; Wei, S.-S.; Li, W.-Q. Anticancer Activity and Mechanism of Xanthohumol: A Prenylated Flavonoid From Hops (Humulus lupulus L.). Front. Pharmacol. 2018, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Dostálek, P.; Karabín, M.; Jelínek, L. Hop Phytochemicals and Their Potential Role in Metabolic Syndrome Prevention and Therapy. Molecules 2017, 22, 1761. [Google Scholar] [CrossRef]
- Vicente de Andrade Silva, G.; Demaman Arend, G.; Antonio Ferreira Zielinski, A.; Di Luccio, M.; Ambrosi, A. Xanthohumol Properties and Strategies for Extraction from Hops and Brewery Residues: A Review. Food Chem. 2023, 404, 134629. [Google Scholar] [CrossRef]
- Kamiński, D.M.; Gawęda, K.; Arczewska, M.; Senczyna, B.; Gagoś, M. A Kinetic Study of Xanthohumol Cyclization to Isoxanthohumol—A Role of Water. J. Mol. Struct. 2017, 1139, 10–16. [Google Scholar] [CrossRef]
- Luo, J.; Pan, Q.; Chen, Y.; Huang, W.; Chen, Q.; Zhao, T.; Guo, Z.; Liu, Y.; Lu, B. Storage Stability and Degradation Mechanism of Xanthohumol in Humulus lupulus L. and Beer. Food Chem. 2024, 437, 137778. [Google Scholar] [CrossRef]
- Carvalho, D.O.; Guido, L.F. A Review on the Fate of Phenolic Compounds during Malting and Brewing: Technological Strategies and Beer Styles. Food Chem. 2022, 372, 131093. [Google Scholar] [CrossRef]
- Aggarwal, S.; Jain, T. Modern Pretreatment Techniques for Phytochemical Extraction. Nutr. Food Sci. 2019, 49, 441–454. [Google Scholar] [CrossRef]
- Debs, E.; Abi-Khattar, A.-M.; Rajha, H.N.; Abdel-Massih, R.M.; Assaf, J.-C.; Koubaa, M.; Maroun, R.G.; Louka, N. Valorization of Olive Leaves through Polyphenol Recovery Using Innovative Pretreatments and Extraction Techniques: An Updated Review. Separations 2023, 10, 587. [Google Scholar] [CrossRef]
- Mat Husin, M.A.; Mohd Yasin, N.H.; Takriff, M.S.; Jamar, N.H. A Review on Pretreatment Methods for Lipid Extraction from Microalgae Biomass. Prep. Biochem. Biotechnol. 2024, 54, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, L.; Li, X.; Shi, J.; Scanlon, M.; Xue, S.; Nosworthy, M.; Vafaei, N. Canola Seed Protein: Pretreatment, Extraction, Structure, Physicochemical and Functional Characteristics. Foods 2024, 13, 1357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Baik, O.-D.; Choi, Y.J.; Kim, S.-M. Pretreatments for the Efficient Extraction of Bioactive Compounds from Plant-Based Biomaterials. Crit. Rev. Food Sci. Nutr. 2014, 54, 1283–1297. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhu, X.; Jambrak, A.R.; Sun, D.-W.; Tiwari, B.K. Mechanistic and Synergistic Aspects of Ultrasonics and Hydrodynamic Cavitation for Food Processing. Crit. Rev. Food Sci. Nutr. 2023, 64, 8587–8608. [Google Scholar] [CrossRef]
