Quality and Content of Bioactive Compounds in Muffins with Residue After Isolation of Starch from Unripe Apples (Malus domestica Borkh)
Abstract
1. Introduction
2. Results and Discussion
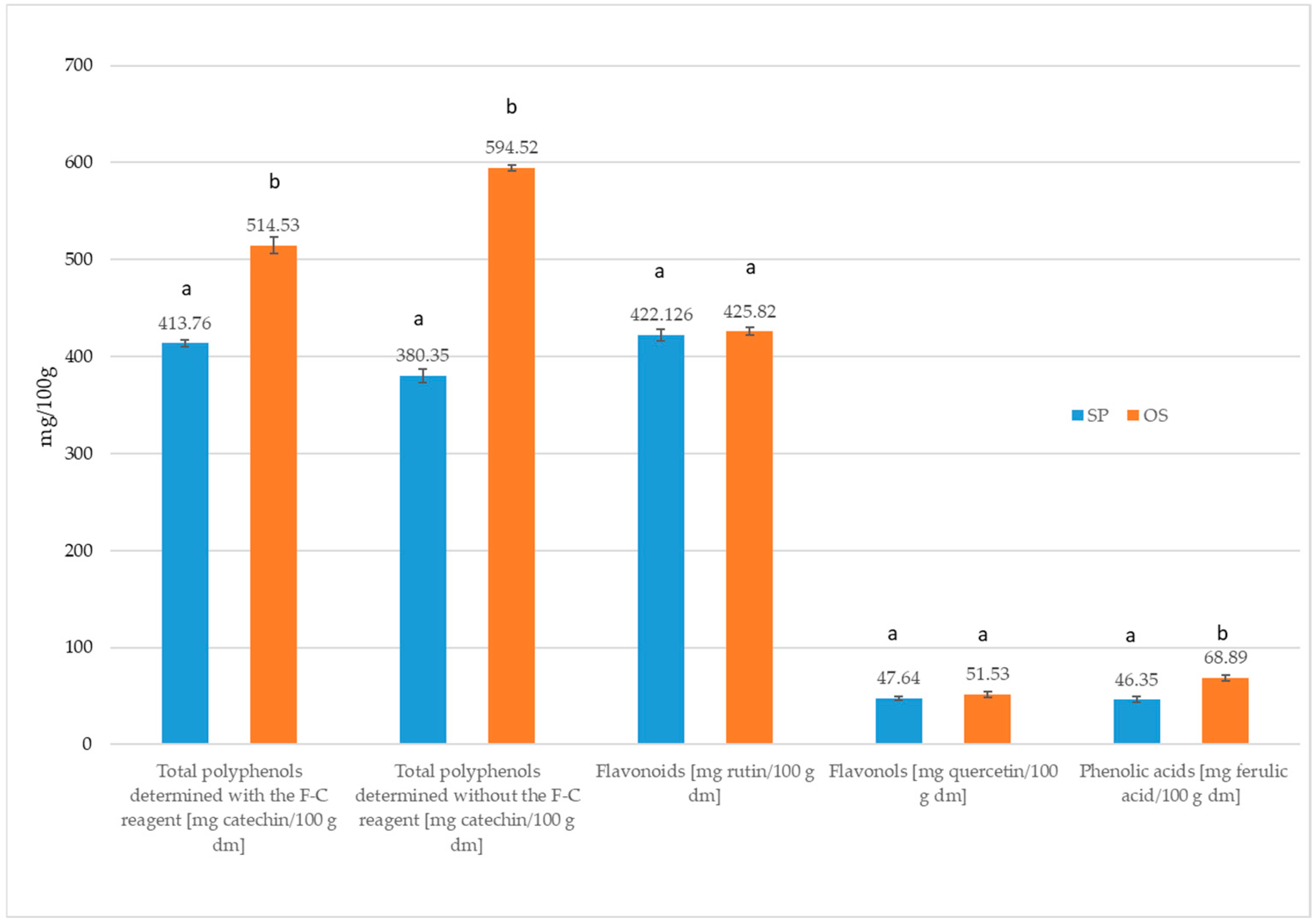
2.1. Characterization of the Polysaccharide Fraction Residue Resulting from the Isolation of Starch from Unripe Apple Varieties—Oliwka and Pyros
2.2. The Influence of Polysaccharide Fraction Residue Derived from Unripe Apple Oliwka and Pyros Varieties on the Polyphenol Contents in Muffins
2.3. The Influence of Polysaccharide Fraction Residue Derived from Unripe Apple Oliwka and Pyros Varieties on the Antioxidant Capacity of Muffins
2.4. The Influence of Polysaccharide Fraction Residue Derived from Unripe Apple Oliwka and Pyros Varieties on the Tocopherols and Phytosterols Content in Muffins
2.5. Influence of Polysaccharide Fraction Residue Derived from Unripe Apple Oliwka and Pyros Varieties on Quality Parameters of Muffins
3. Materials and Methods
3.1. Research Material
3.1.1. Reagents
3.1.2. Materials
3.1.3. Isolation of Polysaccharide Fraction Residue from Unripe Apples
3.1.4. Preparation of Muffins
3.2. Methods
3.2.1. Preparation of Extracts for Analysis of Bioactive Compounds (Polyphenols)
3.2.2. Total Phenolic Content (TPC)
3.2.3. Determination of Total Polyphenols (TPC), Phenolic Acids, and Flavonols
3.2.4. Flavonoid Content Determination
3.2.5. Antioxidant/Antiradical Activities
Determination of Antiradical Activity Using Synthetic Radical ABTS
Determination of Antiradical Activity Using Synthetic Radical DPPH
Determination of Antioxidant Activity Using Molybdenum Reducing Antioxidant Power (FOMO)
Determination of Tocopherols and Phytosterols in Food by Gas Chromatography
3.2.6. Quality Parameter Determination of Muffins
Texture Profile Analysis
Volume Measurement
Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| K | control muffins |
| 5% MSP | muffins with 5% share of polysaccharide fraction residue from Pyros variety of unripe apples |
| 10% MSP | muffins with 10% share of polysaccharide fraction residue from Pyros variety of unripe apples |
| 5% MSO | muffins with 5% share of polysaccharide fraction residue from Oliwka variety of unripe apples |
| 10% MSO | muffins with 10% share of polysaccharide fraction residue from Oliwka variety of unripe apples |
| TPC | Total phenolic content |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2-azino-bis(3-ethylobenzothiazoline-6-sulphonic acid-diamonium salt |
