Physical, Chemical, and Enzymatic Pretreatment of Spent Hops and Its Impact on Xanthohumol Extraction Yield
Abstract
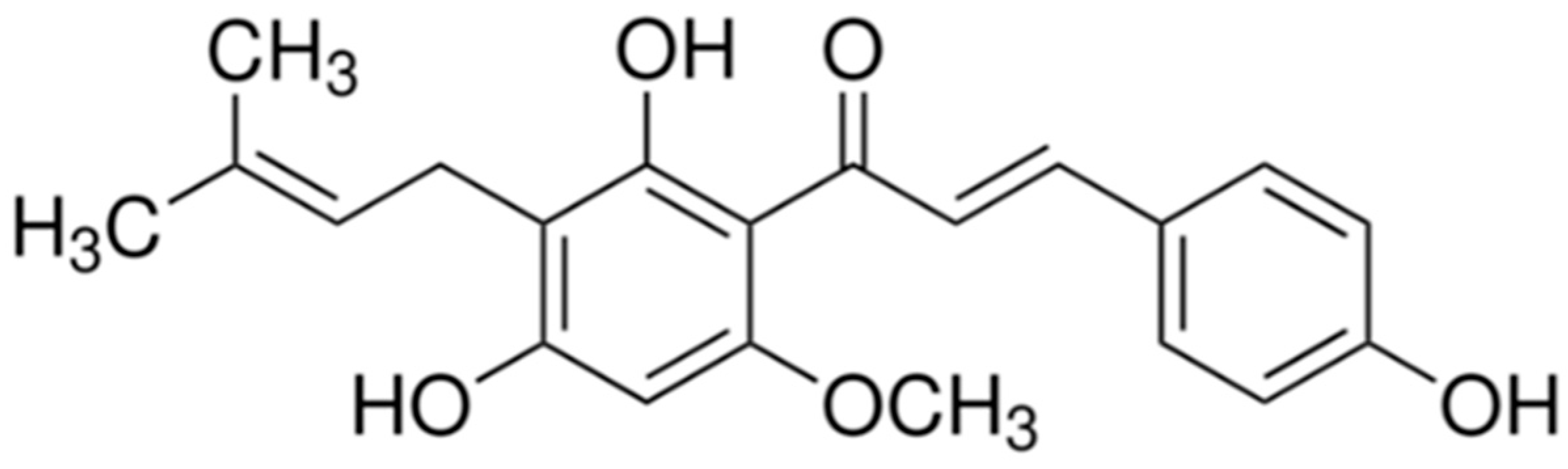
1. Introduction
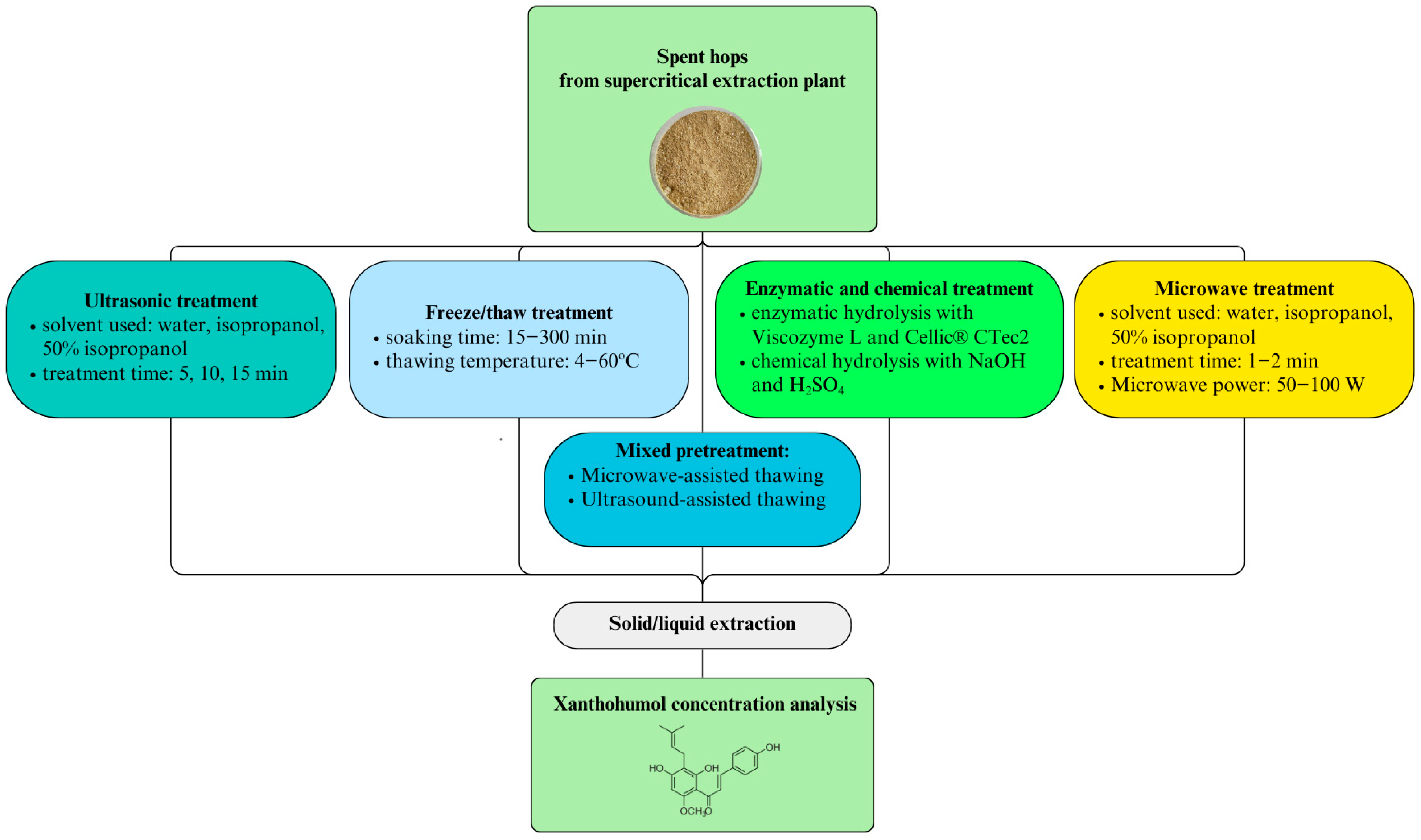
2. Results
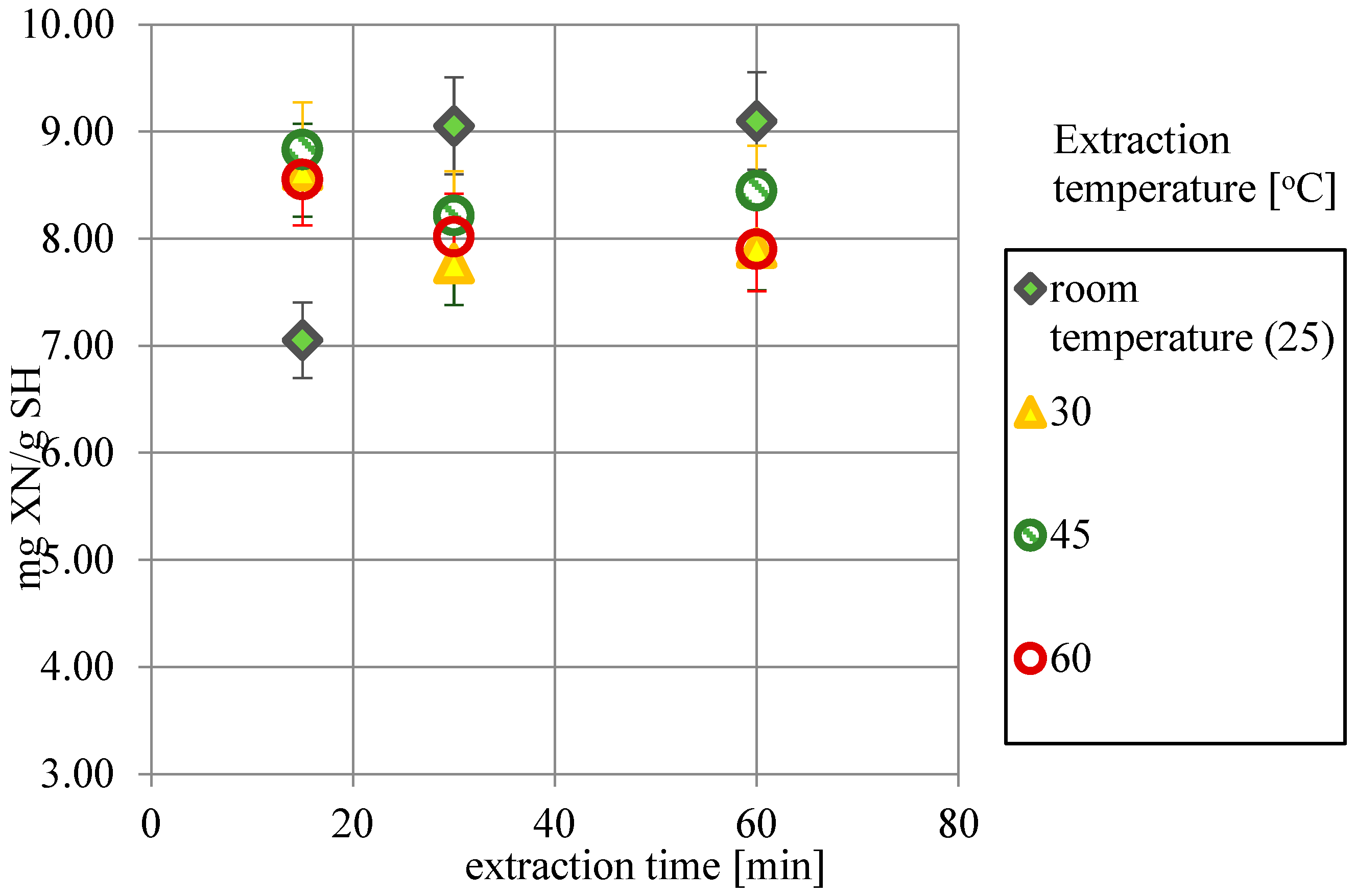
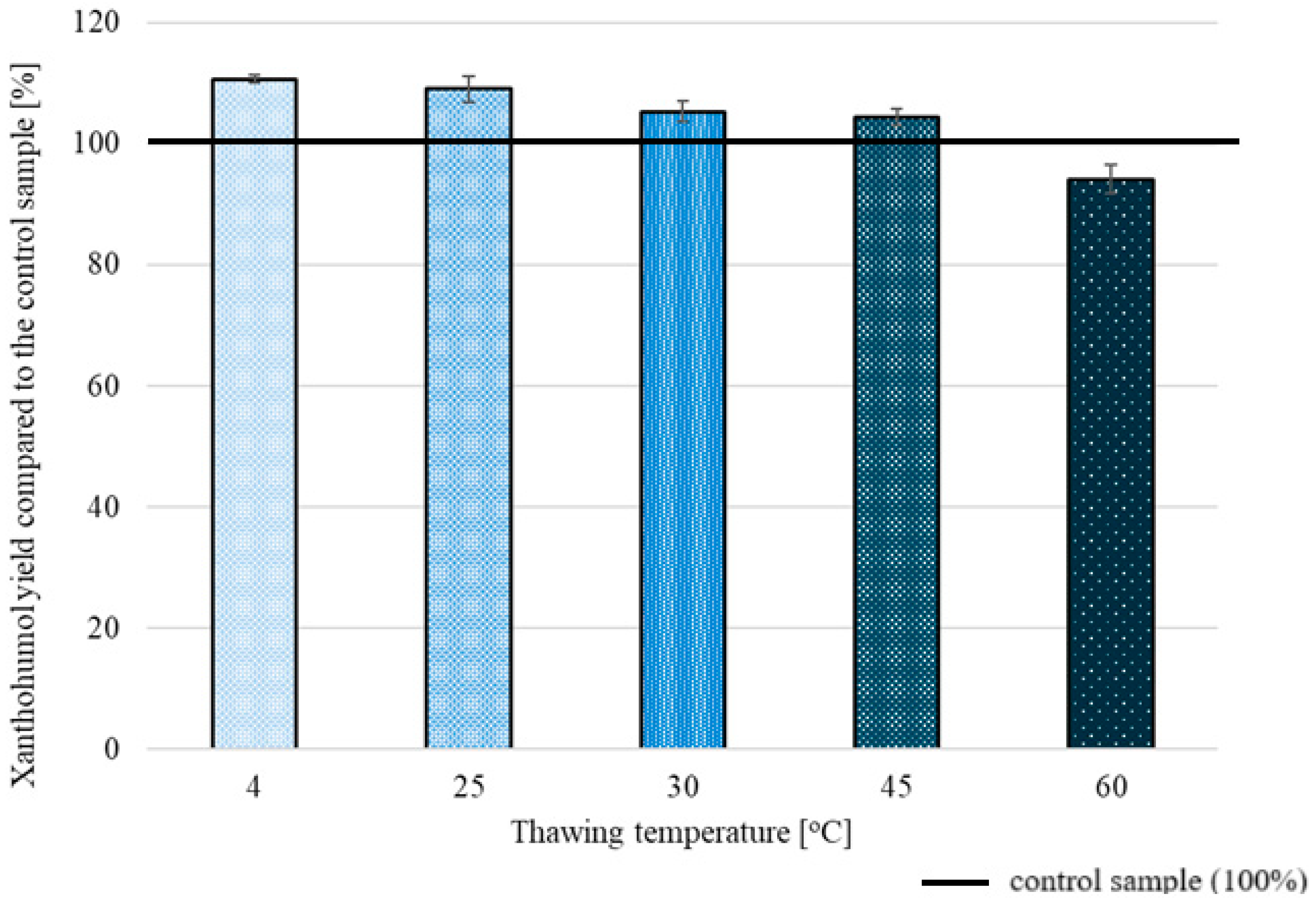
2.1. The Influence of Time and Temperature on XN Extraction Yield
2.2. Physical Pretreatment
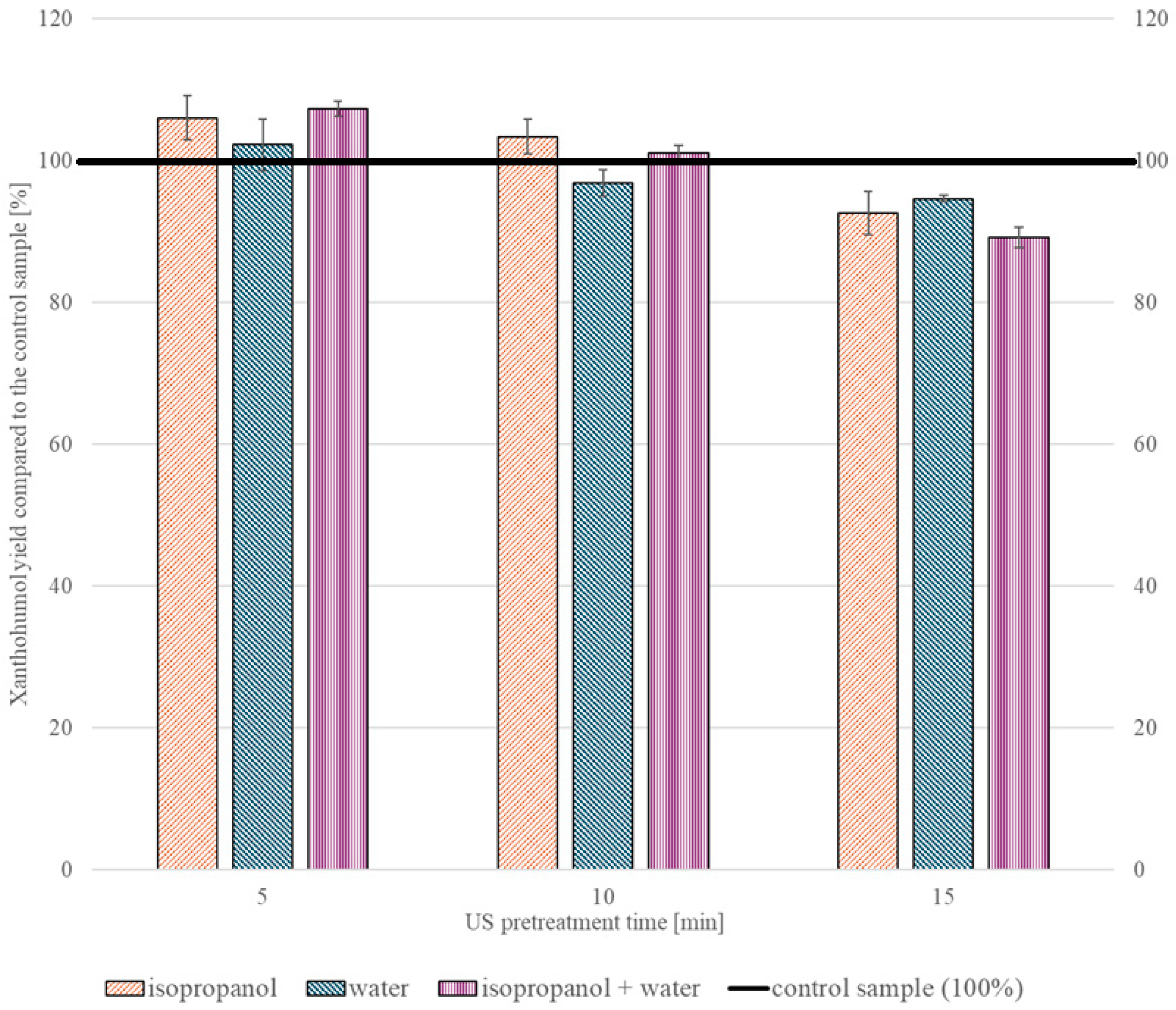
2.2.1. Ultrasonic Treatment
2.2.2. Microwave Treatment
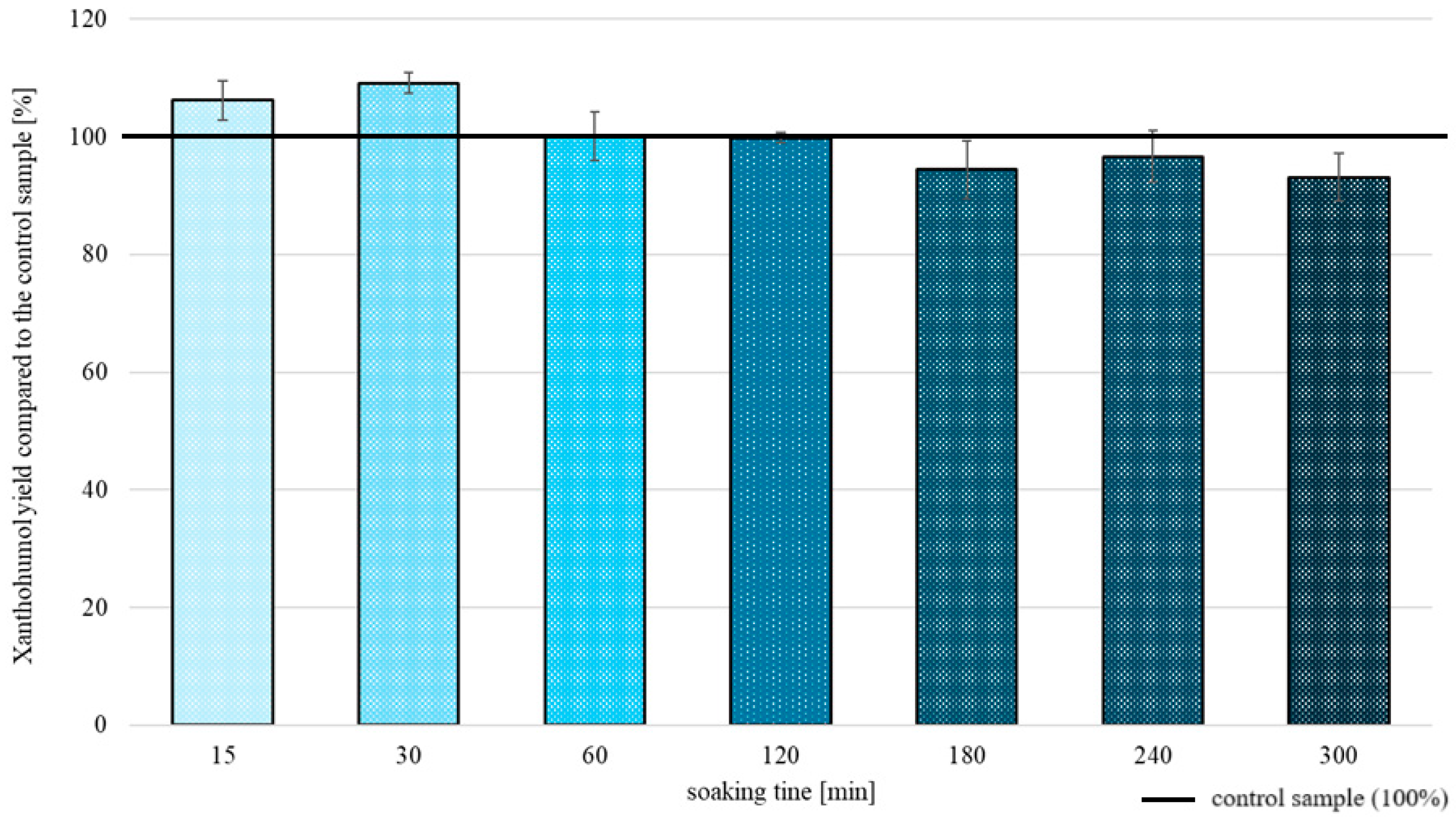
2.2.3. Freeze/Thaw
2.2.4. Ultrasound and Microwave Thawing
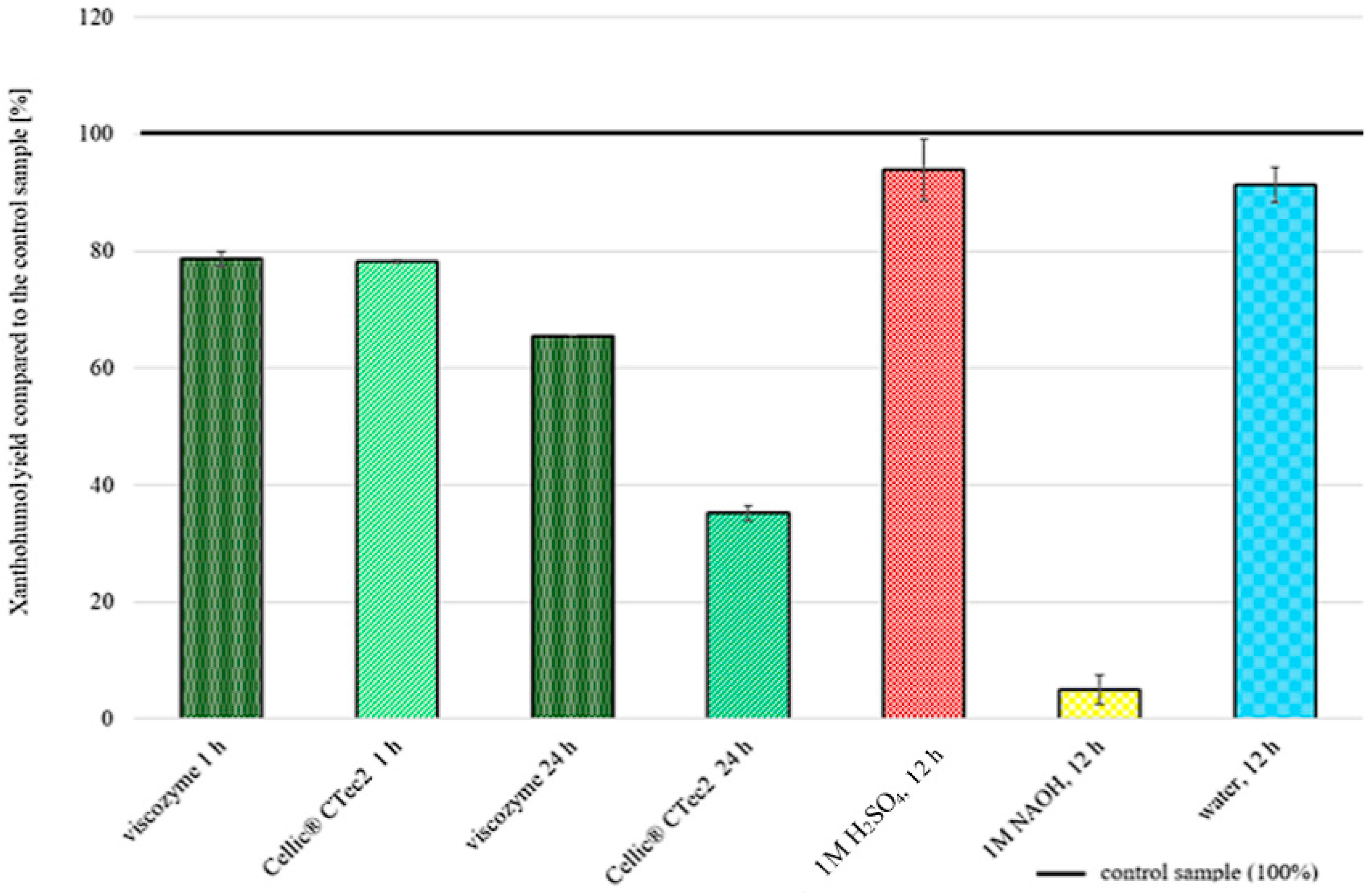
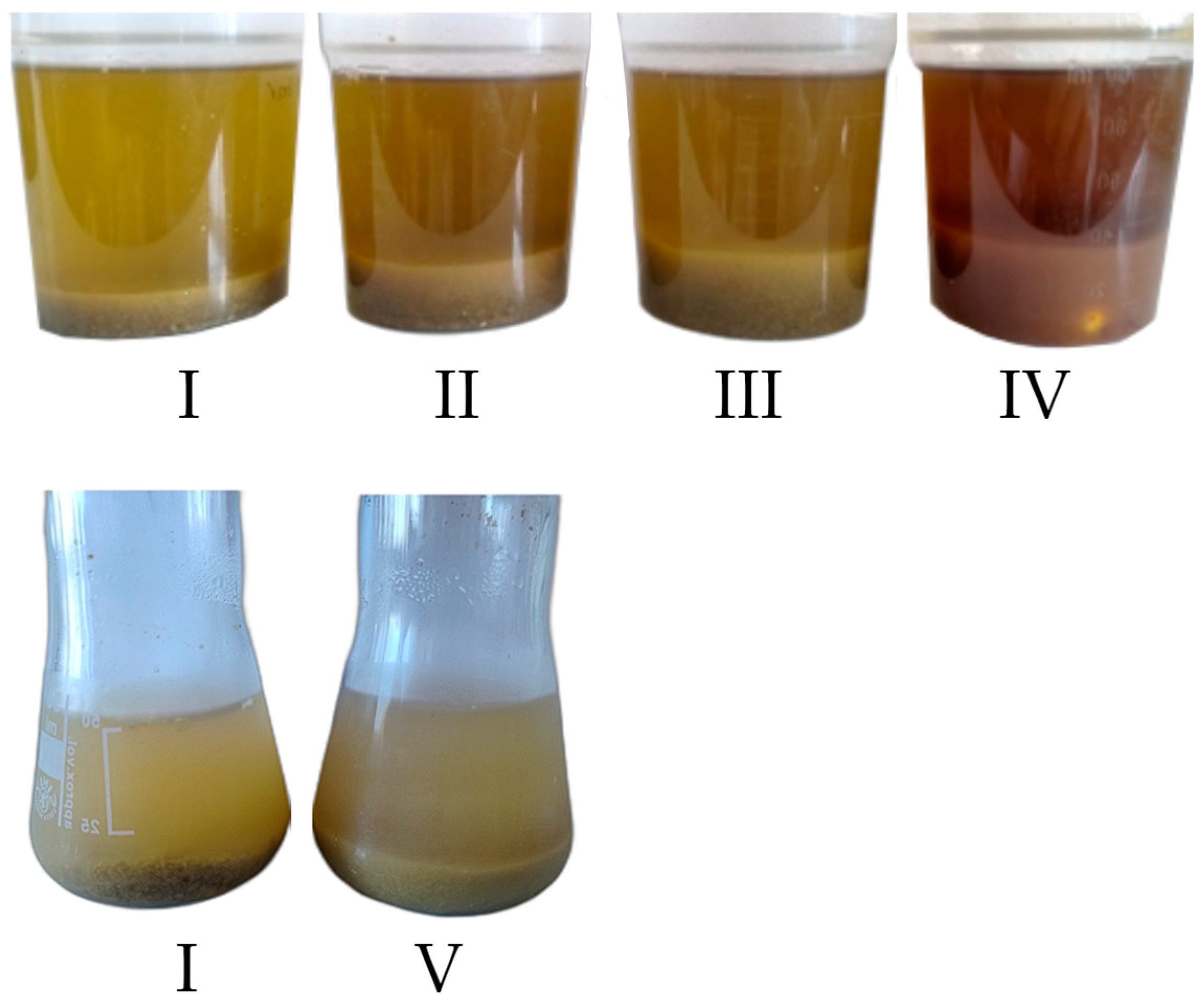
2.3. Chemical and Enzymatic Pretreatment
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanz, V.; Torres, M.D.; López Vilariño, J.M.; Domínguez, H. What Is New on the Hop Extraction? Trends Food Sci. Technol. 2019, 93, 12–22. [Google Scholar] [CrossRef]
- Kostrzewa, D.; Dobrzyńska-Inger, A.; Rój, E. Experimental Data on Xanthohumol Solubility in Supercritical Carbon Dioxide. Fluid Phase Equilibria 2013, 360, 445–450. [Google Scholar] [CrossRef]
- Hieronymus, S. For the Love of Hops the Practical Guide to Aroma, Bitterness and the Culture of Hops; Brewers Association: Boulder, CO, USA, 2012; ISBN 1-938469-01-1. [Google Scholar]
- Patil, Amol Persistence Market Research—Hop Extracts Market. Available online: https://www.persistencemarketresearch.com/market-research/hop-extracts-market.asp (accessed on 1 April 2025).
- Liu, M.; Hansen, P.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological Profile of Xanthohumol, a Prenylated Flavonoid from Hops (Humulus lupulus). Molecules 2015, 20, 754–779. [Google Scholar] [CrossRef]
- Gomes, D.; Rodrigues, L.R.; Rodrigues, J.L. Perspectives on the Design of Microbial Cell Factories to Produce Prenylflavonoids. Int. J. Food Microbiol. 2022, 367, 109588. [Google Scholar] [CrossRef]