- Panda, D.; Manickam, S. Cavitation Technology—The Future of Greener Extraction Method: A Review on the Extraction of Natural Products and Process Intensification Mechanism and Perspectives. Appl. Sci. 2019, 9, 766. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A Comprehensive Review of Ultrasonic Assisted Extraction (UAE) for Bioactive Components: Principles, Advantages, Equipment, and Combined Technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Yuan, J.; Li, H.; Tao, W.; Han, Q.; Dong, H.; Zhang, J.; Jing, Y.; Wang, Y.; Xiong, Q.; Xu, T. An Effective Method for Extracting Anthocyanins from Blueberry Based on Freeze-Ultrasonic Thawing Technology. Ultrason. Sonochem. 2020, 68, 105192. [Google Scholar] [CrossRef]
- Olivares-Galván, S.; Marina, M.L.; García, M.C. Extraction of Valuable Compounds from Brewing Residues: Malt Rootlets, Spent Hops, and Spent Yeast. Trends Food Sci. Technol. 2022, 127, 181–197. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Carbone, K.; Macchioni, V.; Petrella, G.; Cicero, D.O. Exploring the Potential of Microwaves and Ultrasounds in the Green Extraction of Bioactive Compounds from Humulus lupulus for the Food and Pharmaceutical Industry. Ind. Crops Prod. 2020, 156, 112888. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Feng, C.; Liu, X.; Qin, F.; Liang, C.; Bian, H.; Qin, C.; Yao, S. Green, Efficient Extraction of Bamboo Hemicellulose Using Freeze-Thaw Assisted Alkali Treatment. Bioresour. Technol. 2021, 333, 125107. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.; Bao, G.; Qian, H.; Song, Z.; Qi, Z.; Zhang, M.; Chen, W.; Dong, W. Physiological Response Characteristics in Medicago Sativa Under Freeze-Thaw and Deicing Salt Stress. Water. Air. Soil Pollut. 2018, 229, 196. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, W.; Chen, Z.; Wu, T. Freeze-Thaw Induced Gelation of Alginates. Carbohydr. Polym. 2016, 148, 45–51. [Google Scholar] [CrossRef]
- Ando, Y.; Hagiwara, S.; Nabetani, H.; Okunishi, T.; Okadome, H. Impact of Ice Crystal Development on Electrical Impedance Characteristics and Mechanical Property of Green Asparagus Stems. J. Food Eng. 2019, 256, 46–52. [Google Scholar] [CrossRef]
- Liu, M.Q.; Yang, X.Q.; Qi, B.; Li, L.H.; Deng, J.C.; Hu, X. Study of Ultrasonic-Freeze-Thaw-Cycle Assisted Extraction of Polysaccharide and Phycobiliprotein from Gracilaria lemaneiformis. Adv. Mater. Res. 2013, 781–784, 1818–1824. [Google Scholar] [CrossRef]
- Wang, X.M.; Wang, L.J.; Yu, M.; Chen, H. Freeze-Thaw and Sulfuric Acid Pretreatment of Wheat Straw for Fermentable Sugar Release. Adv. Mater. Res. 2013, 724–725, 257–260. [Google Scholar] [CrossRef]
- Li, B.; Sun, D.-W. Novel Methods for Rapid Freezing and Thawing of Foods—A Review. J. Food Eng. 2002, 54, 175–182. [Google Scholar] [CrossRef]