| FOMO | Molybdenum Reducing Antioxidant Power |
| SO | polysaccharide fraction residue from Oliwka unripe apples |
| SP | polysaccharide fraction residue from Pyros unripe apples |
References
- USDA Foreign Agricultural Service. Fresh Apples, Grapes, and Pears: World Markets and Trade. Available online: https://www.fas.usda.gov/data/fresh-apples-grapes-and-pears-world-markets-and-trade-12122024 (accessed on 6 March 2025).
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple Pomace as a Functional and Healthy Ingredient in Food Products: A Review. Processes 2020, 8, 319. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Olšteine, A.; Krasnova, I.; Pugajeva, I.; Lācis, G.; Siger, A.; Michalak, M.; Soliven, A.; Segliņa, D. Phenolic Compounds in Different Fruit Parts of Crab Apple: Dihydrochalcones as Promising Quality Markers of Industrial Apple Pomace by-Products. Ind. Crops Prod. 2015, 74, 607–612. [Google Scholar] [CrossRef]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple Pomace as Food Fortification Ingredient: A Systematic Review and Meta-Analysis. J. Food Sci. 2020, 85, 2977–2985. [Google Scholar] [CrossRef]
- Arnold, M.; Gramza-Michalowska, A. Recent Development on the Chemical Composition and Phenolic Extraction Methods of Apple (Malus domestica)—A Review. Food Bioprocess Technol. 2024, 17, 2519–2560. [Google Scholar] [CrossRef]
- Kauser, S.; Murtaza, M.A.; Hussain, A.; Imran, M.; Kabir, K.; Najam, A.; An, Q.U.; Akram, S.; Fatima, H.; Batool, S.A.; et al. Apple Pomace, a Bioresource of Functional and Nutritional Components with Potential of Utilization in Different Food Formulations: A Review. Food Chem. Adv. 2024, 4, 100598. [Google Scholar] [CrossRef]
- Shalini, R.; Gupta, D.K. Utilization of Pomace from Apple Processing Industries: A Review. J. Food Sci. Technol. 2010, 47, 365–371. [Google Scholar] [CrossRef]
- Bhushan, S.; Kalia, K.; Sharma, M.; Singh, B.; Ahuja, P.S. Processing of Apple Pomace for Bioactive Molecules. Crit. Rev. Biotechnol. 2008, 28, 285–296. [Google Scholar] [CrossRef]
- Waldbauer, K.; McKinnon, R.; Kopp, B. Apple Pomace as Potential Source of Natural Active Compounds. Planta Med. 2017, 83, 994–1010. [Google Scholar] [CrossRef]
- Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://www.unfpa.org/resources/transforming-our-world-2030-agenda-sustainable-development (accessed on 19 December 2022).