- Jiang, C.-H.; Sun, T.-L.; Xiang, D.-X.; Wei, S.-S.; Li, W.-Q. Anticancer Activity and Mechanism of Xanthohumol: A Prenylated Flavonoid From Hops (Humulus lupulus L.). Front. Pharmacol. 2018, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Dostálek, P.; Karabín, M.; Jelínek, L. Hop Phytochemicals and Their Potential Role in Metabolic Syndrome Prevention and Therapy. Molecules 2017, 22, 1761. [Google Scholar] [CrossRef]
- Vicente de Andrade Silva, G.; Demaman Arend, G.; Antonio Ferreira Zielinski, A.; Di Luccio, M.; Ambrosi, A. Xanthohumol Properties and Strategies for Extraction from Hops and Brewery Residues: A Review. Food Chem. 2023, 404, 134629. [Google Scholar] [CrossRef]
- Kamiński, D.M.; Gawęda, K.; Arczewska, M.; Senczyna, B.; Gagoś, M. A Kinetic Study of Xanthohumol Cyclization to Isoxanthohumol—A Role of Water. J. Mol. Struct. 2017, 1139, 10–16. [Google Scholar] [CrossRef]
- Luo, J.; Pan, Q.; Chen, Y.; Huang, W.; Chen, Q.; Zhao, T.; Guo, Z.; Liu, Y.; Lu, B. Storage Stability and Degradation Mechanism of Xanthohumol in Humulus lupulus L. and Beer. Food Chem. 2024, 437, 137778. [Google Scholar] [CrossRef]
- Carvalho, D.O.; Guido, L.F. A Review on the Fate of Phenolic Compounds during Malting and Brewing: Technological Strategies and Beer Styles. Food Chem. 2022, 372, 131093. [Google Scholar] [CrossRef]
- Aggarwal, S.; Jain, T. Modern Pretreatment Techniques for Phytochemical Extraction. Nutr. Food Sci. 2019, 49, 441–454. [Google Scholar] [CrossRef]
- Debs, E.; Abi-Khattar, A.-M.; Rajha, H.N.; Abdel-Massih, R.M.; Assaf, J.-C.; Koubaa, M.; Maroun, R.G.; Louka, N. Valorization of Olive Leaves through Polyphenol Recovery Using Innovative Pretreatments and Extraction Techniques: An Updated Review. Separations 2023, 10, 587. [Google Scholar] [CrossRef]