- Parthiba Karthikeyan, O.; Trably, E.; Mehariya, S.; Bernet, N.; Wong, J.W.C.; Carrere, H. Pretreatment of Food Waste for Methane and Hydrogen Recovery: A Review. Bioresour. Technol. 2018, 249, 1025–1039. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Liu, Y.; Zhang, H. Effects of Multiple Freeze–Thaw Cycles on the Quality of Frozen Dough. Cereal Chem. 2018, 95, 499–507. [Google Scholar] [CrossRef]
- Cao, L.; Zhu, J.; Deng, B.; Zeng, F.; Wang, S.; Ma, Y.; Qin, C.; Yao, S. Efficient Swelling and Mercerization of Bagasse Fiber by Freeze-Thaw-Assisted Alkali Treatment. Front. Energy Res. 2022, 10, 851543. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Rahman, M.S.; Kim, A.-N.; Son, Y.; Gu, S.; Lee, M.-H.; Kim, J.I.; Ha, T.J.; Kwak, D.; Kim, H.-J.; et al. Effect of Freeze-Thaw Pretreatment on Yield and Quality of Perilla Seed Oil. LWT 2020, 122, 109026. [Google Scholar] [CrossRef]
- Tan, H.T.; Khong, N.M.H.; Khaw, Y.S.; Ahmad, S.A.; Yusoff, F.M. Optimization of the Freezing-Thawing Method for Extracting Phycobiliproteins from Arthrospira sp. Molecules 2020, 25, 3894. [Google Scholar] [CrossRef]
- Sulistiawati, E.; Rochmadi, R.; Hidayat, M.; Budiman, A. Enhancement of Phycocyanin Extraction from Dry Spirulina Platensis Powder by Freezing-Thawing Pre-Treatment. Int. J. Technol. 2023, 14, 780. [Google Scholar] [CrossRef]
- Wang, B.; Bai, X.; Du, X.; Pan, N.; Shi, S.; Xia, X. Comparison of Effects from Ultrasound Thawing, Vacuum Thawing and Microwave Thawing on the Quality Properties and Oxidation of Porcine Longissimus Lumborum. Foods 2022, 11, 1368. [Google Scholar] [CrossRef] [PubMed]
- Holzwarth, M.; Korhummel, S.; Carle, R.; Kammerer, D.R. Evaluation of the Effects of Different Freezing and Thawing Methods on Color, Polyphenol and Ascorbic Acid Retention in Strawberries (Fragaria×ananassa Duch.). Food Res. Int. 2012, 48, 241–248. [Google Scholar] [CrossRef]
- Li, M.; Zhou, C.; Wang, B.; Zeng, S.; Mu, R.; Li, G.; Li, B.; Lv, W. Research Progress and Application of Ultrasonic- and Microwave-assisted Food Processing Technology. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3707–3731. [Google Scholar] [CrossRef]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Effects of Enzymatic Pretreatment of Seeds on the Physicochemical Properties, Bioactive Compounds, and Antioxidant Activity of Pomegranate Seed Oil. Molecules 2021, 26, 4575. [Google Scholar] [CrossRef]
- Dzięcioł, M. Influence of Enzymatic Pretreatment on Yield and Chemical Composition of Rosmarinus Officinalis Essential Oil. Pol. J. Chem. Technol. 2022, 24, 61–66. [Google Scholar] [CrossRef]