- Balasuriya, N.; Rupasinghe, H.P.V. Antihypertensive Properties of Flavonoid-Rich Apple Peel Extract. Food Chem. 2012, 135, 2320–2325. [Google Scholar] [CrossRef]
- Makarova, E.; Górnaś, P.; Konrade, I.; Tirzite, D.; Cirule, H.; Gulbe, A.; Pugajeva, I.; Seglina, D.; Dambrova, M. Acute Anti-Hyperglycaemic Effects of an Unripe Apple Preparation Containing Phlorizin in Healthy Volunteers: A Preliminary Study. J. Sci. Food Agric. 2015, 95, 560–568. [Google Scholar] [CrossRef]
- Rodríguez-Muela, C.; Rodríguez, H.E.; Arzola, C.; Díaz-Plascencia, D.; Ramírez-Godínez, J.A.; Flores-Mariñelarena, A.; Mancillas-Flores, P.F.; Corral, G. Antioxidant Activity in Plasma and Rumen Papillae Development in Lambs Fed Fermented Apple Pomace. J. Anim. Sci. 2015, 93, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.-M.; Tou, J.C. A Comprehensive Analysis of the Composition, Health Benefits, and Safety of Apple Pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Skinner, T.; Bridges, B.; Weber, J.T. The Pathology of Parkinson’s Disease and Potential Benefit of Dietary Polyphenols. Molecules 2020, 25, 4382. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.S.; Kaur, S.; Brar, S.K. Perspective of Apple Processing Wastes as Low-Cost Substrates for Bioproduction of High Value Products: A Review. Renew. Sustain. Energy Rev. 2013, 27, 789–805. [Google Scholar] [CrossRef]
- Macagnan, F.T.; Santos, L.R.d.; Roberto, B.S.; de Moura, F.A.; Bizzani, M.; da Silva, L.P. Biological Properties of Apple Pomace, Orange Bagasse and Passion Fruit Peel as Alternative Sources of Dietary Fibre. Bioact. Carbohydr. Diet. Fibre 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Richel, A. Fractionation of Apple By-Products as Source of New Ingredients: Current Situation and Perspectives. Trends Food Sci. Technol. 2014, 1, 99–114. [Google Scholar] [CrossRef]
- Leyva-Corral, J.; Quintero-Ramos, A.; Camacho-Dávila, A.; de Jesús Zazueta-Morales, J.; Aguilar-Palazuelos, E.; Ruiz-Gutiérrez, M.G.; Meléndez-Pizarro, C.O.; de Jesús Ruiz-Anchondo, T. Polyphenolic Compound Stability and Antioxidant Capacity of Apple Pomace in an Extruded Cereal. LWT—Food Sci. Technol. 2016, 65, 228–236. [Google Scholar] [CrossRef]
- Kruczek, M.; Gumul, D.; Kačániová, M.; Ivanišová, E.; Mareček, J.; Gambuś, H. Industrial Apple Pomace By-Products as A Potential Source of Pro-Health Compounds in Functional Food. J. Microbiol. Biotechnol. Food Sci. 2017, 7, 22–26. [Google Scholar] [CrossRef]
- Faillie, J.-L. Pharmacological Aspects of the Safety of Gliflozins. Pharmacol. Res. 2017, 118, 71–81. [Google Scholar] [CrossRef]
- Olawoye, B.; Fagbohun, O.F.; Popoola-Akinola, O.; Adetola, D.B.; Gbadamosi, S.O.; Akanbi, C.T. That We May Eat and Be Healthy: A Case of Slowly Digestible Cookies from Cardaba Banana Starch. Appl. Food Res. 2023, 3, 100342. [Google Scholar] [CrossRef]
- Turksoy, S.; Özkaya, B. Pumpkin and Carrot Pomace Powders as a Source of Dietary Fiber and Their Effects on the Mixing Properties of Wheat Flour Dough and Cookie Quality. Food Sci. Technol. Res. 2011, 17, 545–553. [Google Scholar] [CrossRef]
- Yates, M.; Gomez, M.R.; Martin-Luengo, M.A.; Ibañez, V.Z.; Martinez Serrano, A.M. Multivalorization of Apple Pomace towards Materials and Chemicals. Waste to Wealth. J. Clean. Prod. 2017, 143, 847–853. [Google Scholar] [CrossRef]