- Mat Husin, M.A.; Mohd Yasin, N.H.; Takriff, M.S.; Jamar, N.H. A Review on Pretreatment Methods for Lipid Extraction from Microalgae Biomass. Prep. Biochem. Biotechnol. 2024, 54, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, L.; Li, X.; Shi, J.; Scanlon, M.; Xue, S.; Nosworthy, M.; Vafaei, N. Canola Seed Protein: Pretreatment, Extraction, Structure, Physicochemical and Functional Characteristics. Foods 2024, 13, 1357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Baik, O.-D.; Choi, Y.J.; Kim, S.-M. Pretreatments for the Efficient Extraction of Bioactive Compounds from Plant-Based Biomaterials. Crit. Rev. Food Sci. Nutr. 2014, 54, 1283–1297. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhu, X.; Jambrak, A.R.; Sun, D.-W.; Tiwari, B.K. Mechanistic and Synergistic Aspects of Ultrasonics and Hydrodynamic Cavitation for Food Processing. Crit. Rev. Food Sci. Nutr. 2023, 64, 8587–8608. [Google Scholar] [CrossRef]
- Panda, D.; Manickam, S. Cavitation Technology—The Future of Greener Extraction Method: A Review on the Extraction of Natural Products and Process Intensification Mechanism and Perspectives. Appl. Sci. 2019, 9, 766. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A Comprehensive Review of Ultrasonic Assisted Extraction (UAE) for Bioactive Components: Principles, Advantages, Equipment, and Combined Technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Yuan, J.; Li, H.; Tao, W.; Han, Q.; Dong, H.; Zhang, J.; Jing, Y.; Wang, Y.; Xiong, Q.; Xu, T. An Effective Method for Extracting Anthocyanins from Blueberry Based on Freeze-Ultrasonic Thawing Technology. Ultrason. Sonochem. 2020, 68, 105192. [Google Scholar] [CrossRef]
- Olivares-Galván, S.; Marina, M.L.; García, M.C. Extraction of Valuable Compounds from Brewing Residues: Malt Rootlets, Spent Hops, and Spent Yeast. Trends Food Sci. Technol. 2022, 127, 181–197. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Carbone, K.; Macchioni, V.; Petrella, G.; Cicero, D.O. Exploring the Potential of Microwaves and Ultrasounds in the Green Extraction of Bioactive Compounds from Humulus lupulus for the Food and Pharmaceutical Industry. Ind. Crops Prod. 2020, 156, 112888. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Feng, C.; Liu, X.; Qin, F.; Liang, C.; Bian, H.; Qin, C.; Yao, S. Green, Efficient Extraction of Bamboo Hemicellulose Using Freeze-Thaw Assisted Alkali Treatment. Bioresour. Technol. 2021, 333, 125107. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.; Bao, G.; Qian, H.; Song, Z.; Qi, Z.; Zhang, M.; Chen, W.; Dong, W. Physiological Response Characteristics in Medicago Sativa Under Freeze-Thaw and Deicing Salt Stress. Water. Air. Soil Pollut. 2018, 229, 196. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, W.; Chen, Z.; Wu, T. Freeze-Thaw Induced Gelation of Alginates. Carbohydr. Polym. 2016, 148, 45–51. [Google Scholar] [CrossRef]
- Ando, Y.; Hagiwara, S.; Nabetani, H.; Okunishi, T.; Okadome, H. Impact of Ice Crystal Development on Electrical Impedance Characteristics and Mechanical Property of Green Asparagus Stems. J. Food Eng. 2019, 256, 46–52. [Google Scholar] [CrossRef]
- Liu, M.Q.; Yang, X.Q.; Qi, B.; Li, L.H.; Deng, J.C.; Hu, X. Study of Ultrasonic-Freeze-Thaw-Cycle Assisted Extraction of Polysaccharide and Phycobiliprotein from Gracilaria lemaneiformis. Adv. Mater. Res. 2013, 781–784, 1818–1824. [Google Scholar] [CrossRef]
- Wang, X.M.; Wang, L.J.; Yu, M.; Chen, H. Freeze-Thaw and Sulfuric Acid Pretreatment of Wheat Straw for Fermentable Sugar Release. Adv. Mater. Res. 2013, 724–725, 257–260. [Google Scholar] [CrossRef]
- Li, B.; Sun, D.-W. Novel Methods for Rapid Freezing and Thawing of Foods—A Review. J. Food Eng. 2002, 54, 175–182. [Google Scholar] [CrossRef]
- Parthiba Karthikeyan, O.; Trably, E.; Mehariya, S.; Bernet, N.; Wong, J.W.C.; Carrere, H. Pretreatment of Food Waste for Methane and Hydrogen Recovery: A Review. Bioresour. Technol. 2018, 249, 1025–1039. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Liu, Y.; Zhang, H. Effects of Multiple Freeze–Thaw Cycles on the Quality of Frozen Dough. Cereal Chem. 2018, 95, 499–507. [Google Scholar] [CrossRef]