- Yang, C.; Liu, W.; Zhu, X.; Zhang, X.; Wei, Y.; Huang, J.; Yang, F.; Yang, F. Ultrasound-Assisted Enzymatic Digestion for Efficient Extraction of Proteins from Quinoa. LWT 2024, 194, 115784. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Akbarovich, S.A.; Kim, C.K.; Lee, W.Y. Effect of Combined Ultrasound-enzyme Treatment on Recovery of Phenolic Compounds, Antioxidant Capacity, and Quality of Plum (Prunus salicina L.) Juice. J. Food Process. Preserv. 2021, 45, e15074. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C.F.R. Enzyme-Assisted Extractions of Polyphenols—A Comprehensive Review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Magalhães, P.J.; Carvalho, D.O.; Cruz, J.M.; Guido, L.F.; Barros, A.A. Fundamentals and Health Benefits of Xanthohumol, a Natural Product Derived from Hops and Beer. Nat. Prod. Commun. 2009, 4, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Zołnierczyk, A.K.; Mączka, W.K.; Grabarczyk, M.; Wińska, K.; Woźniak, E.; Anioł, M. Isoxanthohumol-Biologically Active Hop Flavonoid. Fitoterapia 2015, 103, 71–82. [Google Scholar] [CrossRef]
- Hassan, N.S.; Badri, K.H. Lignin Recovery from Alkaline Hydrolysis and Glycerolysis of Oil Palm Fiber. AIP Conf. Proc. 2014, 1614, 433–438. [Google Scholar]
- Gonçalves, M.D.P.; Silveira Junior, V. Energy Consumption Reduction Strategy for Freezing of Packaged Food Products. Food Sci. Technol. 2017, 38, 341–347. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Shao, L.; Yu, C.; Yu, H.; Li, Y. Effect of Freezing/Thawing Temperature on the Viscoelastic and Nutritional Qualities of Carrots. Int. J. Food Prop. 2016, 19, 1413–1424. [Google Scholar] [CrossRef]
- Moon, J.H.; Park, J.K.; Park, B.Y.; Jeon, H.J.; Choi, G.S.; Lee, G.M. Extraction of the Outer Membrane Protein Pertactin from Bordetella Pertussis with Urea for the Production of Acellular Pertussis Vaccine. Biotechnol. Bioprocess Eng. 2024, 29, 505–512. [Google Scholar] [CrossRef]
- Motta, L.B.; Lopes, J.C.; Zanotti, R.F.; Bernardes, P.M.; Silva, J.D. Cryostorage of Sunflower Seeds. Biosci. J. 2014, 30, 312–391. [Google Scholar]
- Gebre-Egziabher, A.; Thomson, B.; Blankenagel, G. Destruction of Microorganisms During Thawing of Skim Milk. J. Food Prot. 1982, 45, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Calinescu, I.; Lavric, V.; Asofiei, I.; Gavrila, A.I.; Trifan, A.; Ighigeanu, D.; Martin, D.; Matei, C. Microwave Assisted Extraction of Polyphenols Using a Coaxial Antenna and a Cooling System. Chem. Eng. Process. Process Intensif. 2017, 122, 373–379. [Google Scholar] [CrossRef]
- Femenia, A.; García-Marín, M.; Simal, S.; Rosselló, C.; Blasco, M. Effects of Supercritical Carbon Dioxide (SC-CO2 ) Oil Extraction on the Cell Wall Composition of Almond Fruits. J. Agric. Food Chem. 2001, 49, 5828–5834. [Google Scholar] [CrossRef]