- Curutchet, A.; Trias, J.; Tárrega, A.; Arcia, P. Consumer Response to Cake with Apple Pomace as a Sustainable Source of Fibre. Foods 2021, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Alongi, M.; Melchior, S.; Anese, M. Reducing the Glycemic Index of Short Dough Biscuits by Using Apple Pomace as a Functional Ingredient. LWT—Food Sci. Technol. 2019, 100, 300–305. [Google Scholar] [CrossRef]
- Masooi, F.A.; Sharma, B.; Chauhan, G.S. Use of Apple Pomace as a Source of Dietary Fiber in Cakes. Plant Foods Hum. Nutr. 2002, 57, 121–128. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Wang, L.; Huber, G.M.; Pitts, N.L. Effect of Baking on Dietary Fibre and Phenolics of Muffins Incorporated with Apple Skin Powder. Food Chem. 2008, 107, 1217–1224. [Google Scholar] [CrossRef]
- Gumul, D.; Korus, J.; Orczykowska, M.; Rosicka-Kaczmarek, J.; Oracz, J.; Areczuk, A. Starch from Unripe Apples (Malus domestica Borkh) as an Alternative for Application in the Food Industry. Molecules 2024, 29, 1707. [Google Scholar] [CrossRef]
- Burda, S.; Oleszek, W.; Lee, C.Y. Phenolic Compounds and Their Changes in Apples During Maturation and Cold Storage. J. Agric. Food Chem. 1990, 38, 945–948. [Google Scholar] [CrossRef]
- Akiyama, H.; Sato, Y.; Watanabe, T.; Nagaoka, M.H.; Yoshioka, Y.; Shoji, T.; Kanda, T.; Yamada, K.; Totsuka, M.; Teshima, R.; et al. Dietary Unripe Apple Polyphenol Inhibits the Development of Food Allergies in Murine Models. FEBS Lett. 2005, 579, 4485–4491. [Google Scholar] [CrossRef]
- BeMiller, J.N.; Whistler, R.L. Starch Chemistry and Technology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 978-0-12-746275-2. [Google Scholar]
- Candrawinata, V.I.; Golding, J.B.; Roach, P.D.; Stathopoulos, C.E. Optimisation of the Phenolic Content and Antioxidant Activity of Apple Pomace Aqueous Extracts. CyTA-J. Food 2015, 13, 293–299. [Google Scholar] [CrossRef]
- Adil, İ.H.; Çetin, H.İ.; Yener, M.E.; Bayındırlı, A. Subcritical (Carbon Dioxide + Ethanol) Extraction of Polyphenols from Apple and Peach Pomaces, and Determination of the Antioxidant Activities of the Extracts. J. Supercrit. Fluids 2007, 43, 55–63. [Google Scholar] [CrossRef]
- Bai, X.-L.; Yue, T.-L.; Yuan, Y.-H.; Zhang, H.-W. Optimization of Microwave-Assisted Extraction of Polyphenols from Apple Pomace Using Response Surface Methodology and HPLC Analysis. J. Sep. Sci. 2010, 33, 3751–3758. [Google Scholar] [CrossRef] [PubMed]
- Persic, M.; Mikulic-Petkovsek, M.; Slatnar, A.; Veberic, R. Chemical Composition of Apple Fruit, Juice and Pomace and the Correlation Between Phenolic Content, Enzymatic Activity and Browning. LWT—Food Sci. Technol. 2017, 82, 23–31. [Google Scholar] [CrossRef]
- Gumul, D.; Ziobro, R.; Korus, J.; Kruczek, M. Apple Pomace as a Source of Bioactive Polyphenol Compounds in Gluten-Free Breads. Antioxidants 2021, 10, 807. [Google Scholar] [CrossRef]
- Mureșan, A.E.; Man, S.; Socaci, S.A.; Pușcaș, A.; Tanislav, A.E.; Pall, E.; Mureșan, V.; Cerbu, C.G. Functionality of Muffins Fortified with Apple Pomace: Nutritional, Textural, and Sensory Aspects. Appl. Sci. 2024, 14, 6439. [Google Scholar] [CrossRef]