- Cao, L.; Zhu, J.; Deng, B.; Zeng, F.; Wang, S.; Ma, Y.; Qin, C.; Yao, S. Efficient Swelling and Mercerization of Bagasse Fiber by Freeze-Thaw-Assisted Alkali Treatment. Front. Energy Res. 2022, 10, 851543. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Rahman, M.S.; Kim, A.-N.; Son, Y.; Gu, S.; Lee, M.-H.; Kim, J.I.; Ha, T.J.; Kwak, D.; Kim, H.-J.; et al. Effect of Freeze-Thaw Pretreatment on Yield and Quality of Perilla Seed Oil. LWT 2020, 122, 109026. [Google Scholar] [CrossRef]
- Tan, H.T.; Khong, N.M.H.; Khaw, Y.S.; Ahmad, S.A.; Yusoff, F.M. Optimization of the Freezing-Thawing Method for Extracting Phycobiliproteins from Arthrospira sp. Molecules 2020, 25, 3894. [Google Scholar] [CrossRef]
- Sulistiawati, E.; Rochmadi, R.; Hidayat, M.; Budiman, A. Enhancement of Phycocyanin Extraction from Dry Spirulina Platensis Powder by Freezing-Thawing Pre-Treatment. Int. J. Technol. 2023, 14, 780. [Google Scholar] [CrossRef]
- Wang, B.; Bai, X.; Du, X.; Pan, N.; Shi, S.; Xia, X. Comparison of Effects from Ultrasound Thawing, Vacuum Thawing and Microwave Thawing on the Quality Properties and Oxidation of Porcine Longissimus Lumborum. Foods 2022, 11, 1368. [Google Scholar] [CrossRef] [PubMed]
- Holzwarth, M.; Korhummel, S.; Carle, R.; Kammerer, D.R. Evaluation of the Effects of Different Freezing and Thawing Methods on Color, Polyphenol and Ascorbic Acid Retention in Strawberries (Fragaria×ananassa Duch.). Food Res. Int. 2012, 48, 241–248. [Google Scholar] [CrossRef]
- Li, M.; Zhou, C.; Wang, B.; Zeng, S.; Mu, R.; Li, G.; Li, B.; Lv, W. Research Progress and Application of Ultrasonic- and Microwave-assisted Food Processing Technology. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3707–3731. [Google Scholar] [CrossRef]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Effects of Enzymatic Pretreatment of Seeds on the Physicochemical Properties, Bioactive Compounds, and Antioxidant Activity of Pomegranate Seed Oil. Molecules 2021, 26, 4575. [Google Scholar] [CrossRef]
- Dzięcioł, M. Influence of Enzymatic Pretreatment on Yield and Chemical Composition of Rosmarinus Officinalis Essential Oil. Pol. J. Chem. Technol. 2022, 24, 61–66. [Google Scholar] [CrossRef]