- Xu, B.; Azam, S.M.R.; Feng, M.; Wu, B.; Yan, W.; Zhou, C.; Ma, H. Application of Multi-Frequency Power Ultrasound in Selected Food Processing Using Large-Scale Reactors: A Review. Ultrason. Sonochem. 2021, 81, 105855. [Google Scholar] [CrossRef]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Brunton, N.P.; Rai, D.K. Recent Advances on Application of Ultrasound and Pulsed Electric Field Technologies in the Extraction of Bioactives from Agro-Industrial By-Products. Food Bioprocess Technol. 2018, 11, 223–241. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, J.; Wu, H.; Gou, M.; Jing, L.; Zhao, K.; Zhang, B.; Zhang, G.; Li, W. Molecular, Crystal and Physicochemical Properties of Granular Waxy Corn Starch after Repeated Freeze-Thaw Cycles at Different Freezing Temperatures. Int. J. Biol. Macromol. 2019, 133, 346–353. [Google Scholar] [CrossRef]
- Smichi, N.; Messaoudi, Y.; Moujahed, N.; Gargouri, M. Ethanol Production from Halophyte Juncus Maritimus Using Freezing and Thawing Biomass Pretreatment. Renew. Energy 2016, 85, 1357–1361. [Google Scholar] [CrossRef]
- Cai, L.; Cao, M.; Regenstein, J.; Cao, A. Recent Advances in Food Thawing Technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 953–970. [Google Scholar] [CrossRef]
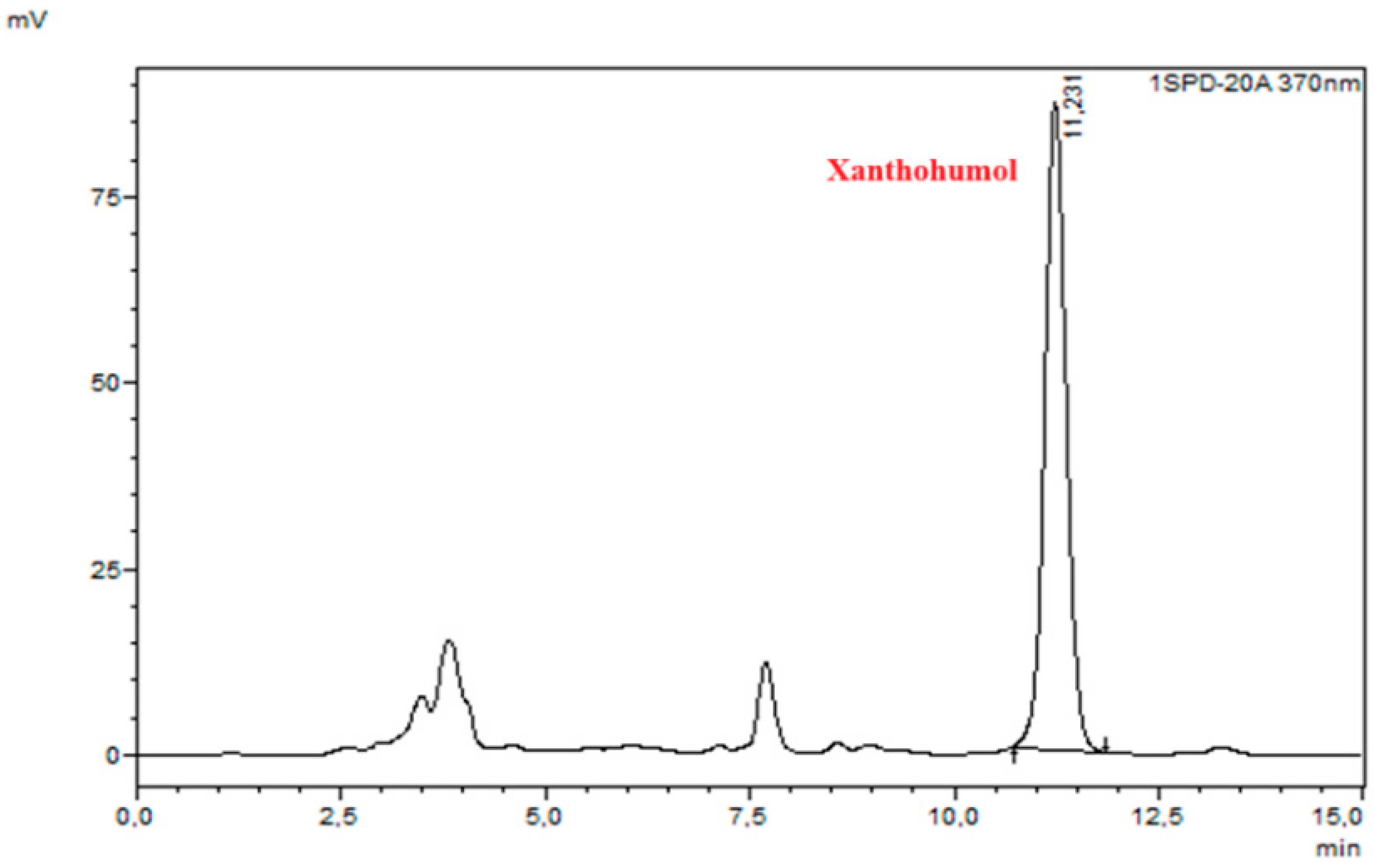
| Microwave power [W] | 50 | 100 | ||
| Treatment time [min] | 1 | 2 | 1 | 2 |
| Solvent composition: | XN extraction yield [% of control sample] | |||
| 50 mL water | 94.0 ± 0.3 | 100.1 ± 0.3 | 98.4 ± 0.5 | 95.8 ± 4.8 |
| 50 mL isopropanol | 102.9 ± 1.0 | Not performed due to solvent evaporation/mixture boiling | ||
| 50 mL water + 50 mL isopropanol | 90.6 ± 5.9 | |||
| Thawing Method | Parameters | XN Extraction Yield |
|---|---|---|
| [% of Control Sample] | ||
| Ultrasound-assisted thawing | 15 min, 35 kHz | 99.5 ± 2.2 |
| 30 min, 35 kHz | 99.2 ± 0.9 | |
| Microwave-assisted thawing | 50 W, 8 min | 86.8 ± 2.3 |
| 80 W, 4 min | 90.2 ± 6.7 | |
| 100 W, 3 min | 89.9 ± 1.7 | |
| Unassisted thawing at 25 °C | 109.02 ± 0.13 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modzelewska, A.; Jackowski, M.; Trusek, A. Physical, Chemical, and Enzymatic Pretreatment of Spent Hops and Its Impact on Xanthohumol Extraction Yield. Molecules 2025, 30, 2200. https://doi.org/10.3390/molecules30102200
Modzelewska A, Jackowski M, Trusek A. Physical, Chemical, and Enzymatic Pretreatment of Spent Hops and Its Impact on Xanthohumol Extraction Yield. Molecules. 2025; 30(10):2200. https://doi.org/10.3390/molecules30102200
Chicago/Turabian StyleModzelewska, Aleksandra, Mateusz Jackowski, and Anna Trusek. 2025. "Physical, Chemical, and Enzymatic Pretreatment of Spent Hops and Its Impact on Xanthohumol Extraction Yield" Molecules 30, no. 10: 2200. https://doi.org/10.3390/molecules30102200
APA StyleModzelewska, A., Jackowski, M., & Trusek, A. (2025). Physical, Chemical, and Enzymatic Pretreatment of Spent Hops and Its Impact on Xanthohumol Extraction Yield. Molecules, 30(10), 2200. https://doi.org/10.3390/molecules30102200