- Ćetković, G.; Čanadanović-Brunet, J.; Djilas, S.; Savatović, S.; Mandić, A.; Tumbas, V. Assessment of Polyphenolic Content and In Vitro Antiradical Characteristics of Apple Pomace. Food Chem. 2008, 2, 340–347. [Google Scholar] [CrossRef]
- Grajek, W. Przeciwutleniacze w Żywności—Aspekty Zdrowotne, Technologiczne, Molekularne i Analityczne, 1st ed.; Wydawnictwo Naukowo-Techniczne: Warsaw, Poland, 2007; ISBN 83-204-3277-0. [Google Scholar]
- Woźniak, Ł.; Szakiel, A.; Pączkowski, C.; Marszałek, K.; Skąpska, S.; Kowalska, H.; Jędrzejczak, R. Extraction of Triterpenic Acids and Phytosterols from Apple Pomace with Supercritical Carbon Dioxide: Impact of Process Parameters, Modelling of Kinetics, and Scaling-Up Study. Molecules 2018, 23, 2790. [Google Scholar] [CrossRef]
- Radenkovs, V.; Kviesis, J.; Juhnevica-Radenkova, K.; Valdovska, A.; Püssa, T.; Klavins, M.; Drudze, I. Valorization of Wild Apple (Malus spp.) By-Products as a Source of Essential Fatty Acids, Tocopherols and Phytosterols with Antimicrobial Activity. Plants 2018, 7, 90. [Google Scholar] [CrossRef]
- Buchner, N.; Krumbein, A.; Rohn, S.; Kroh, L.W. Effect of Thermal Processing on the Flavonols Rutin and Quercetin. Rapid Commun. Mass Spectrom. 2006, 20, 3229–3235. [Google Scholar] [CrossRef]
- Barnes, J.S.; Foss, F.W., Jr.; Schug, K.A. Thermally Accelerated Oxidative Degradation of Quercetin Using Continuous Flow Kinetic Electrospray-Ion Trap-Time of Flight Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2013, 24, 1513–1522. [Google Scholar] [CrossRef]
- Ajila, C.M.; Leelavathi, K.; Rao, U.J.S.P. Improvement of Dietary Fiber Content and Antioxidant Properties in Soft Dough Biscuits with the Incorporation of Mango Peel Powder. J. Cereal Sci. 2008, 2, 319–326. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, M.; Kaur, H. Apple Peel as a Source of Dietary Fiber and Antioxidants: Effect on Batter Rheology and Nutritional Composition, Textural and Sensory Quality Attributes of Muffins. Food Meas. 2022, 16, 2411–2421. [Google Scholar] [CrossRef]
- Acun, S.; Gül, H. Effects of Grape Pomace and Grape Seed Flours on Cookie Quality. Qual. Assur. Saf. Crops Foods 2014, 6, 81–88. [Google Scholar] [CrossRef]
- Ojha, P.; Thapa, S. Quality Evaluation of Biscuit Incorporated with Mandarin Peel Powder. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2017, 18, 19–30. [Google Scholar]
- Caponio, G.R.; Miolla, R.; Vacca, M.; Difonzo, G.; De Angelis, M. Wine Lees as Functional Ingredient to Produce Biscuits Fortified with Polyphenols and Dietary Fibre. LWT—Food Sci. Technol. 2024, 198, 115943. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive Value and Chemical Composition of Pseudocereals as Gluten-Free Ingredients. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. S4), 240–257. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Quek, S.; Perera, C.O. Properties of Bread Dough with Added Fiber Polysaccharides and Phenolic Antioxidants: A Review. J. Food Sci. 2010, 75, R163–R174. [Google Scholar] [CrossRef]
- Şensoy, Í.; Rosen, R.T.; Ho, C.-T.; Karwe, M.V. Effect of Processing on Buckwheat Phenolics and Antioxidant Activity. Food Chem. 2006, 99, 388–393. [Google Scholar] [CrossRef]