- Yang, C.; Liu, W.; Zhu, X.; Zhang, X.; Wei, Y.; Huang, J.; Yang, F.; Yang, F. Ultrasound-Assisted Enzymatic Digestion for Efficient Extraction of Proteins from Quinoa. LWT 2024, 194, 115784. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Akbarovich, S.A.; Kim, C.K.; Lee, W.Y. Effect of Combined Ultrasound-enzyme Treatment on Recovery of Phenolic Compounds, Antioxidant Capacity, and Quality of Plum (Prunus salicina L.) Juice. J. Food Process. Preserv. 2021, 45, e15074. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C.F.R. Enzyme-Assisted Extractions of Polyphenols—A Comprehensive Review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Magalhães, P.J.; Carvalho, D.O.; Cruz, J.M.; Guido, L.F.; Barros, A.A. Fundamentals and Health Benefits of Xanthohumol, a Natural Product Derived from Hops and Beer. Nat. Prod. Commun. 2009, 4, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Zołnierczyk, A.K.; Mączka, W.K.; Grabarczyk, M.; Wińska, K.; Woźniak, E.; Anioł, M. Isoxanthohumol-Biologically Active Hop Flavonoid. Fitoterapia 2015, 103, 71–82. [Google Scholar] [CrossRef]
- Hassan, N.S.; Badri, K.H. Lignin Recovery from Alkaline Hydrolysis and Glycerolysis of Oil Palm Fiber. AIP Conf. Proc. 2014, 1614, 433–438. [Google Scholar]
- Gonçalves, M.D.P.; Silveira Junior, V. Energy Consumption Reduction Strategy for Freezing of Packaged Food Products. Food Sci. Technol. 2017, 38, 341–347. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Shao, L.; Yu, C.; Yu, H.; Li, Y. Effect of Freezing/Thawing Temperature on the Viscoelastic and Nutritional Qualities of Carrots. Int. J. Food Prop. 2016, 19, 1413–1424. [Google Scholar] [CrossRef]
- Moon, J.H.; Park, J.K.; Park, B.Y.; Jeon, H.J.; Choi, G.S.; Lee, G.M. Extraction of the Outer Membrane Protein Pertactin from Bordetella Pertussis with Urea for the Production of Acellular Pertussis Vaccine. Biotechnol. Bioprocess Eng. 2024, 29, 505–512. [Google Scholar] [CrossRef]
- Motta, L.B.; Lopes, J.C.; Zanotti, R.F.; Bernardes, P.M.; Silva, J.D. Cryostorage of Sunflower Seeds. Biosci. J. 2014, 30, 312–391. [Google Scholar]
- Gebre-Egziabher, A.; Thomson, B.; Blankenagel, G. Destruction of Microorganisms During Thawing of Skim Milk. J. Food Prot. 1982, 45, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Calinescu, I.; Lavric, V.; Asofiei, I.; Gavrila, A.I.; Trifan, A.; Ighigeanu, D.; Martin, D.; Matei, C. Microwave Assisted Extraction of Polyphenols Using a Coaxial Antenna and a Cooling System. Chem. Eng. Process. Process Intensif. 2017, 122, 373–379. [Google Scholar] [CrossRef]
- Femenia, A.; García-Marín, M.; Simal, S.; Rosselló, C.; Blasco, M. Effects of Supercritical Carbon Dioxide (SC-CO2 ) Oil Extraction on the Cell Wall Composition of Almond Fruits. J. Agric. Food Chem. 2001, 49, 5828–5834. [Google Scholar] [CrossRef]
- Xu, B.; Azam, S.M.R.; Feng, M.; Wu, B.; Yan, W.; Zhou, C.; Ma, H. Application of Multi-Frequency Power Ultrasound in Selected Food Processing Using Large-Scale Reactors: A Review. Ultrason. Sonochem. 2021, 81, 105855. [Google Scholar] [CrossRef]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Brunton, N.P.; Rai, D.K. Recent Advances on Application of Ultrasound and Pulsed Electric Field Technologies in the Extraction of Bioactives from Agro-Industrial By-Products. Food Bioprocess Technol. 2018, 11, 223–241. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, J.; Wu, H.; Gou, M.; Jing, L.; Zhao, K.; Zhang, B.; Zhang, G.; Li, W. Molecular, Crystal and Physicochemical Properties of Granular Waxy Corn Starch after Repeated Freeze-Thaw Cycles at Different Freezing Temperatures. Int. J. Biol. Macromol. 2019, 133, 346–353. [Google Scholar] [CrossRef]
- Smichi, N.; Messaoudi, Y.; Moujahed, N.; Gargouri, M. Ethanol Production from Halophyte Juncus Maritimus Using Freezing and Thawing Biomass Pretreatment. Renew. Energy 2016, 85, 1357–1361. [Google Scholar] [CrossRef]