- Maillard, M.-N.; Berset, C. Evolution of Antioxidant Activity During Kilning: Role of Insoluble Bound Phenolic Acids of Barley and Malt. J. Agric. Food Chem. 1995, 43, 1789–1793. [Google Scholar] [CrossRef]
- Renard, C.M.; Baron, A.; Guyot, S.; Drilleau, J.F. Interactions Between Apple Cell Walls and Native Apple Polyphenols: Quantification and Some Consequences. Int. J. Biol. Macromol. 2001, 29, 115–125. [Google Scholar] [CrossRef]
- Arnao, M.B. Some Methodological Problems in the Determination of Antioxidant Activity Using Chromogen Radicals: A Practical Case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Ampomah-Dwamena, C.; Bhargava, N.; Tomes, S.; Lin-Wang, K.; Elborough, C.; Deng, C.H.; Rebstock, R. Elevating Fruit Carotenoid Content in Apple (Malus x domestica Borkh). Methods Enzymol. 2022, 671, 63–98. [Google Scholar] [CrossRef] [PubMed]
- Vita, J.A. Polyphenols and Cardiovascular Disease: Effects on Endothelial and Platelet Function. Am. J. Clin. Nutr. 2005, 81, 292S–297S. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and Beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, B.M.; de Ancos, B.; Sánchez-Moreno, C.; Fernández-Ruiz, V.; de Cortes Sánchez-Mata, M.; Cámara, M.; Tardío, J. Wild Blackthorn (Prunus spinosa L.) and Hawthorn (Crataegus monogyna Jacq.) Fruits as Valuable Sources of Antioxidants. Fruits 2014, 69, 61–73. [Google Scholar] [CrossRef]
- von Gadow, A.; Joubert, E.; Hansmann, C.F. Comparison of the Antioxidant Activity of Aspalathin with That of Other Plant Phenols of Rooibos Tea (Aspalathus linearis), α-Tocopherol, BHT, and BHA. J. Agric. Food Chem. 1997, 45, 632–638. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Parpinel, M. Influence of Processing on the Antioxidant Properties of Fruit and Vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Sakač, M.; Torbica, A.; Sedej, I.; Hadnađev, M. Influence of Breadmaking on Antioxidant Capacity of Gluten Free Breads Based on Rice and Buckwheat Flours. Food Res. Int. 2011, 9, 2806–2813. [Google Scholar] [CrossRef]
- Lindenmeier, M.; Hofmann, T. Influence of Baking Conditions and Precursor Supplementation on the Amounts of the Antioxidant Pronyl-L-Lysine in Bakery Products. J. Agric. Food Chem. 2004, 52, 350–354. [Google Scholar] [CrossRef]
- Mala, T.; Piayura, S.; Itthivadhanapong, P. Characterization of Dried Pineapple (Ananas comosus L.) Peel. Powder and Its Application as a Novel Functional Food Ingredient in Cracker Product. Future Foods 2024, 9, 100322. [Google Scholar] [CrossRef]
- Piironen, V.; Varo, P.; Koivistoinen, P. Stability of Tocopherols and Tocotrienols in Food Preparation Procedures. J. Food Compos. Anal. 1987, 1, 53–58. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr. Metab. Insights 2016, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Brandolini, A. Tocols Stability During Bread, Water Biscuit and Pasta Processing from Wheat Flours. J. Cereal Sci. 2010, 52, 254–259. [Google Scholar] [CrossRef]
- Leenhardt, F.; Lyan, B.; Rock, E.; Boussard, A.; Potus, J.; Chanliaud, E.; Remesy, C. Wheat Lipoxygenase Activity Induces Greater Loss of Carotenoids Than Vitamin E During Breadmaking. J. Agric. Food Chem. 2006, 54, 1710–1715. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Bajerska, J.; Górnaś, P.; Segliņa, D.; Pilarska, A.; Jesionowski, T. Physical and Bioactive Properties of Muffins Enriched with Raspberry and Cranberry Pomace Powder: A Promising Application of Fruit By-Products Rich in Biocompounds. Plant Foods Hum. Nutr. 2016, 71, 165–173. [Google Scholar] [CrossRef]