- Cai, L.; Cao, M.; Regenstein, J.; Cao, A. Recent Advances in Food Thawing Technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 953–970. [Google Scholar] [CrossRef]
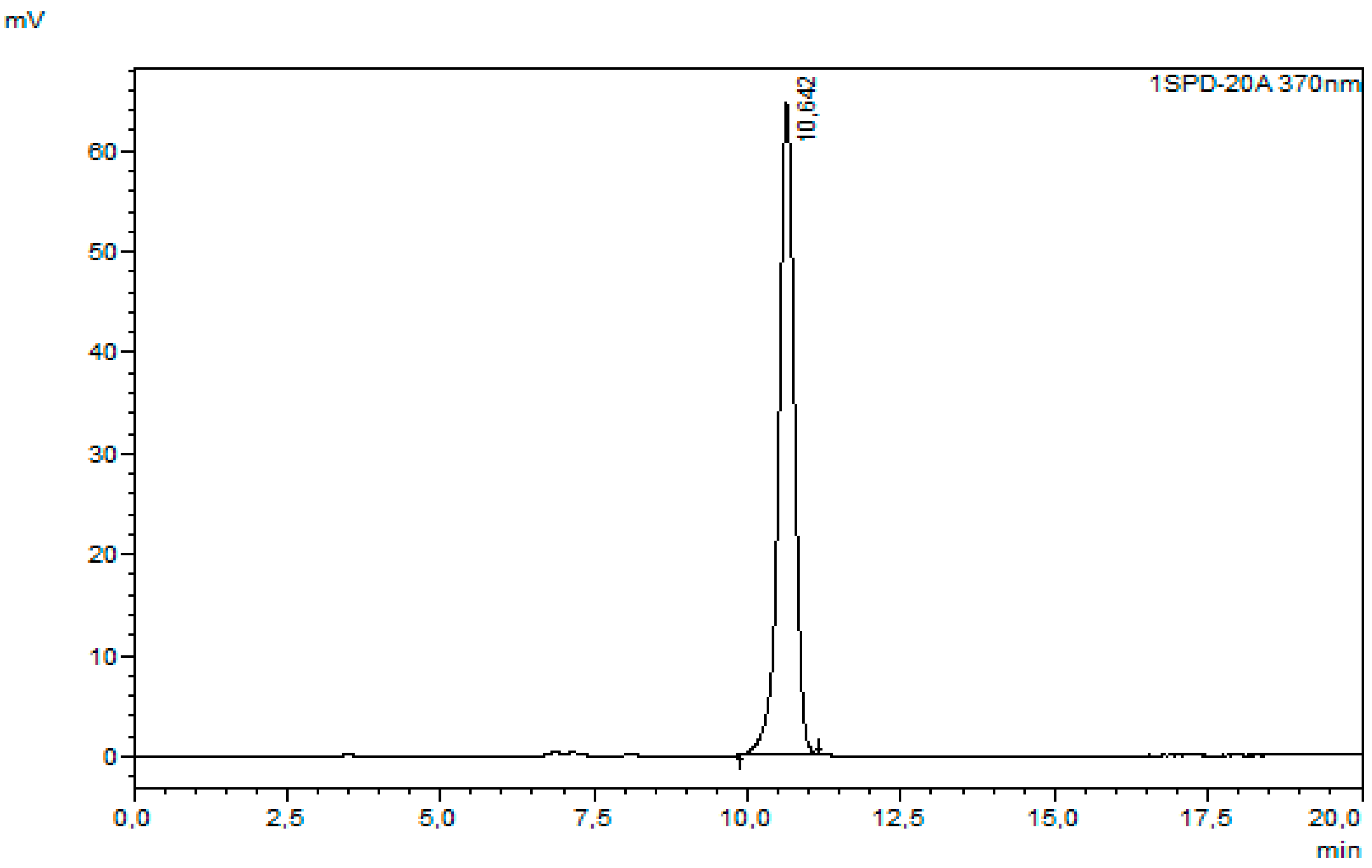
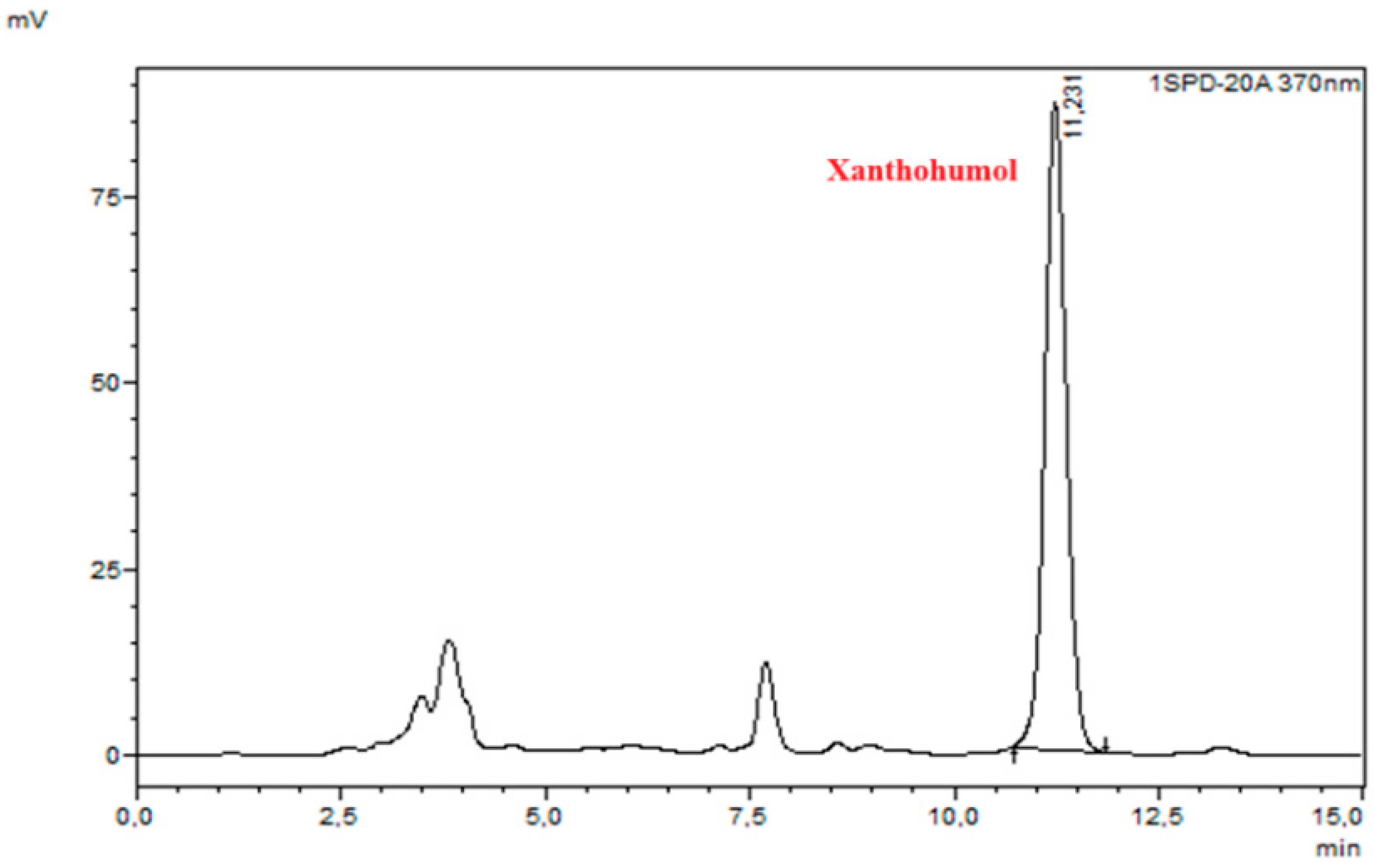
| Microwave power [W] | 50 | 100 | ||
| Treatment time [min] | 1 | 2 | 1 | 2 |
| Solvent composition: | XN extraction yield [% of control sample] | |||
| 50 mL water | 94.0 ± 0.3 | 100.1 ± 0.3 | 98.4 ± 0.5 | 95.8 ± 4.8 |
| 50 mL isopropanol | 102.9 ± 1.0 | Not performed due to solvent evaporation/mixture boiling | ||
| 50 mL water + 50 mL isopropanol | 90.6 ± 5.9 | |||
| Thawing Method | Parameters | XN Extraction Yield |
|---|---|---|
| [% of Control Sample] | ||
| Ultrasound-assisted thawing | 15 min, 35 kHz | 99.5 ± 2.2 |
| 30 min, 35 kHz | 99.2 ± 0.9 | |
| Microwave-assisted thawing | 50 W, 8 min | 86.8 ± 2.3 |
| 80 W, 4 min | 90.2 ± 6.7 | |
| 100 W, 3 min | 89.9 ± 1.7 | |
| Unassisted thawing at 25 °C | 109.02 ± 0.13 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modzelewska, A.; Jackowski, M.; Trusek, A. Physical, Chemical, and Enzymatic Pretreatment of Spent Hops and Its Impact on Xanthohumol Extraction Yield. Molecules 2025, 30, 2200. https://doi.org/10.3390/molecules30102200
Modzelewska A, Jackowski M, Trusek A. Physical, Chemical, and Enzymatic Pretreatment of Spent Hops and Its Impact on Xanthohumol Extraction Yield. Molecules. 2025; 30(10):2200. https://doi.org/10.3390/molecules30102200
Chicago/Turabian StyleModzelewska, Aleksandra, Mateusz Jackowski, and Anna Trusek. 2025. "Physical, Chemical, and Enzymatic Pretreatment of Spent Hops and Its Impact on Xanthohumol Extraction Yield" Molecules 30, no. 10: 2200. https://doi.org/10.3390/molecules30102200
APA StyleModzelewska, A., Jackowski, M., & Trusek, A. (2025). Physical, Chemical, and Enzymatic Pretreatment of Spent Hops and Its Impact on Xanthohumol Extraction Yield. Molecules, 30(10), 2200. https://doi.org/10.3390/molecules30102200