- Giri, N.A.; Gaikwad, P.; Gaikwad, N.N.; Manjunatha, N.; Krishnakumar, T.; Kad, V.; Raigond, P.; Suryavanshi, S.; Marathe, R.A. Development of Fiber-Enriched Muffins Using Pomegranate Peel Powder and Its Effect on Physico-Chemical Properties and Shelf Life of the Muffins. J. Sci. Food Agric. 2024, 104, 2346–2358. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Mazza, G.; Fukumoto, L.; Delaquis, P.; Girard, B.; Ewert, B. Anthocyanins, Phenolics, and Color of Cabernet Franc, Merlot, and Pinot Noir Wines from British Columbia. J. Agric. Food Chem. 1999, 47, 4009–4017. [Google Scholar] [CrossRef]
- Oomah, B.D.; Cardador-Martínez, A.; Loarca-Piña, G. Phenolics and Antioxidative Activities in Common Beans (Phaseolus vulgaris L). J. Sci. Food Agric. 2005, 85, 935–942. [Google Scholar] [CrossRef]
- El Hariri, B.; Sallé, G.; Andary, C. Involvement of Flavonoids in the Resistance of Two Poplar Cultivars to Mistletoe (Viscum album L.). Protoplasma 1991, 162, 20–26. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Jabeen, Z.; Li, Y.; Chen, M.; Li, Z.; Guo, W.; Shamsi, I.H.; Chen, X.; Jiang, L. Detection of Tocopherol in Oilseed Rape (Brassica napus L.) Using Gas Chromatography with Flame Ionization Detector. J. Integr. Agric. 2013, 12, 803–814. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Effect of Roasting Conditions on the Fat, Tocopherol, and Phytosterol Content and Antioxidant Capacity of the Lipid Fraction from Cocoa Beans of Different Theobroma cacao L. Cultivars. Eur. J. Lipid Sci. Technol. 2014, 116, 1002–1014. [Google Scholar] [CrossRef]
- Zhang, R.; Shen, W.; Wei, X.; Zhang, F.; Shen, C.; Wu, B.; Zhao, Z.; Liu, H.; Deng, X. Simultaneous Determination of Tocopherols and Tocotrienols in Vegetable Oils by GC-MS. Anal. Methods 2016, 8, 7341–7346. [Google Scholar] [CrossRef]

| Sample | α-Tocopherol | β-Tocopherol | γ-Tocopherol | δ-Tocopherol | Campesterol | Cholesterol | Stigma-Sterol | Beta- Sitosterol | Cleosterol |
|---|---|---|---|---|---|---|---|---|---|
| [mg/100 g d.m.] | |||||||||
| SP | 1.62 ± 0.1 B | n.d. | 3.05 ± 0.05 B | 0.9 ± 0.08 A | 100.54 ±1.34 A | 2.3 ± 0.2 A | 2.32 ± 0.3 A | 297 ± 0.29 B | 2.0 ± 0 A |
| SO | 0.78 ± 0 A | n.d. | 2.73 ± 0.02 A | 1.7 ± 0 B | 111.28 ± 3.27 B | 2.2 ± 0 A | 2.3 ± 0.3 A | 259 ± 0.72 A | 2.1 ± 0 B |
| Scheme 100 | Total Polyphenols with F-C Reagent (Catechin Equivalent) | Total Polyphenols Without F-C Reagent (Catechin Equivalent) | Flavonoids (Rutin Equivalent) | Phenolic Acids (Ferulic Acid Equivalent) | Flavonols (Quercetin Equivalent) |
|---|---|---|---|---|---|
| [mg/100 g d.m.] | |||||
| K | 50.15 ± 0.00 A | 64.86 ± 1.82 A | 13.39 ± 1.76 A | 1.34 ± 0.73 A | 0.88 ± 0.87 A |
| 5% MSP | 67.66 ± 0.00 B | 75.22 ± 14.66 B | 23.82 ± 0.49 B | 3.74 ± 0.72 B | 1.68 ± 0.28 B |
| 10% MSP | 68.75 ± 0.09 C | 82.13 ± 3.45 C | 26.63 ± 1.69 C | 4.89 ± 0.28 C | 3.24 ± 0.41 C |
| 5% MSO | 71.31 ± 0.63 D | 87.31 ± 1.17 D | 27.2 ± 2.43 C | 5.17 ± 0.32 D | 1.74 ± 0.77 B |
| 10% MSO | 92.09 ± 0.63 E | 118.40 ± 18.17 E | 30.86 ± 1.14 C | 6.67 ± 0.28 E | 3.24 ± 0.96 C |
| Sample | ABTS [mg TX/g d.m.] | DPPH [mg TX/g d.m.] | FOMO [mg TX/g d.m.] |
|---|---|---|---|
| K | 4.95 ± 0.19 A | 1.99 ± 0.00 A | 10.82 ± 1.12 A |
| 5% MSP | 6.35 ± 0.23 B | 2.01 ± 0.10 A | 57.86 ± 059 B |
| 10% MSP | 7.17 ± 0.00 C | 2.09 ± 0.15 A | 107.03 ± 0.80 D |
| 5% MSO | 6.18 ± 0.08 B | 2.21 ± 0.00 B | 80.45 ± 1.10 C |
| 10% MSO | 7.55 ± 0.07 C | 2.02 ± 0.07 A | 141.66 ± 1.52 E |
| Sample | Tocopherols | |||
|---|---|---|---|---|
| [mg/100 g d.m.] | ||||
| α-tocopherol | β-tocopherol | γ-tocopherol | δ-tocopherol | |
| K | 34.44 ± 0.28 C,* | 5.99 ± 0.14 C | 2.29 ± 0.1 A | 3.57 ± 0.23 C |
| 5% MSO | 19.87 ± 0.89 A | 2.15 ± 0.31 A | 2.4 ± 0.15 A | 0.97 ± 0.11 A |
| 10% MSO | 20.58 ± 1.21 B | 3.3 ± 0.57 B | 3.89 ± 0.13 B | 1.89 ± 0.13 B |
| Phytosterols | ||||
| [mg/100 g d.m.] | ||||
| Campesterol | Stigmasterol | β-sitosterol | Cleosterol | |
| K | 0.79 ± 0.15 A | 1.35 ± 0.04 B | 0.65 ± 0.2 A | 18.99 ± 0.11 A |
| 5% MSO | 16.88 ± 0.21 C | 0.64 ± 0.25 A | - | 30.82 ± 0.53 C |
| 10% MSO | 9.24 ± 0.34 B | 0.86 ± 0.17 A | - | 21.53 ± 0.74 B |
| Sample | Hardness [N] | Volume [mL] | Specific Volume [mL/g] | Volume per 100 g Flour [mL] |
|---|---|---|---|---|
| K | 2.18 ± 0.25 A | 71.37 ± 1.31 C | 1.85 ± 0.05 A | 142.73 ± 2.63 A |
| 5% MSP | 2.49 ± 0.36 A | 70.88 ± 1.52 C | 1.82 ± 0.01 A | 141.77 ± 3.04 A |
| 10% MSP | 2.37 ± 0.29 A | 63.06 ± 5.48 B | 1.80 ± 0.03 A | 126.11 ± 10.95 A |
| 5% MSP | 2.36 ± 0.12 A | 68.68 ± 1.61 B | 1.84 ± 0.01 A | 137.36 ± 3.22 A |
| 10% MSO | 2.34 ± 0.04 A | 59.96 ± 3.22 A | 1.75 ± 0.07 A | 119.92 ± 6.44 A |
| Ingredients: | Standard | 5% Polysaccharide Fraction Residue | 10% Polysaccharide Fraction Residue |
|---|---|---|---|
| [g] | |||
| Flour | 150 | 142.5 | 135 |
| Baking powder | 5 | 5 | 5 |
| Crystal sugar | 75 | 75 | 75 |
| Salt | 5 | 5 | 5 |
| Lemon peel | 13.5 | 13.5 | 13.5 |
| Vanilla sugar | 8 | 8 | 8 |
| Natural yogurt | 125 | 125 | 125 |
| Whole egg | 1 | 1 | 1 |
| Grape seed oil | 62.5 | 62.5 | 62.5 |
| Apple polysaccharides fraction residue | - | 7.5 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumul, D.; Kowalski, S.; Mikulec, A. Quality and Content of Bioactive Compounds in Muffins with Residue After Isolation of Starch from Unripe Apples (Malus domestica Borkh). Molecules 2025, 30, 2189. https://doi.org/10.3390/molecules30102189
Gumul D, Kowalski S, Mikulec A. Quality and Content of Bioactive Compounds in Muffins with Residue After Isolation of Starch from Unripe Apples (Malus domestica Borkh). Molecules. 2025; 30(10):2189. https://doi.org/10.3390/molecules30102189
Chicago/Turabian StyleGumul, Dorota, Stanisław Kowalski, and Anna Mikulec. 2025. "Quality and Content of Bioactive Compounds in Muffins with Residue After Isolation of Starch from Unripe Apples (Malus domestica Borkh)" Molecules 30, no. 10: 2189. https://doi.org/10.3390/molecules30102189
APA StyleGumul, D., Kowalski, S., & Mikulec, A. (2025). Quality and Content of Bioactive Compounds in Muffins with Residue After Isolation of Starch from Unripe Apples (Malus domestica Borkh). Molecules, 30(10), 2189. https://doi.org/10.3390/molecules30102189









